Abstract
Our previous in vivo and ex vivo studies suggested that coexistence of two or more troponin T (TnT) isoforms in adult cardiac muscle decreased cardiac function and efficiency (Huang QQ, Feng HZ, Liu J, Du J, Stull LB, Moravec CS, Huang X, Jin JP, Am J Physiol Cell Physiol 294: C213–C22, 2008; Feng HZ, Jin JP, Am J Physiol Heart Circ Physiol 299: H97–H105, 2010). Here we characterized Ca2+-regulated contractility of isolated adult cardiomyocytes from transgenic mice coexpressing a fast skeletal muscle TnT together with the endogenous cardiac TnT. Without the influence of extracellular matrix, coexistence of the two TnT isoforms resulted in lower shortening amplitude, slower shortening and relengthening velocities, and longer relengthening time. The level of resting cytosolic Ca2+ was unchanged, but the peak Ca2+ transient was lowered and the durations of Ca2+ rising and decaying were longer in the transgenic mouse cardiomyocytes vs. the wild-type controls. Isoproterenol treatment diminished the differences in shortening amplitude and shortening and relengthening velocities, whereas the prolonged durations of relengthening and Ca2+ transient in the transgenic cardiomyocytes remained. At rigor state, a result from depletion of Ca2+, resting sarcomere length of the transgenic cardiomyocytes became shorter than that in wild-type cells. Inhibition of myosin motor diminished this effect of TnT function on cross bridges. The length but not width of transgenic cardiomyocytes was significantly increased compared with the wild-type controls, corresponding to longitudinal addition of sarcomeres and dilatative remodeling at the cellular level. These dominantly negative effects of normal fast TnT demonstrated that chronic coexistence of functionally distinct variants of TnT in adult cardiomyocytes reduces contractile performance with pathological consequences.
Keywords: calcium transient, dilatative remodeling, sarcomere length
during cardiac muscle contraction, the rise of cytosolic Ca2+ results in binding of Ca2+ to troponin and induces a series of allosteric changes in the myofilaments, thereby allowing the formation of strong actin-myosin cross bridges to produce force and shortening of the sarcomere (10). Troponin T (TnT) is the tropomyosin-anchoring subunit of troponin and interacts with troponin C (TnC), troponin I (TnI), tropomyosin, and F-actin in the Ca2+ regulation of muscle contraction (16, 22, 31). With its central position in this Ca2+ signaling pathway, TnT functions as a molecular organizer of the thin filament regulatory system of striated muscles (22, 27). Extensive studies have shown the role of TnT structural and functional variations in altering muscle contractility (16, 31).
Three homologous genes have evolved in vertebrates to encode cardiac and skeletal muscle fiber type-specific TnT isoforms. Alternative RNA splicing also generates protein variants from the transcripts of cardiac, slow, and fast skeletal muscle TnT genes (6, 16, 31). These TnT isoforms and splicing variants are highly conserved in their primary structure in the middle and COOH-terminal regions but significantly different in the NH2-terminal region (31). The structure of the NH2-terminal variable region of TnT is regulated by alternative splicing during heart and skeletal muscle development (14, 29) and hind limb unloading (32). Abnormal splicing in the NH2-terminal region of cardiac TnT is related to dilated cardiomyopathy (3, 4). It has been shown that different TnT isoforms (12) or splicing variants (3, 9) result in different Ca2+ responsiveness of the myofilament. Nonetheless, the functional significance of these TnT isoforms is not fully understood.
Most skeletal muscles contain mixed fiber types and express both slow and fast isoforms of TnT, and multiple alternatively spliced variants are present in all skeletal muscle fibers (5). The coexistence of multiple functionally distinct TnT variants in the Ca2+ regulatory system of skeletal muscle thin filaments is consistent with the ability to execute prolonged (tetanic) contractions. Multiple TnT variants with different Ca2+ responsiveness would broaden the twitches to facilitate the production of steady tetanic force. In contrast, a single form of cardiac TnT is present in adult cardiac muscle of human and many other vertebrates after the completion of the alternative splicing-generated isoform switch during perinatal heart development (16, 31). This feature corresponds logically with a uniform Ca2+ activation of the thin filaments suitable for generating rhythmic contractions of the heart. Based on this hypothesis, we have previously demonstrated that the coexistence of functionally distinct TnT isoforms or splicing variants in adult cardiac muscle such as that found in turkey and dog hearts with dilated cardiomyopathy (3, 4) resulted in decreased pumping function and energetic efficiency of the heart (7). These findings support a pathological significance of the abnormally spliced cardiac TnT found in failing human hearts (1, 20).
To further test the hypothesis that coexistence of functionally distinct TnT variants in adult cardiac muscle reduces heart function due to desynchronized Ca2+ activation of the myofilaments, the present study characterized adult cardiomyocytes isolated from transgenic mice overexpressing a wild-type skeletal muscle TnT in the heart (12). Beyond showing the functional defect of a pathological mutation, this model coexpresses a nonmutant fast TnT with the endogenous cardiac TnT to provide an informative experimental system for examining the consequence of coexistence of two functional variants of TnT, other than that of overexpressing a particular mutation, in the heart. Contractility and intracellular Ca2+ transient were simultaneously measured. The results showed dominantly negative effects of the coexistence of fast skeletal muscle TnT and cardiac TnT on contractile kinetics absent of the influence of extracellular matrix. A dilatative remodeling at the cellular level was observed, supporting a pathological significance.
MATERIALS AND METHODS
Transgenic mice overexpressing fast skeletal muscle TnT in the adult heart.
The construction, genotyping, and breeding of transgenic mice bearing a chicken fast skeletal muscle TnT transgene driven by an α-myosin heavy chain promoter (25) have been described previously (12). Six- to eight-month-old mice of both sexes in C57BL/6 background were used in the study. As shown previously (12), the size difference between the chicken fast skeletal muscle TnT and the adult mouse cardiac TnT is minimal (287 vs. 288 amino acids) while their main structural difference lies in the NH2-terminal variable region. Wild-type litter mates of similar age were used as controls.
The mice were maintained on a 12:12-h light-dark cycle (6:00 AM/6:00 PM) and standard pellet diet. The experimental procedures were approved by the Wayne State University School of Medicine Institutional Animal Care and Use Committee.
Isolation of adult mouse ventricular cardiomyocytes.
