SUMMARY
Background
Haemoglobinopathies variously reduce the risk of developing malaria syndromes. Quantifying these relationships may strengthen the foundation for translational studies of malaria pathogenesis and immunity.
Methods
The databases of MEDLINE and Embase (1950 – September 9, 2011) were searched to identify studies that estimated the risk of malaria in patients with and without haemoglobinopathies. Additional studies were identified from reference lists. Included outcomes were Plasmodium falciparum-related outcomes of severe malaria, uncomplicated malaria, asymptomatic parasitaemia, or pregnancy-associated malaria, and P. vivax malaria. Two independent reviewers identified studies, assessed their quality, and extracted data; data were meta-analyzed when outcomes were reported in more than one study.
Findings
Of 62 identified studies, 44 reported on HbAS, 19 on HbAC and HbCC, and 18 on α-thalassaemia. Case-control studies showed a decreased risk of severe malaria for HbAS (summary Odds Ratio [OR] 0.09; 95% confidence interval [CI] 0.06 – 0.12), HbCC (summary OR 0.27; 95% CI 0.11 – 0.63), homozygous α-thalassaemia (summary OR 0.63; 95% CI 0.48 – 0.83), HbAC (summary OR 0.83; 95% CI 0.74 – 0.92), and heterozygous α-thalassaemia (summary OR 0.83; 95% CI 0.74 – 0.92). Only HbAS was consistently associated with protection from uncomplicated malaria (summary Incidence Rate Ratio 0.69; 95% CI 0.61 – 0.79); none demonstrated protection from asymptomatic parasitaemia. There was a paucity of clinical studies investigating β-thalassaemia, HbE, P. vivax malaria, and pregnancy-associated malaria.
Interpretation
Protection from severe malaria syndromes is significant for HbAS, HbCC, HbAC, and homozygous and heterozygous α-thalassaemia, but these haemoglobinopathies differ substantially in the degrees of protection. Protection from uncomplicated malaria and asymptomatic parasitaemia is mild or absent. By attenuating the severity of malaria, haemoglobinopathies serve as a model for investigating the mechanisms of malaria pathogenesis and immunity.
INTRODUCTION
Haemoglobinopathies are highly prevalent in some human populations currently or historically exposed to the malaria parasite Plasmodium falciparum. In the major haemoglobinopathies, adult haemoglobin – normally composed of two α-globin and two β-globin chains – is altered by genetic polymorphisms that encode single amino acid substitutions in β-globin (as in HbS, HbC, and HbE) or reduce production of α- and β-globin chains (in α- and β-thalassaemia, respectively).1 These haemoglobin variants and thalassaemias are postulated to have been naturally selected for their protection from malaria, as evidenced by a broad spectrum of investigations. These include experimental P. falciparum infection protocols, in vitro laboratory experimentation, ecological epidemiologic studies, and cartographic modeling.2 Nevertheless, confirmation and quantification of malaria risk reductions due to haemoglobinopathies requires clinical studies.
Correlates of both malaria pathogenesis and immunity to disease can be identified by studying patterns of differential susceptibility to malaria. Investigations of increased susceptibility to P. falciparum malaria during pregnancy3,4 and resistance to P. vivax infection in West Africans lacking erythrocyte expression of Duffy Antigen Receptor for Chemokines (DARC)5,6 have unearthed fundamental mechanisms of both malaria pathogenesis and acquired immunity. These molecular mechanisms – adumbrated by careful epidemiologic studies – are foundations for leading vaccine candidates against pregnancy-associated malaria7 and vivax malaria.8 While some falciparum malaria vaccines are showing partial efficacy,9,10 malaria’s pathogenic mechanisms are not understood sufficiently to inform the rational design of future therapeutics and preventive measures.
The clinical manifestations of P. falciparum malaria display a broad spectrum of severity from asymptomatic parasitaemia to severe malaria syndromes.11 Differential protection from specific syndromes owing to genetic resistance may constitute a “natural experiment” that helps to identify the mechanisms of malaria pathogenesis that cause clinical morbidity. Toward this end, we conducted a systematic review of published studies to estimate the direct clinical effects of haemoglobinopathies on malaria syndromes.
METHODS
Search strategy & review criteria
We performed our review and meta-analysis in accordance with the PRISMA guidelines (Supplementary methods, Table S1).12 Two authors (SMT and CMP) independently performed the database searches (through September 9, 2011), appraised study quality, and extracted study data. Additional references were selected from the reference lists of identified studies. To appraise the quality of the observational studies, we adapted the principles of the Newcastle-Ottawa scale;13 in order to base analyses on robust data, we only included studies that scored at least seven stars on the scale’s assessment of patient selection, comparability, and exposure/outcome. When reported data were not sufficient for estimation of desired comparisons, we contacted study authors. Overall, we selected studies that reported the frequency of clinical outcomes in patients with and without a haemoglobinopathy.
Study participants
We included studies that principally enrolled children; the exceptions were studies that investigated pregnancy-associated malaria. We included studies conducted in any level of malaria endemicity, but did not consider studies of non-immune travelers.
Study designs
For the incident outcomes of severe malaria, uncomplicated malaria, asymptomatic parasitaemia, and vivax malaria, we included data from both prospective cohort and case-control studies. For asymptomatic parasitaemia (with either Plasmodium species), we also included data from cross-sectional studies. For pregnancy-associated malaria outcomes, we included data from cross-sectional studies of pregnant women. For case-control studies, we required a clear description of the selection of controls. We excluded case reports.
Exposure assessment
We only considered papers in which haemoglobin typing employed electrophoresis, chromatography, or DNA analysis.
Outcome assessment
We investigated clinical outcomes owing to infection with either P. falciparum or P. vivax. P. falciparum-related outcomes were severe malaria (including cerebral malaria and severe malarial anaemia),14 uncomplicated malaria, asymptomatic parasitaemia, and pregnancy-associated malaria; vivax malaria was also included (Supplementary methods).
Definitions
The human genome normally contains four copies of the α-globin gene and two copies of the β-globin gene. Individuals with deletions of one a-globin gene (–α/αα) and two α-globin genes (–α/-α or αα/--) are referred to as α-thalassaemia heterozygotes and homozygotes, respectively. β-thalassaemia refers to individuals with impaired production of a single β-globin gene (β-thalassaemia trait, or β-thalassaemia minor). We did not investigate HbSS, HbSC, the deletion of three α-globin genes (α−/−-), or the impaired production of two β-globin genes (β-thalassaemia major) because these genotypes typically manifest severe clinical sequelae which complicate any assessment of malaria-specific clinical morbidity. Additionally, we did not explore haemoglobin mutations with low global population prevalences, including haemoglobins D, Constant Spring, and Lepore. Odds Ratios (ORs) and Incidence Rate Ratios (IRRs) reflect comparisons between patients with haemoglobin variants and those with HbAA, or between patients with thalassaemias and those without.
Data analysis
For studies that did not report comparisons of interest, we extracted raw data to either 1) compare prevalences of parasitaemia between patient groups with the chi-squared test (in cross-sectional studies); 2) compare prevalences of haemoglobin variants between groups of patients with malaria syndromes with unadjusted ORs (in case-control studies); or 3) compute Risk Ratios (RRs) or IRRs of malaria syndromes between groups of patients with and without haemoglobinopathies (in prospective studies). All comparisons were calculated with exact confidence intervals.
Because case-control and prospective cohort studies estimate relative risk using distinct statistical methodologies, we employed separate analyses to meta-analyze ORs and IRRs. When individual-level case-control data were available for two or more studies that compared the prevalence of a haemoglobinopathy for the same case and control groups, we meta-analyzed the data to produce summary ORs. Meta-analyses were computed using random-effects models employing the DerSimonian & Laird method (metan in Stata/IC); the I2 statistic for heterogeneity was calculated using the Mantel-Haenszel method for meta-analyzed data within subgroups (haemoglobinopathy and malaria syndrome). Similarly, when data were available for two or more prospective studies which compared incidence rates of the same outcome, we meta-analyzed the data to produce summary IRRs. Meta-analyses of IRRs were computed using random-effects Poisson meta-regression.15. We assessed publication bias in case-control studies using funnel plots and Begg’s test (Supplementary methods). All single-study and summary analyses were calculated with Stata/IC (version 11, Stata Corp, College Station, TX).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (SMT) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
The search strategy identified 2664 studies for review, and we selected 62 for inclusion (Figure 1): 44 studies of HbAS, 19 of HbAC, eight of HbCC, 18 of α-thalassaemia (all except one included homozygotes), three of HbE, and two of β-thalassaemia (some studies examined more than one haemoglobinopathy). Of the 62 studies, 18 were prospective cohorts, 15 were case-control, and 31 were cross-sectional studies (two studies reported more than one design). Five studies investigated pregnancy-associated malaria, and two studies included patients with P. vivax malaria. There was no evidence of reporting bias amongst comparable studies (Supplementary methods, Figure S1).
