Abstract
Polyamines regulate multiple signaling pathways and are implicated in many aspects of cellular functions, but the exact molecular processes governed by polyamines remain largely unknown. In response to environmental stress, repression of translation is associated with the assembly of stress granules (SGs) that contain a fraction of arrested mRNAs and are thought to function as mRNA storage. Here we show that polyamines modulate the assembly of SGs in normal intestinal epithelial cells (IECs) and that induced SGs following polyamine depletion are implicated in the protection of IECs against apoptosis. Increasing the levels of cellular polyamines by ectopic overexpression of the ornithine decarboxylase gene decreased cytoplasmic levels of SG-signature constituent proteins eukaryotic initiation factor 3b and T-cell intracellular antigen-1 (TIA-1)-related protein and repressed the assembly of SGs induced by exposure to arsenite-induced oxidative stress. In contrast, depletion of cellular polyamines by inhibiting ornithine decarboxylase with α-difluoromethylornithine increased cytoplasmic eukaryotic initiation factor 3b and TIA-1 related protein abundance and enhanced arsenite-induced SG assembly. Polyamine-deficient cells also exhibited an increase in resistance to tumor necrosis factor-α/cycloheximide-induced apoptosis, which was prevented by inhibiting SG formation with silencing SG resident proteins Sort1 and TIA-1. These results indicate that the elevation of cellular polyamines represses the assembly of SGs in normal IECs and that increased SGs in polyamine-deficient cells are crucial for increased resistance to apoptosis.
Keywords: ornithine decarboxylase, intestinal epithelial homeostasis, ribonucleic acid-binding proteins, processing bodies, mitochondrial ribonucleic acid degradation, posttranscriptional regulation
in response to environmental stress, eukaryotic cells reprogram their translation patterns to conserve anabolic energy for the repair of stress-induced damage (2). The expression of proteins responsible for damage repair is increased, whereas translation of mRNAs encoding for “housekeeping” functions is repressed by redirection of these mRNAs from polysomes to discrete cytoplasmic foci known as stress granules (SGs) and processing bodies (PBs) for storage and degradation (2, 4). SGs and PBs are visible as phase-dense particles that are functional by-products of mRNA metabolism, sharing substrate mRNA, dynamic properties, and many proteins but also housing separate components and performing independent function. Several proteins, including eukaryotic initiation factor (eIF)3, eIF4, poly(A)-binding protein, T-cell intracellular antigen-1 (TIA-1), and TIA-1-related protein (TIAR), are involved in SG assembly, and this process also requires eIF2α phosphorylation through protein kinase R and other kinases (3, 26, 41). The eIF2α phosphorylation decreases the assembly of the active ternary preinitiation complex eIF2/GTP/tRNAmet and inhibits translation initiation and polysome assembly (1, 2, 4, 6, 25), and TIA-1 and TIAR bind to these inactive translation initiation complexes and promote the assembly of SGs (11, 12). PBs are observed side by side with SGs after exposure to stress, but PBs and SGs differ in size, shape, and functions and in the mechanisms of their assembly (1, 4, 35). PB contains components of the 5′-3′ mRNA degradation pathway and microRNA-dependent silencing protein GW182 (34), and its formation does not require eIF2α phosphorylation (5, 16). The positions of SGs are relatively fixed, but PBs are highly motile (2, 16, 35). SGs store nontranslated mRNAs and deliver them to associated PBs for degradation.
Several studies reveal that the rapid formation of SGs in the cytoplasm is an important protective action to prevent damage of vital cellular processes required for homeostasis (2, 4, 7), but the exact mechanism by which SG assembly is regulated remains to be fully investigated. The natural polyamines (spermidine, spermine, and their precursor putrescine) are organic cations found in all eukaryotic cells and are implicated in the control of multiple signaling pathways and distinct cellular functions (9, 13). The levels of cellular polyamines are tightly regulated and depend on the dynamic balance among polyamine biosynthesis, degradation, and transport (9, 36, 37). Cellular polyamine content rapidly increases in cells stimulated to grow and divide, whereas decreasing cellular polyamines stops cell cycle progression, resulting in the arrest in the G1 phase. An increasing body of evidence indicates that polyamines potently modulate gene expression posttranscriptionally (24, 39, 40, 44, 46) besides their roles in regulating gene transcription (10, 28). Increased levels of cellular polyamines by ectopic overexpression of ornithine decarboxylase (ODC), the first rate-limiting enzyme in polyamine biosynthesis, inhibit the expression of growth-inhibitory genes including p53 (21, 44), nucleophosmin (44, 45), JunD (46), activating transcription factor-2 (ATF2) (40), and TGF-β (27) by increasing the degradation of their mRNAs, thus contributing to the stimulation of intestinal epithelial cell (IEC) proliferation. In contrast, polyamine depletion by inhibiting ODC increases these protein levels through the stabilization of their gene transcripts, leading to growth arrest. Recently, one study suggests that the polyamine pathway is also involved in the assembly of SGs and PBs in certain type of cancer cells such as human osteosarcoma (U2OS) and RDG3 cells (18).