Cardiomyocytes were enzymatically isolated from wild-type (n = 15) and transgenic (n = 12) mice. After euthanization, the heart was rapidly excised together with the large vessels attached and immediately immersed in Ca2+-free Joklik medium containing 10 mM HEPES and 10 mM NaHCO3, pH 7.2 at 37°C. The heart was cannulated through the aorta and perfused at 37°C on a Langendorff apparatus with a constant pressure of 80 cmH2O or constant flow at 3 ml/min. The heart was first perfused for 3 min with noncirculating plain Joklik solution and then with circulating digestion Joklik solution containing 0.05% collagenase I (Sigma) and 0.1% BSA (Sigma) for ∼15 min until the tissue color was turning pale. The heart was further perfused with noncirculating plain Joklik solution for 3 min.
The ventricles were dissected, cut into small pieces, and gently agitated in Ca2+-free Joklik media to disperse cardiomyocytes. The cell suspension was filtered through a 100-mesh screen to remove tissue debris, spun at 400 g at room temperature for 3 min, and resuspended in Ca2+-free Joklik media containing 1% BSA. After standing at room temperature and being oxygenated with 100% oxygen gas for 30 min, CaCl2 from a 1-M stock was gradually added to the cell suspension in six steps in 35 min to the final concentration of 1.25 mM. The cardiomyocytes were maintained with continuous oxygenation and used for functional analysis within 6 h after isolation; 10 mM butanedione monoxime (BDM) were included in the perfusion and cell isolation media in some experiments measuring cell and sarcomere lengths.
The following criteria were applied to identify healthy cells for reliable and reproducible functional studies: 1) rod-shaped with clear outline and striation, 2) quiescent without stimulation, 3) stably contractile when paced at 8.0 Hz, 4) no significant change in shortening amplitude after loading of fura-2 AM (see below), and 5) no change in morphology at the end of the experiment.
Measurement of cardiomyocyte contractility and intracellular calcium transient.
The isolated adult mouse cardiomyocytes in Joklik media after restoration of 1.25 mM Ca2+ were loaded in a 0.3-ml superfusion chamber mounted on the stage of a Zeiss Axiovert 100 inverted fluorescence microscope using a temperature-controlled heating adapter. The cells were allowed to settle on the uncoated bottom glass coverslip of the chamber at 37°C for 10 min. After the cells were settled, continuous perfusion was performed with oxygenated physiological buffer containing 132 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 10 mM HEPES, 5 mM pyruvic acid, 0.5 mM probenecid acid, and 1.25 mM CaCl2 pH 7.2. The flow rate was controlled at 0.3 ml/min by a flow controller (cF-8VS Valve assembly; Cell MicroControls, Norfolk, VA). The cardiomyocytes were paced at 1, 2, and 5 Hz with 15 volts/cm square pulses of 5-ms duration using a field stimulator (Myopacer; IonOptix, Milton, MA) through two platinum electrodes placed on the sides of the perfusion chamber.
An IonOptix cell imaging system was used for simultaneously recording the change in the length of cardiomyocytes by edge detection and Ca2+ transient by fura-2 AM fluorescence. The cell image signal from a charge-coupled device camera (model 200, IonOptix) was fed to video-edge or sarcomere length detector software (IonOptix) for data acquisition at a sampling rate of 240/s. The data were stored and analyzed to determine the resting cell length, cell width, shortening amplitude, and the rates of shortening (+dL/dt) and relengthening (−dL/dt). The sarcomere length was determined using Fourier transform and calibrated with a precision diffraction grating standard (Richardson Grating Laboratory, Rochester, NY).
To monitor the intracellular Ca2+ transient in the cardiomyocytes, the isolated cells in Joklik media containing 1.25 mM CaCl2 were incubated with 2 μM fura-2 AM (Molecular Probes) in the dark at room temperature for 10 min. After fura-2 was loaded, the cells were washed twice with Joklik media containing 1.25 mM CaCl2 and 500 μM probenecid and kept in the same media at room temperature for 30 min. Loaded in the superfusion chamber as above, the cardiomyocytes were paced same as in the contractility studies for examination of the intracellular calcium transient through a photomultiplier tube (PMT-300, IonOptix). Intracellular free Ca2+ was measured as the ratio of 360-to-380 nm-excited fura-2 fluorescence at 510 nm.
SDS-PAGE and Western blotting.
As described previously (12), cardiomyocytes isolated from each of the mouse hearts studied were examined with SDS-PAGE and Western blotting to verify the coexistence and relative levels of fast skeletal muscle TnT and endogenous cardiac TnT. The cells were washed twice with PBS and lyzed in SDS-gel sample buffer containing 2% SDS. The samples were run on 14% Laemmli gels with acrylamide:bisacrylamide ratio of 180:1. The protein bands resolved were electrically transferred to nitrocellulose membranes. The membranes were blocked with 1% BSA in TBS (150 mM NaCl and 50 mM Tris·HCl pH 7.5) and incubated with monoclonal antibody (mAb) 6B8 recognizing the chicken fast TnT (30) and mAb CT3 recognizing cardiac TnT (6) diluted in TBS containing 0.1% BSA. The subsequent washes, incubation with alkaline phosphatase-labeled anti-mouse IgG second antibody (Sigma), and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate reaction were performed as described previously (12). Consistent with our previous studies (12, 21), ∼50% incorporation of the exogenous fast skeletal muscle TnT into the cardiac myofibrils of the transgenic mice was observed in the adult transgenic mouse cardiomyocytes, justifying the use of these cells as a model system to test the physiological and pathological effects of coexistence of two distinct TnT isoforms.
Pro-Q Diamond staining of phosphoproteins in mouse cardiac muscle.
Phosphorylation of cardiac TnI, TnT, and myosin-binding protein C (MBP-C) was examined in vivo. Cardiac muscle tissues were sampled while the animals were under anesthesia (100 mg/kg body wt ip). Both baseline and isoproterenol-stimulated (0.2 mg/kg body wt to produce increases of heart rate from ∼480 to ∼520 beats/min) samples were examined. The tissue samples were immediately homogenized in SDS-gel sample buffer containing 2% SDS and heated at 80°C to rapidly inactivate phosphatases and proteases. The samples were resolved in SDS-PAGE gel as above.
