Abstract
Large conductance (BK) calcium activated potassium channels (Slo) are ubiquitous and implicated in a number of human diseases including hypertension and epilepsy. BK channels consist of a pore forming α-subunit (Slo) and a number of accessory subunits. In hair cells of nonmammalian vertebrates these channels play a critical role in electrical resonance, a mechanism of frequency selectivity. Hair cell BK channel clusters on the surface and currents increase along the tonotopic axis and contribute significantly to the responsiveness of these hair cells to sounds of high frequency. In contrast, messenger RNA levels encoding the Slo gene show an opposite decrease in high frequency hair cells. To understand the molecular events underlying this paradox, we used a yeast two-hybrid screen to isolate binding partners of Slo. We identified Rack1 as a Slo binding partner and demonstrate that PKC activation increases Slo surface expression. We also establish that increased Slo recycling of endocytosed Slo is at least partially responsible for the increased surface expression of Slo. Moreover, analysis of several PKC phosphorylation site mutants confirms that the effects of PKC on Slo surface expression are likely indirect. Finally, we show that Slo clusters on the surface of hair cells are also increased by increased PKC activity and may contribute to the increasing amounts of channel clusters on the surface of high-frequency hair cells.
Keywords: large conductance channels, receptor for activated protein kinase C, surface expression and kinetics, Slo
large conductance (bk) potassium channels are ubiquitous and have been implicated in a number of diseases including hypertension, epilepsy, movement disorders, and deafness (16, 17, 19, 35–37). In auditory hair cells of nonmammalian vertebrates BK channels play a critical role in electrical tuning, a mechanism of frequency selectivity (4–7, 18, 20, 23). Electrical tuning occurs when the intrinsic oscillation in membrane potential coincides with the frequency of sound to which that hair cell best responds (characteristic frequency) (15). In hair cells responding to increasingly higher frequency of sound there is a progressively higher membrane oscillation frequency (20). The intrinsic oscillation in membrane potential is brought about by an inward depolarizing Ca2+ current and the consequent activation of an outward K+ current carried through large conductance Ca2+-activated K+ channels (7, 20). The faster oscillation in membrane potential in higher frequency hair cells is brought about by an increasing density of channels and by faster kinetics of the BK channel (7, 20, 23, 25).
Most BK channels exist at the basolateral aspect of hair cells, where they cluster and colocalize with L-type voltage gated Ca2+ channels (25, 26, 40). Currents carried by BK channels and the number of BK channel clusters increase in high-frequency hair cells (7, 40). The increase in channel clusters is at variance with the amounts of Slo transcripts that show an opposite decrease in high-frequency hair cells (21, 32). The seeming paradox between mRNA and Slo surface expression gradients along the tonotopic axis is likely to be mediated by complex mechanisms. For instance, work from the Hudspeth and Duncan labs have shown that some of this paradox could be explained by alternative splicing with COOH-terminal isoforms at the low frequency end having retention signals that prevent surface expression (28, 32). Similarly, we have shown β1 and β4 subunits that are expressed in low-frequency hair cells to decrease surface expression of Slo in these hair cells (9). Furthermore, we and others have shown phosphorylation to affect Slo surface expression (8, 11, 43). Both CDK5 and GSK3 bind to Slo and decrease its surface expression. CDK5 mediates its effects by direct phosphorylation while the effects of GSK3 phosphorylation are also mediated by interactions with β-catenin, which, in turn, promotes Slo surface expression (8, 11, 43). In other work we have inferred gradients in kinase activity along the tonotopic axis with increasing PKA in low-frequency hair cells and increasing PKC activity in high-frequency hair cells (21). In seeking explanations for the gradients in BK channel surface expression along the tonotopic axis, we identified the WD40 adapter protein receptor for activated PKC (Rack1) as a binding partner of Slo using a yeast two-hybrid screen. Here we explore the role of PKC on the surface expression of Slo.
MATERIALS AND METHODS
Materials.
PMA and bisindolylmaleimide I (BIM1) were purchased from Calbiochem, BAPTA/AM was purchased from Santa Cruz, and monensin (4-[2-[5-ethyl-5-[5-[6-hydroxy-6-(hydroxymethyl)-3,5-dimethyl-oxan-2-yl]- 3-methyl-oxolan-2-yl]oxolan-2-yl]- 9-hydroxy-2,8-dimethyl-1,6-dioxasp iro[4.5]dec-7-yl]-3-methoxy-2-methyl-pentanoic acid was purchased from Biolegend. Stock solutions of all chemicals were prepared in DMSO, stored at −20°C, and diluted in DMEM right before use.
Yeast two-hybrid.
Yeast two-hybrid experiments were done as described previously (34). Briefly, the COOH terminus of Slo (aa 746-1114) was subcloned into pGBKT7 and used as bait to interrogate a chick cochlea cDNA library in pGAD T7 (Clontech, CA). AH109 cells were serially transfected with both constructs and plated on drop out medium lacking histidine, adenine, tryptophan and leucine. Single colonies were isolated and serially replated in similar conditions on plates containing X-Gal. Cultures from single isolated colonies were used for yeast mini preps. Plasmid was electroporated into DH101 Escherichia coli, grown in ampicillin and plasmid DNA isolated for sequencing.
DNA constructs (constructs information).
Chicken Slo cDNA tagged with FLAG (NH2 terminus) and yellow fluorescent protein (YFP-COOH terminus) was subcloned in frame into pcDNA6B vector using NheI and NotI restriction enzymes. Rack1 cDNA was subcloned using XhoI and EcorI restriction enzymes and inserted in-frame into the pECFP N1 vector. All constructs were confirmed by DNA sequencing, including the entire coding region.
Cell culture, transfections, and stable cell line generation.
HEK (human embryonic kidney) cells (American Type Culture Collection, ATCC, Manassas, VA) were cultured in DMEM (high glucose) containing 50 units/ml each of penicillin and streptomycin and 10% FBS at 37°C in 5% CO2. Initial transfections of FLAGcSlo-YFP in pcDNA6B were performed using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Stable transfection and positive clones were selected in the presence of 8 μg/ml blasticidin and screened by fluorescence-activated cell sorting.
Coimmunoprecipitations.
