Abstract
Hydrogen sulfide (H2S) has recently been identified as a regulator of various physiological events, including vasodilation, angiogenesis, antiapoptotic, and cellular signaling. Endogenously, H2S is produced as a metabolite of homocysteine (Hcy) by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST). Although Hcy is recognized as vascular risk factor at an elevated level [hyperhomocysteinemia (HHcy)] and contributes to vascular injury leading to renovascular dysfunction, the exact mechanism is unclear. The goal of the current study was to investigate whether conversion of Hcy to H2S improves renovascular function. Ex vivo renal artery culture with CBS, CSE, and 3MST triple gene therapy generated more H2S in the presence of Hcy, and these arteries were more responsive to endothelial-dependent vasodilation compared with nontransfected arteries treated with high Hcy. Cross section of triple gene-delivered renal arteries immunostaining suggested increased expression of CD31 and VEGF and diminished expression of the antiangiogenic factor endostatin. In vitro endothelial cell culture demonstrated increased mitophagy during high levels of Hcy and was mitigated by triple gene delivery. Also, dephosphorylated Akt and phosphorylated FoxO3 in HHcy were reversed by H2S or triple gene delivery. Upregulated matrix metalloproteinases-13 and downregulated tissue inhibitor of metalloproteinase-1 in HHcy were normalized by overexpression of triple genes. Together, these results suggest that H2S plays a key role in renovasculopathy during HHcy and is mediated through Akt/FoxO3 pathways. We conclude that conversion of Hcy to H2S by CBS, CSE, or 3MST triple gene therapy improves renovascular function in HHcy.
Keywords: homocysteine, hydrogen sulfide, 3-mercaptopyruvate sulfurtransferase, cystathionine β-synthase, cystathionine γ-lyase, mitophagy
homocysteine (hcy) is an established vascular risk factor and promotes vascular diseases at an elevated level, known as hyperhomocysteinemia (HHcy), including endothelial dysfunction, smooth muscle proliferation, and matrix remodeling. While the exact mechanisms of these pathophysiological conditions are not clear and may be multifaceted, many laboratories have identified oxidative stress (57), epigenetic modification of proteins (47), and cellular apoptosis and autophagy (36, 55) as being involved in HHcy-associated vascular disorders. Recently, a growing body of evidence suggested that depletion of hydrogen sulfide (H2S) in the body during HHcy is one of the possible mechanisms of vasculopathies (2, 50).
H2S is a gaseous molecule of immense physiological importance that extends from antioxidant properties (28), vasorelaxation (32), neurotransmitter (33), angiogenic agent (48), and many more, too numerous to name. Physiologically, Hcy metabolizes by three enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercatopyruvate sulfurtransferase (3MST), to produce H2S. These three H2S-producing enzymes have a wide range of tissue distributions in common, such as brain, liver, and kidney (22, 27, 44, 45), in addition to their organ specificity. One of the important physiological roles of these three transsulfuration enzymes is to generate H2S in the body using either Hcy or cysteine as a substrate. While the main source of H2S is cysteine under normal physiological conditions, Hcy becomes a preferred source of H2S production in HHcy (9). Thus it is expected that HHcy will result in a higher level of H2S in the body. Paradoxically, during pathological level, HHcy causes downregulation of H2S leading to vascular disease, such as impairment of endothelium (14). The mechanism(s) of such a paradoxical effect of HHcy in H2S regulation and vascular disorders are not clear.
In the recent years, several mechanisms have been proposed to define depletion of H2S during HHcy to explain Hcy-associated vasculopathies. For example, we and others (5, 39) previously reported attenuation of CSE in HHcy as a regulatory mechanism of H2S depletion. The exact mechanism, however, is far from clear. Additionally, in an Hcy-independent study, we (19) recently reported the regulatory role of H2S in vascular endothelial growth factor (VEGF) and their inhibitors during heart failure. However, the role of H2S in Hcy-induced modulation of these factors in the vascular bed and the combined effects of CBS, CSE, and 3MST enzymes in modulation of Hcy-associated renal vasculopathy has never been tested.
Another signature of vascular disease underlies the extracellular matrix (ECM) components in HHcy (59, 60). Proteinases and their tissue inhibitors play major roles in the formation and degradation of ECM under physiological and pathological conditions (53, 56). Matrix metalloproteinases (MMPs) maintain tissue homeostasis in the matrix and, therefore, contribute to ECM modulation. Among MMPs, we (39) previously reported that MMP-2 and -9 are involved in renal matrix remodeling, in particular collagen IV modulation, associated with HHcy. On the other hand, MMP-13 is an interstitial collagenase that degrades collagen I and II (3) and has significant renal expression related to ECM remodeling during progressive renal diseases (1, 30). Although MMPs are directly involved in matrix degradation, endogenously their activities are tightly regulated by tissue inhibitors of metalloproteinases (TIMPs), of which TIMP-1 inactivates most MMPs (43, 52, 59). It has been reported that reduction of Hcy level is directly associated with mitigation of MMP activity (40) and matrix accumulation (59); however, the precise role of H2S in regulation of MMP-13 and TIMP-1 in HHcy related renovascular remodeling is not clearly defined.
While the MMP/TIMP balance maintains ECM homeostasis, autophagic degradation of cellular components, such as mitochondria, also plays role in vascular remodeling (54). Autophagic degradation of mitochondria denotes mitophagy. This is a normal physiological process to remove debris or recycle cellular components. Although the existence of mitophagy is well known, the phenomena whether the mitochondria are randomly or selectively targeted are unclear. Mitochondria are the site of oxidative phosphorylation, and respiration produces reactive oxygen species (ROS; Refs. 11, 26). As a major source of ROS production, mitochondria are especially prone to oxidative damages. Hcy is also known to cause oxidative damage of cellular organelles (34). However, to our knowledge, the effect of HHcy on mitophagy, particularly in the vascular mitochondria, and the possible modulatory role of H2S on renal vascular dysfunction are not elicited.
Reports (18) are also available that oxidative stress induces cell death through dephosphorylation of Akt signaling pathways. Hcy induces oxidative stress and HHcy reduces H2S production, which is an antioxidant molecule (36). Akt in the downstream pathway activates transcription factor FoxO. Importantly, FoxO plays a role in cell survival and is negatively regulated by Akt-activation during oxidative stress (25). It is well reported that H2S minimizes oxidative stress; however, the roles of H2S in Akt activation and FoxO regulation in the downstream pathway during HHcy are not defined.
Taking into account of Hcy pathobiology and the beneficial role of H2S in health and disease, the current study was undertaken to address the mechanism of renovascular dysfunction in HHcy and ameliorating role of H2S, if any. Since the renal artery plays an important role in regulating renal function in normal and pathophysiological conditions, we chose to delineate the functional status of the renal artery in HHcy and defined the implications of triple gene therapy (CBS, CSE, and 3MST) to modulate this function through H2S generation. Additionally, the involvement of Akt/FoxO signaling pathway in this mechanism was explored in cellular level.
MATERIALS AND METHODS
Animal model.
C57BL/6J [wild type (WT)] mice of ages 12–16 wk were used for this study. Mice were obtained from Jackson Laboratories and housed in the animal care facility of the University of Louisville. The mice were anesthetized with tribromoethanol (240 mg/kg body wt) and killed to collect renal artery. All animal procedures were in accordance with the National Institutes of Health's guidelines for animal research and were approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Antibodies and reagents.
CBS, CSE, and 3MST antibodies were purchased from Novus Biologicals (Littleton, CO). Anti-CD31, anti-VEGF, and anti-endostatin antibodies were purchased from Abcam (San Francisco, CA). Antibodies against microtubule-associated protein light chain 3 (LC3)AI/II, mammalian target of rapamycin (mTOR), BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3), and Beclin 1 were from Cell Signaling. Anti-phospho-Akt, anti-phospho FoxO3a, anti-MMP-13, anti-TIMP-1, anti-β-actin, anti-GAPDH, and horseradish peroxidase-conjugated secondary antibodies were from Millipore. ROS, particularly hydrogen peroxide (H2O2), detection reagent DCFDA [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester; CM-H2DCFD] was from Invitrogen. All other reagents were used from commercially available highest grade.
