Abstract
Background
Sudden unexplained nocturnal death syndrome (SUNDS) has been reported worldwide. SUNDS is endemic in Southeast Asia and is colloquially known as Bangungut in the Philippines, Lai Tai in Thailand, and Pokkuri in Japan. Although SUNDS in Thailand and Japan have been determined to be phenotypically, genetically and functionally identical to the Brugada syndrome, the relationship between Bangungut/SUNDS in the Philippines and the Brugada syndrome has not been clarified. This paper explores the concordance between Bangungut/SUNDS and the Brugada syndrome.
Methods
We summarized autopsy studies on Bangungut retrieved from PubMed since 1917 and current epidemiological data on Philippine SUNDS to clarify its diagnostic features. We also reviewed current hypotheses of the pathophysiological mechanism of the Brugada syndrome to explore its applicability to Bangungut/SUNDS.
Results
The use of the term Bangungut is confusing as it includes many diseases that may cause SUNDS. However, our review reveals a notable subset of Bangungut, identified as Bangungut/SUNDS with no gross cardiac pathology that conforms to the clinical picture of the folk-belief of Bangungut and of the Brugada syndrome, namely: predominance among male in the 20-40 age range; sudden death during sleep or at rest, usually following ingestion of a large meal at night; and victims were in apparent good health prior to their demise. Current pathophysiological mechanisms of Brugada syndrome seemed plausible explanations for a majority of this subset of Bangungut/SUNDS.
Conclusion
Bangungut/SUNDS and the Brugada syndrome appear closely related. Pathophysiological mechanisms of the Brugada syndrome may explain the enigma of Bangungut/SUND. Whether Bangungut/SUNDS is phenotypically, genetically and functionally an allele of the Brugada syndrome remains inconclusive due to lack of research data. We therefore proposed a research agenda including genetic testing and pharmacological challenge of probands and their family members suspected of SUNDS to conclusively establish the relationship between these two syndromes.
Keywords: SUNDS, Bangungut, Brugada syndrome, the Philippine, Prevalence, Pathophysiology, Research Agenda
Introduction
Sudden unexplained nocturnal death syndrome (SUNDS) has been reported worldwide.1 In the Philippines, SUNDS, colloquially known as Bangungut (Ban-gun-gut) has been reported as early as 1917.2 Typically, the victims are male adults (average age 33 years old), who are in apparent good health, and after eating a heavy meal at night, would wake up in the middle of the night, with symptoms of rising (“bangun”) and/or moaning (“ungul”). If not awakened from sleep, the individual is found dead.3 Bangungut appears to primarily afflict Filipinos and Filipino emigrants to Hawaii, the Pacific Islands and the United States.4,5 A 2007 report of a Philippine National Nutrition and Health survey showed a prevalence of 43/100,000 persons/year dying from SUNDS6 which extrapolates to 39,163 persons/year based on the 2008 Philippine census figure of 91,077,287 (2008 CIA World Fact book: July 2007 est.). Of these, 11,975 persons (61.3%) would be males in the 15-64 years age group. It is unclear how many of these cases of SUNDS were attributable to typical Bangungut or other disease entities such as myocardial infarction, acute pancreatitis, cardiomyopathy or cardiac channelopathy that may cause sudden death. Because death due to Bangungut primarily afflicts men in the prime of their life (average age, 33 years old), the disease is devastating. Even those who are resuscitated from SUNDS must deal with the terror and fear that it can recur again. This exacts a huge psychological and socio-economic burden on both the surviving victims and their families. Clearly, Bangungut poses a serious public health challenge in the Philippines. The search for its etiology remains elusive.
In recent years, the Brugada syndrome, a cardiac channelopathy related to the mutation of sodium channel gene SCN5A encoding for cardiac channel proteins, is increasingly recognized worldwide as a cause for SUNDS. SUNDS caused by Brugada syndrome appears to offer the intriguing possibility of unraveling the enigma of Bangungut. In the 1980s, the United States Centers for Disease Control (CDC) has reported a rise of cases of SUNDS among young Southeast Asian refugees to the US.7 SUNDS has been found to be endemic in South Asia. It is colloquially called Lai-Tai (“died during sleep”), in Thailand;8 and Pokkuri (“sudden unexpected death at night”), in Japan.9 The prevalence is 26 to 38 per 100,000 men (ages 20-49 years old) in Thailand.10,11 SUNDS in Thailand and Japan have been determined to be phenotypically, genetically, and functionally identical to the Brugada syndrome.12 However, the relation between Bangungut/SUNDS in the Philippines and Brugada syndrome has not been established. Thus, we feel timely to revisit the Bangungut/SUNDS phenomenon and explore its concordance with the Brugada syndrome. In this report, we first summarized autopsy studies on Bangungut in the Philippines retrieved from PubMed since 1971 as well as recent epidemiological studies on Philippine SUNDS to clarify Bangungut’s clinical and diagnostic features. Next, we summarized current pathophysiological mechanisms of the Brugada syndrome to determine whether they are plausible explanations for Bangungut/SUNDS. Then we infer how treatment guidelines of the Brugada syndrome could guide the treatment of Bangungut/SUNDS. Finally, we proposed a research agenda to conclusively establish the association of Bangungut / SUNDS with the Brugada syndrome.
