Abstract
A novel vasodilatory influence of endothelial cell (EC) large-conductance Ca2+-activated K+ (BK) channels is present after in vivo exposure to chronic hypoxia (CH) and may exist in other pathological states. However, the mechanism of channel activation that results in altered vasoreactivity is unknown. Previously, we demonstrated that inhibition of either BK channels or heme oxygenase (HO) restores vasoconstrictor reactivity after CH. Additionally, administration of the scaffolding domain of caveolin (Cav)-1 inhibits EC BK activity and restores vasoconstrictor reactivity in this setting. These results led us to hypothesize that CH exposure results in a loss in Cav-1 inhibition of EC BK channels, resulting in their activation by HO-derived carbon monoxide (CO). Experiments were conducted on freshly dispersed aortic ECs from control and CH-exposed (barometric pressure: 380 mmHg for 48 h) rats. In electrophysiology experiments, outward currents were greater in cells from CH rats as well as from cells from control rats treated with the cholesterol-depleting agent methyl-β-cyclodextrin. These enhanced currents were returned to control by HO inhibition. Channel activity could be restored by the CO donor CO-releasing molecule (CORM)-2 during HO inhibition. Administration of the Cav-1 scaffolding domain eliminated BK currents in cells from CH rats, and current was not restored by the addition of CORM-2. Colocalization experiments in ECs from control and CH rats demonstrated an association between HO-2, Cav-1, and BK. We conclude that EC BK channel activity is HO dependent in the absence of the inhibitory effect of the Cav-1 scaffolding domain.
Keywords: electrophysiology, methyl-β-cyclodextrin, scaffolding domain, large-conductance calcium-activated potassium channels
chronic hypoxia (CH) results from pathological conditions or prolonged residence at high altitude that impair oxygenation. Patients with hypoxemia resulting from obstructive lung diseases have lower calf vascular resistance (6) and blunted reflex vasoconstriction in response to a challenge of lower body negative pressure (15). In experimental models of chronic hypoxemia, systemic vasoconstrictor responsiveness is attenuated after a prolonged exposure to either normobaric or hypobaric hypoxia (1, 10). In addition, CH reduces total peripheral resistance responses to vasoconstrictor agonists (10) and diminishes vasoconstrictor reactivity within several systemic vascular beds (7, 14, 20, 27). Upon return to normoxic conditions, the blunted vasoconstrictor reactivity persists (10) and remains for up to 96 h (20). This response is associated with vascular smooth muscle (VSM) and endothelial cell (EC) membrane potential hyperpolarization that involves the activation of endothelial large-conductance Ca2+-activated K+ (BK) channels (17, 24). Consistent with these observations, removal of the endothelium restores agonist-induced and myogenic vasoconstrictor reactivity within the mesenteric and gracilis circulations after CH (11, 12, 17) and contractile responsiveness in aortic rings (7). These observations are paralleled by the demonstration of BK currents in ECs from the aorta and gracilis arterioles (17).
Activity of endothelial BK channels is inhibited by the scaffolding domain of caveolin (Cav)-1 (17, 29, 35) and may be enhanced by a variety of endothelial vasoactive substances. Indeed, nitric oxide (NO), carbon monoxide (CO), and the cytochrome P-450 product 11,12-epoxyeicosatrienoic acid all activate BK channels (2, 4, 9, 18, 38). Previous work (14) from our laboratory has demonstrated a role of the CO-producing enzyme heme oxygenase (HO) as a hyperpolarizing influence after CH that diminishes agonist-induced vasoconstrictor reactivity. Additionally, HO inhibition or administration of the BK channel blocker iberiotoxin (IBTX) similarly restores VSM membrane potential (17, 25) in arteries from CH rats. There is also evidence in other cell types of association between HO and BK channels (22, 36, 39), and the HO product CO activates VSM BK channels (19, 31–34). Since EC BK channels appear to be tonically active after CH, we hypothesized that HO-derived CO serves as an endogenous stimulus under conditions in which Cav-1 inhibition of the channel is impaired. This novel mode of endothelium-dependent vascular regulation would explain the HO and BK dependency of diminished vasoconstrictor reactivity in this setting.
METHODS
Animals
Experiments were performed on male Sprague-Dawley rats (Harlan). All procedures were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Hypoxic Exposure
CH rats were exposed to hypobaric hypoxia at a barometric pressure of 380 mmHg for 48 h. Normoxic control rats were housed in identical cages at ambient pressure (∼630 mmHg).
Isolation of ECs
Freshly dispersed aortic ECs were used for patch-clamp and immunofluorescence imaging experiments. The aorta was chosen as the source of cells based on earlier results showing parallel endothelium-dependent attenuation of vasoconstrictor reactivity after CH in aortic rings and resistance vessels that was similarly reversed by HO inhibition (7, 14). Furthermore, cells from the aorta and gracilis resistance arteries demonstrate identical effects of CH to unmask functional BK channels (17). Aortae were removed and placed in ice-cold HEPES-buffered physiological saline solution (HBSS), which contained 150 mmol/l NaCl, 6 mmol/l KCl, 1 mmol/l MgCl2, 1.5 mmol/l CaCl2, 10 mmol/l HEPES, and 10 mmol/l glucose and was adjusted to pH 7.4 with NaOH. Thoracic aortae were cut longitudinally and subsequently incubated for 2 h in basal endothelial growth medium with 4% BSA and 10 μg/ml of the endothelium-specific probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine percholorate (Ac-LDL-Dil) at 37°C. Immediately after the endothelial labeling procedure previously described (16, 17, 28), aortae were cut into 2-mm strips and exposed to mild digestion solution containing 0.2 mg/ml DTT and 0.2 mg/ml papain in HBSS for 45 min at 37°C. Vessel strips were removed from the digestion solution and placed in 1 ml of HBSS containing 2 mg/ml BSA. Single ECs were released by gentle trituration with a small-bore Pasteur pipette and were stored at 4°C between experiments for up 5 h. One to two drops of the cell suspension were seeded on a glass coverslip mounted on an inverted fluorescence microscope (Olympus IX71) for 30 min before superfusion. Single ECs and EC clusters were identified by the selective uptake of fluorescently labeled Ac-LDL-Dil with a rhodamine filter before each electrophysiological experiment.
Patch-Clamp Experiments
Single freshly dispersed ECs were superfused under constant flow (2 ml/min) at room temperature (22–23°C) in an extracellular solution (conaining 141 mmol/l NaCl, 4.0 mM KCl, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 10 mmol/l HEPES, and 10 mmol/l glucose and buffered to pH 7.4 with NaOH). Whole cell current data were generated with an Axopatch 200B amplifier (Axon Instruments) after a 5-min dialysis period using 4- to 6-MΩ patch electrodes filled with an intracellular solution (containing 140 mmol/l KCl, 0.5 mmol/l MgCl2, 5 mmol/l Mg2ATP, 10 mmol/l HEPES, and 1 mmol/l EGTA and adjusted to pH 7.2 with KOH). CaCl2 was added to yield a free Ca2+ concentration of 1 μM, as calculated using WinMAXC chelator software. Whole cell currents were measured in response to voltage steps applied from −60 to +150 mV in 10-mV increments from a holding potential of −60 mV. All experiments used a conventional whole cell patch-clamp configuration with the following biophysical criteria: seal resistance > 1 GΩ and series resistance < 25 MΩ. These criteria were confirmed after membrane rupture and monitored through the course of the experiment.
Protocols
Role of HO-derived CO in EC BK activity.
Experiments were performed on freshly dispersed ECs from control and CH rats. Whole cell macroscopic currents were recorded before and after superfusion with the HO inhibitors zinc protoporphryin (ZnPPIX; 500 nM) or chromium mesoporphyrin (CrMP; 100 μM). Additional experiments were conducted to test the effect of the HO substrate hemin (100 μM) on transmembrane currents as well as the effect of the CO donor CO-releasing molecule (CORM)-2 (100 μM) or its inactive form (iCORM-2). The identity of BK currents was confirmed in some experiments by use of the specific inhibitor IBTX (100 nM) or the BK activator NS-1619 (100 μM).
Role of Cav-1 and cholesterol in EC BK activity.