Cell culture.
Mouse aortic endothelial cells (MAECs) were from Cellbiologics (Chicago, IL). Cells were cultured and maintained in DMEM/F-12 (50/50) medium containing 10% FBS, 2 mM l-glutamine, and antibiotics (Mediatech, Herndon, VA). T-25 flasks were grown in a tissue culture incubator, trypsinized (0.25% trypsin, 0.1% EDTA in HBSS without Ca2+, Mg2+, and sodium bicarbonate; Mediatech), and plated onto 12-well TPP (Techno Plastic Products, Trasadingen, Switzerland) cell culture plate. For the transfection study, cells were allowed to grow ∼50% confluence and then transfected with either CBS, CSE, 3MST, or triple genes. Transfection efficacy was measured by GFP vector in separate experiments. After 48 h of transfection, cells either were lysed to measured CBS, CSE, and 3MST protein expression and/or to detect H2S generation capability or treated with or without Hcy (75 μM) to collect mitochondria. Cells were also cultured for 48 h in the presence of H2S (30 μM) and Hcy (75 μM) and immunostained with LC3AI/II antibody secondarily conjugated with FITC. For flow cytometry, analysis mitochondria were isolated from the cells, immunostained with LC3AI/II, and analyzed by flow cytometry. ROS production was measured in the isolated mitochondria treated with Hcy (75 μM) for 48 h using CM-H2DCFDA as a substrate. For thephosphorylation study, cells pretreated with or without H2S (30 μM) were treated with Hcy for 30 min, and proteins were analyzed. Empty plasmid was used as a control for all transfection study.
Renal artery culture, overexpression of genes, and myobath study.
The renal artery was used for ex vivo gene transfection and culture and to measure functional status. The artery was isolated from anesthetized mice and placed in ice-cold physiological salt solution (PSS) containing the following (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2 PO4, 1.2 MgSO4, 12.5 NaHCO3, and 11.1 glucose pH 7.4. Fat and connective tissues were cleaned off, and the vessels were cut into 2-mm pieces. Four to five arterial rings were placed in each well of a 12-well cell culture plate (TPP) containing 1 ml complete cell culture medium (DMEM/F-12 50/50, 10% FCS, 1% l-glutamine, and 1% penicilin-streptomycin solution; Cellgro; Mediatech). Arterial explants were transfected with plasmid having CBS, CSE, and 3MST genes (0.4 μg DNA/cm2 of growth area) or empty plasmid using jetPRIME transfection reagent (Polyplus Transfection) following the manufacturer's instructions. After 6 h, arterial rings were gently mounted on the bottom of Matrigel well (BD Biosciences). For effective mounting, a very thin layer of Matrigel (BD Biosciences) was applied to the outer surface of vessel to be attached to the well and immediately placed on the well. One milliliter of complete cell culture medium was added to the wells, and the plates were kept in 37°C cell culture incubator for 48 h. The transfection efficiency was 30–40% as determined by the percentage of GFP-positive cells through flow cytometry 24 h after transfection. After 48 h, arterial rings were taken off the matrigel, and any excess Matrigel was removed. Rings were mounted with two tungsten wires (Scientific Instruments Services, Ringoes, NJ) of same diameter (0.002 in.) attached to the myobath and were placed in 25-ml organ bath filled with PSS at 37°C. The PSS in the myobath was constantly aerated with 95% O2-5% CO2, respectively. Rings were stretched gradually to obtain 0.5-g optimal resting tensions and were equilibrated for an hour. After equilibration, phenylephrine (Phe) of 10−6 to 10−2 M was added in the organ bath to make a final concentration of 10−9 to 10−5 M, respectively. Acetylcholine (Ach) was added to the organ bath in similar manner as described for Phe to detect endothelial-dependent vasorelaxation. The tissue responses were recorded graphically using mp100 software for 10 min of each for each drug concentration.
Detection of tissue capability to generate H2S.
The capability of renal arterial tissue to generate H2S was determined according to the previously adopted method (41).
Tissue sectioning.
At the end of experiment, cultured renal arterial tissue were placed in tissue freezing media (Triangle Biomedical Sciences, Durham, NC) and were frozen in liquid nitrogen. Frozen blocks with the molds were placed in a −70°C freezer until serial sections were made. Cryosections (Leica CM1850) of 3-μm thicknesses were put on glass slides and immunostained with anti-CD31, anti-VEGF, anti-endostatin, and anti-CSE antibodies with appropriate secondary fluorescence antibodies to measure expression of these molecules under laser scanning confocal microscopy (Olympus FluoView 1000).
Immunostaining.
Cryosections on the slide or MAECs grown in chamber slides (Lab-Tek II; Thermo Fisher Scientific, Rockford, IL) were washed with PBS (pH 7.4), fixed with 3.7% paraformaldehyde containing 0.25% l-α-lysophosphatidylcholine for 30 min followed by three washes with PBS 5 min each. Tissues were then blocked with 1% BSA for 15 min and washed with PBS (3×, 5 min each), the appropriate primary antibody (1:100 dilutions in 1% BSA) was added, and they were incubated for overnight at 4°C with gentle agitation. Excess antibody was washed by PBS (3×, 5 min each) wash, and secondary fluorescence-conjugated antibody (1:500 dilutions in 1% BSA) was added and incubated for 2 h at room temperature. Unbound secondary antibodies were removed by PBS wash (3×, 5 min each), tissues were stained with nuclear stain DAPI wherever mentioned in results, and fluorescence was visualized in a laser scanning confocal microscope (Olympus Fluoview 1000) with the appropriate filter.
Immunoblotting.
Protein was isolated from cells using RIPA lysis buffer (Thermo Scientific), containing protease inhibitors and PMSF. The protein content in the samples was estimated by BCA assay, and an equal amount of total protein was loaded in each well of SDS-PAGE gels. Protein was separated by electrophoresis, transferred to a PVDF membrane, and incubated with primary antibody followed by secondary horseradish peroxidase-conjugated antibody. An ECL plus Western blotting reagent (GE Health Care, Little Chalfont, Buckinghamshire) was used to detect the protein of interests. To normalize expressed protein in the Western blot, membranes were stripped with membrane-stripping buffer (Boston BioProducts, Worcester, MA) and reprobed with either β-actin or GAPDH antibody. The intensity of bands was detected by Gel -Doc software and was normalized with their corresponding β-actin/GAPDH control.
Measurement of ROS.
ROS, in particular H2O2, hydroxyl (HO−•) and peroxyl (ROO−•) radicals in the isolated mitochondria, were detected by CM-H2DCFDA reagent following manufacturer's instructions. This dye is nonfluorescent in reduced form but after cellular oxidation becomes fluorescent. Briefly, mitochondria were isolated from experimental cells, resuspended in PBS containing 10 μM CM-H2DCFDA, and maintained at 37°C in the dark for 30 min. Cells washed to remove excess dye and analyzed by fluorescence spectrophotometer.
Flow cytometry.
At the end of experiments, mitochondria were isolated and immunolabeled with LC3AI/II antibody. Mitochondria were then washed and labeled with FITC conjugated secondary antibody. Cells were washed, and 10,000 events were analyzed by flow cytometry (Accuri 6). Isotype control was used for this study.
Statistical analysis.
Values were taken as means ± SE of measurement. The number of experiment was carried out for each of the experiment is mentioned in results. The difference between mean values of multiple experiments was analyzed by one-way ANOVA (unless otherwise mentioned) followed by Scheffé's post hoc analysis. Paired t-test was used to determine significance difference between groups, and P < 0.05 was considered significant.
RESULTS
Overexpression of genes, tissue generation of H2S and vascular reactivity of renal artery in ex vivo condition.
