Abstract
Muscle atrophy can be triggered by systemic illnesses that are associated with elevated proinflammatory/catabolic cytokines, which, in turn, are thought to contribute to muscle wasting. In this study, we found that the prototypical NF-κB transcription factor, Rel A (p65), is required for NF-κB activation in C2C12 and L6 myotubes due to treatment with exogenous TNF-α, IL-1α, IL-1β, TNF-related weak inducer of apoptosis, but not IL-6. All five cytokines induced atrophy in C2C12 myotubes, and inhibition of p65 reversed atrophy due to TNF-α, IL-1α, IL-1β, TNF-related weak inducer of apoptosis, but not IL-6 treatment. p65 was also required for TNF-α-induced increase in atrophy and inflammatory gene expression. TNF-α- and IL-1β-treated myotubes increased IL-6 protein expression, but use of an IL-6 blocking antibody showed that the IL-6 production did not contribute to atrophy. These data show that p65 is a required transcription factor mediating the catabolic effects of four different cytokines in cultured myotubes, but IL-6 works by a different mechanism.
Keywords: nuclear factor-κB, muscle, cachexia, helenalin, p65 short interfering RNA
muscle atrophy can be triggered by systemic illnesses, such as cancer, sepsis, acquired immunodeficiency syndrome, and chronic lung and kidney diseases. These conditions are associated with inflammatory responses and elevated proinflammatory and catabolic cytokines, such as TNF-α, IL-1, and IL-6, which, in turn, are thought to contribute to muscle wasting (2, 9, 32, 33). Catabolic/proinflammatory cytokines have been shown to exert many of their cellular effects by activation of NF-κB and mitogen-activated protein kinase signaling pathways (4). Treatment of cultured myotubes with the catabolic cytokines TNF-α, IL-1β, IL-1α, and TNF-related weak inducer of apoptosis (TWEAK) induce atrophy or myofibrillar protein loss (12, 15, 18, 21, 24). Genetic inhibition of NF-κB signaling by overexpression of the IκB superrepressor attenuates TNF-α- and TWEAK-induced myotube protein loss (12, 15, 18, 23), but the identity of the members of the NF-κB transcription factor family involved in TNF-induced atrophy has not been established. Furthermore, a role of NF-κB-mediated transcription in myotube atrophy for cytokines other than TNF-α or TWEAK is unknown.
Although the atrophy-inducing effect of the catabolic cytokine IL-6 has been shown in vivo (3, 31), to our knowledge it has not been shown in cultured myotubes. IL-6-treated myotubes show activated STAT3 phosphorylation (7), but a role of NF-κB in mediating any effect of IL-6 on myotubes has not been studied. Furthermore, at least TNF-α and IL-1β induce IL-6 protein expression in cultured myotubes (10, 13, 26), but the extent to which this autocrine production of IL-6 contributes to atrophy is unknown. While in vivo studies to investigate the mechanism of catabolic cytokines in muscle wasting are necessary, the effects of exogenous cytokine action on muscle cells in culture will help to elucidate the mechanism of cytokine-induced muscle loss in the absence of host and immune cell interactions.
-This study tested several hypotheses. First, we tested whether the prototypical NF-κB transcription factor p65 (Rel A) is required for NF-κB activation and myotube atrophy due to treatment with exogenous TNF-α, IL-1α, IL-1β, TWEAK, or IL-6. Second, we tested whether p65 is required for TNF-α-induced increases in the expression of atrophy and inflammatory genes. Third, we tested whether the autocrine production of IL-6 protein by TNF-α or IL-1β treatment of C2C12 cells contributes to myotube atrophy. Of the five cytokines tested, only IL-6 did not induce NF-κB reporter activation, but TNF-α, IL-1α, IL-1β, and TWEAK activation of the reporter were reversed by pharmacological inhibition of p65 in C2C12 and L6 muscle cell lines. All five cytokines induced myotube atrophy, but only IL-6-induced atrophy was not reversed by p65 inhibition. Genetic inhibition of p65 in TNF-α-treated myotubes confirmed these findings. Inhibition of p65 fully reversed atrophy induced by TNF-α, IL-1α, IL-1β, and TWEAK, but not IL-6, showing that p65 is necessary for most, but not all, catabolic cytokine-induced atrophy.
METHODS
Chemicals.
Mouse TNF-α, IL-1α, IL-1β, and IL-6 were purchased from Sigma (St. Louis, MO), and mouse TWEAK from R&D Systems (Minneapolis, MN). Helenalin was purchased from Enzo (Plymouth Meeting, PA). Control small interfering RNA (siRNA) and p65 siRNA were purchased from Ambion (Austin, TX). HiPerfect and Effectene transfection reagents were purchased from Qiagen (Valencia, CA). The soluble IL-6 blocking antibody was purchased from eBioSciences (San Diego, CA). Anti-p65 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-GAPDH was from Sigma (St. Louis, MO). Alexa Fluor 680 donkey anti-rabbit IgG was from Invitrogen (Carlsbad, CA). The mouse IL-6 ELISA kit was purchased from eBioscience (San Diego, CA).
Cell culture.
C2C12 mouse myoblasts and L6 rat myoblasts (American Type Culture Collection, Manassas, VA) were maintained in DMEM with 10% fetal bovine serum (Invitrogen) at 37°C in 5% CO2. For myoblast differentiation, cells were grown in DMEM with 2% horse serum (Invitrogen) at 37°C in 5% CO2. All cytokine treatments were 10 ng/ml, unless noted. Myotubes treated with helenalin were pretreated for 1 h before cytokine treatments. All controls and cytokine-treated wells in helenalin experiments receive the same volume of DMSO in each well. For all variables measured, three independent wells were used to calculate mean values for control and treated myotubes.
NF-κB reporter assays.
The activity of NF-κB was measured with an NF-κB-GL3 luciferase reporter plasmid described previously (17). Cells were grown in 24-well plates before plasmid transfection. On the day of transfection, cells were switched to differentiation medium and transfected with 500 ng reporter plasmid per well using Effectene. Three days after transfection, cells were treated with cytokine for various amounts of time and lysed with 200 μl Passive Lysis Buffer (Promega, Madison, WI). Cell lysates were transferred to Eppendorf tubes and centrifuged, and 5-μl cell lysate was mixed with 95 μl Luciferase Assay Reagent (Promega). Emission was read on a Turner Designs luminometer.