Our previous studies (27, 28, 36, 37) and others (13, 33, 42) have shown that polyamines are essential for maintaining normal intestinal epithelial homeostasis, an effect that predominantly relies on their ability to regulate IEC proliferation and apoptosis. Normal IEC proliferation in the intestinal mucosa is dependent on the supply of polyamines to the dividing cells in the crypts, and decreasing cellular polyamines inhibits cell renewal in vivo as well as in vitro (21, 37, 45). Polyamines also modulate IEC survival, since depletion of cellular polyamines decreases apoptosis, whereas increasing cellular polyamines promotes susceptibility of IECs to cell death (40, 43). In the current study, we sought to investigate the role of cellular polyamines in the regulation of SG formation in IECs. The data presented here indicate that in normal IECs (IEC-6 line), increased levels of cellular polyamines by ectopic ODC overexpression repressed SG assembly induced by exposure to arsenite-induced oxidative stress. In contrast, the depletion of cellular polyamines by inhibiting ODC enhanced arsenite-induced SG formation. Polyamine-deficient cells also exhibited an increase in resistance to tumor necrosis factor-α (TNF-α)/cycloheximide (CHX)-induced apoptosis, and this protective effect was prevented by inhibiting SG formation, suggesting that polyamines regulate apoptosis by altering the assembly of SGs in IECs.
MATERIALS AND METHODS
Chemicals and supplies.
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture medium and dialyzed fetal bovine serum were from Invitrogen (Carlsbad, CA), and biochemicals were from Sigma (St. Louis, MO). The antibodies recognizing eIF3b, TIAR, Sort1, TIA-1, and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA), and the secondary antibody conjugated to horseradish peroxidase was purchased from Sigma. α-Difluoromethylornithine (DFMO) was from Genzyme (Cambridge, MA).
Cell culture.
The IEC-6 cell line, derived from normal rat intestinal crypt cells (31), was purchased from the American Type Culture Collection at passage 13 and used at passages 15–20 (39, 46). Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum, 10 μg/ml insulin, and 50 μg/ml gentamicin. ODC-overexpressing IEC-6 (ODC-IEC) cells were developed as described in our previous studies (22, 44) and expressed a more stable ODC variant with full enzyme activity (14).
RNA interference.
Expression of Sort1 or TIA-1 was silenced by transfection of specific small interfering RNA (siRNA). The siRNAs that specifically targets the coding region of Sort1 (siSort1) or TIA-1 (siTIA-1) and the corresponding C-siRNA were synthesized and purchased from Dharmacon. For each 60-mm cell culture dish, 15 μl of the 20 μM stock duplex siSort1, siTIA-1, or C-siRNA were mixed with 300 μl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 15 μl of LipofectAMINE 2000 in 300 μl of Opti-MEM. The solution was incubated for 20 min at room temperature and gently overlaid onto monolayers of cells in 3 ml of medium, and the cells were harvested for various assays after a 48-h incubation.
Assay for ODC enzyme activity and polyamine analysis.
ODC activity was determined by radiometric technique in which the amount of 14CO2 liberated from l-[1-14C]ornithine was estimated (32). Sample collection and analysis were carried out as described previously (19, 21). Enzymatic activity was expressed as picomoles of CO2 per milligram of protein per hour.
The cellular polyamine content was analyzed by high-performance liquid chromatography analysis as previously described (19). Briefly, after 0.5 M perchloric acid was added, the cells were frozen at −80°C until ready for extraction, dansylation, and high-performance liquid chromatography analysis. The standard curve encompassed 0.31–10 μM. Values that fell >25% below the curve were considered undetectable. The results are expressed as nanomoles of polyamines per milligram of protein.
Preparation of cytoplsamic proteins and Western blot analysis.
Whole cell lysates were prepared using 2% SDS, sonicated, and centrifuged (12,000 rpm) at 4°C for 15 min. Cytoplasmic proteins were prepared using NE-PER Cytoplasmic and Nuclear Extraction Reagents from Pierce Biotechnology (Rockford, IL), and it was performed following recommendations by the manufacture. Briefly, 50 μl packed cells were suspended in cytoplasmic extract reagent I and incubated on ice for 10 min. After an addition of cytoplasmic extract reagent II and subsequent vortex and incubation, cell lysates were centrifuged. The supernatant containing cytoplasmic extract was transferred and stored at −80°C until use. The protein samples were boiled for 5 min and size fractionated by SDS-PAGE (7.5% acrylamide). After the proteins were transferred onto nitrocellulose filters, the blots were incubated with primary antibodies recognizing eIF3b, TIAR, Sort1, or TIA-1 proteins; following incubations with secondary antibodies, immunocomplexes were developed by using chemiluminescence.
Immunofluorescence staining.
Immunofluorescence was performed as described (17) with minor changes (39). Cells were fixed using 3.7% formaldehyde, and the rehydrated samples were incubated overnight at 4°C with primary antibody against eIF3b or TIAR diluted 1:500 in blocking buffer and then incubated with secondary antibody conjugated with Alexa Fluor-633 and Alexa Fluor-488 (Molecular Probes, Eugene, OR) for 2 h at room temperature. After being rinsed and mounted, they were viewed through a Zeiss confocal microscope (model LSM510). Images were processed using PhotoShop software (Adobe, San Jose, CA).