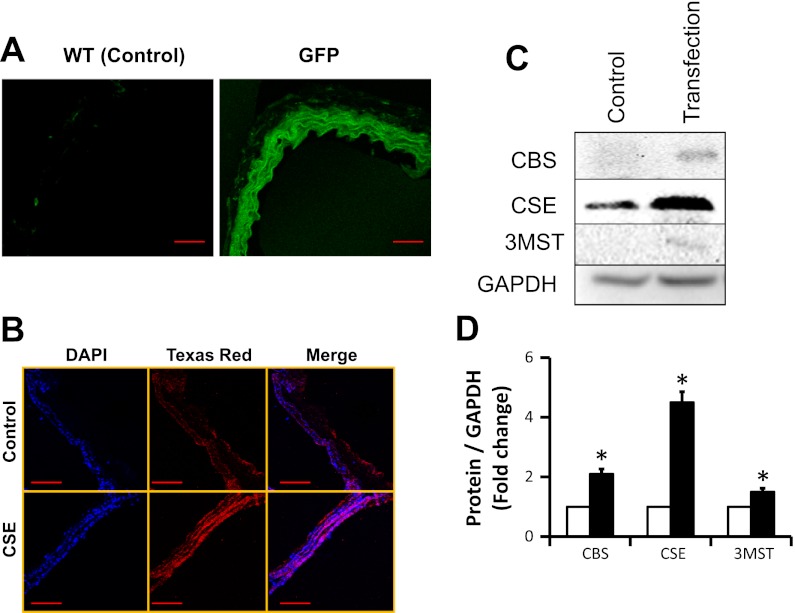
Arterial explants overexpressed with CBS, CSE, 3MST, or triple genes were used for this study. Transfection efficacy of gene was determined by GFP assay (Fig. 1A). Briefly, a similar group of tissue that were overexpressed with CBS, CSE, 3MST, or triple genes was transfected with pcDNA3.1/GFP plasmid vector and after 48 h of transfection tissue sections were observed under confocal microscope. The results indicated that the tissues expressed GFP (Fig. 1A). Since CSE mainly localizes in the vascular smooth muscle cells (VSMCs), we confirmed its overexpression in VSMCs by immunostaining (Fig. 1B). Furthermore, tissue protein was analyzed by immunoblotting to confirm overexpression of CBS, CSE, and 3MST (Fig. 1, C and D).
Fig. 1.
A: transfection efficacy in arterial explants. Renal arterial explants from wild type (WT; C57BL/6J) were transfected with pcDNA3.1/GFP, pcDNA3.1/CBS, pME18S-CSE-HA, pME18S-3MST, or triple genes of cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST) as described in materials and methods and an earlier report (38). After 48 h, GFP-transfected artery was cryosectioned and transfection efficacy was determined by examining pcDNA/GFP fluorescence in fluorescent microscope (scale bar = 50 μm). B: CSE overexpression in vascular smooth muscle cell (VSMC). Since CSE is mainly localized in the VSMC, enhanced expression of CSE in the arterial section after gene delivery was confirmed by immunostaining. Red fluorescence indicated expression of CSE, and blue fluorescence indicated nuclear stain with DAPI (scale bar = 100 μm). C and D: immunoblotting and protein expression levels. Enhanced expression of CBS, CSE, and 3MST protein in the renal arterial explant was further confirmed by immunoblotting following gene transfection (data are means ± SE; n = 3; *P < 0.05 vs. control).
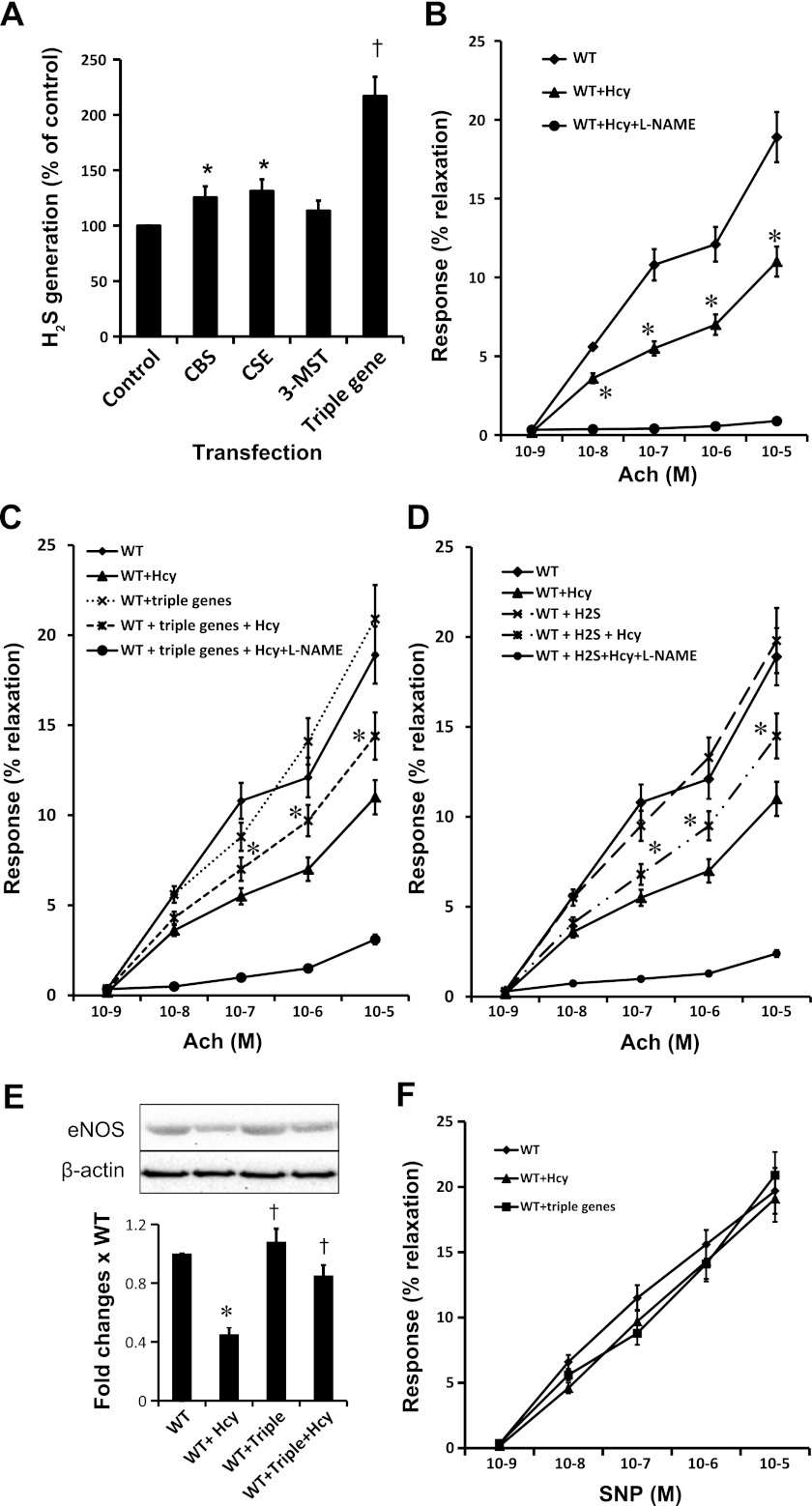
Next, we measured tissue ability to generate H2S. Results indicated that the tissues that overexpressed CBS, CSE, or 3MST had greater ability to generate H2S compared with nontransfected tissue (Fig. 2A). Interestingly, triple gene-delivered tissue generated almost double the amount of H2S compared with control and ∼70–80% more than individual gene therapy (Fig. 2A).
Fig. 2.
