Abstract
Recent developments in proteomic technologies have enabled the high-throughput, multiplex measurement of large panels of antibodies in biological fluids of patients with immune-driven diseases. Antigen microarrays are increasingly being used to delineate the natural history of autoantibody formation and epitope spread, and thus gain insight into the pathogenesis of autoimmune diseases, as well as into host immunity and its shortcomings. Characterization of autoimmunity that precedes the onset of clinically apparent disease has the potential to guide disease prevention using either conventional immunosupression or novel, antigen-specific tolerizing therapies. In addition, autoantibody profiling has the potential to identify molecular subtypes of a disease, which could allow for prediction of disease outcomes such as severity, tissue damage, and response to therapy.
Keywords: Autoantibody, Protein Microarray, Autoimmunity, Rheumatoid Arthritis, Multiplex, Proteomics, Autoantigen, Review
2. INTRODUCTION
The humoral immune response to both foreign and self antigens involves antibody targeting of a specific but often broad set of epitopes, starting with the epitope that elicited the initial immune response and spreading to other epitopes on the same antigen (a process termed intramolecular epitope spread) or to structurally similar or physically associated epitopes on different antigens (a process termed intermolecular epitope spread) (1–3). Epitope spreading likely evolved as a mechanism that combats pathogens’ attempts to evade the host immune system by mutating (4): it increases the chance of immune recognition of an antigen, augments opsonization, and allows cross-linking of antibodies on the antigen’s surface, all of which promote immune clearance of the antigen. Although beneficial during the response to infection, epitope spreading is thought to contribute to the pathogenesis, and perhaps precipitate the onset of symptoms, of autoimmune diseases, allowing an antigen that is in itself innocuous to induce eventual autoimmune-mediated destruction (5–7). Epitope spreading in autoimmunity may arise owing to antibody targeting of self antigens that are newly formed as a result of inflammation-induced post-translational modification (8) or of self antigens that are newly exposed as a result of immune-mediated apoptosis (9).
Because the humoral response diversifies over time, insight into the specificity and timing of epitope spreading is critical to our understanding of an immune response, as well as to the implementation of therapeutic intervention in immune-driven disease. In addition, the fine specificity of the humoral response is not identical in all patients with a given disease, and thus autoantibody profiling has potential as a tool for stratifying disease into distinct subtypes, paving the way for determining disease prognosis and targeting therapy.
3. ANTIGEN MICROARRAYS
Autoantibodies are a hallmark of many autoimmune diseases, and the presence of specific autoantibodies is included in many sets of classification criteria, such as the ones for systemic lupus erythematosus (SLE), mixed connective tissue disease, Sjögren’s syndrome, and RA (10, 11). Currently, routine measurement of autoantibodies in the clinical laboratory generally relies on enzyme-linked immunosorbent assays (ELISA) or fluorescence immunoassays that are laborious, require large quantities of biological fluids, and detect only one antibody at a time. Recent advances in proteomic technologies aim to overcome these limitations. Riding on the success of DNA microarray technology, protein microarrays have been developed that enable high-throughput, simultaneous measurement of multiple proteins in small volumes of biological fluid (12–14). Moreover, protein microarrays have the potential to provide more accurate and comprehensive information about a disease than do conventional immunoassays by identifying multi-component signatures of disease, instead of simply detecting the levels of only a very limited set of specified antigens. Although protein microarrays can be used to study diverse types of interactions, including protein-protein interactions and protein-DNA interactions (15), this review focuses on the use of a particular type of protein microarray—the antigen microarray—to profile autoantibody specificities, and thereby identify clinically informative autoantibody signatures associated with humoral immune responses, in particular in the setting of autoimmunity.
In addition to their use in autoimmune diseases, antigen microarrays have also been used to monitor the antibody response to vaccination (16), infection (17), organ transplantation (18), and allergens (19) and as a screen for occult cancer (20). Moreover, although they most commonly comprise protein antigens, antigen microarrays have now been adapted to allow identification of antibodies against lipid (21) or carbohydrate (22) antigens. Thus, there are clearly many potential uses for, and permutations of, antigen microarrays.
3.1. Planar microarrays
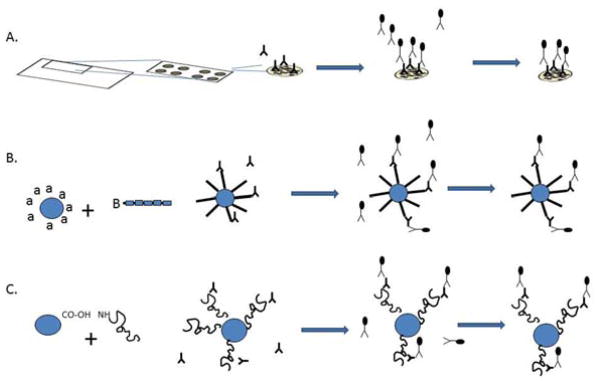
The most commonly used antigen microarrays are the solid-phase, planar arrays. These are essentially miniaturized, multiplexed immunoassays. Antigens are immobilized in ordered arrays (commonly on nitrocellulose membranes or derivatized glass microscope slides), probed with antibody-containing solution (e.g. sera, plasma, culture supernatants), and then incubated with a secondary antibody conjugated either to an enzyme (e.g. horseradish peroxidase) or directly to a fluorophore that allows visualization and quantification of antigen-specific antibodies following imaging of the array (Figure 1). Evolving, though less common, technologies being developed for readout of antibody binding on planar microarrays include surface plasmon resonance (23) and electrochemiluminescence (24).