Determination of apoptosis.
After various experimental treatments, cells were photographed with a Nikon inverted microscope before fixation (40, 43). Annexin-V staining of apoptosis was carried out by using a commercial apoptosis kit (Clontech, Palo Alto, CA) and performed according to the protocol recommended by the manufacturer. Briefly, cells were rinsed with 1× binding buffer and resuspended in 200 μl of 1× binding buffer. Five microliters of annexin V were added on a slide and incubated at room temperature for 10 min in the dark. Annexin-stained cells were visualized and photographed under fluorescence microscope using a dual-filter set for FITC and rhodamine, and the percentage of apoptotic cells was determined.
Statistics.
Values are means ± SE from six samples. Immunofluorescence staining and immunoblotting results were repeated three times. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using Duncan's multiple range test (15).
RESULTS
Polyamines represses the formation of SGs in normal IECs in response to stress.
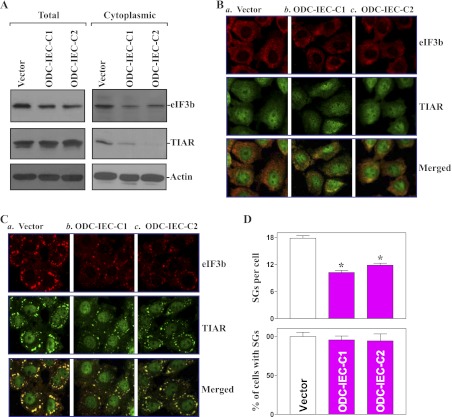
SGs assemble in heat-shocked plant and mammalian cells exposed to environmental stresses or after overexpression of some translational repressors; they continuously rearrange with time and with pieces of granules detaching and joining a neighboring granule and inhibit translation initiation (2, 4). To define the role of cellular polyamines in the assembly of SGs in the intestinal epithelium, we determined whether increasing cellular polyamines alter the formation of SGs by using two clonal populations of IECs stably expressing ODC (ODC-IEC) that were recently developed in our laboratory (22). As reported previously (39, 44), these ODC-IECs expressed high levels of ODC protein, exhibited >50-fold ODC enzyme activity, and had increased cellular polyamines putrescine (by ∼12-fold), spermidine (by ∼2-fold), and spermine (by ∼25%) compared with control cells transfected with the vector lacking ODC cDNA. Because eIF3b and TIAR are the signature constituents of SGs and are linked to SG formation (12, 26), we sought to examine changes in whole cell and cytoplasmic levels of these two proteins in stable ODC-IECs. Increasing the levels of cellular polyamines by ODC overexpression reduced both total and cytoplasmic eIF3b levels, and it only decreased cytoplasmic TIAR abundance (Fig. 1A). There were no significant changes in the levels of total TIAR protein in stable ODC-IECs. To determine the influence of increased cellular polyamines on the formation of SGs in response to stress, sodium arsenite (a potent inducer of oxidative stress) was used to elicit SG assembly in this study. There were no visible SG foci in both control and stable ODC-IECs before an application of sodium arsenite (Fig. 1B), but treatment with sodium arsenite at the concentration of 0.5 mM for 45 min induced the formation of SGs, as illustrated by the aggregation of the SG markers, eIF3b and TIAR (Fig. 1C). However, stable ODC-IECs exhibited a significant reduction in arsenite-induced SGs compared with control populations of IEC-6 cells (Fig. 1, C and D). The effect of increasing cellular polyamines by ODC overexpression on arsenite-induced assembly of SGs were not simply due to clonal variation, since two stable clones, ODC-IEC-C1 and ODC-IEC-C2, showed identical responses. These results indicate that increasing cellular polyamines not only decreases the cytoplasmic accumulation of eIF3b and TIAR proteins but also represses stress-induced SG assembly.
Fig. 1.
Increasing the cellular polyamines by ornithine decarboxylase (ODC) overexpression represses the assembly of stress granules (SGs). A: levels of total and cytoplasmic eukaryotic initiation factor 3b (eIF3b) and T-cell intracellular antigen-1 (TIA-1)-related protein (TIAR) proteins in stable ODC-intestinal epithelial cells (ODC-IECs). IEC-6 cells were infected with either the retroviral vector containing the sequence encoding mouse ODC cDNA or control retroviral vector. The clones resistant to the selection medium were isolated and screened for ODC expression. Whole cell lysates and cytoplasmic proteins were prepared for Western immunoblotting analysis. Twenty micrograms of total or cytoplasmic proteins were applied to each lane, and immunoblots were hybridized with the antibody specific for eIF3b or TIAR. After the blot was stripped, actin immunoblotting was performed as an internal control for equal loading. B: cellular distribution of eIF3b and TIAR as measured by immunofluorescence staining assays in stable ODC-IECs: cells infected with control vector (a) and clones (C) of cells infected with ODC expression vector (b and c). Cells were permeabilized and incubated with the anti-eIF3b or -TIAR antibody and then with anti-IgG conjugated with Alexa Fluor. eIF3b was shown as red, whereas TIAR was shown as green. Original magnification, ×250. C: changes in arsenite-induced assembly of SGs in cells described in B. After stable ODC-IECs were treated with 0.5 mM arsenite for 45 min, the formation of SGs was examined by staining eIF3b and TIAR. D: quantification of the percentage of cells containing stress granules (bottom) and the numbers of stress granules per cell (top) in cells described in C. Values are means ± SE of data from 6 samples. *P < 0.05 compared with the vector alone.
