Abstract
Cellular activity of the myosin light chain phosphatase (MLCP) determines agonist-induced force development of smooth muscle (SM). CPI-17 is an endogenous inhibitor protein for MLCP, responsible for mediating G-protein signaling into SM contraction. Fluctuations in CPI-17 expression occur in response to pathological stresses, altering excitation-contraction coupling in SM. Here, we determined the signaling pathways regulating CPI-17 expression in rat aorta tissues and the cell culture using a pharmacological approach. CPI-17 transcription was suppressed in response to the proliferative stimulus with platelet-derived growth factor (PDGF) through the ERK1/2 pathway, whereas it was elevated in response to inflammatory, stress-induced and excitatory stimuli with tranforming growth factor-β, IL-1β, TNFα, sorbitol, and serotonin. CPI-17 transcription was repressed by inhibition of JNK, p38, PKC, and Rho-kinase (ROCK). The mouse and human CPI-17 gene promoters were governed by the proximal GC-boxes at the 5′-flanking region, where Sp1/Sp3 transcription factors bound. Sp1 binding to the region was more prominent in intact aorta tissues, compared with the SM cell culture, where the CPI-17 gene is repressed. The 173-bp proximal promoter activity was negatively and positively regulated through PDGF-induced ERK1/2 and sorbitol-induced p38/JNK pathways, respectively. By contrast, PKC and ROCK inhibitors failed to repress the 173-bp promoter activity, suggesting distal enhancer elements. CPI-17 transcription was insensitive to knockdown of myocardin/Kruppel-like factor 4 small interfering RNA or histone deacetylase inhibition. The reciprocal regulation of Sp1/Sp3-driven CPI-17 expression through multiple kinases may be responsible for the adaptation of MLCP signal and SM tone to environmental changes.
Keywords: smooth muscle contraction and differentiation, excitation-transcription coupling, protein phosphatase-1, inflammation
excitation-contraction coupling in smooth muscle (SM) is governed through the signaling pathways determining reversible phosphorylation of the myosin regulatory light chains (MLC20). This coupling is altered through a subset of gene expression regulated in response to environmental cues, such as physiological stimuli and pathological stresses. The specific MLC20 phosphatase (MLCP) is highly expressed in SM cells (18, 19). G-protein activation suppresses MLCP activity, causing augmented MLC20 phosphorylation and force development, named Ca2+ sensitization (28). Conversely, MLCP activity is enhanced in response to cGMP elevation, causing Ca2+ desensitization (31, 63). Cellular MLCP activity is regulated through the phosphorylation of myosin phosphatase targeting protein 1 regulatory subunit (MYPT1) (54) and a switch in specific splicing variants of MYPT1 confers the functional transition of MLCP during SM development (24). In addition, CPI-17, an endogenous inhibitor protein for MLCP (15), is predominantly expressed in SM, where it mediates agonist stimuli into MLCP inhibition. Several kinases, such as PKC, Rho-kinase (ROCK), integrin-linked kinase, zipper-interacting protein kinase, and p21-activated kinase, are identified to phosphorylate CPI-17 at Thr38, converting this protein into a potent inhibitor of MLCP (15). Conversely, CPI-17 is dephosphorylated and inactivated in response to nitric oxide production, leading to SM relaxation, playing a key role in determining cellular MLCP activity and modulating SM responsiveness (29).
CPI-17 expression varies among SM tissues. The expression is higher in arteries with the estimated concentration at 7 μM, which is above the level of MLCP (1–3 μM; Ref. 61). Less CPI-17 is expressed in phasic SMs, such as intestines and urinary detrusor SM, at < 1 μM (61). CPI-17 expression is regulated in response to environmental factors. During embryonic development, CPI-17 expression increases in arteries in parallel with a gain of the cardiac output and decreases in response to SM de-differentiation in the tissue cultures and neointimal cells (16, 27). Furthermore, lines of evidence suggest alterations in CPI-17 expression in response to pathological stresses. An increase in CPI-17 expression occurs in hypoxic pulmonary artery (9), hyperglycemic or obstructed urinary bladder (1, 4), bronchus and airway under inflammation (38, 47), and kidney cortex treated with testosterone (51). By contrast, the downregulation of CPI-17 expression occurs in inflamed ileum and colon SM, suggesting the change in CPI-17 expression depending on the SM tissue types (39, 40). In addition to SM tissues, CPI-17 expression is low in embryonic heart, neurons, endothelium, and epithelium, but it is not detectable in mature skeletal and cardiac muscles (27, 61). CPI-17 expression is elevated in epithelium-derived cancer cells, in which the proliferation is upregulated through the inhibition of MLCP (22, 53). Thus it is evident that CPI-17 expression is tightly regulated through tissue-specific signaling pathways and determines cellular MLCP activity, whereas little is known about mechanisms controlling the expression.
Alterations in the transcription of SM-specific genes, including ion channels, cytoskeletal, and signaling proteins trigger a reversible transition between proliferative and contractile phenotypes of SM cells in response to environmental stimuli (42). The majority of SM-specific gene expression are driven through the binding of a ubiquitous transcription factor, serum-response factor (SRF), complexed with a SM-specific transcription cofactor myocardin or myocardin-related transcription factors, to the specific DNA motif CArG box, which is found at the 5′-flanking region of most SM-specific genes with TATA-box (32, 35, 37, 42, 45). The proliferative PDGF stimulus suppresses the myocardin/SRF action and causes a repression of SM-specific gene transcription. This PDGF-induced SM gene repression is mediated through multiple signal pathways, such as ERK1/2; Kruppel-like factor 4 (KLF4), which is a repressor of myocardin gene; the de-acetylation of histone H4; microRNA-145/143; and various environmental stimuli, related to pathological conditions, such as oxidized phospholipid, collagen composition, tranforming growth factor (TGF)-β, and inflammatory cytokines (32, 35, 45, 58). In addition, certain genes expressed in SM, such as ACLP and CRP2, are regulated by the ubiquitous transcription factors Sp1/Sp3 but not by myocardin binding (30, 64). PDGF stimulus or vascular injury causes an increase and decrease in the expression of ACLP and CRP, respectively, suggesting the role of Sp1/Sp3 in the transcription of certain genes expressed in SM (30, 64). Yet, non-myocardin pathways remain to be resolved.
To understand the mechanisms by which MLCP activity and SM tone are regulated through the expression of CPI-17 in response to environmental changes, we determined the molecular basis of CPI-17 transcription in SM. The mouse and human CPI-17 gene promoters and the regulatory circuit controlling the activity in rat aorta tissue and cell culture were defined using quantitative (q)RT-PCR, chromatin immunoprecipitation (ChIP), EMSA, and luciferase-reporter gene assay. The results suggest that myocardin-independent Sp1/Sp3 pathways regulate the CPI-17 transcription through multiple kinases, such as ERK1/2, p38, JNK, PKC, and ROCK in response to environmental changes.
MATERIALS AND METHODS
Cell culture.
Rat aorta smooth muscle cell (AoSMC) was prepared from inbred Sprague-Dawley rats (4- to 6-wk-old, male) using the explant method (13), following the protocol approved by the Thomas Jefferson University Insitutional Animal Care and Use Committee. AoSMC was cultured with the growth medium DMEM/Ham's F-12 (DMEM/F12, Mediatech) supplemented with 10% FBS (Atlanta Biologicals) and penicillin-streptomycin (Mediatech). The endogenous CPI-17 was stably expressed in the cell culture until passage 15. AoSMC was also prepared using the enzymatic method with a mixture of collagenase and elastase (Worthington Biologicals; Ref. 62). The expression of CPI-17 was higher in the dispersed cells, and the expression declined during passage, as described previously (62). The response of the CPI-17 transcription was essentially indistinguishable between the two preparations (data not shown). The quiescent AoSMC was prepared by overnight conditioning with MCDB 131 medium (Mediatech) in the presence of 1% FBS. Drosophila S2 cells were gifted by Dr. James B. Jaynes in the institution and harvested in Schneider's Drosophila medium (Invritogen) with 12% FBS and penicillin/streptomycin. COS1 and HEK293 cells were purchased from ATCC and harvested in DMEM with 5% FBS.
qRT-PCR.