Figure 1.
Schematic representation of planar and bead-based microarrays. A. Planar antigen microarrays, B. Bead-based peptide antigen array, C. Bead-based protein antigen array. In all cases, antigen is bound to the experimental surface, probed with antibody-containing fluid sample, washed, incubated with secondary detection antibody, and visualized for quantitation of primary antibody reactivity.
The first group to develop antigen microarrays expressly for analyzing autoantibodies was that of Hämmerle and colleagues (25), who used planar microarrays to profile serum antibodies against serial dilutions of 18 autoantigens known to be targeted in various autoimmune diseases. In our initial antigen microarray work (26), we adapted and expanded previously developed microarray technologies (25, 27, 28) to enable profiling of autoantibody responses against over 1000 features representing 196 proteins or peptides that have been implicated in the autoimmune response in one or more connective tissue diseases (26). We have since refined these microarrays for disease-specific indications, developing arrays containing putative antigens present in the synovial lining of the joint for profiling of rheumatoid arthritis (RA) (29), arrays containing myelin peptides for profiling of multiple sclerosis (MS) (21), and arrays containing nuclear antigens for profiling of systemic lupus erythematosus (SLE) (30). Similar disease-specific antigen microarrays have been developed for characterizing autoantibody profiles in animal models of these diseases (3, 31, 32).
The success of early protein microarrays has engendered considerable interest in adapting these or similar technologies for use in the clinical laboratory. For instance, Meso Scale Diagnostics (Gaithersburg, MD) has developed a planar protein microarray that utilizes electrochemiluminescence to detect antibody-antigen binding events on ordered arrays contained within a standard-sized 96- or 386-well plate (24). In the field of autoimmunity, this technology has been adopted by Crescendo Bioscience (South San Francisco, CA) for development of multiplex biomarker assays for the diagnosis and prognostication of RA (33). Likewise, Roche Diagnostics (Penzburg, Germany) is developing a multiplex, semi-automated, planar microarray. Although aimed initially at profiling of autoantibodies in RA, similar projects are focused on developing this technology for autoantibody profiling of SLE and the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides. Two other platforms recently made commercially available are Mikrogen’s recomLine (Neuried, Germany) (34) and Phadia ImmunoCap (Uppsala, Sweden), which have been marketed primarily as assays for the detection of infectious and allergic conditions, respectively, but are now being adapted for the characterization of autoimmunity.
Despite the advantages they have over traditional immunoassays, antigen microarrays have their own share of limitations. One of the most important limitations is selection bias. Although the number of antigens that can be analyzed per assay has increased from only 18 in the earliest versions of protein microarrays (25) to well over 500 in our current versions, standard microarrays are not best suited to the discovery of previously unknown antigens: they allow analysis only of a priori selected proteins. The recently developed high-density protein microarrays, however, are a step in the direction of unbiased proteomics. The most widely used of the high-density arrays are the ProtoArrays developed by Invitrogen; these contain over 8000 recombinant human proteins and have been used to identify previously unrecognized autoantigens targeted in RA (35). Likewise, high-density protein microarrays containing 37200 redundant recombinant human proteins (German Resource Center for Genome Research GmbH) have been used to identify autoantigens targeted in alopecia areata (36) or dilated cardiomyopathy (37). Putative autoantigens discovered by high-density protein microarrays are subsequently validated by standard antigen microarrays and by immunoblotting. Nevertheless, current high-density microarrays still do not contain the whole human proteome. Furthermore, because they are usually expressed in insect or bacterial cells, the recombinant proteins used in the high-density arrays may lack some relevant post-translational modifications or may be misfolded, both of which could affect their antigenicity. Another approach to reducing the bias in selecting the putative autoantigens contained in an antigen microarray is to use mass spectrometry to identify proteins bound to immune complexes isolated from diseased tissue or to identify proteins with which autoimmune disease sera react in immunoblots (38, 39).
As with selection of putative autoantigens, selection of epitopes or of epitope orientation is fraught with difficulty, either because the exact epitope of the antigen is unknown or because the orientation in which it can be recognized on planar microarrays is unknown. To address this issue, several groups have attempted to sterically restrict peptide orientation and/or improve presentation of the epitope to the antibodies. One approach to standardizing peptide orientation is the use of fmoc chemistry to generate peptides with a biotin molecule at one end; the biotin-containing peptides are then plated onto an avidin-coated surface, thus ensuring that the non-bound end is free to interact with the antibody. However, this approach requires knowing which end of the peptide is critical, a fact often determined only by trial and error. Even when the “correct end” of the epitope is presented, there is no guarantee that the critical residues will be freely available for interaction with the epitope’s cognate antibody. This issue can be partly overcome by the addition of linker molecules (such as polyethylene glycol) to the epitope-containing peptide, which makes the peptide project out from the surface of the planar array or particle, or by cyclization (the addition of flanking cysteines to the sides of the critical residues), which may help create epitopes represented by tertiary structures.