Experiments were performed on freshly dispersed cells from control and CH rats. Cells from control animals were superfused with the cholesterol-depleting agent methyl-β-cyclodextrin (MBCD; 100 μM) to mildly disrupt Cav-1 microdomains and unmask BK currents, as we have previously reported (17, 29). After this treatment, cells were superfused with ZnPPIX to assess the role of HO on channel activity. Cells from CH rats were first dialyzed with the cell-permeant Cav-1 scaffolding domain peptide (AP-CAV; 10 mM) to inhibit channel activity, as previously reported (29), and then superfused with the CO donor CORM-2 as described above to test if exogenous CO restores current.
Immunofluorescence of Isolated EC Clusters
Small sheets of ECs were freshly dispersed from the aorta (see detailed methods above) and used for immunofluorescent detection of BK-α, HO-1, HO-2, and Cav-1. One to two drops of the cell suspension were seeded on a glass coverslip for 30 min before fixation in 4% formaldehyde-PBS at room temperature for 15 min. After fixation, cells were permeabilized in 0.01% Triton-X-PBS for 10 min and blocked in 4% donkey serum in PBS for 1 h. Cells were incubated with primary antibodies for HO-1 (Stressgen) or HO-2 (Stressgen) and Cav-1 (BD Biosciences) for association of the HO isoforms with Cav-1. Other sections were treated with primary antibodies for BK-α (Alamone) and HO-1 or HO-2. All primary antibodies were detected with secondary antibodies conjugated to fluorescent dyes. Isolated cells were visualized with a confocal laser microscopy (LSM 510 Zeiss, ×63 oil-immersion lens). Colocalization of HO-1 and BK or HO-2 and BK was analyzed by calculating the Manders correlation coefficient. Images underwent nearest-neighbor deconvolution, and individual channels were thresholded to normalize intensity between channels, as we have previously described (29). Colocalization (pixel overlap between channels) was defined by Manders correlation coefficient values (23) ranging from 0 to 1, with values close to 0 indicating nonoverlapping images and values close to 1 reflecting colocalization. A similar analysis was performed to assess the association of Cav-1 with HO-1 and HO-.2.
RESULTS
Role of HO-Derived CO in EC BK Activity
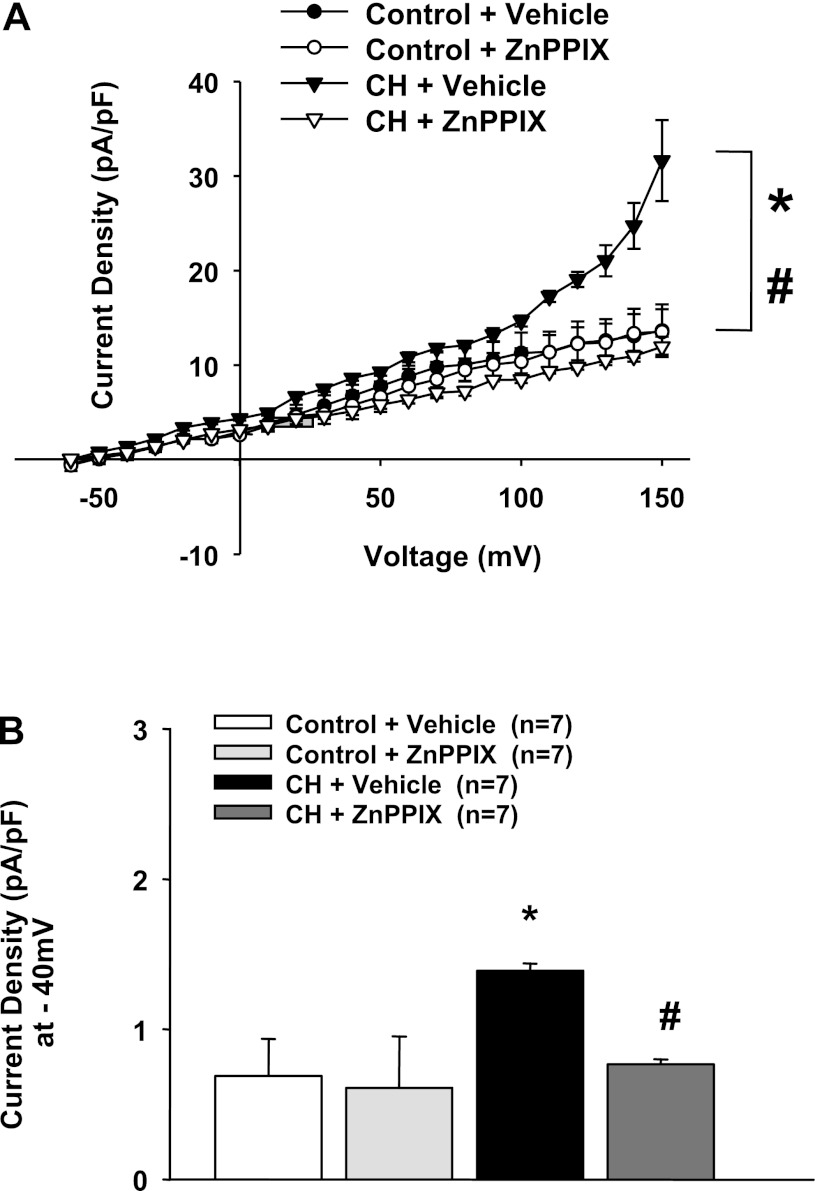
Endothelial transmembrane currents in cells from CH rats were significantly larger than controls (Fig. 1), as previously reported (17). This enhanced current in cells from CH rats has been previously shown to be blocked by IBTX (17), as shown in the present study (Fig. 2A). Interestingly, blockade of HO activity in ECs from normal animals similarly normalized currents compared with IBTX (Figs. 2, A and B, and 3, A and B), without affecting control cells (Figs. 1, A and B, and 2B). Additionally, the combination of the HO inhibitor ZnPPIX and IBTX did not significantly decrease outward currents more than ZnPPIX alone (Fig. 3A), suggesting that ZnPPIX- and IBTX-sensitive currents are derived from the same pathway. Furthermore, the effect of ZnPPIX is very likely a specific inhibitory action on HO activity and the production of CO, since we have previously shown specificity at even higher concentrations (7). Inhibition of BK activity by HO blockade was reversed by the BK channel opener NS-1619 (Fig. 4, A and B). We (17) have previously shown that NS-1619 is without effect in control cells. Thus, tonic BK channel activity is dependent on an HO product after CH; however, functional channels are still present in these cells and can be activated by other stimuli.
Fig. 1.
A: outward currents in endothelial cells (ECs) from chronic hypoxic (CH) rats were significantly greater than cells from control rats. Heme oxygenase (HO) inhibition with zinc protoporphryin (ZnPPIX) decreased outward currents only in ECs from CH rats. *P < 0.05 vs. vehicle-treated control rats over the range of −40 to +150 mV; #P < 0.05 vs. vehicle-treated CH rats over the range of −50 to +150 mV. B: current density at −40 mV in all groups. *Different from the vehicle-treated control group; #differed from the vehicle-treated control group.
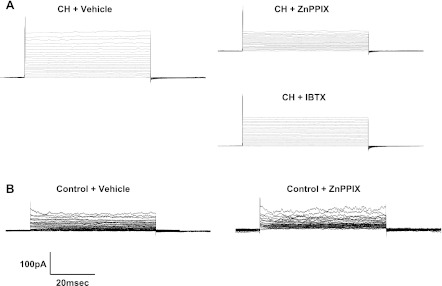
Fig. 2.
A: representative traces from patch-clamp step protocols in cells from CH rats under control conditions (vehicle) and after treatment with either ZnPPIX or iberiotoxin (IBTX). B: representative traces from patch-clamp step protocols in cells from control rats under vehicle and ZnPPIX treatments.
