Abstract
Intermediate filaments (IFs), composed of desmin and keratins, link myofibrils to each other and to the sarcolemma in skeletal muscle. Fast-twitch muscle of mice lacking the IF proteins, desmin and keratin 19 (K19), showed reduced specific force and increased susceptibility to injury in earlier studies. Here we tested the hypothesis that the number of malformed myofibers in mice lacking desmin (Des−/−), keratin 19 (K19−/−), or both IF proteins (double knockout, DKO) is increased and is coincident with altered excitation-contraction (EC) coupling Ca2+ kinetics, as reported for mdx mice. We quantified the number of branched myofibers, characterized their organization with confocal and electron microscopy (EM), and compared the Ca2+ kinetics of EC coupling in flexor digitorum brevis myofibers from adult Des−/−, K19−/−, or DKO mice and compared them to age-matched wild type (WT) and mdx myofibers. Consistent with our previous findings, 9.9% of mdx myofibers had visible malformations. Des−/− myofibers had more malformations (4.7%) than K19−/− (0.9%) or DKO (1.3%) myofibers. Confocal and EM imaging revealed no obvious changes in sarcomere misalignment at the branch points, and the neuromuscular junctions in the mutant mice, while more variably located, were limited to one per myofiber. Global, electrically evoked Ca2+ signals showed a decrease in the rate of Ca2+ uptake (decay rate) into the sarcoplasmic reticulum after Ca2+ release, with the most profound effect in branched DKO myofibers (44% increase in uptake relative to WT). Although branched DKO myofibers showed significantly faster rates of Ca2+ clearance, the milder branching phenotype observed in DKO muscle suggests that the absence of K19 corrects the defect created by the absence of desmin alone. Thus, there are complex roles for desmin-based and K19-based IFs in skeletal muscle, with the null and DKO mutations having different effects on Ca2+ reuptake and myofiber branching.
Keywords: calcium, muscular dystrophy, skeletal muscle
the muscular dystrophies are a group of inherited degenerative muscle disorders, many of which result from defects in structural proteins. Muscular dystrophies are characterized by progressive muscle weakness and degeneration of skeletal muscles (24, 38), as well as by increased susceptibility to contraction-induced damage that, in many instances, can be proportional to the severity of the disease (10, 18, 19). An increase in the number of malformed (branched) muscle fibers occurs in several murine muscular dystrophies (12, 18). These branched myofibers are defined as any myofiber with two or more cytoplasmically continuous strands (9). Branched and “split” muscle fibers are commonly seen in transverse sections of pathological skeletal muscle (14); split myofibers are also seen in hypertrophic muscle of power lifters (14). Branching most likely reflects the imperfect fusion of myogenic cells during regeneration following necrotic cell death (30), consistent with the frequent occurrence of centrally located nuclei in branched myofibers (10, 23). Malformed myofibers contribute to a decrease in muscle-specific force (18), as well as an increase in susceptibility to contraction-induced injury (10). Indeed, the age-dependent increase in branched myofibers may account, in part, for the age-dependent increase in muscle damage (16) and decrease in specific force seen in dystrophic muscle (10).
Intermediate filaments (IFs), composed of desmin and keratins, link contractile elements to each other and to the sarcolemma in striated muscle. Desmin forms the major IF system in adult striated muscle, which surrounds the contractile apparatus at Z-disks and links Z-disks to costameres at the sarcolemma (7). Although present in smaller amounts than desmin, keratins 8 and 19 (K8 and K19) are also present in mature striated muscle (36), where they coassemble into structures enriched around Z-disks and at costameres, even when desmin is absent as a result of homologous recombination (25, 32). K19 binds directly to the actin-binding domain of dystrophin (31), the 427-kDa sarcolemmal protein that is missing in Duchenne muscular dystrophy (DMD) (24).
Recent studies with knockout mice that lack the IFs desmin (Des−/−), keratin 19 (K19−/−), or both proteins in a double knockout (DKO), demonstrated differences in maximal contractile force, membrane fragility, and susceptibility to injury depending on the missing IF, or combination of missing IFs (22). These phenotypes of IF knockout muscle resemble the phenotypes observed in mdx (10, 16, 18, 19, 23), albeit to a milder extent. Thus, the aim of this study was to investigate the occurrence of malformed myofibers in muscles of Des−/−, K19−/−, and DKO mice compared with wild-type (WT) mice. We also wanted to learn if the increase in extent and morphology are coincident with altered excitation-contraction (EC) coupling-dependent Ca2+ kinetics, similar to that reported for the muscles of mdx mice (mice lacking dystrophin) in a previous study (23). We determined the prevalence of malformed myofibers cultured from the flexor digitorum brevis (FDB) muscles of Des−/−, K19−/−, or DKO mice, compared with age-matched WT and mdx mice. We found distinct morphological anomalies, which we classified and described for all three genotypes. Similar to mdx muscles (10, 23), Des−/− muscles had a significantly increased number of malformed myofibers compared with age-matched WT, but K19−/− and DKO muscles did not. We also examined membrane and internal structures, the presence and location of neuromuscular junctions (NMJs), as well as the Ca2+ kinetics involved in EC coupling in these myofibers. As with mdx myofibers (23), the membrane-associated and internal structures in the branched region of IF-null myofibers resembled the unbranched regions, as well as the regions of normal, unbranched myofibers of the same genotype. We did, however, observe significant differences in the rate of Ca2+ uptake after EC coupling in the smaller branches of branched DKO myofibers compared with the trunks or to large-diameter branches of the same myofiber, and to the small-diameter branches of WT, K19−/−, or Des−/− abnormal myofibers. Taken together, there are complex roles for desmin-based and K19-based IFs in skeletal muscle, with the null and DKO mutations having different effects on Ca2+ reuptake and myofiber branching, consistent with an earlier report (23).
MATERIALS AND METHODS
Animals.
We used WT FVB/N mice and mice lacking keratin 19 (K19−/−), desmin (Des−/−), or both IFs (DKO), also in the FVB/N strain, as described (22), as well as mdx mice (lacking dystrophin) in the C57BL/10ScSn background. A total of 40 adult mice (8–12 mo; N = 8 animals per group) were used. All experimental procedures were approved by the University of Maryland Institutional Animal Care and Use Committee.