One-step qRT-PCR was performed using the Brilliant-II qRT-PCR kit with SyBr green (Stratagen). Total RNA was prepared using RNeasy mini kit (Qiagen). An aliquot (200 ng) was mixed with the mixture of the master mix and specific primers listed in Table 1. The reaction was performed using Mx3005P real-time PCR instrument (Stratagene). Cycle number at threshold (Ct) of real-time PCR was determined using MaxPro software equipped with the instrument. Triplicate assay was run to obtain mean value ± SE of Ct. β-Tubulin was used for internal control. ΔΔCt method was used to determine relative extent of each mRNA, which was defined as 2^-(ΔΔCt) (34). Upper and lower limits of each data point were obtained using the method described by Yuan et al. (66).
Table 1.
List of primers/probes for qRT-PCR and qChIP
| Target | Sequence |
|---|---|
| qRT-PCR | |
| CPI-17 CDS* | ACATGCCAGATGAGGTCAACATCG |
| AAGTCCTCTGTGGGATTCAGGCAA | |
| Tub-ß CDS | AGATGGCAGTCACCTTCATCGGAA |
| TGTTGCTCTCAGCTTCGGTGAACT | |
| Myocardin CDS | GTCGAGTCCAACAGTTCCGGA |
| CTCACTGTCGGTGGCATAGTG | |
| KLF4 CDS | CTTTCCTGCCAGACCAGATG |
| GGTTTCTCGCCTGTGTGAGT | |
| sm22α CDS | AGGCAGCTGAGGATTATGGA |
| CTGCCCAAAGCCATTACAGT | |
| sm-α-actin CDS | CATCAGGAAGGACCTCTATGC |
| CTGATCCACATCTGCTGGAAG | |
| Rel-A CDS | CCACTAAGACGCACCCCACC |
| GCTTGCTCCAGGTCTCGCTT | |
| ChIP | |
| CPI-17 proximal | TGCACGTCTCTGTAGGAAGG |
| CTTGCGCTTGGGTCTCTAAC | |
| FAM-CGGGTCCCAGAACACGAGCC-IABFQ | |
| CPI-17 distal | GCCAGAGGGCGTAACAGG |
| GTCCTGCGGGGATACAGAT | |
| 5HEX-CGGAGCCCTCTGAGATGGGC-IABFQ |
ChIP, chromatin immunoprecipitation; q, quantitative; KLF4, Kruppel-like factor 4; sm, smooth muscle.
CDS is coding sequence. Primers and Tacman probes were designed using PrimerQuest software at www.IDTDNA.com.
Promoter assay.
Cells were seeded in 24-well plate and transiently transfected using 2 μl of FuGeneHD (Roche) with 0.5 μg of the promoter DNA segment cloned in pGL4.20 reporter gene vector (Promega) plus 0.5 μg of pCMV-SEAP, encoding secreted embryonic alkaline phosphatase (SEAP) cDNA driven by a CMV promoter (Addgene plasmid24595). After 48-h transfection with the growth medium, the cells were further incubated for 24 h under the desired conditions. The culture medium was transferred to a tube and subjected to SEAP assay. Remained cells were rinsed once with PBS and then lysed for 5 min at room temperature with 75 μl of One-Glo lysis buffer (Promega). The cell lysate (50 μl) was transferred into 96-well black plate and mixed with 50 μl of One-Glo assay buffer (Promega). After a 5-min incubation, the luminescence was determined using the plate reader with luminometer module (Safire2, Tecan). In parallel, the culture medium was heated for 5 min at 65°C. An aliquot (2 μl) of the medium was incubated with 100 μl of SEAP reagent, including 50% PhosphaGLO, 0.1 M Na2CO3, 0.5 mM MgCl2, and 10 mM homoarginine, pH 10 in 96-well black plate and subjected to luminometry. Promoter activity was defined as change in relative fluorescence units (ΔRFU) (Luciferase assay)/ΔRFU (SEAP assay), where ΔRFU = RFU (sample) − RFU (background). Background value was obtained using the empty vector of pGL4.20 and pCMV.
EMSA.
Nonradioactive EMSA was performed using LightShift chemiluminescence EMSA kit (Pierce) with biotinylated DNA duplexes as probes, following the manufacturer's protocol. The 5′-biotinylated primers were used for PCR to amplify the desired portion. Biotinylated PCR products were purified using MinElute PCR purification kit (Qiagen). For the preparation of nuclear protein extracts, AoSMC or HEK293 cells were homogenized with the buffer including 0.32 M sucrose, 3 mM MgCl2, and 10 mM HEPES, pH 7.2, with 0.5 mM Tris(2-carboxyethyl)phosphine (TCEP), 4 mM Pefabloc, 1 mM microcystin LR, and 0.3 mM sodium orthovanadate. Nuclei were collected by centrifugation for 10 min at 700 g and washed three times with the same buffer. The nuclear proteins were extracted for 30 min on ice with the buffer including 0.4 M NaCl, 3 mM MgCl2, 0.1% Tween20, 10% glycerol, 0.1 mM EGTA, and 10 mM HEPES buffer pH 7.2, supplemented with 0.5 mM TCEP, 4 mM Pefabloc, 1 mM microcystin LR, and 0.3 mM sodium orthovanadate. The homogenates were spun for 15 min at 20,000 g, and the supernatant was used as the nuclear extract. The optimized conditions for EMSA were 10 μg of nuclear extracts mixed with 100 fmol of biotinylated DNA probe, 1 μg of poly(deoxyinocinic-deoxycytidilic) acid, 40 μM ZnCl2, 0.2 mM EGTA, 100 mM NaCl, 0.1% Tween20, and 20 mM HEPES buffer, pH 7.2. After a 30-min incubation on ice, the reaction mixture was loaded to 0.5 × TBE-5% acrylamide gel (Bio-Rad), and the labeled DNA was visualized on nylon membrane using chemiluminescence method. For the competition and the supershift assay, nonlabeled DNA (2.5 pmol) and specific antibody (1 μg) were added to the mixture, respectively.
ChIP assay.
ChIP assays were carried out using rat aorta tissues or AoSMC. Briefly, for rat aorta tissue, a cleaned fresh rat aorta (∼15 mg) was immediately fixed for 1 h at room temperature with 10 ml of 1% formaldehyde in PBS, quenched for 5 min with 2% glycine in PBS, and then pulverized under liquid nitrogen. The frozen tissue powder was homogenized with the lysis buffer [0.1 M NaCl, 50 mM MOPS-NaOH (pH 7.0), 1 mM EGTA, 0.1% Tween20, 5% glycerol, 2 mM MgCl2, 0.5 mM TCEP, and 4 mM Pefabloc, using Polytron mixer. For AoSMC, the cells in a 10-cm dish were fixed with 1% formaldehyde, quenched with 2% glycine, and homogenized with the lysis buffer using Dounce homogenizer. The crosslinked chromatin fraction was collected by centrifugation, washed twice using the lysis buffer, and treated for 20 min at room temperature with mung bean nuclease (New England Biolab). The homogenates were suspended using sonicator and the sheared chromatin was subjected to immunoprecipitation. An aliquot (100 μl) of the sheared chromatin was diluted with RIPA buffer, supplemented with 10 μg/ml salmon sperm DNA and 0.1 mg/ml BSA, and mixed with 4 μg of immunoprecipitation-grade antibodies as indicated in results. The mixture was rocked overnight at 4°C and mixed with protein A/G magnetic beads (Pierce) to capture the immunocomplex. After being extensively washed with the buffer with RIPA buffer supplemented with 0.25 M LiCl, the DNA fragments were released from the complex by incubation for 2 h at 65°C with proteinase K (New England Biolab). The DNA fragments were purified using QIA Quick PCR purification kit, and the eluant was subjected to PCR or qPCR analysis. For qPCR analysis, we used Tacman primer/probe (IDT) with the primers above. Preimmune IgG (Millipore) was used as blank, and the extent of DNA was normalized against the blank as fold enrichment. All PCR primers and Tacman probes used in the assay are listed in Table 1.
Others.
Mouse and human genomic DNA fragments were prepared by PCR using the mouse genomic BAC clone (RP23–400K20) and human genomic DNA preparation (Clontech) as templates, respectively. Small interfering (si)RNA knockdown was performed using synthetic siRNA fragments for rat myocardin, and KLF4 and Rel-A were obtained from Silencer library of Ambion. AoSMC in 35-mm dish was incubated for 3 days with the mixture of 50 pmol of siRNA and 2 μl of Lipofectamine2000 (Invitrogen) and then subjected to qRT-PCR. Immunoblotting was done as described previously (25). Antibodies for Sp1 and Sp3 and Ac-histone H4 were obtained from Millipore. Anti-GAPDH, anti-SRF, anti-phospho-Sp1, and recombinant Sp1 were from Novus, SantaCruz, Abcam, and Promega, respectively. Anti-CPI-17 was prepared previously (50). Statistical analyses of Student's t-test and ANOVA were performed using Microsoft Excel, and P < 0.05 was considered as significant.
