Abstract
This perspective examines how hundreds of pigment molecules in purple bacteria cooperate through quantum coherence to achieve remarkable light harvesting efficiency. Quantum coherent sharing of excitation, which modifies excited state energy levels and combines transition dipole moments, enables rapid transfer of excitation over large distances. Purple bacteria exploit the resulting excitation transfer to engage many antenna proteins in light harvesting, thereby increasing the rate of photon absorption and energy conversion. We highlight here how quantum coherence comes about and plays a key role in the photosynthetic apparatus of purple bacteria.
Keywords: exciton, FRET, excitation transfer, purple bacteria
Life on Earth is sustained mainly by the energy of sunlight harvested through photosynthesis.1 Photosynthetic bacteria, algae, and plants employ for this purpose a molecular machinery that captures and transforms solar energy into a proton gradient across the cellular membrane which is then used to drive a multitude of cellular processes. The photosynthetic systems seen today likely evolved into enormous diversity from a common ancestor,1 since the physical principles employed by these systems are universal in many respects.
Photosynthesis converts light energy into successively more stable forms of energy storage, from light absorption into a nanosecond-lived electronic excitation to a charge-separated state, and finally to a membrane proton gradient. Light harvesting starts with the absorption of a photon by chlorophyll or carotenoid pigments that are embedded within an ensemble of proteins; the systems of pigment and protein are called light harvesting complexes.2 The most crucial step in light harvesting converts the electronic excitation into a charge-separated state across the cellular membrane. This conversion is done at a pigment-protein complex known as the reaction center (RC).3 Emerson and Arnold established in 1932 that hundreds of pigment molecules cooperate in light-harvesting.4 This cooperation has fascinated biophysicists since then, but only today do we know the molecular structures involved so that the physical mechanism behind the cooperation can be resolved.5–9
The cooperating pigments display a hierarchical pattern of tight packing and, as a result, exhibit a system of strong and weak electronic interactions that is essential for efficient light harvesting. Within the most strongly interacting groups of pigments, electronic excitation is spread coherently following light absorption;10–18 however, between pigment groups that are weakly coupled, electronic excitation is shared incoherently, namely through random excitation transfer.5,7,19–21 The coherent spread is known as exciton dynamics22–25 and the incoherent spread as Förster resonant energy transfer (FRET).9,25–29 Photosynthetic light harvesting interweaves both behaviors. Additionally, some pigments fall into an intermediate coupling regime.30–33 In this regime there is a small amount of coherent spread of electronic excitation, though it is not as well understood how much this influences the efficiency of light harvesting in purple bacteria.
Recently, the role that quantum coherence may play for efficient light harvesting has caught some notoriety (discussed also in a prior Perspective34), particularly due to experiments results by Fleming, Engel and Scholes.35–39 In time-resolved two-dimensional spectroscopy it is possible to see oscillations of exciton state populations, special initial states prepared by carefully chosen laser pulses. The oscillations, lasting up to a few hundred femtoseconds, are attributed to quantum coherence emerging as a result of the initially prepared coherent quantum state and decay rapidly (compared to the typical lifetime of excitation in photosynthetic systems of one nanosecond). It is not presently known how much this phenomenon contributes to efficient light harvesting.40 The quantum coherence that we discuss in this Perspective arises through strong coupling between chlorophyll molecules making close contact with each other in the proteins of the photosynthetic apparatus. The focus of this review is to explain how this quantum coherence increases efficiency41 and architectural flexibility8 in the light harvesting system of a fundamental photosynthetic life form, namely purple bacteria.1,2,42
The Reaction Center
The crucial step in photosynthetic light harvesting is the conversion of short-lived excitation energy, resulting from photon absorption, to a form that can be more leisurely used by a living cell, namely that of a charge gradient. This step takes place in a protein complex known as the reaction center (RC). The function of the RC is to receive excitation energy, either by absorbing a solar photon directly, or by excitation transfer from the pigment molecules of nearby light harvesting complexes, and to convert the excitation into a charge-separated state.43–46
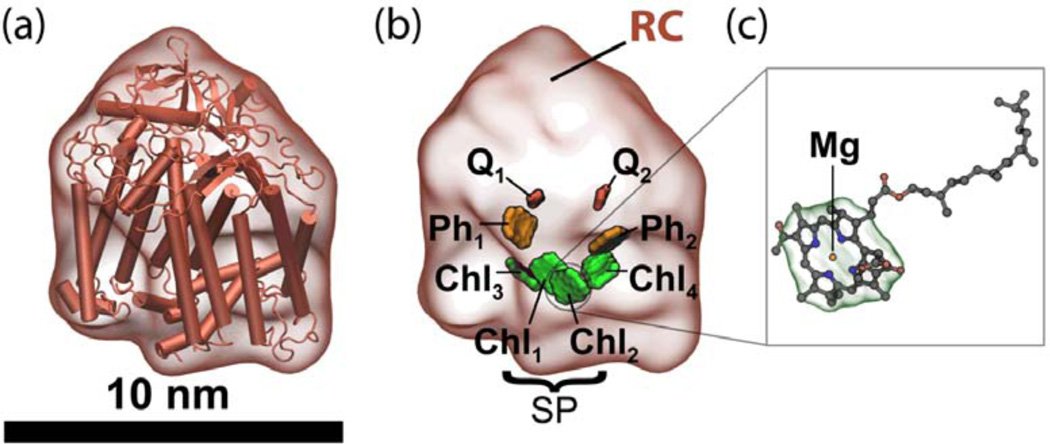
The reaction center contains four bacteriochlorophyll (BChl) pigments and two bacteriopheophytins (akin to a BChl without its magnesium) that absorb light (Figure 1). The light energy absorbed by all six pigments is eventually delivered as an excitation to the central pair of the four BChls, the so-called special pair (SP = Chl1 + Chl2, Figure 1). Light absorbed by pigments with higher electronic excitation energy (Chl3, Chl4, Ph1, Ph2) leads to coherent (excitonic) oscillations between excited states involving these pigments, as shown in recent experiments,36 before settling into pigments with lower electronic excitation energy, namely Chl1 and Chl2 of the SP. The SP initializes charge separation by transferring an excited electron through Chl3 to a nearby pigment, a bacteriopheophytin (Ph1). The electron subsequently transfers to a permanently bound molecule of quinone, Q1, and, finally, to a second, exchangeable quinone, Q2. The series of electron transfers establish within about a hundred microseconds a charge separated state .