Myofiber preparation.
WT, K19−/−, Des−/−, DKO, or mdx mice were anesthetized with isoflurane (2% with oxygen flow rate of 0.5 l/min). Once anesthesia was confirmed by lack of a deep tendon reflex (no foot withdrawal in response to pinching the foot), the mice were euthanized by cervical dislocation and FDB muscles were harvested bilaterally. Single myofibers were enzymatically isolated in DMEM supplemented with 0.2% BSA, 1 μl/ml gentamycin, and 4 mg/ml type I collagenase (Sigma, C0130, Sigma-Aldrich, St. Louis, MO) for 1–3 h at 37°C, as described (4, 11). Myofibers were plated on extracellular matrix (ECM; Sigma E1270)-coated imaging dishes (P35G-1.0–14-C, Matek) and incubated for 12–24 h at 37°C and 5% CO2 in DMEM supplemented with 0.2% BSA and 1 μl/ml gentamycin before imaging or fixation with 4% paraformaldehyde or electron microscopy (EM) fixative, described below. FDB myofibers can be maintained in culture for over 10 days (5), but all of the myofibers in this study were imaged or fixed for immunolabeling within a 12-h period to avoid potential changes from prolonged incubation (27). Canato et al. (6) suggest that FDB enzymatic dissociation may favor survival of a subset of the myofiber population, such that those fibers that are most fragile and/or necrotic do not survive dissociation and the first day in culture (6). If this were the case for the current study, the significance of our findings in these in vitro studies may be underestimated; the number of myofibers with altered morphology may be even larger in in vivo studies with intact muscle.
Examination of ultrastructure with electron microscopy.
Single myofibers were isolated from FDB muscles as above, plated on an ECM-coated coverslip, and fixed in a 0.1 M phosphate buffer (pH 7.2) with 2% paraformaldehyde and 2.5% glutaraldehyde at 25°C for 1 h and stored at 4°C before processing. Fixed cultures were washed and postfixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h and washed again. Some coverslips were dehydrated in a graded series of ethanols, chemically dried with hexamethyldisilazane (HMDS, Electron Microscopy Sciences, Fort Washington, PA), mounted on scanning electron microscopy (SEM) pin mounts, and sputter-coated with Platinum/Palladium for SEM. SEM images were acquired in a Quanta200 variable pressure SEM (FEI.Co). Other specimens, processed for transmission EM (TEM), were dehydrated serially in 50% ethanol, 70% ethanol, 70% ethanol containing 1% uranyl acetate, 90% ethanol, and 100% ethanol (10 min each). This was followed by two more 100% ethanol washes and infiltration with increasing concentrations of Spurr resin (Electron Microscopy Sciences). After two exchanges of pure resin, specimens were embedded in Spurr resin and polymerized at 60°C overnight. Silver colored (∼70 nm) ultrathin sections were cut and collected with a Leica UC6 ultramicrotome (Leica Microsystems, Bannockburn, IL), counterstained with uranyl acetate and lead, and examined in a TEM (Tecnai T12, FEI) operated at 80 kV. Digital images were acquired with an AMT bottom mount CCD camera and AMT600 software.
Localization of cellular constituents.
After fixation with 4% paraformaldehyde for 20 min, myofibers (n > 100) were permeabilized with 0.1% Triton X-100 in PBS, incubated in 3% BSA in PBS, and labeled with primary antibodies to dystrophin (rabbit, Thermo Scientific RB-9024, Fremont, CA) or α-actinin (mouse, Sigma A7811), followed by species-specific secondary antibodies (Jackson Immunoresearch, West Chester, PA). 4′,6-Diamidino-2-phenylindole (DAPI) or propidium iodide was used to label nuclei, and α-bungarotoxin conjugated to Alexa-594 (Molecular Probes B13423, Eugene, OR) was used to identify the neuromuscular junctions. Myofibers were washed and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Digital images were obtained with a Zeiss 510 confocal laser-scanning microscope (Carl Zeiss, Poughkeepsie, NY) with pinhole settings calibrated to 1.0 Airy disk.
Electrically evoked Ca2+ transients.
Dishes of myofibers were loaded with the dual emission, ratiometric, fluorescent Ca2+ indicator, Indo-1 PE AM (5 μM in 20% pluronic/DMSO), for 45 min in a normal rodent Ringer (NR; 140 NaCl, 4 KCl, 1.8 CaCl, 1 MgSO4, 10 HEPES, 5 glucose, pH to 7.4, osmolarity ∼300 mosM). Dishes were washed and incubated for 30 min in NR to allow Indo-1 to deesterify within the myofibers. During this period, 1 μM N-benzyl-p-toluene sulfonamide (BTS), a myosin ATPase inhibitor, was added to the NR to limit myofiber movement during field stimulation. Myofibers were imaged on an IonOptix imaging platform mounted to an IX-70 inverted fluorescence microscope equipped with a Sutter DG-4 excitation source and with an Ion Optix (Milton, MA) PMT imaging platform for Indo UV experimentation. The Indo-1 PE emission ratio was assayed as an estimate of the myoplasmic Ca2+ concentration ([Ca2+]) flux (excitation: 340 nm; emission: 405/485 nm). Myofibers were field stimulated with single, supramaximal square pulses (500 μs) to assess amplitude or with a double pulse (10-ms interval), after which the decay in the Ca2+ signal, reflecting Ca2+ uptake from the myoplasm into the sarcoplasmic reticulum (SR) (8), was measured and fitted to a single exponential to determine tau (OriginLab 8.5, OriginLab, Northampton, MA) (37). The peak of the single, induced transient was taken as the magnitude of Ca2+ release (8). Resting [Ca2+] was assessed in each myofiber before analysis of Ca2+ release and reuptake. Global fluorescent transients were assayed in WT (n = 21 normal, n = 6 abnormal), K19−/− (n = 14 normal, n = 3 abnormal), Des−/− (n = 34 normal, n = 6 abnormal), and DKO (n = 37 normal, n = 9 abnormal) unbranched myofibers and bifurcated myofibers (trunk, small branch, and large branch).