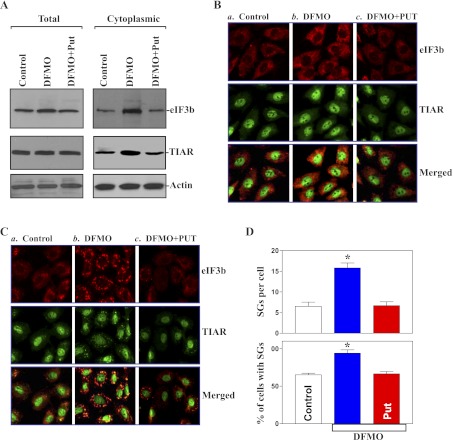
To examine changes in the assembly of SGs in the absence of cellular polyamines, DFMO (a specific inhibitor of ODC) was used to deplete cellular polyamines in this study. Exposure of IEC-6 cells to 5 mM DFMO for 6 days completely inhibited ODC enzyme activity and almost totally depleted cellular polyamines. The levels of putrescine and spermidine were undetectable on day 6 after treatment with DFMO, and spermine was decreased by ∼60%. Interestingly, polyamine depletion by DFMO increased total and cytoplasmic eIF3b levels and induced cytoplasmic TIAR abundance (Fig. 2A, right), although it failed to alter whole cell TIAR protein (Fig. 2A, left). Putrescine (10 μM) given together with DFMO prevented the increase in levels of total and cytoplasmic eIF3b and cytoplasmic TIAR; the addition of spermidine (5 μM) had an effect equal to putrescine on eIF3b content and TIAR subcellular reorganization when it was added to cultures containing DFMO (data not shown). We also examined changes in the levels of eIF3b and TIAR proteins in stable ODC-IECs after exposure to DFMO. Consistent with the findings observed in parental IEC-6 cells, both total and cytoplasmic eIF3b levels and cytoplasmic TIAR content significantly increased after treatment with DFMO, but there were no changes in whole cell TIAR protein in DFMO-treated ODC-IECs (data not shown). Decreasing cellular polyamines alone did not directly induce SG formation without treatment with arsenite (Fig. 2B) or serum starvation (data not shown). However, the depletion of cellular polyamines enhanced the assembly of SGs when polyamine-deficient cells were exposed to arsenite as indicated by increases in percentage of cells containing SGs and numbers of SGs per cell (Fig. 2, C and D). The combined treatment with putrescine and DFMO prevented the induction in arsenite-induced SG assembly in polyamine-deficient cells, rendering the imaging patterns of SGs and the percentage of cells containing SGs similar to those observed in control cells exposed to the same dose of arsenite. These results indicate that decreasing cellular polyamines increase the assembly of SGs in response to oxidative stress.
Fig. 2.
Polyamine depletion enhances the assembly of SGs. A: levels of total and cytoplasmic eIF3b and TIAR proteins in control IEC-6 cells and cells treated with α-difluoromethylornithine (DFMO; 5 mM) alone or DFMO plus putrescine (Put, 10 μM) for 6 days. B: cellular distribution of eIF3b and TIAR: control (a), DFMO (b), and DFMO plus Put treatment (c). eIF3b was shown as red, whereas TIAR was shown as green. Original magnification, ×250. C: changes in arsenite-induced assembly of stress granules in cells described in B. Cells were treated with 0.5 mM arsenite for 45 min to induce SG formation before fixation and staining for eIF3b and TIAR. D: quantification of the percentage of cells containing SGs and the numbers of SGs per cell in cells described in C. Values are means ± SE of data from 6 samples. *P < 0.05 compared with controls and cells treated with DFMO plus Put.
Induced SGs protect IECs against apoptosis.