As previously described (8), SDS gels were prefixed in 50% methanol/10% acetic acid for 45 min and kept in fresh fixer overnight. After being washed three times in deionized water for 10 min each, the gels were stained for 90 min in a dark container with Pro-Q Diamond reagent (Invitrogen) freshly equilibrated to room temperature. After being destained three times in 20% acetonitrile, 50 mM sodium acetate, pH 4.0, for 30 min each, the gels were washed for 5 min twice in deionized water and scanned on a Typhoon 9410 fluorescence imager (GE Healthcare) using excitation at 532 nm and recording emission at 560 nm.
Data analysis.
The contractile and fura-2 fluorescence traces recorded using the IonOptix system were analyzed for the measurements of contractile and Ca2+ transient parameters. The data were exported to Origin or Excel computer program for graphing. Gel densitometry analysis was carried out on images at 600 dpi resolution using the ImageJ software. Statistic analysis of the significance of difference was done using Student's t-test.
RESULTS
Coexistence of fast TnT and cardiac TnT in adult transgenic mouse cardiomyocytes decreased the amplitude of contraction.
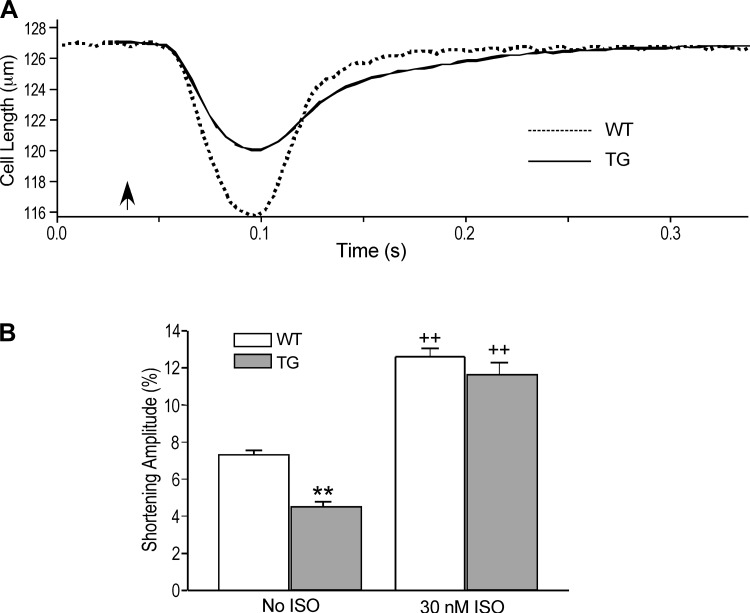
During paced contractions at 2.0 Hz, the shortening amplitude of the transgenic mouse cardiomyocytes overexpressing fast skeletal muscle TnT was significantly smaller than that of wild-type cells (Fig. 1). Treatment with 30 nM isoproterenol significantly increased the shortening amplitudes of both groups and the difference diminished (Fig. 1B). The preserved capacity of contractile response to β-adrenergic-stimulated augmentation of intracellular Ca2+ handling in the transgenic cardiomyocytes suggested that the decreased baseline contractility was due to altered myofilament responsiveness to Ca2+ activation from the coexistence of fast skeletal muscle TnT and cardiac TnT while the potential capacity of force generation was preserved.
Fig. 1.
Weaker contraction of transgenic mouse cardiomyocytes coexpressing fast troponin T (TnT) and cardiac TnT. A: representative shortening and relaxation traces demonstrated lower shortening amplitude with longer duration of the transgenic (TG) vs. wild-type (WT) cardiomyocytes. Arrow indicates the electrical stimulation of contraction. B: quantitative analysis found significantly smaller shortening amplitude of the TG cardiomyocytes than the WT control (**P < 0.01). Treatment of 30 nM isoproterenol (ISO) increased the shortening amplitudes of both groups (++P < 0.01) and diminished the difference seen at baseline.
Decreased shortening and relengthening velocities of the transgenic cardiomyocytes.
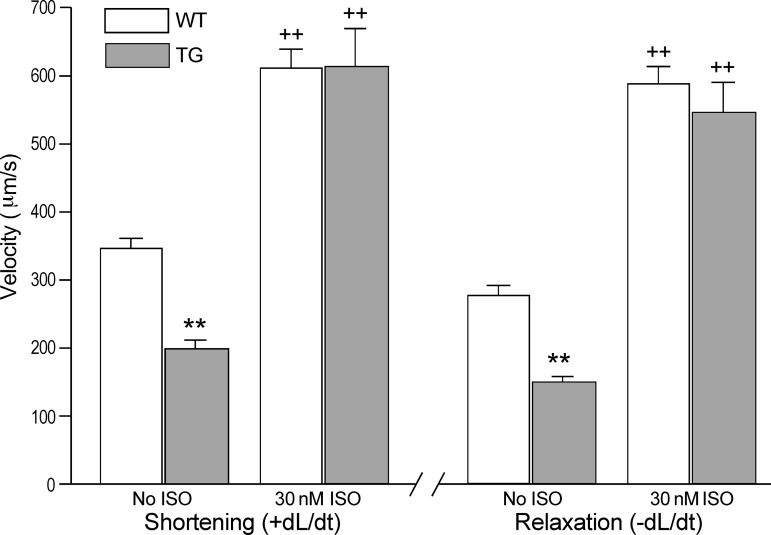
The transgenic mouse cardiomyocytes exhibited lower maximum shortening and relengthening velocities (±dL/dt) compared with wild-type controls (Fig. 2). Isoproterenol stimulation significantly increased +dL/dt and −dL/dt in both groups and the differences diminished (Fig. 2). Similar to the shortening amplitude responses, the preserved potential of contractile velocity of the transgenic cardiomyocytes suggested a reduced thin filament responsiveness to Ca2+ activation in the transgenic mouse cardiomyocytes coexpressing fast TnT and cardiac TnT.
Fig. 2.
Decreased shortening and relengthening velocities of transgenic mouse cardiomyocytes coexpressing fast TnT and cardiac TnT. Contractions paced at 2 Hz showed significantly decreased the rates of shortening (+dL/dt) and relengthening (−dL/dt; **P < 0.01) of the TG vs. WT mouse cardiomyocytes. ISO treatment augmented the contractile and relaxation velocities in both groups (++P < 0.01) and diminished the differences.
Prolonged contractile and relaxation durations of the transgenic mouse cardiomyocytes.
