INTRODUCTION
Auditory neuropathy spectrum disorder (ANSD) is a unique type of hearing loss generally characterized by present outer hair cell function and dys-synchronous auditory neural activity. Clinically, the diagnosis is defined by present otoacoustic emissions and/or cochlear microphonic with absent auditory brainstem response and middle ear reflexes. The category of ANSD encompasses several etiologies that give rise to dys-synchronous neural responses, with potential sites of lesion including the inner hair cells, their synapse with the auditory nerve, and the auditory nerve fibers themselves (Starr et al. 1996; Starr et al. 2000; Berlin et al. 2005). Whether pre- or post-synaptic in origin, the dys-synchrony in ANSD primarily results in a temporal impairment.
Patients with ANSD demonstrate impaired performance on temporal tasks such as gap detection, modulation detection, and encoding of low frequency phase-locking cues compared to those with normal hearing and sensorineural hearing loss (SNHL) (Zeng et al. 1999; Rance et al. 2004; Zeng et al. 2005; Yalcinkaya et al. 2009). In addition, in ANSD the severity of temporal impairment on psychophysical measures has a direct relationship to deficits in speech perception performance (Zeng et al. 1999; Rance et al. 2002; Rance et al. 2004). In contrast, patients with SNHL have impaired loudness discrimination, high-frequency discrimination, and frequency resolution, while these same abilities are normal in patients with ANSD (Rance et al. 2004; Zeng et al. 2005). Unlike SNHL, in ANSD temporal impairment renders audiometric thresholds unreliable in estimating the degree of impairment. In addition, the degree of hearing loss in ANSD often underestimates the difficulties of speech recognition in quiet and particularly in noise (Kraus et al. 1984; Kraus et al. 2000; Zeng and Liu 2006). The converse impairment patterns in ANSD and SNHL precludes clinically managing these patients in the same way, and optimal clinical management of patients with ANSD is currently being explored.
Current clinical intervention protocols for ANSD typically include a trial with acoustic amplification. At a minimum, hearing aids fit to the severity of the hearing loss will provide audibility of acoustic input; however, this does not guarantee adequate development of auditory and speech and language skills. A subset of children with ANSD may have speech perception in quiet and spoken language abilities comparable to matched SNHL controls (Rance et al. 2002; Rance et al. 2007; Rance and Barker 2008), however speech perception performance in noise remains significantly poorer in ANSD (Rance et al. 2007). In a retrospective analysis of 260 patients with ANSD, hearing aid outcomes were reported for 85 patients (Berlin et al. 2010). It was surmised that 14% of patients gained benefit from amplification as manifested by increased functional interactions or language acquisition, while the remaining patients reportedly showed little (25%) or no benefit (61%) with hearing aids. It appears that for many patients with ANSD amplification does not provide adequate benefit, and in these cases cochlear implantation is often recommended as the next treatment option.
The implanted pediatric ANSD population is unique in that there is higher likelihood for better hearing thresholds in the contralateral ear compared to pediatric SNHL cochlear implantees, which tend to have bilateral severe-to-profound hearing loss as a criterion for candidacy. In patients with SNHL who are unilaterally implanted, contralateral acoustic input can lead to improved speech perception, particularly when low-frequency information is available through the hearing aid (Ching et al. 2004; Qin and Oxenham 2006; Kong and Carlyon 2007; Cullington and Zeng 2010; Mok et al. 2010). In cases of unilateral implantation in patients who have ANSD and who have aidable contralateral hearing, there are questions as to management of the contralateral ear. This is particularly an issue because ANSD is characterized by impaired processing of low frequency information (Zeng et al. 1999; Rance et al. 2004; Zeng et al. 2005; Yalcinkaya et al. 2009), hence there is question as to whether the bimodal advantages found in patients with SNHL would similarly be observed in those with ANSD. In ANSD, the potential exists for acoustic input to deliver misinformation that would interfere with speech perception from the cochlear implant. Currently, clinical guidelines regarding intervention of the contralateral ear are lacking, with the choices including no intervention, amplification, occlusion, or implantation.
The purpose of this study was to examine acute effects of contralateral intervention in implanted children with ANSD and aidable contralateral hearing. Specifically, the contralateral ear was either fit with a hearing aid or occluded with an ear plug. It was hypothesized that contralateral acoustic input would interfere with speech perception achieved with the cochlear implant alone; therefore speech perception performance would decline with amplification and improve with occlusion.