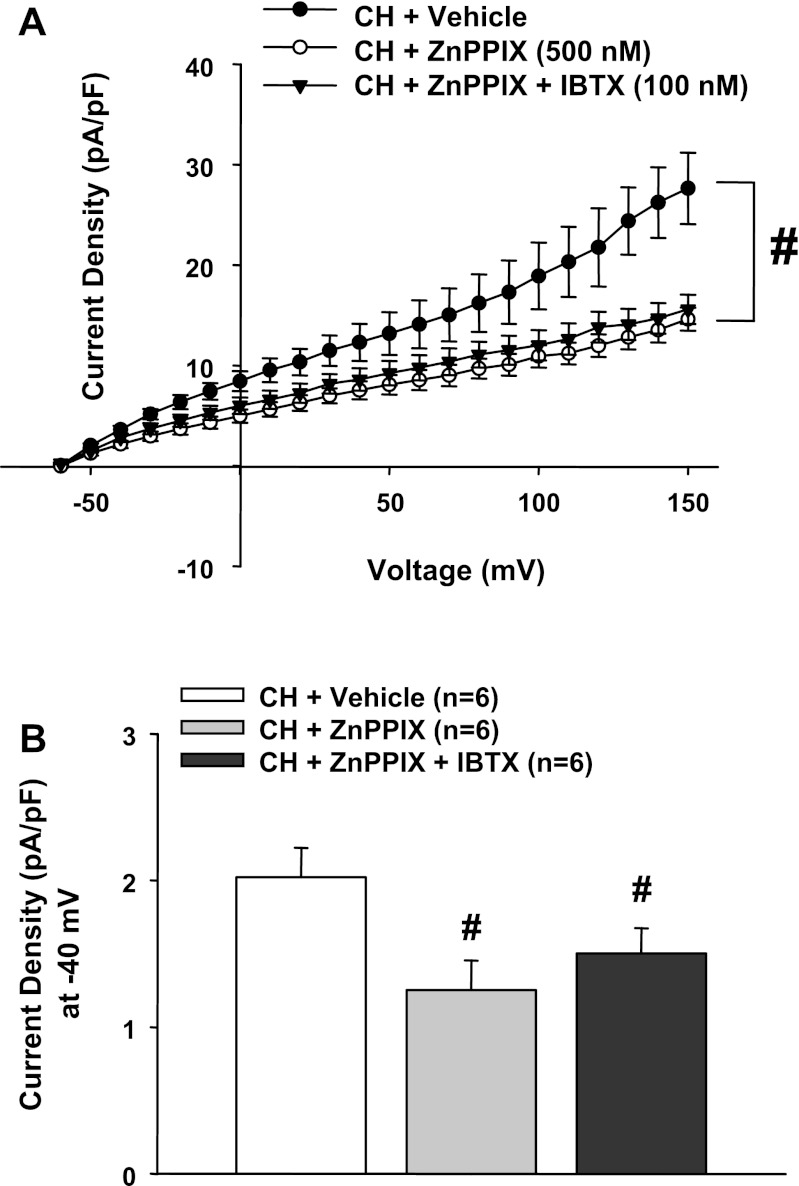
Fig. 3.
A: the inhibitory effect of ZnPPIX on outward current in CH cells was not different from the effect of combining ZnPPIX and the large-conductance Ca2+-activated K+ (BK) channel-specific inhibitor IBTX. B: current density at −40 mV in each group. n = 5–7 cells/group. #P < 0.05 vs. the vehicle-treated CH group.
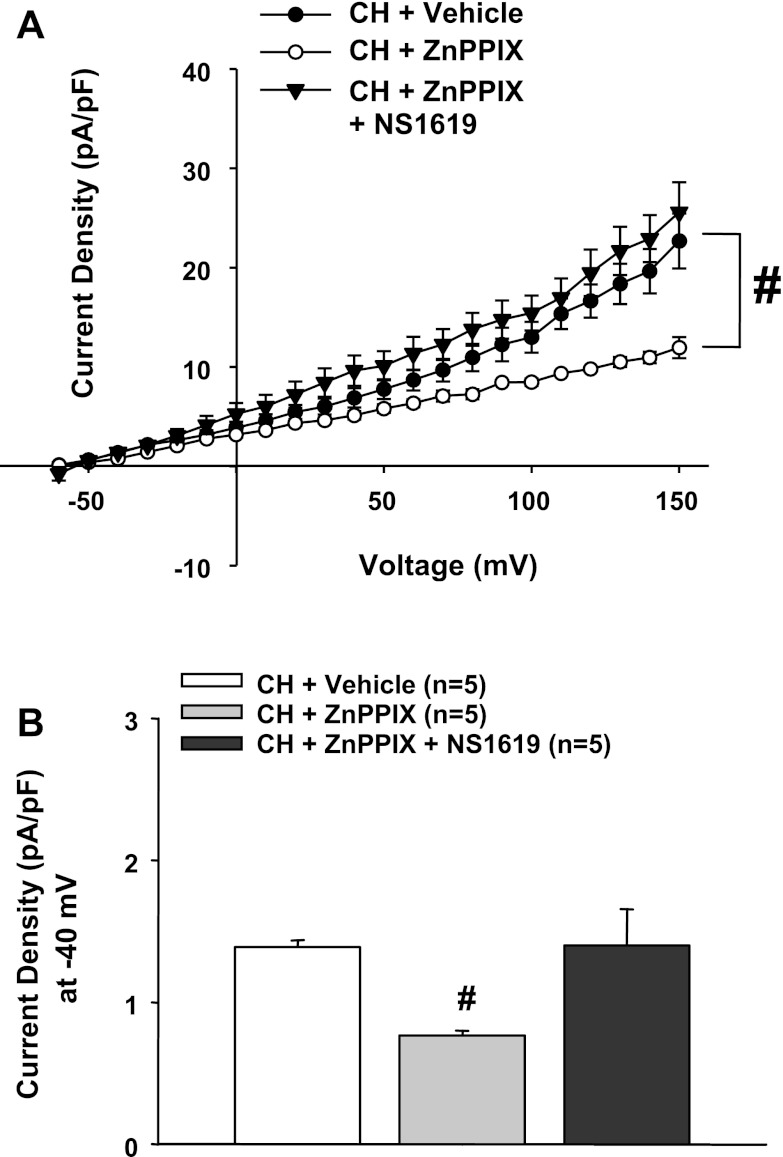
Fig. 4.
A: during HO inhibition with ZnPPIX, the BK activator NS-1619 restored outward currents to vehicle-treated CH levels. NS-1619 was without effect in control cells (17, 29). #P < 0.05 vs. the vehicle-treated CH group over the range of −30 to +150 mV. B: current density at −40 mV in all groups. #Different from the vehicle-treated CH group.
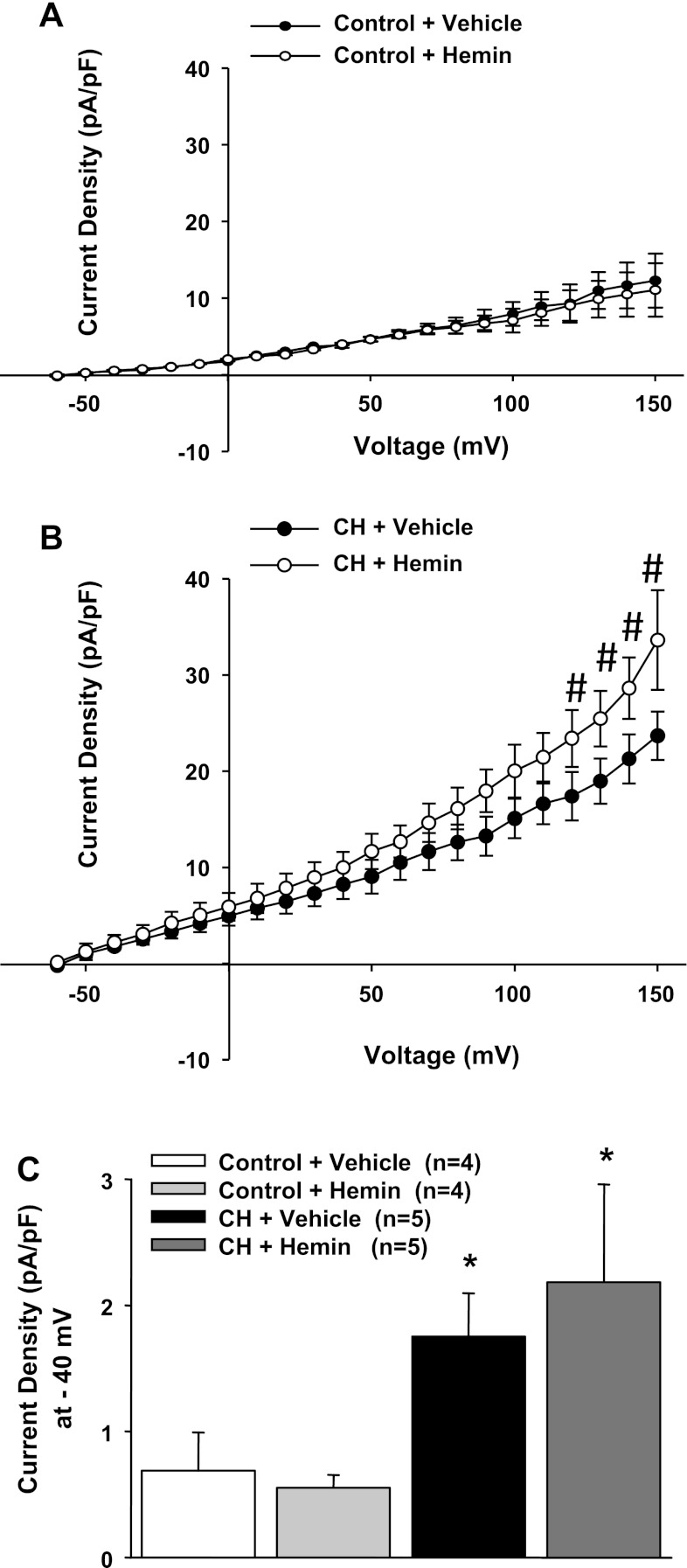
We also observed that the addition of excess substrate for the HO enzyme caused further enhancement of outward current only in cells from CH rats. Figure 5 shows this differential effect of hemin in cells from the two groups of animals; however, this augmentation was only observed at very positive membrane potentials and may not be relevant under physiological conditions. Nevertheless, these data suggest that HO activity may be mildly substrate limited in this preparation and that further stimulation of HO is associated with greater channel activity.