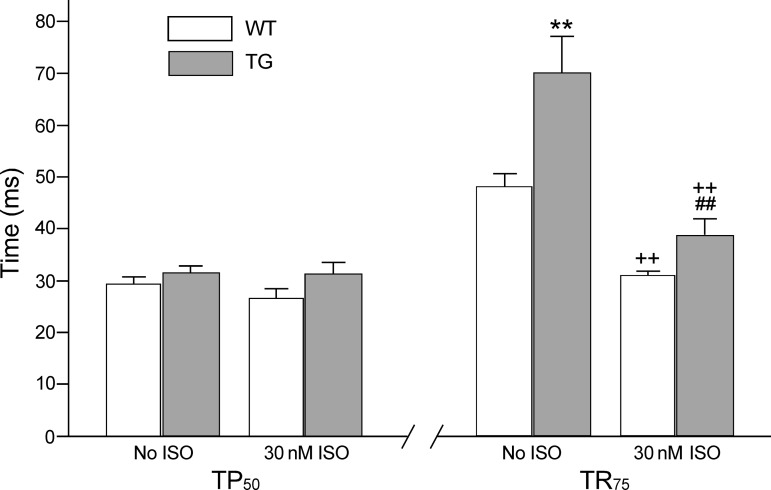
The transgenic mouse cardiomyocytes showed no significant difference in the time for the development of 50% peak shortening (TP50) compared with the wild-type control. Isoproterenol treatment did not have significant effect on TP50 (Fig. 3). The diastolic time parameter, time from peak shortening to 75% relengthening (TR75), was significantly prolonged in the transgenic cardiomyocytes compared with that of wild-type cells before isoproterenol treatment (Fig. 3). Isoproterenol significantly shortened the TR75 of both groups but the transgenic cardiomyocytes retained longer time of relaxation than the wild-type control (Fig. 3).
Fig. 3.
Prolonged relaxation time of transgenic cardiomyocytes coexpressing fast TnT and cardiac TnT. Contractions paced at 2 Hz showed that time to half peak shortening (TP50) was not significantly changed in the TG vs. the WT cardiomyocytes in the absence or presence of 30 nM ISO. However, time from peak shortening to 75% relengthening (TR75) was significantly prolonged in the TG vs. WT cardiomyocytes (**P < 0.01). TR75 was shortened by ISO treatment in both groups (++P < 0.01) but remained longer in the TG cardiomyocytes than the WT control (##P < 0.01).
Altered intracellular calcium transient in the transgenic mouse cardiomyocytes.
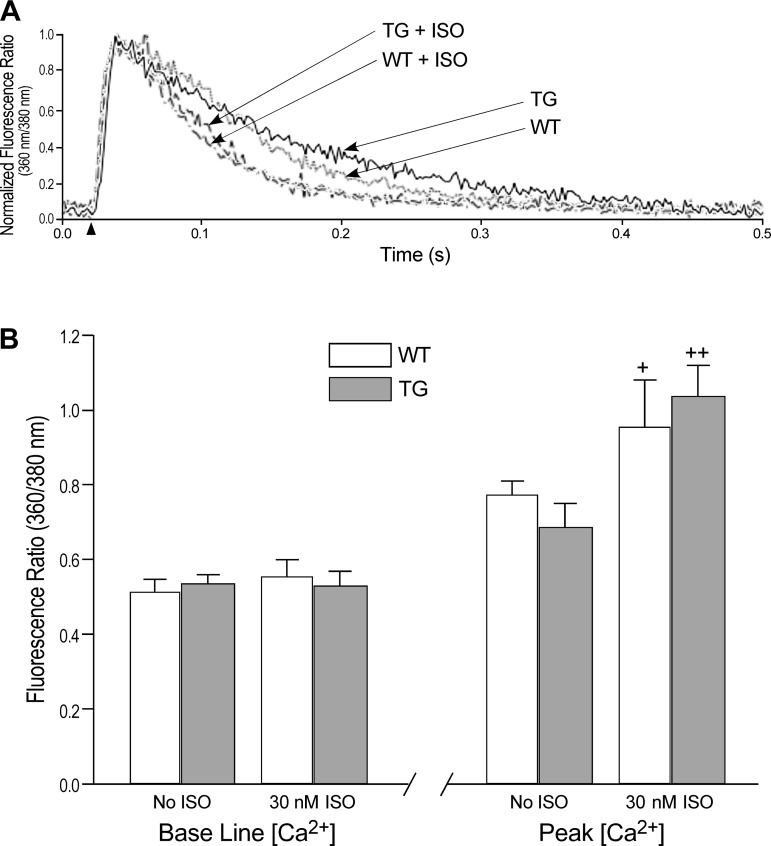
Simultaneous recording of the fura-2 AM fluorescence during the contractile measurements indicated intracellular Ca2+ transients in the transgenic and wild-type cardiomyocytes. The results showed no difference between the baseline cytosolic Ca2+ in the transgenic cardiomyocytes and in wild-type cells. Treatment with 30 nM isoproterenol did not have significant effect on the baseline Ca2+ in either group (Fig. 4). The peak of cytosolic Ca2+ transient following the electrical stimulation showed no significant difference between the transgenic cardiomyocytes and the wild-type cells. Isoproterenol treatment increased the peak Ca2+ transient similarly in both groups (Fig. 4).
Fig. 4.
Intracellular Ca2+ transient in TG and WT cardiomyocytes. A: representative fura-2 AM fluorescence (the ratio of 360-to-380 nm-excited emission at 510 nm) traces of intracellular Ca2+ transient in TG and WT cardiomyocytes at baseline and under ISO treatment. B: quantitative analysis showed no difference in the levels of resting Ca2+ between TG and WT cardiomyocytes. Treatment with ISO did not result in significant change in resting Ca2+. Peak intracellular Ca2+ transient was also similar in the TG and WT cardiomyocytes and isoproterenol treatment increased peak Ca2+ transient in both groups (+P < 0.05, ++P < 0.01).