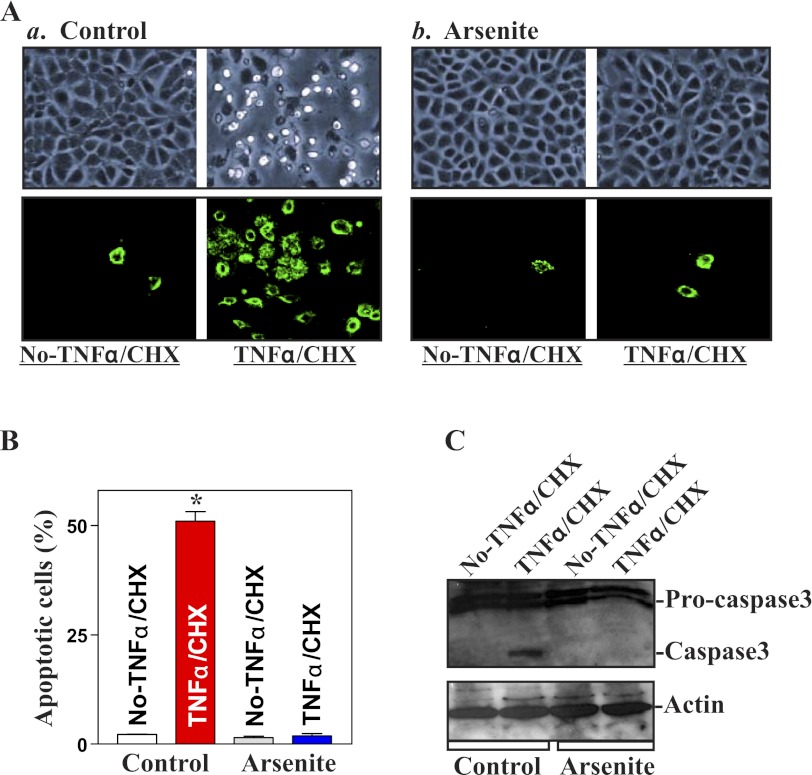
To define the biological consequences of inducing SGs in IECs, we examined its possible implication in regulating apoptosis. Induced formation of SGs by arsenite failed to directly induce apoptosis without any challenge of apoptotic stimulators (Fig. 3A, a and b, left, and B). There were no apparent differences in cell viability in arsenite-treated cells compared with the control cells as measured by Trypan blue staining assay. Neither morphological features of apoptosis nor detectable levels of active caspase-3 (Fig. 3C) were obtained in cells after exposure to arsenite alone. We further determined whether arsenite-induced SGs altered the susceptibility of IECs to apoptosis induced by exposure to TNF-α/CHX. This apoptotic model was used in this study because TNF-α/CHX-induced apoptosis is widely accepted as a form of programmed cell death induced by a biological apoptotic inducer (8, 43). When control cells were exposed to TNF-α/CHX for 4 h, morphological features characteristic of apoptosis were observed (Fig. 3A,a, right, and B); annexin V staining also showed significant phosphatidylserine presence in the cell membrane, a classic indicator of apoptotic cells (Fig. 3A,a, bottom). These morphological assessments of apoptosis were confirmed by changes in active caspase-3 (Fig. 3C). Importantly, when cells were pretreated with arsenite to induce SGs, they exhibited a strong tolerance to TNF-α/CHX-induced apoptosis. Exposure of arsenite-treated cells to the same doses of TNF-α/CHX caused no apoptosis (Fig. 3A,b, right, and C). These results suggest that induced SGs by exposure to oxidative stress protect IECs against apoptosis.
Fig. 3.
Effect of arsenite-induced SG formation on the susceptibility of IECs to apoptosis induced by TNFα in combination with cycloheximide (CHX). A: images of apoptotic cell death and ApoAlert annexin-V staining after treatment with TNFα/CHX for 4 h: control (a) and cells pretreated with arsenite for 45 min to induce SG formation (b). Original magnification, ×150. B: percentage of apoptotic cells as described in A. Values are means ± SE of data from 6 samples. *P < 0.05 compared with No-TNFα/CHX. C: representative Western immunoblots of procaspase-3 and caspase-3 in cells described in A. Whole cell lysates were harvested for Western immunoblotting analysis, and equal loading was monitored by β-actin.
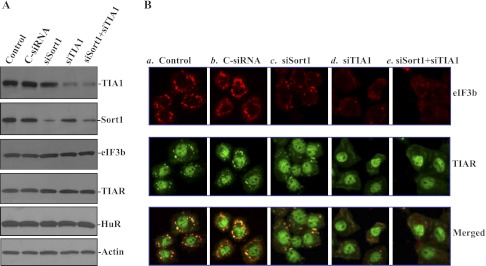
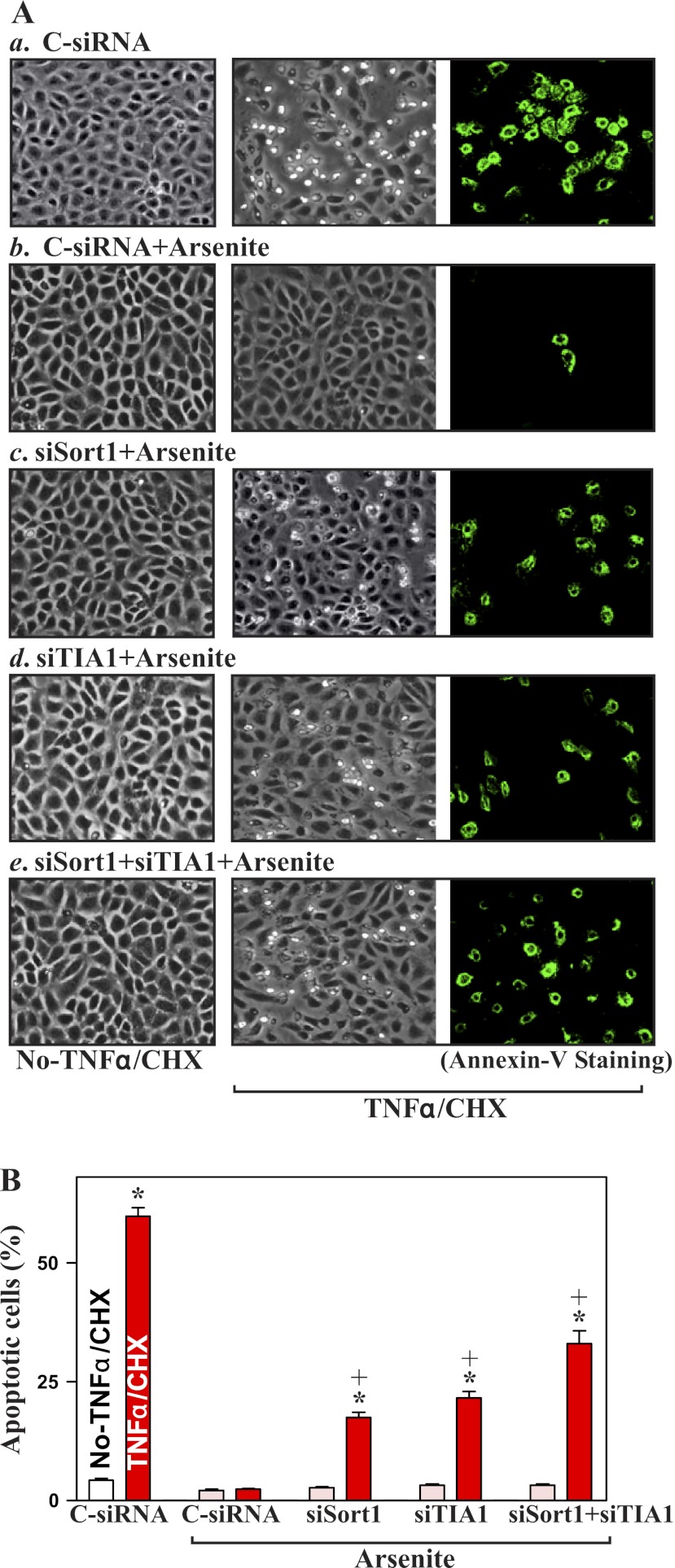
To determine whether inhibiting SG formation alters the susceptibility to apoptosis, the expression of SG-resident proteins, Sort1 and TIA-1, was silenced by transfection with siRNAs targeting the Sort1 (siSort1) or TIA-1 (siTIA-1) mRNA coding region. As shown in Fig. 4A, transfection with siSort1 and siTIA-1 for 48 h specifically decreased the levels of Sort1 and TIA-1 proteins, respectively, but they did not affect the expression of other proteins such as eIF3b, TIAR, and human antigen R (HuR). Silencing