Statistical analysis.
Independent variables collected for morphology and Ca2+ measurements were analyzed by single-factor analysis of variance (ANOVA) followed by the Holm-Sidak post hoc analyses for multiple comparisons (SigmaStat, San Rafael, CA). Significance was set at P < 0.05 and all results are reported as means ± SE (N is the number of mice; n is the number of myofibers).
RESULTS
Muscle fiber morphology.
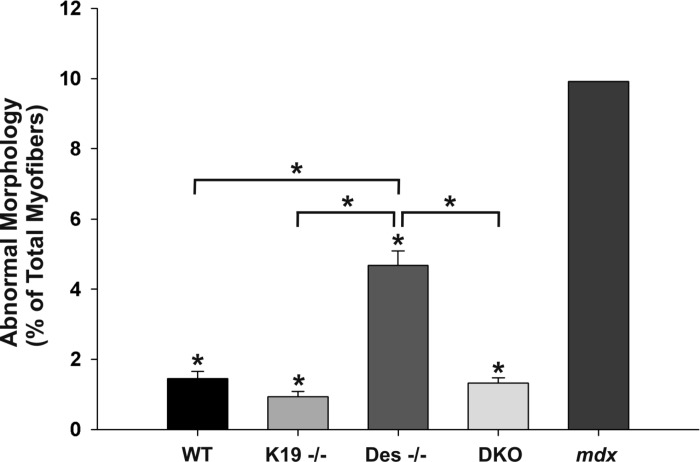
Previous reports have demonstrated that malformed myofibers can be readily observed in enzymatically isolated myofibers in culture (18, 19). We used this approach to examine myofiber morphology in FDB muscles from adult mice lacking IFs compared with WT and mdx mice (Fig. 1). In myofibers dissociated from 8 WT muscles (n = 4 animals) from mice > 8 mo old, 1.45 ± 0.21% of the FDB myofibers were identified visually as being split, having a thin process, or one or more branches of both small and large diameter (Fig. 1; black bar). Age-matched K19−/− and DKO myofibers were similar to WT, with 0.94 ± 0.15% and 1.33 ± 0.14% showing these abnormal structures, respectively (Fig. 1; medium-light gray bar and light gray bar, respectively). In contrast, 4.68 ± 0.41% of Des−/− myofibers had malformations (Fig. 1; medium gray bar). For comparison, we examined FDB myofibers cultured from one mdx mouse, which we and others have shown to have higher levels of branched or split myofibers (19, 23). These mdx muscles yielded a high number of myofibers with branching, with an average of 9.92% per muscle (Fig. 1; dark gray bar), which is consistent with our previous study (23). Thus, the absence of desmin, but not of K19 or both K19 and desmin, results in increased branching of FDB myofibers with a frequency that is approximately half that seen in FDB myofibers lacking dystrophin.
Fig. 1.
Fiber branching in flexor digitorum brevis (FDB) muscles lacking intermediate filaments vs. mdx muscles. FDB muscles were enzymatically dissociated and morphological abnormalities were categorized according to their appearance. The bar graph shows percentages of wild type (WT; n = 1,835), keratin 19-deficient (K19−/−; n = 5,272), desmin-deficient (Des−/−; n = 4,816), double-knockout (DKO; n = 5,915), and mdx (n = 121) myofibers with abnormal morphology. Data are means ± SE. *Statistical significance, P < 0.01 vs. mdx muscles or differences as indicated with brackets.
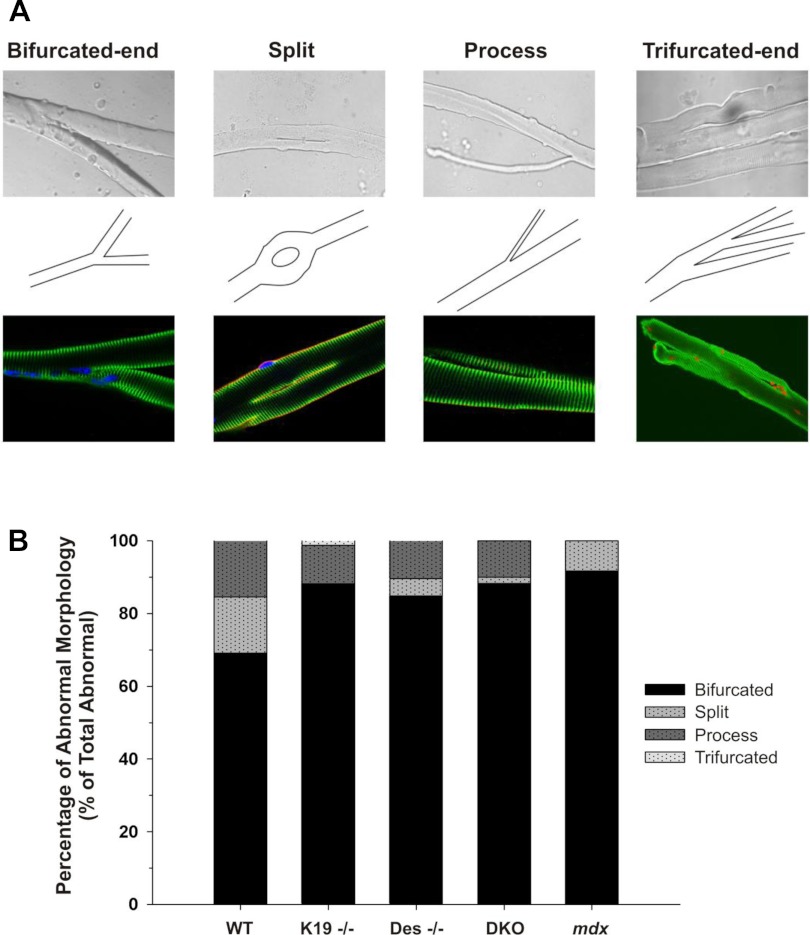
In each genotype above, branching patterns had several distinct morphologies (Fig. 2). Figure 2A shows the four distinct morphologies identified (bifurcated-end, split, process, or trifurcated-end) in bright field and fluorescence images (top and bottom, respectively) with the corresponding schematics (middle). The composite bar graph in Fig. 2B shows the percentage of abnormal myofibers of each phenotype for WT, K19−/−, Des−/−, DKO, and mdx myofibers. Within the population of bifurcated, split, myofibers with thin processes, and trifurcated, the majority (70% in WT; ∼85% in all IF mutants; 92% in mdx) were bifurcated at one end, with only two branches. A smaller number of myofibers (16% in WT; ∼10% in all IF mutants; 0% in mdx) had a single long narrow process. Less common were myofibers that had a split in the center (16% in WT; ∼4% in Des−/− and DKO; 0% in K19−/−; 8% in mdx) or with multiple branches at the ends (0% in WT, DKO, and mdx; ∼1% in K19−/− and Des−/−).
