Summary
Background
The most common source of hematopoietic progenitor cells (HPCs) for hematopoietic reconstitution comprises granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSCs). It has been proposed that endothelial progenitor cells (EPCs) share precursors with HPCs, and that EPC release may accompany HPC mobilization to the circulation following G-CSF administration.
Objective
To investigate EPC activity following HPC mobilization, and the direct effects of exogenous G-CSF administration on human umbilical vein endothelial cells (HUVECs) and endothelial outgrowth cells (EOCs), using in vitro and in vivo correlates of angiogenesis.
Patients/Methods
Heparinized venous blood samples were collected from healthy volunteers and from cord blood at parturition. G-CSF-mobilized samples were collected before administration, at apheresis harvest, and at follow-up. PBSCs were phenotyped by flow cytometry, and cultured in standard colony-forming unit (CFU)-EPC and EOC assays. The effect of exogenous G-CSF was investigated by addition of it to HUVECs and EOCs in standard tubule formation and aortic ring assays, and in an in vivo sponge implantation model.
Results
Our data show that G-CSF mobilization of PBSCs produces a profound, reversible depression of circulating CFU-EPCs. Furthermore, G-CSF administration did not mobilize CD34+CD133− cells, which include precursors of EOCs. No EOCs were cultured from any mobilized PBSCs studied. Exogenous G-CSF inhibited CFU-EPC generation, HUVEC and EOC tubule formation, microvessel outgrowth, and implanted sponge vascularization in mice.
Conclusions
G-CSF administration depresses both endothelial cell angiogenesis and monocyte proangiogenic activity, and we suggest that any angiogenic benefit observed following implantation of cells mobilized by G-CSF may come only from a paracrine effect from HPCs.
Keywords: angiogenesis, endothelial progenitor cells, granulocyte colony-stimulating factor, stem cell therapy
Introduction
The recent discovery that endothelial progenitor cells (EPCs), which play a role in de novo vascularization (vasculogenesis), circulate in adult blood and reside in the bone marrow [1,2] prompted a range of studies based on localized implantation of autologous cells, aimed at vascularizing ischemic tissue, particularly in myocardial and critical limb ischemias. EPCs have not yet been definitively characterized, but have been linked with hematopoietic progenitor cells (HPCs). EPCs and HPCs share a common ancestor, the hemangioblast, in the developing fetus that may be retained in adult life [3]. HPCs derived from bone marrow [bone marrow stem cells (BMSCs)] or peripheral blood (PB) [peripheral blood stem cells (PBSCs)] following granulocyte colony-stimulating factor (G-CSF) administration have been utilized as sources of EPCs for regenerative vascularization. A recent meta-analysis showed that BMSC treatment generally improves short-term measurements of cardiac function after myocardial infarction. However, there is, as yet, little evidence with which to assess the long-term clinical effects of this treatment [4]. Although most studies so far reported have used BMSCs for therapeutic angiogenesis, those in which G-CSF-mobilized PBSCs were used gave comparable, mild improvements in cardiovascular lesions [5], and both sources are generally regarded as adequate for therapeutic angiogenesis, just as they are for hematopoiesis. To date, some studies have shown that EPCs, as well as HPCs, are demonstrably mobilized by G-CSF [6-8]. However, this depends on how EPCs are defined and interpreted: we can measure increases or decreases in EPC numbers following G-CSF administration, depending on how EPCs are defined [9].
The current characterizations of EPCs have been based on phenotype and on colony assays. HPCs are routinely defined for clinical use by their expression of CD34 or CD133 [10]. A link between CD34/CD133 expression and the EPC phenotype was proposed almost from the initial discovery of circulating EPCs [11,12], but recent studies have indicated that cells expressing CD133 and their progeny remain hematopoietic, and only CD34+CD133− cells are true EPCs [13,14]. True EPCs are defined as cells that, in culture over 3–4 weeks on collagen, can give rise to endothelial outgrowth cells (EOCs) [14-16], whereas cells that give rise over 5–6 days to colonies on fibronectin (colony-forming unit endothelial progenitor cells) (CFU-EPCs), formerly proposed to be EPCs [17], are now recognized to be generated by monocytes [16,18,19]. CFU-EPCs stain for many endothelial markers [20,21] but also retain CD14 expression [22]. Monocytes can themselves mimic endothelial cells (ECs) by upregulating expression of many markers held to be endothelial, and have probably been mistaken for ECs in many investigations [22,23]. Although it is implicit in many studies that the observed clinical benefit is delivered by EPCs, which are ultimately incorporated as ECs into new vasculature, it is becoming apparent that neovascularization can also be promoted indirectly by cells that release paracrine factors that promote angiogenesis without being incorporated as ECs [24]. Although such cells may not be true EPCs, their proangiogenic effect may be important, and this may be why so many different cell phenotypes have been proposed to be EPCs [25,26].
To date, G-CSF PBSC mobilization has been generally regarded as a practical and feasible source of cells for therapeutic angiogenesis [27-29]. However, a recent meta-analysis reported that G-CSF infusion alone had no significant clinical benefit in myocardial infarction [30], and it was reported that G-CSF-mobilized PBSCs were less effective in inducing ulcer healing than were BMSCs [31]. The mechanism by which G-CSF may mobilize EPCs and/or enhance angiogenesis is still unknown. Similarly, the effects of G-CSF on ECs and the vasculature have not been extensively studied [32]. In this study, we have investigated circulating EPC activity following HPC mobilization by G-CSF administration, and the direct effects of G-CSF on in vitro and in vivo correlates of angiogenesis.