Finally, many planar microarrays are beset by lot-to-lot and platform-to-platform variability, which relates in part to differences in the microarray surface chemistry, spotting technique, and readout used. Our initial microarrays relied on capillary action, using spotting pins to dispense protein solution onto the microarray, usually in quadruplicate (26). However, this approach is limited by imprecision of liquid metering, because the pins deliver a slightly, but significantly, higher volume to the initial spot and slightly less to each replicate spot. To address this issue, several groups are developing piezoelectric “ink-jet” technology (40), imprint lithography (41), photolithography techniques that allow protein deposition to be more precisely controlled. A different approach to populating the microarray is in situ synthesis, in which PCR products or plasmid DNA serve as templates for the generation of proteins directly on the array by cell-free transcription-translation systems (42); this technique also avoids the issue of protein instability associated with long-term storage of protein microarrays. Newer modifications of protein arrays microarrays include microfluidomic platforms, which may improve the performance of multiplex microarray profiling, though cost of materials for such technology is still limiting (43).
3.2. Novel non-planar microarrays
For circumvention of some of the problems encountered with planar microarrays, focus is increasingly turning to fluid-phase antigen arrays. Three fluid-phase antigen assays that are commercially available, Quanta Plex (Inova Diagnostics, San Diego, CA), BioPlex 2200 (Bio-Rad Laboratories, Hercules, CA), and recomBead (Mikrogen), are based on bead-array technology initially developed by Luminex. In this type of array, antigens are immobilized on fluorescently addressable beads and then probed with biological fluids in fluid phase; a differentially fluorescent secondary antibody is then added and antibody binding is quantified by flow cytometry. Both the Quanta Plex and the BioPlex 2200 assays enable reliable measurement of autoantibodies relevant to various rheumatic diseases (44, 45), though the BioPlex 2200 system is a more fully automated and higher-throughput system (45, 46). We are using Luminex technology to develop a multiplexed bead array for profiling autoantibodies in RA, based on a select panel of biomarkers identified by our synovial planar microarrays (47).
Other emerging technologies include the recently commercialized non-planar microarray UltraPlex (SmartBead Technologies Ltd), in which antigens are attached to barcoded microparticles and which has been used to profile 9 ANA specificities (48), and three-dimensional (3D) gel microarrays, in which 3D surface chemistry results in fluid-phase reaction kinetics because the attached antigen is held at a distance from the slide’s surface (15, 49). 3D-gel microarrays are reportedly about ten times as sensitive as planar microarrays and are commercially available (GE HealthCare Co. and PerkinElmer Inc); however, they are expensive and their use and validation has so far been limited.
4. CLINICAL APPLICATION OF ANTIGEN ARRAYS IN AUTOIMMUNITY
As discussed, autoimmune diseases are characterized by the production of diverse repertoires of autoantibodies. The ability to delineate patients’ autoantibody profiles could potentially yield benefit in 3 areas: (i) improved diagnosis; (ii) identification of molecular subtypes of disease, which could aid in differential prognosis of disease or treatment outcome; and (iii) identification of autoantigens targeted in the lead up to clinically apparent disease, which could provide insight into the mechanisms underlying disease pathogenesis and enable preventive intervention, including antigen-specific tolerizing therapy (50) (Table 1).
Table 1.
Potential deployment of antigen microarrays in autoimmune disease
| Potential Application | Examples | Reference |
|---|---|---|
|
| ||
| Improved diagnostic accuracy | Currently used commercial CCP assay for RA composed of a pool of citrullinated peptides. | 51 |
| Potential for increased sensitivity with additional antigenic targets. | Robinson Lab, Work in progress | |
|
| ||
| Molecular subtyping of phenotypically similar conditions | High numbers of anti-citrullinated protein antibodies are associated markers of increased RA severity including TNF, IL-6, and the presence of the HLA shared epitope. | 29 |
| Identification of interferon high signature associated with anti-ribonuclear, but not nucleosomal autoantibodies. | 84 | |
| Panel of nine autoantibodies associated with increased interferon gene expression in those with incomplete lupus syndromes potentially identifying those at risk for full blown disease. | 85 | |
|
| ||
| Pre-clinical disease identification and intervention | Identification of increased fine epitope spread in those with undifferentiated arthritis who progress to clinical RA compared with those who remit | 7 |
| Expansion of epitope spreading may identify those at imminent risk for progression from preclinical autoimmunity to clinical RA. | Robinson Lab, Work in progress | |
| Guided antigen-specific tolerizing therapy | In a murine model of MS, increased diversity of autoantibody responses in the early phase of disease predicted a more severe clinical course and tolerizing vaccines encoding a greater number of array-determined targets proved superior in treating established disease. | 3 |
4.1. Use of autoantibody profiles for improved diagnosis
Detection of autoantibodies has long been used in the diagnosis of rheumatic and non-rheumatic autoimmune conditions (11) and has received greater prominence in the recently revised RA classification criteria (10). The increased reliance on autoantibodies in the diagnosis of RA is based largely on the observation that RA patients possess autoantibodies against citrullinated proteins (51). Citrullinated proteins are those that have undergone a post-translational modification in which peptidyl-arginine is converted to the non-standard amino acid peptidyl-citrulline. The antibodies targeting these proteins are termed anti-citrullinated protein antibodies (ACPA). Taking advantage of antibody targeting of citrullinated proteins in RA, a commercial diagnostic test has been developed that is based on antibody reactivity to a synthetic, citrullinated, filaggrin-derived cyclic peptide. This assay is known as the anti-cyclic citrullinated peptide (CCP) test and has a diagnostic sensitivity of approximately 67% and a specificity of 95% (52). Because filaggrin is not in fact present in the synovium, much effort has focused on identifying the true citrullinated antigens that are targeted in RA (35, 38, 53). For instance, isolation of immune complexes from arthritic joints and subsequent mass-spectrometric analysis of the IgG-bound proteins revealed that several extracellular matrix components (38) and citrullinated proteins such as fibrinogen (39) are targeted in RA.