A: gene delivery increased generation of hydrogen sulfide (H2S) in arterial explants. WT arterial explants after 48 h of gene transfection were homogenized, and tissue capability to generate H2S was determined by our previously adopted method (41) using homocysteine (Hcy) as a substrate. Data represent means ± SE; n = 4–5 independent experiments. *P < 0.05 vs. control; †P < 0.01 vs. individual gene transfection. B: Hcy impaired endothelial-dependent vasorelaxation. Renal arterial explants of WT mice were cut into 2-mm pieces and cultured with or without Hcy (75 μM) for 48 h following protocol as described in materials and methods. Arterial rings were mounted in a myobath containing physiological salt solution as described materials and methods. A group of rings that were incubated with Hcy mounted in myobath containing NG-nitro-l-arginine methyl ester (l-NAME; 10 μM), an endothelial nitric oxide synthase (eNOS) inhibitor. Rings were precontracted with phenylephrine (Phe; 10−5M) and later challenged with dose-dependent acetylcholine (Ach) as indicated. Vessels that received l-NAME were virtually unresponsive to Ach, indicating blocked eNOS activity. Data were analyzed by two-way ANOVA and represented as means ± SE; n = 4–5. *P < 0.05 vs. WT at same dose of Ach. C: triple gene therapy improved endothelial-dependent vasorelaxation in HHcy. Renal arterial explants of WT mice were cut into 2-mm pieces, transfected with triple genes and cultured with or without Hcy (75 μM) as indicated for 48 h. Arterial rings of WT and WT + triple genes served as controls for WT + Hcy and WT + triple genes + Hcy, respectively. Rings were mounted between 2 tungsten wires and hung in myobath as described earlier. In another set of experiment, WT arterial rings received l-NAME (10 μM) in the myobath in addition to triple genes and Hcy treatment. Endothelial-dependent vasorelaxation was measured in cumulative Ach doses. Data were analyzed by two-way ANOVA and represent means ± SE; n = 4–5 independent experiments. *P < 0.05 WT + triple genes + Hcy vs. WT + Hcy. D: H2S improved endothelial-dependent vasorelaxation in HHcy. Arterial rings were incubated with Hcy and with or without H2S (30 μM, in the form of NaHS). Endothelial-dependent vasorelaxation was measured as described earlier. Rings that received l-NAME in myobath were unresponsive to Ach, indicating blocked eNOS activity. Data were analyzed by two-way ANOVA and represent means ± SE; n = 4–5 independent experiments. *P < 0.05 WT + H2S + Hcy vs. WT + Hcy. E: expression of eNOS. After myobath study, arterial rings were homogenized in RIPA lysis buffer and eNOS expression was measured by immunoblotting. Data represent means ± SE; n = 4–5. *P < 0.01 vs. WT and †P < 0.05 vs. WT + Hcy. F: no changes in endothelial-independent vascular relaxation were observed among the groups. Phe (10−5 M)-precontracted vessels were challenged with dose-dependent (10−9-10−5 M) sodium nitroprusside (SNP), a direct NO donor. No differences were recorded among WT, WT + Hcy, and WT + triple gene vessels, indicating unchanged vascular smooth muscles reactivity among the groups. Data were analyzed by two-way ANOVA and represent means ± SE; n = 4.
Since Hcy is known to cause impaired endothelial-dependent vascular relaxation and H2S causes vasodilation, we measured vascular endothelial reactivity (Ach induced endothelial dependent) of Phe (10−5 M) precontracted vessels of Hcy-, H2S-, and H2S-generating genes transfected arterial rings. In the myobath, Phe precontracted renal arterial rings were challenged with cumulative doses of Ach (10−9-10−5 M) to measure endothelial-dependent relaxation. Results indicated that arterial rings incubated with Hcy (WT + Hcy) had impaired endothelial-dependent vasorelaxation compared with control groups (WT; Fig. 2B). Interestingly, endothelial-dependent vasorelaxation was improved in the arterial rings incubated with Hcy that were overexpressed triple genes (WT + triple genes + Hcy; Fig. 2C). A similar result was obtained from the group of WT + H2S + Hcy (Fig. 2D). No significant changes in vascular reactivity were recorded in the control (WT) vs. WT + triple gene-delivered groups (Fig. 2C) or WT vs. WT + H2S supplemented groups (Fig. 2D) without Hcy treatment.
We suspected inhibition of endothelial nitric oxide synthase (eNOS) by Hcy led to diminished endothelial-dependent vascular relaxation and improvement of relaxation was due to either the increase of eNOS by triple genes or through the protective mechanism by H2S in HHcy. To examine this, we preincubated (and incubation continued until end of the experiments) vessels, as shown in Fig. 2, B–D, with NG-nitro-l-arginine methyl ester (l-NAME; 10 μM), a specific inhibitor of eNOS. These vessels were precontracted with Phe (10−5 M), and dose-dependent responses to Ach were measured. Results as shown in Fig. 2, B–D, indicated that l-NAME incubated vessels were unresponsive to Ach-induced relaxation.
After vascular reactivity study, we analyzed tissues to detect eNOS expression through immunoblotting. The results indicated that Hcy diminished eNOS expression, whereas triple genes ameliorated eNOS expression in HHcy (Fig. 2E).
To determine whether endothelial-independent relaxation was impaired in HHcy and gene therapy had any role to ameliorate vasorelaxation, we performed endothelial-independent vasorelaxation by sodium nitroprusside. The results indicated that there were virtually no differences of endothelial-independent relaxation, as detected by cumulative sodium nitroprusside (10−9-10−5 M) challenge (Fig. 2F).
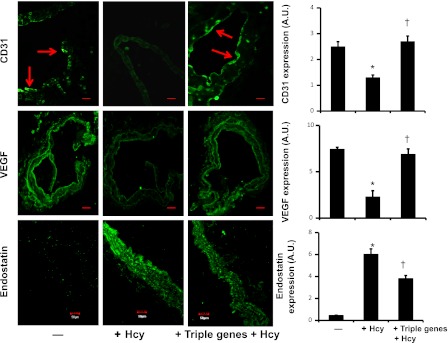
Triple gene delivery improved CD31, VEGF, and diminished endostatin expressions in hyperhomocysteinemia.
Immunostaining of arterial explants indicated that HHcy attenuated CD31 and VEGF, whereas endostatin expression was abrogated (Fig. 3). Overexpressed genes of CBS, CSE, and 3MST reversed the effect of Hcy on the expression of these angiogenic and antiangiogenic factors (Fig. 3).
Fig. 3.
Gene therapy induced CD31 and VEGF and diminished endostatin in renal artery explants in HHcy. CBS, CSE, and 3MST genes were transfected in WT arterial explants, and explants were cultured in matrigel for 48 h in the presence of Hcy (75 μM). Explants were cryosectioned and immunostained with appropriate antibodies secondarily conjugated with FITC. Fluorescence images were taken under confocal microscope (red arrows indicated endothelial lining; scale bar = 50 μm; A.U., arbitrary unit). Bar diagram: data represent means ± SE, n = 7. *P < 0.01 vs. control and †P < 0.01 vs. triple genes + Hcy.
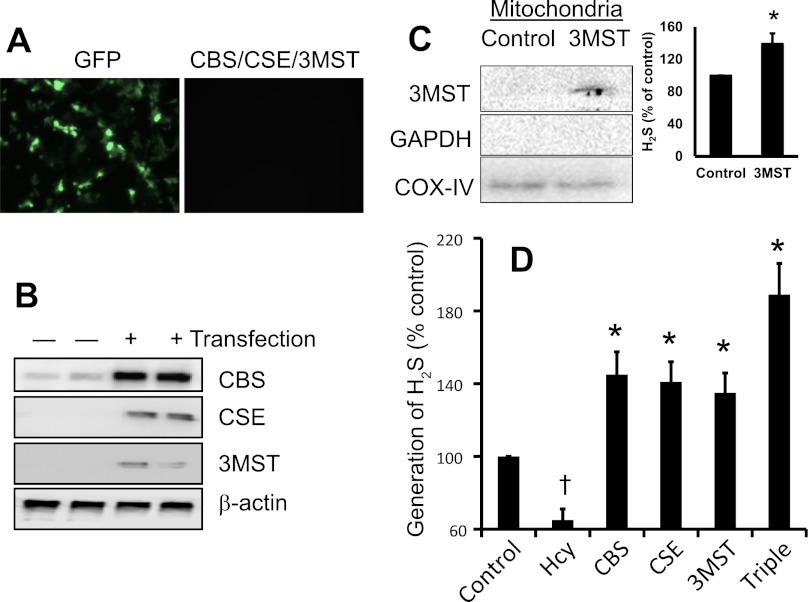
Overexpression of triple genes increased H2S generation in HHcy.