SUBJECTS, MATERIALS AND METHODS
Subjects
Inclusion criteria for this study were children diagnosed with ANSD who: 1) were unilaterally implanted; 2) had aidable hearing the contralateral ear, defined as a 3-frequency pure-tone average of ≤ 80 dB HL; 3) had at least one year of cochlear implant experience; and 4) were able to perform the speech perception task. Of 20 children with ANSD being treated at the Medical College of Wisconsin Koss Cochlear Implant Program, 8 fit these criteria and all participated in the study. One subject, S3, had a pure-tone average of 97 dB HL; however, he continued to wear a hearing aid in the contralateral ear after implantation, and because he was a bimodal user was included in the study. The demographic data for the 9 participants are shown in Table 1, including pre- and post-implant electrophysiological results of these subjects. Additional clinical data are included in Table 2, including speech perception word scores obtained clinically with the cochlear implant alone, core language standard scores from the Clinical Evaluation of Language Fundamentals 4th Edition (CELF-4), primary communication mode, risk factors, and other diagnoses.
Table 1.
Demographic variables of subjects with ANSD and aidable contralateral hearing.
| Subject | Age at Test (years) |
Cochlear Implant Type |
Age at Activation (years) |
Length of Implant Use (years) |
Pre-Implant Electrophysiology (ABR) |
Post-Implant Electrophysiology (EABR or ECAP) |
|---|---|---|---|---|---|---|
| 1 | 7.6 | Med El C40+ | 1.6 | 6 | Absent | +EABR |
| 2 | 8.3 | Cochlear N24 | 3 | 5.3 | Absent | +EABR |
| 3 | 16.2 | Cochlear Freedom |
13.3 | 2.9 | Abnormal | +ECAP |
| 4 | 18.5 | Med El C40+ | 12.2 | 6.3 | Absent | −EABR |
| 5 | 7.7 | AB HiRes 90k | 3.5 | 4.2 | Original ABR waveform not available |
+ECAP |
| 6 | 6.8 | Med El Pulsar | 3.3 | 3.5 | Abnormal (R) Absent (L) |
+ECAP |
| 7 | 8.4 | AB HiRes 90k | 3.0 | 5.4 | Absent | +ECAP |
| 8 | 8.8 | AB HiRes 90k | 3.3 | 5.5 | Absent | +ECAP |
| 9 | 8.9 | AB HiRes 90k | 7.8 | 1.1 | Absent | +ECAP |
Table 2.
Clinical characteristics of subjects.
| Subject | Clinical Speech Perception Test |
Clinical Speech Perception Score (% correct) |
Chronological Age at Language Testing (years) |
CELF-4 Core language standard score |
Primary Communication Mode |
ANSD Risk Factors | Other Diagnoses |
|---|---|---|---|---|---|---|---|
| 1 | CNC recorded |
80 | 5.8 | 109 | Auditory oral | Hyperbilirubinemia (phototherapy), NICU for 3 weeks |
None |
| 2 | PBK recorded |
70 | 7.0 | 93 | Total communication |
None | Beckwith Weidemann Syndrome |
| 3 | CNC recorded |
48 | 15.2 | DNT core language |
Auditory oral | Anoxia, family history of hearing loss |
Seizure disorder |
| 4 | LNT – hard live voice |
48 | 14.2 | DNT core language |
Total communication |
Encephalopathy, family history of hearing loss, high fever with loss of developmental skills at age 4 |
None |
| 5 | PBK recorded |
80 | 6.7 | CNT – could not attend to formal eval |
Auditory oral | Born at 25 wks, 4 months in NICU, extended ventilator use |
Autism, ADHD |
| 6 | PBK recorded |
44 | 6.5 | 59 | Total communication |
None | None |
| 7 | PBK recorded |
60 | 8.1 | 78 | Total communication |
Brain bleed, NICU for 6 wks, hyperbilirubinemia with blood exchange |
None |
| 8 | PBK recorded |
36 | 9.3 | 111 | Auditory oral | Born at 32 wks, on ventilator for several wks, grade 1 intraventricular hemmorhage |
Autism |
| 9 | PBK monitored live voice |
36 | 7.8 | CNT - fatigue | Total communication |
Born at 36.5 wks, low birth weight, jaundice, blood transfusion |
Twin-to-twin transfusion disorder |
Audiometric Threshold Testing
As hearing in ANSD may show fluctuations, audiometric thresholds were measured in the acoustic ear on the day of testing using pure-tone stimuli via insert earphones (E-A-RTONE® 5A Insert Earphones (E-A-R Auditory Systems, Indianapolis, IN). The pure tone thresholds were used to create the target fitting levels for the acutely-fit hearing aid.
Cochlear Implant Settings
Subjects listened with their daily-use cochlear implant programs for all experimental conditions. Based on recent clinical records, the cochlear implant soundfield audiometric thresholds for all subjects ranged between 15-30 dB HL across frequencies 250-6000 Hz.