Materials and methods
Animals
Male C57B6J mice aged 10–12 weeks were purchased from Charles River Laboratories (Tranent, UK) or Harlan Olac Ltd (Loughborough, UK). Experimental procedures were approved by the University of Edinburgh ethics committee, and were authorized by the Home Office under the Animals (Scientific Procedures) Act 1986.
Cell sources and sampling
Peripheral venous blood samples from healthy adults (normal PB) were collected into heparin and from cord blood (CB) following elective caesarean section. For sequential studies, healthy PBSC donors (mobilized PB donors) and PBSC transplant patients (mobilized PB patients) donated 10 mL of venous PB before G-CSF mobilization (pre-G-CSF); at apheresis harvest (post-G-CSF), and 1–2 months after harvest (follow-up). The G-CSF protocol used in this work was the standard local clinical mobilization regimen. The G-CSF (lenograstim) dose for healthy donors was 10 μg kg−1 d−1, given for four consecutive days before collection of post-G-CSF cells (at apheresis) on day 5. Patient samples were subjected to chemotherapy — salvage chemotherapy (lymphoma patients) or cyclophosphamide (multiple myeloma patients) — followed by G-CSF (lenograstim), starting at least 24 h following the last dose of chemotherapy — 5 μg kg−1 d−1 (lymphoma patients) or 10 μg kg−1 d−1 (multiple myeloma patients) — and given for 6–7 days before collection of post-G-CSF cells when CD34+ counts exceeded 10 × 106 L−1. Pre-G-CSF treatment cells were collected 10–30 days prior to G-CSF administration. Healthy adult donors (for allogeneic transplant) are the primary study subjects, and results for patients are included for comparison. Further clinical, hematologic and laboratory data are reviewed elsewhere (J. Crawford, MD thesis, University of Edinburgh, submitted). Appropriate ethical informed consent was obtained from subjects in all cases. Mononuclear cells (MNCs) were isolated by buoyant density centrifugation over Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden).
Isolation of short-term (2 h) plastic-adherent MNCs
MNCs (30 × 106) in 5 mL of IMDM (Invitrogen, Paisley, UK) containing 10% fetal bovine serum (Sigma, Dorset, UK) were plated in 25-cm2 Corning tissue culture flasks (Fisher Scientific, Loughborough, UK) and incubated at 37 °C. After 2 h, adherent cells were detached using 1 mL of trypsin–EDTA in saline (Sigma). Harvested cells were resuspended in IMDM and characterized by flow cytometry for use in further experiments.
Flow cytometry analysis and sorting
Cells were directly stained and analyzed on a FACS Calibur flow cytometer (Becton Dickinson, Oxford, UK), using CellQuest Pro software for phenotypic expression of surface markers, and analyzed using FCS Express (DeNovo Software, Los Angeles, CA, USA), as described previously [9]. Cells were sorted using a FACS Aria flow cytometer (Becton Dickinson), using Diva software. Sorted populations were recovered and characterized by further analysis. The anti-human monoclonal antibodies used for flow cytometry included anti-CD34–fluorescein isothiocyanate, anti-CD14–phycoerythrin, anti-CD45–PerCP (Becton Dickinson), and anti-CD133–allophycocyanin (Myltenyi Biotec, Bisley, UK).
CFU-EPCs
This assay, based on the method of Hill et al. [17], was performed using a commercial kit according to the manufacturer’s recommendations (Stem Cell Technologies, Grenoble, France). As previously described [9,20,21], 5 × 106 unmodified MNCs were resuspended in EndoCult Liquid Medium (Stem Cell Technologies) and plated on fibronectin-coated six-well plates (Becton Dickinson) for 2 days. The non-adherent cells were recovered, resuspended in fresh medium, and transferred to a fibronectin-coated 24-well plate (Becton Dickinson) at 1 × 106 per well in the presence or absence of G-CSF (100 ng mL−1) for a further 3 days; the colonies per well were then counted, and the EPC frequency was calculated. The concentration of G-CSF used in this and other in vitro assays described below was based on a previously determined optimal dose for CD34+ PBSC expansion/differentiation to neutrophils [33].
Culture of EOCs
EOC culture was performed as described by Ingram et al. [34]. Briefly, 30 × 106 MNCs from normal PB or 10 × 106 MNCs from CB were resuspended in endothelial growth medium (EBM-2; Lonza, Slough, UK) and plated onto type 1 rat tail collagen-coated six-well tissue culture plates (Becton Dickinson). The cells were incubated at 37 °C with 5% CO2 for 3–4 weeks. The medium was changed every 2 days for 7 days, and then twice a week until first passage. Colonies were counted when they became evident but before they became confluent.
In vitro vascular tubule formation assay
Matrigel matrix (Becton Dickinson) solution was thawed overnight at 4 °C, and all plasticware was precooled. Human umbilical vein endothelial cells (HUVECs) (Lonza) and EOCs were resuspended at 1 × 105 mL−1 in EBM-2 in the presence or absence of G-CSF (100 ng −1mL). Five hundred microliters of cells were added to duplicate wells precoated with 250 μL of Matrigel that had been allowed to solidify for 1 h at 37 °C. Capillary structures and EC networks were examined by phase contrast microscopy (× 40 lens), using an inverted microscope (Nikon Eclipse TS100-F, Nikon Instruments, Kingston Upon Thames, UK). Pictures were taken at 4 h and at 22 h. The EC network was quantified from the image fields by scoring the number of cell–cell connections.