RESULTS
ERK1/2 mediates PDGF stimulus into the repression of the CPI-17 gene.
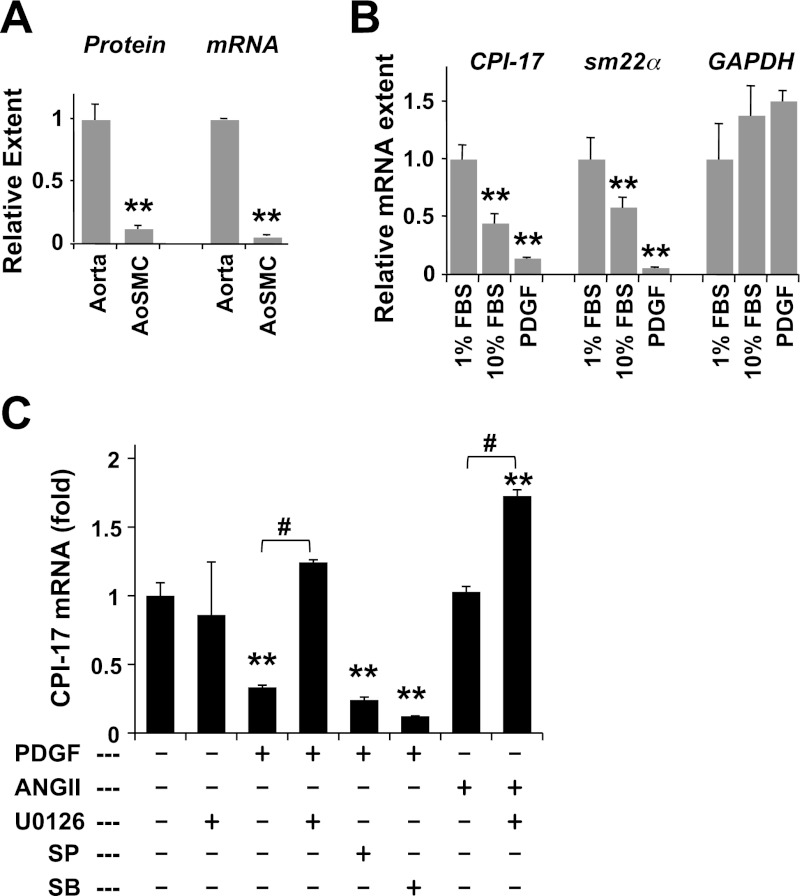
Previous studies (27, 62) using immunoblotting and immunohistochemistry showed a decrease in CPI-17 protein in response to de-differentiation of SM cell culture and in neointimal cells. Figure 1A shows the simultaneous analysis of the levels of CPI-17 protein and mRNA in rat aorta SM tissue and the primary cell culture. The extent of the protein and mRNA was normalized against that of GAPDH as an internal control. The reduction in CPI-17 protein coincided with the level of mRNA, indicating the transcriptional regulation of CPI-17 expression (Fig. 1A). The CPI-17 mRNA level in rat aorta cell culture was reduced after a 24-h incubation under the growth condition (10% FBS), compared with the quiescent condition (1% FBS). PDGF-BB, a potent repressor of SM-specific genes, suppressed the CPI-17 transcription in parallel to a SM-marker gene, sm22α, but not a housekeeping gene, GAPDH (Fig. 1B). Because PDGF stimulation showed a tendency of GAPDH gene activation, we used β-tubulin as an internal control for the rest of assays. PDGF-BB is known to activate MAPK signaling, including ERK1/2, p38, and JNK. As shown in Fig. 1C, treatment of the quiescent AoSMC with U0126, a MEK inhibitor, did not affect the basal level of CPI-17 expression, whereas it cancelled PDGF-induced repression (Fig. 1C). Neither inhibitor of JNK [SP600125 (SP)] or p38 [SB203580 (SB)] eliminated the action of PDGF. Angiotensin-II (ANG II) is known to induce SM cell proliferation through MAPK pathways in parallel with the contraction. CPI-17 transcription was not affected by ANG II stimulation but was enhanced in the presence of U0126 (Fig. 1C). These results suggest that PDGF-induced activation of ERK1/2 causes the repression of CPI-17 gene under growth condition, whereas ANG II stimulus induced both positive and negative signals in the gene regulation.
Fig. 1.
Platelet-derived growth factor (PDGF)-induced repression of CPI-17 gene. A: total protein extracts (20 μg) and total RNA preparation (0.2 μg) of rat aorta tissues and the cell culture [rat aorta smooth muscle cell (AoSMC)] were subjected to immunoblotting (left) and quantitative (q)RT-PCR (right), respectively. CPI-17 protein extent was normalized against GAPDH, and the ratio in aorta tissues was set as 1 (n = 6). Relative extent of CPI-17 mRNA was obtained by ΔΔCt method using GAPDH as an internal control (n = 9). B: subconfluent AoSMC was incubated for overnight with MCDB medium supplemented with 1% FBS. Quiescent cells were restimulated for 24 h with 10% FBS or 50 ng/ml PDGF-BB, subjected to qRT-PCR analysis, using β-tubulin as an internal control (n = 6). C: quiescent AoSMC was stimulated with 50 ng/ml PDGF or 1 μM ANG II for 24 h in the presence of kinase inhibitors (10 μM U1026, 10 μM SP600125, and 10 μM SB203580), which are added 30 min before the stimulation (n = 3–6). **P < 0.05 and #P < 0.05, compared with unstimulated and no-inhibitor controls, respectively.
JNK, p38, ROCK, and PKC are involved in CPI-17 transcription.
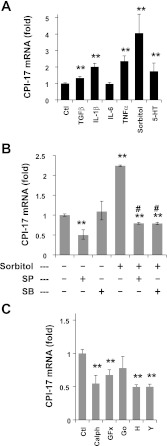
We tested whether inflammatory cytokines, stress, and excitatory stimulus activate CPI-17 transcription in AoSMC (Fig. 2). As shown in Fig. 2A, CPI-17 transcription in the quiescent AoSMC was elevated moderately by TGFβ stimulus and prominently in response to the inflammatory cytokines IL-1β and TNFα but not IL-6. Osmotic stress induced by the addition of sorbitol caused fourfold elevation of CPI-17 mRNA (Fig. 2B), reaching nearly 50% of that in aorta tissues. Sorbitol-induced increase in CPI-17 mRNA was eliminated in the presence of SP600125 and SB203580, whereas these inhibitors had minimal impacts on the basal transcription (Fig. 2B). These results suggest that sorbitol stress activates JNK/p38, inducing CPI-17 transcription. The inflammatory signals are also known to activate NF-κB, a transcription factor complex consisting of Rel-A and p50, in parallel to JNK and p38. However, the contribution of NF-κB in the CPI-17 expression was negligible, because the relative CPI-17 expression was unchanged in cells treated with siRNA of Rel-A, a subunit of NF-κB (1.3 ± 0.20 fold; P > 0.05 vs. control; n = 3).
Fig. 2.
Excitation-transcription coupling of CPI-17 mRNA in AoSMC. Extent of CPI-17 mRNA in AoSMC was determined by qRT-PCR using β-tubulin as reference. A: quiescent cells were stimulated overnight with TGFβ (5 ng/ml), IL-1β (20 ng/ml), IL-6 (10 ng/ml), TNFα (10 ng/ml), and sorbitol (0.2M) (n = 8–9) and subjected to qRT-PCR assay. B: unstimulated and sorbitol stimulated cells were treated overnight with SP600125 (SP; 10 μM) and SB203580 (SB; 10 μM) (n = 6). C: cells in the growth medium supplemented with 10% FBS were harvested with calphostin-C (Cal; 50 nM), GF109203x (GFx; 3 μM), Go6976 (Go; 1 μM), H1152 (H; 3 μM), and Y27632 (Y; 10 μM; n = 9). **P < 0.05 and #P < 0.05, compared with untreated and stimulated cell, respectively.