Myotube diameter measurement and immunocytochemistry.
Cells were grown in a six-well plate overnight before they were switched to differentiation medium. Starting on day 3 of differentiation, cells were treated with cytokine for 48 h. Control cells were given fresh medium every 24 h. For experimental cells, fresh medium and cytokine were changed every 24 h in the presence or absence of helenalin (1 μM), or in the presence or absence of the IL-6 blocking antibody (1 μg/ml). Myotubes were photographed at ×20 magnification at 0, 24, and 48 h posttreatment using a Nikon TS-500 inverted fluorescent microscope. Six to ten randomly selected fields per well were photographed by a Spot RT camera and Spot Software (Diagnostic Instruments). At least 200 diameters were measured per group using MetaMorph Imaging software (Universal Imaging). For immunocytochemistry, myotubes were fixed in 1.5% formaldehyde in HBSS for 30 min, washed in PBS, permeabilized in 1% Triton X-100, washed in PBS-Tween and then blocked in 3% BSA in PBS-Tween. Myotubes were incubated with mouse monoclonal anti-myosin MF20 (Developmental Studies Hybridoma Bank, Iowa City, IA) antibody, washed, and then incubated with goat anti-mouse fluorescein-conjugated Alexa Fluor 488. Myotubes were visualized through a FITC-HYQ filter, and images were taken as described above.
RNA isolation and quantitative RT-PCR.
Cells were grown in a 24-well plate overnight before they were switched to differentiation medium. On day 3 of differentiation, myotubes received cytokine treatment in the presence or absence of helenalin. After cytokine treatment, total RNA was isolated using the RNeasy Micro Kit (Qiagen). RNA was quantitated by spectrophotometry, and the integrity of 18S and 28S RNA was checked by formaldehyde-agarose gel electrophoresis. To make cDNA, 2.5 μg of total RNA were reverse transcribed in a 50-μl reaction using the High Capacity Reverse Transcription Kit (Applied Biosystems), according to manufacturer's instructions. cDNA was used as template for quantitative RT-PCR using the TaqMan method. TaqMan probes with the following assay IDs were used: atrogin-1/Fbxo32 (F-box-only protein 32; Mm00499523_m1), CARP (cardiac ankyrin repeat protein; Mm00496512_m1), Runx1 (runt-related transcription factor-1; Mm01213405_m1), C3 (Mm01232779_m1), MCP-1 (monocyte chemotactic protein-1; Mm00441242_m1), IP-10 (IFN-γ-inducible protein 10; Mm99999072_m1), Atg7 (autophagy-related 7; Mm00512209_m1), Bnip3 (Bcl-2/adenovirus E1B 19-kDa protein-interacting protein 3; Mm01275601_g1), Gabarapl1 (γ-aminobutyric acid receptor-associated protein-like 1; Mm00457880_m1), and GAPDH (Mm03302249_g1). All samples were measured in triplicate with an Applied Biosystems 7500 Real-Time PCR system and quantitated against a gene-specific standard curve constructed by fivefold cDNA serial dilutions. Each cytokine treatment value was then normalized to a GAPDH value from the same sample and plotted as the fold change relative to the noncytokine-treated control.
siRNA knockdown of p65 protein expression.
For knockdown experiments, cells were plated at a density of ∼50% and grown overnight. The next day, cells were switched to differentiation medium and transfected with siRNA against p65 or control siRNA at a concentration of 10 nM using HiPerFect (Qiagen) transfection reagent.
Western blot and ELISA.
Cells were grown in a six-well plate and lysed with 800 μl Passive Lysis Buffer. Cell lysates were isolated, and 20 μg of total protein were electrophoresed on an SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane for immunoblotting. The protein signal (p65 or GAPDH) was detected on the blot by indirect immunostaining with infrared fluorescence imaging using a LiCor Odyssey imager. An ELISA was used to measure IL-6 protein expression in cell culture medium using methods as directed by the manufacturer.
Statistical analysis.
At each time point of treatment for each dependent variable, control vs. cytokine values were compared using Student's t-test. Multiple comparisons were assessed by one-way ANOVA followed by Tukey's honestly significant difference test. Differences between mean values were considered significant at P < 0.05.
RESULTS
Cytokine induction of a NF-κB reporter gene.