Fig. 2.
Distribution of abnormal morphology in FDB muscles lacking intermediate filaments. A: micrographs (top), corresponding schematics (middle), and immunofluorescence images (bottom) depicting variations of abnormal morphology. Four general morphologies were present, which included a bifurcated end, a mid-myofiber split, a single small-diameter appendage/process, and a multisplit end (“trifurcated”). B: composite bar graph showing the percentage of abnormal myofibers in each of the four morphologies for WT, K19−/−, Des−/−, DKO, and mdx myofibers.
Myofiber ultrastructure and cytoskeletal architecture.
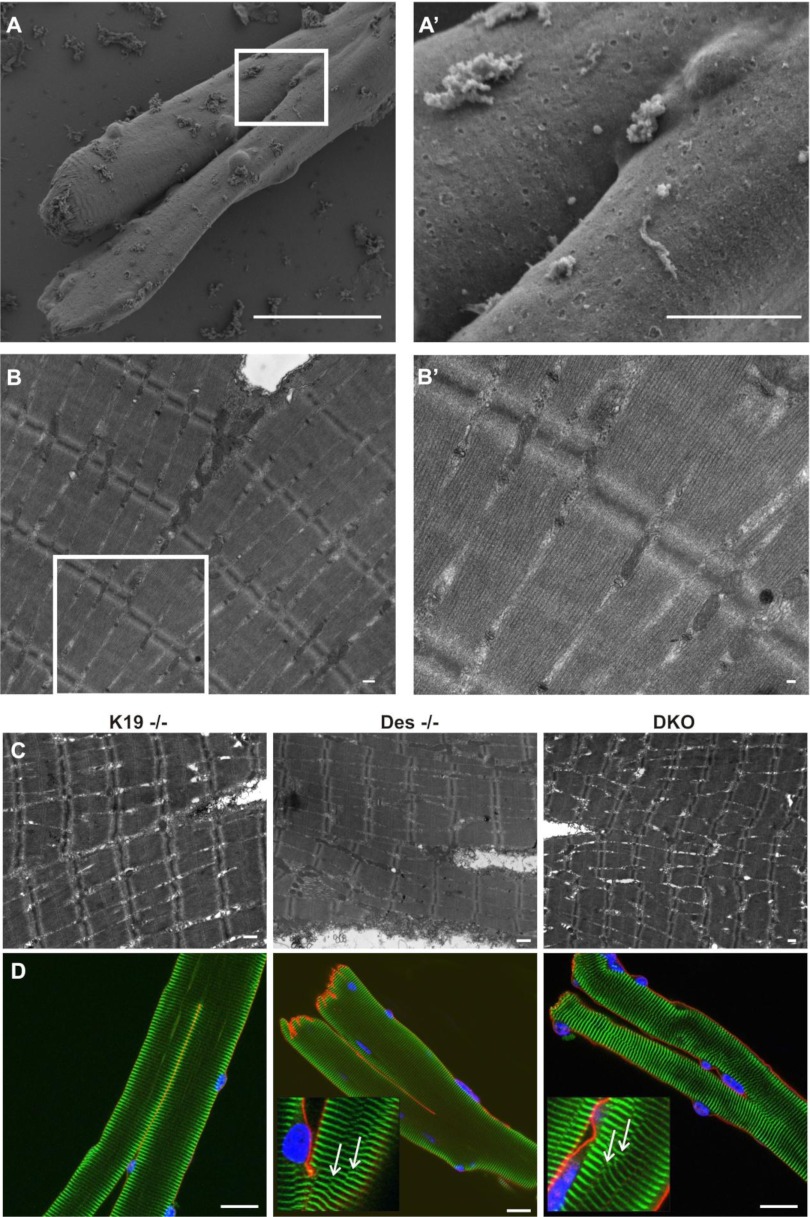
We used scanning and transmission EM to investigate possible differences in the ultrastructure of branched K19−/−, Des−/−, and DKO myofibers, specifically at the point of branching. Single myofibers were isolated and fixed as indicated in materials and methods. A myofiber with a single trunk that bifurcates into two branches of comparable size is shown in Fig. 3A, and, at higher magnification in Fig. 3A′. Another branched myofiber, imaged by TEM, is shown in Fig. 3B, and, at higher magnification in Fig. 3B′. Surprisingly, there was little disruption to the myofibrils near the branch points. The TEM images shown in Fig. 3C are representative of branched myofibers in K19−/−, Des−/−, and DKO, respectively. We previously reported that the myofibrils in tibialis anterior (TA) muscles of Des−/− and K19−/− muscles were misaligned, and that the myofibrils in the DKO showed the greatest misalignment (22). However, this misalignment was minimal in the IF-null FDB myofibers, which had sarcomeres that appeared to be nearly in register with one another, even at the branch points.
Fig. 3.