In vitro angiogenesis: aortic ring assay
C57Bl6 mice were killed by asphyxiation in CO2. The thoracic aorta was removed, washed in serum-free MCDB 131 medium (Invitrogen), cleaned of periadventitial tissue, and divided into 1-mm rings. Aortic rings were embedded in 200 μL of Matrigel (Becton Dickinson) and incubated at 37 °C in serum-free MCDB 131, with heparin, ascorbic acid and GA1000 (Cambrex Biosciences, Wokingham, UK) in the presence or absence of G-CSF (100 ng mL−1). The medium was changed every 48 h. All assays were performed in triplicate. The growth of new vessels was counted at day 4 and day 8 by light microscopy.
Subcutaneous sponge implantation assay for in vivo vascularization
Mice were anesthetized with halothane, and a sterilized sponge cylinder (0.5 cm diameter, 1 cm long) (Caligen Foam, Accrington, UK) was implanted subcutaneously on each flank. Each animal had an intervention-impregnated sponge [growth-factor-reduced (GFR)-Matrigel + G-CSF] on one side and a control, vehicle-impregnated sponge (GFR-Matrigel alone) on the other side. Twenty days after implantation, mice were killed, and sponges were excised. Sponges were fixed in 4% formalin and embedded in paraffin wax. Sections (5 μm) were stained with hematoxylin–eosin for identification of blood vessels, as previously described [35]. Vessel density within sponges was determined using the mean of triplicate Chalkley counts on each of two sections per sponge [36].
Statistical analysis
Unless otherwise stated, continuous variables are reported as mean ± standard error of the mean. Statistical analyses were performed with GraphPad Prism 4 (Graph Pad Software, La Jolla, CA, USA), using two-tailed Student’s t-test, Mann–Whitney U-test or Wilcoxon paired tests where appropriate. A P-value of < 0.05 was considered to indicate statistical significance.
Results
The effects of in vivo administration of a PBSC-mobilizing G-CSF regimen on indicators of angiogenesis
The induced depression of CFU-EPC capacity in PB MNCs following G-CSF administration is profound but transient
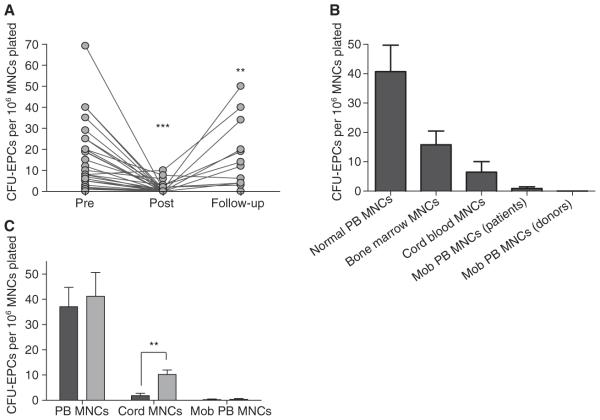
When individual healthy subjects (PBSC donors) were followed sequentially, G-CSF administration caused a profound decline in CFU-EPC activity from normal levels, which recovered with time (Fig. 1A). The CFU-EPC activity was virtually abolished after G-CSF administration as compared with the pre-G-CSF sample (P < 0.001, paired t-test, n = 21). CFU-EPC activity returned to almost basal levels in follow-up samples after 1–2 months following completion of G-CSF treatment (P < 0.01, paired t-test, n = 13). Similar depression and recovery of CFU-EPC activity following G-CSF mobilization of PBSCs (for autologous transplantation) was seen in a large series of hematology patients (data not shown).
Fig. 1.
Influence of administration of granulocyte colony-stimulating factor (G-CSF) for hematopoietic progenitor cell (HPC) mobilization (Mob) on colony-forming unit endothelial progenitor cells (CFU-EPCs) in peripheral blood (PB) mononuclear cells (MNCs). (A) There was a fall in CFU-EPCs following G-CSF administration to healthy adult HPC donors and a subsequent rise in CFU-EPCs at 1–2-month follow-up after mobilization. (B) CFU-EPCs in normal PB MNCs and in HPC-rich MNC sources. (C) CFU-EPCs in MNCs (black) and following monocyte (CD14) enrichment by plastic adherence (grey). A P-value of < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001). Pre, pre-G-CSF administration; Post, post-G-CSF administration.
G-CSF-mobilized PB MNCs are unable to generate CFU-EPCs and these are not recovered by monocyte enrichment
Confirming what we have previously reported [9], CFU-EPCs were most prevalent in normal PB MNCs and were virtually absent from G-CSF-mobilized PB MNCs, whether from patient or healthy donor sources (Fig. 1B). We found that CFU-EPCs were slightly increased (e.g. from 21 to 25 per 106 MNCs, n = 6) when normalPB MNCs were enriched for monocytes (to around 80% CD14+) by 2 h of adherence on uncoated culture plates. Further enrichment of monocytes (to 98%) by CD14+ selection by FACS further increased CFU-EPCs (e.g. from 25 to 41 per 106 MNCs, n = 6) and completely removed CFU-EPCs from the CD14-depleted adherent cells. CFU-EPCs were not found in CD34-enriched (> 90%) or CD133-enriched (> 90%) MNCs from different HPC-rich sources (Table 1).
Table 1.
