Abstract
Mice deficient for the apical membrane oxalate transporter SLC26A6 develop hyperoxalemia, hyperoxaluria, and calcium oxalate stones due to a defect in intestinal oxalate secretion. However, the nature of the basolateral membrane oxalate transport process that operates in series with SLC26A6 to mediate active oxalate secretion in the intestine remains unknown. Sulfate anion transporter-1 (Sat1 or SLC26A1) is a basolateral membrane anion exchanger that mediates intestinal oxalate transport. Moreover, Sat1-deficient mice also have a phenotype of hyperoxalemia, hyperoxaluria, and calcium oxalate stones. We, therefore, tested the role of Sat1 in mouse duodenum, a tissue with Sat1 expression and SLC26A6-dependent oxalate secretion. Although the active secretory flux of oxalate across mouse duodenum was strongly inhibited (>90%) by addition of the disulfonic stilbene DIDS to the basolateral solution, secretion was unaffected by changes in medium concentrations of sulfate and bicarbonate, key substrates for Sat1-mediated anion exchange. Inhibition of intracellular bicarbonate production by acetazolamide and complete removal of bicarbonate from the buffer also produced no change in oxalate secretion. Finally, active oxalate secretion was not reduced in Sat1-null mice. We conclude that a DIDS-sensitive basolateral transporter is involved in mediating oxalate secretion across mouse duodenum, but Sat1 itself is dispensable for this process.
Keywords: SLC26A6, SLC26A1, sulfate, bicarbonate, basolateral membrane
nephrolithiasis is a common disease with increasing prevalence in the United States, and calcium oxalate accounts for 70–80% of kidney stones (19). Supersaturation of urine is the underlying driving force for stone formation (19), and urine concentration of oxalate is an important risk factor for calcium oxalate nephrolithiasis (16). The level of urinary oxalate is dependent on metabolic production, enteric absorption, and renal excretion (17).
The role of the intestine in oxalate homeostasis has been underscored by studies in transgenic mice. Specifically, mice with targeted disruption of the gene encoding the apical membrane oxalate transporter SLC26A6 develop hyperoxalemia and hyperoxaluria (4, 7) and a high incidence of calcium oxalate urolithiasis (7). The predominant source of the excess oxalate in the plasma and urine of Slc26a6-null mice is dietary oxalate (7), and Slc26a6-null mice have a defect in oxalate secretion in small intestine (4, 7). These findings support the concept that SLC26A6-mediated oxalate secretion limits net intestinal absorption of dietary oxalate, which would otherwise result in hyperoxalemia and increased glomerular filtration of oxalate, thereby causing hyperoxaluria and increased risk for calcium oxalate stones.
Mouse duodenum has served as a useful model system to study mechanisms of intestinal oxalate transport. SLC26A6 expression in mouse intestinal tissue is highest in duodenum (18), and active oxalate secretion in this tissue is completely ablated in Slc26a6-null mice (7, 9). In contrast to the evidence for active oxalate secretion in mouse duodenum, oxalate absorption in this tissue is predominantly, if not exclusively, passive and paracellular (9). However, no studies to date have addressed the nature of the basolateral membrane oxalate transport process that operates in series with SLC26A6 to mediate active oxalate secretion across mouse duodenum.
Sulfate anion transporter-1 (Sat1 or SLC26A1) is an anion exchanger for sulfate and bicarbonate (HCO3−) that is capable of mediating oxalate transport and that is expressed on the basolateral membrane of epithelial cells in liver, kidney, and intestine (2, 3, 8, 10, 12, 14, 15, 20). Moreover, Sat1-null mice have recently been reported to have a kidney stone phenotype remarkably similar to Slc26a6-null mice with hyperoxalemia and hyperoxaluria (3). This finding has been interpreted to indicate that Sat1 is the basolateral oxalate transporter that collaborates with apical SLC26A6 to mediate intestinal oxalate secretion (3, 5, 17).
Accordingly, the purpose of the present study is to test the hypothesis that the basolateral step of oxalate secretion in mouse duodenum is mediated by Sat1 and represents a process of disulfonic stilbene-sensitive exchange of oxalate with the Sat1 substrates sulfate and HCO3−. We successfully demonstrate the role of a DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate)-sensitive basolateral transporter in mediating oxalate secretion across mouse duodenum, but unexpectedly find that Sat1 itself is dispensable for this process.
MATERIALS AND METHODS
Measurement of oxalate and mannitol fluxes across mouse duodenum.