Importantly, the anti-CCP test misses over 25% of RA cases that are identified clinically. Thus, the question arises: do 25% of RA patients lack ACPA or do they possess ACPA that do not react with the commercial CCP peptides (a proprietary mixture of pooled citrullinated peptides)? Can screening for antibody reactivity to additional peptides or proteins increase the diagnostic sensitivity of the CCP test without significantly reducing its specificity? Because autoimmune diseases are characterized by the production of not one but multiple autoantibodies, simultaneous measurement of an array of autoantibodies should yield a more comprehensive picture of the disease and possibly greater diagnostic sensitivity. Indeed, the number of autoantibodies detected has been shown to be important in predicting the development of diabetes (54–56) and RA (7). Multiplex antigen microarrays have been shown to have similar or improved sensitivity and specificity compared to conventional single assays in the detection of autoantibodies in serum from patients with autoimmune disease (25, 26) or of allergen-specific IgE (57). Our preliminary data, obtained using a bead-based antigen microarray, suggest that nearly one third of anti-CCP-negative patients have autoantibodies against 2 or more citrullinated peptides, and that screening using additional citrullinated peptides adds significant diagnostic sensitivity to the current CCP assay with minimal loss of specificity (Robinson Lab, unpublished work).
4.2. Use of autoantibody profiles for disease stratification and prognosis
Any given autoimmune disease is heterogeneous, varying from patient to patient in rate of progression, severity, and underlying molecular dysregulation. Conventional single immunoassays do not adequately predict the course of the disease or the response to therapy, since detection of one or two autoantibodies cannot differentiate between the multiple, distinct subtypes of disease. Simultaneous profiling of multiple autoantibodies is increasingly being used to identify ‘autoantibody signatures’ of disease subtypes, and stratification of diseases on the basis of such signatures is hoped to enable more accurate diagnosis, as well as differential prognosis.
Over the last five years, antigen microarrays have begun to be used in identifying autoantibody profiles that reflect distinct subtypes of several autoimmune diseases. In RA, different autoantibody profiles have been shown to be associated with different HLA classes (29), and specific autoantibody profiles to be associated with increases in levels of certain inflammatory cytokines (47). In multiple sclerosis (MS), antigen microarray profiling identified autoantibody signatures that distinguished relapse-remitting MS and primary progressive MS from each other and from other neurologic or autoimmune-driven disease (58). Moreover, different autoantibody profiles were associated with different brain pathology. Autoantibody profiling has also been used to stratify disease into subsets associated with different disease manifestations of SLE (59, 60), predict the disease course in so-called incomplete lupus (60), and define a subpopulation of patients with dilated cardiomyopathy of autoimmune etiology (37).
One potential prognostic application of disease stratification is the prediction of disease severity. The presence of anti-CCP antibodies, as detected by the commercial CCP test, has been shown to be associated with a higher risk of erosive joint destruction and to predict a disease course of greater severity (61). Screening of sera derived from a cohort of patients with early RA by using an antigen microarray containing 225 putative peptide or protein antigens revealed that a specific ACPA profile was associated with features predictive of more severe RA (29, 47, 62), and work is ongoing to develop autoantibody profiles as biomarkers that can predict disease severity and joint damage even better than the current anti-CCP assay alone. In addition, information about autoantibody isotype may be useful in predicting disease severity. Antigen microarrays can be used to analyze the relative amounts of antibody isotypes present (26, 63). The isotype dictates the effector function of the antibody and yields clues as to the polarization of the T-helper cell that may have activated the autoreactive B cell (64). Besides providing insight into disease pathogenesis, isotype profiling holds promise for prediction of disease severity: a recent study showed that ACPA isotypes served as predictors of RA severity, with the presence of more isotypes being associated with a higher risk of radiographic damage (65).