Effect of subpopulation enrichment/depletion on colony-forming unit endothelial progenitor cells (CFU-EPCs) in mononuclear cell (MNC) populations
| Source | Number of CFU-EPCs per 106 MNCs plated |
|---|---|
| Unfractioned MNCs* | 21 |
| 2-h adherent cells (> 80% CD14+)* | 25 |
| 2-h non-adherent cells* | 2 |
| CD14-enriched (> 98%)* | 41 |
| CD14-depleted* | 0 |
| CD34-enriched (> 90%)† | 0 |
| CD34-depleted† | 3 |
| CD133-enriched (> 90%)† | 0 |
| CD133-depleted† | 8 |
CFU-EPCs are slightly increased when normal peripheral blood MNCs are enriched for monocytes (to about 80% CD14+) by 2 h of adherence on uncoated culture plates. Further enrichment of monocytes (to 98%) by CD14+ selection by fluorescence-activated cell sorting further increased CFU-EPCs and completely removed CFU-EPCs from the CD14-depleted plastic-adherent cells. CFU-EPCs were not found in CD34-enriched (> 90%) or CD133-enriched (> 90%) MNCs from different HPC-rich cell sources. Their depleted column eluates tend to show reduced CFU-EPC activity as compared with the unfractioned starting MNCs, which may imply some loss of CFU-EPC activity by retention on columns by adhesion, implying these cells are adherent.
Paired normal peripheral blood samples (n = 6).
Unpaired HPC-rich samples (bone marrow, cord blood, mobilized blood).
In a larger series studied by plastic adherence enrichment alone, normalPB MNCs generated a mean of 37 CFU-EPCs per 106 cells plated, CB MNCs generated fewer than five CFU-EPCs per 106 cells plated, and G-CSF-mobilized PB MNCs were virtually unable to form any CFU-EPCs (0.6 CFU-EPCs per 106 cells plated (Fig. 1C). Whereas enrichment of CD14+ cells by plastic adherence increased the number of CFU-EPCs slightly in normal PB MNCs and significally in CB MNCs, no CFU-EPCs were seen when CD14-enriched cells from G-CSF-mobilized MNCs were cultured.
Mobilization of PB HPCs with G-CSF alters the phenotype proportions of CD34+ cell subpopulations
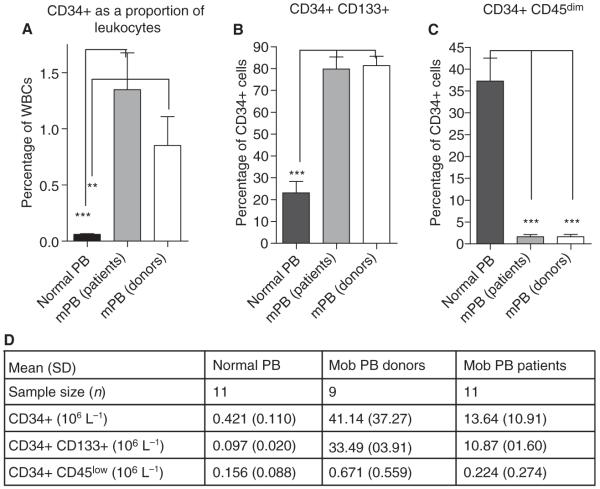
G-CSF-mobilized PB samples had a more than 10-fold higher proportion of CD34+ cells than normal PB samples (Fig. 2A). In agreement with our earlier studies [9], G-CSF-mobilized PB samples had markedly higher coexpression of CD133 by CD34+ cells (81.4% ± 10.5%) than normal PB samples (23.1% ± 18.2%). This was true for both healthy donors (P < 0.001, Mann-Whitney U-test, n = 9) and for autologous patients (Fig. 2B). Furthermore, in contrast to normal PB, in which a mean of 37% of CD34+ cells were CD45low, G-CSF-mobilized PB samples contained very low proportions of CD34+CD45low cells (mean G-CSF-mobilized PB autologous, 1.64%; mean G-CSF-mobilized PB allogeneic, 1.63%) (P < 0.001, Mann-Whitney U-test, n = 9) (Fig. 2C), and their absolute numbers were little increased in G-CSF-mobilized PB as compared with normal PB, in contrast to total CD34+ numbers (Fig. 2D).
Fig. 2.
Influence of administration of granulocyte colony-stimulating factor (G-CSF) on hematopoietic progenitor cell (HPC) mobilization on CD34+ cells and CD34+ subpopulations in peripheral blood (PB) leukocytes. (A) CD34+ cells as a proportion of total leukocytes in normal PB and in G-CSF-mobilized PB from healthy donors and from hematologic malignancy patients in remission (mPB). (B) The proportion of CD34+ cells coexpressing CD133 in normal PB and in G-CSF-mobilized PB from healthy donors and from hematologic malignancy patients in remission. (C) The proportion of CD34+ cells with low to negligible CD45 expression in normal PB and in G-CSF-mobilized PB from healthy donors and from hematologic malignancy patients in remission. (D) Absolute numbers of circulating HPC subpopulations per liter of PB: all CD34+ cells; CD34+ cells coexpressing CD133; and CD34+ cells low in CD45 expression. The results are calculated from research laboratory determination of total leukocyte counts and proportional subpopulations, expressed as mean ± standard deviation (SD). A P-value of < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001). Mob, mobilized; WBC, white blood cell.