Transport of oxalate and mannitol across mouse duodenum was performed as previously described (9). In brief, duodenal segments were mounted as intact sheets in a modified Ussing chamber that had an exposed surface area of 0.30 cm2. Mucosal and serosal surfaces of the duodenal segments were bathed with oxygenated Ringer solution (140 mM Na+, 119.8 mM Cl−, 5.2 mM K+, 1.2 mM Ca2+, 1.2 mM Mg2+, 25 mM HCO3−, 2.4 mM HPO42−, 0.4 mM H2PO4−, and 10 mM glucose at pH 7.4). Secretory fluxes of oxalate and mannitol were measured by adding 2 μM [14C]oxalate (specific activity 117 mCi/mmol, Amersham Biosciences, Piscataway, NJ) and 0.08 μM [3H]mannitol (specific activity 20 Ci/mmol, MP Biomedicals, Santa Ana, CA) to the serosal bath, and unlabeled 2 μM oxalate and 0.08 μM mannitol in the opposite bath. After a 150-min equilibration period to achieve steady-state flux rates, we collected samples before and after the subsequent 60-min period to calculate unidirectional serosa-to-mucosa flux. All flux studies were performed under short-circuited conditions using a Multichannel Voltage/Current Clamp-Model VCC MC6 (Physiologic Instruments, San Diego, CA). The apparent permeability coefficients (Papp) for oxalate and mannitol were calculated using the following equation:
where J is the flux, A is the cross-sectional tissue area of the Ussing chamber, and [S] is the substrate concentration of [14C]oxalate or [3H]mannitol.
For experiments evaluating sensitivity to basolateral DIDS, 1 mM DIDS (Sigma-Aldrich, St. Louis, MO) was dissolved in oxygenated Ringer solution. For experiments evaluating effects of apical or basolateral sulfate, 1 mM NaSO4 was dissolved in oxygenated Ringer solution and added to either the mucosa or serosa compartment. High basolateral HCO3− was prepared by adding 50 mM NaHCO3 to the Ringer solution, with final pH 7.9. Low basolateral HCO3− buffer was prepared by titrating Ringer solution containing 25 mM HCO3− with 6 N HCl to pH 6.9 and 7.5 mM HCO3−. CO2/HCO3− free buffer was prepared by replacing 25 mM HCO3− in Ringer solution with 25 mM HEPES and adjusting pH to 7.4 with NaOH. For experiments involving carbonic anhydrase inhibition, a stock solution of acetazolamide (Sigma-Aldrich, St. Louis, MO) dissolved in DMSO was added to both the mucosa and serosa for the final concentration of 0.5 mM in each compartment, and equivalent DMSO was added to the control solutions (0.05%).
The generation of Sat1-null mice was previously described (3). Mice homozygous and heterozygous for disruption of Sat1 were compared with wild-type mice of the same C57BL background. Genotyping was confirmed by PCR, as reported (3). All other experiments (e.g., effects of DIDS, sulfate, HCO3−) were performed using wild-type 129 mice, the background strain for Slc26a6-null mice generated in our laboratory (7).
All animal protocols were approved by the Yale University Institutional Animal Care and Use Committee.
RT-PCR of Sat1 transcript in mouse intestine and kidney.
Total RNA samples from different segments of mouse intestine and kidney were extracted with RNeasy Mini Kit (Qiagen) from 6-mo-old wild-type 129S6/SvEv mice. Residual genomic DNA contamination was removed from total RNA samples with the DNA-free kit (Ambion, Austin, TX), and RNA was subjected to reverse transcriptase PCR using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The resultant cDNA was used to conduct real-time PCR on the Applied Biosystems 7300 Real Time PCR System (Applied Biosystems, Foster City, CA). Mouse SLC26A1 specific oligonucleotide primers (5′-ACAACACTGATCATTGGGCTACA; 5′-GCCGGAGGATACCCATGAG) were designed with Primer Express 3.0 software from mouse SLC26A1 nucleotide sequence (NM_174870) retrieved from GenBank. A pair of mouse hypoxanthine phosphoribosyltransferase-1 primers (5′-CAGTACAGCCCCAAAATGGT; 5′-CAAGGGCATATCCAACAACA) was used as an internal control. Real-time PCR was performed using the Power SYBR Green PCR Master Mix and PCR Reagents Kit (Applied Biosystems, Foster City, CA). All PCRs were run in triplicate. Data are expressed using the comparative threshold cycle (dCt) method, and mRNA ratios of SLC26A1 to histone are given by 2−dCT. After quantitative PCR, the PCR product was directly subcloned into the pCR4-TOPO plasmid by using the TOPO TA cloning Kit (Invitrogen, Carlsbad, CA). Sequencing was performed using the M13 forward primer by the Yale Keck DNA Sequencing Facility.
Data analysis and statistical evaluation.
Data are presented as means ± SE. Student t-test was used to test for statistical significance of the difference in means. JMP 9.0.0 (SAS Institute) was used for statistical analysis. A P value of ≤0.05 was considered as a statistically significant difference.
RESULTS
Expression of Sat1 in mouse intestine and kidney.