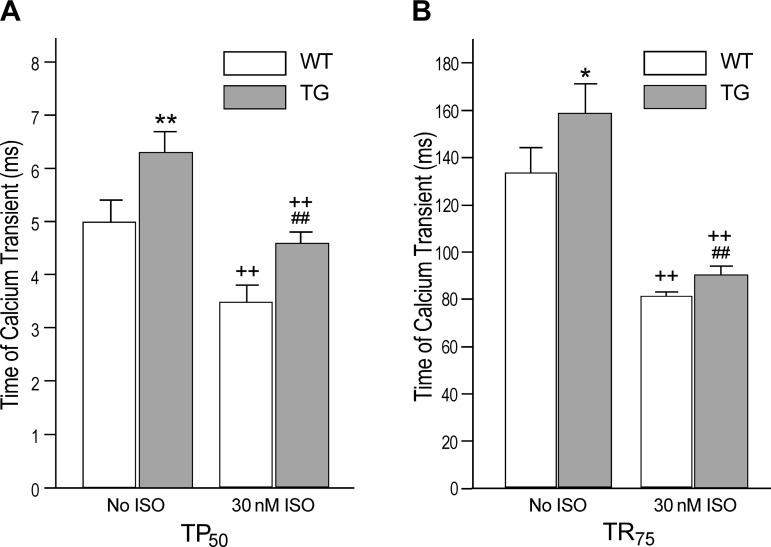
The time to develop 50% peak cytosolic Ca2+ (TP50) was longer in the transgenic cardiomyocytes than that in wild-type cells (Fig. 5A). Isoproterenol treatment significantly shortened TP50 of Ca2+ transient in both groups but the difference remained (Fig. 5A). The time from peak Ca2+ transient to 75% decay (TR75) was also longer in the transgenic cardiomyocytes than that in wild-type cells at baseline (Fig. 5B). Isoproterenol treatment significantly shortened TR75 of Ca2+ transient in both groups, but the difference remained (Fig. 5B).
Fig. 5.
Prolonged time parameters of intracellular Ca2+ transient in transgenic mouse cardiomyocytes. Time to a half peak cytosolic Ca2+ (TP50; A) and time from peak Ca2+ to 75% decay (TR75; B) were longer in the TG vs. WT cardiomyocytes (**P < 0.01, *P < 0.05). Time parameters were effectively shortened by ISO treatment in both groups (++P < 0.01) but were still longer in the transgenic cardiomyocytes (##P < 0.01 compared with the ISO-treated WT control).
Coexpressing fast TnT and cardiac TnT did not affect PKA-catalyzed phosphorylation of cardiac TnI and MBP-C.
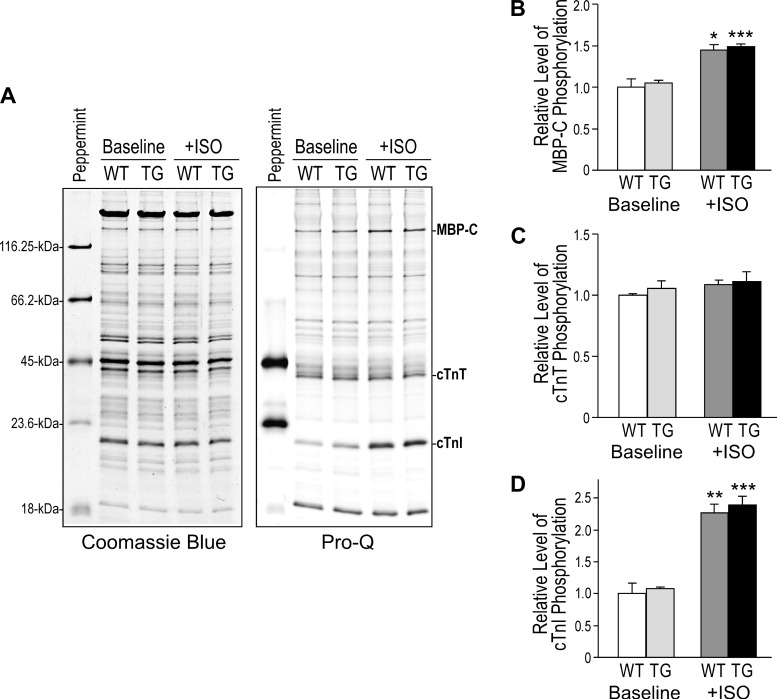
Pro-Q stain of phosphoproteins and densitometry quantification (Fig. 6) showed that isoproterenol treatment in vivo significantly increased the phosphorylation levels of MBP-C and cardiac TnI, both are known PKA substrate in the cardiomyocytes. The baseline levels and isoproterenol effects were similar in WT and TG hearts. The levels of phosphorylated cardiac TnT were similar in WT and TG mouse hearts at baseline and were not changed in the isoproterenol-treated groups. Considering that the decreased cardiac TnT due to the replacement by fast TnT that was not detected with significant phosphorylation at baseline or treated with isoproterenol, the result indicated an increase in the relative level of cardiac TnT phosphorylation in the TG hearts. The functional significance remains to be investigated.
Fig. 6.
Coexistence of fast TnT and cardiac TnT did not affect PKA-catalyzed phosphorylation of cardiac TnI and myosin-binding protein C (MBP-C). A: Pro-Q stain of phosphoproteins in SDS-gel was used to detect phosphoproteins in the TG and WT hearts with or without ISO treatment in vivo. PeppermintStick phosphoprotein markers were used as background control. Densitometry quantification showed that ISO treatment significantly increased the phosphorylation of MBP-C (B) and cardiac TnI (D) similarly in WT and TG hearts. C: levels of phosphorylated cardiac TnT were similar in WT and TG mouse hearts and were not different in the ISO-treated group. Considering that the presence of fast TnT that showed no significant phosphorylation, the result indicated an increase in the relative level of cardiac TnT phosphorylation in the TG hearts. A total of 12 mice were examined (n = 3 mice each in the baseline and ISO-treated WT and TG groups). Values are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the baseline level in Student's test.
Transgenic mouse cardiomyocytes overexpressing fast skeletal muscle TnT had shorter resting sarcomere length under rigor condition.