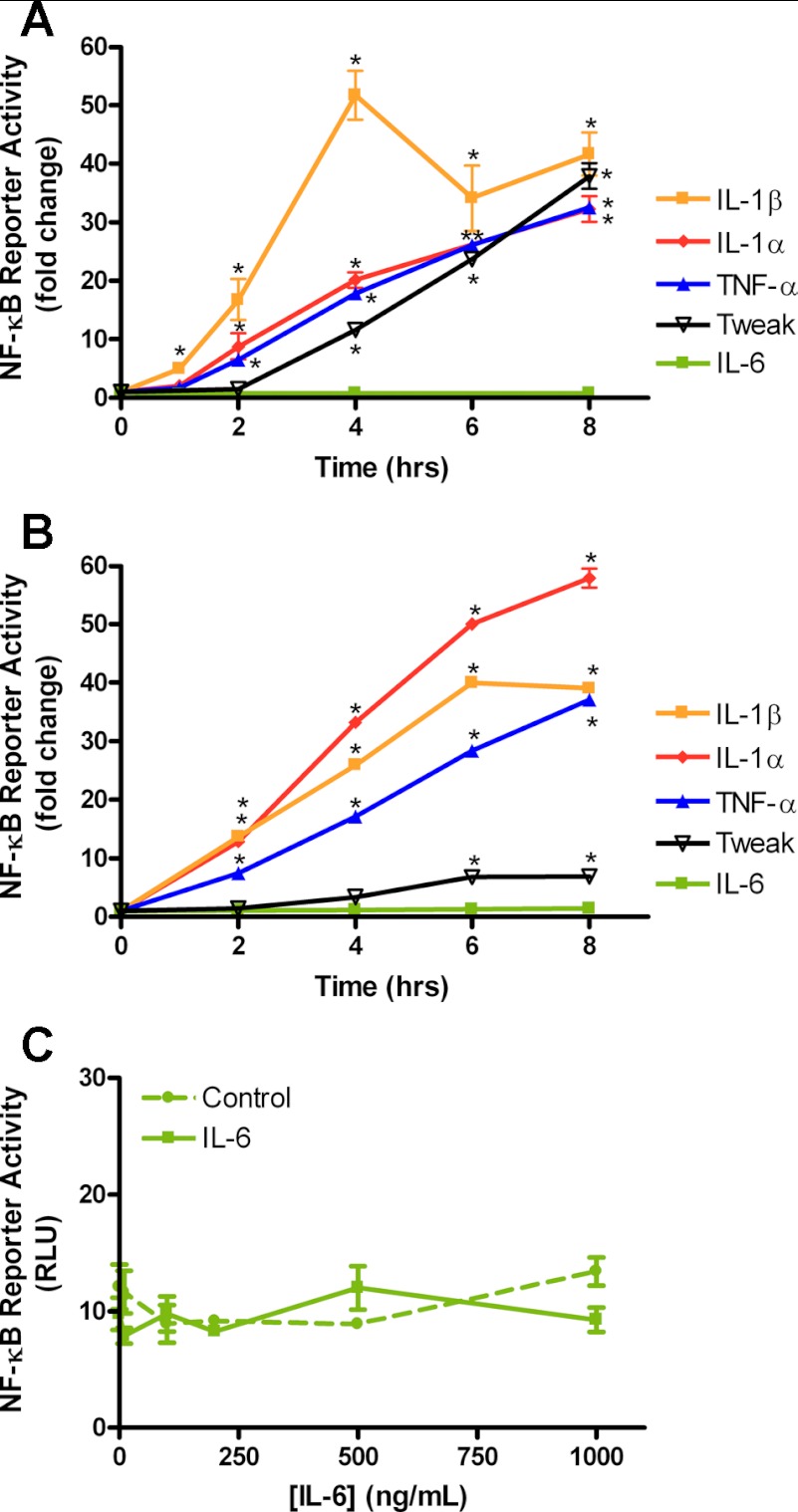
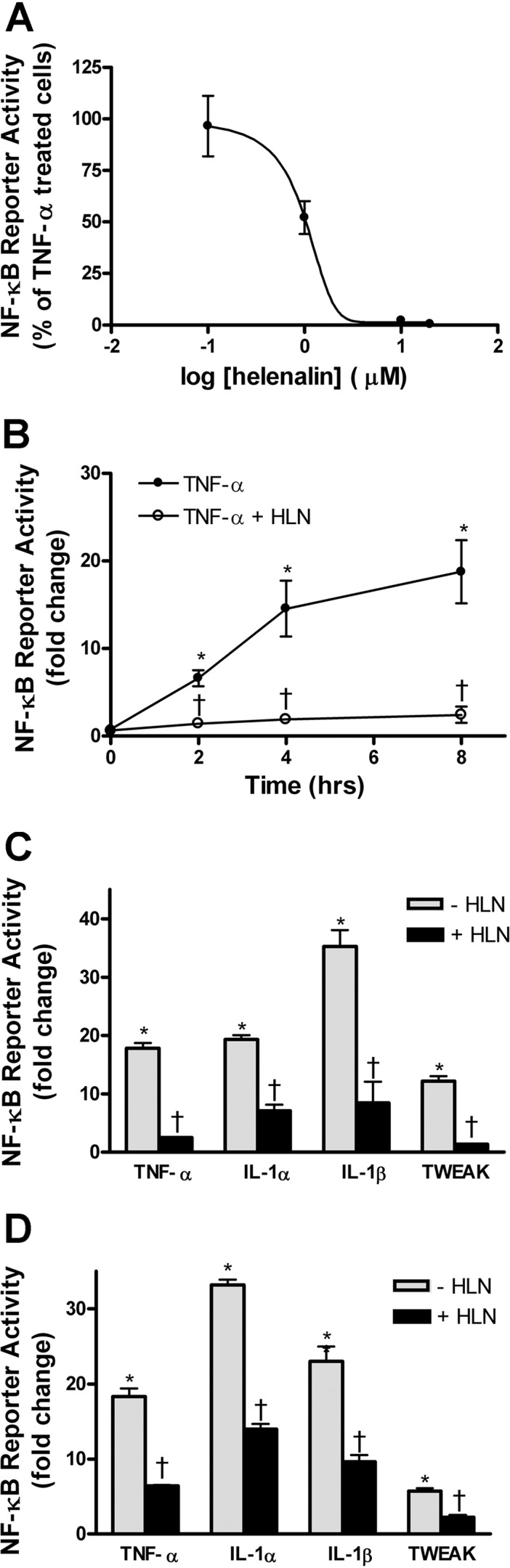
In 3-day differentiated C2C12 myotubes, four of the cytokines tested, TNF-α, IL-1α, IL-1β, and TWEAK, showed a time-dependent activation of a transiently transfected NF-κB reporter gene, but IL-6 treatment did not (Fig. 1A). The rat L6 muscle cell line was also tested with the same cytokines, and similar results were found, except that the response to TWEAK was much less robust (Fig. 1B). Increases in the dose of IL-6 up to 1 μg/ml did not activate the NF-κB reporter in C2C12 myotubes after 4 h of treatment (Fig. 1C). Thus, while the IL-6 gene is strongly regulated by NF-κB (25), the reverse does not appear to be the case. Pharmacological inhibition of the Rel A (p65) member of the NF-κB family of transcription factors using helenalin (27) showed a dose-dependent inhibition of TNF-α-induced NF-κB activity in C2C12 myotubes (Fig. 2A). A time course of TNF-α-induced NF-κB activity and helenalin treatment showed consistent inhibition of the reporter from 2 to 8 h (Fig. 2B). The other cytokines that activated the NF-κB reporter were also inhibited by helenalin after 4 h of treatment in C2C12 myotubes (Fig. 2C). Similar inhibitory effects of helenalin were seen in L6 myotubes after 4 h of treatment (Fig. 2D).
