Abstract
The role of the cystic fibrosis transmembrane conductance regulator (CFTR) in lysosomal acidification has been difficult to determine. We demonstrate here that CFTR contributes more to the reacidification of lysosomes from an elevated pH than to baseline pH maintenance. Lysosomal alkalinization is increasingly recognized as a factor in diseases of accumulation, and we previously showed that cAMP reacidified alkalinized lysosomes in retinal pigmented epithelial (RPE) cells. As the influx of anions to electrically balance proton accumulation may enhance lysosomal acidification, the contribution of the cAMP-activated anion channel CFTR to lysosomal reacidification was probed. The antagonist CFTRinh-172 had little effect on baseline levels of lysosomal pH in cultured human RPE cells but substantially reduced the reacidification of compromised lysosomes by cAMP. Likewise, CFTR activators had a bigger impact on cells whose lysosomes had been alkalinized. Knockdown of CFTR with small interfering RNA had a larger effect on alkalinized lysosomes than on baseline levels. Inhibition of CFTR in isolated lysosomes altered pH. While CFTR and Lamp1 were colocalized, treatment with cAMP did not increase targeting of CFTR to the lysosome. The inhibition of CFTR slowed lysosomal degradation of photoreceptor outer segments while activation of CFTR enhanced their clearance from compromised lysosomes. Activation of CFTR acidified RPE lysosomes from the ABCA4−/− mouse model of recessive Stargardt's disease, whose lysosomes are considerably alkalinized. In summary, CFTR contributes more to reducing lysosomal pH from alkalinized levels than to maintaining baseline pH. Treatment to activate CFTR may thus be of benefit in disorders of accumulation associated with lysosomal alkalinization.
Keywords: chloride channel, macular degeneration, vacuolar H+-ATPase, lipofuscin, phagocytosis, autophagy, retinal pigmented epithelial cells
lysosomes are a major site for the clearance of engulfed material in phagocytic cells (7, 25). This degradative activity is particularly important for retinal pigmented epithelial (RPE) cells, as the internalization of outer segments shed daily by photoreceptors makes them among the most phagocytotically active cells in the body (19). As postmitotic cells, a high degradative load makes them particularly susceptible to factors that decrease lysosomal enzyme activity, as the increased retention of partially degraded material may be pathological. In this regard, lysosomal enzymes function optimally over a narrow range of acidic pH values and the predominant lysosomal enzymes of the RPE reflect this sensitivity (11). This sharp pH dependence implies that alkalinizing lysosomes of RPE cells will lower enzyme efficiency and interfere with the degradation of internalized outer segments.
Disruption of lysosomal pH (pHL) slows outer segment degradation by RPE cells. Administration of the lysosmotic compound chloroquine leads to the incomplete digestion of photoreceptor outer segments within RPE cells, an increase in lysosomal-associated organelles, and the accumulation of extracellular debris around adjacent Bruch's membrane and choriocapillaris (24, 29). Similarly, tamoxifen raises lysosomal pH and slows outer segment clearance in RPE cells (20). In autosomal recessive Stargardt's disease, a mutation in the ABCA4 transporter leads to the accumulation of N-retinylidene-N-retinylethanolamine (A2E) in RPE cells (1, 27). Addition of A2E to cultured RPE cells increases the lysosomal pH, slows degradation of proteins, and inhibits the translocation of protons into the lysosomes and the degradation of outer segments (3, 15). Lysosomal pH in the RPE cells of ABCA4−/− mice is elevated compared with age-matched controls (20), consistent with the alkalinizing effect of A2E.
As alkalinization of RPE lysosomes leads to incomplete degradation of photoreceptor outer segments, it follows that restoring an acidic pH to the organelle may improve degradation and preserve cell health. A previous study found that stimulation of A2A adenosine receptors or β-adrenergic receptors reacidified compromised RPE cell lysosomes; the coupling of both receptors to the Gs protein implicated the second messenger cAMP (20). Cell-permeant chlorophenylthio-cyclic AMP (cpt-cAMP) reacidified lysosomes, and myristoylated protein kinase inhibitor prevented acidification, strongly implying a role for protein kinase A. Furthermore, activation of these pathways increased the degradation of photoreceptor outer segments, providing functional verification. Of note, cAMP was able to reacidify the lysosome in RPE cells from ABCA4−/− mice, suggesting that the pathway remained intact in diseased cells.
While the ability of cAMP to restore lysosomal pH in RPE cells and improve outer segment degradation is clear, the underlying mechanisms remain to be determined. The basic physiology of lysosomal acidification identifies two likely candidates. First, the delivery of protons to lysosomes and late endosomes is primarily mediated by the vacuolar proton pump vHATPase (33). Insertion of vHATPase into the plasma membrane of clear cells is enhanced by cAMP acting through soluble adenylate cyclase (32). While the transport of protons by the vHATPases lowers organelle pH, the accumulation of positive charge eventually establishes an unfavorable driving force across the membrane and makes further acidification energetically unfavorable (28, 30). This excess positive charge is often neutralized by the influx of Cl− into the vesicle, making anion transport a second key mechanism in lysosomal pH regulation (10). The most widely distributed cAMP-activated Cl− channel is the cystic fibrosis transmembrane conductance regulator (CFTR), where phosphorylation of protein kinase A activates CFTR (9). As functional and immunochemical evidence indicates that CFTR is present in RPE cells (4, 36, 37), this study asked whether CFTR contributed to the cAMP-mediated acidification of lysosomes in RPE cells.
EXPERIMENTAL PROCEDURES
Cell culture.
The human ARPE-19 cell line was obtained from American Type Culture Collection (Manassas, VA) and processed as described previously (20). In brief, cells were grown to confluence in 25-cm2 primary culture flasks (Becton Dickinson) in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 medium with 3 mM l-glutamine, 100 μg/ml streptomycin, and 2.5 mg/ml Fungizone and/or 50 μg/ml gentamicin and 10% fetal bovine serum (all Invitrogen, Carlsbad, CA). Cells were incubated at 37°C in 5% CO2 and subcultured weekly with 0.05% trypsin and 0.02% EDTA.
Lysosomal pH measurement from ARPE-19 cells.
Measurement of lysosomal pH from ARPE-19 cells was performed using a ratiometric lysosomal pH indicator dye (LysoSensor Yellow/Blue DND-160; Invitrogen). The procedure has been described in detail previously (20). Given the temperamental nature of the dye, three key parameters were optimized to identify conditions giving the best signal-to-noise level using the least amount of dye. Dye concentration from 1 to 25 μM was tested, with 5 μM the lowest concentration giving a consistently bright signal. The dye was incubated from 1 to 30 min, with 3 min identified as the shortest incubation time to provide a sufficiently bright signal. To evaluate the possible alkalinizing effects of the dye and the possible light effects on the fluorescence readings, each of the fluorescence measurements was repeatedly conducted every 40 s for 15 min. While an increased ratio was observed at higher dye concentrations, this effect was minimal with 3–5 μM dye. The stability of the dye was examined with a single set of readings at 5, 10, 30, and 60 min after incubation. The signal slowly decreased, presumably owing to diffusion of the dye. Given this information, we consistently incubated cells with 5 μM LysoSensor Yellow/Blue dye for 3 min at room temperature (RT), washed to remove any residual dye, and light emitted >520 nm in response to excitation at 340 nm and 380 nm was measured for 20 ms every 30 s. Fluorescent levels were the mean of four measurements taken after the removal of the dye; while most measurements were made ∼16.5–18.5 min after dye removal, delays occasionally occurred. Cells were bathed in 100 μl isotonic solution (IS; in mM: 105 NaCl, 5 KCl, 6 HEPES acid, 4 Na HEPES, 5 NaHCO3, 60 mannitol, 5 glucose, 0.5 MgCl2, 1.3 CaCl2). Drugs tested for their ability to reverse the effects of alkalinizing compounds were added to cells 5 min before the addition of the alkalinizing agents. Drugs were tested in the absence of dye and found to have no significant effect on the light excited at 340 or 380 nm.