Figure 1.
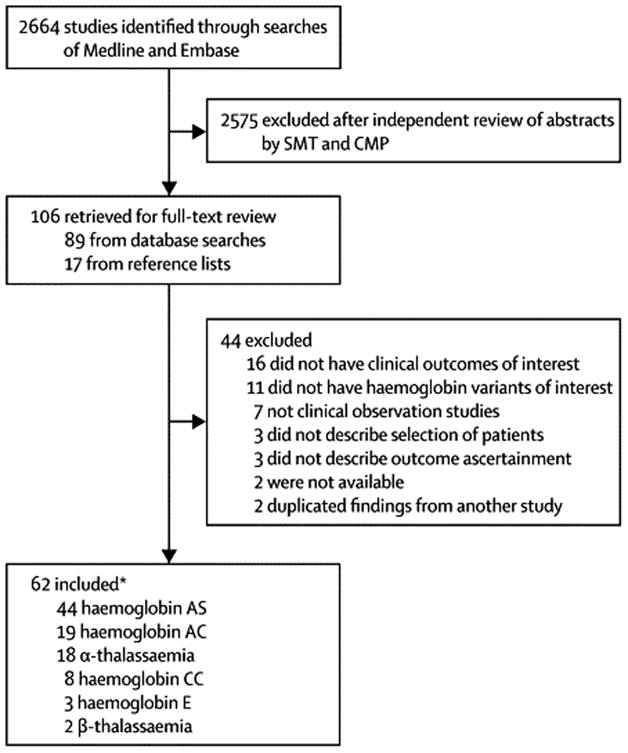
Study identification flowchart
Severe P. falciparum malaria syndromes
Haemoglobin S
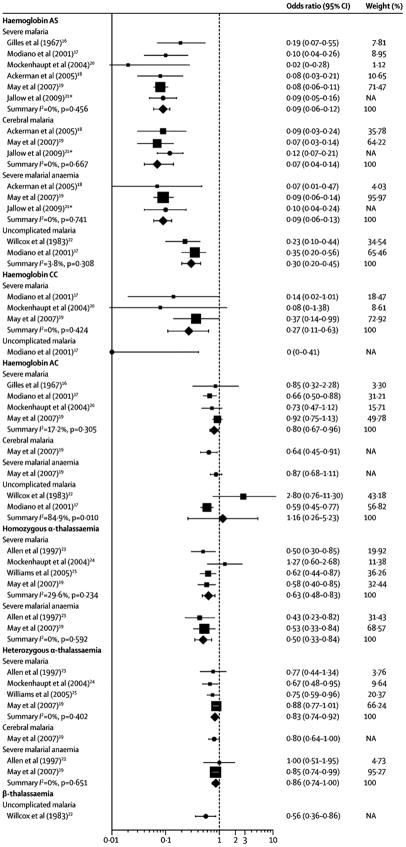
Compared to healthy controls, the summary OR for severe malaria was 0.09 (95% CI 0.06 – 0.12; I2 10.6%) across five studies which together enrolled more than 10,000 patients,16–20 and was similar to summary ORs for the specific syndromes of cerebral malaria (0.07; 95% CI 0.04 – 0.14; I2 0%)18,19 and severe malarial anaemia (0.09; 95% CI 0.06 – 0.13; I2 0%)(Figure 2, Table 1).18,19 Compared to children with uncomplicated malaria, ORs for severe malaria in three studies17,21,22 were heterogeneous and a summary OR estimate was non-significant (0.52; 95% CI 0.20 – 0.38; I2 50.0%).
Figure 2.
Unadjusted individual and summary Odds Ratios for specific malaria syndromes by haemoglobin variants
Abbreviations: CI, confidence interval; Hb, haemoglobin
a Not included in meta-analyses because prevalences of HbAS in cases and controls were not reported. All Odds Ratios (ORs) are for comparison with healthy controls. The data markers represent either ORs from individual studies (circles) or summary ORs for subgroup data (diamonds) that were generated by random-effects meta-analysis of individual studies (squares). For individual studies included in meta-analyses, the size of the square data marker is relative to the weight of the study. The I2 statistic is a measure of the heterogeneity of the individual study estimates which were meta-analyzed, and was calculated using the Mantel-Haenszel method. ORs for individual studies may differ from those in Table S1 or in the original published studies because they were calculated from raw data and are thus unadjusted.
Table 1.
Case-control studies investigating the effect of haemoglobinopathies on P. falciparum malaria
| Source | Location | Syndromea | No. patients | Age | Comment | % Haemoglobin opathy |
|---|---|---|---|---|---|---|
| Haemoglobin AS | ||||||
| Gilles et al (1967)16 | Nigeria | Severe malaria | 100 | Range 6m - 4y | 4.0 | |
| Healthy | 100 | Range 6m - 4y | Aparasitaemic outpatients | 18.0 | ||
| Willcox et al (1983)33 | Liberia | Uncomplicated malaria | 518 | Range 2m - >15y | Outpatients | 1.9 |
| Healthy | 1027 | Range 2m - >15y | Community surveys | 7.9 | ||
| Hill et al (1991)22 | The Gambia | Severe malaria | 619 | Median 3.0y Range <10y |
1.2 | |
| Severe non-malaria illness | 332 | Median 2.2y Range <10y |
Inpatients with severe non- malaria illness | 10.9 | ||
| Mild non-malaria illness | 510 | Median 1.9y Range <10y |
Mild outpatient illness without malaria parasites | 12.9 | ||
| Uncomplicated malaria | 354 | Median 3.0y Range <10y |
2.8 | |||
| Agarwal et al (2000)21 | Mali | Severe malaria | 67 | Mean 4.3y | 4.5 | |
| Cerebral malaria | 34 | Mean 3.8y | Subset of severe malaria patients with coma, obtundation, or convulsions | 5.9 | ||
| Severe malarial anaemia | 12 | Mean 1.0y | Subset of severe malaria patients with Hct <15% | 0 | ||
| Uncomplicated malaria | 391 | Mean 9.1y | 2.7 | |||
| Modiano et al (2001)17 | Burkina Faso | Severe malaria | 359 | Mean 4y Range 0.5 -15y |
1.1 | |
| Uncomplicated malaria | 476 | Mean 4y Range 0.5 -15y |
4.0 | |||
| Healthy | 3513 | Mean 11y Range 1- 40y |
Community cross-sectional survey | 9.5 | ||
| Mockenhaupt et al (2004)20 | Ghana | Severe malaria | 290 | Median 24m Range 6 - 102m |
0 | |
| Healthy, parasitaemic | 290 | NA | Community cross-sectional survey, matched to severe cases | 7.9 | ||
| Healthy aparasitaemic | 290 | NA | Community cross-sectional survey, matched to severe cases | 8.3 | ||
| Ackerman et al (2005)18 | The Gambia | Severe malaria | 315 | NA | 1.1 | |
| Cerebral malaria | 235 | NA | Subset of severe malaria patients with BCS <3 | 1.3 | ||
| Severe malarial anaemia | 80 | NA | Subset of severe malaria patients with Hb <5g/dL | 0.6 | ||
| Newborns | 583 | NA | Cord blood specimens | 8.7 | ||
| May et al (2007)19 | Ghana | Severe malaria | 2591 | Median 20m | 1.4 | |
| Cerebral malaria | 581 | NA | Subset of severe malaria patients with BCS <3 | 1.2 | ||
| Severe malarial anaemia | 1649 | NA | Subset of severe malaria patients with Hb <5g/dL | 1.6 | ||
| Healthy | 2048 | Median 30m | Community cross-sectional survey, matched to severe cases | 14.8 | ||
| Jallow et al (2009)99 | The Gambia | Severe malaria | 1060 | NA | NA | |
| Cerebral malaria | 869 | NA | Subset of severe malaria patients with BCS <3 | NA | ||
| Severe malarial anaemia | 318 | NA | Subset of severe malaria patients with Hb <5g/dL | NA | ||
| Newborns | 1500 | NA | Cord blood specimens | 8.0 | ||
| Haemoglobin C | ||||||
| Gilles et al (1967)16 | Nigeria | Severe malaria | 100 | Range 6m - 4y | He: 6.0 | |
| Healthy | 200 | Range 6m - 4y | Clinic attendance, aparasitaemic | He: 7.0 | ||
| Willcox et al (1983)33 | Liberia | Uncomplicated malaria | 518 | Range 2m - >15y | He: 1.3 | |
| Healthy | 1027 | Range 2m - >15y | Community surveys | He: 0.4 | ||
| Guinet et al (1997)26 | Mali | Severe malaria | 110 | Range 6m - 9y | 10.0 | |
| Severe malarial anaemia | 6 | Range 6m - 9y | Subset of severe malaria patients with Hct <15% or Hb <5g/dL | 0 | ||
| Uncomplicated malaria | 55 | Range 6m - 9y | Matched to severe cases | 9.0 | ||
| Agarwal et al (2000)21 | Mali | Severe malaria | 67 | Mean 4.3y | Ho: 0 He: 4.5 |
|
| Cerebral malaria | 34 | Mean 3.8y | Subset of severe malaria patients with coma, obtundation, or convulsions | Ho: 0 He: 2.9 |