Fig. 5.
A: addition of the HO substrate hemin was without effect on outward currents in cells from control rats. B: hemin increased outward currents in cells from CH rats, but only over the range of +120 to +150 mV. #Different from the vehicle-treated CH group. C: summary data at −40 mV. *P < 0.05 vs. the vehicle-treated control group.
Role of CO in BK Channel Activation
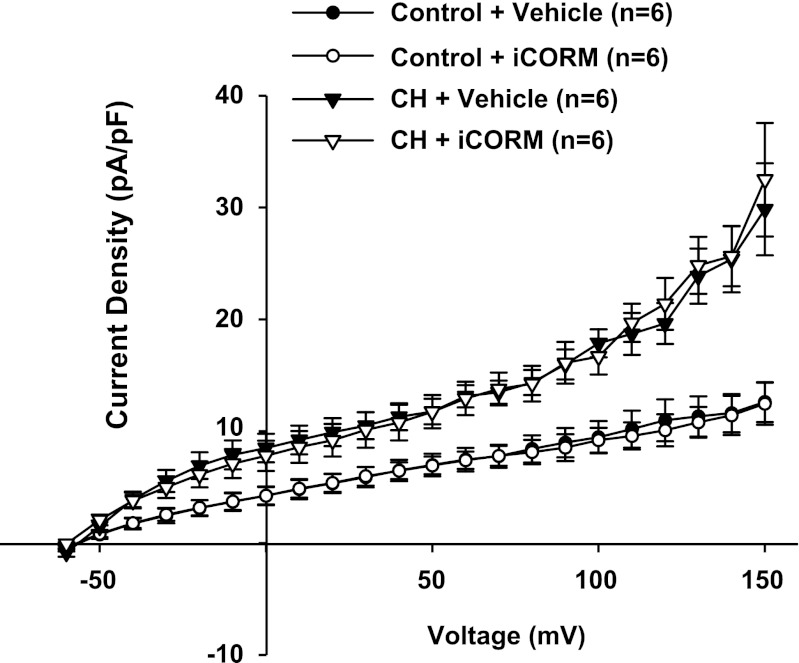
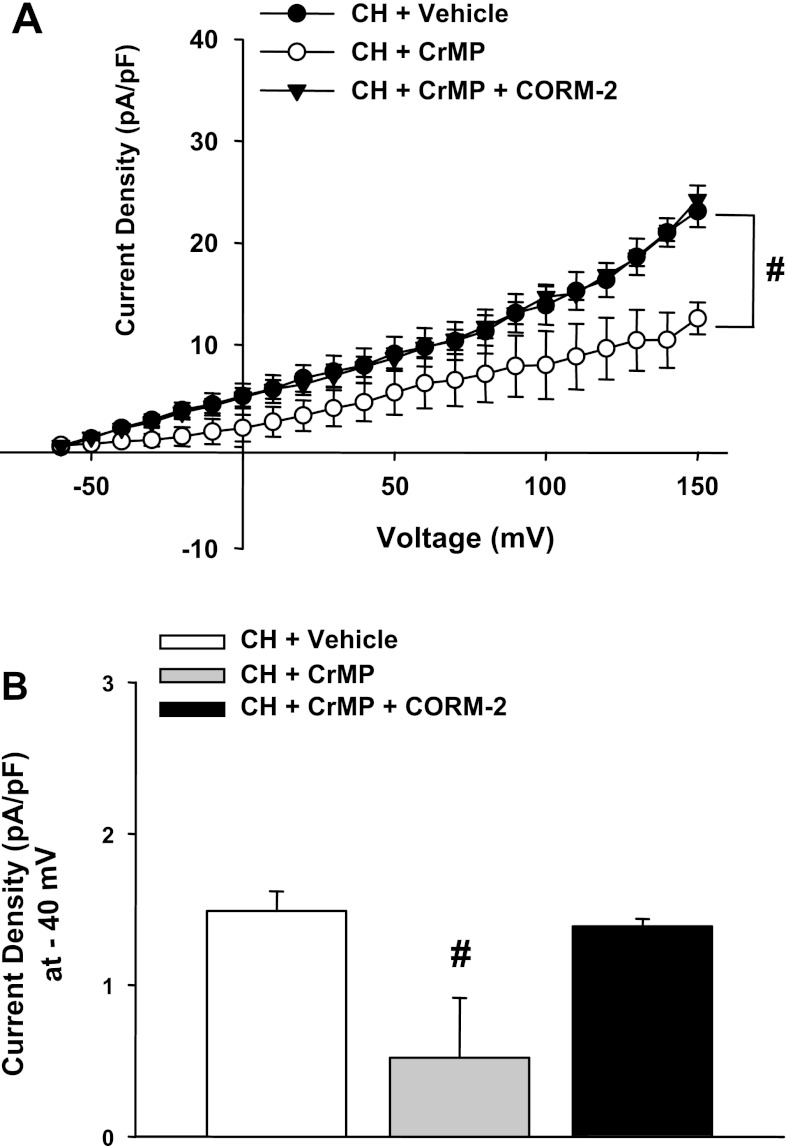
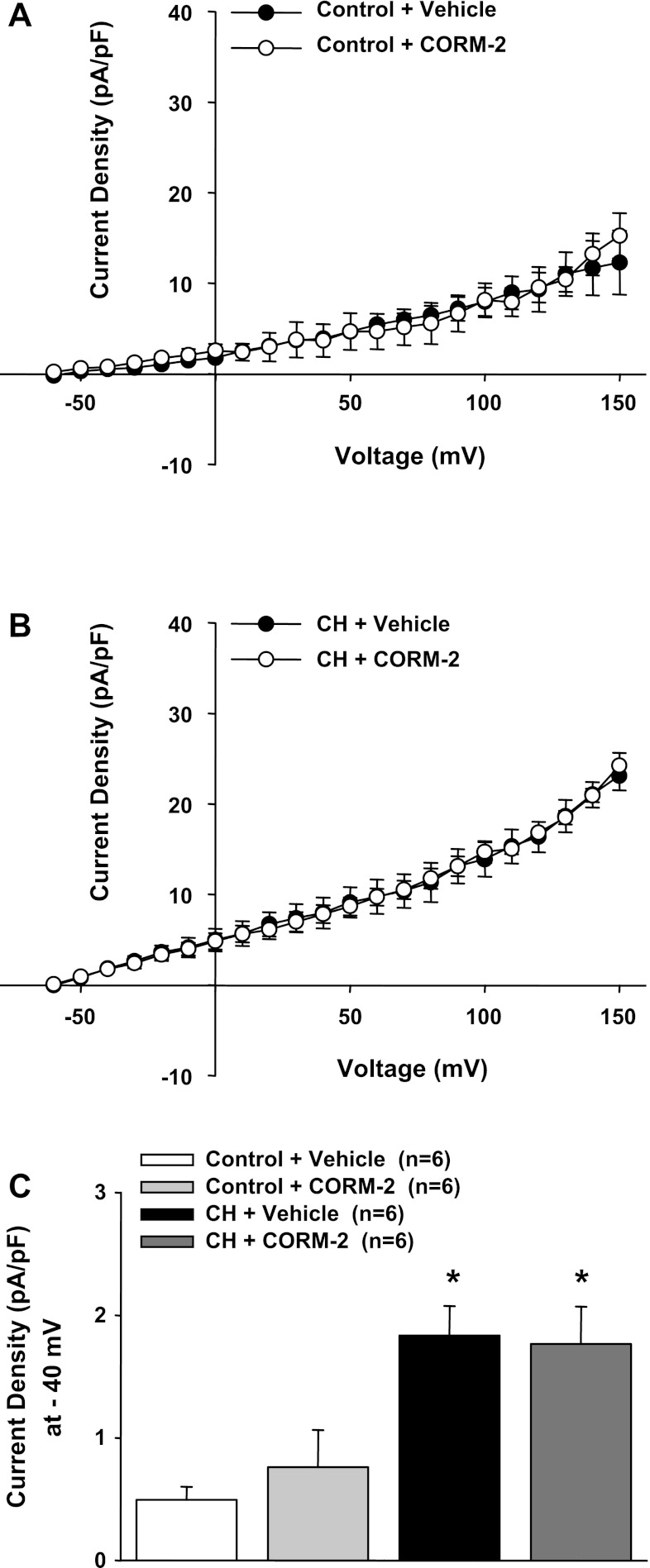
CO is a product of HO and has been shown to activate VSM BK channels (19). We performed experiments to test whether administration of a CO donor could reverse the effects of HO inhibition. iCORM was found to have no effect on outward currents in ECs from control and CH animals (Fig. 6). In another series of experiments, we tested the effect of CrMP to inhibit outward current and the efficacy of the CO donor CORM-2 to reverse this effect in cells from CH rats (Fig. 7, A and B). Although CORM-2 reversed the effects of CrMP, it was without further effect in the absence of HO blockade in cells from either control or CH rats (Fig. 8, A and B). These data support the hypothesis that HO-derived CO is responsible for tonic activation of EC BK channels in cells from CH animals and that endogenously produced CO is sufficient to provide near maximal activation under these experimental conditions.
Fig. 6.
Treatment with the inactivated form of carbon monoxide (CO)-releasing molecule (CORM)-2 (iCORM; 100 μM) had no effect on outward currents in cells from either control or CH rats.
Fig. 7.