Additional steps were taken to minimize variation. For example, measurements were performed on parallel wells in a 96-well plate, and the fluorescence was measured with a fluorescence plate reader at room temperature (Fluroskan; Thermo Fisher Scientific, Waltham, MA). Despite these precautions, variation in ratio and pH values did occur somewhat between preparations. While changes in passage number, cell age, measurement time, and investigator may have increased variation, other unknown factors may have led to small differences. However, the relative levels within a plate were consistent.
Initial experiments were undertaken to confirm that pH measurements made with the ratiometric dye LysoSensor Yellow/Blue DND-160 were an accurate reflection of the lysosomal pH (pHL). The LysoSensor dye stained small punctuate regions in ARPE-19 cells that showed considerable overlap with the single wavelength acidic stain LysoTracker Red DND-99 (Fig. 1A). This colocalization confirms the ability of dyes to specifically signal from acidic organelles. While readout is attributed to lysosomes, it should be noted that the output likely originates from a mixture of organelles including lysosomes, phagolysosomes, and perhaps late endosomes. The ratiometric signal from LysoSensor Yellow/Blue was converted into absolute pH values following calibration with ionophores monensin and nigericin (Fig. 1B). Independent pH calibrations were performed simultaneously with each experimental plate to minimize potential confounding of these data by LysoSensor dye instability. The ratio of light excited at 340/380 nm was converted to pH by calibration performed at precisely the same time as the other readout in adjacent wells of the same plate; the extracellular solution was buffered to pH 4.0 to 6.0 in the presence of 10 μM H+/Na+ ionophore monensin and 20 μM H+/K+ ionophore nigericin dissolved in 20 mM 2-(N-morpholino)ethanesulfonic acid (MES), 110 mM KCl, and 20 mM NaCl and adjusted to pH 4.0 to 6.0 with HCl/NaOH.
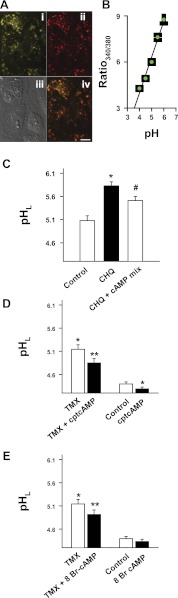
Fig. 1.
cAMP reacidifies compromised lysosomes. Lysosomal pH (pHL) was measured.A: labeling of retinal pigmented epithelial (RPE) cell lysosomes. i and ii: lysosomal pH indicator LysoSensor DND-160 Yellow/Blue (i) and the red fluorescent lysosomal stain LysoTracker Red DND-99 (ii) demonstrated considerable colocalization when simultaneously delivered to cultured RPE cells. iii: differential interference contrast image. iv: overlap of i and ii. A slight shift occurred between images. Scale bar, 20 μm. B: typical calibration of lysosomal pH from ratios in the presence of ionophores monensin and nigericin. F340/380, ratio of fluorescence excited at 340 nm/380 nm and emitted at 520 nm. The curve is a mean of 11 separate measurements. The calibration curve was well fit with a linear regression (r2 = 0.98). Calibration was performed separately on each plate throughout the study to minimize variation. C: while chloroquine (CHQ, 10 μM) elevated lysosomal pH of retinal pigment epithelial (RPE) cells, a cAMP-stimulating mix (cAMP mix) of the cell-permeant analog chlorophenylthio (cpt)-cAMP (100 μM), forskolin (10 μM), and IBMX (100 μM) acidified cells exposed to chloroquine (n = 10). D: cpt-cAMP (500 μM) acidified cells treated with tamoxifen (TMX, 10 μM) although cpt-cAMP induced a smaller acidification of untreated cells (n = 27). E: a second cell-permeant analog, 8-Br-cAMP (500 μM), also acidified cells treated with tamoxifen (10 μM) more than cells at baseline pH levels (n = 27). Here and elsewhere, bars on data plots represent means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. chloroquine; **P < 0.05 vs. tamoxifen.
Isolation and measurement of lysosomal pH from fresh ABCA4−/− mouse RPE cells.
ABCA4−/− mice were a kind gift from Dr. Gabriel Travis of the Jules Stein Eye Institute (Univ. of California, Los Angeles). All animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Mice were reared at 5–15 lux and euthanized with a CO2 overdose. Mouse RPE cells were isolated and processed as described (20). In brief, after enucleation, intact eyes were incubated in 2% dispase and 0.4 mg/ml collagenase IV for 45 min, rinsed, and incubated in growth medium for 20 min (containing DMEM with 1× MEM + nonessential amino acids, 3 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 mg/ml Fungizone and/or 50 μg/ml gentamicin, plus 10% fetal bovine serum; all Invitrogen). Sheets of RPE cells were separated from the choroid and triturated to single cell level. Cells from four to six eyes were pooled, loaded with 2–5 μM LysoSensor Yellow/Blue for 5 min at room temperature, rinsed, and distributed into wells of 384-well UV Star plates (Greiner Bio-One, Monroe, NC) and measured as described above. Although eyes from ABCA4−/− mice were slightly autofluorescent, the signal from the dye was 100-fold greater, validating the measurements (20). Lysosomal pH was measured within 3 h postmortem. CFTRAct11 was added to the bath 20 min before measurements were taken. Because of the reduced number of cells, measurements from fresh RPE cells were not calibrated but were expressed as ratio of fluorescence excited at 340 vs. 380 nm and emitted >540 nm. As the numbers of cells from each eye were too low to give acceptable signal to noise, three pairs of each genotype were pooled in one trial and two pairs from each genotype were pooled in another.
Outer segment degradation assay.
Bovine photoreceptor outer segments were isolated on the basis of published protocols (1). As described previously (20), outer segments were labeled with the fluorescent dye calcein to overcome the pH-dependent loss of signal that occurs with FITC; the negative log of acidic dissociation constant (pKa) of 6.8 means that fluorescence of FITC is lost in acidic organelles, making actual clearance hard to determine. In contrast, calcein is unaffected by pH or calcium levels. Outer segments were loaded with 5 μM calcein-acetoxymethyl (AM; Invitrogen) in PBS for 10 min and spun twice at 14,000 rpm. The AM form of the dye is nonfluorescent until cleaved by intracellular esterases, reducing background fluorescence. The fluorescence signal reached a maximum within 20 min and was evenly distributed, although its localization in disk membranes compared with cytoplasm could not be distinguished. Outer segments labeled with calcein were added to cells in 96-well plates for 2 h. Cells were then washed vigorously and incubated with growth medium for a further 2 h. This 2-h “chase” of cells in the absence of extracellular outer segments was designed to allow sufficient time for the transport of outer segments to lysosomes before drugs were added, to ensure that drugs were targeting the degradation and not internalization or binding steps. Microscopic examination confirmed that the majority of outer segments were in lysosomes after this chase period (see Fig. 5A in results). Baseline fluorescence levels excited at 485 nm and emitted >520 nm were read at the end of the 2-h chase period. Cells were then incubated with alkalinizing drugs, putative treatments, or control solution for 20 h, after which the cells were washed twice and the final fluorescence reading was taken. The time course was chosen on the basis of previous kinetic analyses showing that most outer segment degradation is complete within 18 h (21). The “%POS” (percentage of photoreceptor outer segments) was defined as the change in fluorescence between final (t20) and initial (t0) readings: %POS = 100 × [(t20)/(t0)]. The time course is summarized in results (see Fig. 5B).