Excitatory stimulation with serotonin (5-HT) also enhanced CPI-17 transcription by 1.7 fold (Fig. 2A). 5-HT receptors dominating in rat aorta SM cells (type-2A and 2B) are coupled with Gq/11 and trigger the activation of PKC and ROCK (55). We further determined whether PKC and ROCK also activate CPI-17 transcription. The treatment with the pan PKC inhibitors calphostin C (Calph) and GF109203x (GFx) or with the ROCK inhibitors H1152 (H) and Y27632 (Y), significantly reduced CPI-17 transcription in AoSMC under the growth conditions (Fig. 2C), whereas the PKCα/β-specific inhibitor Go6976 (Go) failed to block the transcription. These results suggest that a subset of PKC isoforms and ROCK positively regulate CPI-17 expression.
Proximal GC-rich motifs are necessary for the CPI-17 promoter.
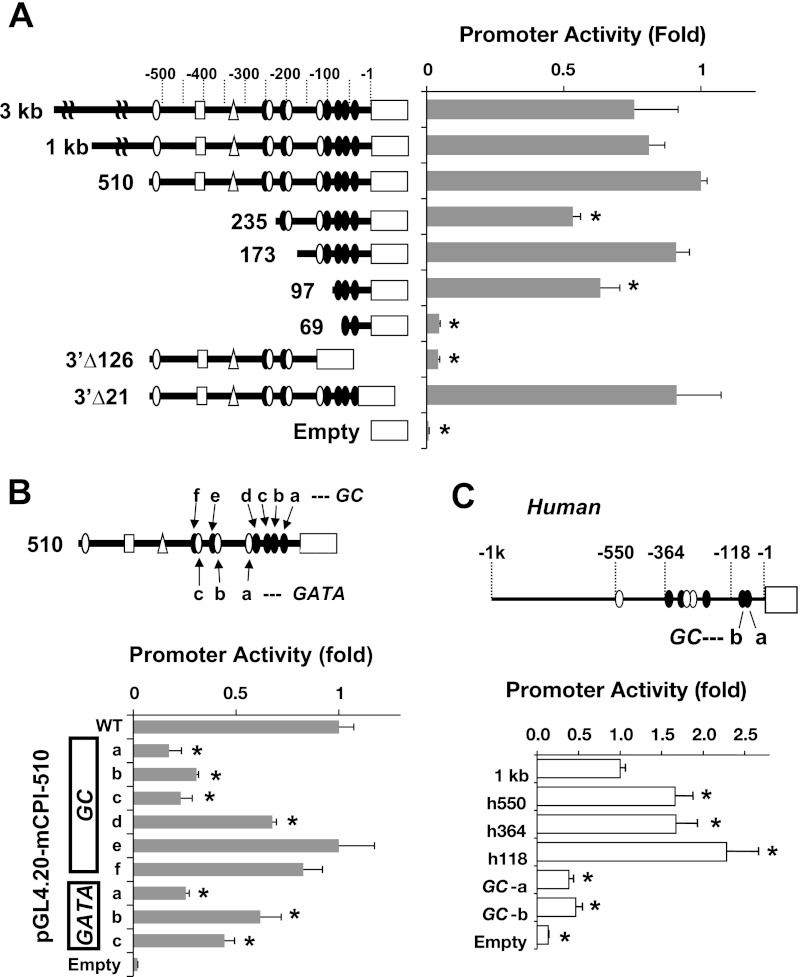
The primary structures of 5′-untranslational region (5′-UTR) of mouse, rat, and human CPI-17 genes were analyzed using TRANSFAC, the database for transcription factor binding sites (Fig. 3 and see Fig. 6). There is no evidence of TATA-box or CArG box at the proximal region. CCAT sequence that obeys the initiation motif for TATA-less promoter was found within 50 bp from the initiation codon. A cluster of GC-rich motifs (GC box), which is often found in TATA-less promoter, exists within 100 bp at the proximal region, adjacent to a pair of GATA motifs.
Fig. 3.
Luciferase reporter gene assay for CPI-17 gene 5′-flanking region. pGL4.20 luciferase reporter gene vectors used in the assay are illustrated at left. pGL4.20 vector and CMV-secreted embryonic alkaline phosphatase (SEAP) were cotransfected for 72 h under the growth condition and subjected to luciferase and SEAP assay. Promoter activity was defined as luciferase activity/SEAP activity and normalized against mouse 510 bp (A and B) and human 1 kb (C). WT, wild type. *P < 0.05 against mouse 510 bp or human 1 kb (n = 6–12).
Fig. 6.
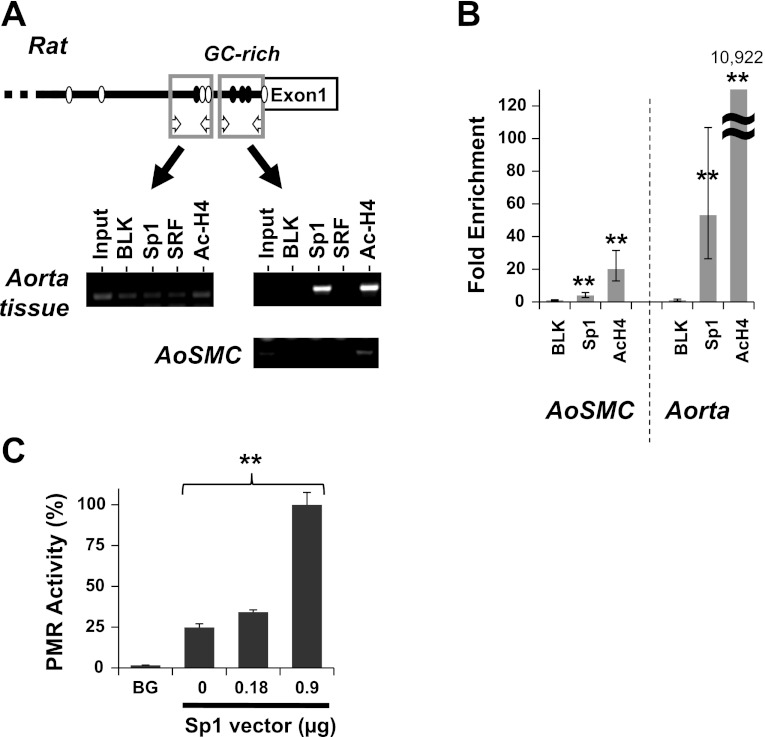
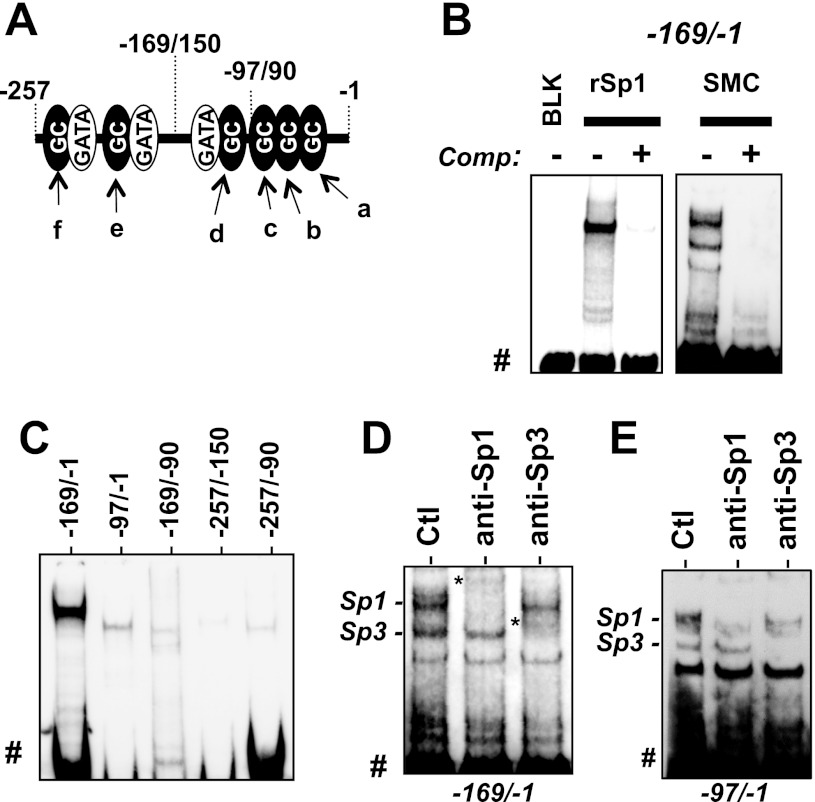
Binding of Sp1 to CPI-17 promoter. Chromatin immunoprecipitation (ChIP) assay was performed using rat aorta tissues and AoSMC with antibodies listed, followed by Conventional PCR (A) using two sets of primers for distal (left) and proximal (right) region of the CPI-17 promoter, and qPCR analysis for the proximal region (B). Preimmune IgG was used as blank and the ChIP signal was normalized against blank and shown as fold enrichment. **P < 0.05, compared with blank (n = 6). C: promoter assay (PMR) was performed using S2 cells transfected with the reporter vector for the mouse CPI-17 173-bp segment in the presence of the Sp1 vector at the indicated amount. Background (BG) was determined using the empty vector. **P < 0.05, by one-way ANOVA analysis (n = 3).