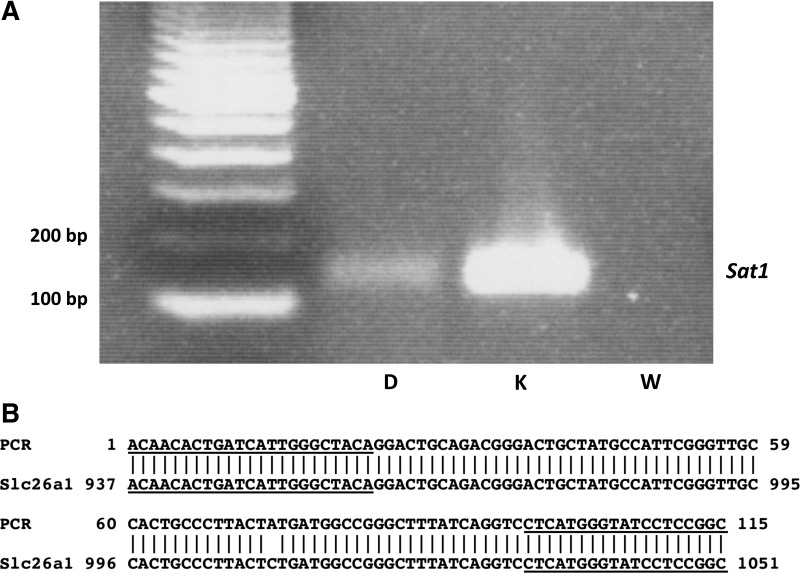
As illustrated in Fig. 1A, amplification of cDNA from mouse duodenum by RT-PCR using primers based on the mouse SLC26A1 sequence resulted in a product of the expected size, as found in kidney. Sequencing of the PCR product from mouse duodenum confirmed that it represented SLC26A1 (Fig. 1B). Shown in Table 1, relative expression of Sat1 by quantitative PCR was found to be highest in the kidney, followed by proximal colon, duodenum, ileum, and distal colon. We were unable to detect active oxalate secretion in proximal colon (data not shown) and, therefore, tested the role of Sat1 in the duodenum, a segment with a high rate of oxalate secretion (7, 9).
Fig. 1.
Sulfate anion transporter-1 (Sat1) is expressed in mouse duodenum. A: expression of Sat1 (SLC26A1) was verified in mouse duodenum (D) and kidney (K) using RT-PCR. W, water control. B: sequence of the PCR product had 99% identity with mouse Sat1 (NCBI Reference Sequence: NM_174870.3). Primer sequences are underlined.
Table 1.
Relative expression of Sat1 in mouse intestine and kidney
| Tissue | Relative Expression, ×10−3 |
|---|---|
| Duodenum | 0.28 ± 0.04 |
| Ileum | 0.13 ± 0.04 |
| Proximal colon | 6.36 ± 1.99 |
| Distal colon | 0.06 ± 0.02 |
| Kidney | 1370 ± 40 |
Values are means ± SE (n = 6). Expression levels of sulfate anion transporter-1 (Sat1) relative to hypoxanthine phosphoribosyltransferase-1 were determined by quantitative PCR.
Effect of DIDS.
As the initial approach to test the hypothesis that the basolateral step of oxalate secretion in mouse duodenum is mediated, we examined the effect of the anion exchange inhibitor DIDS. Sat1 is highly sensitive to DIDS, with an IC50 of 30 μM (2). We tested the effect of 1 mM DIDS, a concentration that inhibits mouse Sat1 >90% (11). To specifically identify the effect of DIDS on transcellular oxalate secretion, we measured the Papp for secretion of [14C]oxalate simultaneously with that of [3H]mannitol, a marker for passive flux through the paracellular pathway. We previously demonstrated that the Papp for secretion of oxalate across mouse duodenum exceeds that of mannitol. However, the excess secretory Papp of oxalate compared with mannitol is abolished by luminal DIDS and is absent in duodenum of Slc26a6-null mice. These findings indicated that the secretory flux of oxalate has two components: a paracellular flux with identical apparent permeability to mannitol, and a transcellular flux that is inhibited by luminal DIDS and is completely dependent on apical membrane SLC26A6 activity (9).
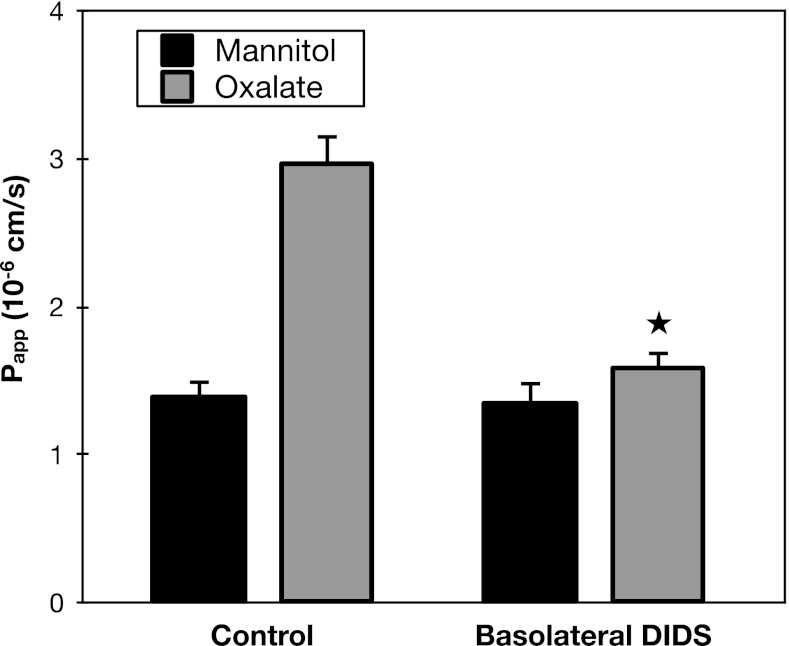
Shown in Fig. 2, addition of 1 mM DIDS to the serosal medium had no effect on mannitol flux, but significantly inhibited oxalate secretion across mouse duodenum. Indeed, serosal DIDS almost completely abolished the higher secretory Papp of oxalate compared with mannitol, indicating >90% inhibition of the transcellular component of oxalate secretion. The results of this experiment indicate that activity of a DIDS-sensitive basolateral transporter is required for transcellular oxalate secretion across mouse duodenum.