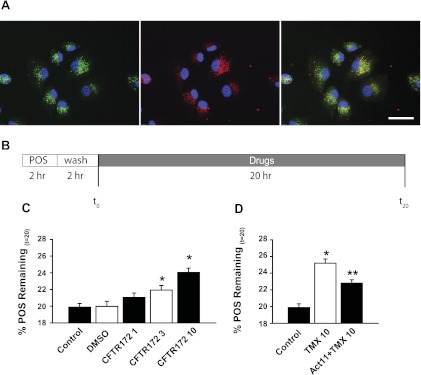
Fig. 5.
CFTR and clearance of photoreceptor outer segments (POS). A: colocalization of the green calcein-labeled POS (left) and red fluorescent lysosomal stain (middle) in ARPE-19 cells after 2-h POS challenge and 2-h wash to allow POS internalization. Overlap (right) indicates that most of the outer segments were in lysosomes at this point. Scale bar, 40 μm. B: timeline illustrating the experimental outline. C: fewer outer segments remained after 20 h in control than after 20 h with CFTRinh-172 (n = 10). DMSO vehicle (0.1%) had no effect. The “%POS” was defined as percentage change in fluorescence from POS between final (t20) and initial (t0) readings: %POS = 100 × [(t20)/(t0)]. D: tamoxifen (10 μM) also reduced the proportional reduction in outer segments after 20 h, in accordance with the effect of tamoxifen on lysosomal pH. The selective CFTR agonist CFTRAct11 (30 μM) reduced outer segment retention by tamoxifen, consistent with an increased enzymatic function with lowered lysosomal pH. *P < 0.05 vs. control, **P < 0.05 vs. tamoxifen alone, ANOVA on ranks, Holms-Sidak post hoc test (n = 15).
To localize outer segments (see Fig. 5A), cells were cultured on glass coverslips. Cells were exposed to calcein-labeled outer segments for 2 h, followed by a 2-h wash. Cells were then costained with 100 nM LysoTracker Red (Invitrogen). The red fluorescent lysosomal stain was imaged at 540/>590 nm (excitation/emission), whereas labeled outer segments were imaged at 480/>535 nm on a Nikon microscope (Eclipse 600; Nikon USA, Melville, NY) and imaged with a 3-CCD digital camera (Toshiba America, Irvine, CA). Images were processed using Image Pro Plus software (Media Cybernetics, Silver Spring, MD).
Molecular manipulation of CFTR.
One day before transfection, 2 × 105 cells/ml ARPE-19 cells were cultured in 96-well black plates (Corning, Corning, NY) using 100 μl of growth medium without antibiotics. The plasmid DNA contained the wild-type CFTR construct used previously (34). The plasmid (0.4 μg) was diluted in 25 μl serum-free Opti-MEM medium and incubated for 5 min at RT. Lipofectamine 2000 (1 μl) was subsequently mixed in 25 μl Opti-MEM medium and incubated for 20 min at RT to allow complex formation. This mixture was added to the same volume of growth medium and loaded into ARPE-19 cells. Plasmids were incubated with cells at 37°C for 4–6 h, after which the transfection mix was replaced with growth medium. Cells were grown for a further 48 h before measurements. Western blotting using the anti-CFTR antibody (M3A7; Upstate Biotechnology) was performed using standard protocols (21). In brief, ARPE-19 cells were lysed in RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0 and protease inhibitor cocktail) and centrifuged at 13,000 g for 10 min at 4°C. Protein concentrations were determined using the bicinchoninic acid protein assay kit. Protein lysate were loaded at 0.5 μg/ml in each lane in sample buffer (2% SDS, 10% glycerol, 0.001% bromophenol blue, and 0.05 M Tris·HCl, pH 6.8), separated on SDS-PAGE (Bio-Rad), and transferred to polyvinylidene difluoride membrane (Millipore).
Approaches to CFTR depletion were analogous; 0.5 μl of small interfering (si)RNA (20 μM, siGenome SMART Pool, catalog no. M-006425-01, Dharmacon/ThermoFisher) or nontargeting siRNA (si CONTROL, catalog no. D-001210-01) was mixed with 0.2 μl DharmaFECT in 50 μl Opti-MEM medium without serum. The mixture was added to cells and incubated at 37°C in a CO2 incubator for 24 h, after which it was replaced with growth medium incubated for a further 48 h. Depletion of CFTR mRNA was shown using quantitative PCR (QPCR) with standard approaches. Total RNA was extracted using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed with 1 μg of total RNA, 200 units reverse transcriptase (Superscript II), and 500 ng oligo(dT) primers in a 20 μl reaction volume. Real-time RT-PCR was performed on a 7500 real-time PCR system (Applied Biosystems, Foster City, CA), using a SYBR Green master mix, and the quantitative assessment of gene levels was analyzed by the ΔΔCt approach. Primer pair sequences used for QPCR are CFTR forward 5′-TCGGCTCCAAGTTCTGGGA-3′, reverse 5′-GAGGATGATGTAGCCACGACA-3′, GAPDH forward 5′-TCACCACCATGGAGAAGGC-3′, reverse 5′-GCTAAGCAGTTGGTGGTGCA-3′.
Isolation of lysosomes.
Trypsinized ARPE-19 cells were centrifuged at 1,000 rpm for 5 min and resuspended in 0.25 M sucrose with 5 mM ATP in 10 mM Tris buffer (pH 7.4 with HCl). After homogenization, samples were spun at 1,000 g (10 min). The supernatant was then centrifuged (20,000 g, 10 min) and the pellet was resuspended in a 0.25 M sucrose buffer with 8 mM CaCl2 in Tris·HCl buffer (pH 7.4) to lyse mitochondria (15 min, 35°C). After a subsequent centrifugation (5,000 g, 15 min), the supernatant was placed on top of a discontinuous sucrose gradient (45%, 34.5%, and 14.3%, Tris·HCl buffer). The lysosomal fraction was collected in the 34.5%–14.3% interface after an ultracentrifugation at 77,000 g for 2 h in a SW71 rotor. After isolation, lysosomes were diluted 1:10 in a 150 mM KCl solution in Tris·HCl (pH 7.4) and pelleted at 25,000 g. This pellet was resuspended in 5 μM LysoSensor Yellow/Blue dye. Lysosomes were washed 2 × by centrifugation (25,000 g, 15 min), resuspended in test or control solutions including 5 mM MgATP, and plated into a 96-well plate (50 μl/well), and the pH was determined.
Immunohistochemistry.
Colocalization of CFTR and Lamp1 in ARPE-19 cells was determined with a Nikon A1R confocal microscope system at the University of Pennsylvania Live-Cell Imaging Core. ARPE-19 cells were cultured on 12-mm glass coverslips (Fisher Scientific) for 24 h. After washing in PBS the cells were treated with and without a cAMP-stimulating mix of forskolin (10 μM), cpt-cAMP (500 μM), and IBMX (100 μM) for 30 min at 37°C and then fixed in 4% paraformaldehyde-PBS, 10 min at RT followed by permeabilization with 0.05% saponin for 10 min at RT. Thereafter, all steps were in the continuous presence of 0.1% saponin. After three washes in PBS the nonspecific binding was blocked with 10% goat serum in PBS for 1 h, and the cells were then incubated overnight with anti-CFTR (mouse monoclonal antibody 570, from J. Riordan, University of North Carolina, Chapel Hill, through the Cystic Fibrosis Foundation Therapeutics antibody distribution program) and anti-Lamp1 (rabbit polyclonal antibody, Abcam) at 1:400. After rinsing, cells were labeled with Alexafluor 488 (goat anti-mouse IgG) and Alexafluor 568 (goat anti rabbit IgG; both Invitrogen) at 1:4,000 and 1:8,000, respectively, for 1 h at RT. Finally, the cells were washed, mounted in Vectashield H-1000 (Vector Laboratories), and stored at 4°C in the dark until visualized. Related localization of CFTR and Lamp1 was imaged with a Nikon A1 inverted confocal microscope using 488 nm and 594 nm lasers, a Plan Apo ×100 oil differential interference contrast N2 objective lens (numerical aperture 1.4), and an emission bandwidth of 450–595 nm. Images were acquired and processed with NIS-Elements software AR (Nikon). The z-stack with the greatest Pearson's coefficient in each field was used for calculations.