Figure 1.
(a) Cartoon representation of the photosynthetic reaction center protein with surface outline. (b) Surface outline of the reaction center showing bacteriochlorophylls (Chl1, Chl2, Chl3 and Chl4) in green, bacteriopheophytins (Ph1 and Ph2) in orange and quinones (Q1 and Q2) in red. The central bacteriochlorophylls, Chl1 and Chl2, form the so-called special pair (SP). (c) Atomic structure of a BChl.
A chlorophyll under bright daylight conditions would absorb about 10 photons every second, fewer still in the actual dark habitat of purple bacteria, e.g., at the bottom of ponds. As a result, the RC would be idling most of the time, had biological photosynthesis not evolved a feeder system of pigments. This feeder system comprises mainly of an array of external BChls that funnel electronic excitation to the RC through the FRET mechanism at a high enough rate to keep the RC busy forming charge separated states, keeping the bacterium from starving. However, there is a principle obstacle to this feeder strategy, namely the tendency of BChls too close to the RC to intercept electron transfers towards the charge separated state and lead the captured electrons astray.
Fortunately, the rate of electron transfer to BChls has a shorter range than FRET from BChls. This is because electron transfer involves tunneling and decays exponentially with SP-BChl separation, while FRET involves (induced dipole – induced dipole) Coulomb interaction and thus the transfer rate decays as 1/R6.9,29 As a result, the latter process has a wider effective range. This range difference permits a corridor around the RC, i.e., a circular region where BChls are far enough to prevent electron transfer away from the RC, yet close enough for efficient energy transfer to the SP.2,6 Such a corridor, seen in a wide range of photosynthetic organisms, can be realized apparently only because the SP exhibits a unique FRET potential, without which the FRET range would potentially be shorter than the electron transfer range.
FRET is widely employed in the modern physics laboratory for single molecule measurement of distances.47,48 The well known expression for the rate of FRET between a donor (D) and an acceptor (A) molecule, reviewed recently,9 is
| (1) |
Here is a geometrical factor, usually near unity, accounting for the orientation of the two molecules; JDA is the spectral overlap between donor emission and acceptor absorption; RDA is the center-center distance between donor and acceptor molecules. The key molecular properties determining the range over which the nanosecond-lived electronic excitation can be transferred are the so-called transition dipole moments dD and dA characterizing the relevant donor and acceptor emission and absorption processes. Excitation transfer described by Eq. (1) occurs between weakly interacting pigments, where the point-dipole approximation is valid (with inter-pigment separations > 1 nm). In the intermediate or strong interaction regimes, more complex formalisms are needed to describe excitation transfer.21,32 As such, Eq. (1) cannot be used to calculate excitation transfer within any individual light-harvesting complex, but is valid for excitation transfer between complexes.21
The RC utilizes quantum coherence to achieve a particularly high |dA| value to enhance its potential for FRET. For this purpose the RC poses Chl1 and Chl2 of the SP within a close (8 Å) Mg–Mg distance of each other such that their excited states are strongly coupled, namely by about 500 cm−1 (8066 cm−1 = 1 eV).49 As a result, electronic excitation of one of the BChls becomes coherently shared among Chl1 and Chl2, even under the circumstances of high physiological temperature T (kBT = 209 cm−1). The excited SP is then found in the states
| (2) |
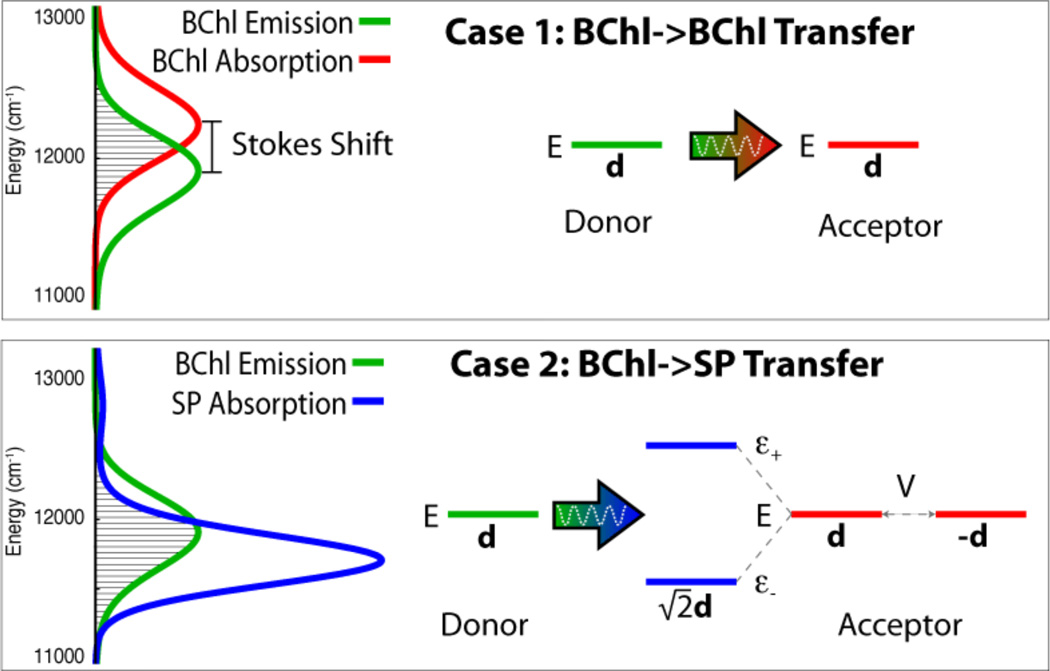
where |1〉, |2〉 represent the excited states of Chl1, Chl2. The states that share the BChl excitations coherently with each other are called exciton states; according to basic quantum physics, the excitons have an energy difference of 1000 cm−1; as a result the absorption spectrum of the SP is split into two lines. A rather straightforward calculation reveals that, given the sign of the coupling energy and the directions of d1 and d2, the transition dipole moment of the lowest energy exciton state is , where |d| = |d1| = |d2|, while the upper exciton state has an almost vanishing transition dipole moment. From this calculation, one can conclude that the lower energy exciton has a stronger FRET potential with a 12% further FRET range than that a single BChl has (dA = d), e.g., a range of 56 Å for the SP instead of only 50 Å for an individual BChl. The comparison assumes that the shifted exciton state has at least the same spectral overlap with feeder BChls as the individual SP BChls. Actually, energy shifts arising through exciton formation in light harvesting systems usually improve spectral overlap (see Figure 2).