Another prognostic application is the prediction of a patient’s response to a particular therapeutic agent. Antigen microarray profiling of patients with RA identified a panel of markers that could predict patients’ response to the anti-TNF therapeutic etanercept (66). Notably, no single marker was able to distinguish responders from non-responders—it was only a panel of markers that provided such predictive value. Antigen microarrays have similarly been used for the identification of autoantibodies that predict renal transplant rejection (18, 67), and work is ongoing, in our lab and that of others, to identify autoantibody profiles that predict response to other biologic and non-biologic therapies in RA and related rheumatic conditions. Antigen microarrays could also be used to detect the generation of antibodies against drugs used to treat disease. For instance, high levels of antibodies against the anti-TNF therapeutic infliximab develop in some RA patients, resulting in reduced clinical efficacy and the need for dose escalation of infliximab (68). Given that the clinical efficacy of infliximab is often not determined until several months into therapy, the detection of antibodies against infliximab early in the course of therapy could potentially be used as an indicator of the need for dose escalation or an alternative therapeutic agent.
A use of autoantibody signatures that is only just starting to be explored is in expediting the approval of investigational drugs. Disease stratification based on autoantibody profiles could conceivably be useful in reducing cohort size in, and duration of, clinical trials by allowing pre-selection of patients who are likely to respond to the investigational drug, as well as by serving as pharmacodynamic, surrogate markers of therapeutic efficacy (69) that can substitute for endpoints that often take longer to manifest.
4.3. Use of pre-clinical autoantibody profiles for disease prevention
Individuals who develop RA often first seek medical attention when they develop clinical symptoms that do not yet meet the classification criteria for RA (70), at which time they are given a diagnosis of undifferentiated arthritis. Several studies have demonstrated the benefits of treating RA aggressively and early (71–74), even during the phase of undifferentiated arthritis (75). Moreover, RA patients develop anti-CCP antibodies many years before the onset of clinical RA (76–78), indicating the existence of a protracted period of autoimmunity that precedes the onset of arthritic symptoms. The positive predictive value of anti-CCP antibodies in predicting the onset of RA is estimated to be 5–16% in the general population (77, 78), though it is likely to be higher in at-risk populations. A substantial proportion of RA patients never develop anti-CCP antibodies, such that different tests are needed for predicting the development of RA in these individuals. Even in individuals who do possess anti-CCP antibodies, it is currently not possible to predict when they might develop RA—an important factor given that anti-CCP antibodies can be detected up to 10 years before the onset of arthritic symptoms and that treatments for RA are costly and can have serious adverse side effects. Thus, more accurate and all-encompassing predictors of the imminent onset of RA are needed to allow for potential intervention at the earliest possible time point.
Preclinical autoimmunity is characterized by the progressive accumulation of a series of autoantibodies in RA (7, 79–81) and in SLE (5), presumably reflecting epitope spread. Investigating the specificities of the autoantibodies produced, as well as the order in which the different autoantibodies are produced, during this preclinical phase should provide insight into the pathogenesis of autoimmune disease and could potentially allow for the implementation of early therapeutic intervention or even disease prevention. In one study examining a relatively small number of antigens, patients with undifferentiated arthritis who went on to develop RA were shown to have a greater number of ACPA than patients with undifferentiated arthritis who did not progress to RA (7). In this study, autoantibody reactivity was screened by single ELISA assays and was therefore limited to analysis of only five citrullinated antigens. Seeking to obtain a more comprehensive and detailed picture of the autoimmunity preceding the onset of RA, we have begun delineating the fine specificity of the ACPA immune response in sequential serum samples obtained before the onset of symptoms. Using bead-based antigen microarrays, we found that the preclinical phase of RA is characterized by a progressive accumulation of multiple autoantibodies targeting a variety of citrullinated epitopes, which correlate closely with a rise in anti-CCP titer and, at the later stages, preclinical inflammation, as defined by increases in serum cytokine levels (Robinson, unpublished work). This finding is consistent with results from autoantibody profiling of murine models of RA and MS, which demonstrated a similar pattern of gradual epitope spread occurring up to the time of clinical disease onset (31). Additionally, our preliminary characterization of the ACPA immune response within the time period just before the onset of RA revealed a profile of autoantibodies highly predictive of the onset of clinical RA within two years, thus potentially identifying a window for very early intervention with a goal of preventing the onset of clinical disease. These findings are similar to those in other diseases, such as findings showing that preclinical detection of autoantibodies can predict onset of type I diabetes in first-degree relatives of patients with the disease (54–56) and onset of clinically definite MS in patients with an otherwise clinically isolated syndrome (82). Finally, identification of the first autoantigen targeted by the immune response, or of a critical antigen that characterizes the transition to an active inflammatory process and clinical disease, could uncover a target for antigen-specific tolerizing therapy similar to that being developed for MS (50, 69, 83).
5. CONCLUSIONS
The use of antigen microarrays has opened many doors into facilitating characterization of the multiple reactivities associated with autoimmunity and other immunologic events, including infection, vaccination, transplantation, allergy, and malignancy. Limitations, such as high levels of inter-assay variability and lack of consistency and standards between experimental platforms, are being addressed by academic and industry-driven innovations, efforts that should provide the catalyst for the transition of multiplex autoantibody profiling from the academic benchtop to the clinical laboratory and patient care.