Fig. 2.
Basolateral DIDS inhibits oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across wild-type (WT) mouse duodenum in the absence and presence of 1 mM DIDS added to the serosal solution. Papp, apparent permeability coefficient. Values are means ± SE (n = 4). *P value < 0.05 vs. oxalate secretion in control group.
Effects of sulfate.
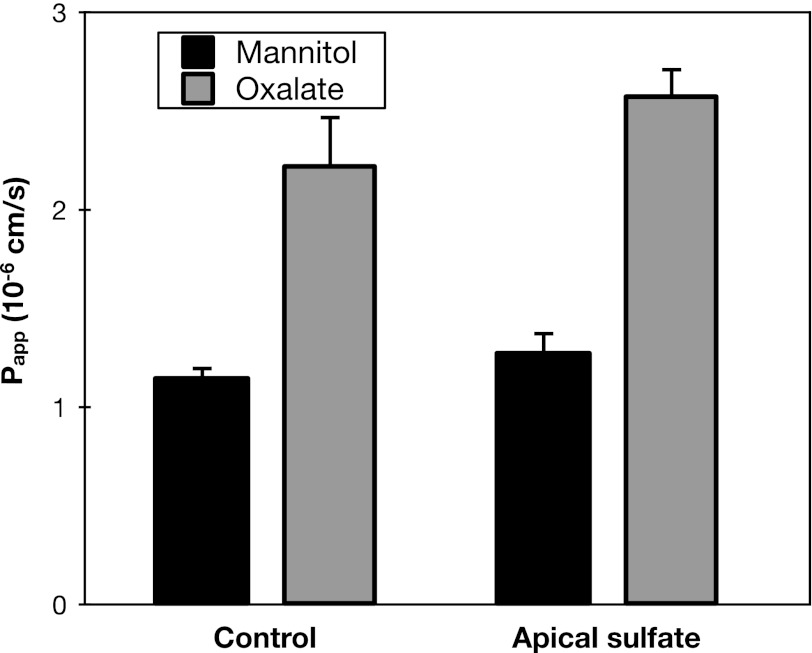
To additionally test the hypothesis that the basolateral step of oxalate secretion in mouse duodenum is mediated by Sat1, we evaluated the effects of its high-affinity substrate sulfate (2). The Michaelis constant (Km) of mouse Sat1 for sulfate is 0.31 mM (11). A potential mechanism by which Sat1 could mediate active oxalate uptake across the basolateral membrane is by exchange for intracellular sulfate. If the basolateral step in transcellular oxalate secretion is exchange for intracellular sulfate, then it is possible that oxalate secretion would be stimulated by addition of sulfate to the luminal solution. However, as seen in Fig. 3, addition of 1 mM apical sulfate failed to significantly stimulate oxalate secretion across mouse duodenum.
Fig. 3.
Apical sulfate does not affect oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in the absence and presence of 1 mM sulfate added to the luminal solution. Values are means ± SE (n = 4).
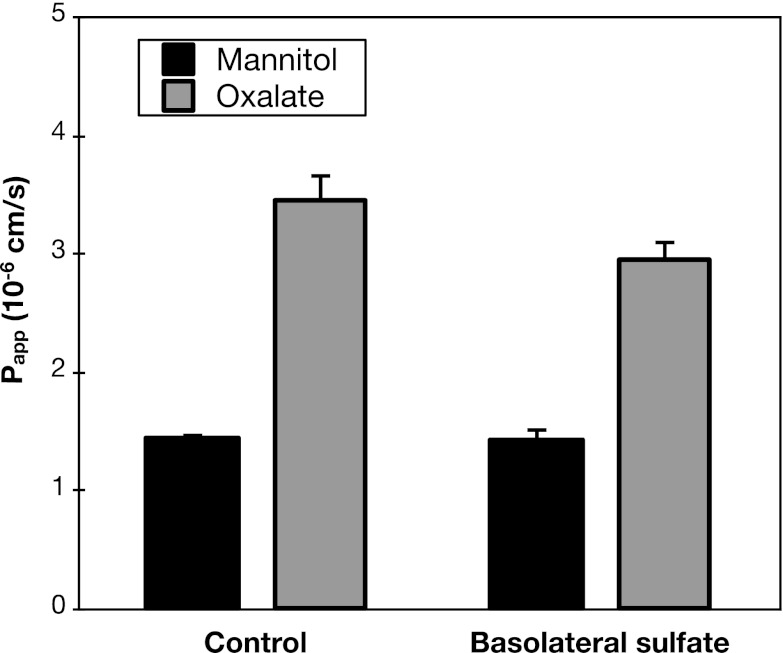
The inability of apical sulfate to stimulate oxalate secretion does not rule out a role for Sat1; in the absence of added sulfate, it is possible that another substrate of Sat1 (such as HCO3−, see below) is at sufficient intracellular concentration to drive oxalate uptake across the basolateral membrane. Regardless of the specific intracellular exchanger partner for oxalate, it is predicted that adding sulfate to the serosal solution at a concentration several fold higher than its Km should compete with oxalate and thereby inhibit any oxalate secretion mediated by Sat1. As illustrated in Fig. 4, addition of 1 mM sulfate to the serosal medium did not significantly inhibit oxalate secretion across mouse duodenum. Although a small inhibitory effect of serosal sulfate cannot be excluded, the results in Fig. 4 strongly argue that the major fraction of oxalate secretion across mouse duodenum is sulfate insensitive and thus unlikely to be mediated by Sat1.