Figure 2.
When excitation is transferred from a donor bacteriochlorophyll (BChl) to an acceptor BChl, the Stokes shift between the emission and absorption spectra causes an imperfect energy overlap, as shown in Case 1 (see filled area illustrating overlap JDA). This results in a reduced rate of excitation transfer. The reaction center can counter the reduced overlap by introducing a second acceptor BChl that is strongly coupled to the first, forming the special pair (SP). Strong coupling, accounted for by an interaction energy of V = 500 cm−1 (V is determined in Ref. 50), coherently spreads excitation between the two acceptor SP BChls, shifting also the SP exciton energies from the single BChl excited state energy E to energies ε− and ε+. This shift alters the absorption spectrum, as shown in Case 2, and accordingly increases the overlap, JDA, between emission (green line) and absorption (blue line) spectra (see filled area). Furthermore, due to the anti-parallel orientation of the SP BChl’s transition dipole moments, the lower energy exciton state attains a transition dipole moment of . The combination of better spectral overlap JDA and a stronger dA value increases the rate of excitation transfer for Case 2 over that of Case 1.
Light Harvesting Complex 1
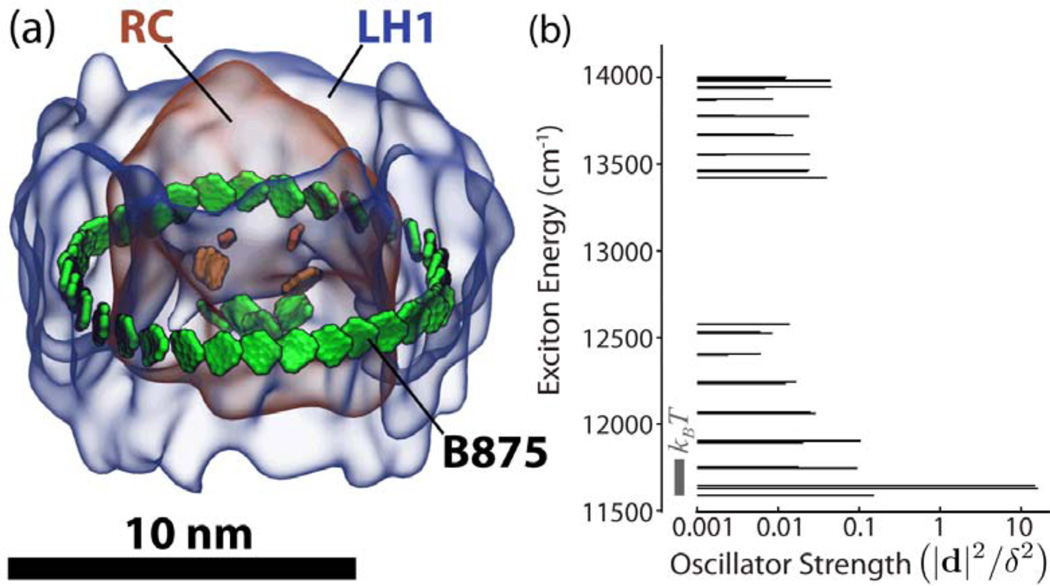
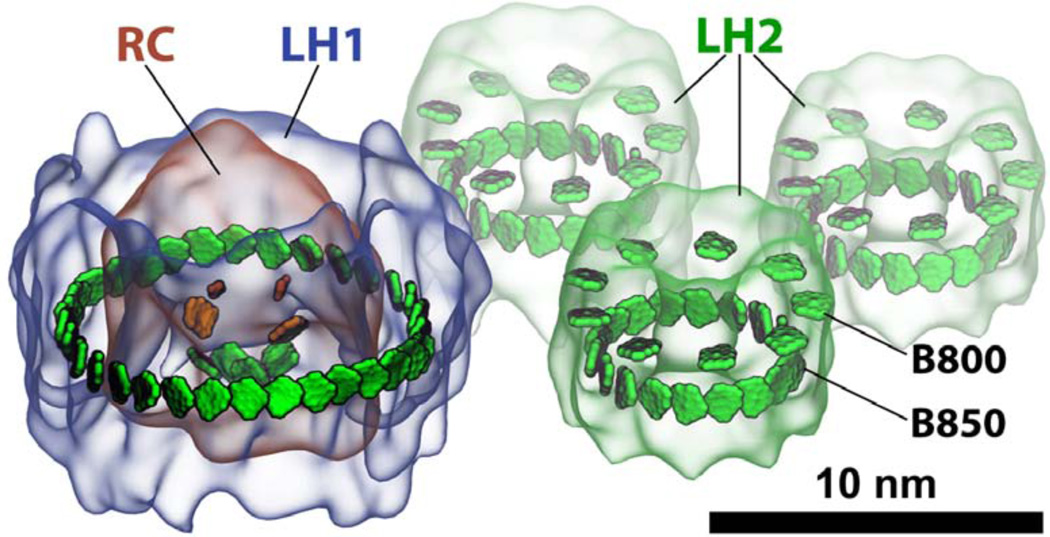
The purple bacterial light harvesting system, indeed, places feeder BChls in the expected ring-shaped corridor around the SP of the RC. The feeder system is an awesome structure,51 having 32 BChls stacked as close as feasible in the ring as shown in Figure 3. The BChls are held by a protein complex, called light harvesting complex 1 (LH1), that is made of 32 separate transmembrane α-helices forming a scaffold for the BChls and 16 carotenoids, the latter important light harvesting partners,50 not further considered here.