BANGUNGUT: A REVIEW OF STUDIES
Autopsy studies
Since its first report in the Philippine Medical Journal in 1917,13 Bangungut has been the focus of study by pathologists.14,15,16 Sta. Cruz (1960) provided a summary of autopsy report of 460 cases diagnosed as “Bangungut” from 1915 to 1938 that showed a preponderance of males, ages 25-40 years who were in apparent good health and died suddenly at night, “often with a history of a heavy meal or a jovial disposition the night before.” The autopsy findings revealed usual post-mortem findings in the heart, lung, pleura, peritoneum and visceral blood vessels. Some findings of acute hemorrhagic pancreatitis were associated with a history of abdominal pain the night before the demise. The author suspected that cardiac etiology; with or without avitaminosis B1 (Beri-Beri) and nightmare may contribute to Bangungut.17
Majoska (1948) summarized 81 cases of sudden unexplained death among Filipinos in Hawaii in 1937 to 1948. Since the diagnosis of acute hemorrhagic pancreatitis was not recognized as a probable etiologic factor until March 1, 1944, Majoska divided the cohort into two groups: 51 cases in 1937-1944 and 30 cases in 1945-1948. Both groups showed a predominance of single, male Filipino, of the laborer occupation, who died in the evening after sleep. In the pre-1944 group, 28 cases were attributed to heart disease, 10 cases to acute pancreatitis, and the rest to various pulmonary and kidney diseases. The post-1944 group showed 21 cases of acute hemorrhagic pancreatitis and the rest due to various pulmonary, cardiac and other causes. Malnutrition, food poisoning, parasitic infection, hypoglycemia were ruled out through physical examination and chemical tests. Although the chief post-mortem finding is that of acute hemorrhagic pancreatitis, it is not known whether this is the cause of death or the result of post-mortem changes.18
Aponte (1960) studied 11 autopsied cases (10 Filipinos and I Guamanian) of Bangungut in Guam. The autopsy findings showed acute cardiac dilatation with blood clots, pulmonary edema with scattered subpleural areas of ecchymosis, and cerebral edema. Five cases of interstitial pancreatitis were noted. The author implicated acute cardio-pulmonary failure to Bangungut.19
Park and Weinstein (1990) conducted 14 autopsy cases of SUNDS (14 Filipinos, and I native of the Island of Yap) in the Mariana Islands in 1972 to 1989. Their findings revealed deceased were workers of low socio-economic status, most lived in barracks, and new arrival from Southeast Asia. Ages ranged from 23 to 55, (average 38 years). All were in good health before deaths. All deaths occurred at night with colleagues reported symptoms of “convulsing, gagging, gurgling, frothing, gasping or tongue-biting.” Autopsy findings showed no anatomic findings to account for death. None had significant coronary atherosclerosis. Four had cardiomegaly (heart weight >350 g) but were not standardized according to body size. Four were noted to have stomachs distended by partially ingested food. Histology and toxicological studies were either non-contributing or negative. The authors suggested ventricular fibrillation associated with an abnormal cardiac conduction defect to be the cause of death in SUNDS.20
Munger and Booton (1998) surveyed 772 death certificates with diagnoses of sudden and unexplained death in sleeps (SUDS) in Manila from 1948-1982. The findings revealed 81 cases of acute cardio-respiratory failure (ACRF) of the Bangungut type, 168 cases of ACRF with congestion and petechial hemorrhages of internal organs, 98 cases of ACRF in sleep, 73 cases of ACRF with acute hemorrhagic pancreatitis, and 77 cases of “pending diagnosis.” The authors concluded that various causes of sudden and unexplained nocturnal death, including Bangungut were included in the sample. They attributed the immediate cause of death in Bangungut to ventricular fibrillation, but were unclear about the underlying mechanism.21
Summary of Philippine Autopsy Studies
Gross autopsy findings revealed mostly usual postmortem cardiac, pulmonary, visceral and brain findings. Many disease entities, including acute pancreatitis, were included under the diagnosis of SUNDS and Bangungut. The finding of acute pancreatitis is not generalized and consistent. Whether it is the cause of Bangungut and is likely the result of post-mortem pancreatic changes remains unclear. Many reports lack microscopic findings. And the unavailability of modern diagnostic techniques at the time rendered the diagnosis of SUNDS inconclusive. However, most authors of these studies suspected a cardio-pulmonary etiology.
Despite these limitations, clearly, there was a notable subset of Bangungut in which no gross evidence of pathological findings on the heart was found. This subset of Bangungut, identified heuristically in this paper as Bangungut/SUNDS seemed to conform to the widely-held folk belief of Bangungut, namely: predominance among male in the 20-40 age range; high incidences of sudden death during sleep or at rest, usually following ingestion of a large meal at night; and victims were in apparent good health prior to their demise. Folklore of causation about Bangungut exists among the population.
Epidemiological Study of SUNDS in the Philippines
Gervacio-Domingo et al. (2007) conducted a stratified randomized sampled of 4,747 households in a nation-wide sampling using a questionnaire validated in a previous study with autopsy findings.22 They estimated a prevalence of 43/100,000 per year with deaths primarily among the male population and concluded that sudden unexplained death during sleep occurred commonly in the general Philippine population.23
Subsequently, Gervacio-Domingo et al., using a stratified multistage sampling design, measured the prevalence of Brugada type 1 ECG pattern with J joint and coved ST elevation in the right precordial leads among subjects from all regions and provinces in the Philippines. Among the population, the results showed a prevalence of 0.2% (95% Confidence Interval [CI] 0.03% −0.36%) with Brugada ECG type 1 pattern, 0.3% among males, and 2% with any type 1, 2 and 3 Brugada ECG pattern. The authors concluded that Brugada type 1 ECG pattern is common among Filipinos.24
A recent study that tracked over four years the natural course of 7 initially identified and asymptomatic Filipino patients with Brugada type 1 ECG pattern confirmed what is generally known as the clinical features of Bangungut. The study revealed that the subjects were predominantly male in their third or fourth decades of life. Three subjects reported a major cardiac event (syncope, seizure, and/or prolonged non-arousability). Although the sample size is small, the frequency of cardiac events translates to a crude mean rate of 43%.25
The relatively high prevalence of Brugada type 1 ECG pattern among Filipinos strongly supports autopsy findings that this subset of Bangungut/SUNDS without gross cardiac pathology may be closely linked to the Brugada syndrome.