Reciprocal immunoprecipitations (IPs) were performed with mouse monoclonal anti-FlagM2 antibody (Sigma) or mouse monoclonal anti-Rack1 B3 (Santa Cruz). Membrane-enriched protein lysates were generated 48 h after transfection with Rack1-CFP into a stable cell line expressing FLAG-cSlo-YFP. For Slo IPs 50 μl anti-Flag M2-agarose was added to the lysates. The mixtures were incubated with constant agitation for 1 h at 4°C. For Rack1 IPs 500 μl of cell lysates were incubated with 5 μg of Rack1 B3 antibody on overhead shaker overnight. The immune complexes were captured using protein G-agarose columns loaded with 25 μl of slurry and incubated with end-over mixing for 1 h. After the binding step, the beads were washed three times with washing buffer. To elute the proteins of interest 80 μl of 2× sample buffer was added to the resin. The samples were boiled for 5 min and collected by centrifugation. The eluted samples were separated on a precast 4–15% Tris-Glycine SDS-PAGE gel (Bio-Rad). Proteins were transferred by wet transfer to polyvinylidene fluoride membrane (Roche). Western blots were probed with anti-FLAG antibody (M2, 1:1,000) followed by goat anti-mouse antibody-peroxidase conjugate (1:5,000) or mouse Rack1 antibody (BD) (1:2,000), followed by goat anti-mouse IgM (1:2,000), and followed by bovine anti-goat antibody-peroxidase conjugate (1:3,000) for 1 h at room temperature. Immunoreactive proteins were detected by using SuperSignal West Dura Extended Duration Substrate (Thermo scientific/Pierce).
Recycling assay.
To assess the recycling of internalized cSlo channels (31), HEK cells stably expressing FLAG-cSlo-YFP were incubated with anti-FLAG (M2) mouse antibody (Sigma) at 1 μg/ml for 1 h to allow antibody-labeled channels to undergo internalization. After a quick PBS wash, the surface-bound antibody was removed with ice-cold stripping buffer containing 0.5 m NaCl and 0.5% acetic acid (pH 2.7). The cells were then reintroduced back in the culture medium ± PMA for the appropriate time periods (20, 40, and 60 min) to allow the recycling of the antibody-labeled internalized channels. After a brief PBS wash, the cells were briefly fixed with 2% paraformaldehyde in PBS. The surface (recycled) cSlo channels were then labeled with the secondary goat anti-mouse antibody conjugated to Alexa 647 (Invitrogen) for 30 min at RT, washed, and subjected to fluorescence-activated cell sort (FACS) analysis as described below.
In experiments using transferin, FLAG-Slo-YFP expressing HEK cells were incubated with Alexa-647-labeled human transferin for 45 min at 37°C. Cells were fixed in 4% paraformaldehyde and visualized using confocal microscopy.
Immunofluorescence detection of Rack1 and Slo.
Immunofluorescence detection including quantitative immunofluorescence was done as described previously (12), with modifications. We used an LSM 510 meta confocal microscope (Zeiss) to obtain images and used the attendant Zeiss LSM software to analyze and extract data. Chickens were euthanized by CO2 asphyxiation, and their cochlea were removed and microdissected. The Institutional Animal Care and Use Committee (IACUC) at Yale University specifically approved this study (protocol number 2010-10439 “Studies on Hair Cell BK Channels”). For whole mount experiments the cochlea were fixed in washed in PBS (×3) and placed in blocking solution (PBS, 1% BSA, 5% horse serum, 0.1% Tween 20). The tissue was washed again in wash buffer (×3) and incubated with a 1:50 dilution of mouse anti-Slo antibody (BD/ Transduction labs) for 1 h at RT. Alexa 488 goat anti-mouse antibody (1:1,000) was added after washing the tissue (×3) and incubated for an additional 1 h. Tissue was then incubated in 1:500 Alexa 546-conjugated anti-Rack1 antibody (Transduction labs) in blocking solution overnight at 4°C. After being washed in PBS, 0.1% Tween 20 (×3), the tissue was mounted in Vectashield (Vector) and viewed using a Zeiss 510 meta confocal microscope. Sixteen bit images were acquired using a 64× water immersion lens (numerical aperture 1.2), with fixed laser settings, a scan rate of 1.6 microseconds per pixel, a pinhole aperture of 1.0 Airy units, and fixed detector gain. Regions of interest of tall hair cells from fixed distances from the apical end of the cochlea were identified, and fluorescence data extracted. We established that the fluorescence intensity was within the linear range and used mean fluorescence density as a measure of protein concentration. Surrounding supporting cells where there is minimal Slo expression were used to subtract background fluorescence. Cells from three individual cochlea were used for these analyses. The specificity of the antibodies was established by Western blots of cell lysates from cells with the respective constructs. Both antibodies identified bands of the expected size with minimal additional bands. For experiments using PMA and monensin, chick cochlea were kept in culture in the presence of PMA, monensin, or DMSO (control) and labeled Slo detected as described previously (11) in hair cells ∼1.5 mm from the apical end.
Fluorescence resonance energy transfer.
HEK cells were transfected with human Slo (hSlo)-YFP and Rack1-CFP (or CFP), and fluorescence resonance energy transfer (FRET) was evaluated 48 h later. For these experiments, cells were fixed in ice-cold acetone, rehydrated, and mounted in Vectashield. Sixteen bit images were acquired with a Zeiss 510 confocal microscope using a 64× water immersion lens (numerical aperture 1.2), with fixed laser settings, a scan rate of 1.6 microseconds per pixel, a pinhole aperture of 1.0 Airy units, and fixed detector gain. Regions of interest were demarcated, and emission spectra from each of these areas were determined using a Zeiss LSM 510 meta detector while exciting at 458 nm and separately at 514 nm. Photobleaching was done at 514 nm with the excitation of the 40W argon laser set at maximum until the YFP emission decreased 50% (∼20 s). To minimize photo damage and bleaching of adjacent sections in the z-axis the aperture was kept at 1 Aiery unit. Immediately after bleaching, the emission spectra from the regions of interest were again recorded while exciting at 458 nm. The amounts of emission in the CFP window (474-485) before and after photobleaching were quantified using the LSM 510 software and used to calculate FRET efficiency. The percentile efficiency of FRET was defined as fret efficiency (percentile) = (Ea − Eb)/Ea × 100, where Ea and Eb are the emissions of CFP (at 474-494 nm) after and before photobleaching, respectively (27).
FACS analysis.
To investigate whether the activation or inhibition of PKC influence the membrane surface expression of cSlo, the cells were treated with the chemicals listed below, and the effects were analyzed by FACS. The constructs used for these experiments had an (extracellular) epitope tagged to its NH2 terminus (FLAG for chick Slo) and Myc for hSlo HEK cells stably expressing FLAG-cSlo-YFP were preincubated in culture media lacking serum for 3 h and treated with either 15 nM PMA (PKC activator) or 2 μM monensin for 2–4 h. Where 30 nM BIM-1 (PKC inhibitor) and 50 μM BAPTA (Calcium chealator) were used, the cells were pretreated with these chemicals at the indicated concentrations for 30 min before PMA was added. After the incubation, live cells were harvested, fixed in 1% formaldehyde, and stained with anti-FLAG (M2) mouse antibody (Sigma) in PBS at 4°C. After a brief wash with PBS, the secondary goat anti-mouse antibody conjugated to Alexa 647 (Invitrogen) was used. After another PBS wash cells were briefly fixed in 1% paraformaldehyde. FACS was performed on a two laser Calibur (BD) machine using the supplied software. FACS data were subsequently analyzed with FlowJo software (Tree Star, Ashland, OR).