Fig. 4.
Basolateral sulfate does not affect oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in the absence and presence of 1 mM sulfate added to the serosal solution. Values are means ± SE (n = 4).
Effects of HCO3−.
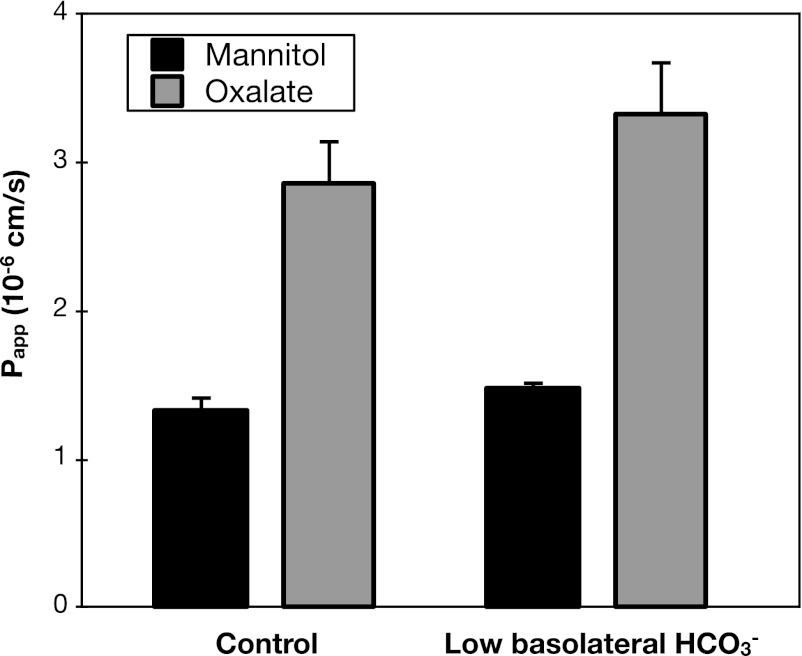
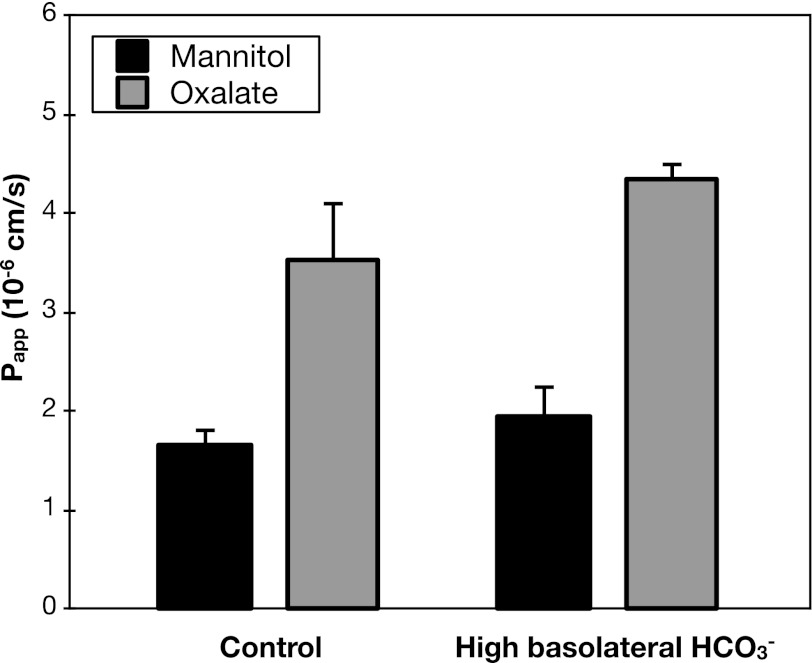
In addition to sulfate, HCO3− is a major substrate for anion exchange mediated by Sat1 (10). Thus another potential mechanism by which Sat1 could mediate active oxalate uptake across the basolateral membrane is by exchange for intracellular HCO3−. If the basolateral step in transcellular oxalate secretion is exchange for intracellular HCO3−, then it is possible that the rate of oxalate secretion would be affected by the HCO3− gradient across the basolateral membrane. However, as shown in Figs. 5 and 6, respectively, neither lowering serosal HCO3− from 25 to 7.5 mM, nor raising serosal HCO3− from 25 to 75 mM, significantly affected the rates of oxalate secretion.
Fig. 5.