Statistical analysis.
Data are reported as means ± SE; n values represent the number of independent wells. Statistical analyses used a one-way ANOVA with post hoc test on differences for intragroup comparisons. Results with P < 0.05 were considered significant.
RESULTS
cAMP acidifies compromised lysosomes.
The regulation of lysosomal pH is complex, with particular mechanisms active only over a particular pH range. As a key goal of these studies is to restore an acidic lysosomal pH in disease states where lysosomal pH is more alkaline than normal, we experimentally elevated the pHL and tested the ability of various candidates to restore acidity. Chloroquine is a weak base which alkalinized the lysosomes of RPE cells (Fig. 1C). This lysosomal alkalinization was reversed by elevating intracellular cAMP with a mixture including cell-permeant cpt-cAMP. Tamoxifen was used as a second approach to alkalinize the pH of lysosomes. Tamoxifen is thought to elevate pHL through its actions as a weak base and as a mediator of coupled transport of proton/hydroxide and chloride (6); the alkalinizing effects of tamoxifen on lysosomal pH are independent of the estrogen receptor (20). While tamoxifen alkalinized the lysosomal pH of RPE cells, addition of cpt-cAMP mix also acidified cells treated with tamoxifen. Interestingly, the magnitude of lysosomal acidification produced by cpt-cAMP in cells at baseline was substantially smaller (Fig. 1D). Likewise, the cell-permeant 8-Br-cAMP acidified lysosomes, with the magnitude of this acidification greater in alkalinized lysosomes (Fig. 1E).
Pharmacological identification of CFTR in lysosomal acidification.
The ability of cAMP to acidify lysosomal pH over the working range of lysosomal enzymes suggested the activation of a cAMP-dependent transport mechanism. Given that anion entry into the lysosomal lumen will balance the positive charge brought by proton entry, this acidification might be due to activation of a cAMP anion channel and/or transporter. The role of CFTR in lysosomal acidification was thus probed. The sulfonylurea glibenclamide inhibits conductance through CFTR (2). Glibenclamide produced a small elevation in baseline pHL (Fig. 2A). A newer generation of drugs with enhanced specificity for CFTR includes the inhibitor CFTRinh-172, which produces a voltage-independent block of CFTR that is not influenced by cAMP levels (23). CFTRinh-172 (10 μM) led to a small but significant elevation in pHL from baseline levels of 4.50 ± 0.02 to 4.58 ± 0.02 (Fig. 2B). However, CFTRinh-172 raised the lysosomal pH substantially more when the cAMP mix was used to treat cells challenged with chloroquine, from 5.07 ± 0.16 to 5.80 ± 0.08 (Fig. 2C). The blocker also raised the pH of cells treated with tamoxifen + cAMP mix from 5.31 ± 0.07 to 5.63 ± 0.11 (Fig. 2D). The magnitude of the effect of 10 μM CFTRinh-172 was thus larger in alkalinized cells treated with cAMP mix.
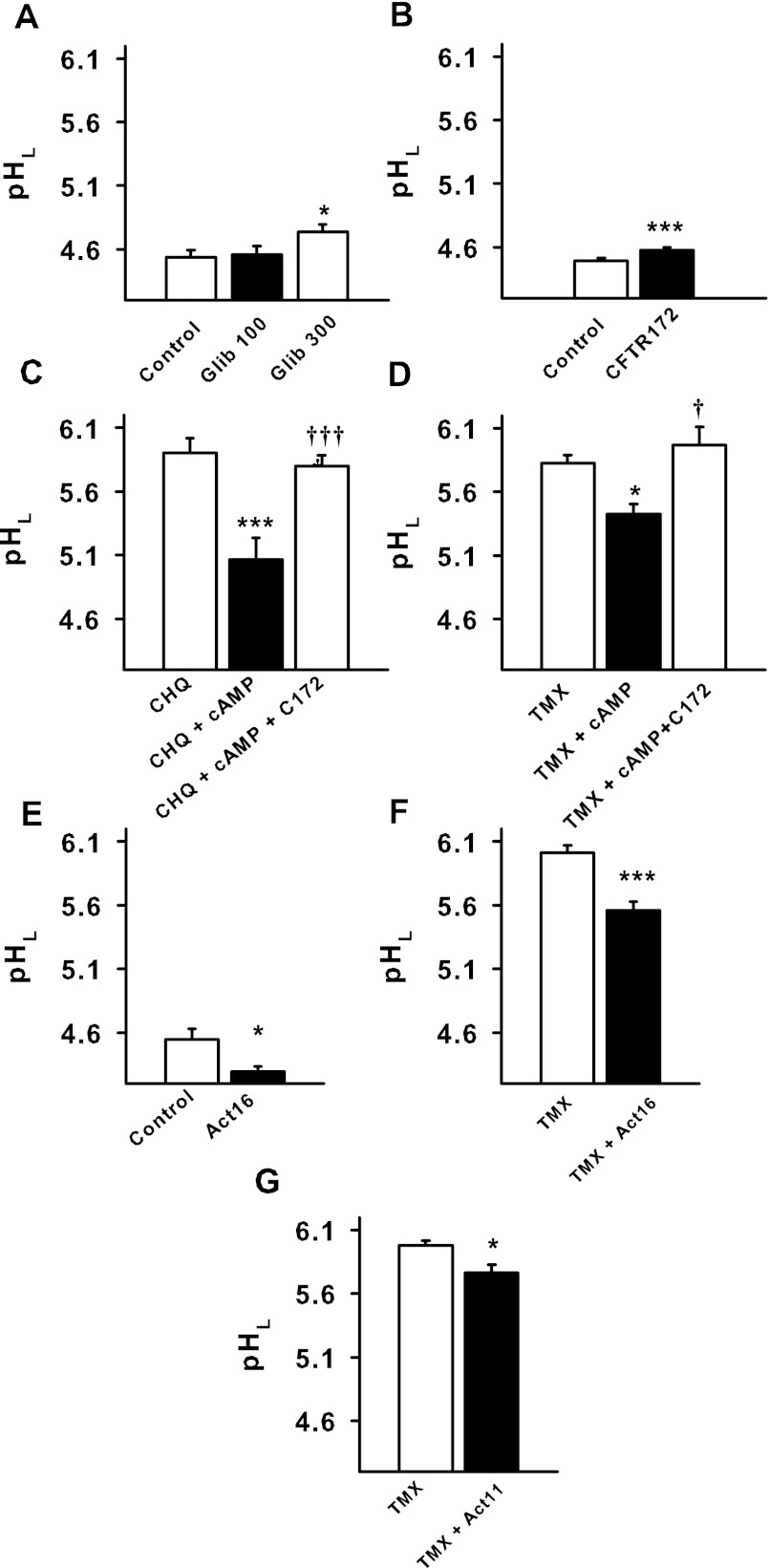
Fig. 2.