Figure 3 shows luciferase-reporter gene activities driven by the 5′-flanking DNA segments of mouse (A and B) and human (C) CPI-17 genes. The sequential 5′-deletion from 3 kb to 510 bp did not alter the activity of mouse CPI-17 promoter (Fig. 3A). The further deletion from 510 to 235 bp, which eliminated a pair of GATA (open circle) and GC-box (closed circle), partially reduced the activity to 50%. The deletion of next pair of GATA/GC box, yielding the 173 bp, restored the promoter activity, suggesting a repressor element between −235/−173 bp. The activity was significantly lower in the 97-bp fragment, and the deletion of another GC box (97 bp) eliminated the promoter activity. Consistently, the deletion of the 126 bp at the proximal region (Δ126), including the GC-box cluster, but not 21 bp (Δ21), diminished the promoter activity (Fig. 3A). Figure 3B shows effects of adenine substitutions at each GC box and GATA in the mouse 510-bp promoter activity. The mutation at one of three proximal GC boxes (GC-a, -b, and -c) or the proximal GATA adjacent to GC-d reduced the activity to almost basal levels, compared with the mutations at others, such as GC-d, -e, and -f, suggesting the dominant role of the proximal GC boxes and a GATA motif in the CPI-17 promoter activity. Consistent with the mouse gene, a pair of the proximal GC boxes in human promoter region (Fig. 3C) was necessary for the maximum promoter activity, suggesting that the binding of transcription factors to the conserved GC-box cluster drives the CPI-17 transcription. In the human gene, the promoter activity was significantly enhanced when the 1-kb fragment was deleted from 5′-end, to 550, and 118 bp, suggesting repressor elements at −1 kb/−550 bp and −364/−118 bp. There is no apparent GATA motif in the proximal human promoter, unlike the mouse gene (Fig. 3, B and C).
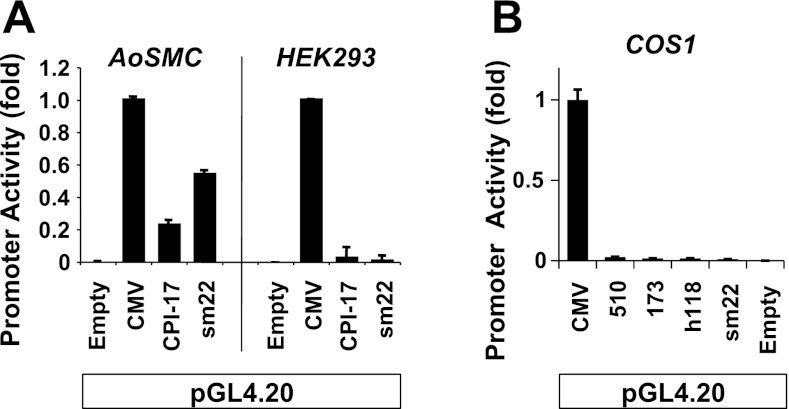
As shown in Fig. 4, the mouse 510-bp promoter was active in SM cells but not in non-SM cells, such as HEK293 and COS1 cells. It was similar to the sm22a promoter, which was used as a conventional SM gene maker promoter. Although the GC-rich motif ubiquitously functions in many gene expressions, neither the proximal GC-box cluster in mouse 173-bp nor human 118-bp CPI-17 gene was active in COS1 cells. These results suggest that the CPI-17 promoter is selectively activated in SM cells through the proximal GC-box cluster.
Fig. 4.
Specific activity of the CPI-17 promoter in AoSMC. AoSMC, HEK293, and COS1 cells were transfected with a pair of vectors of pGL4.20 and CMV-SEAP. Mouse 510-bp construct (A and B) and 173-bp (B) and human 118-bp reporter constructs (B) were used for the assay.
Sp1 transcription factor binds to the proximal GC boxes.
The binding of specific transcription factors to the proximal GC boxes was examined by nonradioactive EMSA using biotinylated DNA probes illustrated in Fig. 5A, and the supershift assay with specific antibodies (Fig. 5). Addition of recombinant Sp1 protein or the nuclear extracts from AoSMC resulted in upward mobility shifts of the DNA fragment of the −169/−1 region (Fig. 5B), which was eliminated with unlabeled DNA competitor, indicating specific binding to the DNA probe. Neither shorter fragment, −97/−1 nor −169/−90, showed prominent binding to Sp1 (Fig. 5C). Recombinant Sp1 also failed to recognize the GC boxes between −257/−150 bp (Fig. 5C). Thus the intact DNA structure at −169/−1 region (Fig. 5A) is required for the strong binding of Sp1 to the proximal GC-box cluster. Addition of anti-Sp1 and anti-Sp3 antibodies to the AoSMC nuclear extracts mixed with the −169/−1 (Fig. 5D) or −97/−1 (Fig. 5E) fragment caused supershift of the dominant band at the top and the band below, respectively (Fig. 5, D and E), indicating the specific binding of Sp1 and Sp3 to the DNA probes containing the proximal GC boxes. Unlike 32P-labeled EMSA, the signals of the complex with antibodies (labeled with an asterisk in Fig. 5D but undetectable in Fig. 5E) were faint due to poor transfer to the membrane.
Fig. 5.
EMSA for Sp1/Sp3 binding to the CPI-17 promoter. A: scheme of mouse 169-bp DNA probe. B: EMSA for the biotinylated 169-bp probe (100 fmol) with recombinant Sp1 (rSp1, 100 ng) or the nuclear extracts from AoSMC (2 μg of proteins). Unlabeled DNA probe (2.5 pmol) was used as competitor. C: recombinant Sp1 binding to various DNA fragments, indicated in A. D: EMSA Supershift was performed using AoSMC nuclear extracts (2.4 μg) and the biotynylated 169-bp or 97-bp probe (100 fmol) in the presence of antibodies for Sp1 and Sp3 (1 μg). DNA-protein complexes are indicated at left. BLK, blank. Free probe (#) and supershift complex (*) with antibody are indicated.
The proximal GC boxes are present in the rat CPI-17 gene promoter (Fig. 6A). Sp1 binding to the CPI-17 promoter in the mature SM cells in rat aorta tissues and the AoSMC culture was determined by ChIP assay (Fig. 6, A and B). Two pairs of PCR primers that detect the DNA segments including the proximal and the distal region were used to detect the specific binding of Sp1 and SRF and acetylated histone H4 (Ac-H4; Fig. 6A). When the chromatin mixtures were prepared from aorta tissue homogenates, the DNA segment including the proximal region was effectively enriched in the immunocomplexes with anti-Sp1 and anti-Ac-H4 antibodies (Fig. 6A). In the AoSMC expressing less CPI-17, the binding of Sp1 and Ac-H4 to the proximal region was marginal. The quantitative analysis of the ChIP assay using real-time PCR showed 4.0-fold and 53-fold enrichment of the DNA segment with anti-Sp1 beads in the chromatin from AoSMC and aorta tissues, respectively (Fig. 6B). Ac-H4 binding to the CPI-17 promoter was also lower in AoSMC, compared with aorta tissue. These results suggest that Sp1 binding to the proximal GC-box cluster causes remodeling of the chromatin and activates CPI-17 transcription. We further determined the role of Sp1 in CPI-17 transcription using drosophila S2 cells, which lack endogenous Sp1 and Sp3 (Fig. 6C) (8). The mouse CPI-17 173-bp fragment was activated in S2 cells, as the extent of ectopic Sp1 cDNA increased (Fig. 6C). These results strongly suggest the positive regulation of the CPI-17 promoter through the proximal binding of Sp1. Sp1 is likely activated in the mature SM cells via posttranslational modifications and/or binding of cofactors, because Sp1 expression was unchanged between aorta tissues and AoSMC (data not shown).
Sp1-dependent elements of the CPI-17 promoter are controlled through MAPK pathways but not by PKC or ROCK.