Acknowledgments
We would like to acknowledge our valued collaborators and the current and prior members of the Robinson Lab who have contributed to the projects described in this review. This work was supported by NIH NIAMS RC1 AR058713, NIAMS R01 AR-054822, an American College of Rheumatology Research and Education Foundation Within-Our-Reach Award, and Veterans Affairs Health Care System funding to W.H.R. J.S. received salary support from an American College of Rheumatology Research and Education Foundation Physician Scientist Development Award.
References
- 1.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu E, Hemmerich PH, Peter HH, Krawinkel U, von Mikecz AH. Characteristic epitope recognition pattern of autoantibodies against eukaryotic ribosomal protein L7 in systemic autoimmune diseases. Arthritis Rheum. 1997;40:661–671. doi: 10.1002/art.1780400411. [DOI] [PubMed] [Google Scholar]
- 3.Robinson WH, Fontoura P, Lee BJ, de Vegvar HE, Tom J, Pedotti R, DiGennaro CD, Mitchell DJ, Fong D, Ho PP, Ruiz PJ, Maverakis E, Stevens DB, Bernard CC, Martin R, Kuchroo VK, van Noort JM, Genain CP, Amor S, Olsson T, Utz PJ, Garren H, Steinman L. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 4.Milich DR, McLachlan A, Thornton GB, Hughes JL. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987;329:547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- 5.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, Drijfhout JW, Huizinga TW, Toes RE, Pruijn GJ. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–1561. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 8.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 9.Rosen A, Casciola-Rosen L, Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–1561. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 11.von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995;24:323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 12.Ekins RP. Multi-analyte immunoassay. J Pharm Biomed Anal. 1989;7:155–168. doi: 10.1016/0731-7085(89)80079-2. [DOI] [PubMed] [Google Scholar]
- 13.Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Klemic JF, Chang S, Casamayor A, Klemic KG, Smith D, Gerstein M, Reed MA, Snyder M. Analysis of yeast protein kinases using protein chips. Nat Genet. 2000;26:283–289. doi: 10.1038/81576. [DOI] [PubMed] [Google Scholar]
- 15.Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov. 2006;5:310–320. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuman de Vegvar HE, Robinson WH. Microarray profiling of antiviral antibodies for the development of diagnostics, vaccines, and therapeutics. Clin Immunol. 2004;111:196–201. doi: 10.1016/j.clim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Neuman de Vegvar HE, Amara RR, Steinman L, Utz PJHL, Robinson, Robinson WH. Microarray profiling of antibody responses against simian-human immunodeficiency virus: postchallenge convergence of reactivities independent of host histocompatibility type and vaccine regimen. J Virol. 2003;77:11125–11138. doi: 10.1128/JVI.77.20.11125-11138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM. Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76:1277–1283. doi: 10.1038/ki.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchêne M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Müller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Müller MW, Valenta R. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–416. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 20.Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009;276:6880–6904. doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L, Robinson WH. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 22.Gyorgy B, Tothfalusi L, Nagy G, Pásztói M, Géher P, Lörinc Z, Polgár A, Rojkovich B, Ujfalussy I, Poór G, Pócza P, Wiener Z, Misják P, Koncz A, Falus A, Buzás EI. Natural autoantibodies reactive with glycosaminoglycans in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R110. doi: 10.1186/ar2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson R. SPR for molecular interaction analysis: a review of emerging application areas. J Mol Recognit. 2004;17:151–161. doi: 10.1002/jmr.660. [DOI] [PubMed] [Google Scholar]
- 24.Hu L, Xu G. Applications and trends in electrochemiluminescence. Chem Soc Rev. 2010;39:3275–3304. doi: 10.1039/b923679c. [DOI] [PubMed] [Google Scholar]
- 25.Joos TO, Schrenk M, Hopfl P, Kröger K, Chowdhury U, Stoll D, Schörner D, Dürr M, Herick K, Rupp S, Sohn K, Hämmerle H. A microarray enzyme-linked immunosorbent assay for autoimmune diagnostics. Electrophoresis. 2000;21:2641–2650. doi: 10.1002/1522-2683(20000701)21:13<2641::AID-ELPS2641>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, de Vegvar HE, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 27.Haab BB, Dunham MJ, Brown PO. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2001;2:RESEARCH0004. doi: 10.1186/gb-2001-2-2-research0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacBeath G, Schreiber SL. Printing proteins as microarrays for high-throughput function determination. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 29.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, Sønderstrup G, Monach P, Drijfhout JW, van Venrooij WJ, Utz PJ, Genovese MC, Robinson WH. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 30.Graham KL, Robinson WH, Steinman L, Utz PJ. High-throughput methods for measuring autoantibodies in systemic lupus erythematosus and other autoimmune diseases. Autoimmunity. 2004;37:269–272. doi: 10.1080/08916930410001710686. [DOI] [PubMed] [Google Scholar]