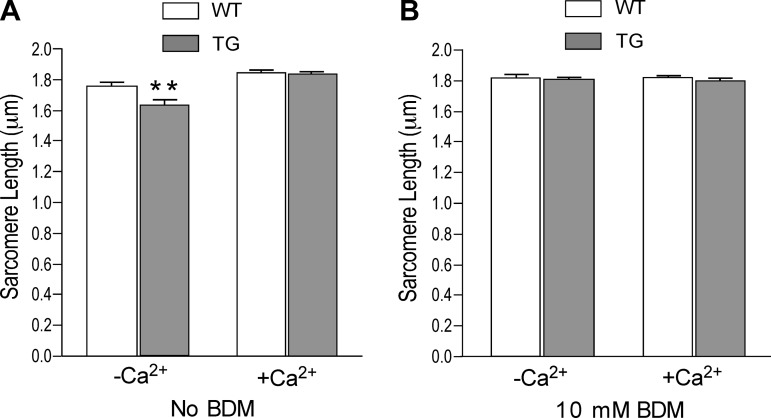
Before restoration of Ca2+ in the isolation media, the transgenic cardiomyocytes exhibited shorter resting sarcomere length than that of wild-type cells (1.66 ± 0.01 and 1.76 ± 0.02 μm, respectively; Fig. 7A). After restoration of extracellular Ca2+ to 1.25 mM, the resting sarcomere lengths of both transgenic and wild-type cardiomyocytes became longer and the difference between them disappeared (Fig. 7A). The normal resting sarcomere length of the transgenic cardiomyocytes at the physiological condition allowed comparable functional studies.
Fig. 7.
Effects of the presence of fast TnT on resting sarcomere length of transgenic mouse cardiomyocytes. A: in Ca2+-depleted media, resting sarcomere length in the TG cardiomyocytes was shorter than that of the WT cells (**P < 0.01). After restoration of physiological concentration of extracellular Ca2+, sarcomere length increased slightly in WT but significantly in TG groups and their difference disappeared. B: 10 mM butanedione monoxime (BDM) also increased the resting sarcomere lengths and diminished the different effects of Ca2+ depletion on TG and WT cardiomyocytes.
On the other hand, the effect of restoration of Ca2+ on increasing resting sarcomere length reflected the role of Ca2+ in the function of mitochondria for the production of ATP (2), which is critical to releasing the myofibrils from the rigor state generated due to ATP depletion during the cell isolation procedure (18). The effect of fast TnT on the rigor resting sarcomere length in the transgenic mouse cardiomyocytes suggested a role of TnT function in the state of actin-myosin cross bridges. Supporting this notion, inhibition of myosin ATPase by BDM to maintain a state of weak cross bridges before Pi release (33) retained normal resting sarcomere length before and after the restoration of extracellular Ca2+ and diminished the difference between transgenic and wild-type cardiomyocytes (Fig. 7B).
Chronic coexistence of fast skeletal muscle TnT and cardiac TnT resulted elongation of transgenic mouse cardiomyocytes.
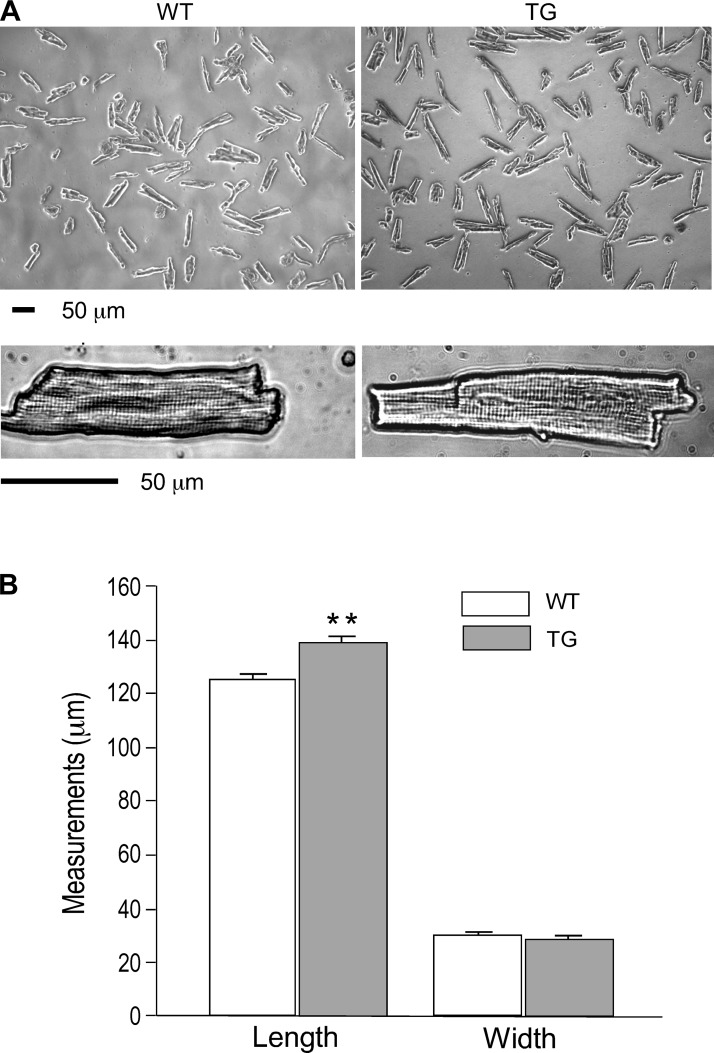
At physiological level of extracellular Ca2+, the overall morphology of the isolated transgenic mouse cardiomyocytes was similar to that of wild-type cells (Fig. 8A). However, the resting transgenic cardiomyocytes were about 11% longer than age-matched wild-type controls (139.16 ± 2.29 and 125.48 ± 1.77 μm, respectively) whereas the cell widths were unchanged (Fig. 8B). The results indicated a longitudinally increased number of sarcomeres in the transgenic mouse cardiac myofibrils. This change in cellular structure suggested a dilatative remodeling of the transgenic mouse cardiomyocytes as a pathological consequence of the decreased contractility due to the chronic coexistence of two TnT variants in the adult cardiomyocytes.
Fig. 8.
Increased length of transgenic mouse cardiomyocytes coexpressing fast TnT and cardiac TnT. A: phase contrast microscopic images of isolated adult WT and TG mouse ventricular cardiomyocytes. B: cell length and width measured after restoration of Ca2+ in the extracellular medium showed increased lengths of the TG cardiomyocytes (**P < 0.01) with no change in cell width.
DISCUSSION
Coexistence of nonmutant fast TnT and endogenous cardiac TnT in the myofilaments of adult cardiomyocytes decreased contractility.