Transient transfections of hSlo and its phosphorylation site mutants were used for FACS analysis as previously described. We determined surface expression of control and mutants by using a live staining method. Briefly, live cells were harvested, fixed, and incubated with anti-Myc conjugated with Alexa 647 (Cell Signaling) in PBS and 1% BSA at room temperature for 30 min. The cells were then fixed in 1% paraformaldehyde before analysis on a Facscalibur machine (Becton-Dickinson).
To control for changes in intracellular expression of Slo channels affecting surface expression levels in specific phosphorylation mutants of Slo, we also determined total expression of Slo by intracellular staining for Slo in the same batch of cells. In brief, harvested cells were fixed and permeabilized by perm/fix buffer (BD Biosciences) at 4°C for 15 min. The cells were then incubated with mouse anti-Slo-antibody directed against its intracellular COOH terminus (Cat No.: 611248 BD Biosciences) at a concentration of 1 μg/ml in PBS and 1% BSA at 4°C. The primary antibody was detected in turn with a secondary anti-mouse antibody conjugated to Alexa 647. The cells were fixed briefly in 1% paraformaldehyde before analysis. FACS analysis was carried out with FlowJo software (Tree Star, Ashland, OR) as described previously (33). Because instrument settings could vary between experiments, we normalized all data to surface-labeled hSlo expression and separately intracellular hSlo expression. Relative surface expression was determined by dividing the mean fluorescence intensity of surface-labeled Slo by the mean fluorescence intensity of total labeled Slo.
Data analysis.
All results are given as means ± SE. Where appropriate, ANOVA was used to test for significance in differences.
RESULTS
Rack1 interacts with Slo.
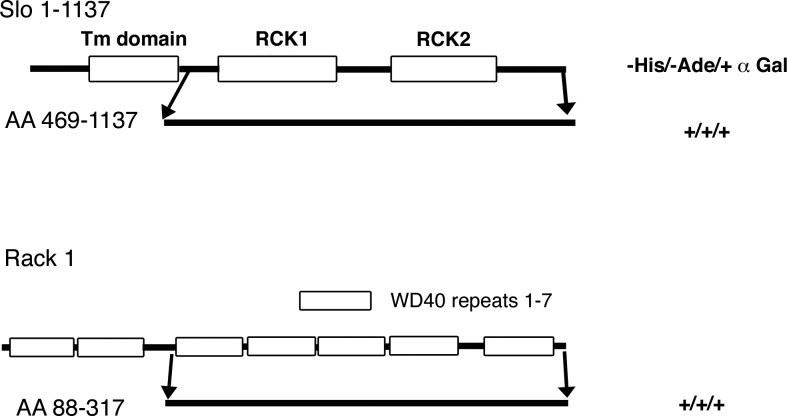
We used yeast two-hybrid screening to identify Slo interacting proteins (Fig. 1). For these experiments we used the COOH terminus of Slo from amino acids 746-1114 (NP_989555.1KCNMA1). Yeast AH109 cells were sequentially transformed with Slo in the pGBKT7 vector and then with a chick cochlea cDNA library in the PGADT7 vector. Double transfectants were then plated on dropout medium lacking tryptophan, leucine, histidine, and adenine (-Trp/-Leu/-His/-Ade). We identified several clones (27) of which three contained a partial cDNA sequence for Rack1 (Fig. 1). Rack1 is an adaptor protein that targets PKC to its targets, but recent data have also shown that Rack1 can affect physiological functions independent of PKC (1). All three of the clones contained the identical cDNA sequence, suggesting that they were derived from the same clone before amplification of the cDNA library. The cDNA encoded amino acids 88-317 of chick Rack1 (gene id 417044 GNB2L1).
Fig. 1.
Slo interacts with receptor for activated PKC (Rack1) in yeast 2-hybrid experiments. The schematic figure shows the COOH-terminal region of Slo used for the yeast 2-hybrid experiments. The region extends from amino acid 746-1114 and includes the RCK domains 1 and 2 immediately after the transmembrane domain. Also shown is a schematic of chick Rack1 including the amino acids 88-317 identified in the yeast 2-hybrid experiment. The interaction of these regions occurred under stringent conditions (-His/-Ade/+α-galactosidase).
We sought to confirm the interaction between Slo and Rack1, since yeast two-hybrid screens can identify nonspecific interactions (particularly in nonstringent conditions, which, however, did not apply in our particular instance). We used reciprocal immunoprecipitation after transfection of Rack1-CFP into a stable cell line expressing FLAG-Slo-YFP to confirm interactions between Slo and Rack1. As shown in Fig. 2, we were able to reciprocally immunoprecipitate both proteins, confirming a strong interaction between the two proteins.
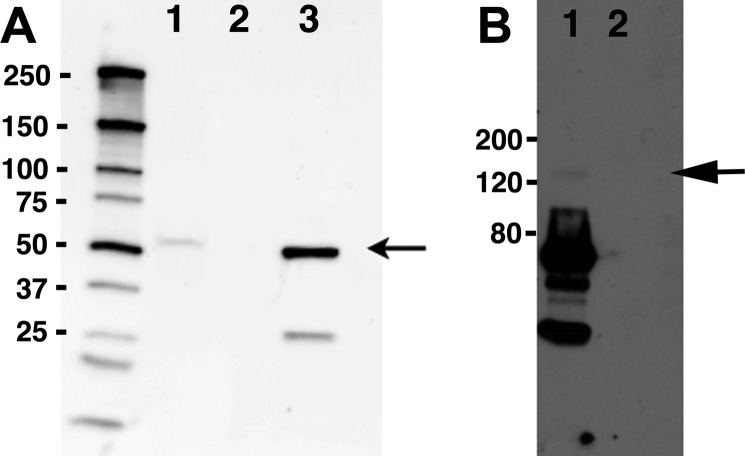
Fig. 2.
Reciprocal immunoprecipitation experiments confirm an interaction between cSlo and Rack1. Rack1 was immunoprecipitated with Rack1 (B3) antibody (Santa Cruz) from lysates of a stable cell line expressing FLAG-cSlo-yellow fluorescent protein (YFP) transfected with Rack-1-CFP. Slo was immunoprecipitated using FLAG M2 antibody (Sigma). The immunoprecipitates were separated on SDS-PAGE gels, and the reciprocal protein was detected by Western blotting. A: imunoprecipitates of FLAG contain Rack1-CFP [arrow 62 kDa (35 kDa RACK1 + 27 kDa CFP)] detected using anti-Rack1 antibody (lane 1). Immunoprecipitates using mouse serum served as a negative control (lane 2). The lysates from stable cell line expressed FLAG-cSlo-YFP (lane 3) and served as the positive controls. The molecular weight marker sizes are shown on the left. B: immunoprecipitates of Rack contain FLAG-cSlo-YFP (arrow) detected using anti-FLAG M2 antibody (Sigma) (lane 1). Immunoprecipitates using mouse serum served as a negative control (lane 2). Protein molecular weight markers are indicated on the left.