Low basolateral bicarbonate (HCO3−) does not affect oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in the presence of 25 mM (control) or 7.5 mM HCO3− in the serosal solution. Values are means ± SE (n = 4).
Fig. 6.
High basolateral HCO3− does not affect oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in the presence of 25 mM (control) or 75 mM HCO3− in the serosal solution. Values are means ± SE (n = 4).
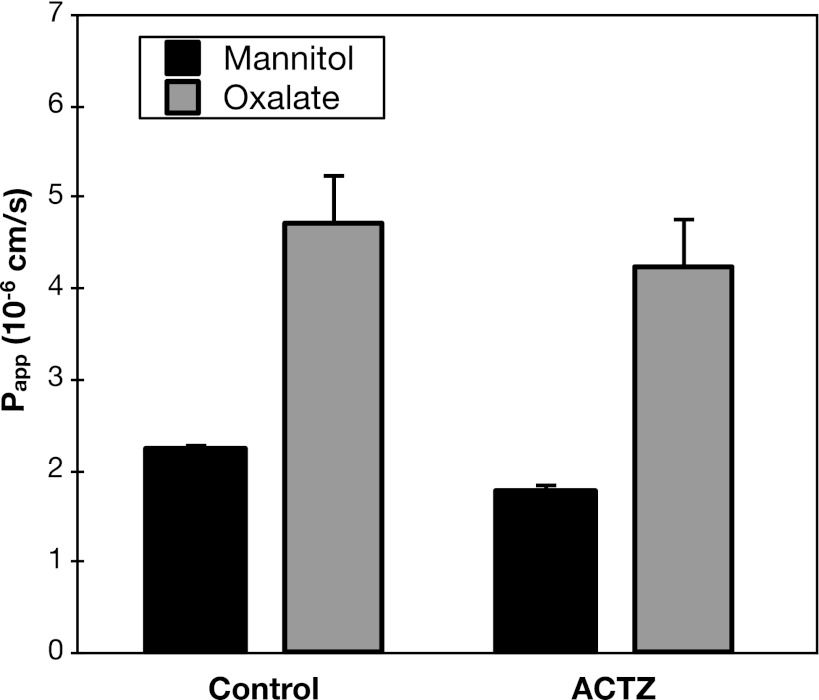
Carbonic anhydrases catalyze the hydration of intracellular carbon dioxide to form HCO3− in many tissues (13). Inhibition of carbonic anhydrase reduces duodenal HCO3− secretion, an effect attributed, in part, to blunted generation of intracellular HCO3− (6). Accordingly, as another strategy to evaluate the role of HCO3− in duodenal oxalate secretion, we tested the effect of the carbonic anhydrase inhibitor acetazolamide. Illustrated in Fig. 7, addition of acetazolamide to the luminal and serosal bathing solutions failed to significantly alter the rate of oxalate secretion.
Fig. 7.
Acetazolamide (ACTZ) does not affect oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in the absence and presence of 0.5 mM ACTZ added to the bathing solutions. Values are means ± SE (n = 4).
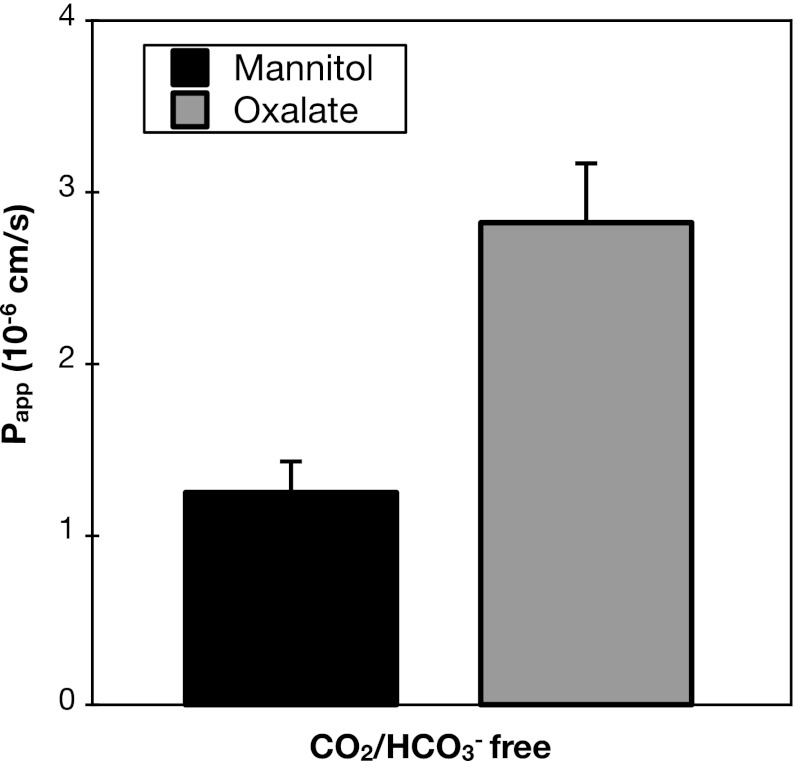
As a final test for the HCO3− dependence of oxalate secretion, we evaluated whether transcellular oxalate secretion would persist in the nominal absence of carbon dioxide and HCO3− from the bathing media. Remarkably, as indicated in Fig. 8, even in the complete absence of adding CO2/HCO3− to the bathing media, transcellular oxalate secretion was still present, as shown by the excess secretory Papp of oxalate compared with mannitol. Although permeability to both mannitol and oxalate were reduced in the absence of CO2/HCO3− (comparison of Fig. 8 to previous figures), the ratio of the Papp for oxalate relative to that of mannitol was similar in the absence and presence of CO2/HCO3−.