Myofiber ultrastructure and cytoskeletal architecture across the branch points of bifurcated myofibers. A–C: electron microscopy (EM) images of single bifurcated myofibers. Single myofibers were isolated from FDB muscle, plated on a gel-coated dish, and fixed as indicated in materials and methods. A: representative scanning EM (SEM) image of a typical myofiber that has altered morphology, with a single trunk that bifurcates at the midpoint of the myofiber length. Scale bar, 40 μm. A′: higher magnification of the boxed region in A to emphasize the branch point. Scale bar, 10 μm. B: representative transmission EM (TEM) image of another typical myofiber with altered morphology (as described in A). Scale bar, 500 nm. B′: higher magnification of the boxed region in B to emphasize ultrastructure of individual sarcomeres at and across the branch point. Scale bar, 100 nm. C: representative TEM images of typical K19−/−, Des−/−, or DKO myofibers that have altered morphology. Scale bar, 500 nm. Images show a surprisingly normal ultrastructure in malformed myofibers at and across branch points. D: representative images of fluorescent labeling of cellular structure in branched K19−/−, Des−/−, or DKO myofibers reveal no gross abnormalities. FDB myofibers were enzymatically dissociated, plated, fixed, and double-labeled with antibodies against α-actinin (green) and dystrophin (red). Nuclei were identified with DAPI staining (blue). Scale bar, 20 μm. In some instances, there were examples of altered cytoskeletal structure in Des−/− and DKO (insets) in the form of “Y-shaped” structures (white arrows) visualized in different focal planes of the myofibers shown. Although this occurrence suggests that the myofibrils, which are normally in register with one another, were disrupted in some locations, the Y-shaped structures were distributed similarly throughout the myofibers with no increased occurrence at the branch points.
To further evaluate features of cellular organization, we labeled fixed myofibers with antibodies against dystrophin (red) and α-actinin (green) in Fig. 3D. Dystrophin is a 427-kDa protein at the sarcolemmal membrane (absent in mdx) and α-actinin concentrates at the Z-disks, where it cross-links the thin filaments in adjacent sarcomeres. In most myofibers of all genotypes, the dystrophin at the sarcolemma appeared continuous and α-actinin at the Z-disks appeared organized as normally where myofibers branched (Fig. 3D). As noted above in our TEM studies, the Z-disks of branched myofibers, labeled with α-actinin, were well aligned.
The neuromuscular junction in branched myofibers.
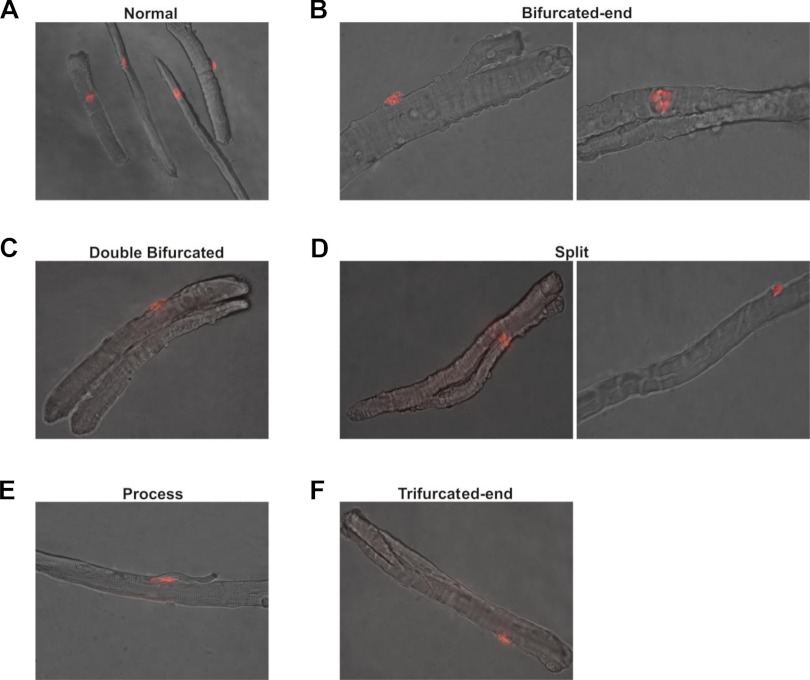
A normal skeletal muscle myofiber has a single NMJ responsible for synchronizing action potential (AP) propagation during muscle contraction so that all parts of the myofiber are activated synchronously, avoiding any regional sarcomere overstretching. The presence of two or more NMJs on a myofiber could lead to unsynchronized AP propagation and result in aberrant mechanical stress during muscle contraction (17). To learn if branched myofibers have single or multiple NMJs, we fixed over 1,000 myofibers from WT, K19−/−, Des−/−, DKO, and mdx FDB muscles, and labeled them with α-bungarotoxin conjugated to Alexa-594 to identify the acetylcholine receptors in the motor end plate. In all of the branched myofibers we examined, we found only a single motor end plate, similar to unbranched myofibers (Fig. 4). Motor end plates were found almost exclusively at the center of myofibers with normal morphology (Fig. 4A). Interestingly, the motor end plate was often located at other regions within malformed myofibers (Fig. 4, B–F), including on the trunk, on both small- and large-diameter branches, on the process, as well as above the split or at either end of a split myofiber. However, we detected no consistent pattern of localization of these postsynaptic structures in any of the four phenotypes.
Fig. 4.
Neuromuscular junctions (NMJs) in bifurcated myofibers. Single myofibers were isolated from FDB muscle and fixed as indicated in materials and methods. Myofibers were labeled with α-bungarotoxin conjugated to Alexa-594 to identify the NMJs (red). A single neuromuscular junction was observed in all myofibers examined, even when branches were present. A: typical location of the postsynaptic region (motor end plate) of NMJs at the middle of the myofiber in several normal adult myofibers. B–F: locations of the single postsynaptic region (motor end plate) of the NMJ in each of the four morphological phenotypes identified for abnormal myofibers. The postsynaptic region in these abnormal myofibers from intermediate filament (IF)-lacking adult mice was often located at various regions within the myofiber, not just at the myofiber center as found in normal myofibers.
Global EC-coupling.