We also sought to determine if the interaction occurred in more physiological conditions. We used FRET to confirm interactions between the two proteins in the cell. We transfected Rack1-CFP (the entire open reading frame of Rack1 to which CFP was fused at the very COOH terminus of Rack1) into a permanent cell line expressing Slo-YFP (the entire open reading frame of Slo to which YFP was fused at the COOH terminus of Slo). We used acceptor photobleaching to confirm FRET. As shown in Fig. 3, acceptor (YFP) photobleaching resulted in an increase in donor (CFP) fluorescence in cells expressing Slo-YFP and Rack1-CFP. In contrast, cells expressing Slo-YFP and CFP showed a decrease in donor fluorescence after acceptor photobleaching. These data confirm protein-protein interactions between Slo and Rack1.
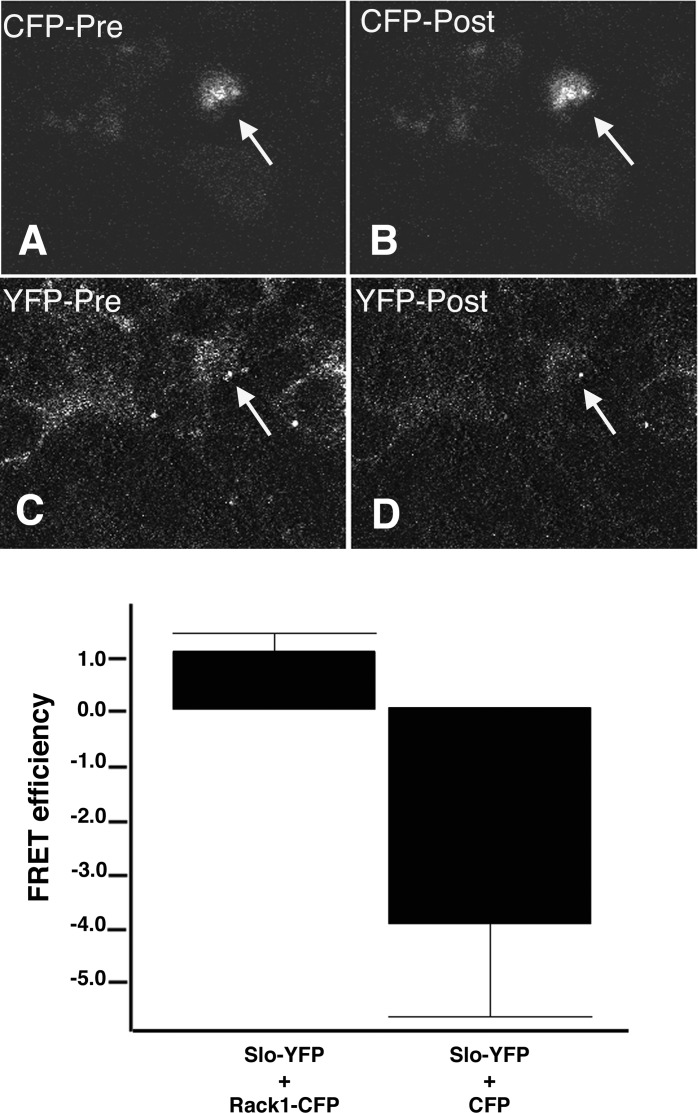
Fig. 3.
Fluorescence resonance energy transfer (FRET) confirms cellular interactions between Slo and Rack1. CFP and YFP fluorescence detected with a LSM510 confocal with a meta detector of a stable cell line expressing Slo-YFP transfected with Rack1-CFP. CFP (A, B) and YFP (C, D) fluorescence before (A, C) and after (B, D) YFP photobleaching is shown. There is an increase in CFP fluorescence that accompanies a decrease in YFP fluorescence after YFP photobleaching (arrow). Shown are the FRET efficiencies of cells expressing Slo-YFP and Rack1-CFP and separately Slo-YFP and CFP. An increase in FRET efficiency after photobleaching is observed in cells expressing Slo-YFP and Rack1-CFP (1.1 ± 0.3 SE, n = 42). In contrast, FRET efficiency decreases after photobleaching in cells expressing Slo-YFP and CFP (−4.0 ± 1.7 SE, n = 15).
We then sought to determine if Slo and Rack1 colocalize in hair cells. For these experiments we used antibody labeling in chick cochlea and then used confocal microscopy to detect the presence of these proteins in hair cells. As shown in Fig. 4, Slo is colocalized with Rack1 in these hair cells. In particular we note the presence of Rack1 in clusters of Slo at the surface of the cell.
Fig. 4.
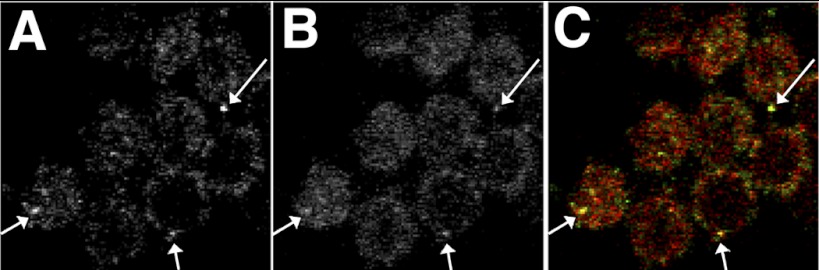
Rack1 and Slo are colocalized on the surface of hair cells. Shown are confocal images of tall hair cells from chick cochlea fixed and labeled with anti-Slo and anti-Rack1 antibodies viewed in the X-Y plane. Slo channels (A) are clustered on the surface of hair cells, where they colocalize (arrows) with Rack1 (B). C: merged images. Mean fluorescence intensity of RACK1 and Slo were 21.2 ± 4.1 SD and 14.4 ± 2.0 SD (arbitrary units), respectively. Colocalization analysis using JACOP plugin in ImageJ gave a Pearson's Coefficient (r) of 0.738 (0.0 below thresholds determined by Coste's automatic thresholds); Manders M1 and M2 coefficients of 0.951 and 0.961, respectively; and Li's intensity coefficient correlation of 0.295, all consistent with highly significant colocalization.
Increasing PKC activity increases Slo surface expression.
We sought to determine the effects of increasing protein kinase activity on Slo surface expression. Recent data have shown increasing PKC activity to bring about variable surface expression of a number of receptors and ion channels (13, 29, 31). For these experiments we used the stable cell line expressing FLAG-Slo-YFP. The FLAG tag at its NH2 terminus lies on its extracellular surface and allows us to identify Slo expressed on the surface of the cell.