Figure 3.
(a) Light harvesting complex 1 (LH1) surrounding the photosynthetic reaction center. The 32 LH1 bacteriochlorophylls forming the B875 ring are shown in green. (b) Exciton spectrum and oscillator strengths of the B875 ring. The transition dipole moment of a single bacteriochlorophyll is given by δ = 6.3 Debye. The gray bar indicates the amount of thermal energy at 300 Kelvin.
The 32 BChls and 16 carotenoids greatly increase the cross section for light absorption of the RC, but the BChl ring, known as the B875 ring for its absorption peak at 875 nm, should be far enough away from the SP to prevent electron tunneling from the excited SP, but close enough to transfer electronic excitation faster than the excitation lifetime, τ0, of a nanosecond; an acceptable transfer time, τ1, is 50 picosecond or less (corresponding to a transfer efficiency of τ0/(τ0 + τ1) = 95 %). To reach such quick FRET does not only require a large FRET potential on the SP side, but also on the side of the LH1 BChl ring.
The BChls in the LH1 ring are packed together nearly as tightly as the two SP BChls with an average Mg–Mg distance of 1 nm. The tight packing results again in strong (compared to kBT) electronic interaction between nearest-neighbors, such that excitation is coherently shared between the LH1 BChls despite thermal disorder,10,18,41,52–54 just as it is in the SP of the RC. The coherent spread of excitation over single BChl states |j〉, j = 1, …, 32, is described by exciton states for μ = 1, …, 32, where αμj are the expansion coefficients of the exciton states in the site basis and are calculated from the B875 Hamiltonian, as shown in Ref. 30.
The exciton spectrum of the B875 ring is shown in Figure 3 along with the squared transition dipole moment values of each state, the so-called oscillator strengths. One can recognize that the exciton energies are spread over 2450 cm−1, a value that exceeds kBT 12-fold, such that thermalization among the exciton states, which arises within 1–2 picoseconds after light absorption into the B875 ring,21 leads to a significant population gradient among the exciton states.53,54
After fast thermalization, the B875 excitons transfer their energy to the SP states as described by a generalization of the FRET rate expression (Eq. (1))
| (3) |
where Vμν is the interaction energy between LH1 exciton state and SP exciton state and Jμν is the two states’ spectral overlap density.9,25,27,28 Accounting for the thermal populations pμ of the LH1 exciton states, the overall transfer rate is
| (4) |
Calculation of the FRET rate kLH1→SP, accounting for the actual geometry of the LH1-RC complex shown in Figure 3, yields a value of 1/(30 ps) which is high enough compared to the excitation decay rate of 1/ns. The corresponding FRET rate for a single LH1 BChl and a single SP Chl (average taken over Chl1 and Chl2) is calculated to be 1/(480 ps). The comparison shows that quantum coherence greatly accelerates LH1 → SP FRET, making it feasible to extend light harvesting capacity by engaging 32 LH1 BChls.
Light Harvesting Complex 2
It turns out that in the dark habitat of many purple bacteria, even the LH1 pigments do not feed enough excitation to the RC for cells to thrive. Purple bacteria evolved a further pigment pool to absorb passing photons more completely and increase excitation feed to the RC. For this purpose the bacteria simply extend the exciton mechanism described for LH1. One might guess at this point that the bacteria simply add more “empty” LH1 rings next to the LH1-RC complex, but that solution would leave gaping holes in the middle of the additional LH1 rings, i.e., would not amount to a maximal pigment density. Also, LH1 rings are only stable when they surround an RC. Instead, the bacteria add around the LH1-RC complexes smaller, stable ring proteins. These proteins are highly homologous to the LH1 protein, but they form rings of only about half the size. The smaller rings, called light harvesting complex 2 (LH2) and shown in Figure 4, involve a scaffold of 18 (in some cases 16) separate trans-membrane helices with 27 (24) BChls and 9 (8) carotenoids.55,56
Figure 4.
One LH1-RC complex with three LH2 complexes nearby. The upper and lower rings of BChls in LH2 are the B800 and B850 rings, respectively.
The LH2 BChls form actually two BChl rings, a B850 ring and a B800 ring, named after their absorption peaks at 850 nm and 800 nm.57 The B850 ring contains 18 (16) BChls that are, in the same fashion as the BChls of the B875 ring, tightly packed with strong nearest-neighbor interactions. The B800 BChls, also seen in Figure 4, are not spaced as tightly and, as a result, do not form thermally stable excitons. Indeed, they also do not contribute to long-range FRET, their role being to add more absorption power; with an orientation perpendicular to the B850 BChls, the B800 BChls optimally absorb photons polarized in the membrane plane. Once a B800 BChl absorbs light, it transfers the excitation quickly (within 1 ps58) to the B850 ring for further excitation transfer. The coupling between B800-carotenoid couplings and the B850-carotenoid couplings fall into an intermediate regime,30,59 enhancing the rate of excitation transfer from B800 to B850 BChls.30,60 Although the B800 to B850 transfer remains to be fully understood,32,61,62 it’s influence on light harvesting efficiency, due to the short B800 to B850 transfer time, is simply optimal and cannot be improved further..
Quantum coherence of the B850 excitons benefits LH2→LH2 and LH2→LH1 FRET in the same way as it benefits LH1→RC FRET.20,63 In fact, one can employ the same description, using expressions (Eq. (3), Eq. (4)), except that indices μ refer to LH2 excitons and ν to LH1 excitons. Calculations8 show that through exciton coherence the FRET rates increase so much that large LH2→LH1 and LH2→LH2 transfer distances become feasible, i.e., large distances with FRET rates higher than 1/(20 ps). The distances correspond to a maximum edge-to-edge separation between LH1 and LH2 proteins of 12 Å and between two LH2s of 8 Å. Such distances and short transfer times make it possible for cells to add, depending on the ambient light level, numerous LH2s to LH1-RC complexes into a functioning cellular membrane, in fact, up to five or more LH2s for each LH1-RC. Due to excitonic coupling, FRET rates become high enough to ensure that the energy of every photon absorbed reaches a RC within about 100 ps, achieving thus an efficiency of 90 %. To understand how such light harvesting systems are engineered one needs to employ experimental imaging methodologies and theoretical descriptions that work at the level of hundreds of proteins.