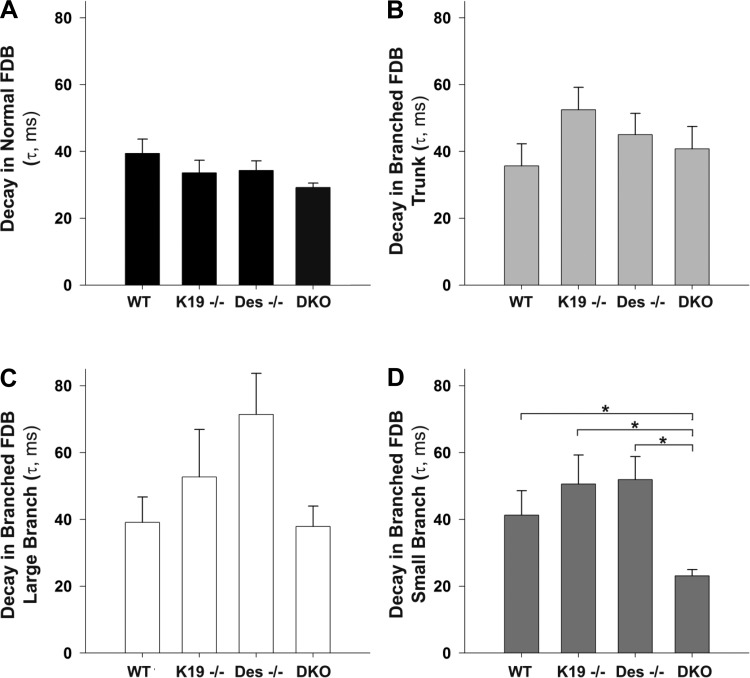
Several groups have identified significant deficits in EC coupling in mdx myofibers, including both a reduction in evoked Ca2+ release from the SR and Ca2+ reuptake into the SR (1, 20, 28, 29, 39, 40). However, all of these studies have been conducted in myofibers with normal morphology. Here we investigated global, electrically evoked Ca2+ signals in WT, K19−/−, Des−/−, and DKO myofibers, with or without bifurcated ends (Fig. 5). This morphological variant was selected as it was the most frequent in the myofibers we cultured (Fig. 2B). In Indo-1 PE-loaded myofibers, we took the Indo-1 ratio as a surrogate of [Ca2+] and assayed resting myoplasmic [Ca2+] (before field stimulation), as well as the magnitude and kinetics of the evoked Ca2+ transient. In branched myofibers, the main trunk, as well as the branches (classified as the small-diameter branch and the large-diameter branch) were imaged independently. Normal and bifurcated myofibers of every genotype showed no differences in resting [Ca2+], as measured by the ratio of Indo-1 PE emission at 405 nm (Ca2+ bound) to 485 nm (Ca2+ free) or in the amplitude of the Ca2+ transient (data not shown). The decay rate of the fluorescent transient was also indistinguishable among normal myofibers (Fig. 5A), as well as the trunk (Fig. 5B) and large branches (Fig. 5C) of branched myofibers from WT, K19−/−, Des−/−, or DKO. The decay rate of the small-diameter branches from the bifurcated end of DKO myofibers was significantly smaller (faster) than the WT, K19−/−, and Des−/− (Fig. 5D).
Fig. 5.
Myofiber Ca2+ handling is altered in myofibers lacking IFs. Global fluorescent transients were assayed in myofibers loaded with Indo-1 Leakage Resistant (5 μM for 45 min). A fixed size imaging window was placed on the myofiber for sampling a normal myofiber or several regions of a branched myofiber including: the trunk portion, the small-branched end, or the large-branched end. A–D: decay tau summary data for WT, K19−/−, Des−/−, and DKO myofibers with normal morphology (A) and for specific regions within bifurcated myofibers including the trunk (B), large branch (C), or small branch (D). Basal Ca2+ concentration ([Ca2+]), estimated as the Indo-1 fluorescence ratio, as well as the amplitude of the Indo-1 ratio were not different between groups. Data are means ± SE. *Statistical significance, P < 0.01 indicated with brackets.
DISCUSSION
IFs resist mechanical stress (13) and are likely to play important mechanical roles in muscle (2, 7). We have been studying the IFs in skeletal muscle to learn how they contribute to the subcellular architecture required for contraction and the pathways along which contractile force is transmitted from the sarcomeres to the extracellular matrix. Previous results indicate that fast-twitch muscle of mice lacking the IF proteins desmin and K19 showed lower specific force and greater susceptibility to injury than controls (22). These physiological differences could be due to the decrease in the IFs needed to stabilize the sarcoplasm to maintain EC coupling (12, 21, 39, 40) and to transmit force radially, from the contractile apparatus to the sarcolemma (3). Alternatively, these differences might be an indirect result of the changes that follow muscle degeneration and regeneration, leading to the formation of branched myofibers, which have been linked to similar phenotypes in the mdx mouse (10, 16, 18, 19, 23). We used FDB muscles to test the latter hypothesis. Although we report significant changes in both myofiber morphology and the decay rate of the Ca2+ transient in branches of FDB myofibers lacking desmin or both K19 and desmin, respectively, our results suggest that these two IF proteins play distinct or, in one example, antagonistic roles in muscle structure and function.
The absence of desmin in FDB myofibers resulted in the appearance of more branched or split myofibers than the absence of K19−/− or WT myofibers. Surprisingly, the same relative number of abnormal myofibers seen in the WT was also present in myofibers [DKO (1.3%)] where both sets of IF proteins were absent, providing evidence that the absence of K19 in the DKO corrects the defect caused by the absence of desmin. Thus, the keratin IF network is needed for the absence of desmin to produce this phenotype. We previously reported that desmin is required for mitochondria to accumulate under the sarcolemma of K19−/− myofibers, and that myofibers lacking both proteins do not accumulate more subsarcolemmal mitochondria than WT (22). Thus, the absence of one IF can antagonize the effects created by the absence of the other. These opposing effects may be direct, by changing the structure or mechanical resistance of the myoplasm (3, 12, 21, 39, 40), or indirect, via changes in gene expression (26), ion channel function (41), or signaling cascades (35). For instance, the absence of K19 may lead to the upregulation of other proteins, perhaps even other keratins, as part of a compensatory response that mitigates the effects of K19−/− and DKO on myofiber stability and contributes to the milder branching phenotype observed for both genotypes. This observation is consistent with previous reports that demonstrated the absence of K19 showed no phenotype in developing embryos and in epithelial cells of the gut and kidney (33), as well as a mild myopathy in fast-twitch skeletal muscle from K19−/− mice (32).