Fig. 1.
Proinflammatory cytokine-induced NF-κB reporter activity. A: C2C12 cells were transfected with a NF-κB reporter, differentiated for 3 days, and treated with each cytokine (10 ng/ml) for the times indicated up to 8 h. Fold changes are compared with activity in the absence of cytokine treatment (statistically different from 2- to 8-h treatment, except IL-6). B: L6 myoblasts differentiated for 3 days and treated the same as in A. C: 3-day differentiated C2C12 myotubes were treated with increasing concentration of IL-6 for 4 h. TWEAK, TNF-related weak inducer of apoptosis; RLU, relative light units. Values are means ± SE from 3 separate wells of myotubes. *P < 0.05 vs. control.
Fig. 2.
Helenalin (HLN) inhibits cytokine-induced NF-κB reporter activation in 3-day differentiated C2C12 (A–C) and L6 (D) myotubes. A: dose-response curve of HLN inhibition for TNF-α-induced NF-κB reporter activity at 4 h; 50% inhibition of TNF-α-induced NF-κB activity is at 1 μM HLN. B: time course of TNF-α-induced NF-κB activity, with or without HLN (2 μM). Values plotted are fold change compared with untreated reporter-transfected myotubes. C: NF-κB activity fold change in TNF-α, IL-1α, IL-1β, or TWEAK-treated C2C12 myotubes (4 h), with or without 2 μM HLN. D: L6 myotubes treated as in C. Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. cytokine.
Inhibition of p65 blocks cytokine-induced myotube atrophy.
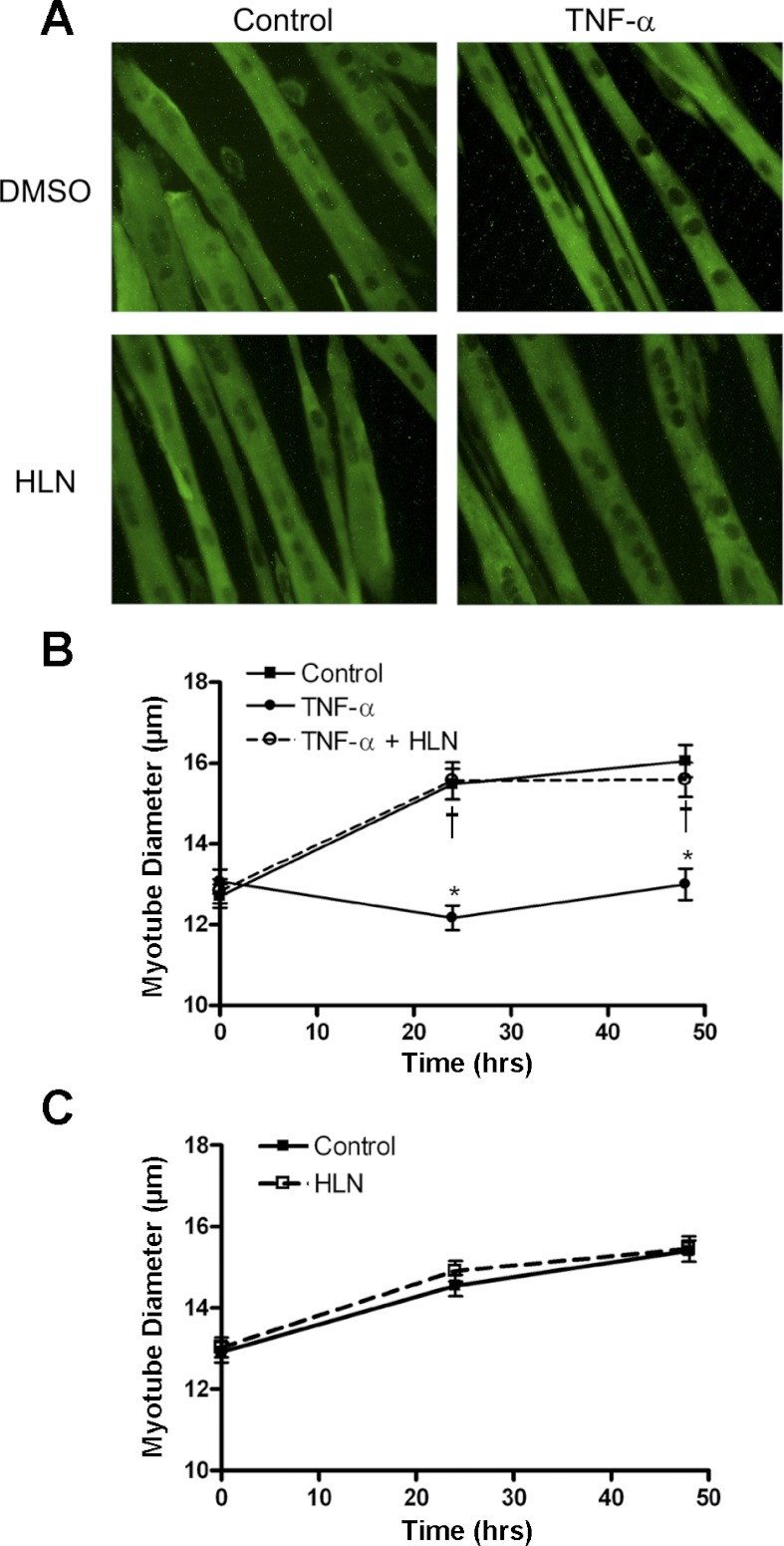
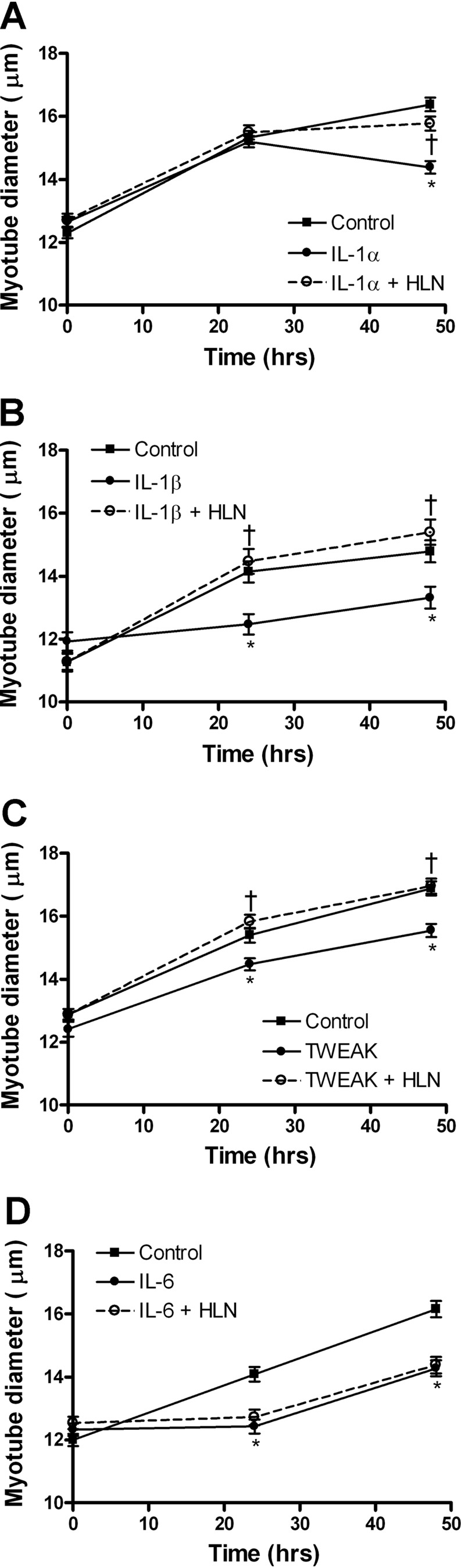