Cells treated with the PKC activator PMA at 15 nM had increased surface expression of Slo. Figure 4 shows FACS analysis of nonpermeabilized cells stained with anti-FLAG antibody, which shows increased surface staining of cells (right shift) with PMA treatment (Fig. 5). This effect was partially blocked by cotreatment with 30 nM BIM1 (Fig. 5). BIM1 is a cell permeable inhibitor of conventional (α, β, γ) and novel (δ, η, ϵ, θ) PKC isoforms (3, 13). Moreover, the effect was also largely blocked by treatment with the membrane permeable Ca2+ chelator BAPTA-AM (Fig. 5). These data confirm that the effect of PMA was likely due to activation of one of the Ca2+-dependent (conventional) PKC isoforms (α, β, γ) (3, 13).
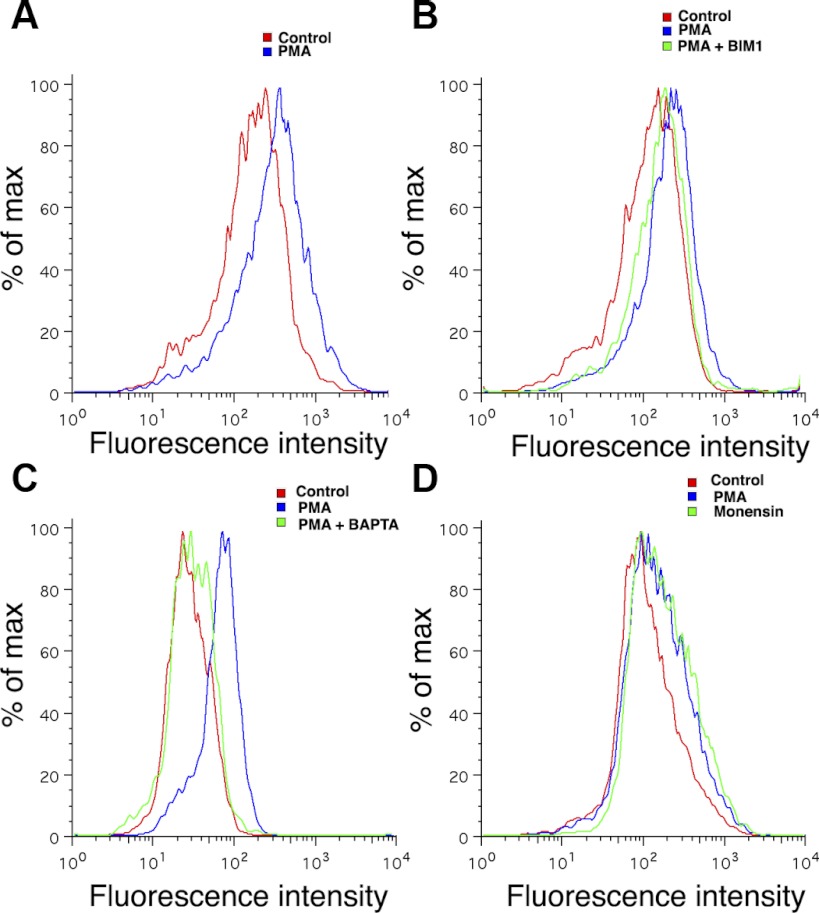
Fig. 5.
Activation of conventional PKC increases Slo surface expression. A: stable human embryonic kidney (HEK) cell line expressing FLAG-Slo-YFP was treated with 15 nM PMA and 0.001% DMSO (control). Cells were fixed after 6 h and labeled with anti-FLAG antibody Alexa 648 and Alexa 648 fluorescence detected by fluorescence-activated cell sort (FACS). There is an increased Slo expressed on the surface of the cell after 6 h of PMA treatment. B and C: effect on increased surface expression produced by PMA is inhibited by bisindolylmaleimide I (BIM1; B) and the cell permeable Ca2+ chelator BAPTA-AM (10 μM; C). These data suggest that PMA activates a conventional PKC isoform (α, β, γ) to produce its effects. D: treatment with monensin resulted in an increase in Slo surface expression similar to that seen after addition of 15 nM PMA. Monensin is an inhibitor of endocytosis at the plasma membrane as well as an inhibitor of the endoplasmic reticulum-Golgi transition.
Increased surface expression of Slo is in part due to membrane recycling.
We sought to determine how increased Slo expression on the surface of the cell was brought about. The increased surface expression was seen within a short period of time and was not accompanied by an increase in total expression of Slo. We sought to determine whether the increased surface expression of Slo brought about by PKC was due to effects on membrane recycling. In initial experiments we determined that addition of 2 μM monensin resulted in an increase in Slo surface expression similar to that seen after addition of 15 nM PMA (Fig. 5). Monensin is a well-known inhibitor of endocytosis at the plasma membrane as well as an inhibitor of the endoplasmic reticulum-Golgi transition (10, 22, 46). Moreover, the effects of monensin and PMA were additive when the two chemicals were used together (data not shown). The latter result suggested that activation of PKC resulted in increased surface expression of Slo at least partially from increased delivery of recycled endosomes.
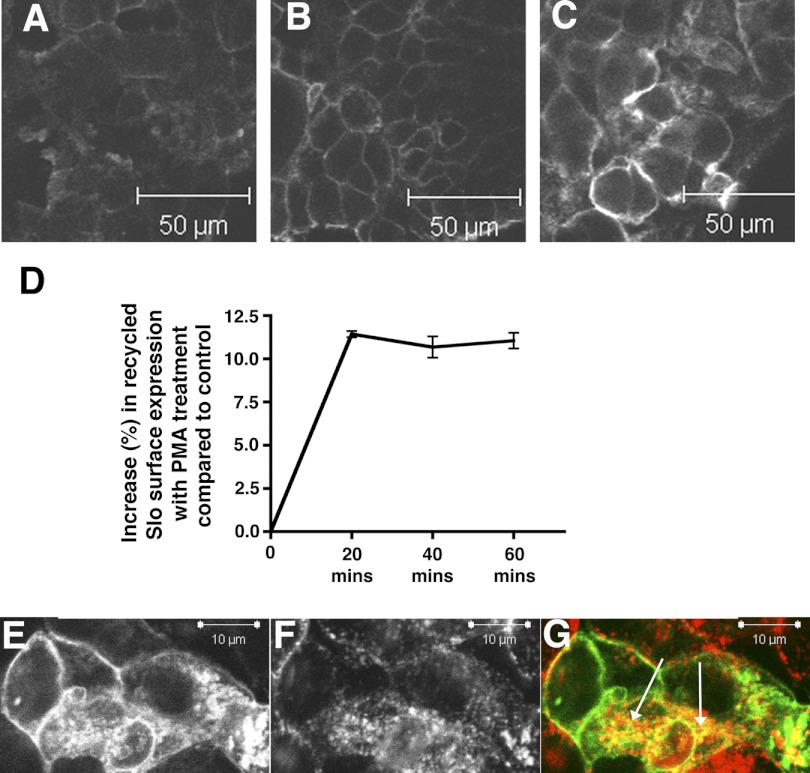
To test the possibility that PKC activation resulted in increased delivery of recycled endosomes we used a recycling assay to determine how PKC activation affected Slo recycling (31). As shown in Fig. 6, there was an increase in endocytosed Slo molecules delivered to the surface of the cell upon treatment with 15 nM PMA. Moreover, we also demonstrate that Slo colocalizes with transferrin receptor confirming that Slo channels are recycled into endosomes (Fig. 6).