This study does not directly address the question of whether the presence of branched myofibers correlates with loss of specific force and greater susceptibility to injury. We previously reported that TA muscles in Des−/− mice have a larger force deficit than K19−/− or WT, and that DKO TA muscles had no greater force deficit than Des−/−. We also showed that only the DKO showed a significantly greater susceptibility to repetitive large strain injuries caused by eccentric contractions relative to WT (22). The previous study used isolated TA muscles to measure specific force (22), whereas the current study focuses on enzymatically dissociated FDB myofibers. Although both muscles are predominantly composed of fast-twitch myofibers, the TA sustains much greater forces and stresses than the FDB. In this regard, the disparate functional demands between these two types of muscles may contribute to the differences seen across different studies and may account for the fact that the FDB myofibers, examined by EM and confocal fluorescence microscopy, do not exhibit the same shifts in the alignment of myofibrils that we documented for the Des−/−, K19−/−, and DKO TA muscles (22). Functional differences may also make it difficult to generalize about the role of branched myofibers in cultures from FDB muscles in determining particular phenotypes in mutant TA muscles.
To evaluate whether ultrastructural differences are present in myofibers with altered morphology, we first examined the internal ultrastructure within malformed myofibers using thin-section electron microscopy. We reasoned that since there are obvious gross structural variants (Figs. 1 and 2), there might be obvious alterations in the internal ultrastructure that could increase the likelihood of damage secondary to forceful lengthening contractions. TEM images reveal similar distributions of the contractile apparatus in the K19−/−, Des−/−, and DKO with similar levels of misalignment seen throughout the myofibers and not specifically associated with, or worse at, the branch points (Fig. 3, B and C). To further evaluate whether subcellular structural differences are present in myofibers with altered morphology, we examined the cytoskeletal architecture of K19−/−, Des−/−, and DKO myofibers with malformations. Immunolocalization of dystrophin and α-actinin, two critical cytoskeletal proteins providing structural integrity at the sarcolemma and within myofibrils, respectively, revealed no gross disruptions of sarcolemmal continuity or alignment of Z-disks across a branch point; misalignments of the contractile apparatus in the Des−/− and DKO as previously reported (22) appear not to be exacerbated across the branch points (Fig. 3D). We observed some examples of altered cytoskeletal structure in Des−/− and DKO myofibers in the form of “Y-shaped” structures (Fig. 3D insets; white arrows) providing evidence that the myofibrils, which are normally in register with one another, even at the split, were disrupted. These Y-shaped structures have recently been observed by others at the myofiber periphery in WT muscle with an abundance of these irregularities extending deep into the myofiber center in mdx muscle (15), but the prevalence of this structural abnormality is unclear.
A normal skeletal muscle myofiber has a single NMJ and is responsible, in part, for synchronizing AP propagation along a myofiber. A previous study utilized SEM to examine the frequency and innervation patterns of branched myofibers and suggested there is a potential for branched myofibers to have multiple NMJs (34). Another study utilized modeling of action potential transmission in normal and branched myofibers to suggest that myofibers with multiple NMJs results in unsynchronized action potential propagation and contraction of myofibers, which could cause additional stress on the bifurcated myofiber (17). However, neither of those studies used a NMJ-specific marker. To determine directly whether branched myofibers have multiple NMJs, we used α-bungarotoxin conjugated to Alexa-594 to identify the postsynaptic region (motor end plate) of NMJs. In normally shaped myofibers, the motor end plate typically is located halfway along the length of the myofiber (Fig. 4A). In all of the abnormally shaped myofibers we examined, only one motor end plate was observed, even when branches were present. Interestingly, the motor end plate was found at a variety of locations in each of the four distinct branched phenotypes identified (Fig. 4, B–F), including on the trunk, on small or large branches, on processes, as well as at either end of a split myofiber. We were unable to find any clear examples of two or more NMJs on a single muscle fiber, regardless of morphology.
Mdx myofibers have deficits in EC coupling, which are manifested in part as altered electrically elicited calcium release from the SR (12, 21, 39, 40); these are thought to play a role in decreased muscle specific force in mdx myofibers (40). Our previous study showed that in mdx muscle, myofibers with altered morphology have deficits in EC coupling (23). Thus, there appears to be an association between the overall decrease in muscle function in aging mdx muscle and the high prevalence of abnormal myofibers. Since previous studies also showed a decrease in muscle-specific force in mice lacking keratin 19-based and desmin-based IFs (22), we speculated here that EC coupling deficits might also play a role in decreased muscle specific force in these IF-lacking myofibers. In this investigation, we found no differences in the resting intracellular [Ca2+] or SR Ca2+ release amplitude for both normal and abnormal myofibers of every genotype examined (data not shown). The only significant difference we observed was the rate of Ca2+ uptake into the SR after Ca2+ release in small branches of DKO myofibers (Fig. 5D). The Ca2+ transients in the large-diameter branched portions of a bifurcated myofiber appear to share the reduced uptake kinetics (larger tau, slower uptake) typical of developing myofibers, whereas the Ca2+ transients in the small-diameter branch portions of a bifurcated myofiber reflect the faster uptake kinetics (smaller tau) typical of mature myofibers (8).
Summary.
As desmin and keratins resist mechanical stress (13) and are likely to play important mechanical roles in muscle (2, 7), the loss of one or both of these IFs may result in mechanical instability of the sarcolemma and myofiber damage during muscle contraction. The morphology and physiology of FDB myofibers were remarkably close to those from WT muscles, the major exception being the increased number of branched myofibers in the Des−/−. The absence of desmin has a larger effect on branching than K19, which is present in smaller amounts in adult skeletal muscle. However, the absence of K19 appears to compensate for the absence of desmin in the DKO, such that it mitigated the effects and contributed to the milder branching phenotype observed in the DKO. The neuromuscular junctions in the mutant mice, while more variably located, are limited to one per myofiber. The organization of the sarcolemma and the contractile apparatus in the vicinity of the branch appear normal, and even most of the parameters associated with EC coupling that we measured are similar in WT and mutant myofibers. The only significant difference we observed was the rate of Ca2+ uptake in small branches of DKO myofibers. In summary, desmin-based and keratin-based IFs do not serve solely as alternative structures that stabilize the myoplasm and transmit contractile forces.