A and B: similar to treatment with ZnPPIX, outward currents were diminished in cells from CH rats by the HO inhibitor chromium mesoporphyrin (CrMP). However, during continued HO inhibition, current was restored by the CO donor CORM-2. #P < 0.05 vs. the vehicle-treated CH group over the range of −30 to +150 mV. B: summarized data. #Different from the vehicle-treated CH group.
Fig. 8.
In the absence of HO blockade, CORM-2 was without effect on transmembrane currents in either group (A and B). C: summarized data in the absence of HO inhibition. *P < 0.05 vs. the vehicle-treated control group.
HO Dependence of BK Currents in Cholesterol-Depleted ECs
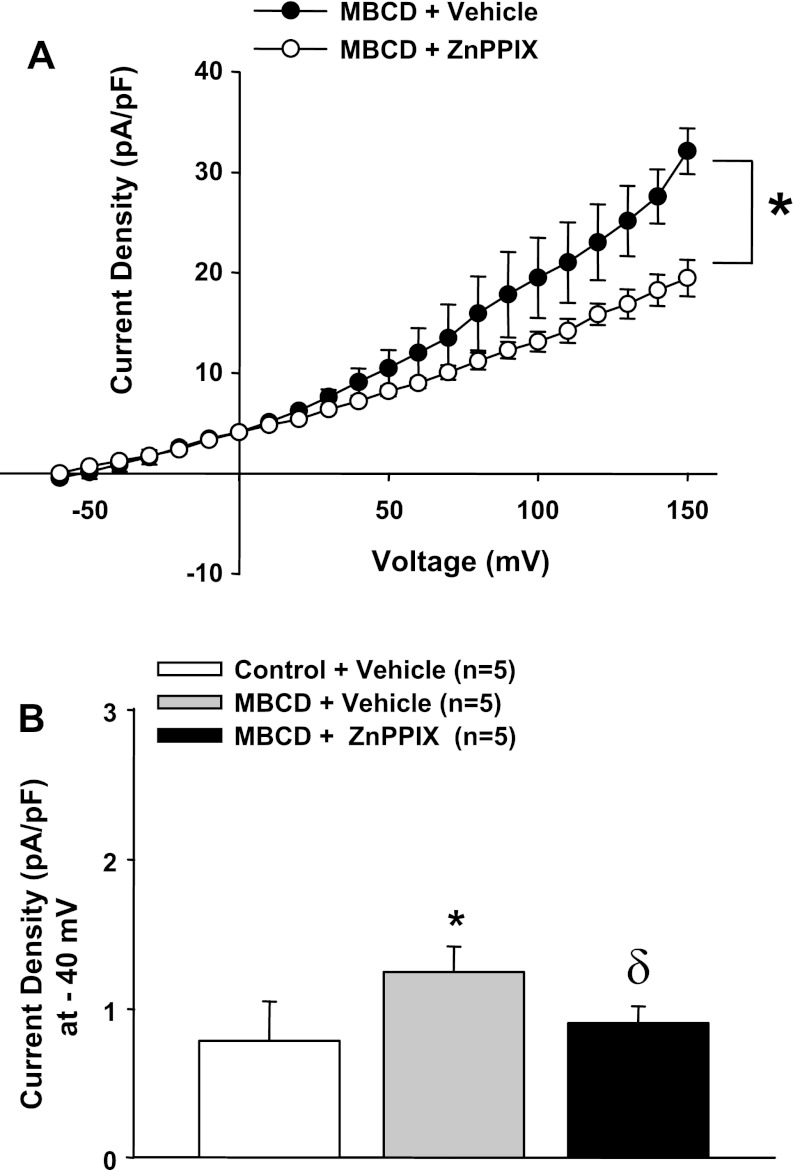
We (17, 29) have previously demonstrated that cholesterol depletion with MBCD (100 μM) unmasks BK currents in ECs from control animals due to a loss in Cav-1 inhibition of the channel. Thus, we hypothesized that removal of Cav-1 inhibition of BK channels by cholesterol depletion would elicit a HO-dependent activation of the channel, similar to results observed in cells from CH animals. Consistent with this hypothesis, MBCD treatment of control ECs elicited HO-dependent currents (Fig. 9, A and B). Thus, like in CH cells, tonic BK channel activity is dependent on HO when caveolar domains are disrupted. These results suggest a tight coupling of HO with BK channels in these cells within lipid-rich microdomains.
Fig. 9.
A: cholesterol depletion with methyl-β-cyclodextrin (MBCD) in control ECs caused enhanced outward current compared with untreated control ECs that was reversed by HO blockade with ZnPPIX. *P < 0.05 vs. control treatment (−40 to +150 mV); δP < 0.05 vs. MBCD (−20 to +150 mV). B: summarized data at −40 mV. *Different from the vehicle-treated control cells; δdifferent from control cells treated with MBCD.