The present study demonstrated decreased contractility of adult transgenic mouse cardiomyocytes coexpressing fast skeletal muscle TnT and the endogenous cardiac TnT. Compared with that of wild-type adult mouse cardiomyocytes, the transgenic cardiomyocytes exhibited smaller amplitude of contraction and lower shortening and relengthening velocities together with prolonged duration of relaxation. The decreased contractile amplitude and kinetics indicated that myofilament activation and deactivation in the transgenic mouse cardiomyocytes were both affected. Although the contractility measured in isolated cardiomyocytes reflected functions in the absence of external load, the data provided cellular level evidence for the role of TnT function in the pathogenesis of heart failure. A particular value of the data is the exclusion of influence of myocardial extracellular matrix that often undergoes significant remodeling in chronic heart failure models.
We previously demonstrated that the total contents of TnT remained normal in transgenic mouse cardiac muscle overexpressing fast skeletal muscle TnT (12, 21) or variants of cardiac TnT (7). We (12, 21) have previously reported that in the transgenic mouse line studied here fast TnT was incorporated in the myofilaments together with the endogenous cardiac TnT at the ratio of appropriately 50:50. Our data showed that this coexistence of fast skeletal muscle TnT and cardiac TnT in the thin filament regulatory system of adult transgenic mouse cardiomyocytes had a negative impact on contractility.
The incorporation of fast skeletal muscle TnT in cardiac myofilaments together with the endogenous cardiac TnT may have two functional consequences: 1) an overall shift in the thin filament Ca2+ responsiveness due to functional differences between the two TnT isoforms; and 2) decreased contractile efficiency due to desynchronization of the thin filament Ca2+ regulatory system in the adult cardiac muscle that normally contains solely cardiac TnT (7). The lower amplitude and wider duration of the contractile curve of the transgenic mouse cardiomyocytes vs. that of wild-type cells in Fig. 1A support the hypothesis of desynchronized contraction and relaxation. In addition to providing evidence for the function of different TnT isoforms in fine-tuning of muscle contractility, the data supported a hypothesis that the presence of two functionally distinct isoforms of TnT in the adult cardiac muscle has dominantly negative effects on heart function (7, 13).
There are two intracellular nontroponin factors that may affect the shortening amplitude and velocity of isolated cardiomyocytes in the absence of external load. One is the restoration force maintaining the slack resting length of the sarcomere, which may determine the contractility of cardiac muscle based on Frank-Starling mechanism. The other is the cytoskeleton stiffness that resists to shortening. There was no change in resting sarcomere length in media containing physiological concentration of Ca2+ as used in the functional analysis. Electron microscopy showed that the sarcomere structure in the transgenic mouse cardiac muscle expressing fast skeletal muscle TnT was apparently normal (data not shown), implying no change in cytoskeleton structure. Therefore, the altered thin filament Ca2+ responsiveness would be the primary cause of the decreased contractile function.
Effect of TnT function on intracellular Ca2+ transient.
The unchanged baseline Ca2+ in the transgenic mouse cardiomyocytes indicated no chronic Ca2+ overloading or underloading in the cells. The peak amplitude of cytosolic Ca2+ transient was also similar in the transgenic and wild-type mouse cardiomyocytes (Fig. 4). The results suggested unchanged resting level Ca2+ that provided normal loading of the sarcoplasmic reticulum and other intracellular reservoirs, corresponding to unchanged Ca2+ release during contraction.
On the other hand, significantly prolonged durations of the Ca2+ transient were found in the transgenic mouse cardiomyocytes (Fig. 5). Isoproterenol corrected the decreases in shortening amplitude and peak amplitude of Ca2+ transient in the transgenic cardiomyocytes (Fig. 1B and Fig. 4) but not the prolonged durations of the Ca2+ transient (Fig. 5). Whereas the change in troponin function could not directly explain the effects on intracellular Ca2+ transient and further investigation is needed, this phenotype may suggest prolonged time for Ca2+ to associate to and dissociate from the transgenic mouse cardiac myofilaments, in which the desynchronized TnT function in the cardiac muscle thin filaments may play a role in broadening the overall time of Ca2+ binding to and releasing from troponin.
Incorporation of fast TnT in the thin filaments of transgenic mouse cardiomyocytes produced a decrease in rigor resting sarcomere length.
An interesting observation of the present study is the shorter resting sarcomere length in the transgenic mouse cardiomyocytes in Ca2+ depletion (Fig. 7A). After restoration of physiological concentration of extracellular Ca2+, the resting sarcomere length became normal (Fig. 7A). The effect of Ca2+ on resuming ATP production in the mitochondria (2) would release the myofilaments from rigor cross bridge attachment. Supporting the correlation between altered TnT function and rigor resting sarcomere length, preventing strong cross bridge formation with BDM (33) produced normal sarcomere length in the transgenic mouse cardiomyocytes and diminished the effect of Ca2+ depletion (Fig. 7B). Supporting the relationship between troponin function and the state of cross bridges, BDM-dependent shorter resting sarcomere length was also reported in a previous study in transgenic mouse cardiomyocytes overexpressing I92Q mutant cardiac TnT (26). Another previous study (11) provided further support by observing effect of rigor cross bridges on the molecular conformation of troponin C in rat cardiac muscle.
Titin restoration force plays a determining role in the slack length of isolated cardiolmyocytes (17). Titin is known to bind F-actin (15). Therefore, the effect of TnT function onresting sarcomere length of the transgenic mouse cardiomyocytes may correlate to the interaction between titin and the thin filament. Fast skeletal muscle TnT and cardiac TnT differ mainly in the NH2-terminal variable region. Structural alterations in the NH2-terminal region of TnT convey conformational and functional changes in the middle and COOH-terminal regions to modulate the function of the thin filament regulatory system (16, 31). The NH2-terminal region of TnT also increases the cooperativity of myosin S1 binding to the thin filament (24). These mechanisms may contribute to the altered rigor resting state of the transgenic mouse cardiac myofilaments containing fast skeletal muscle TnT. Further investigation is needed to better understand the effect of TnT and thin filament function on the rigor resting length of cardiac sarcomeres.
Chronic coexistence of fast TnT and cardiac TnT in adult mouse cardiomyocytes resulted in dilatative remodeling.