G-CSF-mobilized PB MNCs are unable to generate EOCs
CB MNCs (n = 15) reliably generated EOC colonies when 10 × 106 MNCs were plated. For normal PB MNCs (n = 12), at least 30 × 106 MNCs had to be plated. G-CSF-mobilized blood MNCs were unable to form any EOC colonies for up to 30 × 106 MNCs plated (n = 7), either for healthy donors or for patients (Table 2).
Table 2.
Attainment of endothelial outgrowth cell (EOC) colonies from different mononuclear cell (MNC) sources and subpopulations
| Source (cord blood MNCs) | EOC colonies found |
Number of MNCs plated for one EOC colony |
|---|---|---|
| Unfractioned MNCs | Yes | 3 ×106 |
| CD133-enriched (> 90%) | No | (> 1.3 × 106) |
| CD133-depleted | Yes | 0.5 × 106 |
| CD34-enriched (> 90%) | Yes | 1.7 × 106 |
| CD34-depleted | No | (> 3 × 106) |
| CD34+CD133+ (> 90%) | No | (> 1.65 × 106) |
| CD34+CD133-depleted | Yes | 0.002 × 106 |
EOCs can be routinely cultured from cord blood MNCs plated at 5–10 × 106 per well on collagen. For normal adult peripheral blood MNCs, 30 × 106 MNCs per well are required to give approximately one EOC colony. From cord blood MNCs separated by magnetic beads, CD34-enriched cells form EOCs, but their CD34-depleted eluates do not. CD133-enriched cells do not form EOCs, but their CD133-depleted eluates do: if the CD133-depleted cells are further fractionated according to CD34 expression, the CD34-enriched (CD34+CD133−) cells form EOCs but the CD34− eluates do not.
EOC potential is associated with CD34+CD133− cells
Enrichment of the CD34+ fraction of CB MNCs by magnetic bead cell sorting (> 90% purity) showed that this fraction was the source of all EOC colonies. No colonies were found in CD34-depleted MNCs (Table 2). Conversely, the CD133-enriched fraction from CB MNCs (> 90% purity) gave no EOC colonies, and all of the EOC colonies were generated from the CD133-depleted fraction. When the CD133− fraction was further sorted into CD34+ and CD34− fractions, only the CD34+ (CD133−) fraction gave EOC colonies. The number of cells required to produce EOC colonies fell dramatically with enrichment of CD34+CD133− cells. Enrichment for the CD34+CD133− cell population increased the frequency of EOC generation from CB MNCs, but not when G-CSF-mobilized blood MNCs were used.
The effect of direct addition of exogenous G-CSF on in vitro indicators of angiogenesis
The number of CFU-EPCs is reduced by addition of G-CSF to colony cultures
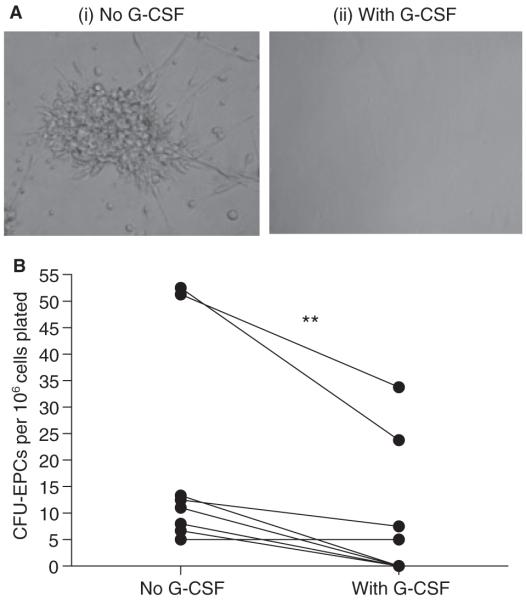
The addition of G-CSF to normal PB MNCs in vitro significantly reduced the CFU-EPC frequency as compared with controls without G-CSF (P < 0.01, paired t-test, n = 8) (Fig. 3). The addition of 100 ng mL−1 vascular endothelial growth factor (VEGF) or stromal cell-derived factor-1 (SDF-1) to the wells to which G-CSF had been added did not rescue colony formation (data not shown).
Fig. 3.
Influence of exogenous granulocyte colony-stimulating factor (G-CSF) in vitro on colony-forming unit endothelial progenitor cells (CFU-EPCs). (A) Representative microscopy images of CFU-EPC formation (i) by normal peripheral blood mononuclear cells (MNCs) and (ii) in the presence of exogenous G-CSF (100 ng mL−1). There was a significant reduction in MNC CFU-EPCs in the presence of G-CSF as compared with paired samples without G-CSF. A P-value of < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Tubule formation by HUVECs and EOCs is reduced by G-CSF
Vascular tubule formation by HUVECs in Matrigel showed a significant reduction at 22 h in the presence of G-CSF as compared with controls (P < 0.01, paired t-test, n = 5) (Fig. 4C); a paired example is shown in Fig. 4A,B. The addition of 100 ng mL−1 VEGF or SDF-1 to the wells with G-CSF did not rescue tube formation (data not shown). EOCs grown on collagen behave like HUVECs in many ways, and form tubules in Matrigel. As with HUVECs, tubule formation by EOCs in Matrigel was inhibited by G-CSF (n = 5) (Fig. 4D–F).
Fig. 4.