Pharmacological modulation of CFTR alters lysosomal pH. A: glibenclamide (Glib) increased the baseline pH of lysosomes in RPE cells (*P < 0.05 vs. control; n = 28 control, n = 21 100 μM Glib, n = 21 300 μM Glib). B: selective CFTR inhibitor CFTRinh-172 (CFTR172, 10 μm) significantly increased baseline pH of lysosomes in the cultured cells (n = 40). ***P < 0.005 vs. control. C: the cell-permeant cAMP analog cpt-cAMP (cAMP, 500 μM) acidified cells exposed to chloroquine (10 μM), while CFTRinh-172 (C172, 10 μm) reduced the reacidification. ***P < 0.001 vs. chloroquine, †††P < 0.001 vs. chloroquine + cpt-cAMP, n = 10. D: likewise, cpt-cAMP (500 μM) acidified cells exposed to tamoxifen (30 μM), but CFTRinh-172 (10 μm) inhibited the reacidification effect of the cpt-cAMP. *P < 0.05 vs. tamoxifen, †P < 0.05 vs. tamoxifen + cpt-cAMP; n = 33. E: CFTRAct16 (Act16, 10 μm) lowered baseline lysosomal pH in ARPE-19 cells (n = 12). *P < 0.05 vs. control. F: CFTRAct16 (10 μm) lowered lysosomal pH in tamoxifen (30 μm)-treated ARPE-19 cells (n = 32). ***P < 0.001 vs. tamoxifen. G: the more selective CFTR activator CFTRAct11 (Act11, 30 μm) also lowered lysosomal pH in tamoxifen (30 μm)-treated ARPE-19 cells (n = 28). *P < 0.05 vs. tamoxifen.
Specific activators of CFTR were examined for their ability to acidify lysosomes (23). CFTRAct16 led to a small but significant acidification of lysosomal pH in ARPE-19 cells from baseline levels; 10 μM CFTRAct16 dropped pHL from 4.49 ± 0.07 to 4.30 ± 0.03 (Fig. 2E). However, the effect of CFTRAct16 was substantially larger in lysosomes alkalinized by tamoxifen; pHL fell from 5.96 ± 0.05 to 5.57 ± 0.07 (Fig. 2F). This increased magnitude of the acidification by CFTRAct16 in tamoxifen-treated cells compared with baseline levels was analogous to the increased alkalinization by CFTRinh-172 on tamoxifen-treated compared with baseline treated cells.
As CFTRAct16 is reported to raise cAMP, at least some of its effects may have resulted from changes to cAMP (23). In contrast, CFTRAct11 activates CFTR without affecting cAMP. CFTRAct11 lowered pHL in cells treated with tamoxifen, from 6.03 ± 0.04 to 5.88 ± 0.05 (Fig. 2G). The smaller effect is consistent with the original characterization of these two drugs, where CFTRAct11 was less efficacious than CFTRAct16 (23). Taken together, the acidifying actions of CFTR activators CFTRAct16 and CFTRAct11, combined with the alkalinizing effects of CFTR inhibitors glibenclamide and CFTRinh-172, strongly support the concept that CFTR contributes to lysosomal acidification in ARPE-19 cells, but that the effect is larger when cells are alkalinized.
Molecular manipulation of CFTR.
While the pharmacologic data above consistently suggest a role for CFTR in lysosomal acidification, manipulation of CFTR using molecular tools was undertaken to further test the hypothesis. When examined in untreated cells, depletion of CFTR with siRNA had a small but insignificant effect on lysosomal pH (Fig. 3A). However, depletion of CFTR with siRNA prevented the acidifying effect of cAMP in cells treated with tamoxifen (Fig. 3B). In the corollary experiment, the effect of CFTR over expression was examined. Transfecting cells with plasmid containing CFTR lowered pHL in cells treated with tamoxifen (Fig. 3C). To confirm the effectiveness of these manipulations, siRNA was shown to reduce CFTR mRNA; levels were 1, 0.93, and 0.59 in nontransfected control, scrambled control, and cells exposed to CFTR siRNA, respectively (Fig. 3D). Likewise, transfecting cells increased CFTR protein as detected by immunoblotting.
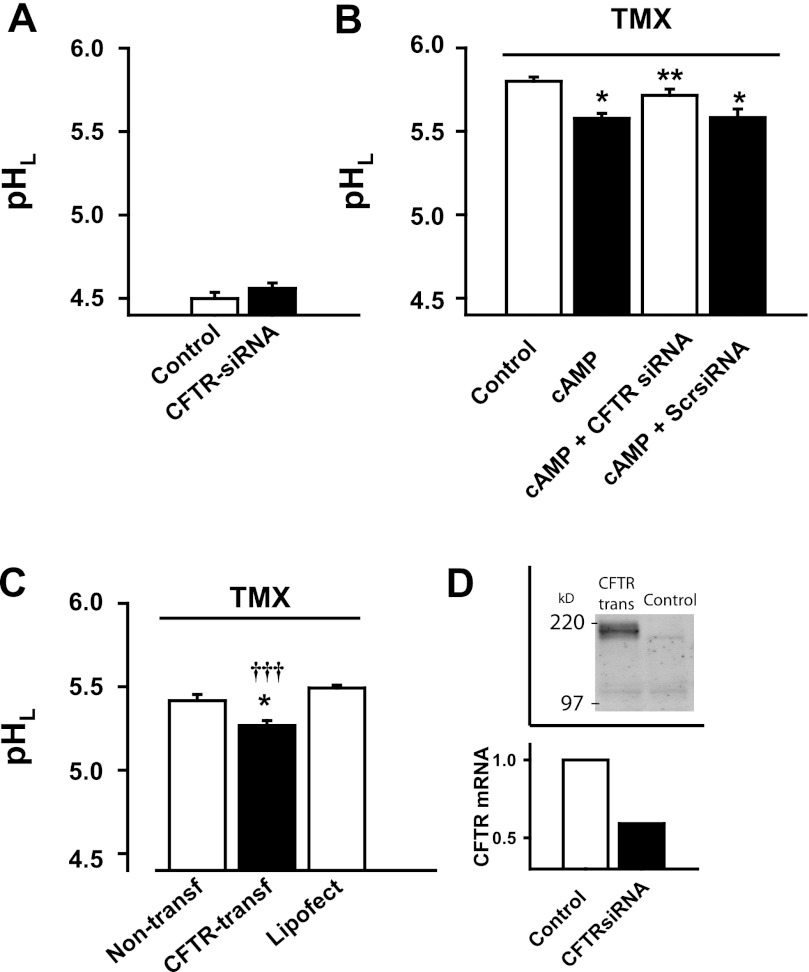
Fig. 3.
Molecular manipulation of CFTR alters lysosomal pH. A: CFTR small interfering (si)RNA leads to a small but insignificant increase in the baseline lysosomal pH in cultured RPE cells (n = 12). B: siRNA against CFTR blocked the acidifying effect of the cAMP cocktail mix [cAMPmix: 10 μM forskolin, 500 μM cpt-cAMP, and 100 μM IBMX (n = 24) on lysosomal pH in ARPE-19 cells treated with tamoxifen, 30 μM, n = 18]. Scrambled siRNA (Scr-siRNA) transfection did not alter lysosomal pH (n = 12). *P < 0.05 vs. tamoxifen control, **P < 0.05 vs. tamoxifen + cAMP mix. C: RPE cells that overexpressed CFTR (CFTR-transf) demonstrated lower lysosomal pH when exposed to tamoxifen (30 μm), compared with either the nontransfected (non-transf) or empty vector-transfected (Lipofect) cells (n = 3). *P < 0.05 vs. non-transf, †††P < 0.001 vs. Lipofect. D, top: Western blot showing light band of CFTR in control, with increased expression after transfection with plasmid. D, bottom: quantitative PCR indicating decrease in mRNA for CFTR after treatment with siRNA.