Chromatophores
Purple bacteria assemble in their cellular membrane about a hundred light harvesting proteins, typically 50–100 LH2s and 10–20 LH1-RC complexes, that achieve light driven charge separated states in the RCs.5,7,8 The quinone Q2 gets charged twice, sequentially, by electron transfer from the extracellular to the intracellular side of the membrane while attracting two protons from the intracellular side, forming hydroquinone, Q → QH2. The QH2 leaves the RC and diffuses through the membrane to proteins called bc1 complex, usually present at a 2:1 ratio of LH1-RC to bc1 complex.64 In the bc1 complex, electrons and protons are taken from the quinone; the electrons are returned to the RC through a shuttling protein called cytochrome c2; the protons are released to the extracellular side, forming thus a membrane potential of the same polarity as the electrons did before.
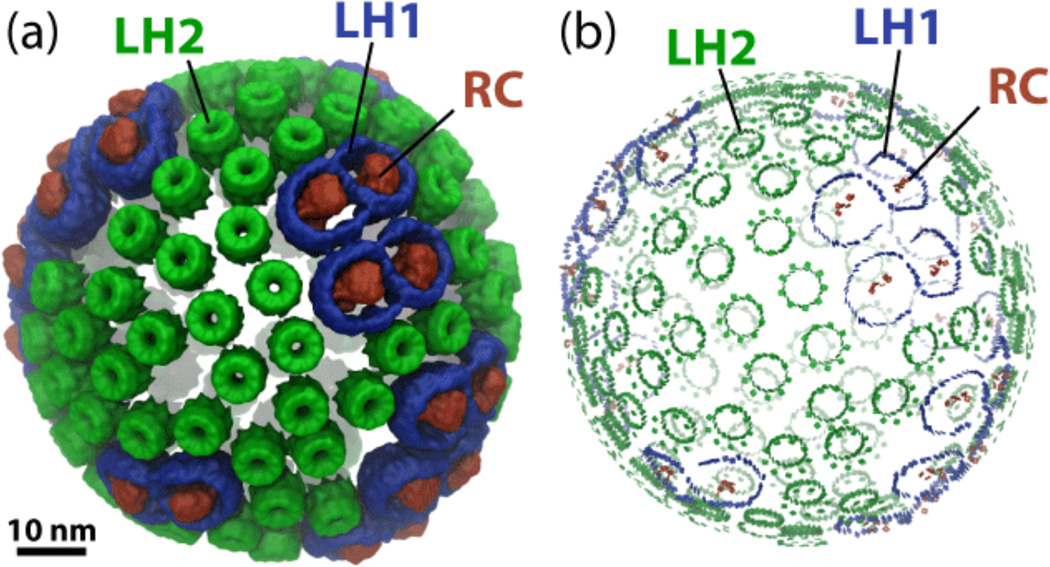
The supramolecular assembly of membrane proteins thus described is called the photosynthetic chromatophore. A purple bacterial cell may contain over a thousand chromatophores,65 each containing over 3000 BChls.5 In many species, chromatophores form spherical vesicles such as the one shown in Figure 5.8 The chromatophore is an amazing biological device whose primary function is light harvesting and the formation of a membrane potential. This function can be traced in great detail across many time scales beginning with the capture and sub-picosecond transfer of light energy among its constituent pigments.
Figure 5.
Spherical chromatophore from Rhodobacter sphaeroides showing (a) proteins and (b) bacteriochlorophylls. Reaction center (RC) is shown in red, light harvesting complex 1 (LH1) in blue and light harvesting complex 2 (LH2) in green. LH1-RC complexes form figure-8 shaped dimers in Rhodobacter sphaeroides.8
The overall efficiency of light harvesting in the chromatophore can be calculated by combining four processes in a so-called stochastic rate equation: (i) light absorption; (ii) excitation migration as described by the FRET rates in Eqs. (Eq. (1), Eq. (3), Eq. (4)); (iii) electron transfer in the RC; (iv) fluorescence or so-called internal conversion that lead to the finite nanosecond life time of BChl electronic excitation. Process (iv) limits the efficiency of light harvesting: the longer the time from light absorption to electron transfer at the RC, the less the efficiency, due to loss of excitation to fluorescence or internal conversion. The solution of the stochastic rate equation5,7,8 permits one to calculate various characteristics of the chromatophore, in particular its light harvesting efficiency of 90%. It should be noted that optimal light harvesting efficiency is not the only relevant constraint to give a photosynthetic organism a competitive advantage. For example, the organism also needs to protect itself from photo-oxidative damage, especially under high light conditions, by dissipating excitation energy across its whole light harvesting apparatus rather than only in the RCs.
The chromatophore of purple bacteria displays a remarkable simplicity compared to its evolutionary competitors in cyanobacteria, algae, and plants; the latter usurped the biosphere by evolving a more complex photosynthetic apparatus that feeds photo-excited electrons into various cellular processes, e.g., synthesis of sugar, and replenishes electrons by splitting water into oxygen gas, electrons, and protons (the purple bacteria just circulate electrons in the chromatophore). Nonetheless, by studying the chromatophore, the simplest known incarnation of biological photosynthesis, the key features of the quantum biology of light harvesting in all of biological photosynthesis are revealed, in particular the role of quantum coherence.
The role of quantum coherence in purple bacteria light harvesting was first established in 1997.30 Quantum coherence manifests itself in exciton states of BChl clusters that bunch up transition dipole moments of individual BChls. Additionally, quantum coherence shifts energy levels and improves resonance (spectral overlap) between BChl clusters. As a result, quantum coherence critically increases FRET rates, which allows additional pigments, placed a distance away from the RC, to capture additional photons and rapidly feed excitation energy to the SP for conversion into an electronic gradient before significant loss of energy occurs. Quantum coherence thus also allows antenna protein complexes to be spaced far enough apart that other processes, such as diffusion of quinone molecules in the chromatophore membrane, can proceed unhindered, whilst maintaining remarkably high light harvesting efficiency.