Hearing Aid Fitting and Occlusion
Seven of the nine subjects were fit with an Oticon Adapto P behind-the-ear (BTE) hearing aid using a Comply Tip disposable earmold. Hearing aids were fit using simulated real-ear measurements (S-REM) in which levels measured in the 2cc-coupler were converted to estimated ear canal levels using age-appropriate average real-ear-to-coupler difference levels (RECD). For these subjects, S-REM approximated DSL i/o prescribed targets for average speech using the Dynamic Stimulus setting on the Audioscan RM500, and although there is error inherent in dynamic stimulus measures for electroacoustic verification of frequency response for nonlinear hearing aid processing, it is comparable to other gain-based correction approaches (Scollie et al. 2002).
Two subjects, S3 and S9, wear a contralateral hearing aid on a daily basis. Both subjects were tested with their own hearing aids at preferred settings. S3 was bilaterally fitted with hearing aids at 3 years of age and had over 10 years of experience with amplification at the time of cochlear implantation. During participation in this study he wore his Phonak Novoforte E4 hearing aid in the contralateral ear, and at the time of testing he had 2.9 years of bimodal listening experience. S9 was bilaterally fit with Oticon Gaia BTE hearing aids 14 months prior to cochlear implantation. She continued to wear her hearing aid after cochlear implantation, and therefore at the time of testing had used bimodal hearing for 13 months.
Real ear measurements were used to quantify the occlusion provided by disposable Radians Foam Earplugs (Radians, Memphis, TN). The average attenuation was found to be 4.3, 11.7, 20, 24, 38.3 and 28.3 dB at 250, 500, 1000, 2000, 3000, and 4000 Hz, respectively, as determined by calculating the difference between the real ear unaided response (REUR) and the real ear occluded response (REOR), which were obtained through probe microphone measurements with and without the earplug placed in the subjects’ ear canals using the Audioscan RM500.
Speech Perception Testing
Speech perception testing was performed using the Children’s Realistic Index for Speech Perception Junior (CRISP Jr.) test (Garadat and Litovsky, 2007). The CRISP Jr. is a closed-set task consisting of 16 words (monosyllable and spondee) that are likely in the vocabulary of an average 2.5 to 3-year old child. All testing was conducted in the soundfield in a carpeted double-walled sound booth. Both the target and competitor stimuli were presented from speakers placed next to each other and in front of the subject (0 degrees azimuth) at a distance of 1.2 m from the center of the subject’s head. The target stimuli were recorded with a male voice and the competitor stimuli were 2-talker babble consisting of sentences spoken by a female voice. All stimuli were calibrated prior to each session (Quest Technologies 2200).
The CRISP Jr. is a 4-alternative forced choice task used to measure each subject’s speech recognition threshold (SRT), defined as 79.4% correct on the psychometric function (Levitt 1971), in both quiet and noise. Therefore, the SRT value reflects the level at which the subjects hear and correctly identify approximately 80% of the target words.
The target level was set to 60 dB SPL-A at the beginning of each adaptive trial and then varied using a modified adaptive three-down/one-up algorithm following the first incorrect response with a maximum target level of 65 dB SPL-A. In the noise condition, the competing stimuli were presented at a constant overall level of 55 dB SPL-A. While this level is somewhat lower than typical real-world challenging noisy environments, preliminary data collection using these stimuli in our laboratory indicated significant difficulties in our implanted ANSD population in achieving the 80% correct threshold with higher noise levels. Since the study aim was to examine effects of contralateral intervention, we chose a noise level that was challenging but not overwhelming, although this may limit the applicability of the results to performance in everyday noisy listening situations. For younger subjects and those not able to attend to a longer task testing was terminated following 3 reversals and SRT determined using an average of the last 2 reversals. For older subjects, testing was terminated after 4 reversals and the average of the last 3 was used to determine SRT.
Prior to testing, the subjects were familiarized with the target words, and all subjects were able to correctly identify all target words. During testing, following the presentation of the target word four randomly selected pictures including the target were displayed on a computer monitor that was placed directly below the speakers. The subjects then identified the word either verbally or by pointing. No feedback regarding the correctness of the response was provided to the subjects during testing. Each subject completed at least one adaptive track in quiet and one adaptive track with background noise in each of the three configurations: CI alone, CI+HA, and CI+plug.
This study was approved by the Children’s Hospital of Wisconsin Human Research Review Board (CHW #06/80, GC 140).