Fig. 6.
Increased Slo surface expression in response to PKC activation results in part from increased recycling of endocytosed Slo. To assess the recycling of internalized cSlo channels, the FLAG-cSlo-YFP stably expressing cells were incubated with anti-FLAG antibody for 1 h to allow labeled channels to undergo internalization. The surface-bound antibody was removed with stripping, and the cells were then reintroduced back in the culture medium ± PMA for varying period(s) to allow the recycling of the antibody-labeled internalized (endocytosed) channels. The cells were fixed with paraformaldehyde. The surface (recycled) cSlo channels were then labeled with secondary AB conjugated to Alexa 647 (Invitrogen). A–C: shown are confocal images of HEK cells expressing FLAG-cSlo-YFP that were subject to a recycling assay and demonstrate recycled Slo on the surface of the cell. Cells treated with PMA for 20 min show an increase in recycled Slo (C), compared with comparable control cells at time 0 (A) and at 20 min (B). D: presence of recycled Alexa 647 labeled cSlo channels were quantified by FACS. Shown is the percentile increase in surface labeled cSlo in PMA-treated cells compared with controls. There was an increase in recycled Slo molecules delivered to the surface of the cell upon treatment with 15 nM PMA compared with control at 20 min that sustained itself for over 1 h (mean fluorescence ( ± SE). E–G: shown are confocal images of HEK cells expressing FLAG-cSlo-YFP that were treated with transferrin (50 μg/ml) for 60 min. Cells were fixed and analyzed by confocal imaging. Slo-YFP (E) partially colocalizes with transferrin (F) with the merged pseudo-colored images shown in G (Green-Slo, Red-transferrin, and colocalized proteins in yellow). Arrows indicate internalized transferrin receptor. The images were obtained in the midplane of the cell.
Increased Slo surface expression in response to PKC activation results from an indirect effect and not due to direct phosphorylation of Slo.
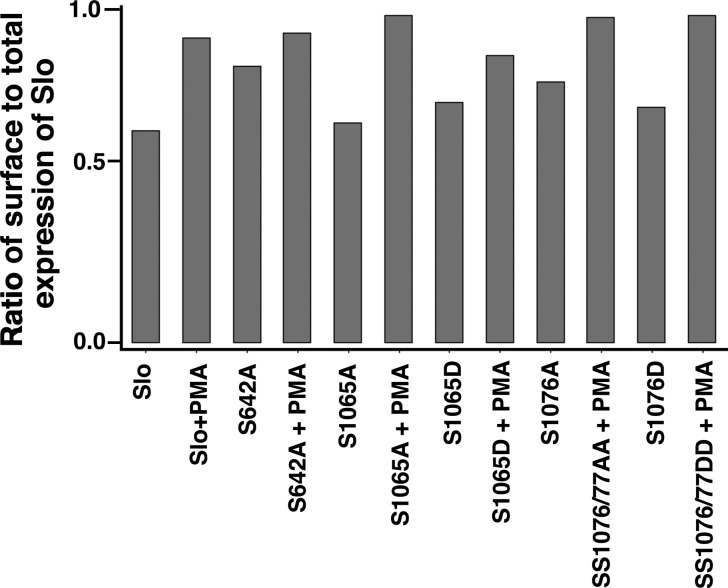
To ascertain if the effects of PKC activation were due to a direct phosphorylation of Slo, we sought to mutate specific PKC phosphorylation sites in Slo and determine whether these had any effect on PMA mediated surface expression. For these experiments we used published data from Dr. Jim Trimmers laboratory that identified specific phosphorylation sites of Slo by mass spectroscopic analysis of Slo purified from rat brain. Of 13 predicted conventional PKC phosphorylation sites [residues T111, S340, S512, S639, S655, S855, T901, S1026, S1065, S1070, S1076, S1077, and S1101 with numbering based on figure in (49)] predicted by two phospho-prediction programs (Netphos 1.0 and GPS 2.1) five residues (655, 855, 1065, 1076, 1077, and 1081) were identified as phosphorylated. One additional residue (642) was identified as a target of PKC ε (ε), which we included in our analysis since BIM1 and BAPTA were able to only partially block the effects of PMA, raising the possibility that some of the PMA effects could be due to unconventional PKCs. Three of these sites (642, 655, and 855) were also identified as potentially phosphorylated by CDK5 and we have presented data previously that phosphorylation of these residues does not increase surface expression of Slo (8). Despite repeated attempts we were unable to make phosphomimetic and phosphodeletional forms of S1081. As before, since the specific phosphorylation sites were initially identified in human Slo, we used hSlo for these experiments. We first made phospho-null mutants and determined whether these mutants showed altered surface expression when treated with PMA. As shown in Fig. 7, all but one of these mutants showed no change in baseline surface expression and all of them responded to PMA treatment with an increase in Slo surface expression. One mutant, S642A, showed a minimally increased surface expression, as we have documented previously (8). Moreover, none of the phospho-mimetic mutations at these sites showed increased surface expression (Fig. 7). Together these data confirm that the effect of PMA treatment is mediated indirectly and not by phosphorylation of Slo [note that 647D shows decreased surface expression consistent with its phosphorylation with CDK5, which decreases Slo surface expression in contrast to PKC (8)].
Fig. 7.
Increased Slo surface expression in response to PKC activation is not due to direct phosphorylation of Slo. The following phospho-null mutants S642A, S1065A, S1076A, and SS1076/77AA were created and tested for Slo surface expression with or without the PMA treatment. All but 1 (S642A) of these mutants showed a change in baseline surface expression, and all of them responded to PMA treatment with an increase in Slo surface expression. In addition the following phospho-mimetic mutants S1065D, S1076/77DD, and S1076D were tested. They also didn′t alter Slo surface expression and responded to PMA treatment with increased Slo expressed on the surface of the cell. These results were replicated in 3 independent experiments.
Slo channel clusters in hair cells increase in response to PKC and show recycling.