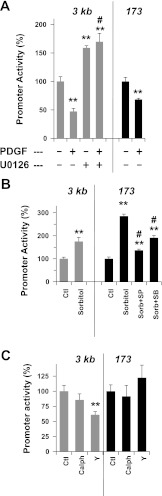
Figure 7 shows the promoter activity of the mouse 3-kb or 173-bp reporter gene constructs in AoSMC. Both the 3-kb and 173-bp segments were negatively and positively regulated in response to PDGF (A) and sorbitol (B), respectively. Inhibition of ERK1/2 activity with U0126 eliminated PDGF-induced repression of the 3-kb segment (Fig. 7A). Interestingly, U0126 treatment elevated the 3-kb promoter activity but not mRNA level (Fig. 1C). These results suggest that additional regulatory elements beyond the 3-kb region or potential differences between rat and mouse genes. Sorbitol-induced activation of the 173-bp CPI-17 promoter was eliminated by the inhibition of p38 or JNK (Fig. 7B). Thus the proliferative stimuli and stress signals are converged onto the proximal Sp1-binding region of the CPI-17 promoter, suggesting the involvement of Sp1 in the pathways. Y27632 significantly reduced the promoter activity of the 3-kb segment, but not the 173-bp of the proximal Sp1-binding region, suggesting that a ROCK-dependent enhancer element exists between −3 kb/−173 bp. By contrast, both 3-kb and 173-bp promoter activities were insensitive to calphostin-C (Fig. 7C), indicating that PKC and ROCK regulate the CPI-17 transcription through different response elements. Potentially, the PKC response elements present in the further distal region and/or the intron of the CPI-17 gene.
Fig. 7.
Reporter gene assay using mouse CPI-17 3-kb and 173-bp constructs. After a 48-h transfection, AoSMC cells were further incubated for 24 h in MCDB-5% FBS and stimulated with agonists and inhibitors indicated in the panels. **P < 0.05 and #P < 0.05, compared with untreated cells and the cells without inhibitor, respectively (n = 3–9).
CPI-17 transcription was independent of KLF4/myocardin/SRF-mediated SM gene regulation.
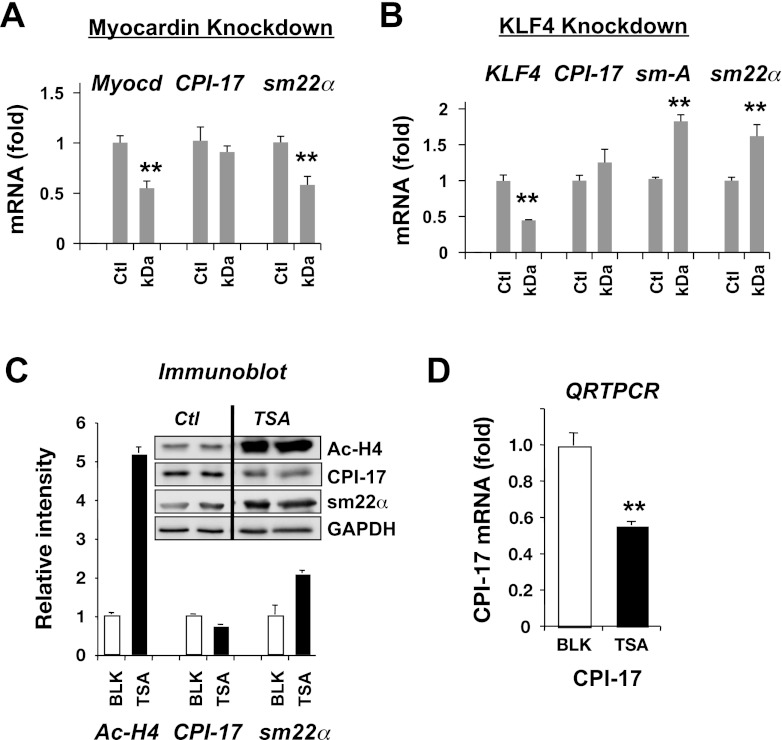
We tested whether the Sp1/Sp3-dependent CPI-17 transcription is regulated through myocardin pathway and histone acetylation using siRNA knockdown and trichostatin-A (TSA), an inhibitor for histone deacetylases (HDACs). Figure 8A shows qRT-PCR analysis in AoSMC treated with siRNA for myocardin. The siRNA knockdown reduced the extent of the endogenous myocardin mRNA to 50%. This reduction in myocardin resulted in the concomitant reduction in sm22α mRNA but no effect on CPI-17 mRNA (Fig. 8A). PDGF stimulus is known to induce the expression of KLF4, the repressor of myocardin gene. Importantly, KLF4 is a member of Sp1 superfamily, recognizing GC box. The silencing of KLF4 caused increases in the transcription of sm-actin (sm-A) and sm22α, suggesting the dis-repression of myocardin. However, KLF4 knockdown did not increase CPI-17 mRNA, indicating that neither myocardin nor KLF4 plays a role in the CPI-17 expression (Fig. 8B). Myocardin also contributes to the regulation of histone acetylation at SM-gene promoters, and this myocardin-promoted histone acetylation can be mimicked by the inhibition of HDACs with TSA (46). The quantitative ChIP assay showed the prominent Ac-H4 binding in aorta tissues (Fig. 6B). Indeed, histone H4 acetylation was significantly higher in aorta tissues (1.0 ± 0.05; n = 3), compared with AoSMC (0.29 ± 0.02; n = 3; P < 0.05 vs. aorta tissue), in parallel with the CPI-17 expression (Fig. 1A). The expression of sm22α was elevated in AoSMC treated with TSA in parallel to the increase in histone H4 acetylation, whereas CPI-17 expression was unchanged in response to histone acetylation (Fig. 8C). The inhibition of CPI-17 gene activity induced by TSA treatment was more evident in the results of qRT-PCR (Fig. 8D). These results suggest that histone H4 acetylation is not the factor determining the CPI-17 promoter activity, because the forced acetylation using the HDAC inhibitor (TSA) did not increase CPI-17 expression.
Fig. 8.
Kruppel-like factor 4 (KLF4)/myocardin-independent expression of CPI-17. qRT-PCR was performed using AoSMC treated with small interfering RNA for myocardin (A) and KLF4 (B; n = 4). Ct value of β-tubulin was used as reference. Ctl indicates cells treated with control small interfering RNA. Myocd and sm-A indicate primer sets for myocardin and sm-α-actin, respectively. Quiescent AoSMC was treated overnight with 0.2 μM trichostatin-A (TSA) and subjected to immunoblotting (C) and qRT-PCR (D; n = 6). **P < 0.05 compared with control.
DISCUSSION
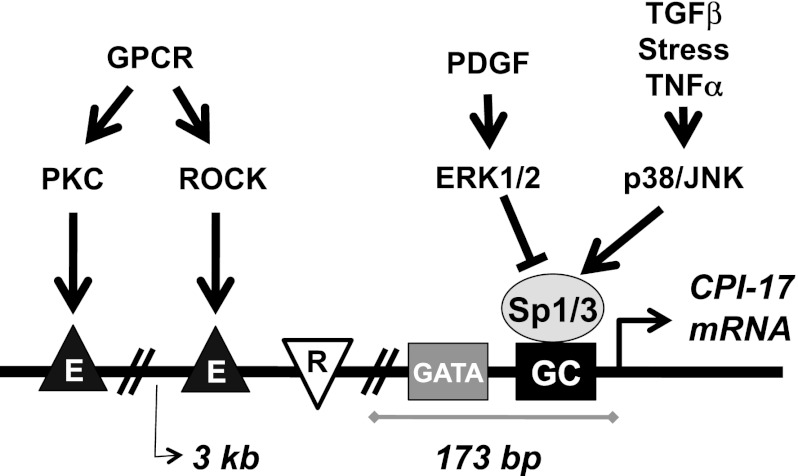
By comparison of rat aorta tissue and the cell culture using a pharmacological approach, we determined the mechanisms controlling CPI-17 transcription in response to stimuli. Agreeing with the previous reports in physiological and pathological studies using arterial tissues (9, 26, 27, 51), the CPI-17 gene promoter in the AoSMC system was positively and negatively regulated in response to proliferative, inflammatory, and excitatory stimuli that alter SM responsiveness. Figure 9 summarizes the proposed model for the reciprocal signaling pathways in CPI-17 transcription. The gene promoter activity is determined through Sp1/Sp3 binding to the GC boxes that cluster at the proximal site.
Fig. 9.