RESULTS
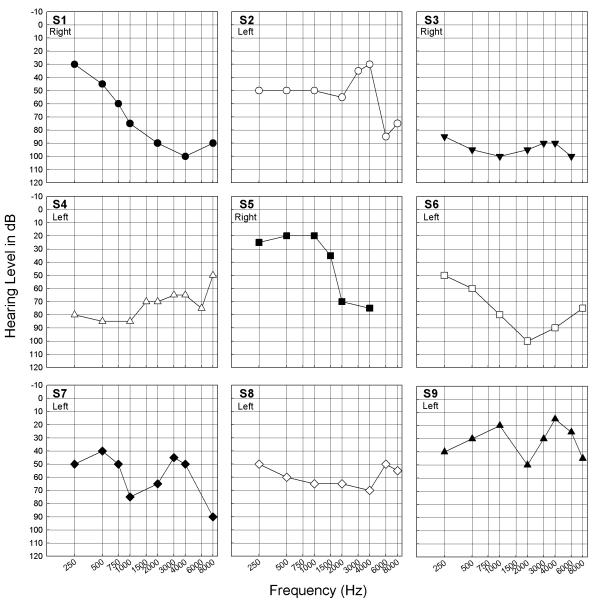
Unaided audiometric thresholds in the non-implanted ear are shown for all subjects in Figure 1. Data for each subject were plotted with unique symbols which are used in subsequent figures.
Figure 1.
Unaided acoustic audiometric thresholds in the non-implanted ear for each subject. Right or left ear is indicated in each panel, and distinct symbols were plotted for individual subjects.
Although all hearing losses were considered to be in the aidable range, there was variability in severity and configuration across subjects. When compared to audiograms obtained clinically, all subjects demonstrated stable audiometric thresholds. To compare CI performance with contralateral audiometric levels, SRTs for the CI alone configuration in quiet were compared to the 3-frequency pure tone average (.5, 1, 2 kHz) for each subject. There was no relationship between contralateral acoustic hearing levels and CI performance in this subject population (r=−.01; slope=0.007; p=.98).
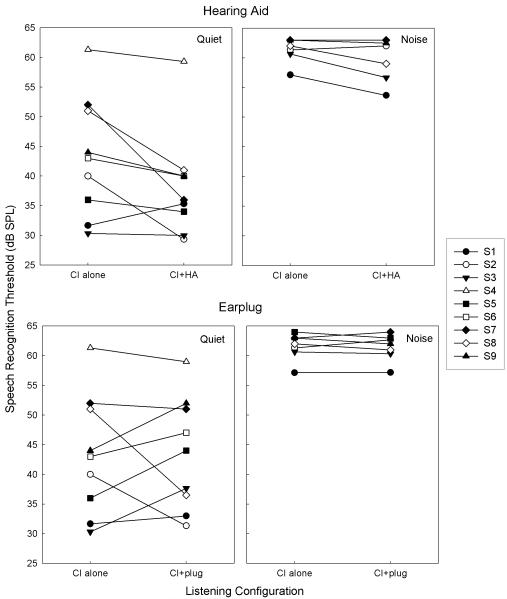
To examine the effects of contralateral intervention on CI performance, SRTs in the CI-alone configuration for each subject were compared to the SRTs for the CI+HA (Figure 2, top) and CI+plug (Figure 2, bottom) configuration. SRTs in quiet for the CI-alone configuration ranged from 30.3 to 61.3 dB, with a mean of 43.2 dB (10.1 SD). SRTs in quiet for the CI+HA configuration in quiet ranged from 29.3 to 59.3 dB with a mean of 38.3 dB (8.9 SD), with 7 of 9 subjects showing an SRT decrease. In both conditions, S4 demonstrated outlying SRT values that were more than 2 SD above the mean SRT for the other subjects. The lower mean SRT for the CI+HA condition for all subjects represented a statistically significant improvement in SRT with contralateral amplification compared to CI-alone (paired t-test, df=8; p=.04).
Figure 2.
Comparison of SRT for the CI-alone to the CI+HA (top) and CI+plug (bottom) configurations for quiet and noise conditions. Smaller SRT values indicate better performance. A statistically significant improvement in SRT with contralateral amplification compared to CI-alone was observed (paired t-test, df=8; p=.04).
In noise, SRTs were measurable for 6 of the 9 subjects, with three subjects (S4, 5, and 6) experiencing floor effects. Specifically, they were unable to achieve 80% correct word identification with background noise of 55 dB SPL and a maximum target level of 65 dB SPL. For the 6 children who were able to reach the word identification threshold in noise, SRTs for CI-alone in noise ranged from 57.2 to 63 dB, with a mean of 61.6 dB (2.2 SD), while SRTs for CI+HA ranged from 53.7 to 63 dB, with a mean of 59.5 dB (3.7 SD). The two subjects who did not show a decrease in SRT with CI+HA in quiet (S1 and S3) demonstrated a decrease with CI+HA in noise. The differences between CI-alone and CI+HA in noise for the group were not statistically significant (paired t-test, df=5; p=.09).