||
| Severe malarial anaemia | 12 | Mean 1.0y | Subset of severe malaria patients with Hct <15% | Ho: 0 He: 0 |
||
| Uncomplicated malaria | 391 | Mean 9.1y | Ho: 1.5 He: 15.9 |
|||
| Modiano et al (2001)17 | Burkina Faso | Severe malaria | 359 | Mean 4y Range 0.5 – 15y |
Ho: 0.3 He: 17.6 |
|
| Uncomplicated malaria | 476 | Mean 4y Range 0.5 – 15y |
Ho: 0 He: 15.6 |
|||
| Healthy | 3513 | Mean 11y Range 1- 40y |
Community cross-sectional survey | Ho: 1.7 He: 21.7 |
||
| Mockenhaupt et al (2004)20 | Ghana | Severe malaria | 290 | Median 24m Range 6 – 102m |
Ho: 0 He: 16.6 |
|
| Healthy, parasitaemic | 290 | NA | Community cross-sectional survey, matched to severe cases | Ho: 1.0 He: 24.5 |
||
| Healthy, aparasitaemic | 290 | NA | Community cross-sectional survey, matched to severe cases | Ho: 1.7 He: 19.0 |
||
| May et al (2007)19 | Ghana | Severe malaria | 2591 | Median 20m | Ho: 0.2 He: 9.4 |
|
| Cerebral malaria | 581 | NA | Subset of severe malaria patients with BCS <3 | Ho: NA He: 7.2 |
||
| Severe malarial anaemia | 1649 | NA | Subset of severe malaria patients with Hb <5g/dL | Ho: NA He: 9.2 |
||
| Healthy | 2048 | Median 30m | Community cross-sectional survey, matched to severe cases | Ho: 0.5 He: 8.7 |
||
| Haemoglobin E | ||||||
| Oo et al (1995)27 | Myanmar | Severe malaria | 200 | 19 - 45y | 28.0 | |
| Uncomplicated malaria | 109 | 19 - 45y | Hospitalized | 27.5 | ||
| Hutagalung et al (1999)28 | Thailand | Severe malaria | 33 | 14 - 45y | 3.0 | |
| Uncomplicated malaria | 184 | 14 - 45y | Hospitalized | 22.3 | ||
| α-thalassaemia | ||||||
| Allen et al (1997)29 | Papua New Guinea | Severe malaria | 249 | NA | Ho: 44.6 He: 37.3 |
|
| Severe malarial anaemia | 155 | Median 2.1y | Subset of severe malaria patients with Hb <5g/dL | Ho: 36.1 He: 43.9 |
||
| Healthy | 249 | NA | Community survey, matched to severe cases | Ho: 57.0 He: 31.3 |
||
| Lell et al (1999)100 | Gabon | Severe malaria | 100 | Mean 44m | Ho: 10.0 He: 37.0 |
|
| Uncomplicated malaria | 100 | Mean 44m | Ho: 10.0 He: 43.0 |
|||
| Mockenhaupt et al (2004)30 | Ghana | Severe malaria | 261 | <5y | Ho: 5.0 He: 23.7 |
|
| Healthy, parasitaemic | 614 | <5y | Community cross-sectional survey, matched to severe cases | Ho: 3.3 He: 33.2 |
||
| Healthy, aparasitaemic | 479 | <5y | Community cross-sectional survey, matched to severe cases | Ho: 3.6 He: 31.9 |
||
| Williams et al (2005)31 | Kenya | Severe malaria | 655 | NA | Ho: 12.7 He: 47.0 |
|
| Fatal malaria | 72 | NA | Death in hospital | Ho: 8.4 He: 10.4 |
||
| Healthy | 648 | <7y | Community survey, matched | Ho: 16.7 He: 50.6 |
||
| May et al (2007)19 | Ghana | Severe malaria | 2591 | Median 20m | Ho: 2.0 He: 25.2 |
|
| Cerebral malaria | 581 | NA | Subset of severe malaria patients with BCS <3 | Ho: 2.2 He: 23.8 |
||
| Severe malarial anaemia | 1649 | NA | Subset of severe malaria patients with Hb <5g/dL | Ho: 1.8 He: 24.7 |
||
| Healthy | 2048 | Median 30m | Community cross-sectional survey, matched to severe cases | Ho: 2.0 He: 27.3 |
||
| β-thalassaemia | ||||||
| Willcox et al (1983)33 | Liberia | Uncomplicated malaria | 526 | 2m - >15y | 5.7 | |
| Healthy | 1132 | 2m - >15y | Community surveys | 9.7 | ||
Abbreviations: Hb: haemoglobin; NA: data not available; Ho: homozygote; He: heterozygote; BCS: Blantyre Coma Score; Hct: hematocrit.
Unless otherwise stated, patients with severe malaria, severe malarial anaemia, cerebral malaria and uncomplicated malaria met definitions described in the Methods section. Healthy patients were recruited as described in the Comments column.
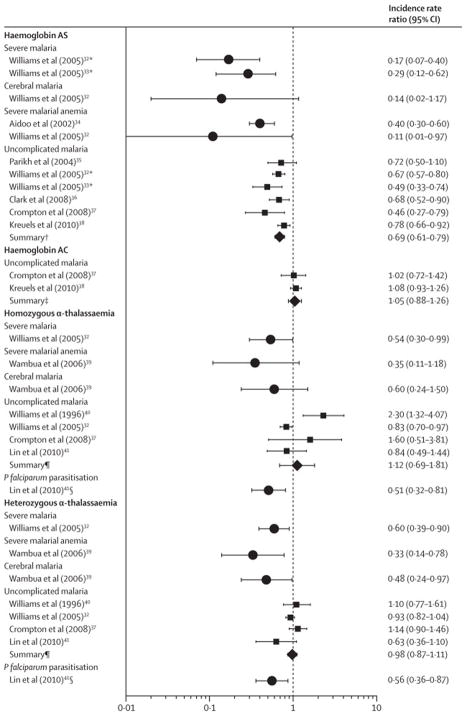
Only two cohort studies have reported the incidence of severe malaria (Figure 3, Table 2). The incidence of severe malaria was reduced by 71% (95% CI 38% – 88%)23 and 83% (95% CI 60% – 93%)24 in similar cohorts of Kenyan children. In the second cohort, the incidence of cerebral malaria was nonsignificantly reduced by 86% (95% CI -17% – 98%), while that of severe malarial anaemia was reduced by 60% (95% CI 40% – 70%)25 and 89% (95% CI 3% – 99%).24 Taken together, data from both case-control and prospective cohort studies indicate that HbAS is consistently associated with large reductions in the risk of severe malaria syndromes.
Figure 3.
Individual and summary incidence rate ratios of P. falciparum syndromes in prospective studies of children with haemoglobinopathies
Abbreviations: CI, confidence interval; Hb, haemoglobin. All incidence rate ratios (IRRs) compare patients with the variant listed to patients with either HbAA or the αα/αα genotype. Summary measures (diamonds) were computed using random-effects Poisson meta-regression of individual studies (squares).
a Studies had overlapping cohorts. Because the cohort in Williams et al (2005)23 subsumes that of Williams et al (2005),24 only data from Williams et al (2005)23 was included in the meta-analyzed summary IRR for uncomplicated malaria in HbAS children.
b Summary IRR of uncomplicated malaria in HbAS compared with HbAA children from five studies.23,34-37
c Summary IRR of uncomplicated malaria in HbAC compared with HbAA children from two studies.35,37
d Summary IRR of uncomplicated malaria in homozygous or heterozygous α-thalassaemic compared with non-thalassaemic children from three studies [Williams 1996, Williams 2005 (NG), Crompton 2008].23,35,39
e Detected by polymerase chain reaction.
Table 2.