Fig. 8.
Transcellular oxalate secretion takes place in the absence of CO2/HCO3−. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across WT mouse duodenum in bathing solutions in which HCO3− was replaced with HEPES and gassed with 100% O2. Values are means ± SE (n = 4).
Taken together, the negative results of the experiments shown in Figs. 5–8 to test the role of HCO3− in oxalate secretion strongly argue against the operation of a basolateral transport mechanism mediating oxalate-HCO3− exchange.
Effect of Sat1 gene deletion.
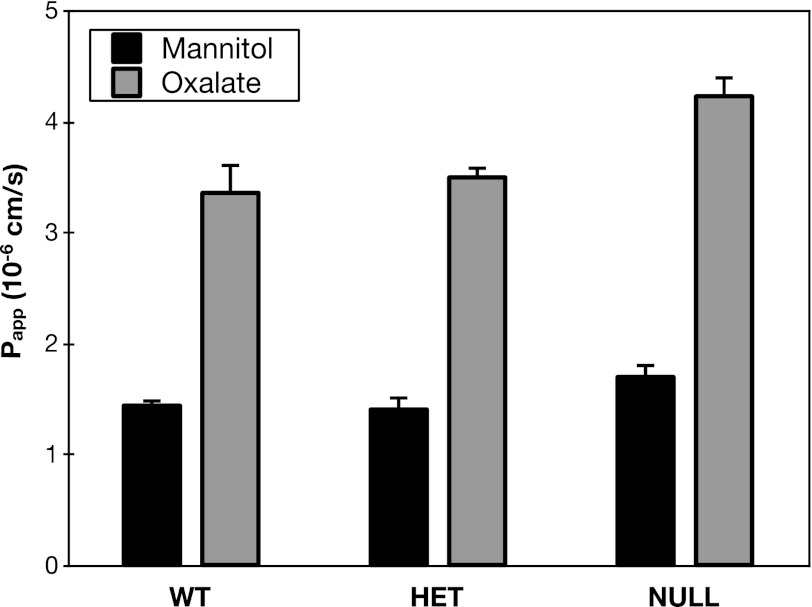
Although we had failed to detect roles for the Sat1 substrates sulfate and HCO3− in duodenal oxalate secretion, it remained possible that Sat1 might nevertheless mediate basolateral oxalate uptake by exchange for an intracellular anion yet to be identified as a Sat1 substrate. Therefore, as the definitive test for the role of Sat1, we examined the effect of Sat1 gene deletion on oxalate secretion across mouse duodenum. As illustrated in Fig. 9, we could detect no change in oxalate secretion across the duodenum of Sat1-null mice. The absence of any defect in oxalate secretion in Sat1-null mice effectively rules out Sat1 as the principal basolateral oxalate transporter that operates in series with apical SLC26A6 to mediate transcellular oxalate secretion across mouse duodenum.
Fig. 9.
Sat1-null mice have no defect in oxalate secretion. Transepithelial secretory fluxes of [14C]oxalate and [3H]mannitol were measured simultaneously across mouse duodenum of WT, heterozygote (HET), and Sat1-null mice. Values are means ± SE (n = 3 for WT, n = 3 for HET, n = 8 for null).
DISCUSSION
Our goal was to test the hypothesis that the basolateral step of oxalate secretion in mouse duodenum is mediated by Sat1 and takes place by exchange of oxalate with one or both of the Sat1 substrates, sulfate and HCO3−. We confirmed expression of Sat1 in this segment of mouse intestine by RT-PCR. However, we could find no significant effects of sulfate and HCO3− on oxalate flux, and we were unable to detect a defect in oxalate secretion across the duodenum of Sat1-null mice.
We focused on duodenum because SLC26A6 expression in mouse intestinal tissue is highest in this segment of the intestine (18), and active oxalate secretion in this tissue is completely ablated in Slc26a6-null mice (7, 9). The observed defect in oxalate secretion in small intestine of Slc26a6-null mice correlates with a phenotype of hyperoxalemia and hyperoxaluria (4, 7) due to enhanced net absorption of dietary oxalate (7).
The observation that mice with disruption of the gene encoding Sat1, a basolateral anion exchanger able to transport oxalate (2, 8, 10, 14, 15, 20), have a similar phenotype of hyperoxalemia and hyperoxaluria as found in Slc26a6-null mice (3) has been interpreted to indicate that Sat1 is involved in the same pathway of oxalate secretion (3, 5, 17). However, our results clearly indicate that Sat1 is dispensable for oxalate secretion across mouse duodenum. We demonstrated that the basolateral step in transcellular oxalate secretion is DIDS sensitive. Given its low expression level, Sat1 may not contribute significantly to oxalate secretion that is predominantly mediated by another DIDS-sensitive basolateral transporter yet to be identified.