THE BRUGADA SYNDROME
Since its first description as a clinical entity in 1992,26 the Brugada syndrome is increasingly recognized worldwide as a channelopathy associated with a high risk for sudden cardiac death. ECG abnormalities constitute the hallmark of Brugada syndrome. A triad of right bundle-branch block, ST elevation in the right pre-cordial leads, and fatal ventricular arrhythmia characterizes Brugada syndrome. Brugada’s signature characteristics include: 1) an accentuated ST segment elevation of ECG or J wave appearing principally in the right precordial leads (V1-V3), often followed by a negative T wave; 2) very closely coupled extrasystoles; and 3) rapid polymorphic ventricular tachycardia (VT) and/ or ventricular fibrillation (VF).27 Three types of ST segment elevation are recognized: Type 1 is characterized by a coved ST-segment elevation > 2 mm (o.2 mV) followed by an inverted T-wave and is considered the most lethal form. Type 2 has a saddle-type configuration induced by a high take-off ST-segment elevation, the J wave amplitude (> 2 mm) giving rise to a gradually descending ST-segment elevation, and followed by a positive or biphasic T-wave. Type 3 is a right precordial ST-segment elevation of <1 mm of saddleback type, coved type or both.28 (Figure 1). Brugada syndrome is definitively diagnosed when a type 1 ST-segment elevation is observed in >1 mm in the right precordial leads (V1 to V3) in the presence or absence of a sodium channel-blocking agent, and in conjunction with one of the following: documented ventricular fibrillation (VF), polymorphic ventricular tachycardia (VT), a family history of sudden cardiac death at <45 years old, coved-type ECGs in family members, inducibility of VT with programmed electrical stimulation, syncope, or nocturnal agonal respiration.29
Figure 1. Electrocardiographic Types of Brugada Syndrome.
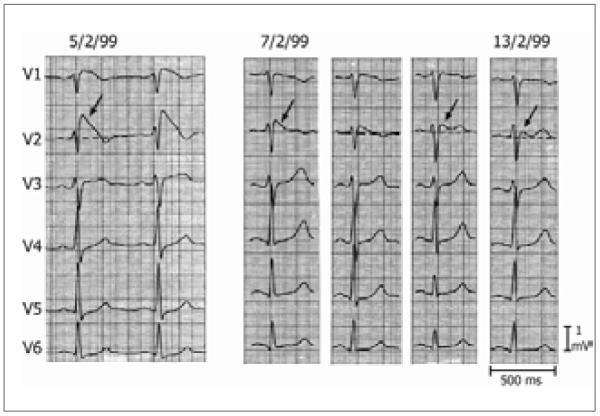
Figure 1. Three Types of ST segment elevation generally observed in patients with the Brugada syndrome. Shown are precordial leads recorded from a patient diagnosed with the Brugada syndrome. The dynamic ECG changes occurring over a period of 2 days are shown. The left panel shows a clear Type 1 ECG, which is diagnostic of the Brugada syndrome. A saddleback ST segment elevation (Type 2) is observed on 2-7-99. The ST segment is further normalized on 2-13-99 showing a Type 3 ECG. Modified from {Wilde, 2002 7380 /id}, with permission.
The ECG sign of the Brugada syndrome is dynamic (Figure 1) and often concealed, exhibiting profound day-to-day and beat-to-beat variation in amplitude and morphology.30 The coved-type is required for the diagnosis while the saddleback type is an intermediate form that requires confirmation using pharmacological challenge to convert it into the coved-type or through genetic analysis. Sodium channel blockers such as ajmaline, flecanide, procainamide, disopyramide, propafenone and pilsicainide can unmask the ECG pattern.31 In addition to sodium channel blockers, tricyclic antidepressants, first generation antihistaminics (dimenhydrinate), alcohol intoxication, cocaine toxicity, insulin plus glucose, α-adrenergic agonists, β-adrenergic blockers, vagotonic agents, and febrile state can also unmask the Brugada sign or lead to accentuation of the ST segment elevation in patients with the syndrome.32,33,34
The Brugada syndrome is inherited through an autosomal dominant mode of transmission.35 It has been associated with mutations in seven different genes encoding inward and outward currents.
The first gene associated with Brugada syndrome was SCN5A, which encodes the α subunit of the sodium channel gene.36 Currently, over 293 mutations in SCN5A have been linked to the Brugada syndrome.37 SCN5A mutations reduce sodium channel current by a variety of mechanisms.38 SCN5A mutations have been shown to result in loss of function due either to: 1) failure of the sodium channel to express; 2) a shift in the voltage- and time-dependence of INa activation, inactivation or reactivation; 3) entry of sodium channel into an intermediate state of inactivation from which it recovers more slowly, or 4) accelerated inactivation of the sodium channel.39 Loss of function of INa leads to an outward shift of the balance of current during the early phase of the action potential, which, if not corrected, can result in arrhythmogenesis (VF/VT).
Mutations in SCN5A (Nav1.5, BrS1)40 have been reported in 11-28% of Brugada syndrome probands,41 CACNA1C (Cav1.2, BrS3) in 6.7%,42 CACNB2b (Cavß2b, BrS4) in 4.8%,43 and mutations in Glycerol-3-phophate dehydrogenase 1-like enzyme gene (GPD1L, BrS2),44 SCN1B (β1-subunit of sodium channel, BrS5),45 KCNE3 (MiRP2; BrS6),46 and SCN3B (Navß3, BrS7)47 are much more rare. These genetic defects lead to development of Brugada syndrome secondary to either a loss of function of sodium (INa) or L-type calcium (ICa) channel current, or a gain of function of transient outward current (Ito). Approximately 75% of Brugada syndrome probands remain genotype-negative.
Owing to a predominance of the transient outward current (Ito) in the right ventricle, especially in the right ventricular outflow tract (RVOT), the Brugada syndrome is considered predominantly a right ventricular disease.