Direct effects of CFTR on lysosomal membrane.
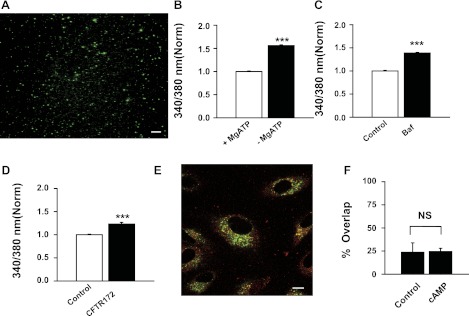
Although activation of CFTR clearly had an acidifying effect on lysosomal pH, the above experiments performed in intact cells could not discriminate between CFTR acting directly on the membrane and CFTR on the plasma membrane acting to modify lysosomal function through indirect means. To determine whether CFTR functioned on the lysosomal membrane, vesicles were isolated and loaded with LysoSensor Yellow/Blue dye (Fig. 4A). While the identity of these vesicles may not be exclusively lysosomal, several controls are consistent with them as acidic lysosomal-like organelles. For example, the pH of the isolated lysosomes was lower in the presence of 5 mM ATP than in its absence (Fig. 4B), consistent with the presence of a vHATPase on the isolated vesicles. As such, ATP was included in subsequent experiments. The pH was elevated by 0.5 μM of the proton pump inhibitor bafilomycin (Fig. 4C), confirming that constitutive activity of the vHATPase lowers the pH in the isolated vesicles. To evaluate the contribution of CFTR to this pH, vesicles were bathed in ATP with and without CFTRinh-172. CFTRinh-172 alkalinized the isolated lysosomes (Fig. 4D). This suggested that CFTR was functioning on the isolated membrane.
Fig. 4.
pH regulation isolated lysosomes from ARPE-19 cells. A: lysosomes isolated from ARPE-19 cells and stained with LysoSensor Yellow/Blue (bar, 10 μm). B: the pH in isolated lysosomes was determined from the ratio of light excited at 340 vs. 380 nm in cells loaded with LysoSensor dye; this ratio provides an index of the pH value in the absence of calibration. The pH ratio was lower in the presence of 5 mM ATP (+ MgATP) than without (− MgATP) (n = 12). C: bafilomycin A (Baf, 0.5 μM, n = 12) increased the pH of isolated lysosomes; together these indicate vacuolar proton pump vHATPase activity. D: CFTRinh-172 also elevated the pH ratio (10 μM, n = 23). E: colocalization of Lamp1 and CFTR in RPE vesicles. The ARPE-19 cells were double-immunostained with anti-CFTR (green) and anti-Lamp1 (red) antibodies (bar, 10 μm). F: cAMP treatment did not increase overlap between CFTR and Lamp1. Analysis of images obtained using confocal microscopy indicated that the percentage of pixels staining green for CFTR that also stained yellow for Lamp1 was 23.7 ± 9.2% in control and 24.5 ± 3.5% after exposure to the cAMP-cocktail (10 μM forskolin, 500 μM cpt-cAMP, and 100 μM IBMX) for 30 min at 37°C (n = 5 for both). NS, not significant. ***P < 0.001.
ARPE-19 cells expressed both CFTR and Lamp1 in organelle-type structures; both antigens were expressed alone and together, suggesting that at least some of the CFTR in RPE cells is associated with acid vesicles (Fig. 4E). However, colocalization was not increased by treating the cells with cAMP (Fig. 4F). It should be stressed that while colocalization of CFTR on the lysosomal membrane is supportive, many proteins eventually end up in the lysosome, and the resolution of light microscopy cannot distinguish between CFTR present on the lysosomal membranes or in the lumen awaiting degradation. However, the functional data on isolated lysosomes implies that CFTR is not only present, but also active in the membrane of isolated lysosomes.
CFTR contributes to lysosomal clearance.
The regulation of lysosomal pH is likely of great importance to RPE cells as many degradative lysosomal enzymes function maximally at a pH between 4 and 5. The lysosomal environment is critical for activity of these enzymes, so lysosomal enzyme activity cannot be determined using standard lysed cell assays where cells are ruptured and enzyme activity measured in a tube. As RPE cells are responsible for the degradation of photoreceptor outer segments in vivo, the contribution of CFTR to this degradation was determined. Cells were incubated with calcein-labeled outer segments for 2 h, followed by a 2-h wash to enable internalization. Images taken after this 2-h chase period indicated that the majority of outer segments colocalized with LysoTracker Red at this point (Fig. 5A). Baseline fluorescence was determined at this point, t = 0. Drugs shown above to alter lysosomal pH were then added, and the fluorescence remaining 20 h later was determined (t = 20, Fig. 5B). The effect of drugs added at this point would primarily reflect their effect on outer segment degradation, as most outer segment binding, internalization, and delivery to the lysosomes has already occurred.
The majority of outer segment fluorescence was eliminated after 20 h in control conditions; remaining fluorescence was <20% of that present at baseline. However, CFTRinh-172 increased the amount of fluorescence remaining in a concentration-dependent fashion, with 3 μM and 10 μM CFTRinh-172 leading to a significant increase in fluorescence (Fig. 5C). Tamoxifen reduced the rate of outer segment clearance, but CFTRAct11 enhanced the clearance of fluorescent outer segments, presumably by activation of CFTR (Fig. 5D). This supports the presumption that an elevation in lysosomal pH reduces the efficiency of lysosomal enzymes, and it implies that CFTR promotes outer segment degradation/clearance by lysosomes.
CFTR activation lowers lysosomal pH in RPE from ABCA4−/− mice.
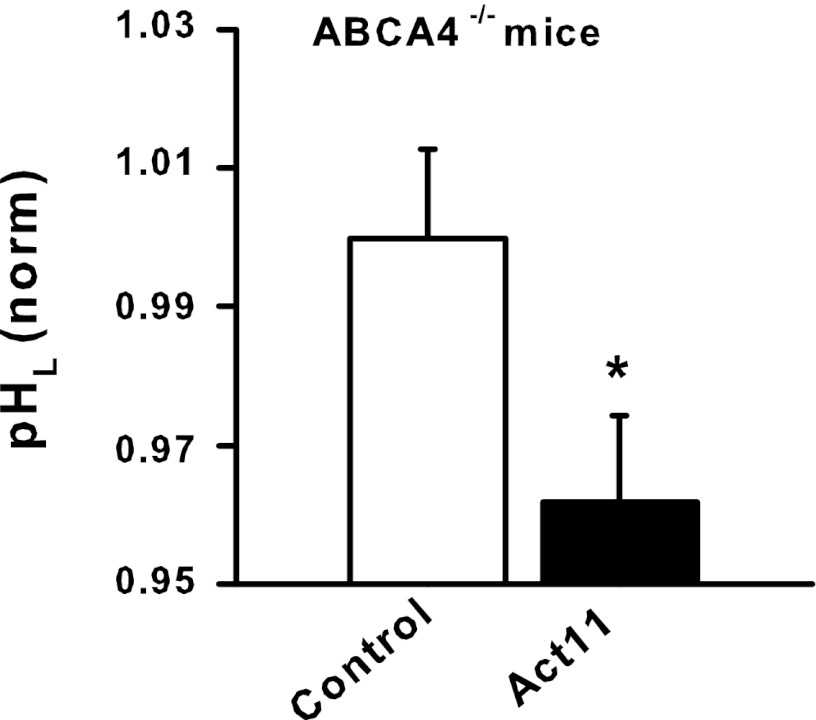
As the findings above suggest that CFTR may be well suited to lower lysosomal pH in conditions where the pH was pathologically elevated, the effects of CFTR activation on RPE cells from the ABCA4 −/− mouse were examined. The ABCA4 gene is mutated in Stargardt's disease, and RPE cells in the mouse have high levels of A2E (1, 26). A2E raises the lysosomal pH of cultured RPE cells (15), and the lysosomal pH of RPE cells from ABCA4 −/− mice increases with animal age (20). We thus asked whether stimulation of CFTR could lower lysosomal pH in RPE cells from ABCA4−/− mice. Stimulation of CFTR with CFTRAct11 lowered the lysosomal pH in RPE cells from ABCA4−/− mice (Fig. 6). Of note, these eyes were from elderly mice, with a mean age of 465 days. This suggests that activation of CFTR may be effective even with older individuals where the lysosomes have been subjected to damages for a prolonged time.