The chromatophore is an amazing opto-electronic device. It amasses pigments, in a hierarchical pattern, as shown in Figure 5b, exploiting quantum coherence in a beautiful and elegant manner.
Quotes from paper.
The editor asked for 2–4 quotes from the perspective. I’m not sure how long or varied they should be, but suggest that we could use the following:
“quantum coherence greatly accelerates LH1 → SP FRET, making it feasible to extend light harvesting capacity by engaging 32 LH1 BChls”
“Due to excitonic coupling, FRET rates become high enough to ensure that the energy of every photon absorbed reaches a RC within about 100 ps, achieving thus an efficiency of 90 %.”
“quantum coherence critically increases FRET rates, which allows additional pigments, placed a distance away from the RC, to capture additional photons and rapidly feed excitation energy to the SP for conversion into an electronic gradient before significant loss of energy occurs.”
Acknowledgments
The authors are supported by grants from the National Science Foundation (MCB-0744057) and (PHY-0822613) as well as from the National Institutes of Health (P41-RR005969).
Biographies
J. Strümpfer received his M.Sc in Computation Chemistry from the University of Cape Town in 2009 and is presently pursuing his Ph.D. studies in the Theoretical and Computational Biophysics Group.
M. Şener received his Ph. D. in Physics at the State University of New York at Stony Brook in 1999 and is presently a postdoctoral researcher in the Theoretical and Computational Biophysics Group.
K. Schulten received his Ph.D. from Harvard University in 1974. He is Swanlund Professor of Physics and is also affiliated with the Department of Chemistry as well as with the Center for Biophysics and Computational Biology. Prof. Schulten is a full-time faculty member in the Beckman Institute, co-director of the Center for the Physics of Living Cells, and directs the Theoretical and Computational Biophysics Group (http://www.ks.uiuc.edu).
References
- 1.Blankenship RE. Molecular Mechanisms of Photosynthesis. Malden, MA: Blackwell Science; 2002. [Google Scholar]
- 2.Cogdell RJ, Gall A, Köhler J. The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes. Quart. Rev. Biophys. 2006;39:227–324. doi: 10.1017/S0033583506004434. [DOI] [PubMed] [Google Scholar]
- 3.Deisenhofer J, Epp O, Mikki K, Huber R, Michel H. Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3Å resolution. Nature. 1985;318:618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 4.Emerson R, Arnold A. The photochemical reaction in photosynthesis. J. Gen. Physiol. 1932;16:191–205. doi: 10.1085/jgp.16.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sener MK, Olsen JD, Hunter CN, Schulten K. Atomic level structural and functional model of a bacterial photosynthetic membrane vesicle. Proc. Natl. Acad. Sci. USA. 2007;104:15723–15728. doi: 10.1073/pnas.0706861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Şener MK, Schulten K. In: The Purple Phototrophic Bacteria. Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. Vol. 28. Springer: Advances in Photosynthesis and Respiration; 2008. pp. 275–294. [Google Scholar]
- 7.Caycedo-Soler F, Rodríguez FJ, Quiroga L, Johnson NF. Interplay between excitation kinetics and reaction-center dynamics in purple bacteria. New. J. Phys. 2010;12 095008. [Google Scholar]
- 8.Sener M, Strumpfer J, Timney JA, Freiberg A, Hunter CN, Schulten K. Photosynthetic Vesicle Architecture and Constraints on Efficient Energy Harvesting. Biophys. J. 2010;99:67–75. doi: 10.1016/j.bpj.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sener M, Strümpfer J, Hsin J, Chandler D, Scheuring S, Hunter CN, Schulten K. Förster energy transfer theory as reflected in the structures of photosynthetic light harvesting systems. ChemPhysChem. 2011;12:518–531. doi: 10.1002/cphc.201000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg A, Ratsep M, Timpmann K, Trinkunas G. Excitonic polarons in quasi-one-dimensional LH1 and LH2 bacteriochlorophyll a antenna aggregates from photosynthetic bacteria: A wavelength-dependent selective spectroscopy study. Chem. Phys. 2009;357:102–112. [Google Scholar]
- 11.Janusonis J, Valkunas L, Rutkauskas D, van Grondelle R. Spectral Dynamics of Individual Bacterial Light-Harvesting Complexes: Alternative Disorder Model. Biophys. J. 2008;94:1348–1358. doi: 10.1529/biophysj.107.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valkunas L, Janusonis J, Rutkauskas D, van Grondelle R. Protein dynamics revealed in the excitonic spectra of single LH2 complexes. J. Luminesc. 2007;127:269–275. [Google Scholar]
- 13.Timpmann K, Trinkunas G, Qian P, Hunter CN, Freiberg A. Excitons in core LH1 antenna complexes of photosynthetic bacteria: Evidence for strong resonant coupling and off-diagonal disorder. Chem. Phys. Lett. 2005;414:359–363. [Google Scholar]
- 14.Freiberg A, Rätsep M, Timpmann K, Trinkunas G, Woodbury NW. Self-Trapped Excitons in LH2 Antenna Complexes between 5 K and Ambient Temperature. J. Phys. Chem. B. 2003;107:11510–11519. [Google Scholar]
- 15.Herman P, Kleinekathöfer U, Barvík I, Schreiber M. Exciton scattering in light-harvesting systems of purple bacteria. J. Luminesc. 2001;94–95:447–450. [Google Scholar]
- 16.Sumi H. Bacterial photosynthesis begins with quantum-mechanical coherence. Chem. Rec. 2001;1:480–493. doi: 10.1002/tcr.10004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Meier T, Zhang W, Chernyak V, Mukamel S. Superradiance Coherence size in single-molecule spectroscopy of LH2 antenna complexes. J. Phys. Chem. B. 1999;103:3954–3962. [Google Scholar]