either Sort1 or TIA-1 alone and silencing both Sort1 and TIA-1 also inhibited the formation of SGs when cells were exposed to arsenite (Fig. 4B). In Sort1- or TIA-1-silenced populations of cells, arsenite-induced SGs were moderately decreased, but they were completely prevented when the expression of both Sort1 and TIA-1 was silenced (Fig. 4B,e). Furthermore, inhibition of SG formation by silencing Sort1 and TIA-1 also reduced the protection of arsenite against TNF-α/CHX-induced apoptosis (Fig. 5). In normal cells transfected with control siRNA (C-siRNA), pretreatment with arsenite totally prevented TNF-α/CHX-induced apoptosis. There were no differences in morphological features and percentage of apoptotic cells between cells treated with arsenite alone and arsenite-treated cells exposed to TNF-α/CHX. However, the arsenite-induced resistance to TNF-α/CHX-induced apoptosis was decreased in Sort1- and TIA-1-silenced cells. Consistently, silencing Sort1 or TIA-1 alone just partially prevented arsenite-induced protection, whereas silencing both Sort1 and TIA-1 dramatically decreased this resistance to apoptosis (Fig. 5A,e and B). These results show that the inhibition of SG formation increases the susceptibility of IECs to apoptosis.
Fig. 4.
Effect of silencing Sort1 and TIA-1 on arsenite-induced assembly of SGs. A: representative immunoblots of TIA-1, Sort1, eIF3b, TIAR, and human antigen R (HuR) in TIA-1-silenced or Sort1-silenced IECs. Cells were transfected with a specific TIA-1-directed small interfering (si)RNA (siTIA-1), Sort1-directed siRNA (siSort1), or control siRNA (C-siRNA), and the levels of various proteins were measured 48 h after the transfection. B: changes in arsenite-induced assembly of SGs after Sort1 or TIA-1 silencing: control (a), cells transfected with C-siRNA (b), cells transfected with siSort1 (c), cells transfected with siTIA-1 (d), and cells cotransfected with both siSort1 and siTIA-1 (e). Forty-eight hours after transfection, cells were treated with arsenite for 45 min; the formation of SGs was examined by staining eIF3b and TIAR.
Fig. 5.
Effect of inhibition of SG formation by silencing Sort1 and TIA-1 on apoptotic sensitivity in normal IECs. A: images of apoptotic cell death after treatment with and without TNFα/CHX for 4 h. After cells were transfected with C-siRNA, siSort1, or siTIA-1 for 48 h, they were treated with arsenite for 45 min before exposure to TNFα/CHX: cells transfected with C-siRNA (a), cells transfected with C-siRNA and then exposed to arsenite (b), cells transfected with siSort1 and treated with arsenite (c), cells transfected with siTIA-1 and treated with arsenite (d), and cells cotransfected with siSort1 and TIA-1 and treated with arsenite (e). Original magnification, ×150. B: percentage of apoptotic cells as described in A. Values are means ± SE of data from 6 samples. *,+P < 0.05 compared with No-TNFα/CHX and cells that were transfected with C-siRNA and then treated with TNFα/CHX, respectively.