Fig. 6.
Lysosomal pH was decreased in RPE cells from ABCA4−/− mice. pH values were normalized to the mean control of each day's measurements to control for experimental variation; absolute pH levels dropped from 6.3 to 5.9. Data represent lysosomal pH measurements from 9–11 wells each from 10 eyes pooled and processed in 2 separate measurements. Eyes were from older animals with a mean age of 465 ± 49 days. *P < 0.05 vs. control.
DISCUSSION
The identification of CFTR as a component of the acidification mechanism in lysosomal-like organelles of RPE cells is supported by multiple observations. First, CFTR inhibitors glibenclamide and CFTRinh-172 led to a small increase in lysosomal pH. When cAMP was used to restore acidity to lysosomes treated with chloroquine or tamoxifen, this lysosomal reacidification was also inhibited by CFTRinh-172. Of note, CFTRinh-172 was effective in cells treated with both tamoxifen and chloroquine, suggesting that the CFTR is involved in a general acidification that is independent of the method used to alkalinize the cells. The ability of CFTR activators CFTRAct16 or CFTRAct11 to acidify the lysosomes of RPE cells strengthens a role for CFTR. CFTRAct16 and CFTRAct11 are more selective activators that were identified using an elegant high-throughput screening approach (23). For instance, CFTRAct16 activated cloned CFTR in Fisher rat thyroid cells, with an approximate EC50 of 200 nM, compared with 500 nM for CFTRAct11, although the former also elevated cAMP. The increased acidification of RPE lysosomes by CFTRAct16 compared with CFTRAct11 is consistent with their relative effects on the cloned CFTR, while the acidification of these organelles by CFTRAct11 implies that the actions may be specific for CFTR. It should be noted that in contrast to the experiments on cloned CFTR, the experiments here were performed in the absence of forskolin, so CFTR activation in RPE cells presumably relies on endogenous levels of cAMP. The overexpression of CFTR acidified lysosomes, while, lastly, the acidifying effects of cAMP were inhibited by CFTR depletion by siRNA. Together, these results provide strong molecular and pharmacological evidence that CFTR contributes to the acidification of lysosomes of RPE cells.
CFTR and organelle pH.
A considerable controversy exists regarding a possible contribution of CFTR to organelle acidification. While defective acidification of intracellular vesicles was originally proposed to contribute to the pathology of cystic fibrosis nearly two decades ago (2), subsequent studies were unable to substantiate a role for CFTR in this process, suggesting that the contribution may vary with cell type and organelle (31, 35). A role for CFTR in vesicle acidification has once again been proposed in recent years, attracting an even more enthusiastic denouncement. Lysosomes were alkalinized in macrophages from CFTR−/− mice and by CFTRinh-172 (8). Using FITC-conjugated probes and dextran-conjugated pHrodo/rhodamine green, lysosomal alkalinization was shown in alveolar macrophages from CFTR−/− mice and, to a lesser extent, in alveolar macrophages from cystic fibrosis mice harboring the ΔF508 and G551D mutants (12). The absence of CFTR impeded phagolysosome fusion (24). However, several other groups were unable to identify a role for CFTR in vesicle acidification (13, 18, 20, 22).
The substantial data from groups both supporting and denying a role for CFTR in organelle acidification suggest that the mechanisms which regulate CFTR's contribution to this process may be quite complex. It is thus likely that the small experimental differences that lead to disparate results actually hold clues to unravel this mystery (21). Cellular differences are clear, with alveolar macrophages displaying a role for CFTR while peritoneal macrophages do not, even in the same experimental system (12). The relative distribution of proton and anion transporters likely varies, with anion influx the rate-limiting step for organelle acidification in some cells, while the non-CFTR counter ion permeability is more substantial (and not limiting) in other cell types (20). Use of CFTRinh-172 reconstituted from frozen as a stock solution in DMSO was significantly less effective than freshly dissolved inhibitor (12). It is also clear that single wavelength probes are problematic given a relationship between pH and vesicle size (22). Probes based on FITC are also of questionable value in measuring the pH of lysosomes, given the pKa of 6.2. The use of pH-sensitive probes selectively attached to CFTR and delivered to organelles through the endocytosis of CFTR is an ideal technique to assess the role of CFTR in recycling endosomes (20), but it may not provide the best assessment of lysosomal pH if CFTR arrives via an alternate route such as straight from the Golgi. Likewise, bulky accessory compounds such as Zymosan or dextran may deliver the pH-dependent probes to one subgroup of organelles, while lysosmotropic probes such as LysoSensor Yellow/Blue may preferentially target a different subgroup. As lysosomes may well be derived from multiple pools (5), the apparent contradictions in regard to an intracellular role of CFTR might actually identify functional differences in subgroup composition. In addition, additional pathways activated by cAMP may also contribute to organelle acidification.
In this regard, our demonstration that the CFTR contributes little to the baseline levels of lysosomal acidity but aids in the regulated acidification in response may explain some of the discrepancy. The alkalinizing effect of CFTRinh-172 was larger in compromised cells treated with cAMP than under baseline conditions, and the effect of activating CFTR was greater in alkalinized cells (Fig. 2). The most likely explanation for this is the reduced open probability of the CFTR channel in acidic solutions. Single channel recording indicated that the open probability of CFTR dropped by 47% when the extracellular pH was decreased from 7.4 to 4.5 (32). While this decreased open probability is consistent with the enhanced contribution of CFTR in more alkaline conditions, other factors may contribute. For example, CFTR may make a larger contribution in cells treated with cAMP, or Cl− influx could be more rate-limiting after treatment with weak bases.
The ability of antagonist CFTRinh-172 to directly raise the pH in isolated organelles (Fig. 4) provides important mechanistic support for the model by which CFTR residing on the lysosomal membrane enables Cl− influx to counterbalance the positive charge accompanying proton import (2). While activation of CFTR on the plasma membrane could increase cytoplasmic Cl− sufficiently to increase the driving force for Cl− into the organelle, localization of CFTR directly on the lysosomal membrane is predicted to be much more effective given the voltage difference across organelle membrane. When trying to understand the action of isolated lysosomes it may be relevant that adenylate cyclase III is localized to endosomal-like organelles (15), and membrane-bound adenylate cyclase is coupled to A-kinase anchoring proteins (AKAPs) (14). As the magnitude of baseline pH was not determined in isolated lysosomes, it is not clear whether the effect would have been larger on alkalinized lysosomes. Regardless, these experiments do identify a direct contribution of CFTR to the regulation of lysosomal pH. However, the inability of cAMP to alter colocalization of CFTR and Lamp implies that the predominant contribution of cAMP is to the gating of CFTR and not in trafficking of CFTR to the lysosome. However, it is likely that cAMP acts to enhance other parallel pathways of organelle pH regulation.