MAECs were used for in vitro transfection study. Transfection efficacy of plasmid vector was determined by GFP expression (Fig. 4A). Overexpression of CBS, CSE, and 3MST was measured by Western blot, and the results indicated that transfected cells were overexpressing these protein compared with control (Fig. 4B). Since 3MST is reported to localize in the mitochondria, the expression of 3MST in the isolated mitochondria after transfection was detected by immunoblotting. Results indicated that 3MST was overexpressed in the mitochondria (Fig. 4C). Not only had that, isolated mitochondria of 3MST-transfected cells produced increased amount of H2S compared with nontransfected cells (Fig. 4C, bar diagram). More importantly, while Hcy significantly attenuated H2S generation in the control cells (nontransfected cells); the overall ability to generate H2S, in the presence of Hcy by the cells overexpressing CBS, CSE, and 3MST genes was significantly increased compared with control (Fig. 4D). The generation of H2S was maximally measured from the triple gene-delivered cells (Fig. 4D).
Fig. 4.
Triple genes expression and H2S generation in endothelial cells. A: mouse aortic endothelial cells (MAECs) were transfected with either CBS, CSE, 3MST, or triple genes. Expression of GFP vector indicating successful transfection. B: after 48 h of transfection, cell were lysed and expression of CBS, CSE, and 3MST protein were measured by Western blot. C: in isolated mitochondria, localized expression of 3MST was confirmed by immunoblotting. Immunoblotting of mitochondria extracted protein with anti-GAPDH antibody indicated isolation of pure mitochondria, which is free from cytosolic fraction of conserved GAPDH protein. Immunoblot was reprobed with cytochrome c oxidase (COX IV) antibody and used as a mitochondrial loading control. The capability of H2S generation by isolated mitochondria from 3MST-transfected cells was measured following protocol as described in materials and methods. Bar diagram showed 3MST-transfected mitochondria generated increased amount of H2S vs. control in presence of Hcy (data are means ± SE; n = 4; *P < 0.05 vs. control). D: cells capability to generate H2S in presence of Hcy was measured as indicated in the method and our previously adopted protocol (37). Representative data from n = 5 independent experiments. Data are means ± SE; n = 4. †P < 0.05 vs. control and *P < 0.05 vs. Hcy.
Triple gene delivery prevented mitophagy in HHcy.
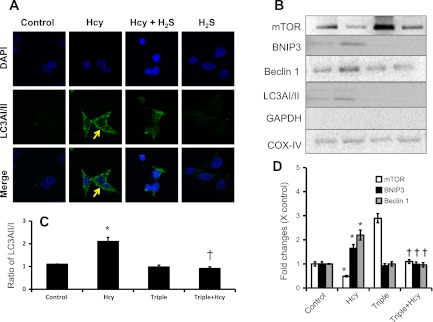
To determine whether H2S mitigates Hcy-induced mitophagy, we incubated MAECs with or without Hcy pretreated with H2S (30 μM). Expression of LC3AI/II, a marker of autophagy/mitophagy, was dramatically increased in cells incubated with Hcy (75 μM) for 48 h (Fig. 5A). Supplementation of H2S (30 μM) along with Hcy diminished expression of LC3AI/II (Fig. 5A). Immunoblotting results of isolated mitochondria extracted protein indicated and further confirmed expression of LC3I/II in the mitochondria in HHcy (Fig. 5, B and C). Triple gene delivery abolished Hcy-induced expression of LC3I/II in mitochondria (Fig. 5, B and C). Additionally, markers of mitophagic molecules, such as BNIP3 and Beclin 1, were upregulated in mitochondria; whereas mTOR was attenuated by Hcy treatment (Fig. 5, B and D). These effects were normalized in the mitochondria of MAECs, which overexpressed CBS, CSE, and 3MST triple genes and received Hcy treatment for 48 h (Fig. 5, B and D).
Fig. 5.
Hcy-induced mitophagy was mitigated by H2S and gene therapy. A: for immunostaining, MAECs were cultured for 48 h in the presence or absence of H2S (30 μM, in the form of NaHS) and Hcy (75 μM) as shown. Cells immunostained with microtubule-associated protein light chain 3 (LC3)AI/II antibody secondarily conjugated with FITC (green). Nucleus stained with DAPI (blue). B: Hcy-induced expression of mitophagy markers, mammalian target of rapamycin (mTOR), Beclin 1, BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3), and ratio of LC3AI/II were mitigated by CBS, CSE, and 3MST gene transfection. MAECs were transfected with genes as indicated and treated with or without Hcy (75 μM) for 48 h. Mitochondria were isolated and lysed in RIPA lysis buffer. Equal amounts of protein were analyzed for mitophagy markers as indicated. GAPDH immunoblotting of mitochondria extracted protein indicated isolation of pure mitochondria, which is free from cytosolic GAPDH. Immunoblot was reprobed with COX IV antibody and used as a mitochondrial loading control. Ratio of LC3AII/I (C) and densitometric analyses (D) of mTOR, BNIP3, and Beclin 1 are shown. Data represent means ± SE; n = 4. *P < 0.01 vs. control and †P < 0.01 vs. Hcy.
Triple gene delivery mitigated Hcy-induced mitophagy and ROS production.
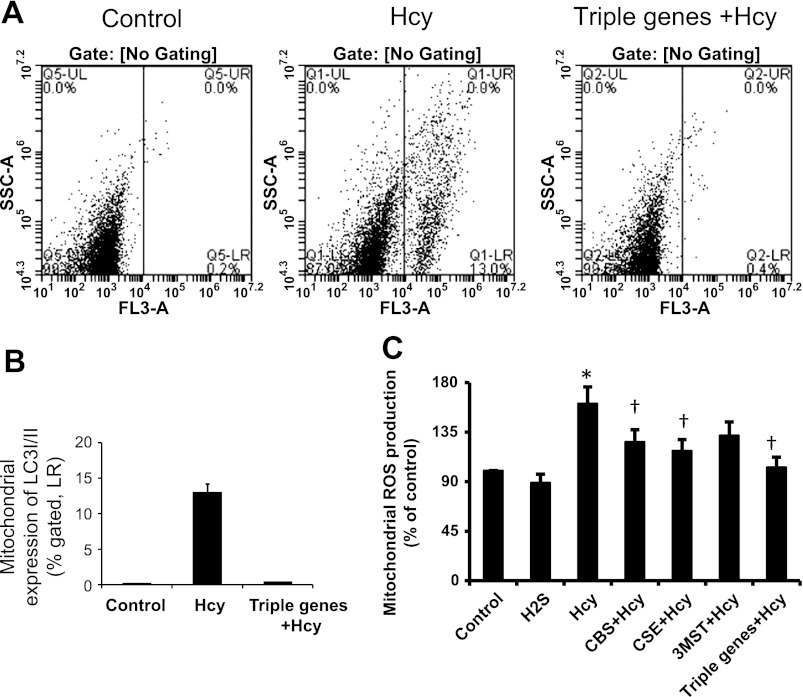
Immunostaining as well as immunoblotting of mitophagic markers as shown in Fig. 5 indicated mitophagy in HHcy and preventive role of H2S from Hcy-induced mitophagy in MAECs. To further verify, we isolated mitochondria from triple gene-delivered MAECs treated with or without Hcy. As shown in the Fig. 6A, flow cytometry data indicated that a significant number of mitochondria (13%; Fig. 6, A and B) expressed LC3AI/II treated with Hcy. Interestingly, the mitochondria of triple gene-delivered cells were not expressing this marker.