Changes in apoptotic response in polyamine-deficient cells after silencing Sort1 and TIA-1.
As reported in our previous studies (38, 40, 43) and others (33), polyamines regulate apoptosis through multiple signaling pathways and that depletion of cellular polyamines by inhibiting ODC activity with DFMO induces the resistance to TNF-α/CHX-induced apoptosis in IECs. Here we determined whether inhibition of SG formation by silencing Sort1 and TIA-1 altered the polyamine depletion-mediated resistance to apoptosis. Consistent with our earlier findings (38, 40, 43), exposure of polyamine-deficient cells to the same doses of TNF-α/CHX failed to induce apoptosis; there were no differences in the percentages of apoptotic cells when comparing cells treated with DFMO alone and DFMO-treated cells exposed to TNF-α/CHX for 4 h (data not shown). This increased protection against TNF-α/CHX-induced apoptosis was not altered when polyamine-deficient cells were transfected with C-siRNA (Fig. 6A,b), but this resistance to TNF-α/CHX-induced apoptosis decreased significantly when either Sort1 or TIA-1 expression was silenced (Fig. 6A, c and d, and B), and it was almost totally lost when expression of both Sort1 and TIA-1 was inhibited (Fig. 6A,e and B). These results indicate that the elevation in SG assembly contributes to the increased resistance to apoptosis after polyamine depletion.
Fig. 6.
Effect of inhibition of SG formation on apoptotic sensitivity in polyamine-deficient IECs. A: images of apoptotic cell death after treatment with and without TNFα/CHX for 4 h. Cells were treated with DFMO for 4 days and then transfected with C-siRNA, siSort1, or siTIA-1 for 48 h in the presence of DFMO. After cells were treated with arsenite for 45 min, they were exposed to TNFα/CHX for 4 h: control cells transfected with C-siRNA (a), DFMO-treated cells transfected with C-siRNA and then exposed to arsenite (b), DFMO-treated cells transfected with siSort1 and treated with arsenite (c), DFMO-treated cells transfected with siTIA-1 and treated with arsenite (d), and DFMO-treated cells cotransfected with siSort1 and TIA-1 and treated with arsenite (e). Original magnification, ×150. B: percentage of apoptotic cells as described in A. Values are means ± SE of data from 6 samples. *,+P < 0.05 compared with No-TNFα/CHX and DFMO-treated cells transfected with C-siRNA and then treated with TNFα/CHX, respectively.
DISCUSSION
Polyamines are implicated in many aspects of cellular physiology, but the exact roles of polyamines at cellular and molecular levels are not well understood. Recent studies indicate that polyamines potently modulate the stability and translation of several mRNAs (24, 38, 39, 44, 46), except their well-defined roles in the control of gene transcription (10, 28). Cytoplasmic SGs rapidly appear in cells subjected to environmental stress and have emerged as important players in the posttranscriptional regulation of gene expression (1, 2, 4, 34), but little is known about the role of cellular polyamines in the regulation of SG assembly. In the present study, we identified a novel function of polyamines in the SG assembly in normal IECs and show that increasing the levels of cellular polyamines by ectopic ODC overexpression represses the formation of SGs in response to arsenite-induced oxidative stress, whereas a depletion of cellular polyamines by inhibiting ODC activity with DFMO enhances SG assembly, thus advancing our understanding of the biological functions of polyamines. Experiments aimed at investigating the functional consequences of inducing SGs further suggest that the induction in SGs protects IECs against apoptosis and that polyamine depletion-induced increase in SG assembly contributes to the resistance of IECs to TNF-α/CHX-induced cell death.
Our results indicate that increasing the levels of cellular polyamines by ODC overexpression decreased the cytoplasmic abundance of eIF3b and TIAR, whereas depletion of cellular polyamines by inhibiting ODC increased cytoplasmic eIF3b and TIAR levels; neither intervention directly induced formation of SGs without challenge with arsenite. However, stable ODC-IECs exhibited a significant reduction in the assembly of SGs when they were exposed to arsenite, whereas DFMO-treated cells displayed an increase in arsenite-induced SGs. The specificity of these effects in DFMO-treated cells was demonstrated by the addition of exogenous putrescine, because it not only completely restored eIF3b levels and TIAR subcellular distribution to normal but also totally prevented the increase in arsenite-induced SG formation. Recently, Li et al. (18) examined the involvement of polyamines' pathway in the assembly of SGs in tumor cells and found that ODC knockdown by transfection with siRNA targeting ODC mRNA (siODC) inhibits the assembly of arsenite-induced SGs in U20S and RDG3 (U20S cells stably transfected with GFP-G3BP and RFP-DCP1a) cells. Although the exact reason for causing the difference in arsenite-induced SG assembly between IEC-6 cells and U20S cells after polyamine depletion remains unknown, it could be relevant to the following facts: 1) IEC-6 cells represent normal IECs that are nontumorigenic and retain the character of epithelial crypt cells; 2) U20S cells are derived from human osteosarcoma; and 3) basal levels of cellular polyamines between IEC-6 and U20S cells could be significantly different, since polyamine biosynthesis and content in tumor cells are much higher than those observed in normal epithelial cells in general (9, 13, 36). In support to this possibility, our ongoing studies show that a depletion of cellular polyamines by DFMO reduced arsenite-induced SG formation in human colon carcinoma cells (Caco-2 line) (data not published).