Changes in SRT with a contralateral earplug compared to CI-alone in quiet were mixed, with increases in SRT for 5 subjects and decreases in SRT for 4 subjects. The range of SRTs for the CI+plug configuration were 31.3 to 59 dB, with a mean of 43.5 dB (9.5 SD). This was not significantly different from the CI-alone SRT values (paired t-test, df=8; p=.93). In noise, 7 of the 9 subjects were able to complete testing with 2 subjects experiencing floor effects. The SRTs ranged from 57.2 to 64 dB, with a mean of 61.4 dB (2.2 SD), with no significant difference from the CI-alone configuration (paired t-test, df=6; p=0.72).
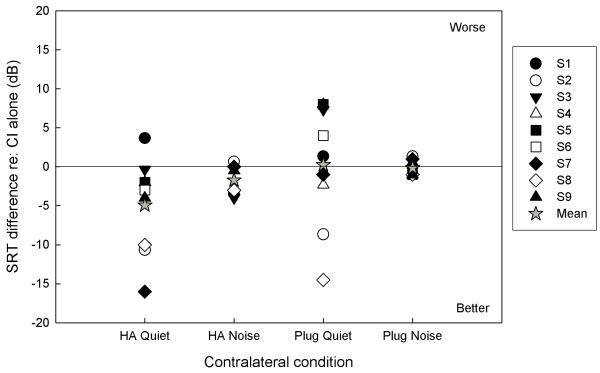
Effects of contralateral intervention for all subjects across conditions are summarized in Figure 3. Changes in performance were determined by subtracting SRTs with contralateral intervention from SRTs with the CI-alone; therefore, negative values indicate improved performance with contralateral intervention. The change in SRT with CI+HA in quiet ranged from +3.7 to −16.0 dB, and the average across-subjects decrease in SRT was 4.92 (SD=6.1). The power to detect this 4.92 decrease on 9 subjects was 56.6%. In noise, the range of SRT change for CI+HA was +0.7 to −4.0 dB, and the average across-subjects decrease in SRT was 1.71 dB (SD=2.0). The power to detect this 1.71 dB decrease on 6 subjects was 61.5%. For the CI+plug configuration, changes in SRT in quiet ranged from +8.0 to −14.5 dB with a mean increase of 0.24 dB (7.8 SD).
Figure 3.
Effects of contralateral intervention across listening configurations and conditions. Differences in SRT were calculated relative to the CI-alone SRT; therefore negative values indicate an improvement with contralateral intervention. Mean values are shown with star symbols.
The effect of the earplug on SRT in noise ranged from +1.34 to −1.0 dB with a mean increase of 0.14 dB (1.0 SD).
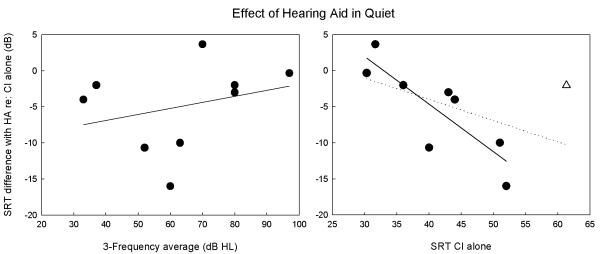
Improvements in SRT were observed most consistently in the CI+HA configuration in quiet. Therefore, data for this configuration were further analyzed to examine factors that may predict decreases in SRT with contralateral amplification. Change in SRT with the hearing aid was plotted against 3-PTA of the amplified ear (Figure 4, left). Regression analyses showed no relationship (r=.29, slope=.08, p=.46), indicating that contralateral acoustic hearing levels were not associated with bimodal effects on SRT in this subject population. Hearing loss configuration was also analyzed by grouping the subjects into those with ‘sloping’ (i.e., thresholds at 4 kHz at least 25 dB higher than 250 and 500 Hz) and ‘flat’ hearing loss (i.e., thresholds at 4 kHz within 25 dB of 250 and 500 Hz). Subjects 1, 5, and 6 fell into the sloping category, and subjects 2,3,4,7, 8, and 9 in the flat category. The mean differences in CI+HA SRT in quiet were −0.44 (3.6 SD) for the sloping group and −7.2 (6.0 SD) for the flat group. Although the flat group tended to have a greater overall decrease in SRT, mean differences were not significant (independent samples t-test, df=7; p=.12).
Figure 4.
Difference in SRT between CI+HA and CI-alone in quiet was plotted against 3-PTA in the contralateral ear (left) and CI-alone performance (right). No association was found between SRT difference and 3-PTA. A statistically significant relationship was found between change in SRT and CI-alone performance when an outlying poor CI performer was excluded (r=−.83, slope=−.66, p=.01), as indicated by the solid regression line. The dotted regression line shows the analysis that included the outlying performer, and this correlation was not significant.