Prospective studies investigating the effect of haemoglobinopathies on P. falciparum malaria
| Source | Outcome | Location | n | Ages | Incidence in unexposeda | Incidence Rate Ratio (95% C.I.) |
|---|---|---|---|---|---|---|
| Haemoglobin AS | ||||||
| Williams et al (2005)23,b | Severe malaria | Kenya | 2655 | < 5y | 0.022/y | 0.17 (0.07 – 0.40) |
| Williams et al (2005)24,b | Severe malaria | Kenya | 2104 | < 5y | 0.020/y | 0.29 (0.12 – 0.62) |
| Williams et al (2005)23 | Cerebral malaria | Kenya | 2655 | < 5y | 0.004/y | 0.14 (0.02 – 1.17) |
| Aidoo et al (2002)25 | Severe malaria anaemia | Kenya | 1022 | < 5y | 0.048/y | 0.40 (0.30 – 0.60) |
| Williams et al (2005)23 | Severe malarial anaemia | Kenya | 2655 | < 5y | 0.005/y | 0.11 (0.01 – 0.97) |
| Parikh et al (2004)34 | Uncomplicated malaria | Uganda | 307 | 6m – 5y | 2.04/y | 0.72 (0.5 – 1.1) |
| Williams et al (2005)23,b | Uncomplicated malaria | Kenya | 370 | < 11y | 2.36/y | 0.67 (0.57 – 0.80) |
| Williams et al (2005)24,b | Uncomplicated malaria | Kenya | 323 | < 8y | 3.06/y | 0.49 (0.33 – 0.74) |
| Migot-Nabias et al (2006)96 | Uncomplicated malaria | Senegal | 169 | 2 – 10y | NA | Reduced incidence in HbAS (IRR not reported) |
| Clark et al (2008)36 | Uncomplicated malaria3 | Uganda | 558 | 1 – 10y | 0.82/y | 0.68 (0.52 – 0.90) |
| Crompton et al (2008)35 | Uncomplicated malaria3 | Mali | 176 | 2 – 10y | 1.76/y | 0.46 (0.27 – 0.79) |
| Enevold et al (2008)40 | Uncomplicated malaria | Tanzania | 159 | 6m – 19y | 35%/6m | RR: 1.55 (0.51 – 4.77) |
| Kreuels et al (2010)37 | Uncomplicated malaria3 | Ghana | 852 | 3m – 2y | 1.2/y | 0.78 (0.66 – 0.92) |
| Summary23,34-37 | Uncomplicated malaria | 0.69 (0.61 – 0.79) | ||||
| Le Hesran et al (1999)59 | P. falciparum parasitaemia | Cameroon | 240 | < 5y | NA | No difference (IRR not reported) |
| Stirnadel et al (1999)58 | P. falciparum parasitaemia | Tanzania | 204 | < 1y | NA | No difference (IRR not reported) |
| Haemoglobin C | ||||||
| Rihet et al (2004)101 | Uncomplicated malaria3 | Mali | 256 | 1 – 24y | NA | Reduced incidence with HbC (IRR not reported) |
| Crompton et al (2008)35 | Uncomplicated malaria3 | Mali | 176 | 2 – 10y | 1.76/y | He: 1.02 (0.72 – 1.42) |
| Kreuels et al (2010)37 | Uncomplicated malaria3 | Ghana | 852 | 3m – 2y | 1.2/y | He: 1.08 (0.93 – 1.26) |
| Summary35,37 | Uncomplicated malaria | He: 1.05 (0.88 – 1.26) | ||||
| α-thalassaemia | ||||||
| Williams et al (2005)31 | Severe malaria | Kenya | 2104 | < 5y | 0.061/y | Ho: 0.54 (0.30 – 0.99) He: 0.60 (0.39 – 0.90) |
| Wambua et al (2006)32 | Severe malarial anaemia | Kenya | 2104 | < 5y | 0.010/y | Ho: 0.35 (0.11 – 1.18) He: 0.33 (0.14 – 0.78) |
| Wambua et al (2006)32 | Cerebral malaria | Kenya | 2104 | < 5y | 0.002/y | Ho: 0.60 (0.24 – 1.50) He: 0.48 (0.24 – 0.97) |
| Williams et al (1996)39 | Uncomplicated malaria3 | Vanuatu | 544 | < 5y | 0.7/y | Ho: 2.30 (1.32 – 4.07) He: 1.10 (0.77 – 1.61) |
| Williams et al (2005)31 | Uncomplicated malaria | Kenya | 370 | < 11y | 2.46/y | Ho: 0.83 (0.70 – 0.97) He: 0.93 (0.82 – 1.04) |
| Migot-Nabias et al (2006)96 | Uncomplicated malaria3 | Senegal | 169 | 2 – 10y | NA | No difference (IRR not reported) |
| Enevold et al (2008)40 | Uncomplicated malaria | Tanzania | 159 | 6m – 19y | 45%/6m | Ho: RR 0.12 (0.02 – 0.83) He: RR 0.30 (0.10 – 0.85) |
| Crompton et al (2008)35 | Uncomplicated malaria3 | Mali | 176 | 2 – 10y | 1.76/y | Ho: 1.60 (0.51 – 3.81) He: 1.14 (0.90 – 1.46) |
| Lin et al (2010)67 | Uncomplicated malaria3 | Papua New Guinea |
206 | 5 – 14y | NA | Ho: 0.84 (0.49 – 1.44) He: 0.63 (0.36 – 1.10) |
| Summary23,35,39 | Uncomplicated malaria |
Ho: 1.12 (0.69 – 1.81) He: 0.98 (0.87 – 1.11) |
||||
| Lin et al (2010)67 | P. falciparum parasitaemia (PCR) | Papua New Guinea |
206 | 5 – 14y | NA | Ho: 0.51 (0.32 – 0.81) He: 0.56 (0.36 – 0.87) |
Abbreviations: NA: data not available; IRR: incidence rate ratio; RR: risk ratio; Ho: homozygote; He: heterozygote; PCR: polymerase chain reaction. No prospective studies investigated the effects of HbE or β-thalassaemia on malaria.
Annual incidence rate in the unexposed population (i.e., HbAA or αα/αα genotype) of the indicated outcome.
Haemoglobin C
HbC appears to protect against severe malaria to a lesser degree than HbAS and in proportion to allele frequency (Figure 2). Compared to healthy children in four studies16,17,19,20 that together enrolled over 9,000 patients, the summary ORs for severe malaria were 0.27 (95% CI 0.11 – 0.63; I2 0%) for HbCC and 0.83 (95% CI 0.74 – 0.92; I2 10.2%) for HbAC (Figure 2; Table S1). Protection from specific severe malaria syndromes has not been fully investigated in HbCC; in one study,19 HbAC showed mild protection from cerebral malaria (OR 0.64; 95% CI 0.45 – 0.91) and severe malarial anaemia (OR 0.87; 95% CI 0.68 – 0.11). When compared to children with uncomplicated malaria, protection from severe malaria is inconsistent: non-significant protection is reported from severe malaria in three studies17,21,26 of HbCC (summary OR 0.12; 95% CI 0.12 – 10.70; I2 0.7%) and HbAC (summary OR 0.76; 95% CI 0.32 – 0.79; I2 60.7%), and from severe malarial anaemia in two studies21,26 that combined homozygotes and heterozygotes (summary OR 0.35; 95% CI 0.04 – 0.73; I2 0%). Significant protection from cerebral malaria was reported in one study of Malian children that combined homo- and heterozygotes (OR 0.15; 95% CI 0.004 – 0.93).21
Prospective studies have not reported the incidence of severe syndromes in HbC children (Table 2). Thus, convincing evidence for protection from severe malaria owing to HbC derives largely from few case-control studies.
Haemoglobin E
Meta-analysis of two studies27,28 in Myanmar and Thailand that compared the prevalence of HbE in severe and uncomplicated malaria cases demonstrated no evidence of protection (summary OR 0.41; 95% CI 0.04 – 0.95), though this should be interpreted cautiously given the significant heterogeneity of the findings (I2 70.5%, p=0.027) and the highly-selected settings of the studies.
α-thalassaemia
Four studies19,29–31 investigated α-thalassaemia in healthy children and children with severe malaria: summary ORs were 0.63 (95% CI 0.48 – 0.83; I2 20.6%) for homozygotes and 0.83 (95% CI 0.74 – 0.92; I2 0%) for heterozygotes. Protection from cerebral malaria was nonsignificant in one study19 for heterozygotes (OR 0.80; 95% CI 0.64 – 1); protection from severe malarial anaemia was reported in two studies,19,29 with summary ORs of 0.50 (95% CI 0.35 – 0.72; I2 0%) for homozygotes and 0.86 (95% CI 0.75 – 0.996; I2 0%) for heterozygotes. One prospective study from Kenya documented a decreased incidence of severe disease in α-thalassaemia homozygotes (IRR 0.54; 95% CI 0.30 – 0.99) and heterozygotes (IRR 0.60; 95% CI 0.39 – 0.90) (Table 2; Figure 3).23 Additionally, protection from severe malarial anaemia among heterozygotes (IRR 0.33; 95% CI 0.14 – 0.78) was similar to protection from cerebral malaria (IRR 0.48; 95% CI 0.24 – 0.97).32
β-thalassaemia
No studies have investigated the risk of severe malaria in patients with β-thalassaemia.