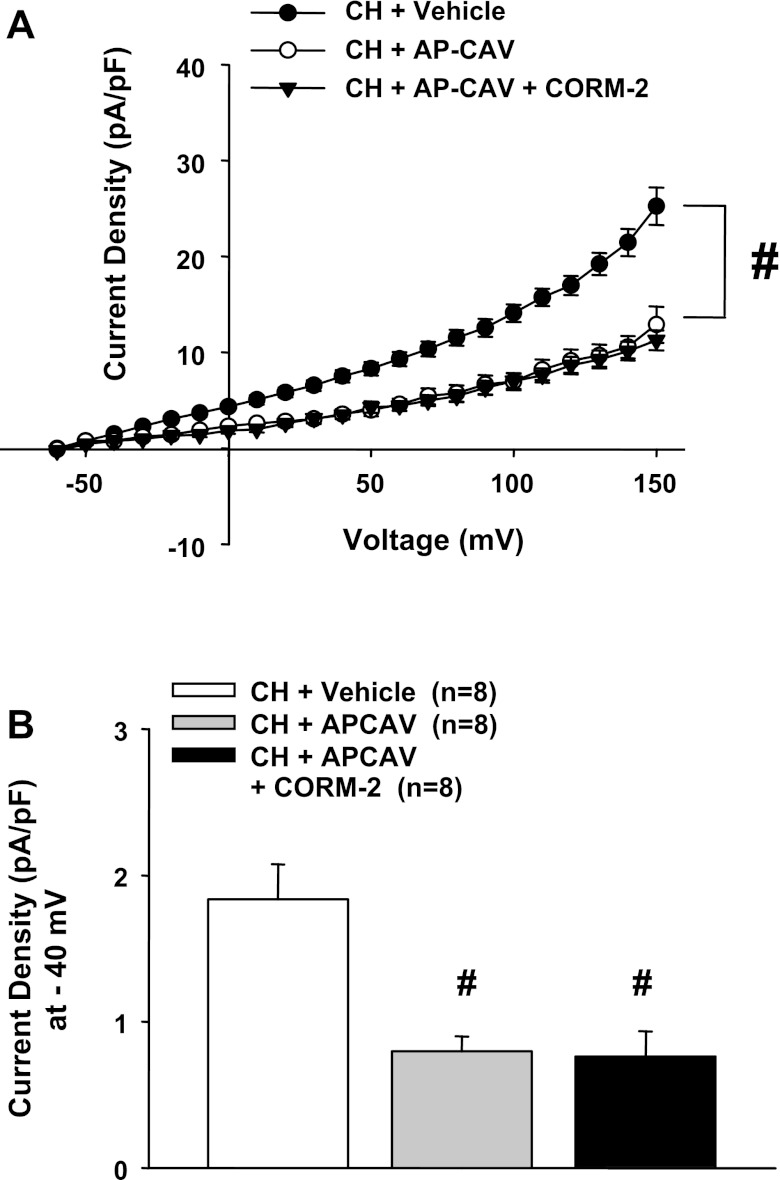
Role of the Scaffolding Domain of Cav-1 on HO-Dependent BK Currents
Similar to our previous results (17, 29), AP-CAV treatment significantly decreased outward currents in ECs from CH rats (Fig. 10, A and B). In contrast to the HO inhibition experiments above, however, exogenous CO had no effect in the presence of AP-CAV. These results support the hypothesis that Cav-1 inhibits BK activity, rendering the channels insensitive to activators such as NS-1619 and CO.
Fig. 10.
A: outward currents in cells from CH rats were significantly reduced after treatment with the scaffolding domain peptide of caveolin (Cav)-1 (AP-CAV). Unlike after HO inhibition (Fig. 6), current was not restored by exogenous CO (CORM-2). #P < 0.05, AP-CAV-treated CH group vs. vehicle-treated CH group (−50 to +150 mV). B: summarized data at −40 mV. #Different from the vehicle-treated CH group.
Colocalization of HO-1 and HO-2 with Cav-1 and Colocalization of BK-α with HO-2
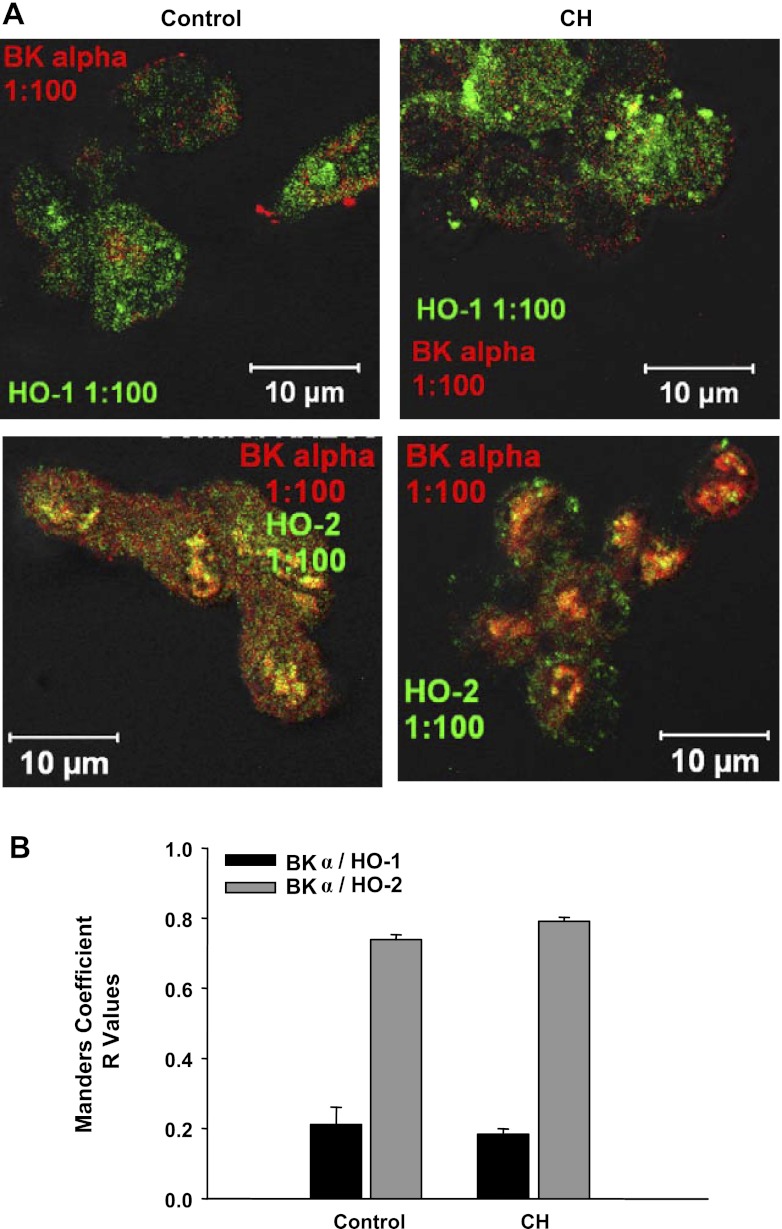
Immunofluorescence imaging of freshly dispersed ECs demonstrated a high incidence of colocalization of HO-2 with BK-α in aortic ECs from CH and control rats (Fig. 11, A and B). However, Manders correlation coefficients, representative of pixel overlap between fluorescence channels, were not different between CH and control groups (Fig. 11B). In contrast to HO-2, HO-1 demonstrated little colocalization with BK in cells from either group of rats.
Fig. 11.
Freshly dispersed rat aortic ECs stained positive for BK-α, HO-1, and HO-2. A and B: HO-2 and BK-α colocalized, whereas little colocalization was found between HO-1 and BK-α. B: colocalization between HO-2 and BK-α did not differ between CH and control groups. n = 4–5 animals; 5–8 images were obtained from each animal.