Another factor analyzed was the baseline performance with the CI. Change in SRT with the hearing aid was plotted against SRT of the CI-alone (Figure 4, right). One subject, S4, was considered an outlier with respect to baseline CI performance, with CI-alone SRT > 2 SD above the mean. Regression analyses performed without S4 showed a significant decrease in SRT with the hearing aid as CI-alone SRT increased (r=−.83, slope=−.66, p=.01; solid line). When S4 was included in the analysis, the correlation was not significant (r=−.49, slope=−.29, p=.18; dotted line). These results indicate that bimodal advantage increased as CI performance decreased, however, there is a floor effect for this relationship at the level of masker chosen for the current study.
DISCUSSION
The purpose of this study was to examine acute effects of amplification and occlusion in the contralateral ear of implanted children with ANSD and aidable contralateral hearing. While it was hypothesized that contralateral acoustic input would interfere with speech perception as achieved with a cochlear implant, the results of this study do not support that hypothesis. Specifically, in quiet, contralateral occlusion did not demonstrate consistent a effect on SRT while contralateral amplification showed a statistically significant improvement for the group.
While the group results did not indicate overall benefit from contralateral occlusion, there were improvements in SRT observed for 4 of the 9 subjects. This may indicate that some implanted children with ANSD may benefit from contralateral occlusion, and clinical intervention should still be considered on an individual basis and reevaluated over time. The consistent improvement in SRT observed across subjects for contralateral amplification was further analyzed in this study.
All subjects demonstrated at least some benefit with acute contralateral amplification either in the quiet or noise conditions, or both. The effects of contralateral amplification on SRT in the present study are consistent with an investigation of adult patients with ANSD who demonstrated bimodal benefit in speech perception performance. In three patients with ANSD who were implanted as adults, Zeng & Liu reported an average improvement of 17% for sentence scores in quiet with the addition of a contralateral hearing aid. In noise, variable effects of bimodal hearing were observed across signal-to-noise ratios within and across subjects, with no significant effect overall (Zeng and Liu 2006).
Effects of bimodal simulation on speech recognition have been more extensively studied in subjects with SNHL. While there tends to be wide variation in methodology among these investigations, bimodal listening tends to demonstrate improved or, at a minimum, equivalent performance compared to that with the cochlear implant alone, particularly when controlling for influential factors such as type of HA, HA fitting procedure, and loudness balancing of the CI with the HA (Ching et al. 2004; Dunn et al. 2005; Luntz et al. 2005; Beijen et al. 2008; Keilmann et al. 2009; Potts et al. 2009; Mok et al. 2010). With a nearly identical test paradigm to the present study [the CRISP test (Litovsky 2005)], Litovsky and colleagues (2006) examined bimodal benefit in 10 children with SNHL by comparing SRTs in the CI alone and CI+HA conditions in quiet and in noise (Litovsky et al. 2006). Overall, they found that SRTs tended to be poorer in the bimodal condition, however there was high inter-subject variability with some children performing much better, and others much worse with the addition of a contralateral HA. Examination of the subjects’ audiograms did not reveal factors that could easily account for these differences. Because the subjects wore their own hearing aids and travelled from other clinics to participate in the study, the authors attributed the high inter-subject variability to lack of control over the types of amplification used in the study and differences in clinical programming of the devices. In addition, several subjects were unable to achieve loudness balancing between the CI and HA. In the present study, all subjects were seen clinically within the same facility, and for 7 of the 9 subjects the HA was the same as was the fitting procedure. While loudness balancing was not performed, the controlled factors may have contributed to the relatively consistent bimodal benefits observed with SRT.
Findings of contralateral hearing aid benefit in implanted patients with ANSD are compelling, primarily because pre-implant hearing aid trial outcomes are often limited to improved sound detection with minimal skill development, thus designating the patients as CI candidates. Electrical stimulation from a cochlear implant elicits highly synchronized neural responses, effectively restoring temporal acuity in implanted patients with ANSD. Comparable levels of temporal processing with CI use have been demonstrated between ANSD and SNHL with electrically-evoked auditory neural responses (Peterson et al. 2003; Runge-Samuelson et al. 2008; Teagle et al. 2010) and speech perception performance (Trautwein et al. 2000; Buss et al. 2002; Peterson et al. 2003; Rance and Barker 2008). Improved temporal resolution with CI use in ANSD may subsequently improve perception of acoustic input from the contralateral ear, resulting in bimodal benefit. As the purpose of this study was to investigate acute effects of contralateral intervention on CI performance, however, we did not measure speech perception for the hearing aid alone and are unable to compare individual ear to bimodal performance over time.