Fig. 6.
Triple gene delivery mitigated mitophagy and mitochondrial reactive oxygen species (ROS) production. A: for flow cytometry, mitochondria were isolated from the treated MAECs as shown, immunostained with LC3AI/II, and analyzed by flow cytometry. Appropriate controls were taken. B: bar diagram showed %mitochondria expressing LC3AI/II marker as an indication of mitophagy. Data represent means ± SE; n = 4/group. *P < 0.05 vs. control and †P < 0.01 vs. Hcy. C: MAECs were transfected either with single gene or triple genes as shown and cultured for 48 h in the presence of Hcy (75 μM). Mitochondria were isolated, and ROS production was detected using DCFDA [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester] as a substrate. Data represent means ± SE; n = 5 experiments. *P < 0.05 vs. control; †P < 0.05 vs. Hcy.
To determine whether Hcy induced ROS in mitochondria that may have played a role in mitophagy, we isolated mitochondria and measured ROS by DCFDA substrate. Figure 6C indicated that Hcy increased ROS production, particularly H2O2, ∼50% more compared with the mitochondria isolated from control cells. Individual gene delivery of CBS, CSE, or 3MST to the cells mitigated ROS production; however, the production of ROS was almost normalized in the mitochondria of triple gene-delivered cells (Fig. 6C).
H2S regulated Akt and Foxo3 activation and MMP/TIMP expression in HHcy.
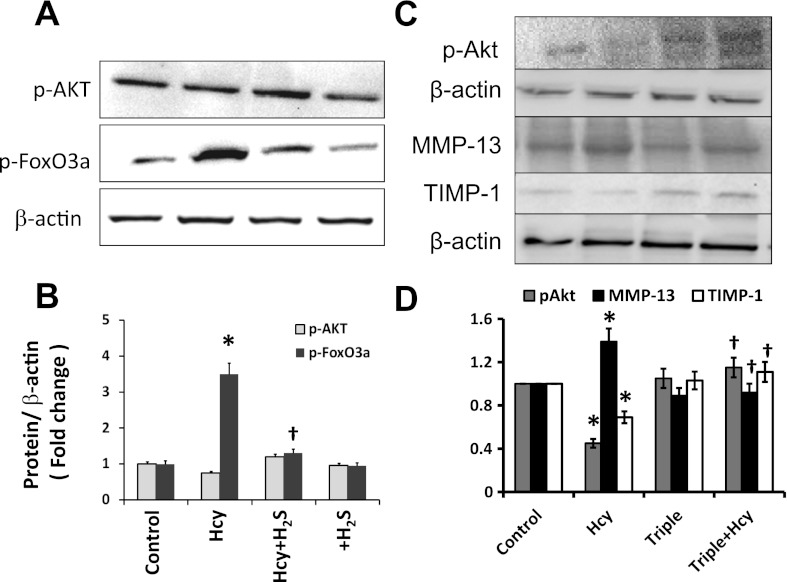
We determined the phosphorylation of survival kinase Akt and its downstream regulator FoxO3a in HHcy, since they are important regulators of mitophagy. Also, any modulatory role of H2S in this pathway was determined. The results indicated that Hcy mitigated activation of Akt and induced phosphorylation FoxO3a (Fig. 7, A and B), whereas, H2S reversed these effects of Hcy. Similarly, triple gene therapy normalized Hcy regulated mitigation of Akt activation (Fig. 7, C and D). Also, increased expression of MMP-13 and attenuated expression of TIMP-1 in Hcy-treated cell were normalized by triple gene therapy (Fig. 7, C and D).
Fig. 7.
A and B: Hcy-induced dephosphorylation of Akt and phosphorylation of FoxO3a mitigated by H2S. A: MAECs pretreated with or without H2S (30 μM, in the form of NaHS) were treated with Hcy (75 μM) for 30 min as indicated. A group without any treatment served as control and a group pretreated with H2S served as control for Hcy + H2S. At the end of experiment, cells were lysed and proteins were analyzed by Western blot. B: bar diagram shows densitometric analyses of phospho-protein expression. Data represent means ± SE; n = 4. *P < 0.05 vs. control and †P < 0.05 vs. Hcy treatment. C and D: gene therapy normalized Akt activation and matrix metalloproteinases (MMP)-13/tissue inhibitors of metalloproteinases (TIMP)-1 imbalance. In a separate experiment (C), cells were transfected with CBS, CSE, and 3MST genes and treated with Hcy (75 μM). Expression of phospho-Akt after 30 min of Hcy treatment and MMP-13 and TIMP-1 after 48 h of Hcy treatment was measured by Western blot. D: bar diagram showed densitometric analyses of protein expression. Data represent means ± SE; n = 4. *P < 0.01 vs. control; †P < 0.05 vs. Hcy treatment.
DISCUSSION
It is well documented that HHcy impairs endothelial function and remodels vascular bed, especially ECM and diminishes vascular function in pathophysiological conditions (4, 13, 15, 16, 24, 39). Here, we demonstrated with evidence that overexpression of CBS, CSE, and 3MST genes mitigated HHcy effect on vascular bed by converting Hcy to H2S. The benefit of gene delivery was achieved by two ways: first, by metabolizing Hcy that minimized stress, and second, by converting Hcy to H2S, which protected tissue from oxidative damage due to HHcy. In addition, involvement of mitophagy, the MMP/TIMP axis, and the Akt/FoxO3a pathway have been demonstrated in endothelial pathobiology during HHcy. Furthermore, the regulatory mechanism of H2S to modulate vascular illness in HHcy was delineated with experimental evidences.
Remodeling of vessel is a dynamic and physiological process, and many factors are involved in this process including angiogenic and antiangiogenic factors. VEGF is an angiogenic factor that promotes endothelial cell proliferation, migration, and tube formation (17). These events are essential for the development of new blood vessels from preexisting ones. This molecule specifically targets endothelial cells and promotes their proliferation, survival, migration, and sprouting (61). On the other hand, an antiangiogenic factor, such as endostatin, inhibits vascular growth by down regulating VEGF (20). Hcy has been reported to inhibit endothelial cell proliferation, migration, and tube formation, suggesting that Hcy inhibit vascular growth (6, 7, 31). Contrary to antiangiogenic effects of Hcy on vasculature, a recent report (35) suggested that H2S, a metabolic product of Hcy, is an endogenous factor of vascular growth and management through VEGF-dependent pathway. In a clinical study, Tepper et al. (51) reported that human endothelial progenitor cells from diabetic subjects exhibited impaired proliferation, adhesion, and subsequent incorporation into vascular structures. This suggested a clear correlation between impaired endothelial proliferation and structural anomaly in a clinical setting. Although this study did not link impaired endothelial proliferation and function, through an in vitro study Tanaka et al. (49) reported a distinct link between improved proliferation and function in endothelial cells using honeycomb-patterned polymer film. Our present report in agreement with this previous study suggested improved endothelial function by triple gene therapy in HHcy through eNOS-dependent pathway (Fig. 2C) and also perhaps through endothelial proliferation mechanism (Fig. 2E and 3). This was most likely achieved through two different mechanisms: first, Hcy toxicity to vascular cells was diminished by accelerated metabolism of Hcy by triple genes; and second, accelerated Hcy metabolism produced angiogenic H2S, which promoted endothelial proliferation as evidenced by CD31 upregulation by triggering VEGF and its inhibitor, endostatin (Fig. 3).