A proposed model for the regulation of CPI-17 transcription. Stress-induced p38/JNK activation enhances Sp1/Sp3 binding to the proximal GC boxes that triggers CPI-17 transcription, whereas PDGF-induced ERK1/2 activation represses the signal. There is a repressor element (R) in mouse and human CPI-17 promoters. PKC and ROCK, downstream kinases in G-protein signaling pathways, positively regulate the CPI-17 promoter through distal enhancer elements (E).
Proliferative stimulus with PDGF reduces CPI-17 transcription, in parallel with repression of sm22α, through the ERK1/2 pathway (Fig. 9). The transcription of CPI-17 in the AoSMC culture was partially reduced by inhibition of PKC and ROCK. Upon osmotic stress with sorbitol, the transcription was enhanced through JNK/p38 pathways. This stress-induced upregulation of CPI-17 transcription likely mimics the signaling pathway induced by TGFβ and inflammatory cytokine stimuli. Therefore, PKC, ROCK JNK, and p38 positively regulate the CPI-17 gene promoter. MAPK signaling pathways, ERK1/2, p38, and JNK are converged onto the proximal Sp1/Sp3 binding region. Sp1/Sp3-induced CPI-17 gene transcription is augmented by the adjacent GATA elements. In the mouse CPI-17 promoter, a repressor exists between −273/−173-bp region. The −510/−235-bp region and GATA-b/c elements likely interfere with the repressor, because the deletion or mutation at these sites causes a reduction in the promoter activity. Furthermore, there are distal enhancer elements responding to PKC and ROCK. Interestingly, these responses to the specific kinases are similar to myocardin-regulated SM genes. For example, ERK1/2 negatively regulates myocardin/SRF signal through Elk-1, KLF4, and Ac-histone pathways (33, 35, 60), whereas ROCK stimulates myocardin and myocardin-related transcription factors, inducing the contractile phenotype (57). Thus CPI-17 expression is synchronized to the SM-specific genes, although the transcription factors regulating the gene promoter are different.
This Sp1/Sp3-induced CPI-17 gene regulation is evolutionally conserved among mouse, rat, and human with some differences in the gene architecture, such as location and number of the regulatory elements. It has been documented that Sp1 is involved in other vascular smooth muscle (VSM) gene regulation, independent from myocardin pathway. ACLP and CRP2 are positively regulated through Sp1/Sp3 binding (30, 64), although the physiological roles of these gene products in the regulation of VSM tone have yet to be established. The gene promoter of cGMP-dependent kinase I (cGK-I) is also regulated through the proximal GC boxes. Sellak et al. (49) originally reported that the TATA-less promoter of cGK-I gene is activated by the proximal binding of Sp1, whereas KLF4 was later reported to bind to the GC boxes of the cGK-I gene promoter and activate the transcription (67). Moreover, Sp1 was reported to negatively regulate myocardin-driven sm22α gene promoter. For example, at atheroplaques, PDGF stimulation causes an increase in Sp1 expression, leading the repression of sm22α gene promoter (59), and activates KLF4 expression, causing repression of the myocardin gene (11). CPI-17 expression is low within the neointima (27) as well as in AoSMC stimulated with PDGF without the downregulation of the Sp1 expression. Apparently, PDGF-induced ERK1/2 signal is capable of ceasing the action of Sp1 to the CPI-17 promoter.
A question arose: how are Sp1/Sp3 activities in SM cells regulated by ERK1/2, p38 and JNK independently of the myocardin pathway? Importantly, Sp1 and Sp3 are ubiquitously expressed in eukaryotic cells (52), whereas CPI-17 expression is restricted in SM and some others. One possible mechanism is the regulation of Sp1 through the phosphorylation. For example, Sp1 phosphorylation at Thr453, an activation site, declined in AoSMC to 25%, compared with aorta tissue (data not shown). A growing body of evidence indicates that at least 24 Ser/Thr sites of Sp1 phosphorylation sites positively and negatively regulate Sp1 transcription activity, protein stability, and DNA binding (52). Posttranscriptional modification of Sp1 at Thr453 and other sites potentially facilitates CPI-17 expression in SM cells. Another possibility involves SM-specific microRNAs, such as miR143/145 and 223, which regulate SM genes (2, 7, 10, 14). Of particular interest, miR143/145 gene ablation causes the repression of CPI-17 and ROCK1 in SM cells (2). Although CPI-17 mRNA does not include miR143/145 recognition sites, it is possible that SM-specific microRNAs play roles in Sp1/Sp3-induced CPI-17 expression in SM cells. In addition, this study does not rule out the possibility that Sp1 activity is driven by an unidentified SM-specific cofactor, like ubiquitous SRF controlled by SM-specific myocardin. GATA4 and GATA6 are highly expressed in SM cells (56, 65), and may play a role in SM-specific expression of CPI-17 through the proximal GATA motifs in the CPI-17 promoter. The SM-specific activation of CPI-17 promoter deserves further investigation.
Upon G-protein activation, PKC and ROCK are sequentially activated and phosphorylate CPI-17 protein at Thr38. This phosphorylation of CPI-17 and the consequential inhibition of MLCP are necessary for the robust and sustained MLC20 phosphorylation (12). Our finding here suggests that this agonist-induced activation of PKC and ROCK augments CPI-17 signal through increases in the gene expression and the phosphorylation at Thr38. During the embryonic development, CPI-17 expression in arterial SM elevates between E10 and E17, in parallel with an increase in cardiac output (27). Likewise, CPI-17 expression is repressed in embryonic SMs, such as bronchi and intestines, but is enhanced in active SM tissues. Thus it is possible that exposure of immature SM cells to excitatory stimuli triggers CPI-17 expression and establishes the Ca2+ sensitization signaling pathway during SM development. This agonist-induced CPI-17 expression reminds us excitation-transcription coupling of myocardin-dependent gene activation in response to depolarization of VSM cells through Ca2+, cAMP response element binding protein, and ROCK pathways (3, 57, 58). Recently, Pagiatakis et al. (44) reported that this ROCK-induced myocardin activation is mediated by CPI-17, although the mechanisms remains unknown. We presume that Sp1/Sp3-induced CPI-17 expression is a part of process in SM cell maturation, adjusting the contractile response and the gene expression to stimuli.
The reciprocal regulation through multiple kinase pathways determines the response of the CPI-17 promoter to specific stimuli. For example, TGFβ and TNFα are known to activate ERK1/2, JNK, and p38 in VSM cells, simultaneously (41, 48). In rat AoSMC we used, both agonists increased the CPI-17 expression, although the extent of the elevation was unique to the stimuli. We presume that the balance of reciprocal activities of the kinases regulating the CPI-17 promoter (Fig. 1C) determines the specific response to each stimulus. The dual regulation was also evident in ANG II stimulus. ANG II is known to activate PKC/ROCK through G-protein activation and JNK/p38 through oxidation pathways in parallel with the cross-activation of PDGF-receptor signaling pathway (36). Inhibition of negative signal through ERK1/2 enhanced CPI-17 transcription driven by PKC/ROCK/p38/JNK. Thus, upon ANG II stimulus, the positive signal is offset by the negative one. A particular side of response may be emphasized when the balance is broken in response to pathological stresses.
An increase in CPI-17 expression has been reported in hypoxic pulmonary artery (9), diabetic bladder (4), urinary obstruction (1), airway inflammation (38, 47), ischemic damage in bladder (23), and subarachnoid hemorrhage (26), which have been linked to hyper-responsiveness of SM contraction, such as asthma attack and vasospasm, in addition to remodeling. Neither the hypoxia-inducible factor-1-response element nor the NF-κB binding element is evident in the mouse CPI-17 promoter region. Also, NF-κB inhibition by Rel-A knockdown did not alter the activity. We presume that these increases in CPI-17 expression occur through the activation of JNK/p38 and/or PKC/ROCK pathways. For example, p38 is activated under hypoxia, causing the cyclin D1 activity in PC12 cells (6). In addition, CPI-17 upregulation occurs in pregnant myometrium (43) and testosterone-treated renal cortex (51), suggesting an involvement of steroid hormone signaling that activates multiple MAPK pathways. By contrast, in intestinal SM, IL-1β stimulus induces the downregulation of CPI-17 expression, which is linked to a loss of SM tone in inflammatory bowel disease (40). Also, CPI-17 is downregulated in rabbit bladder in response to urinary obstruction (5) and aged bladder detrusor SM (17). Consistently, IL-1β stimulation suppresses CPI-17 expression in colon SM cell culture (20). The CPI-17 downregulation also occurs in IL-10-knockout mouse and human patients who suffer from chronic colitis (39). In our study, IL-1β stimulation rather increased CPI-17 expression in AoSMC. We presume that negative signal through ERK1/2 dominates in intestinal SM stimulated with IL-1β, causing CPI-17 downregulation and reduced SM tone. Recently, Ihara et al. (21) reported that CPI-17 expression is unchanged in another colitis model mouse, induced by a 7-day treatment with dextran sodium sulfate, which causes hypercontractility of the circular SM tissue. Likely, the reciprocal regulation of the CPI-17 promoter confers the plasticity in the expression depending on the disease stages. Understanding of the regulatory circuits in CPI-17 transcription through multiple kinase pathways is a key to elucidate an adaptation system of SM tone to pathological challenges.