Physiological implications.
We propose that CFTR does not make a major contribution to the constitutive acidification of lysosomes but plays a more subtle role in rapidly regulating the pH. While the rate-limiting effect of anion counter transport may mask other contributions, it is likely that cAMP also activates alternative mechanisms in parallel to reacidify lysosomes. Members of the ClC family of chloride transporters such as ClC-7 are clearly implicated in lysosomal acidification, while ClC-3, CLC-4, and CLC-5 are involved in endosomal acidification (16, 17). The critical role of organelle acidification in numerous distinct processes may explain the existence of several backup systems. In this case, CFTR may contribute more to receptor-driven acidification in response to certain stimuli than to the basic maintenance to pH. Regardless, this still implies that pharmacological stimulation of CFTR may help boost lysosomal activity under conditions or in disease states where lysosomal pH is compromised. As a primary role of acidic lysosomal enzymes in RPE cells is to degrade photoreceptor outer segments, analysis of outer segment degradation provided quantitation of the functional effects of CFTR manipulation (Fig. 5). The concentration-dependent decrease in outer segment clearance by antagonist CFTRinh-172 strongly implicates a role for CFTR in the normal degradation of outer segments, and the ability of activators CFTRAct16 and CFTRAct11 to enhance turnover of outer segments identifies CFTR as a potential regulatory target.
The ocular response is defective in CFTR−/− mice, with the C-wave, fast oscillations, and light peak reduced (4, 37). While these changes in short-term responses are consistent with presence of a defective Cl− channel in the plasma membrane, examination of RPE cells from older CFTR−/− mice is needed to determine whether they also display effects of lysosomal dysfunction or whether a compensatory mechanism is upregulated. Regardless, the ability of CFTRAct11 to restore lysosomal pH to RPE cells in ABCA4−/− mice suggests that this CFTR anion permeability is still functioning in diseased RPE cells. The mice used in this study were over 15 mo old, and the accumulation of A2E in ABCA4−/− mice will be substantial at this point (20). The actions of CFTRAct11 imply that, despite the sustained insults, the lysosomes retain sufficient CFTR and other transport mechanisms to respond. As such, this identified CFTR activation as a potentially useful target to improve lysosomal clearance in a variety of cells.
GRANTS
This work was supported by National Institutes of Health Grants EY-013434 and EY-015537 (to C. H. Mitchell), EY-017045 (to A. M. Laties), R01 DK-58046 and R01 DK-73185 (to R. C. Rubenstein), and Vision Research Core Grant EY-001583 (to C. H. Mitchell and A. M. Laties), Research to Prevent Blindness (to A. M. Laties), the Paul and Evanina Bell Mackall Foundation Trust (to A. M. Laties), Fight for Sight (to J. Liu and A. M. Laties), and the Jody Sack Fund (to W. Lu).
DISCLOSURES
A. M. Laties and C. H. Mitchell are listed on a patent application that covers some of the material addressed in this article. The IP has not been licensed and no compensation has been received. No other conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L., A.M.L., R.C.R., W.W.R., and C.H.M. conception and design of the research; J.L., W.L., S.G., G.C.B., and E.E.C. performed the experiments; J.L. and C.H.M. analyzed the data; J.L., A.M.L., W.W.R., and C.H.M. edited and revised the manuscript; J.L., W.L., S.G., G.C.B., A.M.L., R.C.R., and W.W.R. approved the final version of the manuscript; W.L., R.C.R., and C.H.M. interpreted the results of the experiments; C.H.M. prepared the figures; C.H.M. drafted the manuscript.
ACKNOWLEDGMENTS
The authors thank Gabriel Travis for the ABCA4−/− mice, HuiLing Hu for assistance with dissections, and the Cystic Fibrosis Foundation therapeutics antibody distribution program. Portions of this work have been previously presented in abstract form (44).
REFERENCES
- 1. Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277: 1805–1807, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature 352: 70–73, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J 18: 562–564, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol 106: 43–50, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem 41: 253–263, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem 274: 18364–18373, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol 35: 119–131, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 8: 933–944, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dulhanty AM, Riordan JR. Phosphorylation by cAMP-dependent protein kinase causes a conformational change in the R domain of the cystic fibrosis transmembrane conductance regulator. Biochemistry 33: 4072–4079, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Faundez V, Hartzell HC. Intracellular chloride channels: determinants of function in the endosomal pathway. Sci STKE 2004: re8, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Geisow MJ, Evans WH. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Exp Cell Res 150: 36–46, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem 282: 31422–31428, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Haggie PM, Verkman AS. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J Biol Chem 284: 7681–7686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall MO, Abrams TA, Mittag TW. The phagocytosis of rod outer segments is inhibited by drugs linked to cyclic adenosine monophosphate production. Invest Ophthalmol Vis Sci 34: 2392–2401, 1993 [PubMed] [Google Scholar]
- 15. Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci 40: 737–743, 1999 [PubMed] [Google Scholar]
- 16. Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 578: 633–640, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104: 205–215, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Lamothe J, Valvano MA. Burkholderia cenocepacia-induced delay of acidification and phagolysosomal fusion in cystic fibrosis transmembrane conductance regulator (CFTR)-defective macrophages. Microbiology 154: 3825–3834, 2008 [DOI] [PubMed] [Google Scholar]
- 19. LaVail MM. Rod outer segment disc shedding in relation to cyclic lighting. Exp Eye Res 23: 277–280, 1976 [DOI] [PubMed] [Google Scholar]
- 20. Liu J, Lu W, Reigada D, Nguyen J, Laties A, Mitchell C. Restoration of lysosomal pH in RPE cells from cultured human and ABCA−/− mice: pharmacological approaches and functional recovery. Invest Ophthalmol Vis Sci 49: 772–780, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu W, Reigada D, Sevigny J, Mitchell CH. Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells. J Pharmacol Exp Ther 323: 157–164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem 277: 37235–37241, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res 28: 277–284, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Massey AC, Zhang C, Cuervo AM. Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol 73: 205–235, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci 42: 1685–1690, 2001 [PubMed] [Google Scholar]
- 27. Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA 97: 7154–7159, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim Biophys Acta 853: 237–265, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt KU, Schraermeyer U. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Res 38: 83–88, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Schneider DL. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem 256: 3858–3864, 1981 [PubMed] [Google Scholar]
- 31. Seksek O, Biwersi J, Verkman AS. Evidence against defective trans-Golgi acidification in cystic fibrosis. J Biol Chem 271: 15542–15548, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Sherry AM, Cuppoletti J, Malinowska DH. Differential acidic pH sensitivity of ΔF508 CFTR Cl− channel activity in lipid bilayers. Am J Physiol Cell Physiol 266: C870–C875, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Sun-Wada GH, Wada Y, Futai M. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct 28: 455–463, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Vais H, Zhang R, Reenstra WW. Dibasic phosphorylation sites in the R domain of CFTR have stimulatory and inhibitory effects on channel activation. Am J Physiol Cell Physiol 287: C737–C745, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Van Dyke RW, Root KV, Schreiber JH, Wilson JM. Role of CFTR in lysosome acidification. Biochem Biophys Res Commun 184: 300–305, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Weng TX, Godley BF, Jin GF, Mangini NJ, Kennedy BG, Yu AS, Wills NK. Oxidant and antioxidant modulation of chloride channels expressed in human retinal pigment epithelium. Am J Physiol Cell Physiol 283: C839–C849, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Wu J, Marmorstein AD, Peachey NS. Functional abnormalities in the retinal pigment epithelium of CFTR mutant mice. Exp Eye Res 83: 424–428, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]