Results from our study (Fig. 4D) and others suggested that there was reduced production of H2S in the presence of high Hcy. It is no surprise to ask a question why the production of H2S reduces during HHcy, when it is a substrate of the transsulfuration enzymes. Previously, we (38) reported possible mechanisms of this paradoxical effect. Briefly, although cysteine is the main substrate of CSE and CSE expresses mainly in vascular bed, during HHcy Hcy competes for binding to CSE with cysteine. Therefore, an increased Hcy level decreases H2S production from cysteine through substrate inhibition (5, 46). In addition, protein homocysteinylation is a major reaction in the presence of thiolactone and homocysteinylation led to protein damage (23). It is possible that, although there is no direct evidence, the activity of CSE may be severely impaired during HHcy resulting in attenuated H2S generation. This is still an active interest in our laboratory; however, presently we do not have supporting evidence to establish this hypothesis. Future rigorous studies are needed to verify this plausibility.
Another mechanism of vessel remodeling is through cellular autophagy and mitophagy. Mitophagy denotes degradation of mitochondria through autophagy, and autophagy is a process whereby cellular components are degraded by engulfment into autophagosomes (8). In this regard several molecules, such as LC3, mTOR, BNIP3, and Beclin 1 play key roles in mitophagic events. While LC3, BNIP3, and Beclin 1 are autophagic markers (58), mTOR regulates cell growth, cell proliferation, cell motility, and cell survival (21). Although mitophagy is finely tuned to reutilize cellular energy during stress, several lines of investigations have reported cellular oxidative stress-mediated mitophagy in the disease pathogenesis (29). Hcy is known to cause oxidative damage of cellular organelles (34). However, the effect of HHcy on mitophagy, particularly in the vascular mitochondria, and its consequences on vascular dysfunction are not well established. Our present report demonstrated that Hcy initiated mitophagy by inhibiting mTOR and inducing LC3AI/II, BNIP3, as well as Beklin 1 (Figs. 5 and 6, A and B). Supplementation of H2S as well as triple gene overexpression mitigated mitophagy (Figs. 5 and 6, A and B). These results suggested two possible different mechanisms of H2S effect on cellular mitophagy in HHcy. In the first mechanism supplemented H2S protected mitochondria from being subjected to mitophagy (Fig. 5A). In the second mechanism, triple gene overexpression of transsulfuration enzymes utilized Hcy as a substrate to produce H2S. This second mechanism therefore minimized the Hcy effect on mitophagy by reducing Hcy stress (Fig. 5B and 6A), as well as by enhancing generation of antioxidant H2S (Figs. 4, C and D). It has been reported that autophagy resulted in necrotic cell death in human umbilical vein endothelial cells (10) and this mechanism may contribute to the deterioration of vascular endothelial function (10). It is possible in our study that diminished endothelial function in HHcy-treated vessels (Fig. 2B) may be, in part, due to autophagic/mitophagic death of functional endothelial cells and/or VSMC. Although we demonstrated here with evidence that Hcy induced mitophagy in in vitro endothelial cells (Figs. 5 and 6, A and B), which may have contributed to impaired endothelial-dependent vascular function in arterial explants (Fig. 2B), further studies are needed to dissect whether only endothelial cells are involved in vascular dysfunction or it is a combined effect of VSMC and endothelial cells. Additionally, in our experiments, gene therapy improved but did not normalize endothelial function. This could be due to partial transfection, as we have reported in materials and methods that ∼30–40% cells was GFP positive.
The signaling molecule of Akt is well recognized for its antiapoptotic activity and is known as a survival kinase (12). FoxO proteins are a group of Forkhead family of transcription factors and studies (42) have indicated that decreased Akt signaling activates autophagy transcription-dependent mechanism involving Foxo3. However, the involvement of Akt/FoxO signaling cascades in Hcy-induced mitophagy and the regulatory role of H2S, if any, are not defined in vascular diseases. Our present report demonstrated that Hcy dephosphorylated Akt, and the downstream pathway upregulated FoxO (Fig. 7). H2S as well as triple gene overexpression ameliorated Akt activation and mitigated FoxO. This result suggested that Akt/FoxO pathway may have a role in the induction of mitophagy/autophagy in HHcy. Contrary to H2S supplementation, which modulated Akt/FoxO pathways, triple gene delivery modulated Hcy effect by metabolizing Hcy to H2S rather than direct interfering of these signaling cascades. This result demonstrated differential role of endogenous vs. exogenous H2S in Hcy signaling.
Chronic HHcy alters ECM components (59, 60) and MMPs maintain ECM homeostasis in the matrix. Among MMPs, MMP-13 have significant renal expression related to ECM remodeling during progressive renal diseases (1, 30). MMP activities are endogenously regulated by TIMPs, and TIMP-1 inactivates most MMPs (43, 52, 59). Although the reduction of Hcy level is directly associated with amelioration of MMP activity (40) and matrix accumulation (59), the mechanism of MMP/TIMP-1 regulation in HHcy and the possible role of H2S are not well known. We (55) previously reported that mitochondrial MMP-9 mediated autophagy/mitophagy in cardiomyocytes during HHcy through a N-methyl-d-aspartate receptor 1-dependent pathway. Here we demonstrated that Hcy induced MMP-13 and mitigated TIMP-1 (Fig. 7C). Interestingly, triple gene delivery normalized the expression of MMP-13, suggesting a triggering mechanism of this metalloproteinase by H2S in HHcy.
In our study, we chose to transfect renal arteries with CBS, CSE, and 3MST genes of transsulfuration enzymes ex vivo rather than in vivo injection for the following reasons: 1) to avoid immune response; 2) to increase efficacy of transfection with minimum amount of DNA; and 3) to avoid potential health problems to the animal, such as toxicity and inflammatory responses with large amount of DNA. Furthermore, in our experiment normal renal arteries, i.e., arteries from animal that are non-HHcy, were used. In ex vivo condition these arteries were treated with high Hcy (75 μM) to measure endothelial dysfunction, and triple genes were delivered to measure any protective role of gene therapy from Hcy threat. It is fact that in our study 3MST did not increase H2S generation significantly in the renal arterial tissue compared with control. However, 3MST transfection significantly increased H2S generation in cultured MAECs mitochondria (Fig. 4C). It is possible that the renal arterial tissue was insufficient to measure 3MST-mediated H2S generation in our experiment. Nonetheless, triple gene therapy significantly increased H2S generation vs. control and improved endothelial-dependent vasorelaxation in the presence of Hcy vs. Hcy alone (Fig. 2, A and C, respectively). Improved endothelial-dependent vasorelaxation in triple gene-delivered vessels were, at least in part, by increased eNOS activity as evidenced by eNOS inhibition (Fig. 2C). Taken together, we believe this functional improvement was achieved as a result of the combined effects of three transsulfuration enzymes. The study could have been considerably strengthened and more clinically relevant if renal arteries from HHcy animals, such as CBS+/−, would have been considered. However, the arteries of CBS+/− animals were already exposed to higher levels of Hcy in their circulation as well as in vasculature, due to impaired metabolism, and may have damaged endothelial integrity. The idea of the present study was to demonstrate the protective role of gene therapy in preserving endothelial function by Hcy metabolism and efficient production of H2S. The study, however, was not designed to regenerate damaged endothelial cells from HHcy animals, such as CBS+/−, ex vivo. Therefore, we used WT arteries instead. Nevertheless, there is an urgent need for a study that aims to repair and regenerate endothelial cells to demonstrate effectiveness of gene therapy to restore endothelial function in HHcy.
In conclusion, the important aspects of our study is that overexpression of Hcy metabolizing genes CBS, CSE, and 3MST improved endothelial function in HHcy by triggering angio- and antiangiogenic factors through H2S generation. In addition, mitophagy and MMP/TIMP were also regulated by gene therapy possibly through H2S regulated Akt/FoxO pathway. This is an interesting finding, where gene delivery could not only be exploited to minimize Hcy toxicity but also to protect cells by the generating antioxidative agent H2S.
Limitations of the study.