The increased resting length but not width of the transgenic mouse cardiomyocytes (Fig. 8B) provided an evidence for the effect of TnT function on the pathogenesis of dilated cardiomyopathy and terminal heart failure. It was previously demonstrated that desynchronized contraction due to the functional difference between fast skeletal muscle TnT and cardiac TnT decreases cardiac function (13) and myocardial energetic efficiency (7). Therefore, whereas the fast TnT incorporated into the transgenic mouse cardiac muscle is a normal TnT protein without any pathological mutation, the chronic coexistence of two functionally distinct TnT variants in the thin filament regulatory system had a negative impact on cardiac function and caused dilatative remodeling in the cardiomyocytes prior to clinical heart failure.
It is worth noting that some vertebrate species naturally express more than one splicing variants of cardiac TnT in the adult hearts [e.g., bovine (27), rat (14, 23), and porcine (19)]. Although no clinical weakness of the heart has been reported in these species, the functional consequences, especially the chronic effect on cardiac efficiency, in those species remain to be studied.
The profound dilatative remodeling in the transgenic mouse hearts was not accompanied by detectable hypertrophic changes in the cardiomyocytes. This observation suggested a primary effect of TnT abnormality on causing dilated cardiomyopathy and heart failure as that seen in turkey (3) and dog (4) models. The cellular signaling mechanism between the altered thin filament Ca2+ responsiveness and the elongation of cardiac myofibrils requires further investigation to fully understand the pathogenesis of dilated cardiomyopathy and congestive heart failure.
GRANTS
This work was supported in part by National Institutes of Health Grants AR-048816, HL-078773, and HL-098945 (to J.-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.-B.Y. and H.W. performed experiments; Z.-B.Y., H.W., and J.-P.J. analyzed data; Z.-B.Y., H.W., and J.-P.J. interpreted results of experiments; Z.-B.Y., H.W., and J.-P.J. prepared figures; Z.-B.Y., H.W., and J.-P.J. drafted manuscript; Z.-B.Y., H.W., and J.-P.J. approved final version of manuscript; J.-P.J. conception and design of research; J.-P.J. edited and revised manuscript.
ACKNOWLEDGMENTS
Z.-B. Yu participated in this study when this research group was at the Case Western Reserve University School of Medicine and the Evanston Northwestern Healthcare Research Institute.
REFERENCES
- 1.Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76: 681–686, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34: 1259–1271, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Biesiadecki BJ, Jin JP. Exon skipping in cardiac troponin T of turkeys with inherited dilated cardiomyopathy. J Biol Chem 277: 18459–18468, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Biesiadecki BJ, Elder BD, Yu ZB, Jin JP. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem 277: 50275–50285, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin JP. Coupled expression of troponin t and troponin i isoforms in single skeletal muscle fibers correlating to contractility. Am J Physiol Cell Physiol 290: C567–C576, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong SM, Jin JP. To investigate protein evolution by detecting suppressed epitope structures. J Mol Evol 68: 448–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng HZ, Jin JP. Coexistence of cardiac troponin T variants reduces heart efficiency. Am J Physiol Heart Circ Physiol 299: H97–H105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng HZ, Chen M, Weinstein LS, Jin JP. Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling. J Biol Chem 283: 33384–33393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes AV, Guzman G, Zhao J, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development: Insights into the role of troponin T isoforms in the heart. J Biol Chem 277: 35341–35349, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Hannon JD, Martyn DA, Gordon AM. Effects of cycling and rigor crossbridges on the conformation of cardiac troponin C. Circ Res 71: 984–991, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Huang QQ, Brozovich FV, Jin JP. Fast skeletal muscle troponin T increases the cooperativity of transgenic mouse cardiac muscle contraction. J Physiol 520: 231–242, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang QQ, Feng HZ, Liu J, Du J, Stull LB, Moravec CS, Huang X, Jin JP. Coexpression of skeletal and cardiac troponin T decreases mouse cardiac function. Am J Physiol Cell Physiol 294: C213–C222, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Jin JP, Lin JJC. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat heart during development. J Biol Chem 263: 7309–73015, 1988 [PubMed] [Google Scholar]
- 15.Jin JP. Cloned rat cardiac titin class I and class II motifs. Expression, purification, characterization, and interaction with F-actin. J Biol Chem 270: 6908–69016, 1995 [PubMed] [Google Scholar]
- 16.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation and functional adaptations of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124, 2008 [DOI] [PubMed] [Google Scholar]
- 17.King NM, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M, Granzier H. Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. J Gen Physiol 137: 81–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kléber AG. Consequences of acute ischemia for the electrical and mechanical function of the ventricular myocardium. A brief review. Experientia 46: 1162–1167, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol 98: 461–467, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mesnard-Rouiller L, Mercadier JJ, Butler-Browne G, Heimburger M, Logeart D, Allen PD, Samson F. Troponin T mRNA and protein isoforms in the human left ventricle: pattern of expression in failing and control hearts. J Mol Cell Cardiol 29: 3043–3055, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Montgomery D, Chandra M, Huang QQ, Jin JP, Solaro RJ. Transgenic incorporation of chicken fast skeletal muscle TnT into cardiac myofilaments blunts the PKC-induced depression of maximal tension. Am J Physiol Heart Circ Physiol 280: H1011–H1018, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ. Tension production and thin-filament protein isoforms in developing rat myocardium. Am J Physiol Heart Circ Physiol 267: H1589–H1596, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Schaertl S, Lehrer SS, Geeves MA. Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry 34: 15890–15894, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A, Gulick J, Neumann J, Knotts S, Robbins J. Transgenic analysis of the thyroid-responsive elements in the a-cardiac myosin heavy chain promoter. J Biol Chem 268: 4331–4336, 1993 [PubMed] [Google Scholar]
- 26.Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, Robbins J, Leinwand LA. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest 104: 469–481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobacman LS. Structure-function studies of the amino-terminal region of bovine cardiac troponin T. J Biol Chem 263: 2668–2672, 1988 [PubMed] [Google Scholar]
- 28.Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol 58: 447–481, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Jin JP. Primary structure and developmental acidic to basic transition of 13 alternatively spliced mouse fast skeletal muscle troponin T isoforms. Gene 193: 105–114, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Jin JP. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry 37: 14519–14528, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: evolution, regulation and function. Arch Biochem Biophys 505: 144–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Naber N, Cooke R. Muscle cross-bridges bound to actin are disordered in the presence of 2, 3-butanedione monoxime. Biophys J 68: 1980–1990, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]