The cellular basis for the Brugada syndrome has come into better focus in recent years. The concept of phase 2 reentry, the presumed trigger for Brugada syndrome, was described in the early 1990’s and evolved in parallel with the clinical discovery of the Brugada syndrome.48,49,50,51 Studies conducted over the past couple of decades indicates that rebalancing of the currents active at the end of phase 1 of the epicardial action potential, leading to an accentuation of the action potential notch in right ventricular epicardium, particularly in the region of the RVOT, is responsible for the accentuated J wave or ST segment elevation associated with the Brugada syndrome.52 Under normal conditions, the appearance of the epicardial action potential notch is due principally to the interaction of the transient outward current (Ito) and L-type calcium channel current (Ica). Amplification of epicardial and transmural dispersion of repolarization (TDR) can result from loss of the action potential dome in RVOT epicardium secondary to the presence of genetic defects, pathophysiological factors as well as pharmacologic influences. The augmented transmural dispersion of repolarization facilitates the development of ST segment elevation and creates a vulnerable window across the right ventricular wall. The epicardial dispersion of repolarization gives rise to phase 2 reentry, thus generating a closely coupled extrasystole capable of invading the vulnerable window and precipitating VF/VT.52 In some cases, particularly in the case of SCN5A mutations, slowing of conduction in RVOT may contribute to these manifestations.53 Recent reports have presented evidence in support of the hypothesis that depolarization forces associated with delayed conduction in the RVOT is the principal mechanism underlying the development of arrhythmias in patients with Brugada syndrome.54 The repolarization vs. depolarization hypotheses is the subject of a scholarly debate between the principal proponents.55,56
HOW WOULD PATHOPHYSIOLOGICAL MECHANISM OF BRUGADA SYNDROME EXPLAIN BANGUNGUT/SUNDS
Brugada syndrome and Bangungut/SUNDS share several characteristics: an ECG pattern, a preponderance in males of the 20-40 age group; more frequent occurrence of sudden death at night or during period of rest, usually after ingestion of a full meal; and usual occurrence in individuals with structurally normal hearts. We shall consider how current cellular, ionic, and genetic findings underlying the Brugada syndrome may explain Bangungut/SUNDS.
1. Predominance in males. Genetic, cellular, ionic and embryonic developmental factors in patients with Brugada syndrome are thought to account for this phenomenon. The clinical phenotype of Brugada syndrome is eight to 10 times more prevalent in males than in females despite equal genetic transmission of the disease.57 Genetic mutation of the SCN5A gene such as G1406R leads to development of the Brugada syndrome phenotype principally in males. A prominent Ito-mediated action potential notch in the right ventricular epicardium of males predisposes them to develop the Brugada phenotype while a smaller Ito-mediated epicardial action potential notch in females relegates them to develop progressive conduction problems.58
The Brugada syndrome is considered a right ventricular disease. This is due to the predominance of Ito in the RV, particularly the RVOT.
2. Nocturnal death and during period of rest and following ingestion of a full meal. Clinical and imaging studies have advanced the postulate that an autonomic imbalance dysfunction exists in the Brugada syndrome resulting in a dominant parasympathetic tone which contributes to arrhythmogenesis.59,60,61,62 Brugada syndrome occurs most frequently at night and after a full meal when high vagal tone predominates. Wichter has pointed out that the typical ECG changes in Brugada can vary over time and can be modulated by exercise, vagal stimulation, or drugs that interact with the cardiac sodium channel (i.e., ajmaline, flecainide, and procainamide) or with the cardiac autonomic nervous system. Indeed, vagal stimulation, parasympathomimetic drugs (e.g., edrophonium), anti-adrenergic drug like β-blockers, or α-adrenergic receptor stimulators (e.g., norephinephrine) can unmask the ECG signs of Brugada. In contrast, the ECG signs of Brugada syndrome may be diminished during exercise and isoproterenol infusion.57
Babaee Bigi et al. used four standard cardiac autonomic function tests (the Valsalva maneuver, beat-to-beat heart rate variation, for parasympathetic function; and postural fall in blood pressure, and the sustained handgrip test, for sympathetic function) to assess cardiac autonomic modulation on 115 subjects with Brugada types 1 (N=28), 2 (N=56) and 3 (N=31) ECG pattern. Cardiac autonomic neuropathy (CAN) was detected in 13 (46%) subjects with type 1 Brugada pattern. Of these, 11 (84%) had previous history of cardiac events, whereas only two of the 15 subjects (13%; P=0.01) without CAN had history of previous cardiac events. None of the type 2 or 3 Brugada subjects had CAN. All type 1 Brugada patients with CAN were male and CAN was not detected in females. The authors concluded that CAN is an important risk factor in the Brugada syndrome. Male gender per se is thought not an independent risk factor for the development of ventricular arrhythmia, but that males with CAN are susceptible to the development of cardiac events.59
When subjects were given a full meal, and twelve-leads ECG were taken before the meal and 30 minutes afterward, Ikeda et al. found a positive test (defined as ST-segment elevation >1 mm at the J point compared to baseline, change from saddle-back pattern to coved pattern followed by a negative T wave, and visible beat-to-beat variation of morphology or amplitude of T waves) among 17 (49%) of the study subjects after a full stomach test. Of these, 14 (82%) of the high-risk group showed a significant (P=0.0002) positive finding as compared to 3 (17%) of the indeterminate risk group. The authors concluded that ingestion of a large meal among identified Brugada-type ECGs individuals and the resultant high parasympathetic tone are associated with spontaneous ST-segment elevation and with arrhythmogenesis.60
Two imaging studies lend further support to the above clinical observations on CAN. Using non-invasive radionuclide technique, Wichter et al. assessed the cardiac autonomic system in patients with Brugada syndrome. They demonstrated regionally impaired adrenergic innervations with [123I] m-iodobenzylguanidine (123I-MIBG) and single-photon emission computed tomography (SPECT). Their results showed an abnormal sympathetic innervation of the heart resulting in a dominance of the parasympathetic tone and subsequent autonomic imbalance.61
Kies et al. further studied the cardiac autonomic nervous system in Brugada by means of quantitative positron emission tomography. Using norepinephrine analog, 11C-hydroxyephedrine (11C-HED) and the non-selective β -blocker 11C-CGP 12177 (11C-CGP), they quantified myocardial pre-synaptic and post-synaptic sympathetic cardiac function. Their results indicated an enhanced pre-synaptic norepinephrine recycling with preserved β adrenoceptor density.62 The authors postulated that a lack of sympathetic drive and preserved parasympathetic stimulation might reduce cAMP production, potentially impacting on protein phosphorylation and spatial heterogeneity of calcium transients. Since the parasympathetic transmitter acetylcholine that is known to affect ion currents such as Ito and ICa is more prominent in the epicardium compared with the endocardium, the autonomic imbalance with reduced adrenergic nerve activity and dominant vagal tone may modulate epicardial ion currents that may result in a loss of action potential and resulting in an increase in the transmural dispersion of refractoriness. This can lead to a higher susceptibility for the emergence of ventricular tachyarrhythmias. As the autonomic imbalance may be more intense during periods of physiological down-regulation of adrenergic activity during periods of rest or sleep, and that a high vagal tone is associated with a full stomach, this postulate for Brugada may well explain the observation that Bangungut/SUNDS occurs at night following the ingestion of a full meal.