Influence of exogenous granulocyte colony-stimulating factor (G-CSF) in vitro on tubule formation. (A, B) Representative microscopy images of (A) normal tubule formation by human umbilical vein endothelial cells (HUVECs) and (B) tubule formation by HUVECs in a paired culture with exogenous G-CSF added. (C) There was a significant reduction in HUVEC tubule connections in the presence of G-CSF as compared with paired samples without G-CSF. (D, E) Representative microscopy images of (D) normal tubule formation by endothelial outgrowth cells (EOCs) and (E) tubule formation by EOCs in a paired culture with exogenous G-CSF added. (F) There was a significant reduction in EOC tubule connections in the presence of G-CSF as compared to paired samples without G-CSF. A P-value of < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Angiogenesis from mouse aortic rings in vitro is reduced by G-CSF
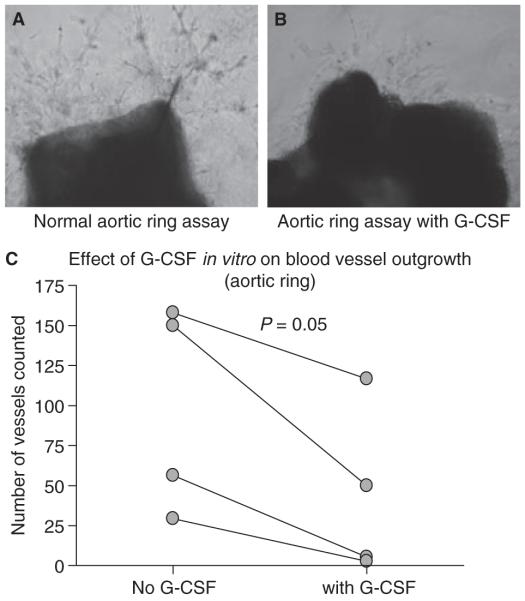
The numbers of vessels formed from murine aortic rings cultured in vitro and scored after 4 and 8 days were reduced in the presence of G-CSF as compared with controls at both time points (P < 0.05, paired t-test, n = 4, means of triplicates). Results scored at 8 days are shown in Fig. 5C. A paired example is shown in Fig. 5A,B.
Fig. 5.
Influence of exogenous granulocyte colony-stimulating factor (G-CSF) in vitro on microvessel outgrowth from mouse aortic rings. Representative microscopy images of (A) normal microvessel outgrowth from mouse aortic ring and (B) microvessel outgrowth from a paired aortic ring sample from the same mouse in the presence of added exogenous G-CSF. (C) There was a significant reduction in microvessel outgrowth from mouse aortic rings in the presence of G-CSF as compared with paired samples without G-CSF.
In vivo spontaneous angiogenesis in subcutaneous sponge implants in mice is inhibited by G-CSF
Control vehicle-impregnated sponges (GFR-Matrigel only) and G-CSF-impregnated sponges (G-CSF in GRF-Matrigel) excised after 20 days following implantation both appeared red on gross inspection, with lace-like coverings of blood vessels. They also both showed infiltration of organized matrix and an abundance of blood vessels. On histologic examination, all sponges exhibited vascularization, but G-CSF-impregnated sponges had significantly fewer blood vessels than controls when scored by Chalkley counts (P < 0.001, Mann–Whitney U-test, n = 4) (Fig. 6). A group of mice (n = 4) implanted with untreated sponges (no GFR-Matrigel vehicle) on both flanks exhibited a similar level of vasculogenesis as that seen in vehicle-impregnated sponges (GFR-Matrigel), demonstrating that GFR-Matrigel as vehicle has no intrinsic effect on vascularization (not shown).
Fig. 6.

Inhibition of angiogenesis in vivo by granulocyte colony-stimulating factor (G-CSF). There was a reduction of spontaneous vascularization of subcutaneously implanted sponges containing G-CSF in growth-factor-reduced Matrigel as compared with contralateral sponges in the same animal containing growth-factor-reduced Matrigel alone. A P-value of < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
It is well established that G-CSF administration successfully mobilizes progenitor cells to PB, and these cells are able to reconstitute the hematopoietic system; therefore, it is thought that G-CSF mobilization might also increase the number of circulating EPCs. In this study, we used PBSCs from subjects receiving G-CSF for HPC mobilization, to examine the possibility that EPCs are mobilized into the circulation concurrently. We were unable to detect EPCs in G-CSF-mobilized PBSCs, using several of the published EPC phenotypes. G-CSF administration for HPC mobilization not only failed to mobilize EPCs, but also inhibited angiogenesis in vitro and in vivo.
Neither G-CSF-mobilized PB MNCs nor enriched monocytes are able to generate CFU-EPCs
Initial observations showed that, following administration of G-CSF to healthy allogeneic PBSC donors, there was a profound depression of CFU-EPC generation [9]. This reduction in endothelial colony potential has been consistently shown by independent observers in our group and in over 70 different samples studied. Longitudinal analysis of sequential PB samples from healthy donors undergoing PBSC mobilization showed that CFU-EPC numbers were severely reduced immediately after G-CSF administration, but returned to almost pretreatment levels within 2 months (Fig. 1A). Similar results have been obtained in hematologic malignancy patients undergoing PBSC mobilization for autologous transplantation, and may indicate that preceding chemotherapy does not markedly affect many putative laboratory measures of PBSC or endothelial function. Although CFU-EPCs were originally proposed by Hill et al. [17] as a correlate of EPC frequency, it is now established that they represent an expression of the activity of CD14+ monocytes [37], which possibly constitute a key proangiogenic monocyte subpopulation, not related to any HPC population. Monocyte enrichment by plastic adherence increased the frequency of CFU-EPC generation from umbilical cord MNCs but did not increase the frequency of CFU-EPC generation from G-CSF-mobilized blood MNCs (Fig. 1C), which, in contrast to CB monocytes, appear to be unresponsive in this assay.