- 18.Meier T, Chernyak V, Mukamel S. Multiple Exciton Coherence Sizes in Photosynthetic Antenna Complexes viewed by Pump?Probe Spectroscopy. J. Phys. Chem. B. 1997;101:7332–7342. [Google Scholar]
- 19.van Brederode M, van Mourik F, van Stokkum I, Jones M, van Grondelle R. Multiple pathways for ultrafast transduction of light energy in the photosynthetic reaction center of Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA. 1999;96:2054–2059. doi: 10.1073/pnas.96.5.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnanto J, Korppi-Tommola J. Modelling excitonic energy transfer in the photosynthetic unit of purple bacteria. Chem. Phys. 2009;357:171–180. [Google Scholar]
- 21.Strümpfer J, Schulten K. Light Harvesting Complex II B850 Excitation Dynamics. J. Chem. Phys. 2009;131:225101. doi: 10.1063/1.3271348. (9 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinekathöfer U. Non-Markovian theories based on a decomposition of the spectral density. J. Chem. Phys. 2004;121:2505. doi: 10.1063/1.1770619. [DOI] [PubMed] [Google Scholar]
- 23.Novoderezhkin VI, Rutkauskas D, van Grondelle R. Dynamics of the Emission Spectrum of a Single LH2 Complex: Interplay of Slow and Fast Nuclear Motions. Biophys. J. 2006;90:2890–2902. doi: 10.1529/biophysj.105.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizaki A, Fleming GR. Unified treatment of quantum coherent and incoherent hopping dynamics in electronic energy transfer: Reduced hierarchy equation approach. J. Chem. Phys. 2009;130:234111–2341110. doi: 10.1063/1.3155372. [DOI] [PubMed] [Google Scholar]
- 25.Damjanović A, Kosztin I, Kleinekathoefer U, Schulten K. Excitons in a Photosynthetic Light-Harvesting System: A Combined Molecular Dynamics, Quantum Chemistry and Polaron Model Study. Phys. Rev. E. 2002;65:031919. doi: 10.1103/PhysRevE.65.031919. (24 pages) [DOI] [PubMed] [Google Scholar]
- 26.Leegwater JA. Coherent versus Incoherent Energy Transfer and Trapping in Photosynthetic Antenna Complexes. J. Phys. Chem. 1996;100:14403–14409. [Google Scholar]
- 27.Sumi H. Theory on rates of excitation-energy transfer between molecular aggregates through distributed transition dipoles with application to the antenna system in bacterial photosynthesis. J. Phys. Chem. B. 1999;103:252–260. [Google Scholar]
- 28.Scholes GD, Jordanides XJ, Fleming GR. Adapting the Förster Theory of Energy Transfer for Modeling Dynamics in Aggregated Molecular Assemblies. J. Phys. Chem. B. 2001;105:1640–1651. [Google Scholar]
- 29.Jang S, Newton MD, Silbey RJ. Multichromophoric Förster Resonance Energy Transfer. Phys. Rev. Lett. 2004;92:218301. doi: 10.1103/PhysRevLett.92.218301. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Ritz T, Damjanović A, Schulten K. Pigment Organization and Transfer of Electronic Excitation in the Purple Bacteria. J. Phys. Chem. B. 1997;101:3854–3871. [Google Scholar]
- 31.Cheng YC, Silbey RJ. Coherence in the B800 Ring of Purple Bacteria LH2. Phys. Rev. Lett. 2006;96:028103. doi: 10.1103/PhysRevLett.96.028103. [DOI] [PubMed] [Google Scholar]
- 32.Jang S, Newton MD, Silbey RJ. Multichromophoric Förster Resonance Energy Transfer from B800 to B850 in the Light Harvesting Complex 2: Evidence for Subtle Energetic Optimization by Purple Bacteria. J. Phys. Chem. B. 2007;111:6807–6814. doi: 10.1021/jp070111l. [DOI] [PubMed] [Google Scholar]
- 33.Ginsberg NS, Cheng Y-C, Fleming GR. Two-Dimensional Electronic Spectroscopy of Molecular Aggregates. Acc. Chem. Res. 2009;42:1352–1363. doi: 10.1021/ar9001075. [DOI] [PubMed] [Google Scholar]
- 34.Scholes G. Quantum-coherent electronic energy transfer: Did Nature think of it first? J. Phys. Chem. Lett. 2010;1:2–8. [Google Scholar]
- 35.Brixner T, Stenger J, Vaswani HM, Cho M, Blankenship RE, Fleming GR. Two-dimensional spectroscopy of electronic couplings in photosynthesis. Nature. 2005;434:625–628. doi: 10.1038/nature03429. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Cheng Y-C, Fleming GR. Coherence Dynamics in Photosynthesis: Protein Protection of Excitonic Coherence. Science. 2007;316:1462–1465. doi: 10.1126/science.1142188. [DOI] [PubMed] [Google Scholar]
- 37.Gregory S, Tessa R, Elizabeth L, Tae-Kyu Ahn T, Mančal T, Cheng Y-C, Blankenship R, Fleming G. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature. 2007;446:782–786. doi: 10.1038/nature05678. [DOI] [PubMed] [Google Scholar]
- 38.Panitchayangkoon G, Hayes D, Fransted KA, Caram JR, Harel E, Wen J, Blankenship RE, Engel GS. Long-lived quantum coherence in photosynthetic complexes at physiological temperature. Proc. Natl. Acad. Sci. USA. 2010;107:12766–12770. doi: 10.1073/pnas.1005484107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collini E, Wong CY, Wilk KE, Curmi PMG, Brumer P, Scholes GD. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature. 2010;463:644–647. doi: 10.1038/nature08811. [DOI] [PubMed] [Google Scholar]
- 40.Hayes D, Wen J, Panitchayangkoon G, Blankenship R, Engel G. Robustness of electronic coherence in the Fenna–Matthews–Olson complex to vibronic and structural modifications. Faraday Discuss. 2011 doi: 10.1039/c0fd00030b. [DOI] [PubMed] [Google Scholar]
- 41.Olaya-Castro A, Lee CF, Olsen FF, Johnson NF. Efficiency of energy transfer in a light-harvesting system under quantum coherence. Phys. Rev. B. 2008;78:085115. [Google Scholar]