Furthermore, Mizumaki et al. have demonstrated that ST-segment elevation in the Brugada syndrome has diurnal and/or daily variation and that the events occurred more frequently at night or early morning (73% vs. 27 % during daytime).63 This further explains why Bangungut/SUNDS occurs more frequently at night.
3. Occurrence in apparently healthy individuals. Most of the cases of Brugada are asymptomatic. But SCN5A mutation and other genetic defects predispose the probands to express the phenotypical patterns of type 1 Brugada ECG patterns leading to VT/VF and death under pathophysiological conditions. Presumed ionic, molecular and cellular anomalies in Bangungut/SUNDS are not detectable under gross autopsy dissection. Molecular autopsy technique (postmortem genetic testing)64 seems to offer a useful means to identify the genetic etiology of many inherited cardiac diseases including arrhythmic syndromes such as congenital long QT syndrome (LQTS), Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia (CPVT). Unless revealed by special molecular techniques and correlated with clinical and ECG findings, these arrhythmogenic pathologies are not demonstrable on gross autopsy; thus giving the appearance of death occurring in structurally normal hearts. Pharmacological challenge with sodium channel blockers and genetic studies are needed to definitely confirm the diagnosis of Bangungut/SUNDS.
Discussion
Autopsy reports on SUNDS in the Philippines revealed that the use of the term Bangungut is confusing as it includes many pathological entities that may show features of sudden unexplained nocturnal death. Unfortunately, many pre-WWII clinical records were destroyed during the war. Nonetheless, our review showed that there is clearly a notable subset of Bangungut/SUNDS that showed no gross structural heart defects. This subset of Bangungut/SUNDS showed clinical characteristics similar to those of the Brugada syndrome; namely, a preponderance in male of the 20-40 age group; death frequently at night death or during periods of rest after the ingestion of a full meal; and usual occurrence in individuals with structurally normal heart. Indeed, the recent finding of a relatively high incidence of type-1 ECG pattern of Brugada syndrome among Filipinos further strengthened the association between Bangungut/SUNDS and the Brugada syndrome. Current pathophysiological mechanisms of the Brugada syndrome also seemed plausible explanations for a majority of the Bangungut phenomenon as well. Can we then conclude that Bangungut/SUNDS is phenotypically, functionally, and genetically an allele of or is identical to the Brugada syndrome as has been asserted for cases of Lai Tai in Thailand and Pokkuri in Japan?
Sudden cardiac death due to ventricular arrhythmias (VF/VT) can result from channelopathies such as the Long QT syndrome (LQTS), Brugada syndrome, and arrhythmogenic right ventricular cardiomyopathies (ARVC). In addition to LQTS (LQT3), and Brugada syndrome, mutation in SCN5A have also been found to cause progressive conduction system disease (Lev-Lenegre syndrome),65 isolated conduction system disease66 and sudden infant death syndrome (SIDS).67 In all these disorders, death most commonly occurs during sleep, suggesting a common mechanism. Without data that clearly differentiated these categories and correlated with clinical information, ECG pattern, and possible genetic and molecular autopsy findings, it may be premature to conclude that Bangungut/SUNDS is identical to the Brugada syndrome. Brugada syndrome most likely can explain a majority of Bangungut/SUNDS. But whether all Bangungut/SUNDS are Brugada syndrome must await further research data.
Research Agenda for Bangungut/SUNDS
A logical progression of the research agenda on Bangungut/SUNDS could proceed along these directions: First, verify the hypothesis that ventricular arrhythmias (VF/VT) indeed are the immediate cause of death in Bangungut/SUNDS. Second, clarify whether Bangungut/SUNDS is phenotypically, genetically, and functionally identical to Brugada syndrome. Studies on the natural history of Bangungut/SUNDS with ECG monitoring and with genetic testing of probands and their relatives will enhance understanding of both the Bangungut/SUNDS and the Brugada syndrome. The use of pharmacological challenges in suspected cases of Brugada syndrome would reveal the diagnosis. The introduction of cardiac molecular autopsy technique would aid the country’s forensic work on SUNDS and confirm the pathologies underlying SUNDS and possibly Bangungut/SUNDS. Third, elucidate the underlying mechanisms and factors that may predispose and precipitate the Bangungut attack. Psychological68,69,70 neurological71 and cultural factors72,73,74 and others have all been reported in SUNDS and should be investigated. (Table I)
Table I. Proposed Research Agenda and Their Rationale in the Characterization of Bangungut/SUNDS.
| Proposed Study | Rationale |
|---|---|
| Cardiac study | To characterize the cardiac conduction pattern |
| Genetic Study | To determine the genetic predispos- ing factor that may account for the electrical conduction system anomaly in the heart |
| Psychological Study | To determine the role of stress and other psychological factor |
| Cultural Study | To map the setting of Bangungut and related cultural factor as contributory factor to stress and coping mecha- nisms of Bangungut survivors and their family |
| Neurological and Sleep Study | To rule out the presence of seizure and/or seizure-like activity |
| Nutritional/Metabolic Study | To rule out the presence of thiamine defciency, hypokalemia and/or other metabolic or dietary factor |
| Infection Study | To rule out the presence of pseu- domonas pseudomallei or other infectious factor |
| Physiological Study | To determine the role of exercise and/ or physical exertion |
| Cardiac Molecular Study | To ascertain the cardiac abnormality on autopsy fnding |
| Epidemiological Study | To determine the community-based incidence and prevalence of Ban- gungut and to provide baseline data for determining the effectiveness of preventive programs |
| Economic Burden Study | To cost out the economic burden of the illness to the country and to provide baseline data to determine the cost-effectiveness of preventive programs |
Establishment of a National Bangungut Registry
The availability of a registry should be considered that systematically collects cases of Bangungut/SUNDS. This will allow tracking of the trend of this illness in the Philippines and facilitate comparative study of SUNDS around the world. It will also provide the database to allow the design of intervention study and for the testing and demonstration of the effectiveness of preventive programs.