A recognized, strong, inverse correlation between CFU-EPC frequency and cardiovascular risk has been reported extensively (reviewed in [20,21]). This accumulation of reports is not trivial, and indicates that CFU-EPC measurement can assess some aspects of angiogenic capacity. Thus, although CFU-EPC measurement seems not to be an indicator of EPC frequency, as originally proposed, depressed CFU-EPC activity in G-CSF-mobilized samples probably reflects reduced monocyte proangiogenic capacity.
G-CSF-mobilized PB MNCs are unable to generate EOCs
There are a number of claims that G-CSF mobilizes EPCs [6-8], but these depend on how EPCs are defined and interpreted. A very few studies are based on a reported increase in CFU-EPC frequency [7], and nearly all are based on putative phenotype characterization of EPCs. Although the proposed phenotypes for EPC have been dominated by variants based on coexpression of CD34 and CD133, the definitive phenotype of an EPC remains elusive. Recently, it has been reported that true circulating EPCs (EOCs) are CD34-positive but CD133-negative and CD45-negative, whereas cells expressing CD133 and CD45 remain hematopoietic and do not give rise to true ECs [13,14,38]. Previous observations showed that the HPCs in G-CSF-mobilized blood are predominantly CD34+CD133+ [9,39], so by phenotype alone there is no evidence of mobilization of EOCs (contained in the CD34+CD133− subpopulation) by G-CSF (Fig. 2B), and nor is there evidence that CD34+ cells with low or negligible expression of the panleukocyte marker CD45 are selectively mobilized by G-CSF (Fig. 2C). Indeed, no EOCs could be cultured from G-CSF-mobilized blood MNCs (30 × 106) from either autologous patients or allogeneic donors, whereas, in most cases, at least one EOC colony can be found in comparable normal PB MNCs, so there is no evidence that EOCs are mobilized by G-CSF. As there is little or no selective mobilization of CD34+CD133–CD45− cells by G-CSF, this may account for the failure to find EOCs in these samples. Furthermore, enrichment for the proposed EOC precursor population (CD34+CD133−) by magnetic beads increased the frequency of EOC generation from umbilical cord MNCs, but not from G-CSF-mobilized blood MNCs. We have no evidence to judge whether EOCs are prsent at low frequency/absent or inactive in G-CSF-mobilized MNCs.
G-CSF has a direct inhibitory effect on angiogenesis
Although some of the effects of G-CSF on CFU-EPCs in vivo might result from alteration of the balance of different cell types in the circulation and dilution of some MNC subpopulations by others, it can be shown that G-CSF has a direct effect in vitro on CFU-EPCs. In paired MNC samples, the addition of G-CSF resulted in a decrease in CFU-EPC frequency (Fig. 3). Furthermore, the addition of known angiogenesis-promoting cytokines such as VEGF or SDF-1 to the G-CSF-treated MNCs did not rescue colony formation. Similarly, it can be shown that G-CSF depresses the expression in vitro of accepted endothelial cell functions, such as the formation of cell–cell links in human EC (HUVEC and EOC) tubule formation in Matrigel (Fig. 4) and in microvessel outgrowth from mouse aortic rings (Fig. 5).
The direct effect of G-CSF on angiogenesis was ultimately confirmed with the use of an in vivo mouse model of angiogenesis (subcutaneous sponge implantation). Localized G-CSF substantially inhibited spontaneous vascularization of sponges in vivo (Fig. 6), in direct contrast to what was seen in paired sponges lacking G-CSF in the same animals. Honold et al. [8] showed that EPCs in G-CSF-mobilized samples were transiently dysfunctional, owing to the cleavage of the chemokine receptor CXCR4, which is directly involved in stem cell homing. Thus, the observed reduction of endogenous blood vessel formation in the G-CSF-treated sponge may reflect a localized decline in the ability to recruit potential murine angiogenic cells. Preliminary evidence from our current work suggests that G-CSF may downregulate the expression of certain cell surface receptors and adherence molecules, which may impair the ability of cells to function in certain environments; this could explain the observed CFU-EPC depression and might be important in endothelial function and/or angiogenesis. This is currently under investigation.
In agreement with our findings, a recent meta-analysis reported that G-CSF infusion alone has no significant clinical benefit in myocardial infarction [30], and G-CSF-mobilized PBSCs were reported to be less effective in inducing ulcer healing than BMSCs [31]. However, a significant number of reports to date have shown that cellular therapies employing G-CSF-mobilized cells have some clinical benefit [27-29]. Thus, although G-CSF may not selectively mobilize true EPCs as defined by EOCs, and although it may inhibit monocyte proangiogenic activity and EC angiogenic activity, G-CSF does induce an increase in the number of circulating HPCs, which might home to ischemic lesions [27] and could therefore provide a paracrine effect without any incorporation into new vessels. These may be equivalent to the cells provided from bone marrow, and if that is the principal effect required in some aspects of therapeutic vascularization, then mobilized PBSCs may be as beneficial as BMSCs in clinical use.