We treated 3-day myotubes with five different catabolic cytokines in the presence or absence of the p65 inhibitor helenalin for 24 and 48 h to determine whether p65 is required for cytokine-induced atrophy. TNF-α-induced significant atrophy in treated myotubes, as measured by myotube diameter at 24 and 48 h, and at both time points this decrease was reversed by helenalin (Fig. 3, A and B). Helenalin alone did not affect myotube diameter over the 48-h experimental period (Fig. 3C). Similar experiments were carried out on myotubes treated with IL-1α or IL-1β. Both IL-1 cytokines induced atrophy that was completely reversed by helenalin (Fig. 4, A and B). However, IL-1α, unlike TNF-α or IL-1β, induced atrophy at 48 but not 24 h, suggesting it has a different mechanism of catabolism in myotubes, but p65 is still required. TWEAK treatment also caused a decrease in myotube diameter, which was reversed by helenalin at both 24 and 48 h (Fig. 4C). Myotubes treated with IL-6 showed atrophy at 24 and 48 h, but, as expected from results in Fig. 1, helenalin did not reverse the atrophy (Fig. 4D), indicating that the catabolic effect of IL-6 is independent of p65.
Fig. 3.
TNF-α-induced myotubes atrophy is reversed by p65 inhibition. C2C12 myotubes were treated for 48-h TNF-α, with or without HLN (1 μM), compared with control. A: immunocytochemistry of myotubes after 48 h of treatment stained by anti-myosin. B: myotube diameter measured at 0, 24, and 48 h of TNF-α treatment, with or without HLN, compared with no cytokine control group. C: HLN treatment alone had no effect on myotube diameter. Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. cytokine.
Fig. 4.
Inhibition of p65 blocks cytokine-induced atrophy. C2C12 myotube diameter due to cytokine treatment for 48 h, with or without HLN (1 μM), is shown. Diameter was measured for myotubes treated with IL-1α (A), IL-1β (B), TWEAK (C), and IL-6 (D). Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. cytokine.