Uncomplicated P. falciparum malaria
Haemoglobin S
In two West African studies,17,33 compared to healthy children the summary OR for children with uncomplicated malaria was 0.30 (95% CI 0.20 – 0.45; I2 0.8%) (Table 1; Figure 2). Multiple prospective studies have characterized the risk reduction in malaria attributable to HbS (Table 2; Figure 3). Meta-analysis of five studies23,34–37 produced a summary IRR estimate of 0.69 (95% CI 0.61 – 0.79), which likely approximates the risk reduction owing to HbAS more closely in these malaria hyperendemic settings.38
Haemoglobin C
Few studies have reported the risk of uncomplicated malaria associated with HbC. Two studies in West Africa compared healthy children and children with uncomplicated malaria: for HbCC, the OR for malaria was 0 (95% CI 0 – 0.41) owing to the absence of HbCC in the case patients,17 and for HbAC the summary OR was 0.16 (95% CI 0.26 – 0.23; I2 80.9%).17,33 Three prospective studies have yielded conflicting results (Table 2; Figure 3): meta-analysis of two studies35,37 yielded a summary OR of 0.05 (95% CI 0.88 – 0.26). Thus, definitive evidence of protection from uncomplicated malaria afforded by HbCC and HbAC has not been established.
Haemoglobin E
No identified studies quantified susceptibility to malaria by HbE.
α-thalassaemia
Several prospective studies have assessed the incidence of uncomplicated malaria in α-thalassaemic children (Table 2; Figure 3), with conflicting results. In Vanuatu, the incidence of falciparum malaria was higher in α-thalassaemia homozygotes (IRR 0.3; 95% CI 0.32 – 0.07) and heterozygotes (IRR 0.1; 95% CI 0.77 – 0.61);39 in contrast, the incidence of uncomplicated malaria was lower in homozygotes (IRR 0.83; 95% CI 0.70 – 0.97) and heterozygotes (IRR 0.93; 95% CI 0.82 – 0.04) in Kenya,23 as well as homozygotes (RR 0.12; 95% CI 0.02 – 0.83) and heterozygotes (RR 0.30; 95% CI 0.10 – 0.85) in Tanzania.40 Meta-analysis of three studies23,35,39 suggests lack of protection for both homozygotes (summary IRR 0.12; 95% C.I. 0.69 – 0.81) and heterozygotes (summary IRR 0.98; 95% C.I. 0.87 – 0.11).
β-thalassaemia
In one case-control study in Liberia, the prevalence of β-thalassaemia was lower in cases of uncomplicated malaria than in community controls (OR 0.56; 95% CI 0.36 – 0.86) (Table 1; Figure 2).33
P. falciparum parasitaemia
Haemoglobin S
Cross-sectional studies have reported conflicting data on the prevalence of P. falciparum parasitaemia in asymptomatic HbAS children (Table S3). Compared with HbAA children, a lower prevalence of parasitaemia in HbAS children was reported in four studies,41–44 similar prevalence in ten studies,45–54 and higher prevalence in two studies.55,56 In these surveys, parasite densities were reported in HbAS children as lower41,46,49,56,57 or similar45,50,52,55 to those in HbAA children. One case-control study reported similar prevalences of HbAS in parasitized (23%) and unparasitized (24%) asymptomatic children (Table 1).20 In two prospective studies,58,59 parasitaemia rates were similar in HbAS and HbAA children (Table 2). Taken together, HbAS does not consistently protect from P. falciparum parasitaemia.
Haemoglobin C
In cross-sectional surveys of adults and of children, HbC has not been associated with a reduced prevalence of P. falciparum parasitaemia45–47,49,55,60 or P. falciparum density.37,45,46,49,55 The incidence of asymptomatic parasitaemia did not differ between HbAC and HbAA children in Mali.37 Thus, HbC does not appear to modify the risk of P. falciparum parasitaemia.
Haemoglobin E
One cross-sectional study in India reported a significantly lower prevalence of P. falciparum parasitaemia in patients with HbE (AE or EE) (0.6%) compared with patients with HbAA (20.5%; p = 0.005 by chi-squared test).61
α-thalassaemia
In cross-sectional studies, α-thalassaemia was not associated with the prevalence of parasitaemia in children32,62–66 or, in several studies, the density of parasitaemias.56,62–64 In one prospective study of children in Papua New Guinea, both α-thalassaemia homozygotes (IRR 0.51; 95% CI 0.32 – 0.81) and heterozygotes (IRR 0.56; 95% CI 0.36 – 0.87) had fewer episodes of PCR-detectable parasitaemia than those without α-thalassaemia,67 though this outcome has not been investigated in other studies.
β-thalassaemia
In one cross sectional study in Liberia, P. falciparum prevalence was similar in children with (78%) and without (82%) β-thalassaemia.68
Pregnancy-associated P. falciparum malaria
Compared to women with HbAA, the prevalence of peripheral P. falciparum parasitaemia was similar in women with HbAS among Nigerian primigravidae69 and Gabonese primi- and secundigravidae,70 and significantly higher in Ugandan women of all gravidities (Table S4).71 In two studies in Ghana there was no association between HbS, HbC, or α-thalassaemia and P. falciparum prevalence.72 In one study in Papua New Guinea that assessed birth outcomes, α-thalassaemia was not associated with placental malaria, birth weight, placental parasite density, maternal peripheral parasitaemia, or maternal anaemia.73 On the whole, there are few data on the effect of haemoglobin variants on pregnancy-associated malaria or placental parasitization.
P.vivax malaria: Is protection species-specific?
No studies investigated an effect of HbAS, HbAC, or HbCC on P. vivax infection. In a prospective study in Vanuatu, the incidence of P. vivax malaria was significantly increased in homozygous α-thalassaemic children less than 5 years old (IRR 0.4; 95% CI 0.40 – 0.30) and nonsignificantly increased in children greater than 5 years old (IRR 0.0; 95% CI 0.42 – 0.14) (a similar pattern of increased malaria susceptibility was reported for P. falciparum malaria).39 In a cross-sectional study investigating HbE in India, P. vivax parasitaemia was significantly less prevalent in HbEE/AE (0.7%) than in HbAA individuals (20.1%; p < 0.001).61
DISCUSSION
Genetic polymorphisms that affect the structure and production of the β- or α-chains of haemoglobin are variously associated with protection from a range of clinical manifestations of P. falciparum infection. The degree of protection varies between haemoglobinopathies, but in general is greatest against severe malaria, moderate against uncomplicated malaria, and absent against asymptomatic P. falciparum parasitaemia. The degrees of protection against severe malaria by HbAS (91%; 95% CI 88 – 94), HbCC (73%; 95% CI 37 – 89), and homozygous α-thalassaemia (37%; 95% CI 17 – 52) compare favorably with those reported for current large-scale malaria-control efforts, including intermittent preventive antimalarial therapy in children (87% to 69%)74,75 or infants (38%)76 and the use of insecticide-treated bed nets (45%).77
HbS and to a lesser extent HbC protect from malaria but not from parasitaemia, suggesting that these haemoglobin variants prevent the transition from asymptomatic parasitaemia to malaria. This transition is poorly understood. This protective effect may derive from the abnormal display of parasite virulence factors on the surface of parasitized HbC and HbS erythrocytes,78,79 possibly owing to the disruption of the parasite’s remodeling of erythrocyte’s intracellular trafficking network by HbS and HbC.80 Additionally, the age-dependent nature of malaria protection owing to HbAS81,82 and α-thalassaemia83 among children in recent reports support a protective mechanism based upon an enhanced acquisition of malaria immunity. Though HbS does not generally enhance IgG responses to a diverse array of P. falciparum proteins,84 HbS may yet enhance IgG responses specifically to the parasite’s major cytoadherence ligand and virulence factor Plasmodium falciparum erythrocyte membrane protein (PfEMP1).85 Additional possible mechanisms for protection owing to haemoglobinopathies include an enhanced clearance of parasitized erythrocytes,86 impaired parasite growth,87 or the induction of protective immunomodulatory mechanisms by parasitized erythrocytes.88 Data supporting these various molecular mechanisms are complex [reviewed in 89,90], and because these possibilities are not mutually-exclusive, the relative contribution of mechanisms may vary between haemoglobinopathies. By allowing parasitization while attenuating the pathogenic mechanisms that lead to disease and fatal outcomes, haemoglobin variants offer a model system to explore the cellular events involved in causing morbidity (Panel 1).