- 31.Kidd BA, Ho PP, Sharpe O, Zhao X, Tomooka BH, Kanter JL, Steinman L, Robinson WH. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther. 2008;10:R119. doi: 10.1186/ar2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thibault DL, Graham KL, Lee LY, Balboni I, Hertzog PJ, Utz PJ. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Res Ther. 2009;11:R112. doi: 10.1186/ar2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastman S, Manning W, Qureshi F, Smith D, Cavet G, Haney D, Shen Y, Alexander C, Hesterberg L. Assay Development for percise measurement of disease activity serum biomarkers. Poster # THU0065. EULAR Congress. 2010 [Google Scholar]
- 34.Hanly JG, Su L, Farewell V, Fritzler MJ. Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Methods. 2010;358:75–80. doi: 10.1016/j.jim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Auger I, Balandraud N, Rak J, Lambert N, Martin M, Roudier J. New autoantigens in rheumatoid arthritis (RA): screening 8268 protein arrays with sera from patients with RA. Ann Rheum Dis. 2009;68:591–594. doi: 10.1136/ard.2008.096917. [DOI] [PubMed] [Google Scholar]
- 36.Lueking A, Huber O, Wirths C, Schulte K, Stieler KM, Blume-Peytavi U, Kowald A, Hensel-Wiegel K, Tauber R, Lehrach H, Meyer HE, Cahill DJ. Profiling of alopecia areata autoantigens based on protein microarray technology. Mol Cell Proteomics. 2005;4:1382–1390. doi: 10.1074/mcp.T500004-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Horn S, Lueking A, Murphy D, Staudt A, Gutjahr C, Schulte K, König A, Landsberger M, Lehrach H, Felix SB, Cahill DJ. Profiling humoral autoimmune repertoire of dilated cardiomyopathy (DCM) patients and development of a disease-associated protein chip. Proteomics. 2006;6:605–613. doi: 10.1002/pmic.200401293. [DOI] [PubMed] [Google Scholar]
- 38.Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, Wright J, Thornhill TS, Monestier M, Ploegh H, Robinson WH, Mathis D, Benoist C. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:15867–15872. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, Tomooka BH, Gregersen PK, Robinson WH. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller ED, Phillippi JA, Fisher GW, Campbell PG, Walker LM, Weiss LE. Inkjet printing of growth factor concentration gradients and combinatorial arrays immobilized on biologically-relevant substrates. Comb Chem High Throughput Screen. 2009;12:604–618. doi: 10.2174/138620709788681907. [DOI] [PubMed] [Google Scholar]
- 41.Truskett VN, Watts MP. Trends in imprint lithography for biological applications. Trends Biotechnol. 2006;24:312–317. doi: 10.1016/j.tibtech.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 42.He M, Stoevesandt O, Taussig MJ. In situ synthesis of protein arrays. Curr Opin Biotechnol. 2008;19:4–9. doi: 10.1016/j.copbio.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Bange A, Halsall HB, Heineman WR. Microfluidic immunosensor systems. Biosens Bioelectron. 2005;20:2488–2503. doi: 10.1016/j.bios.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Martins TB, Burlingame R, von Muhlen CA, Jaskowski TD, Litwin CM, Hill HR. Evaluation of multiplexed fluorescent microsphere immunoassay for detection of autoantibodies to nuclear antigens. Clin Diagn Lab Immunol. 2004;11:1054–1059. doi: 10.1128/CDLI.11.6.1054-1059.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shovman O, Gilburd B, Barzilai O, Shinar E, Larida B, Zandman-Goddard G, Binder SR, Shoenfeld Y. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci. 2005;1050:380–388. doi: 10.1196/annals.1313.120. [DOI] [PubMed] [Google Scholar]
- 46.Binder SR, Genovese MC, Merrill JT, Morris RI, Metzger AL. Computer-assisted pattern recognition of autoantibody results. Clin Diagn Lab Immunol. 2005;12:1353–1357. doi: 10.1128/CDLI.12.12.1353-1357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hueber W, Robinson WH. Proteomic biomarkers for autoimmune disease. Proteomics. 2006;6:4100–4105. doi: 10.1002/pmic.200600017. [DOI] [PubMed] [Google Scholar]
- 48.Smith J, Onley D, Garey C, Crowther S, Cahir N, Johanson A, Painter S, Harradence G, Davis R, Swarbrick P. Determination of ANA specificity using the UltraPlex platform. Ann N Y Acad Sci. 2005;1050:286–294. doi: 10.1196/annals.1313.030. [DOI] [PubMed] [Google Scholar]
- 49.Angenendt P, Glokler J, Konthur Z, Lehrach H, Cahill DJ. 3D protein microarrays: performing multiplex immunoassays on a single chip. Anal Chem. 2003;75:4368–4372. doi: 10.1021/ac034260l. [DOI] [PubMed] [Google Scholar]
- 50.Robinson WH, Garren H, Utz PJ, Steinman L. Millennium Award. Proteomics for the development of DNA tolerizing vaccines to treat autoimmune disease. Clin Immunol. 2002;103:7–12. doi: 10.1006/clim.2002.5185. [DOI] [PubMed] [Google Scholar]
- 51.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura K, Sugiyama D, Kogata Y, Nishimura K, Nakazawa T, Morinobu A, Kumagai S. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo K, Xiang Y, Nakamura H, Masuko K, Yudoh K, Noyori K, Nishioka K, Saito T, Kato T. Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis Res Ther. 2006;8:R175. doi: 10.1186/ar2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maclaren N, Lan M, Coutant R, Schatz D, Silverstein J, Muir A, Clare-Salzer M, She JX, Malone J, Crockett S, Schwartz S, Quattrin T, DeSilva M, Vander Vegt P, Notkins A, Krischer J. Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2beta predict immune-mediated (Type 1) diabetes in relatives. J Autoimmun. 1999;12:279–287. doi: 10.1006/jaut.1999.0281. [DOI] [PubMed] [Google Scholar]