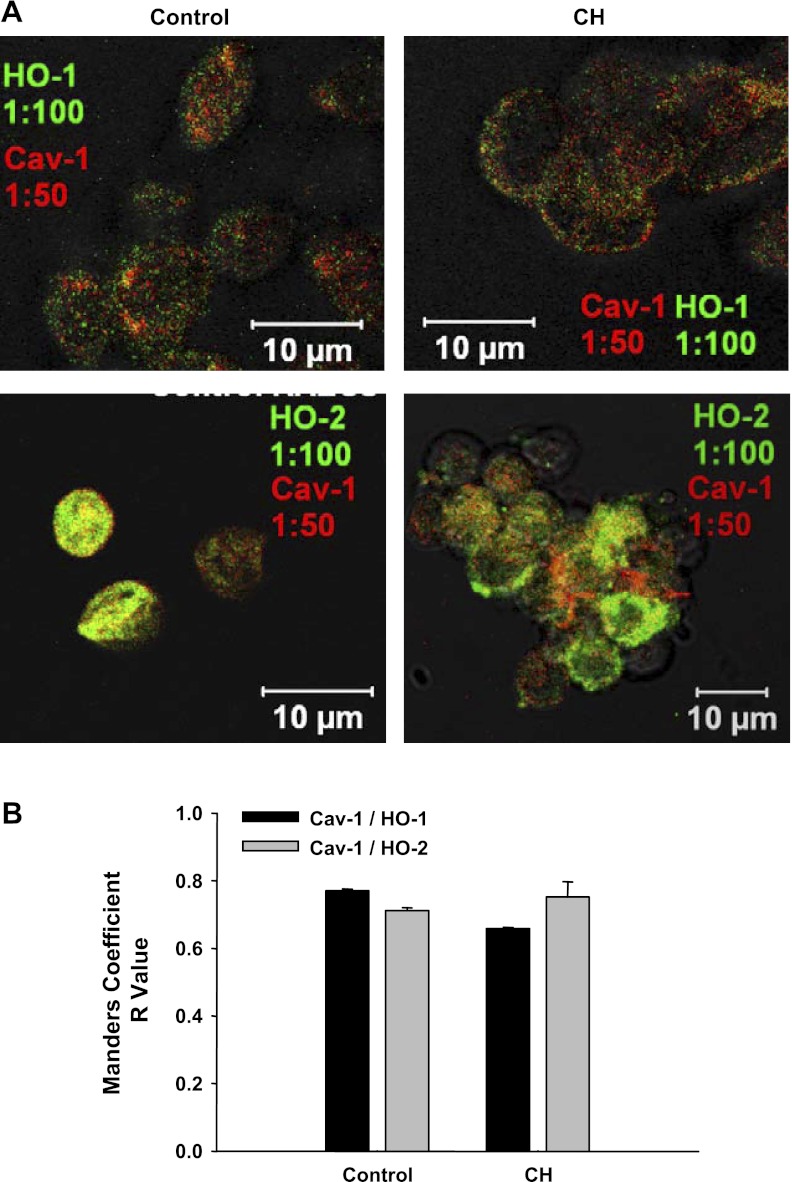
Cav-1 demonstrated similar colocalization with both HO-1 and HO-2 in cells from each group (Fig. 12, A and B). Thus, both isoforms of HO appear to be present in Cav-containing domains, but only HO-2 appears to be associated with the BK channel (21).
Fig. 12.
A: Cav-1 colocalized with both HO-1 and HO-2. B: no differences between HO-1/Cav-1 or HO-2/Cav-1 association were detected in either group. n = 4–5 animals; 5–8 images were obtained from each animal.
DISCUSSION
The major findings from this study are as follows: 1) endothelial BK channels exhibit tonic HO-dependent activation after in vivo exposure to CH or ex vivo cholesterol depletion, 2) BK channel activity can be restored after HO inhibition in cells from CH rats by the HO product CO or by NS-1619, 3) association of BK channels with the scaffolding domain of Cav-1 prevents activation by HO or the HO product CO, and 4) EC BK channels are associated with the HO-2 isoform but not HO-1 in Cav-enriched domains. These findings suggest that BK channels and HO-2 form a functional unit within caveolae that is regulated by the scaffolding domain of Cav-1.
Our findings of a functional association between HO-2 and BK channels in ECs are consistent with prior observations in other cell types. Recent coimmunoprecipitation experiments in human embryonic kidney-293 and glomus type I cells found that BK and HO-2 form oxygen-sensitive complexes (36). Interestingly, knockdown of HO-2 significantly decreased channel activity (36). The authors hypothesized that acute hypoxia inhibits HO production of CO and thus limits channel activity, suggesting that HO-2 is the “oxygen sensor” that regulates glomus cell membrane potential and hence neurotransmitter release in the carotid body. BK channels are hemoproteins and upon binding of heme are potently inhibited. In conditions in which free heme can be degraded by HO, the channel remains active and can be stimulated by the HO product CO (39). However, experiments characterizing the heme-binding domain of BK channels found that decreased oxygen and a reduced state of the heme-binding domain significantly increased the channel's affinity for heme and resulted in potent inhibition (39). In contrast, under normoxic conditions, channel affinity was much higher for CO than heme and permitted channel activity (39). Thus, HO function and heme/CO binding appear to be important regulators of BK activity and associate with the channel to form oxygen-sensitive complexes. In the present study, all experiments were performed under normoxic conditions to eliminate any complicating effects of acute hypoxia. Thus, in vivo during CH, the regulation of EC BK channels could be affected by the influence of acute hypoxia on HO activity. However, the degree of hypoxia used ex vivo to inhibit HO activity is likely much more severe than that observed in the intact vessel wall during CH. The level of hypoxia reported to decrease HO activity is below 10 Torr (5), whereas the arterial Po2 measured in vivo in rats under CH conditions is ∼41 Torr (10). Furthermore, we have observed that the HO-dependent attenuation of vasoconstriction in intact animals is largely unaffected by the acute restoration of normoxia (10). In addition, acute inhibition of HO in conscious rats previously exposed to CH results in profound vasoconstriction that is independent of sympathetic innervation and that is not seen in control animals (27). These latter observations suggest that the HO-dependent pathway defined in the present study has considerable physiological significance after CH. Thus, unlike in the carotid body, oxygen does not likely limit HO activity in the resistance vasculature.
The present study also confirms the important regulatory role of Cav-1 on EC BK function. Under control conditions, Cav-1 potently inhibits HO-dependent activation of BK channels, as evidenced by the lack of BK current under control conditions and the inability to activate channel activity with NS-1619 or CORM-2. However, CH exposure decreases the association between Cav-1 and BK channels (29), enabling channel activation by endogenous gaseotransmitters such as CO and NO (17). A recent study (21) in vascular ECs demonstrated that HO-1 and HO-2 colocalize and are inhibited by association with Cav-1. Thus, a derangement in Cav-1 function after CH exposure may enable not only accessibility of the channel to activators and permit channel opening but could possibly increase endogenous HO activity. Although a previous study (20) found enhanced vascular HO-1 expression after CH, immunofluorescence experiments on native cells suggested that BK channels associate with HO-2 in ECs, and this enzyme isoform is likely responsible for channel activation, although a role for HO-1 cannot be excluded based only on these images. Although increased expression of HO or of BK channels after CH could explain the emergence of HO-dependent BK currents in this setting, the similar effect of CH and acute cholesterol depletion with MBCD suggests that differential channel regulation by Cav-1 is responsible for this effect rather than altered gene expression. Interestingly, colocalization experiments demonstrated no decreases in the association of either HO isoform with Cav-1 in native cells after CH. These results suggest that the BK, Cav-1, and HO-2 complex is intact in control conditions but only becomes active after CH or cholesterol depletion due to decreased Cav-1 inhibition of the channel. In addition, although we did not examine colocalization of the HO isoforms with Cav-1 and BK channels in cells treated acutely with MBCD, we would predict a similar profile as CH due to the similar physiology observed between those groups. Although HO-1 appears to also be associated with Cav-1, it is not part of the BK signaling entity.
There are several established mechanisms by which HO could activate EC BK channels. In human BK channels, the breakdown of heme by HO enables enhanced channel activity from the production of CO, presumably through CO binding of a redox-sensitive domain with the COOH terminus (8, 39). Our results confirm the ability of CO to activate EC BK channels and are consistent with an earlier report (9) in human umbilical vein ECs. In addition to the direct effects of CO on BK channels, reduction of elevated enzymatic activation reduces the local concentration of heme, which exerts an inhibitory effect on the channel (30). Our results also indicate that channel activity is near maximum tonically in a HO-dependent fashion. This latter result may be related to the greater Ca2+ sensitivity that we have observed in EC BK channels compared with similar channels in VSM (29), where localized Ca2+ events (sparks) are required for full activation. In addition, the apparent enhanced Ca2+ sensitivity of EC BK channels could be due to an effect of CO itself, as previously suggested (19). Furthermore, EC intracellular Ca2+ concentration ([Ca2+]i) is greater in arteries from CH rats compared with controls (13), which could also contribute to basal activation. Nevertheless, we propose that HO-BK complexes are inhibited in control conditions due to channel association with Cav-1 and its scaffolding domain. The rapid emergence of HO-dependent BK currents in cells from control animals treated with MBCD illustrates how Cav-1 function either permits or inhibits this unique complex.