Panel 1. Unanswered questions for future clinical and translational research.
Does HbCC protect from uncomplicated malaria and asymptomatic parasitaemia, or only from severe falciparum malaria?
Does α-thalassaemia reduce the risk of disease from specific non-Plasmodium pathogens?
Do haemoglobinopathies influence the risk of uncomplicated or severe P. vivax malaria?
Do haemoglobinopathies influence the risk of pregnancy-associated malaria?
Do HbE and β-thalassaemia confer protection from uncomplicated or severe falciparum malaria?
Does α-thalassaemia exert negative epistatic effects on malaria protection by HbC and HbE?
Do haemoglobinopathies confer malaria protection to non-immune populations?
How do co-inherited G6PD deficiency variants and ABO blood groups influence the malaria-protective effects of haemoglobinopathies? 9. Does HbAS confer protection against falciparum malaria outside of sub-Saharan Africa, (e.g., India)?
The attenuation of malaria by haemoglobinopathies has important implications for non-randomised analyses of clinical malaria studies. While randomised trials may achieve balance of underlying protective polymorphisms, comparisons of non-randomised groups may be compromised by differential prevalences of haemoglobinopathies or other risk modifiers.91 Such potential bias could impact the differential efficacy of therapies, vaccines, or other preventive measures in ecological analyses that compare populations that are not defined by randomisation and in analyses of predictors of individual-level risk. Our data endorse HbS as an important covariate in such analyses owing to its consistent protection from uncomplicated malaria (IRR 0.69; 95% CI 0.61 – 0.79), which is a common outcome in vaccine trials.9,10
Our review highlights several gaps in our basic understanding of how Plasmodium parasites cause the symptoms and life-threatening manifestations of malaria. The paucity of outcome investigations of pregnancy-associated malaria is striking, considering that this disease model has revealed fundamental mechanisms of both parasite virulence and host adaptive immunity.92 Similarly, the effect of haemoglobinopathies on P. vivax parasitaemia and malaria incidence is relatively unknown despite geographic overlap in South Asia. Additionally, given the measurable incidence of severe P. vivax malaria,93 case-control studies may explore associations between haemoglobinopathies and severe vivax malaria syndromes. Finally, clinical investigations have relatively neglected HbE, β-thalassaemia, and HbCC. This is surprising given the high prevalence (up to 50%) of HbE in Cambodia and HbC in parts of West Africa, as well as Haldane’s 60-year-old ‘malaria hypothesis’ that heterozygous β-thalassaemia protects against severe and fatal falciparum malaria.94
Two further points merit attention. First, though our systematic review was specifically designed to assess malaria outcomes, within the identified studies we found some evidence that while HbAS conferred malaria-specific protection22,24 α-thalassaemia protected against other mild and severe infectious syndromes, including pneumonia.29,32 Because malaria itself may counfound the relationship between haemoglobinopathies and other infections – as recently reported for the effect of HbAS on bacteremia95 – myriad individual and epidemiologic factors could account for this difference, in addition to biological differences in the mechanisms of protection. The identification of these mechanisms may be aided if this phenomenon is confirmed by future clinical studies or meta-analyses. Second, the dissimilarity of estimates from prospective studies of the risk of uncomplicated falciparum malaria in homozygous α-thalassaemic children is striking, with significantly increased risk on the southwestern Pacific island of Vanuatu39 but either slightly decreased or unchanged risk in Africa and Papua New Guinea (Table 2).31,35,40,67,96 Other data have suggested an increased Plasmodium prevalence in homozygous α-thalassaemics in Papua New Guinea,97 underscoring that haemoglobinopathies may have variable effects in different settings upon different outcomes. Future studies are needed to more definitively characterize these effects and define their relationship with host genetics, malaria epidemiology, and acquired immunity to malaria.
This systematic review is subject to several limitations. We may have failed to identify relevant studies, though the independent selection of studies by two independent reviewers who each assessed over 2600 studies suggests adequate identification. Secondly, risk estimates for malaria may be influenced by unmeasured or unreported host factors, such as G6PD deficiency and ABO blood groups. Nevertheless, heterogeneity was low for most meta-analyzed comparisons, suggesting a consistent effect of haemoglobinopathies upon malaria risk. Finally, the clinical epidemiology of malaria results from poorly-understood interactions between host, parasite, and environmental factors which vary between included studies. We therefore employed random-effects meta-analysis models, and heterogeneity in risk estimates was generally low.
Despite previous successes in exploiting innate malaria protective-factors to investigate malaria pathogenesis, recent reports highlight the complexity of the co-evolution of host and parasite. P. vivax infection is now recognized in Malagasy individuals who lack DARC expression on their erythrocytes that were previously thought to be resistant to vivax malaria,98 suggesting alternate erythrocyte invasion pathways. Additionally, a-thalassaemia can attenuate the malaria-protective effect of HbAS when co-inherited,23 emphasizing the need to integrate investigations of genetic resistance. Nevertheless, by attenuating the virulence of malaria parasites, haemoglobinopathies offer an attractive “natural experiment” to help elucidate malaria’s pathogenic mechanisms and potentially translate models of pathogenesis and immunity into clinical application.
Supplementary Material
Acknowledgments
Funding. Intramural Research Program, National Institute of Allergy and Infectious Diseases
Funding/Support
This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Role of the Sponsors
Funders had no role in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in preparation, review, or approval of the manuscript.
Footnotes
Author contributions
SMT conceived of and designed the study, conducted the literature search, analyzed and interpreted data, and wrote the manuscript. CMP conducted the literature search, analyzed and interpreted data, and contributed to the drafting of the manuscript. RMF conceived of and designed the study, interpreted the data, drafted the manuscript, and supervised the study conduct. SMT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
All authors declare that they have no conflicts of interest relevant to the subject of this manuscript.
Additional Contributions
We thank Peter Crompton MD MPH (National Institute of Allergy and Infectious Diseases) and Sunil Parikh MD MPH (University of California, San Francisco) for providing access to unpublished data, and Steven Meshnick MD PhD and Jonathan Juliano MD MSPH (each with the University of North Carolina, Chapel Hill) for providing material support. These individuals received no compensation for their assistance. We also thank our anonymous reviewers whose input greatly enhanced this report. Ultimately, we are indebted to the investigators from whose work we have drawn, and the patients for whom they cared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weatherall DJ, Provan AB. Red cells I: inherited anaemias. Lancet. 2000 Apr 1;355(9210):1169–1175. doi: 10.1016/s0140-6736(00)02073-0. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ. Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol. 2008 May;141(3):276–286. doi: 10.1111/j.1365-2141.2008.07085.x. [DOI] [PubMed] [Google Scholar]
- 3.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996 Jun 7;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 4.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998 Oct 29;395(6705):851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 5.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976 Aug 5;295(6):302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 6.King CL, Michon P, Shakri AR, et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8363–8368. doi: 10.1073/pnas.0800371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hviid L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum Vaccin. 2010 Jan;6(1):84–89. doi: 10.4161/hv.6.1.9602. [DOI] [PubMed] [Google Scholar]
- 8.Galinski MR, Barnwell JW. Plasmodium vivax: who cares? Malar J. 2008;7 (Suppl 1):S9. doi: 10.1186/1475-2875-7-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008 Dec 11;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The RTS SCTP. First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children. N Engl J Med. 2011 Oct 18; doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 11.Warrell DA, Gilles HM. Essential malariology. 4. London ; New York, New York: Arnold; U.S.A: Oxford University Press; 2002. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [Accessed 11/7/11, 2011.];2010 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Trans R Soc Trop Med Hyg. 1990;84 (Suppl 2):1–65. [PubMed] [Google Scholar]