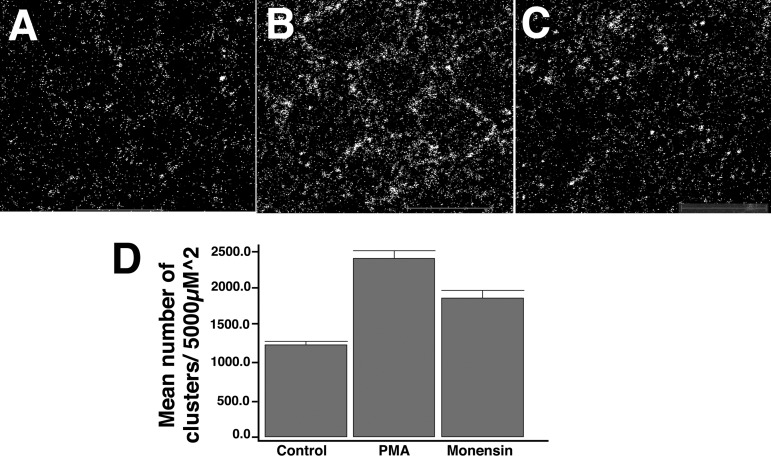
We sought to ascertain if increased PKC activity could bring about an increase in surface expression of BK channels on the surface of hair cells. We treated hair cells in culture with PMA and determined whether there was increased Slo delivered to the surface of the cell. As shown in Fig. 8 hair cells in chick cochlea that were treated with PMA for 3 h showed increased BK channels on their surface evidenced by increased Slo clusters compared with hair cells in untreated cochlea. We have previously noted that Slo channels in hair cells are clustered on the surface of hair cells. Although we were technically unable to determine whether the increase in BK channels in hair cells was due to membrane recycling, we note that monensin produces an increase in hair cell BK channel clusters, suggesting that membrane recycling is an active mechanism in hair cells.
Fig. 8.
Slo channel in hair cells show increased clustering in response to PKC activation. Hair cells in culture were treated with PMA for 3 h and Slo detected by immunofluorescence. Shown are confocal images in the X-Y plane of representative basilar papillae. Hair cells in chick cochlea treated with PMA (B) and monensin (C) show an increase in BK channel clusters compared with hair cells of control cochlea (A). D: shown are the number of BK channel clusters in hair cells from cochlea treated with 100 nM PMA and 2 μM monensin. There is a statistically significant increase in BK channel clusters in hair cells of cochlea treated with PMA and separately monensin compared with controls (P < 0.01 on 1-way ANOVA; n = 3 samples each).
DISCUSSION
In this article we demonstrate two important and interrelated findings. We show that Rack1 interacts with Slo and that PKC induces increased surface expression of Slo likely through effects not mediated by direct phosphorylation of Slo.
The interaction between the two proteins was established with yeast two-hybrid experiments, and subsequently confirmed with reciprocal immunoprecipitations and FRET experiments. These data largely validate a previous study showing an interaction between Slo and Rack1 by yeast two-hybrid experiments (24). That study demonstrated the functional effects of its association with Rack1 on the biophysical properties of Slo.
Our data show a functional effect of PKC on surface expression of Slo. Moreover, we show that the increased surface expression of Slo is mediated in part by increased delivery of recycled Slo. These data are congruent with effects of increased PKC activity on the surface expression of other membrane proteins (13, 29, 31). In one instance the increased surface expression of transient receptor potential vanilloid induced by PKC was mediated by decreased endocytosis (13). We don′t believe this to apply to Slo in HEK cells since we were unable to demonstrate decreased endocytosis of Slo using an endocytosis assay (Bai and Navaratnam, unpublished observations). Moreover, we determined that the addition of PMA and monensin was additive, suggesting an absence of endocytosis of Slo with PMA treatment since monensin brings about increased membrane protein expression largely by decreased endocytosis.
Our data showing sharp colocalization of Rack1 with PKC suggest that Rack1 has a role in mediating Slo function in hair cells. Because Rack1 was first identified as an adaptor protein that mediates interactions between PKC and its target proteins, mediation of PKC effects on Slo seemed to be an obvious effect of its interactions with Slo (38). However, although we were able to demonstrate an effect of PKC activity on Slo surface expression, we were unable to confirm that the effect was mediated by direct phophorylation. None of the phosphomimetic mutations of established PKC phosphorylation sites of Slo showed effects on surface expression that were similar to PMA treatment. Furthermore, PMA treatment of phosphodeletional mutants at all these sites produced an increase in surface expression consistent with an indirect effect. In this context the absence of a direct effect of PKC phosphorylation raises the possibility that Rack1 interactions with other proteins, which mediate other pathways, in turn mediate the surface expression of Slo (1). For instance Rack1 also binds the Src, Fyn, and FAK kinases (as does Slo), and it is possible that the effects on Slo surface expression are mediated through cross talk between PKC affecting any one of these kinase pathways (2, 14, 38, 41, 48). However, an effect of Src, Fyn, and FAK kinases on surface expression of Slo has not been determined. In contrast, we have demonstrated an effect of CDK5 that reduces Slo surface expression by direct phosphorylation (8). It is possible that PKC brings about its effects through affecting CDK5, although these effects are confounding. It is also possible that Rack1 inhibits CDK5 activity although a direct interaction between CDK5 and Rack1 has yet to be demonstrated. Irrespective of these theoretical concerns, it is unlikely that increased Slo surface expression by PMA treatment is brought about by decreased CDK5 activity, since phosphodeletional mutants of CDK5 phosphorylation sites in Slo also show increased surface expression in response to PMA treatment (Bai and Navaratnam, unpublished observation). Finally, more recent data have also shown that Rack1 increases signaling through the planer cell polarity pathway and downregulates Wnt signaling, and we showed that Slo affects Wnt signaling through its interactions with β-catenin (11, 30). Irrespective of these effects, however, it is likely that Rack1 serves as an adapter protein for PKC, owing to the widely demonstrated effects of PKC on the kinetic properties of Slo.
Our data show effects of PKC on increasing Slo surface expression in HEK cells that was paralleled by similar data in hair cells. Because the effects of PKC on Slo surface expression in HEK cells was mediated in part by increased recycling, these data raise the possibility that a similar mechanism operates in hair cells. Our data showing monensin increasing Slo surface expression in hair cells suggest that membrane recycling occurs in hair cells and raise the possibility that PKC brings about increased surface expression of Slo in hair cells by a similar mechanism. Although seemingly obvious as a mechanism that operates in hair cells, our findings nevertheless are the first data supportive of the possibility that membrane recycling occurs in hair cells. In this context it is interesting to note that PKC has been shown to inhibit Slo channel opening by direct phosphorylation of the protein (50, 51). Moreover, conditional phosphorylation of Slo by PKC determines its subsequent susceptibility to both PKA and PKG activation (50, 51). These effects have been observed in both BK channels in vivo as well as heterologously expressed channels (42, 45, 47, 50, 51). Our data on the surface expression of the protein suggest that the effects of PKC are even more complex, on the one hand decreasing currents by a direct effect and yet increasing BK channel expression on the surface of the cell.