TREATMENT OF BRUGADA AND ITS THERAPEUTIC IMPLICATIONS FOR THE TREATMENT OF BANGUNGUT/SUNDS
If the Brugada syndrome is conclusively determined to be identical to the Bangungut/SUNDS, it then follows that treatment of Brugada syndrome may guide treatment for Bangungut/SUNDS. There are many diagnostic and therapeutic considerations. Since the focus of this review is on the relationship between Brugada syndrome and Bangungut/SUNDS, clinicians treating Brugada syndrome should consult standard textbooks and treatment protocol. The following are some highlights of clinical concerns gleaned from the present review of literatures.
Diagnostic Clarification
SUNDS from other causes besides the Brugada syndrome and subsumed under the label of Bangungut should be clearly differentiated. Bangungut/SUNDS should be reserved for syndrome revealing no gross structural heart defects. Table II shows the proposed criteria for the diagnosis of Bangungut/SUNDS in the Philippines. The list of differential diagnosis for Brugada syndrome is long.75 Clinical evaluation should be directed toward excluding other causes contributing to ST-segment elevation. Of particular importance is the exclusion of arrhythmogenic right ventricular cardiomypathy (ARVC), which may mimic Brugada syndrome. Drug challenge with sodium channel blockers may be useful in distinguishing Brugada from ARVC, early repolarization syndrome and from normal degrees of right precordial ST elevation in men.76
Table II. Proposed Diagnostic Criteria for Bangungut/SUNDS.
Clinical features:
|
May include at least one of the following:
|
|
Exclusion criteria: Exclude evidence of another identifable serious medical condition such as myocardial infarction, thyroid disease, infectious disease, severe thiamine defciency, diabetes, electrolyte imbalance and other diseases that may secondarily cause cardiac arrest. |
Therapeutic Considerations
At present, an implantable cardioverter defibrillator (ICD) is the only proven effective treatment of ventricular tachyarrhythmias (VF/VT) caused by Brugada syndrome.77 For seriously ill, symptomatic Brugada and Bangungut/SUNDS patients with a history of aborted sudden death, or presenting with symptom of syncope, seizure, or nocturnal agonal respiration, exhibiting the type 1 Brugada ECG either spontaneously or after sodium channel blockade challenge, ICD should be considered. Ambulatory external defibrillator should be installed in airports, community health units (called Barangay in the Philippines) and in large hotels. This would help the resuscitation of many cardiac arrests due to ventricular tachyarrhythmias.
Current effort in risk stratification of the Brugada syndrome could provide useful guidelines for the treatment of Bangungut/SUNDS as well.
As Brugada syndrome could be manifested during febrile state,78 care should be taken to monitor the patient and aggressively treat both the febrile state and its underlying causes.
A number of antipsychotic and antidepressant drugs are known to increase the risk of ventricular arrhythmia and sudden cardiac death.79 Tricyclic antidepressants amitriptyline, desipramine, nortriptyline; tetracyclic antidepressant, maprotiline; lithium; and antipsychotic trifluoperazine and loxapine have been reported to induce type 1 Brugada ECG patterns. Care should be exercised when prescribing these drugs to Brugada and Bangungut/SUNDS patients. There should be baseline and on-going ECG monitoring.
Genetic counseling should be made available for individuals and family members with confirmed or suspected Brugada and/or Bangungut/SUNDS.
Conclusion
Autopsy studies, ECG and clinical findings in the Philippines lend further credence to the hypothesis that a cardiac event, most likely ventricular arrhythmia (VF/VT) is the immediate cause of death in Bangungut/SUNDS. Both the Brugada syndrome and Bangungut/SUNDS share several characteristics: ECG patterns, increased prevalence among males, and death occurring at rest or at sleep following ingestion of a full meal, usual occurrence in individuals with structurally normal hearts. The relatively high prevalence of Brugada syndrome with type 1 ECG pattern among Filipinos strongly suggests that the Bangungut/SUNDS and the Brugada syndrome share common underlying pathophysiological mechanisms. Genetic mutation in cardiac ionic and resultant altered cellular channels and the possible presence of a cardiac autonomic neuropathy in Brugada syndrome are advanced to account for the Bangungut/SUNDS phenomenon. A research agenda including genetic testing of probands and their pedigree, if proven, would conclusively confirm the association of Brugada syndrome and Bangungut/SUNDS.
References
- 1.Vatta M, Dumaine R, Varghese G, Richard TA, Shimizu W, Aihara N, Nademanee K, Brugada R, Brugada J, Veerakul G, Li H, Bowles NE, Brugada P, Antzelevitch C, Towbin JA. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet. 2002;11(3):337–345. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza-Guazon P. Algunas notas sobre bangungut. Revista Filipina Med Y Fam. 1917;8:437–442. [Google Scholar]
- 3.Aponte GE. The enigma of “bangungut”. Ann Intern Med. 1960 Jun;52:1258–63. doi: 10.7326/0003-4819-52-6-1258. [DOI] [PubMed] [Google Scholar]
- 4.Majoska AV. Sudden death in Filipino men; an unexplained syndrome. Hawaii Med J. 1948 Jul-Aug;7(6):469–73. [PubMed] [Google Scholar]
- 5.Munger RG, Booton EA. Bangungut in Manila: sudden and unexplained death in sleep of adult Filipinos. Int J Epidemiol. 1998 Aug;27(4):677–84. doi: 10.1093/ije/27.4.677. [DOI] [PubMed] [Google Scholar]
- 6.Gervacio-Domingo G, Punzalan FE, Amarillo ML, Dans A. Sudden unexplained death during sleep occurred commonly in the general population in the Philippines: a sub-study of the National Nutrition and Health Survey. J Clin Epidemiol. 2007 Jun;60(6):567–71. doi: 10.1016/j.jclinepi.2006.10.003. Epub 2007 Feb 5. [DOI] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control Sudden, unexpected, nocturnal deaths among Southeast Asian refugees. MMWR. 1981;30:581–584. 589. [PubMed] [Google Scholar]
- 8.Tatsanavivat P, Chiravatkul A, Klungboonkrong V, Chaisiri S, Jarerntanyaruk L, Munger RG, Saowakontha S. Sudden and unexplained deaths in sleep (Laitai) of young men in rural northeastern Thailand. Int J Epidemiol. 1992;21:904–10. doi: 10.1093/ije/21.5.904. [DOI] [PubMed] [Google Scholar]