- 15.Bagos PG, Nikolopoulos GK. Mixed-effects Poisson regression models for meta-analysis of follow-up studies with constant or varying durations. International Journal of Biostatistics. 2009;5(1):1–33. [Google Scholar]
- 16.Gilles HM, Fletcher KA, Hendrickse RG, Lindner R, Reddy S, Allan N. Glucose-6-phosphate-dehydrogenase deficiency, sickling, and malaria in African children in South Western Nigeria. Lancet. 1967 Jan 21;1(7482):138–140. doi: 10.1016/s0140-6736(67)91037-9. [DOI] [PubMed] [Google Scholar]
- 17.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001 Nov 15;414(6861):305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 18.Ackerman H, Usen S, Jallow M, Sisay-Joof F, Pinder M, Kwiatkowski DP. A comparison of case-control and family-based association methods: the example of sickle-cell and malaria. Ann Hum Genet. 2005 Sep;69(Pt 5):559–565. doi: 10.1111/j.1529-8817.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- 19.May J, Evans JA, Timmann C, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007 May 23;297(20):2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 20.Mockenhaupt FP, Ehrhardt S, Cramer JP, et al. Hemoglobin C and resistance to severe malaria in Ghanaian children. J Infect Dis. 2004 Sep 1;190(5):1006–1009. doi: 10.1086/422847. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Guindo A, Cissoko Y, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000 Oct 1;96(7):2358–2363. [PubMed] [Google Scholar]
- 22.Hill AV, Allsopp CE, Kwiatkowski D, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 23.Williams TN, Mwangi TW, Wambua S, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005 Nov;37(11):1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005 Jul 1;192(1):178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002 Apr 13;359(9314):1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 26.Guinet F, Diallo DA, Minta D, et al. A comparison of the incidence of severe malaria in Malian children with normal and C-trait hemoglobin profiles. Acta Trop. 1997 Nov;68(2):175–182. doi: 10.1016/s0001-706x(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 27.Oo M, Tin S, Marlar T, O'Sullivan WJ. Genetic red cell disorders and severity of falciparum malaria in Myanmar. Bull World Health Organ. 1995;73(5):659–665. [PMC free article] [PubMed] [Google Scholar]
- 28.Hutagalung R, Wilairatana P, Looareesuwan S, Brittenham GM, Aikawa M, Gordeuk VR. Influence of hemoglobin E trait on the severity of Falciparum malaria. J Infect Dis. 1999 Jan;179(1):283–286. doi: 10.1086/314561. [DOI] [PubMed] [Google Scholar]
- 29.Allen SJ, O'Donnell A, Alexander ND, et al. alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14736–14741. doi: 10.1073/pnas.94.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mockenhaupt FP, Ehrhardt S, Gellert S, et al. Alpha(+)-thalassemia protects African children from severe malaria. Blood. 2004 Oct 1;104(7):2003–2006. doi: 10.1182/blood-2003-11-4090. [DOI] [PubMed] [Google Scholar]
- 31.Williams TN, Wambua S, Uyoga S, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005 Jul 1;106(1):368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 32.Wambua S, Mwangi TW, Kortok M, et al. The effect of alpha+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med. 2006 May;3(5):e158. doi: 10.1371/journal.pmed.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willcox M, Bjorkman A, Brohult J, Pehrson PO, Rombo L, Bengtsson E. A case-control study in northern Liberia of Plasmodium falciparum malaria in haemoglobin S and beta-thalassaemia traits. Ann Trop Med Parasitol. 1983 Jun;77(3):239–246. doi: 10.1080/00034983.1983.11811704. [DOI] [PubMed] [Google Scholar]
- 34.Parikh S, Dorsey G, Rosenthal PJ. Host polymorphisms and the incidence of malaria in Ugandan children. Am J Trop Med Hyg. 2004 Dec;71(6):750–753. [PubMed] [Google Scholar]
- 35.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008 Nov 1;198(9):1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark TD, Greenhouse B, Njama-Meya D, et al. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis. 2008 Aug 1;198(3):393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 37.Kreuels B, Kreuzberg C, Kobbe R, et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood. 2010 Jun 3;115(22):4551–4558. doi: 10.1182/blood-2009-09-241844. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998 Nov 18;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 39.Williams TN, Maitland K, Bennett S, et al. High incidence of malaria in alpha-thalassaemic children. Nature. 1996 Oct 10;383(6600):522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 40.Enevold A, Lusingu JP, Mmbando B, et al. Reduced risk of uncomplicated malaria episodes in children with alpha+-thalassemia in northeastern Tanzania. Am J Trop Med Hyg. 2008 May;78(5):714–720. [PubMed] [Google Scholar]
- 41.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954 Feb 6;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colbourne MJ, Edington GM. Sickling and malaria in the Gold Coast. Br Med J. 1956;4970(1):784–786. doi: 10.1136/bmj.1.4970.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979 Apr;73(2):161–172. doi: 10.1080/00034983.1979.11687243. [DOI] [PubMed] [Google Scholar]
- 44.Ntoumi F, Mercereau-Puijalon O, Ossari S, et al. Plasmodium falciparum: sickle-cell trait is associated with higher prevalence of multiple infections in Gabonese children with asymptomatic infections. Exp Parasitol. 1997 Sep;87(1):39–46. doi: 10.1006/expr.1997.4173. [DOI] [PubMed] [Google Scholar]
- 45.Edington GM, Laing WN. Relationship between haemoglobins C and S and malaria in Ghana. Br Med J. 1957 Jul 20;2(5037):143–145. doi: 10.1136/bmj.2.5037.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson GR. Significance of haemoglobins S and C in Ghana. Br Med J. 1962 Mar 10;1(5279):682–685. doi: 10.1136/bmj.1.5279.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labie D, Richin C, Pagnier J, Gentilini M, Nagel RL. Hemoglobins S and C in Upper Volta. Hum Genet. 1984;65(3):300–302. doi: 10.1007/BF00286522. [DOI] [PubMed] [Google Scholar]
- 48.Aluoch JR. Higher resistance to Plasmodium falciparum infection in patients with homozygous sickle cell disease in western Kenya. Trop Med Int Health. 1997 Jun;2(6):568–571. doi: 10.1046/j.1365-3156.1997.d01-322.x. [DOI] [PubMed] [Google Scholar]
- 49.Danquah I, Ziniel P, Eggelte TA, Ehrhardt S, Mockenhaupt FP. Influence of haemoglobins S and C on predominantly asymptomatic Plasmodium infections in northern Ghana. Trans R Soc Trop Med Hyg. 2010 Nov;104(11):713–719. doi: 10.1016/j.trstmh.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein SC, Bowman JE, Kaptue Noche L. Population studies in Cameroon: hemoglobin S, glucose-6-phosphate dehydrogenase deficiency and falciparum malaria. Hum Hered. 1980;30(4):251–258. doi: 10.1159/000153138. [DOI] [PubMed] [Google Scholar]
- 51.Willcox MC, Beckman L. Haemoglobin variants, beta-thalassaemia and G-6-PD types in Liberia. Hum Hered. 1981;31(6):339–347. doi: 10.1159/000153235. [DOI] [PubMed] [Google Scholar]
- 52.Motulsky AG, Vandepitte J, Fraser GR. Population genetic studies in the Congo. I. Glucose-6-phosphate dehydrogenase deficiency, hemoglobin S, and malaria. Am J Hum Genet. 1966 Nov;18(6):514–537. [PMC free article] [PubMed] [Google Scholar]
- 53.Bienzle U, Guggenmoos-Holzmann I, Luzzatto L. Plasmodium falciparum malaria and human red cells. I. A genetic and clinical study in children. Int J Epidemiol. 1981;10(1):9–15. doi: 10.1093/ije/10.1.9. [DOI] [PubMed] [Google Scholar]
- 54.Jeremiah ZA, Jeremiah TA, Emelike FO. Frequencies of some human genetic markers and their association with Plasmodium falciparum malaria in the Niger Delta, Nigeria. J Vector Borne Dis. 2010 Mar;47(1):11–16. [PubMed] [Google Scholar]
- 55.Ringelhann B, Hathorn MK, Jilly P, Grant F, Parniczky G. A new look at the protection of hemoglobin AS and AC genotypes against plasmodium falciparum infection: a census tract approach. Am J Hum Genet. 1976 May;28(3):270–279. [PMC free article] [PubMed] [Google Scholar]
- 56.Allen SJ, Bennett S, Riley EM, et al. Morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in children with sickle cell trait in The Gambia. Trans R Soc Trop Med Hyg. 1992 Sep-Oct;86(5):494–498. doi: 10.1016/0035-9203(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 57.Guggenmoos-Holzmann I, Bienzle U, Luzzatto L. Plasmodium falciparum malaria and human red cells. II. Red cell genetic traits and resistance against malaria. Int J Epidemiol. 1981;10(1):16–22. doi: 10.1093/ije/10.1.16. [DOI] [PubMed] [Google Scholar]
- 58.Stirnadel HA, Stockle M, Felger I, Smith T, Tanner M, Beck HP. Malaria infection and morbidity in infants in relation to genetic polymorphisms in Tanzania. Trop Med Int Health. 1999 Mar;4(3):187–193. doi: 10.1046/j.1365-3156.1999.43381.x. [DOI] [PubMed] [Google Scholar]