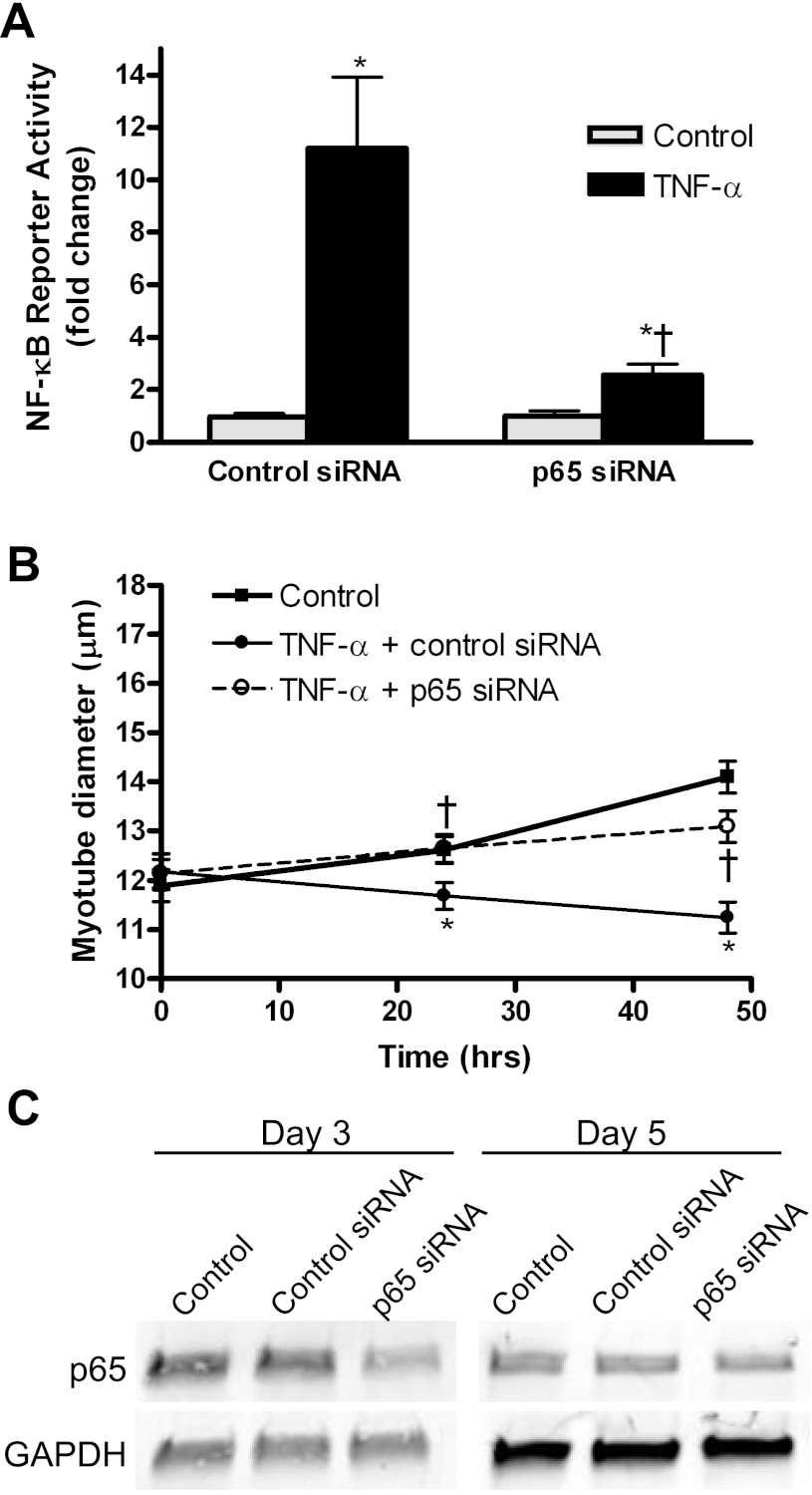
To confirm that the pharmacological effect of helenalin on cytokine-induced NF-κB activity was on inhibition of p65, we transfected myoblasts with a siRNA against p65 plus the NF-κB reporter gene and induced differentiation for 3 days. Myotubes treated with TNF-α for 3 h showed strong activation of the reporter, but this activation was abolished in cells cotransfected with siRNA against p65 (Fig. 5A). The siRNA against p65 was also used to inhibit p65 during TNF-α-induced myotube atrophy. Inhibition of p65 via siRNA knockdown inhibited TNF-α-induced myotube atrophy at 24 and 48 h of treatment (Fig. 5B). Confirmation of p65 knockdown was performed using immunoblotting, which showed a greater than 50% decrease in protein expression at 3 days (day 0 of treatment) and a 50% decrease at 5 days (48-h treatment) postdifferentiation (Fig. 5C).
Fig. 5.
Inhibition of TNF-α-induced atrophy with small interfering RNA (siRNA) against p65. A: C2C12 myoblasts were transfected with control siRNA or siRNA against p65, and then 3-day myotubes were treated with TNF-α for 3 h. B: myotube diameter was measured at 0, 24, and 48 h after TNF-α treatment with control siRNA or p65 siRNA. Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. TNF-α + control siRNA. C: Western blot showing p65 signal in control, control siRNA, and p65 siRNA transfected cells at day 3 posttransfection (day 0 diameter measurements) and day 5 posttransfection (48-h diameter measurements). GAPDH signal shows no change in expression due to siRNA treatment.
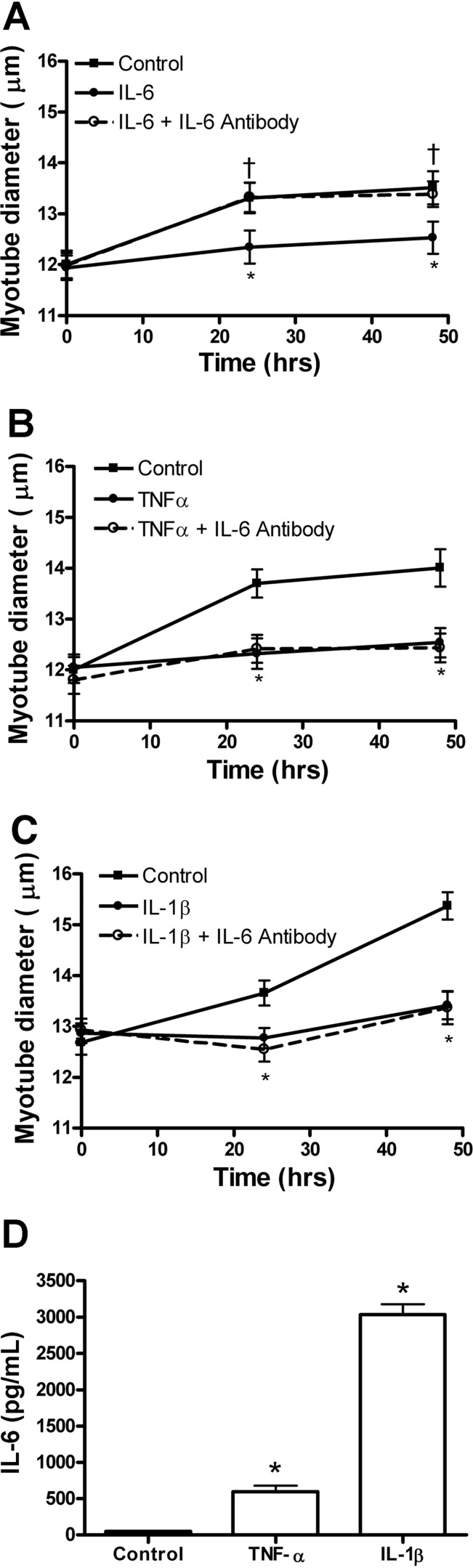
Autocrine IL-6 production by TNF-α or IL-1β does not contribute to atrophy.