Given the observation of bimodal improvement in SRT in quiet for the subjects in the present study, further analyses were performed to examine potential factors that may predict the performance improvements. One potential factor was severity of hearing loss in the aided ear, as more residual hearing may result in greater bimodal benefit. However, no relationship was found between hearing threshold levels and effect of amplification. Configuration of hearing loss in the contralateral ear was also examined. Mok and colleagues (2010) showed frequency-specific aided threshold effects on bimodal benefit in unilaterally implanted children with SNHL. Specifically, a combination of better aided thresholds at 250 and 500 Hz and poorer aided thresholds at 4 kHz resulted in a significantly greater bimodal advantage. Although aided thresholds were not obtained in the present study, the subject group with sloping hearing loss tended to show a smaller bimodal benefit than the group with flat hearing loss. Interestingly, this trend is opposite to the findings for SNHL, presenting the possibility that the mechanisms behind bimodal benefit in ANSD and SNHL may be different. This is preliminary, however, and warrants further investigation. In general, the lack of relationship between bimodal benefit and the variables of hearing level and audiometric configuration may not be surprising given that, with acoustic stimuli, pure tone thresholds are not related to speech perception abilities in the population with ANSD (Kraus et al. 2000; Zeng and Liu 2006).
Another potential predictive variable for bimodal effects was baseline performance with the cochlear implant. A significant relationship was found between improved SRTs in the bimodal condition and performance with the CI alone, indicating that children with poorer CI performance experienced greater benefit from contralateral amplification. There appeared to be a limit to this effect, however, as the outlying poor performer (S4) showed a relatively small decrease in SRT. In cases of poor CI performance with ANSD, it is possible that significant temporal impairment (peripheral or central) persists to the extent that the CI is unable to provide adequate habilitation. The lack of measureable EABR in this subject is indicative of a high degree of dyssynchrony and is consistent with other findings of poor CI performance (Rance et al. 1999; Rance and Barker 2008). As such, encoding of acoustic information may be similarly impaired, and as a result the potential for bimodal effects may be limited. Lack of bimodal benefit in poorly-performing CI users with ANSD has been reported elsewhere. In a clinical report of implanted children with ANSD, Teagle and colleagues described bimodal outcomes for 8 children who wore CI+HA in their everyday listening condition (Teagle et al. 2010). Two children never discontinued HA use after implantation, and six children who had performed poorly with the CI were fit with contralateral amplification once it was determined that the CI provided only minimal benefit. For all subjects, the authors reported no improvement in performance in the bimodal configuration over CI alone, although the children reported benefits in localization and visual communication. It is possible that the 6 children with poor performance in that study were demonstrating floor effects on the CI listening condition similar to S4 in the present study. For the other 2 children, the CI-alone performance was not specified; therefore, it is not known if they might represent either floor or ceiling performance. In addition, all speech perception testing was performed in quiet, leaving open the possibility of observing bimodal benefit if more challenging listening conditions were used, particularly for ceiling performers in quiet.
CONCLUSION
The results of this study indicate that children with ANSD who are experienced cochlear implant users may benefit from contralateral amplification, particularly for moderate CI performers. It is unclear from these data whether long-term contralateral hearing aid use in real-world situations would ultimately benefit this population, however a hearing aid trial is recommended with assessment of bimodal benefit over time. These data may help inform clinical guidelines for determining optimal hearing configurations for unilaterally implanted children with ANSD, particularly when considering candidacy for sequential cochlear implantation.
ACKNOWLEDGEMENTS
The authors are grateful for the participation of the children and their families. The impetus of this study occurred during a cochlear implant team meeting when Sarah Drake, Au.D., initially posed the question as to what to do with the contralateral ear in our implanted patients with ANSD and aidable contralateral hearing. We would like to thank Susan Fulmer, M.D., Laurin Friedland, and Bradley Schow, B.S. for their assistance in data collection. Jane Kellerman contributed significantly to subject recruitment. Two anonymous reviewers contributed highly valuable comments. We acknowledge the contributions of the Masters Family Speech and Hearing Center at the Children’s Hospital of Wisconsin.
Portions of this work were presented orally at the 2010 American Auditory Society Meeting in Scottsdale, Arizona, and the 12th Symposium on Cochlear Implant in Children, Seattle, Washington. Funding was provided by NIDCD K23DC008837.