- 42.Strümpfer J, Hsin J, Sener M, Chandler D, Schulten K. In: Molecular Machines. Roux B, editor. Chapter 2. World Scientific Press; 2011. pp. 19–48. [Google Scholar]
- 43.Breton J, Martin J-L, Migus A, Antonetti A, Orszag A. Femtosecond spectroscopy of excitation energy transfer and initial charge separation in the reaction center of the photo-synthetic bacterium Rhodopseudomonas viridis. Proc. Natl. Acad. Sci. USA. 1986;83:5121–5125. doi: 10.1073/pnas.83.14.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirmaier C, Gaul D, DeBrey R, Holten D, Schenk C. Charge Separation in a Reaction Center Incorporating Bacteriochlorophyll for Photoactive Bacteriopheophytin. Science. 1991;251:922–927. doi: 10.1126/science.2000491. [DOI] [PubMed] [Google Scholar]
- 45.Deisenhofer J, Michel H. The Photosynthetic Reaction Center from the Purple Bacterium Rhodopseudomonas viridis. Science. 1989;245:1463–1473. doi: 10.1126/science.245.4925.1463. [DOI] [PubMed] [Google Scholar]
- 46.Parson WW, Warshel A. In: The Purple Phototrophic Bacteria. Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. Vol. 28. Springer Netherlands: Advances in Photosynthesis and Respiration; 2008. pp. 355–377. [Google Scholar]
- 47.Ha T. Single Molecule Fluorescence Resonance Transfer. Methods. 2001;25:78. doi: 10.1006/meth.2001.1217. [DOI] [PubMed] [Google Scholar]
- 48.Fruhwirth GO, et al. How Förster Resonance Energy Transfer Imaging Improves the Understanding of Protein Interaction Networks in Cancer Biology. ChemPhysChem. 2011;12:442–461. doi: 10.1002/cphc.201000866. [DOI] [PubMed] [Google Scholar]
- 49.Eccles J, Honig B, Schulten K. Spectroscopic Determinants in the Reaction Center of Rhodopseudomonas viridis. Biophys. J. 1988;53:137–144. doi: 10.1016/S0006-3495(88)83075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damjanović A, Ritz T, Schulten K. Energy Transfer Between Carotenoids and Bacteriochlorophylls in a Light Harvesting Protein. Phys. Rev. E. 1999;59:3293–3311. [Google Scholar]
- 50.Hu X, Schulten K. A Model for the Light-Harvesting Complex I (B875) of Rhodobacter sphaeroides. Biophys. J. 1998;75:683–694. doi: 10.1016/S0006-3495(98)77558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinkunas G, Freiberg A. A disordered polaron model for polarized fluorescence excitation spectra of LH1 and LH2 bacteriochlorophyll antenna aggregates. J. Luminesc. 2006;119–120:105–110. [Google Scholar]
- 53.Richter MF, Baier J, Prem T, Oellerich S, Francia F, Venturoli G, Oesterhelt D, Southall J, Cogdell RJ, Köhler J. Symmetry matters for the electronic structure of core complexes from Rhodopseudomonas palustris and Rhodobacter sphaeroides PufX−. Proc. Natl. Acad. Sci. USA. 2007;104:6661–6665. doi: 10.1073/pnas.0611115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richter MF, Baier J, Cogdell RJ, Oellerich S, Köhler J. In: Single Molecule Spectroscopy in Chemistry, Physics and Biology. Gräslund A, Rigler R, Widengren J, Schäfer F, Toennies J, Zinth W, editors. Vol. 96. Springer Berlin Heidelberg: Springer Series in Chemical Physics; 2010. pp. 513–533. [Google Scholar]
- 55.McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ, Isaacs NW. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature. 1995;374:517–521. [Google Scholar]
- 56.Koepke J, Hu X, Muenke C, Schulten K, Michel H. The Crystal Structure of the Light Harvesting Complex II (B800-850) from Rhodospirillum molischianum. Structure. 1996;4:581–597. doi: 10.1016/s0969-2126(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 57.Cory MG, Zerner MC, Hu X, Schulten K. Electronic Excitations in Aggregates of Bacteriochlorophylls. J. Phys. Chem. B. 1998;102:7640–7650. [Google Scholar]
- 58.Herek JL, Fraser NJ, Pullerits T, Martinsson P, Polívka T, Scheer H, Cogdell RJ, Sundström V. B800–>B850 energy transfer mechanism in bacterial LH2 complexes investigated by B800 pigment exchange. Biophys. J. 2000;78:2590–2596. doi: 10.1016/S0006-3495(00)76803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krueger BP, Scholes GD, Fleming GR. Calculation of Couplings and Energy-Tranfer Pathways between the Pigments of LH2 by the ab initio Transition Density Cube Method. J. Phys. Chem. B. 1998;102:5378–5386. [Google Scholar]
- 60.Hu X, Damjanović A, Ritz T, Schulten K. Architecture and Function of the Light Harvesting Apparatus of Purple Bacteria. Proc. Natl. Acad. Sci. USA. 1998;95:5935–5941. doi: 10.1073/pnas.95.11.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hess S, Visscher KJ, Pullerits T, Sundström V, Fowler GJS, Hunter CN. Enhanced Rates of Subpicosecond Energy Transfer in Blue-shifted Light Harvesting LH2 Mutants of Rhodobacter sphaeroides. Biochemistry. 1994;33:8300–8305. doi: 10.1021/bi00193a017. [DOI] [PubMed] [Google Scholar]
- 62.Fowler GJS, Visschers RW, Grief GG, van Grondelle R, Hunter CN. Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature. 1992;355:848–850. doi: 10.1038/355848a0. [DOI] [PubMed] [Google Scholar]
- 63.van Oijen AM, Katelaars M, Köhler J, Aartsma TJ, Schmidt J. Unraveling the Electronic Structure of Individual Photosynthetic Pigment-Protein Complexes. Science. 1999;285:400–402. doi: 10.1126/science.285.5426.400. [DOI] [PubMed] [Google Scholar]
- 64.Joliot P, Vermeglio A, Joliot A. Evidence for supercomplexes between reaction centers, cytochrome c2 and cytochrome bc1 complex. Biochim. Biophys. Acta. 1989;975:336–345. [Google Scholar]
- 65.Vatter A, Wolfe R. The structure of photosynthetic bacteria. J. Bacteriol. 1958;75:480. doi: 10.1128/jb.75.4.480-488.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]