In summary, this study has shown that there is a profound reduction in the number of CFU-EPCs following G-CSF administration, which recovers with time. To the best of our knowledge, we are the first to show that there is no evidence of circulating EOCs following G-CSF administration for mobilization of HPCs. G-CSF-mobilized PBSC were predominantly CD34+CD133+ cells, which are almost certainly hematopoietic cells. The presence in vitro of exogenous G-CSF had a direct antiangiogenic effect that was not abrogated by the addition of proangiogenic factors.
Acknowledgements
We wish to thank the staff of the Scottish National Blood Transfusion Service (SNBTS) Cell Separator Unit in Edinburgh for interviewing and consenting donors and patients and for taking blood samples. This study was supported in part by Project grant PG/06/051 from the British Heart Foundation.
Footnotes
To cite this article: Tura O, Crawford J, Barclay GR, Samuel K, Hadoke PWF, Roddie H, Davies J, Turner ML. Granulocyte colony-stimulating factor (G-CSF) depresses angiogenesis in vivo and in vitro: implications for sourcing cells for vascular regeneration therapy. J Thromb Haemost 2010; 8: 1614–23.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Witzenbichler B, Schatteman G, Isner JM, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 3.Cogle CR, Scott EW. The hemangioblast: cradle to clinic. Exp Hematol. 2004;32:885–90. doi: 10.1016/j.exphem.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Rendon E, Brunskill S, Doree C, Hyde C, Watt S, Mathur A, Stanworth S. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2008:CD006536. doi: 10.1002/14651858.CD006536.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–45. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd RM, Capoccia BJ, Devine SM, DiPersio J, Trinkaus KM, Ingram D, Link DC. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108:3662–7. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 8.Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, Martin H, Haendeler J, Zeiher AM, Dimmeler S. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26:2238–43. doi: 10.1161/01.ATV.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- 9.Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources. J Transl Med. 2007;5:37–46. doi: 10.1186/1479-5876-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, Eades William C, Creer MH, Nolta JA. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–9. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 12.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 13.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 15.Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2. [DOI] [PubMed] [Google Scholar]
- 16.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 18.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–67. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 19.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 20.Mills NL, Tura O, Padfield GJ, Millar C, Lang NN, Stirling D, Ludlam C, Turner ML, Barclay GR, Newby DE. Dissociation of phenotypic and functional endothelial progenitor cells in patients undergoing percutaneous coronary intervention. Heart. 2009;95:2003–8. doi: 10.1136/hrt.2008.163162. [DOI] [PubMed] [Google Scholar]
- 21.Robb AO, Mills NL, Smith IB, Short A, Tura-Ceide O, Barclay GR, Blomberg A, Critchley HO, Newby DE, Denison FC. Influence of menstrual cycle on circulating endothelial progenitor cells. Hum Reprod. 2009;24:619–25. doi: 10.1093/humrep/den411. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577–84. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 23.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–16. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 24.Krenning G, van Luyn MJ, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med. 2009;15:180–9. doi: 10.1016/j.molmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 26.Sieveking DP, Ng MK. Cell therapies for therapeutic angiogenesis: back to the bench. Vasc Med. 2009;14:153–66. doi: 10.1177/1358863X08098698. [DOI] [PubMed] [Google Scholar]
- 27.Schachinger V, Aicher A, Dobert N, Rover R, Diener J, Fichtlscherer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–32. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 28.Meier P, Gloekler S, de Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation. 2009;120:1355–63. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 29.England TJ, Gibson CL, Bath PM. Granulocyte-colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: a systematic review. Brain Res Rev. 2009;62:71–82. doi: 10.1016/j.brainresrev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Ripa RS, Kastrup J. G-CSF therapy with mobilization of bone marrow stem cells for myocardial recovery after acute myocardial infarction – a relevant treatment? Exp Hematol. 2008;36:681–6. doi: 10.1016/j.exphem.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease: meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Park KW, Kwon YW, Cho HJ, Shin JI, Kim YJ, Lee SE, Youn SW, Lee HC, Kang HJ, Shaul PW, Oh BH, Park YB, Kim HS. G-CSF exerts dual effects on endothelial cells – opposing actions of direct eNOS induction versus indirect CRP elevation. J Mol Cell Cardiol. 2008;45:670–8. doi: 10.1016/j.yjmcc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Optimal ex – vivo expansion of neutrophils from PBSC CD34+ cells by a combination of SCF, Flt3-L and G-CSF and its inhibition by further addition of TPO. J Transl Med. 2007;5:53–64. doi: 10.1186/1479-5876-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 35.Andrade SP, Fan TP, Lewis GP. Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br J Exp Pathol. 1987;68:755–66. [PMC free article] [PubMed] [Google Scholar]
- 36.Hague S, MacKenzie IZ, Bicknell R, Rees MC. In-vivo angiogenesis and progestogens. Hum Reprod. 2002;17:786–93. doi: 10.1093/humrep/17.3.786. [DOI] [PubMed] [Google Scholar]
- 37.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–52. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 38.Lu X, Baudouin SV, Gillespie JI, Anderson JJ, Dickinson AM. A comparison of CFU-GM, BFU-E and endothelial progenitor cells using ex vivo expansion of selected cord blood CD133(+) and CD34(+) cells. Cytotherapy. 2007;9:292–300. doi: 10.1080/14653240701247853. [DOI] [PubMed] [Google Scholar]
- 39.de Wynter EA, Buck D, Hart C, Heywood R, Coutinho LH, Clayton A, Rafferty JA, Burt D, Guenechea G, Bueren JA, Gagen D, Fairbairn LJ, Lord BI, Testa NG. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–96. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]