- 59.Le Hesran JY, Personne I, Personne P, et al. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int J Epidemiol. 1999 Aug;28(4):793–798. doi: 10.1093/ije/28.4.793. [DOI] [PubMed] [Google Scholar]
- 60.Storey J, Fleming AF, Cornille-Brogger R, Molineaux L, Matsushima T, Kagan I. Abnormal haemoglobins in the Sudan savanna of Nigeria. IV. Malaria, immunoglobulins and antimalarial antibodies in haemoglobin AC individuals. Ann Trop Med Parasitol. 1979 Aug;73(4):311–315. doi: 10.1080/00034983.1979.11687264. [DOI] [PubMed] [Google Scholar]
- 61.Kar S, Seth S, Seth PK. Prevalence of malaria in Ao Nagas and its association with G6PD and HbE. Hum Biol. 1992 Apr;64(2):187–197. [PubMed] [Google Scholar]
- 62.Mockenhaupt FP, Bienzle U, May J, et al. Plasmodium falciparum infection: influence on hemoglobin levels in alpha-thalassemia and microcytosis. J Infect Dis. 1999 Sep;180(3):925–928. doi: 10.1086/314959. [DOI] [PubMed] [Google Scholar]
- 63.Fowkes FJ, Michon P, Pilling L, et al. Host erythrocyte polymorphisms and exposure to Plasmodium falciparum in Papua New Guinea. Malar J. 2008;7:1. doi: 10.1186/1475-2875-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veenemans J, Andang'o PE, Mbugi EV, et al. Alpha+-thalassemia protects against anemia associated with asymptomatic malaria: evidence from community-based surveys in Tanzania and Kenya. J Infect Dis. 2008 Aug 1;198(3):401–408. doi: 10.1086/589884. [DOI] [PubMed] [Google Scholar]
- 65.Shekalaghe S, Alifrangis M, Mwanziva C, et al. Low density parasitaemia, red blood cell polymorphisms and Plasmodium falciparum specific immune responses in a low endemic area in northern Tanzania. BMC Infect Dis. 2009;9:69. doi: 10.1186/1471-2334-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen SJ, Rowe P, Allsopp CE, et al. A prospective study of the influence of alpha thalassaemia on morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in Gambian children. Trans R Soc Trop Med Hyg. 1993 May-Jun;87(3):282–285. doi: 10.1016/0035-9203(93)90129-e. [DOI] [PubMed] [Google Scholar]
- 67.Lin E, Tavul L, Michon P, et al. Minimal association of common red blood cell polymorphisms with Plasmodium falciparum infection and uncomplicated malaria in Papua New Guinean school children. Am J Trop Med Hyg. 2010 Oct;83(4):828–833. doi: 10.4269/ajtmh.2010.09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willcox M, Bjorkman A, Brohult J. Falciparum malaria and beta-thalassaemia trait in northern Liberia. Ann Trop Med Parasitol. 1983 Aug;77(4):335–347. doi: 10.1080/00034983.1983.11811722. [DOI] [PubMed] [Google Scholar]
- 69.Fleming AF, Harrison KA, Briggs ND, et al. Anaemia in young primigravidae in the guinea savanna of Nigeria: sickle-cell trait gives partial protection against malaria. Ann Trop Med Parasitol. 1984 Aug;78(4):395–404. doi: 10.1080/00034983.1984.11811837. [DOI] [PubMed] [Google Scholar]
- 70.Bouyou-Akotet MK, Ionete-Collard DE, Mabika-Manfoumbi M, et al. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar J. 2003 Jun 25;2:18. doi: 10.1186/1475-2875-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamilton PJ, Gebbie DA, Wilks NE, Lothe F. The role of malaria, folic acid deficiency and haemoglobin AS in pregnancy at Mulago hospital. Trans R Soc Trop Med Hyg. 1972;66(4):594–602. doi: 10.1016/0035-9203(72)90305-7. [DOI] [PubMed] [Google Scholar]
- 72.Mockenhaupt FP, Rong B, Gunther M, et al. Anaemia in pregnant Ghanaian women: importance of malaria, iron deficiency, and haemoglobinopathies. Trans R Soc Trop Med Hyg. 2000 Sep-Oct;94(5):477–483. doi: 10.1016/s0035-9203(00)90057-9. [DOI] [PubMed] [Google Scholar]
- 73.O'Donnell A, Raiko A, Clegg JB, Weatherall DJ, Allen SJ. Alpha+-thalassaemia and pregnancy in a malaria endemic region of Papua New Guinea. Br J Haematol. 2006 Oct;135(2):235–241. doi: 10.1111/j.1365-2141.2006.06274.x. [DOI] [PubMed] [Google Scholar]
- 74.Dicko A, Diallo AI, Tembine I, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(2):e1000407. doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konate AT, Yaro JB, Ouedraogo AZ, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(2):e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aponte JJ, Schellenberg D, Egan A, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009 Oct 31;374(9700):1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- 77.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2):CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 78.Fairhurst RM, Baruch DI, Brittain NJ, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005 Jun 23;435(7045):1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 79.Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008 Jan 22;105(3):991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cyrklaff M, Sanchez CP, Kilian N, et al. Hemoglobins S and C Interfere with Actin Remodeling in Plasmodium falciparumâ]“Infected Erythrocytes. Science. 2011 Nov 10; doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 81.Williams TN, Mwangi TW, Roberts DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005 May;2(5):e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gong L, Maiteki-Sebuguzi C, Rosenthal PJ, et al. Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood. 2012 Feb 10; doi: 10.1182/blood-2011-08-371062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veenemans J, Jansen EJ, Baidjoe AY, et al. Effect of alpha(+)-thalassaemia on episodes of fever due to malaria and other causes: a community-based cohort study in Tanzania. Malar J. 2011;10:280. doi: 10.1186/1475-2875-10-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan X, Traore B, Kayentao K, et al. Hemoglobin S and C Heterozygosity Enhances Neither the Magnitude nor Breadth of Antibody Responses to a Diverse Array of Plasmodium falciparum Antigens. J Infect Dis. 2011 Dec;204(11):1750–1761. doi: 10.1093/infdis/jir638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005 May 15;191(10):1631–1638. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 86.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004 Nov 15;104(10):3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 87.Fairhurst RM, Fujioka H, Hayton K, Collins KF, Wellems TE. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood. 2003 Apr 15;101(8):3309–3315. doi: 10.1182/blood-2002-10-3105. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira A, Marguti I, Bechmann I, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011 Apr 29;145(3):398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 89.Williams TN. How do hemoglobins S and C result in malaria protection? J Infect Dis. 2011 Dec;204(11):1651–1653. doi: 10.1093/infdis/jir640. [DOI] [PubMed] [Google Scholar]
- 90.Lopez C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA. Mechanisms of genetically-based resistance to malaria. Gene. 2010 Nov 1;467(1–2):1–12. doi: 10.1016/j.gene.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Parikh S, Rosenthal PJ. Human genetics and malaria: relevance for the design of clinical trials. J Infect Dis. 2008 Nov 1;198(9):1255–1257. doi: 10.1086/592223. [DOI] [PubMed] [Google Scholar]
- 92.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007 Feb;7(2):105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 93.Bassat Q, Alonso PL. Defying malaria: Fathoming severe Plasmodium vivax disease. Nat Med. 2011 Jan;17(1):48–49. doi: 10.1038/nm0111-48. [DOI] [PubMed] [Google Scholar]
- 94.Haldane JB. The rate of mutation of human genes. Hereditas. 1949;35(S1):267–273. [Google Scholar]
- 95.Scott JA, Berkley JA, Mwangi I, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011 Oct 8;378(9799):1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Migot-Nabias F, Pelleau S, Watier L, et al. Red blood cell polymorphisms in relation to Plasmodium falciparum asymptomatic parasite densities and morbidity in Senegal. Microbes Infect. 2006 Aug;8(9–10):2352–2358. doi: 10.1016/j.micinf.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 97.Oppenheimer SJ, Hill AV, Gibson FD, Macfarlane SB, Moody JB, Pringle J. The interaction of alpha thalassaemia with malaria. Trans R Soc Trop Med Hyg. 1987;81(2):322–326. doi: 10.1016/0035-9203(87)90253-7. [DOI] [PubMed] [Google Scholar]
- 98.Menard D, Barnadas C, Bouchier C, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jallow M, Teo YY, Small KS, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009 Jun;41(6):657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lell B, May J, Schmidt-Ott RJ, et al. The role of red blood cell polymorphisms in resistance and susceptibility to malaria. Clin Infect Dis. 1999 Apr;28(4):794–799. doi: 10.1086/515193. [DOI] [PubMed] [Google Scholar]
- 101.Rihet P, Flori L, Tall F, Traore AS, Fumoux F. Hemoglobin C is associated with reduced Plasmodium falciparum parasitemia and low risk of mild malaria attack. Hum Mol Genet. 2004 Jan 1;13(1):1–6. doi: 10.1093/hmg/ddh002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.