GRANTS
This work was supported by National Institutes of Health Grants K01AR053235 (to R. M. Lovering), 1R01AR059179 (to R. M. Lovering), and T32-AR-007592 (to M. H. Goodall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.H.G., C.W.W., and R.M.L. conception and design of the research; M.H.G. performed the experiments; M.H.G. analyzed the data; M.H.G., C.W.W., R.J.B., and R.M.L. interpreted the results of the experiments; M.H.G. prepared the figures; M.H.G. drafted the manuscript; M.H.G., C.W.W., S.J.P., R.J.B., and R.M.L. edited and revised the manuscript; M.H.G., C.W.W., S.J.P., R.J.B., and R.M.L. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical support of John Strong in the Core Imaging Facility of the University of Maryland Baltimore for EM data acquisition.
REFERENCES
- 1. Allen DG. Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol 31: 485–493, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bloch RJ, Capetanaki Y, O'Neill A, Reed P, Williams MW, Resneck WG, Porter NC, Ursitti JA. Costameres: repeating structures at the sarcolemma of skeletal muscle. Clin Orthop Relat Res S203–S210, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31: 73–78, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Brown LD, Rodney GG, Hernandez-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am J Physiol Cell Physiol 292: C1156–C1166, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LD, Schneider MF. Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol Anim 38: 411–422, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Canato M, Dal Maschio M, Sbrana F, Raiteri R, Reggiani C, Vassanelli S, Megighian A. Mechanical and electropysiological properties of the sarcolemma of muscle fibers in two murine models of muscle dystrophy: Col6a1−/− and Mdx. J Biomed Biotechnol 2010: 1–13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capetanaki Y, Bloch RJ, Kouloumenta A, Mavroidis M, Psarras S. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res 313: 2063–2076, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Capote J, Bolanos P, Schuhmeier RP, Melzer W, Caputo C. Calcium transients in developing mouse skeletal muscle fibres. J Physiol 564: 451–464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan S, Head S. The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy. Exp Physiol 96: 564–571, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Chan S, Head SI, Morley JW. Branched fibers in dystrophic mdx muscle are associated with a loss of force following lengthening contractions. Am J Physiol Cell Physiol 293: C985–C992, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, Lopez JR, Allen PD, Pessah IN. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol 73: 1203–1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collet C, Allard B, Tourneur Y, Jacquemond V. Intracellular calcium signals measured with indo-1 in isolated skeletal muscle fibres from control and mdx mice. J Physiol 520: 417–429, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coulombe PA, Bousquet O, Ma L, Yamada S, Wirtz D. The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol 10: 420–428, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Eriksson A, Lindstrom M, Carlsson L, Thornell LE. Hypertrophic muscle fibers with fissures in power-lifters; fiber splitting or defect regeneration? Histochem Cell Biol 126: 409–417, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Friedrich O, Both M, Weber C, Schurmann S, Teichmann M, von Wegner F, Fink R, Vogel M, Chamberlain J, Garbe C. Microarchitecture is severely compromised but motor protein function is preserved in dystrophic mdx skeletal muscle. Biophys J 98: 606–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayes A, Williams DA. Contractile function and low-intensity exercise effects of old dystrophic (mdx) mice. Am J Physiol Cell Physiol 274: C1138–C1144, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Head S. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp Physiol 95: 641–656, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Head SI, Stephenson DG, Williams DA. Properties of enzymatically isolated skeletal fibres from mice with muscular dystrophy. J Physiol 422: 351–367, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci 248: 163–169, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Hollingworth S, Marshall MW, Robson E. Excitation contraction coupling in normal and mdx mice. Muscle Nerve 13: 16–20, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol 586: 5063–5075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lovering RM, O'Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ. Physiology, structure, and susceptibility to injurty of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 300: C803–C813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lovering RM, Michaelson L, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol 297: C571–C580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lovering RM, Porter NC, Bloch RJ. The muscular dystrophies: from genes to therapies. Phys Ther 85: 1372–1388, 2005 [PMC free article] [PubMed] [Google Scholar]
- 25. O'Neill A, Williams MW, Resneck WG, Milner DJ, Capetanaki Y, Bloch RJ. Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol Biol Cell 13: 2347–2359, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohlendieck K, Campbell KP. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol 115: 1685–1694, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravenscroft G, Nowak KJ, Jackaman C, Clement S, Lyons MA, Gallagher S, Bakker AJ, Laing NG. Dissociated flexor digitorum brevis myofiber culture system–a more mature muscle culture system. Cell Motil Cytoskeleton 64: 727–738, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem 276: 4647–4651, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Schertzer JD, van der PC, Shavlakadze T, Grounds MD, Lynch GS. Muscle-specific overexpression of IGF-I improves E-C coupling in skeletal muscle fibers from dystrophic mdx mice. Am J Physiol Cell Physiol 294: C161–C168, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell 8: 673–692, 1976 [DOI] [PubMed] [Google Scholar]
- 31. Stone MR, O'Neill A, Catino D, Bloch RJ. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell 16: 4280–4293, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone MR, O'Neill A, Lovering RM, Strong J, Resneck WG, Reed PW, Toivola DM, Ursitti JA, Omary MB, Bloch RJ. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci 120: 3999–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamai Y, Ishikawa T, Bosl MR, Mori M, Nozaki M, Baribault H, Oshima RG, Taketo MM. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol 151: 563–572, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamaki T, Sekine T, Akatsuka A, Uchiyama S, Nakano S. Detection of neuromuscular junctions on isolated branched muscle fibers: application of nitric acid fiber digestion method for scanning electron microscopy. J Electron Microsc (Tokyo) 41: 76–81, 1992 [PubMed] [Google Scholar]
- 35. Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ursitti JA, Lee PC, Resneck WG, McNally MM, Bowman AL, O'Neill A, Stone MR, Bloch RJ. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem 279: 41830–41838, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Ward CW, Reiken S, Marks AR, Marty I, Vassort G, Lacampagne A. Defects in ryanodine receptor calcium release in skeletal muscle from post-myocardial infarct rats. FASEB J 17: 1517–1519, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord 19: 241–249, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx mouse muscle fibres. J Physiol 568: 867–880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol 557: 59–75, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeung EW, Whitehead NP, Suchyna TM, Gottleib PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562: 367–380, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]