The physiological role of Sat1 in the duodenum remains to be determined. Since the apical membrane Na+-coupled transporter NaS1 is expressed along the entire small intestine, including the duodenum (1), it is possible basolateral Sat1 is involved in the process of sulfate absorption. Indeed, we found that oxalate secretion is not sensitive to competition by serosal sulfate, implying that the basolateral transporter mediating oxalate secretion does not transport sulfate. Thus distinct basolateral transporters may participate in sulfate absorption and oxalate secretion. Future studies will need to be directed at identifying transporters other than Sat1 that are important for mediating oxalate secretion in the duodenum.
Finally, although Sat1-null mice have a similar phenotype to Slc26a6-null mice with hyperoxalemia and hyperoxaluria (3), the basis for this phenotype must be independent of duodenal oxalate secretion. One possibility is that Sat1 plays an important role in oxalate secretion in another intestinal segment, such as in the proximal colon, a site of high expression (Table 1). Although we were unable to detect oxalate secretion in proximal colon under the conditions of our in vitro experiments using isolated tissues, it is possible that oxalate secretion across mouse colon occurs under physiological conditions in vivo. Another possibility is that the mechanism underlying hyperoxalemia and hyperoxaluria in Sat1-null mice is not enteric in origin. Whereas the predominant source of the excess oxalate in the plasma and urine of Slc26a6-null mice was shown to be dietary oxalate (7), this has not been studied in Sat1-null mice.
In summary, we have demonstrated the contribution of a DIDS-sensitive basolateral transporter to mediating oxalate secretion across mouse duodenum, but cannot detect a role either for the Sat1 substrates sulfate and HCO3−, or for Sat1 itself in this process. We, therefore, conclude that a DIDS-sensitive basolateral transporter other than Sat1 operates in series with apical SLC26A6 to mediate oxalate secretion across mouse duodenum.
GRANTS
The work was funded by National Institutes of Health grant R37DK33793 (P. S. Aronson), a National Kidney Foundation Postdoctoral Fellowship (F. Knauf), American Society of Nephrology Student Scholar Grant (N. Ko), Korean American Medical Association Scholarship (N. Ko), Yale research fellowship (N. Ko), and National Health and Medical Research Council of Australia grants (D. Markovich).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.K., F.K., Z.J., D.M., and P.S.A. conception and design of research; N.K. and Z.J. performed experiments; N.K., F.K., Z.J., and P.S.A. analyzed data; N.K., F.K., Z.J., D.M., and P.S.A. interpreted results of experiments; N.K., F.K., Z.J., and P.S.A. prepared figures; N.K. drafted manuscript; N.K., F.K., D.M., and P.S.A. edited and revised manuscript; N.K., F.K., Z.J., D.M., and P.S.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Thecla Abbiati for assistance with the animal care.
REFERENCES
- 1. Beck L, Markovich D. The mouse Na+-sulfate cotransporter gene Nas1. Cloning, tissue distribution, gene structure, chromosomal assignment, and transcriptional regulation by vitamin D. J Biol Chem 275: 11880–11890, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem 269: 3017–3021, 1994 [PubMed] [Google Scholar]
- 3. Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Invest 120: 706–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Heneghan JF, Alper SL. This, too, shall pass–like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells”. Am J Physiol Cell Physiol 302: C18–C20, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Jacob P, Christiani S, Rossmann H, Lamprecht G, Vieillard-Baron D, Muller R, Gregor M, Seidler U. Role of Na+HCO3− cotransporter NBC1, Na+/H+ exchanger NHE1, and carbonic anhydrase in rabbit duodenal bicarbonate secretion. Gastroenterology 119: 406–419, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of Sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol 275: F79–F87, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krick W, Schnedler N, Burckhardt G, Burckhardt BC. Ability of Sat-1 to transport sulfate, bicarbonate, or oxalate under physiological conditions. Am J Physiol Renal Physiol 297: F145–F154, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Lee A, Beck L, Markovich D. The mouse sulfate anion transporter gene sat1 (Slc26a1): cloning, tissue distribution, gene structure, functional characterization, and transcriptional regulation thyroid hormone. DNA Cell Biol 22: 19–31, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol 69: 361–375, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int 71: 103–115, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Quondamatteo F, Krick W, Hagos Y, Kruger MH, Neubauer-Saile K, Herken R, Ramadori G, Burckhardt G, Burckhardt BC. Localization of the sulfate/anion exchanger in the rat liver. Am J Physiol Gastrointest Liver Physiol 290: G1075–G1081, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Regeer RR, Markovich D. A dileucine motif targets the sulfate anion transporter Sat-1 to the basolateral membrane in renal cell lines. Am J Physiol Cell Physiol 287: C365–C372, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Robertson WG, Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26: 105–110, 1980 [DOI] [PubMed] [Google Scholar]
- 17. Robijn S, Hoppe B, Vervaet BA, D'Haese PC, Verhulst A. Hyperoxaluria: a gut-kidney axis? Kidney Int 80: 1146–1158, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Worcester EM, Coe FL. Clinical practice Calcium kidney stones. N Engl J Med 363: 954–963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]