In conclusion we show that PKC mediates expression of Slo on the surface of hair cells. This effect is likely an indirect one based on our data in HEK cells. Because of our prior data showing increased PKC activity in high-frequency hair cells, these data raise the possibility that increasing expression of Slo on the surface of high-frequency hair cells is brought about by increasing membrane recycling induced by PKC. Given the widespread expression of BK channels in both neurons and smooth muscle cells and the ubiquitous presence of PKC, it is possible that PKC regulation of BK channel expression on the surface of cells is a general mechanism that therefore influences a number of physiological events (39, 42, 44, 47).
GRANTS
This study is supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC-007894.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., J.-P.B., and D.N. conception and design of research; A.S., J.-P.B., P.J., and D.N. performed experiments; A.S., J.-P.B., and D.N. analyzed data; A.S., J.-P.B., and D.N. interpreted results of experiments; A.S., J.-P.B., and D.N. prepared figures; A.S., J.-P.B., and D.N. drafted manuscript; A.S., J.-P.B., and D.N. edited and revised manuscript; A.S., J.-P.B., and D.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jim Trimmer and Jiushiang Yan for providing us with the hSlo phosphomimetic and phosphodeletional (S655, S855, and SS642) constructs used in the experiments described in the article.
REFERENCES
- 1. Adams DR, Ron D, Kiely PA. RACK1, a multifaceted scaffolding protein: structure and function. Cell Commun Signal 9: 22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA 99: 14560–14565, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J Biol Chem 280: 7729–7738, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Art JJ, Crawford AC, Fettiplace R. Electrical resonance and membrane currents in turtle cochlear hair cells. Hearing Research 22: 31–36, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. Journal of Physiology 385: 207–242, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Art JJ, Fettiplace R, Wu YC. The effects of low calcium on the voltage-dependent conductances involved in tuning of turtle hair cells. Journal of Physiology 470: 109–126, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Art JJ, Wu YC, Fettiplace R. The calcium-activated potassium channels of turtle hair cells. Journal of General Physiology 105: 49–72, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai JP, Surguchev A, Joshi P, Gross L, Navaratnam D. CDK5 interacts with Slo and affects its surface expression and kinetics through direct phosphorylation. Am J Physiol Cell Physiol 302: C766–C780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bai JP, Surguchev A, Navaratnam D. β4-Subunit increases Slo responsiveness to physiological Ca2+ concentrations and together with β1 reduces surface expression of Slo in hair cells. Am J Physiol Cell Physiol 300: C435–C446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berg T, Blomhoff R, Naess L, Tolleshaug H, Drevon CA. Monensin inhibits receptor-mediated endocytosis of asialoglycoproteins in rat hepatocytes. Exp Cell Res 148: 319–330, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Bian S, Bai JP, Chapin H, Le Moellic C, Dong H, Caplan M, Sigworth FJ, Navaratnam DS. Interactions between beta-catenin and the HSlo potassium channel regulates HSlo surface expression. PLoS One 6: e28264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blot V, McGraw TE. Use of quantitative immunofluorescence microscopy to study intracellular trafficking: studies of the GLUT4 glucose transporter. Methods Mol Biol 457: 347–366, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Cha SK, Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294: F1212–F1221, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Chang BY, Conroy KB, Machleder EM, Cartwright CA. RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol 18: 3245–3256, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol (Lond) 312: 377–412, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diez-Sampedro A, Silverman WR, Bautista JF, Richerson GB. Mechanism of increased open probability by a mutation of the BK channel. J Neurophysiol 96: 1507–1516, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37: 733–738, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Duncan RK, Fuchs PA. Variation in large-conductance, calcium-activated potassium channels from hair cells along the chicken basilar papilla. Journal of Physiology 547: 357–371, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113: 1032–1039, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annual Review of Physiology 61: 809–834, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Frucht CS, Uduman M, Kleinstein SH, Santos-Sacchi J, Navaratnam DS. Gene expression gradients along the tonotopic axis of the chicken auditory epithelium. J Assoc Res Otolaryngol 12: 423–435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griffiths G, Quinn P, Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol 96: 835–850, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hudspeth AJ, Lewis RS. A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. Journal of Physiology 400: 275–297, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isacson CK, Lu Q, Karas RH, Cox DH. RACK1 is a BKCa channel binding protein. Am J Physiol Cell Physiol 292: C1459–C1466, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA 91: 7578–7582, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Issa NP, Hudspeth AJ. The entry and clearance of Ca2+ at individual presynaptic active zones of hair cells from the bullfrog's sacculus. Proc Natl Acad Sci USA 93: 9527–9532, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karpova TS, Baumann CT, He L, Wu X, Grammer A, Lipsky P, Hager GL, McNally JG. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc 209: 56–70, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kim JM, Beyer R, Morales M, Chen S, Liu LQ, Duncan RK. Expression of BK-type calcium-activated potassium channel splice variants during chick cochlear development. J Comp Neurol 518: 2554–2569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kock K, Koenen A, Giese B, Fraunholz M, May K, Siegmund W, Hammer E, Volker U, Jedlitschky G, Kroemer HK, Grube M. Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization. J Biol Chem 285: 11336–11347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, Parent JL, Fritz A, Chen P. Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci USA 108: 2264–2269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control KATP channel surface density. J Biol Chem 285: 5963–5973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miranda-Rottmann S, Kozlov AS, Hudspeth AJ. Highly specific alternative splicing of transcripts encoding BK channels in the chicken's cochlea is a minor determinant of the tonotopic gradient. Mol Cell Biol 30: 3646–3660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89: 3345–3352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navaratnam DS. Yeast two-hybrid screening to test for protein-protein interactions in the auditory system. Methods Mol Biol 493: 257–268, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Nelson MT, Bonev AD. The beta1 subunit of the Ca2+-sensitive K+ channel protects against hypertension. J Clin Invest 113: 955–957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Pyott SJ, Meredith AL, Fodor AA, Vazquez AE, Yamoah EN, Aldrich RW. Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits. J Biol Chem 282: 3312–3324, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA 91: 839–843, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, Ruth P, Shipston MJ, Knaus HG. Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci 24: 442–454, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca2+ and K+ (BK) channels in chick hair cells are clustered and colocalized with apical-basal and tonotopic gradients. J Physiol 560: 13–20, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, Canel M, Machesky LM, Anderson KI, Frame MC. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol 20: 1086–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Shipston MJ, Armstrong DL. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J Physiol 493: 665–672, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Correction: conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLoS One 7: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 83: 215–242, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA 101: 11897–11902, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uchida N, Smilowitz H, Tanzer ML. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci USA 76: 1868–1872, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Widmer HA, Rowe IC, Shipston MJ. Conditional protein phosphorylation regulates BK channel activity in rat cerebellar Purkinje neurons. J Physiol 552: 379–391, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA 99: 5710–5715, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel a subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics 7: 2188–2198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]