This study has following limitations: 1) the renal arteries were cultured for 48 h, which is longer than standard incubation time. This raises a concern regarding the viability of the vessels such a long time after harvesting. In addition, as shown in Fig. 2B, the level of relaxation of treated renal vessels to Ach reaches 20% at most, which is lower than usually observed in normal vessel. This again raises a concern of whether this related to loss of vasoreactivity due to long incubation time. 2) Ex vivo study, instead of in vivo gene treatment, was performed to avoid immune response and potential health problems to animals. Since the safety and toxicity of the treatment in our present study are yet unproven in vivo, we consider this is a potential limitation of our study. 3) The idea tested in this study was to observe any protective effect of gene therapy in preserving endothelial function, rather than endothelial regeneration. In the discussion, although we have discussed the reason for examining normal arteries, the fact that the treatment works in normal arteries does not guarantee it would succeed under disease conditions. An experimental model close to renal vascular disease would be helpful to explore the effectiveness of the therapy in this area. Last, but not least, 4) in our experiment, reduced ROS was found after the gene therapy. In this case, although we did not measure it, an endothelium-independent increase in NO availability could be another mechanism that warrants future investigation.
GRANTS
This study was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-104103 (to U. Sen) and HL-71010 and HL-88012 (to S. C. Tyagi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: U.S., S.K., P.K.M., and S.C.T. conception and design of research; U.S., P.B.S., S.G., D.C., P.K.M., N.Q., and N.M. performed experiments; U.S., P.B.S., and S.K. analyzed data; U.S., S.K., P.K.M., N.T., and S.C.T. interpreted results of experiments; U.S. prepared Figs.; U.S. drafted manuscript; U.S., N.T., and S.C.T. edited and revised manuscript; U.S. approved final version of manuscript.
REFERENCES
- 1.Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 71: 755–763, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Beard RS, Jr, Bearden SE. Vascular complications of cystathionine β-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am J Physiol Heart Circ Physiol 300: H13–H26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int 51: 1388–1396, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation 99: 1156–1160, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chang PY, Lu SC, Lee CM, Chen YJ, Dugan TA, Huang WH, Chang SF, Liao WS, Chen CH, Lee YT. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ Res 102: 933–941, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Halkos ME, Surowiec SM, Conklin BS, Lin PH, Lumsden AB. Effects of homocysteine on smooth muscle cell proliferation in both cell culture and artery perfusion culture models. J Surg Res 88: 26–33, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Chan DC. Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18: R169–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csordas A, Kreutmayer S, Ploner C, Braun PR, Karlas A, Backovic A, Wick G, Bernhard D. Cigarette smoke extract induces prolonged endoplasmic reticulum stress and autophagic cell death in human umbilical vein endothelial cells. Cardiovasc Res 92: 141–148, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol Cell Physiol 264: C961–C967, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol 183: 101–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demuth K, Drunat S, Girerd X, Moatti N, Paul JL, Safar M, Boutouyrie P. Homocysteine is the only plasma thiol associated with carotid artery remodeling. Atherosclerosis 165: 167–174, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Distrutti E, Mencarelli A, Santucci L, Renga B, Orlandi S, Donini A, Shah V, Fiorucci S. The methionine connection: homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology 47: 659–667, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Edirimanne VE, Woo CW, Siow YL, Pierce GN, Xie JY, O K. Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can J Physiol Pharmacol 85: 1236–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke 35: 345–347, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 22: 8983–8998, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Givvimani S, Munjal C, Gargoum R, Sen U, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure. J Appl Physiol 110: 1093–1100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajitou A, Grignet-Debrus C, Devy L, Berndt S, Blacher S, Deroanne CF, Bajou K, Fong T, Chiang YW, Foidart JM, Noel A. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J 16: 1802-+, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 381: 113–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J 13: 2277–2283, 1999 [PubMed] [Google Scholar]
- 24.Joseph J, Kennedy RH, Devi S, Wang J, Joseph L, Hauer-Jensen M. Protective role of mast cells in homocysteine-induced cardiac remodeling. Am J Physiol Heart Circ Physiol 288: H2541–H2545, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, Hu MC, Reddy MA, Natarajan R. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids 41: 113–121, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Lan A, Liao X, Mo L, Yang C, Yang Z, Wang X, Hu F, Chen P, Feng J, Zheng D, Xiao L. Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells. PLos One 6: e25921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol 11: 574–581, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Loscalzo J. Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology. J Clin Invest 119: 3203–3205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 18: 557–559, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, Weitz JI, Austin RC. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood 94: 959–967, 1999 [PubMed] [Google Scholar]
- 35.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol 297: F410–F419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen U, Givvimani S, Abe OA, Lederer ED, Tyagi SC. Cystathionine beta-synthase and cystathionine gamma-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation. Am J Physiol Cell Physiol 300: C155–C163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen U, Mishra PK, Tyagi N, Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem Biophys 57: 49–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol 31: 442–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen U, Rodriguez WE, Tyagi N, Kumar M, Kundu S, Tyagi SC. Ciglitazone, a PPARγ agonist, ameliorates diabetic nephropathy in part through homocysteine clearance. Am J Physiol Endocrinol Metab 295: E1205–E1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, Metreveli N, Hayden MR, Tyagi SC. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology 82: 201–213, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem 284: 28319–28331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma R, Suzuki K, Nagase H, Savin VJ. Matrix metalloproteinase (stromelysin-1) increases the albumin permeability of isolated rat glomeruli. J Lab Clin Med 128: 297–303, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 146: 623–626, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Stabler SP, Steegborn C, Wahl MC, Oliveriusova J, Kraus JP, Allen RH, Wagner C, Mudd SH. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism 51: 981–988, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Stroylova YY, Zimny J, Yousefi R, Chobert JM, Jakubowski H, Muronetz VI, Haertle T. Aggregation and structural changes of alpha(S1)-, beta- and kappa-caseins induced by homocysteinylation. Biochim Biophys Acta 1814: 1234–1245, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol 164: 853–865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka M, Takayama A, Ito E, Sunami H, Yamamoto S, Shimomura M. Effect of pore size of self-organized honeycomb-patterned polymer films on spreading, focal adhesion, proliferation, and function of endothelial cells. J Nanosci Nanotechnol 7: 763–772, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tang XQ, Shen XT, Huang YE, Chen RQ, Ren YK, Fang HR, Zhuang YY, Wang CY. Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: role of ERK1/2 activation. J Mol Neurosci 45: 60–67, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Torres L, Garcia-Trevijano ER, Rodriguez JA, Carretero MV, Bustos M, Fernandez E, Eguinoa E, Mato JM, Avila MA. Induction of TIMP-1 expression in rat hepatic stellate cells and hepatocytes: a new role for homocysteine in liver fibrosis. Biochim Biophys Acta 1455: 12–22, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Tummalapalli CM, Tyagi SC. Responses of vascular smooth muscle cell to extracellular matrix degradation. J Cell Biochem 75: 515–527, 1999 [PubMed] [Google Scholar]
- 54.Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, Tyagi SC. Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int J Physiol Pathophysiol Pharmacol 3: 210–222, 2011 [PMC free article] [PubMed] [Google Scholar]
- 55.Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Transduct Res 30: 78–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallner EI, Yang Q, Peterson DR, Wada J, Kanwar YS. Relevance of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol Renal Physiol 275: F467–F477, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol 22: 34–41, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Xu P, Das M, Reilly J, Davis RJ. JNK regulates FoxO-dependent autophagy in neurons. Genes Dev 25: 310–322, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang ZZ, Zou AP. Homocysteine enhances TIMP-1 expression and cell proliferation associated with NADH oxidase in rat mesangial cells. Kidney Int 63: 1012–1020, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Yi F, Xia M, Li N, Zhang C, Tang L, Li PL. Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension 53: 90–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zent R, Pozzi A. Antiangiogenic therapy in diabetic nephropathy. J Am Soc Nephrol 17: 325–327, 2006 [DOI] [PubMed] [Google Scholar]