- 55.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Chase HP, Eisenbarth GS. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun. 1996;9:379–383. doi: 10.1006/jaut.1996.0051. [DOI] [PubMed] [Google Scholar]
- 56.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 57.Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, Mueller MW. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33:1443–1449. doi: 10.1046/j.1365-2222.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 58.Quintana FJ, Farez MF, Viglietta V, Iglesias AH, Merbl Y, Izquierdo G, Lucas M, Basso AS, Khoury SJ, Lucchinetti CF, Cohen IR, Weiner HL. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li QZ, Xie C, Wu T, Aranow C, Putterman C, Mohan C. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansen LM, van Schaardenburg D, van der Horst-Bruinsma I, van der Stadt RJ, de Koning MH, Dijkmans BA. The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis. J Rheumatol. 2003;30:1691–1695. [PubMed] [Google Scholar]
- 62.Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, van Venrooij WJ, Metzger AL, Genovese MC, Robinson WH. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66:712–719. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham KL, Vaysberg M, Kuo A, Utz PJ. Autoantigen arrays for multiplex analysis of antibody isotypes. Proteomics. 2006;6:5720–5724. doi: 10.1002/pmic.200600345. [DOI] [PubMed] [Google Scholar]
- 64.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 65.van der Woude D, Syversen SW, van der Voort EI, Verpoort KN, Goll GL, van der Linden MP, van der Helmvan Mil AH, van der Heijde DM, Huizinga TW, Kvien TK, Toes RE. The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1110–1116. doi: 10.1136/ard.2009.116384. [DOI] [PubMed] [Google Scholar]
- 66.Hueber W, Tomooka BH, Batliwalla F, Li W, Monach PA, Tibshirani RJ, Van Vollenhoven RF, Lampa J, Saito K, Tanaka Y, Genovese MC, Klareskog L, Gregersen PK, Robinson WH. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R76. doi: 10.1186/ar2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porcheray F, DeVito J, Yeap BY, Xue L, Dargon I, Paine R, Girouard TC, Saidman SL, Colvin RB, Wong W, Zorn E. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89:1239–1246. doi: 10.1097/TP.0b013e3181d72091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haraoui B, Cameron L, Ouellet M, White B. Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol. 2006;33:31–36. [PubMed] [Google Scholar]
- 69.Garren H, Robinson WH, Krasulova E, Havrdová E, Nadj C, Selmaj K, Losy J, Nadj I, Radue EW, Kidd BA, Gianettoni J, Tersini K, Utz PJ, Valone F, Steinman L BHT-3009 Study Group. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol. 2008;63:611–620. doi: 10.1002/ana.21370. [DOI] [PubMed] [Google Scholar]
- 70.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, James FF, Norman SC, Louis AH, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1998;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 71.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, Zwinderman AH, Ronday HK, Han KH, Westedt ML, Gerards AH, van Groenendael JH, Lems WF, van Krugten MV, Breedveld FC, Dijkmans BA. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–3390. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 72.Landewe RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, van Denderen JC, Westedt ML, Peeters AJ, Dijkmans BA, Jacobs P, Boonen A, van der Heijde DM, van der Linden S. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–356. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 73.Mottonen T, Hannonen P, Leirisalo-Repo M, Nissilä M, Kautiainen H, Korpela M, Laasonen L, Julkunen H, Luukkainen R, Vuori K, Paimela L, Blåfield H, Hakala M, Ilva K, Yli-Kerttula U, Puolakka K, Järvinen P, Hakola M, Piirainen H, Ahonen J, Pälvimäki I, Forsberg S, Koota K, Friman C. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568–1573. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 74.van der Heide A, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, van der Veen MJ, Haanen HC, Hofman DM, van Albada-Kuipers GA, ter Borg EJ, Brus HL, Dinant HJ, Kruize AA, Schenk Y. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996;124:699–707. doi: 10.7326/0003-4819-124-8-199604150-00001. [DOI] [PubMed] [Google Scholar]
- 75.van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, Speyer I, Westedt ML, Peeters AJ, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–1432. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 76.Majka DS, Deane KD, Parrish LA, Lazar AA, Barón AE, Walker CW, Rubertone MV, Gilliland WR, Norris JW, Holers VM. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 78.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 79.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. The number of elevated cytokines/chemokines in pre-clinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 81.Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, de Gast T, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum. 2004;50:2423–2427. doi: 10.1002/art.20431. [DOI] [PubMed] [Google Scholar]
- 82.Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 83.Fontoura P, Garren H, Steinman L. Antigen-specific therapies in multiple sclerosis: going beyond proteins and peptides. Int Rev Immunol. 2005;24:415–446. doi: 10.1080/08830180500379655. [DOI] [PubMed] [Google Scholar]
- 84.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005 May;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 85.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]