- 9.Gotoh K. A histopathological study on the conduction system of the so-called “pokkuri disease” (sudden unexpected cardiac death of unknown origin in Japan) Jpn Circ J. 1976;40:753–68. doi: 10.1253/jcj.40.753. [DOI] [PubMed] [Google Scholar]
- 10.Tungsanga K, Sriboonlue P. Sudden unexplained death syndrome in north-east Thailand. Int J Epidemiol. 1993;22:81–87. doi: 10.1093/ije/22.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Tatsanavivat P, Chiravatkul A, Klungboonkrong V, Chaisiri S, Jarerntanyaruk L, Munger RG, Saowakontha S. Sudden and unexplained deaths in sleep (LaiTai) of young men in rural northeastern Thailand. Int J Epidemiol. 1992;21:904–910. doi: 10.1093/ije/21.5.904. [DOI] [PubMed] [Google Scholar]
- 12.Vatta M, Dumaine R, Varghese G, Richard TA, Shimizu W, Aihara N, Nademanee K, Brugada R, Brugada J, Veerakul G, Li H, Bowles NE, Brugada P, Antzelevitch C, Towbin JA. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet. 2002;11(3):337–345. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Mendoza-Guazon P. Algunas notas sobre bangungut. Revista Filipina Med Y Fam. 1917;8:437–442. [Google Scholar]
- 14.Sta. Cruz JZ. The pathology of “Bangungut.”. J Phil Med Assoc. 1951;27:476–81. [PubMed] [Google Scholar]
- 15.Aponte GE. The enigma of “bangungut”. Ann Intern Med. 1960 Jun;52:1258–63. doi: 10.7326/0003-4819-52-6-1258. [DOI] [PubMed] [Google Scholar]
- 16.Majoska AV. Sudden death in Filipino men: an unexplained syndrome. Hawaii Med J. 1948 Jul-Aug;7(6):469–73. [PubMed] [Google Scholar]
- 17.Sta. Cruz JZ. The pathology of “Bangungut.”. J Phil Med Assoc. 1951;27:476–81. [PubMed] [Google Scholar]
- 18.Majoska AV. Sudden death in Filipino men; an unexplained syndrome. Hawaii Med J. 1948 Jul-Aug;7(6):469–73. [PubMed] [Google Scholar]
- 19.Aponte GE. The enigma of “bangungut”. Ann Intern Med. 1960 Jun;52:1258–63. doi: 10.7326/0003-4819-52-6-1258. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Weinstein SR. Sudden unexpected nocturnal death syndrome in the Mariana Islands. Am J Forensic Med and Pathol. 1990;11(3):205–207. doi: 10.1097/00000433-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Munger RG, Booton EA. Bangungut in Manila: sudden and unexplained death in sleep of adult Filipinos. Int J Epidemiol. 1998 Aug;27(4):677–84. doi: 10.1093/ije/27.4.677. [DOI] [PubMed] [Google Scholar]
- 22.Gervacio-Domingo G, Daez ML, Gloria VI, Lim C, Punzalan FE. Development of a questionnaire to measure the incidence of sudden unexplained death syndrome (Bangungut) in the general population. Phil JC. 2005;33:33–37. [Google Scholar]
- 23.Gervacio-Domingo G, Punzalan FE, Amarillo ML, Dans A. Sudden unexplained death during sleep occurred commonly in the general population in the Philippines: a sub study of the National Nutrition and Health Survey. J Clin Epidemiol. 2007 Jun;60(6):567–71. doi: 10.1016/j.jclinepi.2006.10.003. Epub 2007 Feb 5. [DOI] [PubMed] [Google Scholar]
- 24.Gervacio-Domingo G, Isidro J, Tirona J, Gabriel E, David G, Amarillo ML, Morales D, Dans A. The Brugada type 1 electrocardiographic pattern is common among Filipinos. J of Clin Epidemiol. 2008;61:1067–1072. doi: 10.1016/j.jclinepi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Gervacio-Domingo G, Jocson G, Dans A. Frequency of cardiac events at four years among initially asymptomatic Filipinos with Brugada Type 1 electrocardiographic pattern. Am J Cardiol. 2011 Mar;107(5):714–716. doi: 10.1016/j.amjcard.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 27.Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Ann Med. 2004;36(Suppl 1):5–14. doi: 10.1080/17431380410032553. [DOI] [PubMed] [Google Scholar]
- 28.Wilde A, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 29.Antzelevitch C, Brugada P, Borgreffe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: Report of the second consensus conference. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 30.Hirata K, Takagi Y, Nakada M, Kyushima M, Asato H. Beat-to-beat variation of the ST segment in a patient with right bundle branch block, persistent ST segment elevation, and ventricular fibrillation: a case report. Angiology. 1998;49:87–90. doi: 10.1177/000331979804900113. [DOI] [PubMed] [Google Scholar]
- 31.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 32.Brugada R, Brugada J, Antzelevitch C, Kirsch GF, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–5. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 33.Babaliaros VC, Hurst JW. Tricyclic antidepressants and the Brugada syndrome: an example of Brugada waves appearing after the administration of desipramine. Clin Cardiol. 2002;25:395–8. doi: 10.1002/clc.4950250809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brugada P, Brugada J, Brugada R. Arrhythmias induction by antiarrhythmic drugs. Pacing Clin Electrophysiol. 2000;23:291–2. doi: 10.1111/j.1540-8159.2000.tb06751.x. [DOI] [PubMed] [Google Scholar]
- 35.Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Ann Med. 2004;36(Suppl 1):5–14. doi: 10.1080/17431380410032553. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanisms for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 37.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto M, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AAM, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: physiological, pharmacological and clinical implications. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology: The Heart. Oxford University Press; New York: 2002. [Google Scholar]