Since TNF-α and IL-1β are known to induce IL-6 protein expression in C2C12 myotubes, an IL-6 blocking antibody was used to determine whether IL-6 was contributing to TNF-α or IL-1β-induced myotube atrophy. First we established that treatment of myotubes with the IL-6 blocking antibody was sufficient to inhibit IL-6-induced atrophy at 24 and 48 h of treatment (Fig. 6A). Neither TNF-α nor IL-1β-induced atrophy was inhibited by the IL-6 blocking antibody at 24 or 48 h of treatment (Fig. 6, B and C). Incubation with IL-6 antibody without cytokine treatment had no effect on myotube diameter (not shown). To confirm that shown by others (10, 13, 26), 24 h of TNF-α or IL-1β treatment of C2C12 myotubes significantly increased IL-6 protein expression by 13- and 64-fold respectively (Fig. 6D).
Fig. 6.
Blocking of autocrine IL-6 failed to inhibit TNF-α or IL-1β-induced atrophy. Three-day differentiated C2C12 myotubes were treated with cytokines in the absence or presence of IL-6 blocking antibody. Diameter was measured for myotubes treated with IL-6 (A), TNF-α (B), or IL-1β (C), with or without IL-6 blocking antibody and control myotubes. D: IL-6 protein concentration in cell culture medium (at 24 h of treatment) from control, TNF-α, or IL-1β-treated myotubes. Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. IL-6.
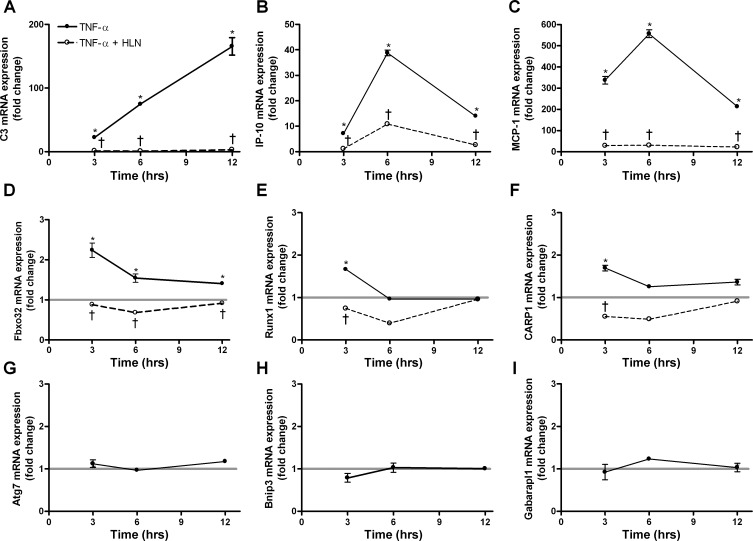
Inhibition of p65 blocks TNF-α-induced gene expression.
We showed upregulation of inflammatory genes by TNF-α: two chemokines, MCP-1 and IP-10, and one complement gene, C3. Since these genes are known to be upregulated by NF-κB p65 (20), we expected their expression would be reversed by helenalin at 3, 6, and 12 h of treatment, which is what was observed (Fig. 7, A–C). With respect to atrophy genes, muscle-specific ubiquitin ligases muscle atrophy F-box (MAFbx; Fbxo32), a gene often found upregulated with atrophy of whole muscle and muscle cells (6, 21, 30), was significantly elevated at 3, 6, and 12 h of TNF-α treatment, and this was reversed by helenalin (Fig. 7D).
Fig. 7.
Inhibition of p65 blocks TNF-α induced mRNA upregulation. Myotubes were treated with TNF-α for 3, 6, or 12 h, with or without HLN. Gene expression fold change is compared with untreated control myotubes. A–C: inflammatory genes, C3, IP-10, and MCP-1 mRNA expression, respectively. D–F: atrophy genes, Fbxo32, Runx1, and CARP1 mRNA expression, respectively. G–I: autophagy genes, Atg7, Bnip3, and Gabarapl1 mRNA expression, respectively. The thick light gray horizontal lines in D–I depict control values for each gene for ease of comparison. Values are means ± SE. *P < 0.05 vs. control. †P < 0.05 vs. TNF-α. See methods for definition of genes.
Two other genes thought to be markers of muscle atrophy in vivo, Runx1 (14, 34, 35) and CARP (19, 35), were significantly elevated at 3 h, and this increase was reversed by helenalin (Fig. 7, E and F). Other common atrophy markers, such as tripartite motif-containing 63 (Trim63) [muscle RING finger 1 (MuRF1)] or Foxo3 (Forkhead box O transcription factor 3) were increased by only 20% at 3 h but were not increased at 6 or 12 h (not shown). Autophagy genes, Atg7, Bnip3, or Gabarapl1, were not significantly increased by TNF-α at any time point (Fig. 7, G, H, I). Expression of GAPDH did not change with 3, 6, or 12 h or TNF-α treatment.
DISCUSSION
During systemic diseases, host-immune cell interactions induce expression of a variety of catabolic cytokines and immune modulators that appear in the circulation (11, 32). Experiments in rodents using blocking antibodies against specific cytokines have suggested that several cytokines can contribute to cachexia (32). To investigate the mechanism of muscle wasting of individual cytokines in the absence of the immune system, infectious agents, or other tissues, muscle cell culture studies exist to address this question. However, the transcription factors required to induce atrophy by catabolic cytokines remain largely unknown.
The novelty of the present work is that we compare five different cytokines over time to study the requirement of p65 for both NF-κB activation and atrophy and, for one cytokine, the role of p65 in gene expression. We show that, with four of the five catabolic cytokines studied, an NF-κB reporter is activated in a time-dependent manner in C2C12 and L6 myotubes, and this activation is reversed by the p65 inhibitor helenalin. Although several laboratories have shown that the IκBα superrepressor inhibited TNF-α and TWEAK-induced NF-κB activation or protein loss (12, 15, 23), there is not primary evidence as to which Rel transcription factors are involved. Here, in two different muscle cell lines, we show that Rel A (p65) is required for NF-κB activation by TNF-α, IL-1α, IL-1β, and TWEAK. C2C12 and L6 myotubes showed similar responses to cytokine treatment and to helenalin, with the exception of TWEAK, which showed a less robust response in the L6 (rat) cell line. It is possible that this is due to the fact that murine cytokines were used. The conservation between mouse and rat TWEAK and TWEAK receptor (TNFrsf12a) is high, 98 and 91%, respectively, but it has been shown that there can be cases of greatly diminished or the absence of species cross-reactivity of TNF superfamily ligands or their receptors (8).