GRANTS
This work was supported by National Institutes of Health Grants HL-083261 and DK-088905, Brandywine Valley Hemophilia Foundation, and Pennsylvania C.U.R.E. (to M. Eto) and Korea Research Foundation (KRF-2007–357-E00004) funded by Korean Government (MOEHRD; to J. I. Kim). Research in this publication includes work carried out by the Kimmel Cancer Center Genomics Facility, supported in part by National Cancer Institute Grant P30-CA-56036.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.I.K. and M.E. conception and design of research; J.I.K., M.U., G.D.Y., and M.E. performed experiments; J.I.K., M.U., G.D.Y., and M.E. analyzed data; J.I.K. and M.E. interpreted results of experiments; J.I.K., M.U., G.D.Y., and M.E. prepared figures; J.I.K. and M.E. drafted manuscript; J.I.K. and M.E. edited and revised manuscript; J.I.K., M.U., and M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate Drs. Satoru Eguchi and James Jaynes for the material support and valuable discussion of AoSMC culture and S2 cell system, respectively.
REFERENCES
- 1.Boberg L, Poljakovic M, Rahman A, Eccles R, Arner A. Role of Rho-kinase and protein kinase C during contraction of hypertrophic detrusor in mice with partial urinary bladder outlet obstruction. BJU Int 109: 132–140, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 119: 2634–2647, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse: Roles of ryanodine receptors and voltage- dependent Ca2+ channels. Circ Res 86: 760–767, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase-β and CPI-17. Am J Physiol Renal Physiol 290: F650–F656, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad PW, Rust RT, Han J, Millhorn DE, Beitner-Johnson D. Selective activation of p38α and p38γ by hypoxia role in regulation of cyclin D1 by hypoxia in PC12 cells. J Biol Chem 274: 23570–23576, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee T, Miano JM, Ivey KN, Srivastava D. MiR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals mutiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55: 887–898, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol 40: 398–407, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284: 3728–3738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deaton RA, Gan Q, Owens GK. Spl-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 296: H1027–H1037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res 100: 121–129, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi S, Hirata Y, Imai T, Kanno K, Marumo F. Phenotypic change of endothelin receptor subtype in cultured rat vascular smooth muscle cells. Endocrinology 134: 222–228, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MVG, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ 16: 1590–1598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem 284: 35273–35277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgijevic S, Subramanian Y, Rollins EL, Starovic-Subota O, Tang AC, Childs SJ. Spatiotemporal expression of smooth muscle markers in developing zebrafish gut. Dev Dyn 236: 1623–1632, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Pinilla PJ, Gomez MF, Hedlund P, Swärd K, Hellstrand P, Camello PJ, Pozo MJ, Andersson K. Effect of melatonin on age associated changes in guinea pig bladder function. J Urol 177: 1558–1561, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys 510: 147–159, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem 279: 37211–37214, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1β. Am J Physiol Cell Physiol 293: C1991–C2000, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihara E, Chappellaz M, Turner SR, Macdonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil 24: e15–e26, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 442: 576–579, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Juan Y, Li S, Levin RM, Kogan BA, Schuler C, Leggett RE, Huang C, Mannikarottu A. The effect of ischemia/reperfusion on rabbit bladder-role of Rho-kinase and smooth muscle regulatory proteins. Urology 73: 1126–1130, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Khromov A, Choudhury N, Stevenson AS, Somlyo AV, Eto M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem 284: 21569–21579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikkawa Y, Matsuo S, Kameda K, Hirano M, Nakamizo A, Sasaki T, Hirano K. Mechanisms underlying potentiation of endothelin-1-induced myofilament Ca2+ sensitization after subarachnoid hemorrhage. J Cereb Blood Flow Metab 32: 341–352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JI, Young GD, Jin L, Somlyo AV, Eto M. Expression of CPI-17 in smooth muscle during embryonic development and in neointimal lesion formation. Histochem Cell Biol 132: 191–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci USA 88: 9307–910, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol 587: 3587–603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layne MD, Yet S, Maemura K, Hsieh C, Liu X, Ith B, Lee M, Perrella MA. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res 90: 728–736, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem 272: 5063–5068, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell 18: 510–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280: 9719–9727, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 35.McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res 100: 1428–1441, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35: 577–593, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Morin C, Fortin S, Cantin AM, Rousseau E. Docosahexaenoic acid derivative prevents inflammation and hyperreactivity in lung: implication of PKC-Potentiated inhibitory protein for heterotrimeric myosin light chain phosphatase of 17 kD in asthma. Am J Respir Cell Mol Biol 45: 366–375, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Ohama T, Hori M, Fujisawa M, Kiyosue M, Hashimoto M, Ikenoue Y, Jinno Y, Miwa H, Matsumoto T, Murata T, Ozaki H. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J Gastroenterol 43: 858–865, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 278: 48794–48804, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Ono H, Ichiki T, Fukuyama K, Iino N, Masuda S, Egashira K, Takeshita A. cAMP-response element-binding protein mediates tumor necrosis factor-α-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol 24: 1634–1639, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Ozaki H, Yasuda K, Kim Y, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol 140: 1303–1312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagiatakis C, Gordon JW, Ehyai S, McDermott JC. A novel RhoA/ROCK- CPI-17 -MEF2C signaling pathway regulates vascular smooth muscle cell gene expression. J Biol Chem 287: 8361–8370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 100: 633–644, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Qiu P, Li L. Histone acetylation and recruitment of serum responsive factor and CREB-binding protein onto SM22 promoter during SM22 gene expression. Circ Res 90: 858–865, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Sakai H, Chiba Y, Hirano T, Misawa M. Possible involvement of CPI-17 in augmented bronchial smooth muscle contraction in antigen-induced airway hyper-responsive rats. Mol Pharmacol 68: 145–151, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 315: 1005–1012, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Sellak H, Yang X, Cao X, Cornwell T, Soff GA, Lincoln T. Sp1 transcription factor as a molecular target for nitric oxide- and cyclic nucleotide-mediated suppression of cGMP-dependent protein kinase-Iα expression in vascular smooth muscle cells. Circ Res 90: 405–412, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Senba S, Eto M, Yazawa M. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. J Biochem 125: 354–362, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Song J, Eyster KM, Kost CK, Kjellsen B, Martin DS. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res 85: 614–621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan NY, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol 29: 2483–2488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurneysen C, Opitz I, Kurtz S, Weder W, Stahel RA, Felley-Bosco E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer 64: 140–147, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Trinkle-Mulcahy L, Ichikawa K, Hartshorne DJ, Siegman MJ, Butler T. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in a-toxin-permeabilized smooth muscle. J Biol Chem 270: 18191–18194, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett 370: 215–221, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, Sasayama S. Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription. J Cell Biol 156: 983–991, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res 95: 406–414, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res 98: 868–878, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Wang D, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci 119: 1769–1780, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Somlyo AV, Somlyo AP. Cyclic-GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Commun 220: 658–663, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Yet S, Folta SC, Jain MK, Hsieh C, Maemura K, Layne MD, Zhang D, Marria PB, Yoshizumi M, Chin MT, Perrella MA, Lee M. Molecular cloning, characterization, and promoter analysis of the mouse Crp2/SmLim gene: Preferential expression of its promoter in the vascular smooth muscle cells of transgenic mice. J Biol Chem 273: 10530–10537, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Yin F, Herring BP. GATA-6 can act as a positive or negative regulator of smooth muscle-specific gene expression. J Biol Chem 280: 4745–4752, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng Y, Zhuang S, Gloddek J, Tseng C, Boss GR, Pilz RB. Regulation of cGMP-dependent protein kinase expression by Rho and Krüppel-like transcription factor-4. J Biol Chem 281: 16951–16961, 2006 [DOI] [PubMed] [Google Scholar]