Although the present patch-clamp data suggest a tonic near-maximal activation of EC BK channels in cells from CH rats, earlier experiments in isolated arteries suggested that channel activity is present at normal physiological membrane potentials and can be further enhanced in response to various stimuli. For example, although tonic activity of EC BK channels likely underlies reduced myogenic and agonist-induced vasoconstriction after in vivo CH exposure (11, 17, 25, 29), endothelium-dependent vasodilator responses to acetylcholine are mediated by an IBTX-sensitive pathway in arteries from these animals that is not seen in controls (17). These data suggest that in the intact artery, EC BK activity is not maximal and is responsive to acute increases in [Ca2+]i elicited by endothelial stimulation. The BK response to transient elevations in [Ca2+]i could be either a direct effect on the channel or through a secondary effect of Ca2+ on HO activity, since the HO-2 isoform shown to associate with BK channels possesses a Ca2+/calmodulin-binding domain (3). This Ca2+ sensitivity of HO activity was recently confirmed in astrocytes, where increased Ca2+ concentration resulted in elevated CO production (37). In addition to acute effects of Ca2+, we (25) have previously observed that administration of the HO substrate heme-l-lysinate results in additional vasodilation in isolated arteries that is IBTX sensitive and that is associated with vessel wall hyperpolarization. These observations differ from the present patch-clamp data in isolated cells, where the addition of hemin elicited only modest effects on BK activity. This discrepancy could be related to the isolation procedures used and the artificially high Ca2+ concentration used in the patch-clamp protocol. In addition, the inability of CORM-2 to further activate the channel even in cells from CH rats is not consistent with the modest effects of hemin in the present study or with the significant effects of hemin in earlier whole vessel experiments. The reason for this discrepancy is not clear and may again be related to the artificial conditions associated with patch clamp. Thus, although studies (17, 29) in intact arteries and isolated ECs have suggested a tonic hyperpolarizing influence of BK channels that affects vasoreactivity, the channel can be further activated to promote additional vasodilation in situ.
In conclusion, CH exposure results in a loss of Cav-1 inhibition of EC BK channels, which are dependent for activation by HO-derived CO. Activation of these normally dormant EC BK channels results in EC hyperpolarization (17, 29) and subsequent vascular wall hyperpolarization and diminished vasoconstrictor reactivity, as previously demonstrated (7, 17, 25, 26).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-95640 and HL-07736 (to B. R. Walker) and by a Predoctoral Fellowship from the South Central Affiliate of the American Heart Association (to M. A. Riddle).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.R. and B.R.W. conception and design of research; M.A.R. performed experiments; M.A.R. analyzed data; M.A.R. and B.R.W. interpreted results of experiments; M.A.R. and B.R.W. prepared figures; M.A.R. drafted manuscript; M.A.R. and B.R.W. edited and revised manuscript; M.A.R. and B.R.W. approved final version of manuscript.
REFERENCES
- 1. Auer G, Ward ME. Impaired reactivity of rat aorta to phenylephrine and KCl after prolonged hypoxia: role of the endothelium. J Appl Physiol 85: 411– 417, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Baron A, Frieden M, Beny JL. Epoxyeicosatrienoic acids activate a high-conductance, Ca2+-dependent K+ channel on pig coronary artery endothelial cells. J Physiol 504: 537– 543, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehning D, Sedaghat L, Sedlak TW, Snyder SH. Heme oxygenase-2 is activated by calcium-calmodulin. J Biol Chem 279: 30927– 30930, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850– 853, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Carraway MS, Ghio AJ, Carter JD, Piantadosi CA. Expression of heme oxygenase-1 in the lung in chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 278: L806– L812, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Casiglia E, Pavan L, Marcato L, Leopardi M, Pizziol A, Salvador P, Zuin R, Pessina AC. Subjects with obstructive pulmonary disease tend to be chronically vasodilated. Clin Sci (Lond) 95: 287– 294, 1998 [PubMed] [Google Scholar]
- 7. Caudill TK, Resta TC, Kanagy NL, Walker BR. Role of endothelial carbon monoxide in attenuated vasoreactivity following chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 275: R1025– R1030, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res 86: 1224– 1229, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Dong DL, Zhang Y, Lin DH, Chen J, Patschan S, Goligorsky MS, Nasjletti A, Yang BF, Wang WH. Carbon monoxide stimulates the Ca2+-activated big conductance K channels in cultured human endothelial cells. Hypertension 50: 643– 651, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Doyle MP, Walker BR. Attentuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol Regul Integr Comp Physiol 260: R1114– R1122, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Earley S, Naik JS, Walker BR. 48-h Hypoxic exposure results in endothelium-dependent systemic vascular smooth muscle cell hyperpolarization. Am J Physiol Regul Integr Comp Physiol 283: R79– R85, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Earley S, Walker BR. Endothelium-dependent blunting of myogenic responsiveness after chronic hypoxia. Am J Physiol Heart Circ Physiol 283: H2202– H2209, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Earley S, Walker BR. Increased nitric oxide production following chronic hypoxia contributes to attenuated systemic vasoconstriction. Am J Physiol Heart Circ Physiol 284: H1655– H1661, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gonzales RJ, Walker BR. Role of CO in attenuated vasoconstrictor reactivity of mesenteric resistance arteries after chronic hypoxia. Am J Physiol Heart Circ Physiol 282: H30– H37, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Heistad DD, Abboud FM, Mark AL, Schmid PG. Impaired reflex vasoconstriction in chronically hypoxemic patients. J Clin Invest 51: 331– 337, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogg DS, McMurray G, Kozlowski RZ. Endothelial cells freshly isolated from small pulmonary arteries of the rat possess multiple distinct K+ current profiles. Lung 180: 203– 214, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol 299: H1439– H1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113– 127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaggar JH, Leffler CW, Cheranov SY, Tcheranova DES, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91: 610– 617, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Jernigan NL, O'Donaughy TL, Walker BR. Correlation of HO-1 expression with onset and reversal of hypoxia-induced vasoconstrictor hyporeactivity. Am J Physiol Heart Circ Physiol 281: H298– H307, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J 18: 1080– 1089, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Kooli A, Kermorvant-Duchemin E, Sennlaub F, Bossolasco M, Hou X, Honore JC, Dennery PA, Sapieha P, Varma D, Lachapelle P, Zhu T, Tremblay S, Hardy P, Jain K, Balazy M, Chemtob S. trans-Arachidonic acids induce a heme oxygenase-dependent vasorelaxation of cerebral microvasculature. Free Radic Biol Med 44: 815– 825, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci 103: 857– 862, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Naik JS, O'Donaughy TL, Walker BR. Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation. Am J Physiol Heart Circ Physiol 284: H838– H845, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Naik JS, Walker BR. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am J Physiol Heart Circ Physiol 285: H220– H228, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Naik JS, Walker BR. Role of vascular heme oxygenase in reduced myogenic reactivity following chronic hypoxia. Microcirculation 13: 81– 88, 2006 [DOI] [PubMed] [Google Scholar]
- 27. O'Donaughy TL, Walker BR. Renal vasodilatory influence of endogenous carbon monoxide in chronically hypoxic rats. Am J Physiol Heart Circ Physiol 279: H2908– H2915, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Paffett ML, Naik JS, Resta TC, Walker BR. Reduced store-operated Ca2+ entry in pulmonary endothelial cells from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 293: L1135– L1142, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol 301: C1404– C1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 425: 531– 535, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol 76: 1– 15, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol 121: 927– 934, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem 272: 8222– 8226, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflügers Arch 434: 285– 291, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem 280: 11656– 11664, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306: 2093– 2097, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar JH, Leffler CW. Glutamate-induced calcium signals stimulate CO production in piglet astrocytes. Am J Physiol Heart Circ Physiol 301: H428– H433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol 286: H610– H618, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Yi L, Morgan JT, Ragsdale SW. Identification of a thiol/disulfide redox switch in the human BK channel that controls its affinity for heme and CO. J Biol Chem 285: 20117– 20127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]