Moreover, the C2C12 myotube atrophy induced by these same cytokines for 24 or 48 h of exposure was completely reversed by helenalin, indicating that p65 is required for atrophy. The Rel A specificity for the activation of NF-κB and C2C12 myotube atrophy was confirmed by p65 knockdown using siRNA against p65 in TNF-α-treated cells. Knockdown of p65 also reversed TNF-α-induced atrophy by 100% after 24 h of treatment and by 75% after 48 h of treatment; the slightly less than complete reversal of atrophy at 48 h of treatment is likely due to less inhibition of p65 protein, as illustrated by the Western blot at 5- vs. 3-day postdifferentiation and posttransfection of the siRNA against p65.
In the case of IL-6, the NF-κB reporter was not activated in C2C12 or L6 myotubes, even using supraphysiological doses. Consistent with this observation, IL-6-induced C2C12 myotube atrophy was not reversed by helenalin. The IL-6 catabolic effect on myotubes involves a NF-κB-independent mechanism, even though the IL-6 gene is strongly regulated by NF-κB (25). Further study is needed to identify the signaling proteins involved in IL-6-induced atrophy, perhaps via STAT-dependent transcription (7). It is well known that TNF-α or IL-1β treatment of cultured muscle cells induces autocrine production of IL-6 (10, 13, 26). It is not known, however, if this autocrine increase in IL-6 synthesis contributes to the myotube atrophy caused by TNF-α or IL-1β. Our data show that increased IL-6 protein expression by myotubes treated with TNF-α or IL-1β does not contribute to the atrophy observed.
The expression of all of the genes measured that were upregulated due to TNF-α treatment were reversed by the p65 inhibitor helenalin. This was not surprising for the proinflammatory genes, as they have been shown to be upregulated by p65 in other cell types (16, 20). Genes commonly upregulated in atrophy were also reversed by helenalin. However, TNF-α does not upregulate Foxo mRNAs in C2C12 myotubes in our hands and as shown by Bhatnagar et al. (5) after 18 h of TNF-α treatment. Autophagy genes, which are upregulated in response to some whole muscle atrophy conditions (28, 29), also show no increase in expression in response to 18 h of TNF-α treatment in whole muscle from mice (1). Thus the atrophy program in cytokine-treated myotubes and in TNF-α-treated mice appears to differ from atrophy due to systemic illness, even though, in the latter, cytokines are thought to be key triggers of atrophy. It has been shown that the TNF-α induction of MAFbx/Atrogin1 is mediated, at least in part, by p38 mitogen-activated protein kinase (22). Our data demonstrate that TNF-α-induced MAFbx expression is also regulated by p65.
Here we reveal the time-dependent activation of NF-κB by proinflammatory/catabolic cytokines in two different muscle cell lines and the complete reversal of myotube atrophy by p65 inhibition in response to TNF-α, IL-1β, IL-1α, and TWEAK, but not IL-6. Autocrine production of IL-6 by TNF-α- or IL-1β-treated C2C12 myotubes does not contribute to myotube wasting. We also show that TNF-α-induced gene expression in myotubes is dependent on p65. Although skeletal muscle cachexia is proving to be a product of numerous molecular factors, the NF-κB arm, activated by multiple catabolic cytokines, is highly dependent on the contribution of Rel A.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R21 AR054446, R01 AR41705, and R01 AR060217.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.Y., C.-L.W., M.G., J.L., and R.W.J. performed experiments; T.Y. and C.-L.W. prepared figures; C.-L.W., R.W.J., and S.C.K. approved final version of manuscript; R.W.J. and S.C.K. conception and design of research; R.W.J. and S.C.K. interpreted results of experiments; R.W.J. and S.C.K. edited and revised manuscript; S.C.K. analyzed data; S.C.K. drafted manuscript.
REFERENCES
- 1. Alon T, Friedman JM, Socci ND. Cytokine-induced patterns of gene expression in skeletal muscle tissue. J Biol Chem 278: 32324–32334, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Argiles JM, Busquets S, Toledo M, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3: 263–268, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflügers Arch 457: 989–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11: 372–377, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A. Tumor necrosis factor-alpha regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLos One 5: e13262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLos One 6: e22538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 281: 13964–13971, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med 37: S354–S367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chevrel G, Granet C, Miossec P. Contribution of tumour necrosis factor alpha and interleukin (IL) 1beta to IL6 production, NF-kappaB nuclear translocation, and class I MHC expression in muscle cells: in vitro regulation with specific cytokine inhibitors. Ann Rheum Dis 64: 1257–1262, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract 21: 68–81, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol 285: R1153–R1164, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez de Aguilar JL, Niederhauser-Wiederkehr C, Halter B, De Tapia M, Di Scala F, Demougin P, Dupuis L, Primig M, Meininger V, Loeffler JP. Gene profiling of skeletal muscle in an amyotrophic lateral sclerosis mouse model. Physiol Genomics 32: 207–218, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J 22: 5530–5539, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J 16: 529–538, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278: 2294–2303, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Laure L, Suel L, Roudaut C, Bourg N, Ouali A, Bartoli M, Richard I, Daniele N. Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J 276: 669–684, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Leung TH, Hoffmann A, Baltimore D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell 118: 453–464, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol 297: C706–C714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 279: R1165–R1170, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12: 871–880, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10: 2327–2334, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-kappa B. Am J Physiol Regul Integr Comp Physiol 284: R1249–R1254, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem 273: 33508–33516, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 29. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298: C542–C549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Strassmann G, Fong M, Freter CE, Windsor S, D'Alessandro F, Nordan RP. Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo. J Clin Invest 92: 2152–2159, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J 31: 492–501, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19: 1715–1722, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu CL, Kandarian SC, Jackman RW. Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PLos One 6: e16171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]