REFERENCES
- Beijen JW, Mylanus EA, Leeuw AR, et al. Should a hearing aid in the contralateral ear be recommended for children with a unilateral cochlear implant? Ann Otol Rhinol Laryngol. 2008;117(6):397–403. doi: 10.1177/000348940811700601. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder) Int J Audiol. 2010;49(1):30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, et al. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy/dys-synchrony. J Am Acad Audiol. 2005;16(8):546–553. doi: 10.3766/jaaa.16.8.3. [DOI] [PubMed] [Google Scholar]
- Buss E, Labadie RF, Brown CJ, et al. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol. 2002;23(3):328–332. doi: 10.1097/00129492-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25(1):9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Cullington HE, Zeng FG. Bimodal hearing benefit for speech recognition with competing voice in cochlear implant subject with normal hearing in contralateral ear. Ear Hear. 2010;31(1):70–73. doi: 10.1097/AUD.0b013e3181bc7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48(3):668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Keilmann AM, Bohnert AM, Gosepath J, et al. Cochlear implant and hearing aid: a new approach to optimizing the fitting in this bimodal situation. Eur Arch Otorhinolaryngol. 2009;266(12):1879–1884. doi: 10.1007/s00405-009-0993-9. [DOI] [PubMed] [Google Scholar]
- Kong YY, Carlyon RP. Improved speech recognition in noise in simulated binaurally combined acoustic and electric stimulation. J Acoust Soc Am. 2007;121(6):3717–3727. doi: 10.1121/1.2717408. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, et al. Consequences of neural asynchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1(1):33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Ozdamar O, Stein L, et al. Absent auditory brain stem response: peripheral hearing loss or brain stem dysfunction? Laryngoscope. 1984;94(3):400–406. doi: 10.1288/00005537-198403000-00019. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(2):467. Suppl 2. [PubMed] [Google Scholar]
- Litovsky RY. Speech intelligibility and spatial release from masking in young children. J Acoust Soc Am. 2005;117(5):3091–3099. doi: 10.1121/1.1873913. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol. 2006;45(Suppl 1):S78–91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz M, Shpak T, Weiss H. Binaural-bimodal hearing: concomitant use of a unilateral cochlear implant and a contralateral hearing aid. Acta Otolaryngol. 2005;125(8):863–869. doi: 10.1080/00016480510035395. [DOI] [PubMed] [Google Scholar]
- Mok M, Galvin KL, Dowell RC, et al. Speech perception benefit for children with a cochlear implant and a hearing aid in opposite ears and children with bilateral cochlear implants. Audiol Neurootol. 2010;15(1):44–56. doi: 10.1159/000219487. [DOI] [PubMed] [Google Scholar]
- Peterson A, Shallop J, Driscoll C, et al. Outcomes of cochlear implantation in children with auditory neuropathy. J Am Acad Audiol. 2003;14(4):188–201. [PubMed] [Google Scholar]
- Potts LG, Skinner MW, Litovsky RA, et al. Recognition and localization of speech by adult cochlear implant recipients wearing a digital hearing aid in the nonimplanted ear (bimodal hearing) J Am Acad Audiol. 2009;20(6):353–373. doi: 10.3766/jaaa.20.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin MK, Oxenham AJ. Effects of introducing unprocessed low-frequency information on the reception of envelope-vocoder processed speech. J Acoust Soc Am. 2006;119(4):2417–2426. doi: 10.1121/1.2178719. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing AIDS or cochlear implants. Otol Neurotol. 2008;29(2):179–182. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ, Sarant JZ, et al. Receptive language and speech production in children with auditory neuropathy/dyssynchrony type hearing loss. Ear Hear. 2007;28(5):694–702. doi: 10.1097/AUD.0b013e31812f71de. [DOI] [PubMed] [Google Scholar]
- Rance G, Beer DE, Cone-Wesson B, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20(3):238–252. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, et al. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25(1):34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Runge-Samuelson CL, Drake S, Wackym PA. Quantitative analysis of electrically evoked auditory brainstem responses in implanted children with auditory neuropathy/dyssynchrony. Otol Neurotol. 2008;29(2):174–178. doi: 10.1097/mao.0b013e31815aee4b. [DOI] [PubMed] [Google Scholar]
- Scollie SD, Steinberg MJ, Seewald RC. Evaluation of electroacoustic test signals II: development and cross-validation of correction factors. Ear Hear. 2002;23(5):488–498. doi: 10.1097/00003446-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11(3):215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- Teagle HF, Roush PA, Woodard JS, et al. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31(3):325–335. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- Trautwein PG, Sininger YS, Nelson R. Cochlear implantation of auditory neuropathy. J Am Acad Audiol. 2000;11(6):309–315. [PubMed] [Google Scholar]
- Yalcinkaya F, Muluk NB, Atas A, et al. Random Gap Detection Test and Random Gap Detection Test-Expanded results in children with auditory neuropathy. Int J Pediatr Otorhinolaryngol. 2009;73(11):1558–1563. doi: 10.1016/j.ijporl.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, et al. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49(2):367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Stickney GS, et al. Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci U S A. 2005;102(7):2293–2298. doi: 10.1073/pnas.0406460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, et al. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10(16):3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]