- 39.Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Ann Med. 2004;36(Suppl 1):5–14. doi: 10.1080/17431380410032553. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanisms for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 41.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto M, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AAM, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr., Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr., Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Norstrand DW, Valdivia CR, Tester DJ, Ueda K, London B, Makielski JC, Ackerman MJ. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation. 2007;116:2253–2259. doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld AC, Pfeufer A, Kaab S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM, Bezzina CR. Sodium channel b1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delpón E, Cordeiro JM, Núñez L, Thomsen PEB, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Burashnikov A, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the b3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 49.Yan GX, Antzelevitch C. Cellular basis for the Brugada Syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 50.Fish JM, Antzelevitch C. Role of Sodium and Calcium Channel Block in Unmasking the Brugada Syndrome. Heart Rhythm. 2004;1:210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fish JM, Antzelevitch C. Cellular and Ionic Basis for the Sex-related Difference in the Manifestation of the Brugada Syndrome and Progressive Conduction Disease Phenotypes. J Electrocardiol. 2003;36:173–179. doi: 10.1016/j.jelectrocard.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 52.Antzelevitch C. Brugada syndrome. PACE. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Postema PG, van Dessel PFHM, Kors JA, Linnenbank AC, van Harpen G, Ritsema van Eck HJ, van Geloven N, De Bakker JMT, Wilde AAM, Tan HT. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in Brugada syndrome: a study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J Am Coll Cardiol. 2010;55:789–797. doi: 10.1016/j.jacc.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 54.Postema PG, van Dessel PF, Kors JA, Linnenbank AC, van Herpen G, Ritsema van Eck HJ, van Geloven N, de Bakker JM, Wilde AA, Tan HL. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in Brugada syndrome: a study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J Am Coll Cardiol. 2010;55:789–97. doi: 10.1016/j.jacc.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 55.Wilde AAM, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.07.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–9. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 57.Fish JM, Antzelevitch C. Cellular and ionic basis for the sex-related difference in the manifestation of the Brugada syndrome and progressive conduction disease phenotype. J of Electrophysiol. 2003;36:173–179. doi: 10.1016/j.jelectrocard.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 58.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Pérez GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis of the predominance of the Brugada syndrome phenotype in males. Circulation. 2001;106:2004–2011. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- 59.Bigi Babaee, Aslani A. Significance of cardiac autonomic neuropathy in risk stratification of Brugada syndrome. Eurospace. 2008;10:821–824. doi: 10.1093/europace/eum272. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda T, Abe A, Yusu S, Nakamura K, Ishiguro H, Mera H, Yotsukura M, Yoshino H. The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome. J Cardiov Electrophysiol. 2006;17:602–7. doi: 10.1111/j.1540-8167.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 61.Wichter T, Matheja P, Eckardt L, Kies P, Schäfers K, Schulze-Bahr E, Haverkamp W, Borggrefe M, Schober O, Breithardt G, Schäfers M. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105:702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 62.Kies P, Wichter T, Schafers M, Paul M, Schäfers KP, Eckardt L, Stegger L, Schulze-Bahr E, Rimoldi O, Breithardt G, Schober O, Camici PG. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation. 2004;110:3017–3022. doi: 10.1161/01.CIR.0000146920.35020.44. [DOI] [PubMed] [Google Scholar]
- 63.Mizumaki K, Fujiki A, Nishida K, Iwamoto J, Sakamoto T, Sakabe M, Tsuneda T, Sugao M, Inoue H. Postprandial augmentation of bradycardia-dependent ST elevation in patients with Brugada syndrome. J Cardiov Electrophysiol. 2007;18(8):839–844. doi: 10.1111/j.1540-8167.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 64.Tester DJ, Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Curr Opin Cardiol. 2006 May;21(3):166–72. doi: 10.1097/01.hco.0000221576.33501.83. [DOI] [PubMed] [Google Scholar]
- 65.Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, Wilde AA, Escande D, Mannens MM, Le Marec H. Cardiac conduction defects associate with mutations in SCN5A. Nat Gent. 1999;23:20–21. doi: 10.1038/12618. [DOI] [PubMed] [Google Scholar]
- 66.Tan HL, Bink-Boelkens MT, Bezzina CR, Viswanathan PC, Beaufort-Krol GC, van Tintelen PJ, van den Berg MP, Wilde AA, Balser JR. A sodium-channel mutation causes isolated cardiac conduction disease. Nature. 2001;409:1043–1047. doi: 10.1038/35059090. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, Richard TA, Berti MR, Bloise R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 68.Melles RB, Katz B. Night terrors and sudden unexplained nocturnal death. Med Hypothesis. 1988;26:149–154. doi: 10.1016/0306-9877(88)90071-0. [DOI] [PubMed] [Google Scholar]
- 69.Binik TM. Psychological predictors of sudden death: a review and critique. Soc Sci Med. 1985;20:667–680. doi: 10.1016/0277-9536(85)90055-3. [DOI] [PubMed] [Google Scholar]
- 70.Taggart P, Critchley H, Lambiase PD. Heart-brain interactions in cardiac arrhythmia. Heart. 2011;97:698–708. doi: 10.1136/hrt.2010.209304. [DOI] [PubMed] [Google Scholar]
- 71.Samuels MA. “Voodoo” death visited: the modern lessons of neurocardiology. Cleve Clin J Med. 2007;74(Suppl1):S8–S16. doi: 10.3949/ccjm.74.suppl_1.s8. [DOI] [PubMed] [Google Scholar]
- 72.Cannon WB. “Voodoo” death. American Anthropologist. 1942;44:169–181. doi: 10.2105/ajph.92.10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan ML. Revisiting Usog, Pasma, Kulam. The University of the Philippines Press; Quezon City: 2008. [Google Scholar]
- 74.Jocano FL. Folk Medicine in a Philippine Municipality. PUNLAD Research House, Inc.; Metro Manila, Philippines: 2003. [Google Scholar]
- 75.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–9. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 76.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–9. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 77.Antzelevitch C, Brugada P, Borgreffe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: Report of the second consensus conference. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 78.Morita H, Zipes DP, Morita ST, Wu J. Temperature modulation of ventricular arrhythmogenecity in canine tissue model of Brugada syndrome. Heart Rhythm. 2007;4:188–197. doi: 10.1016/j.hrthm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert Opin Drug Saf. 2008 Mar;7(2):181–194. doi: 10.1517/14740338.7.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]