The results reported here also show that induced SGs in response to oxidative stress regulate IEC apoptosis, thus playing a role in the control of intestinal epithelial homeostasis. The epithelium of the intestinal mucosa has the most rapid turnover rate of any tissue in the body, and its integrity depends on a dynamic balance of cell proliferation, growth arrest, and apoptosis (23, 33). Apoptosis occurs in the crypt area, where it maintains the critical balance in cell number between newly divided and surviving cells, and at the luminal surface of the colon and villous tips in the small intestine, where differentiated cells are lost (30). It is now well established that IEC apoptosis in the crypt area and the luminal surface of the intestine is tightly regulated by distinct cellular pathways and that an imbalance between epithelial cell survival and apoptosis alters mucosal homeostasis and has significant pathological consequences (30, 33). Our current results show that IEC-6 cells pretreated with arsenite to induce SGs exhibited a strong resistance to TNF-α/CHX-induced apoptosis, and this protective effect was completely prevented by inhibiting SG formation through Sort1 and TIA-1 silencing. Consistent with our findings, induced SG formation following ischemia or cellular hypoxia is also shown to inhibit apoptosis by suppressing stress-responsive MAPK pathways in COS-7 cells and HEK-293 cells (7).
The induced assembly of SGs also plays an important role in the process of increased resistance of IECs to apoptosis after polyamine depletion. Polyamines are implicated in the regulation of apoptosis in different cell types, and their regulatory effects depend on the cell type and death stimuli (33). Our previous studies (20, 36, 40, 43) and others (33, 42) have demonstrated that increased levels of cellular polyamines enhance the susceptibility of IECs to apoptosis, whereas polyamine depletion promotes the resistance of IECs to apoptosis. Polyamines modulate apoptosis in IECs through multiple signaling pathways in the intestinal mucosa. For example, polyamines are shown to downregulate NF-κB activity in IECs, and the depletion of cellular polyamines increases NF-κB nuclear translocation and activates NF-κB/inhibitor of apoptosis protein signaling pathway, leading to the inhibition of apoptosis in polyamine-deficient cells (20, 29). Polyamines also are needed for the inhibition of Akt, MEK-1, JNK, and ATF2 signals, as polyamine depletion induces the kinase activities of Akt, MEK-1, and JNK (33, 38, 43) and increases ATF2 transcriptional activity (40). The data presented in Fig. 6 further show that increased SG assembly following polyamine depletion contributes to the increased resistance to TNF-α/CHX-induced apoptosis, since this tolerance in polyamine-deficient cells was significantly blocked by inhibiting SG formation through silencing Sort1 and TIA-1.
In summary, these results indicate that polyamines are implicated in the regulation of SG assembly in intestinal undifferentiated crypt cells. Increasing the levels of cellular polyamines decreases cytoplasmic abundance of SG resident proteins eIF3b and TIAR and represses SG assembly after exposure to the oxidative stress. On the other hand, polyamine depletion increases the levels of cytoplasmic eIF3b and TIAR and enhances SG formation. Our study further reveals that the rapid formation of SGs in response to oxidative stress regulates apoptosis in IECs. Increased SG formation by pretreatment with arsenite protects IECs against apoptosis, but the inhibition of SG assembly by silencing Sort1 and TIA-1 increases the susceptibility to apoptotic cell death. In addition, polyamine depletion induces the resistance of IECs to TNF-α/CHX-induced apoptosis at least partially by increasing the formation of SGs. These findings suggest that SG assembly is critical for cell survival in the intestinal mucosa in vivo in response to stress environment and plays an important role in the maintenance of intestinal epithelial integrity under physiological and pathological conditions.
GRANTS
This work was supported by Merit Review Grants from the Department of Veterans Affairs (to J.-Y. Wang and J. N. Rao) and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57819, DK-61972, and DK-68491 (to J.-Y. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.Z., J.N.R., L.X., and J.-Y.W. conception and design of research; T.Z., J.N.R., L.L., Y.-H.C., Z.J., and M.O. performed experiments; T.Z., J.N.R., L.L., L.X., Y.-H.C., M.O., J.M.D., and J.-Y.W. analyzed data; T.Z., L.L., and J.-Y.W. prepared figures; J.N.R., L.X., J.M.D., and J.-Y.W. interpreted results of experiments; J.-Y.W. drafted manuscript; J.-Y.W. edited and revised manuscript; J.-Y.W. approved final version of manuscript.
ACKNOWLEDGMENTS
J.-Y. Wang is a Senior Research Career Scientist, Medical Research Service, US Department of Veterans Affairs.
REFERENCES
- 1. Anderson P. Post-transcriptional control of cytokine production. Nat Immunol 9: 353– 359, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Anderson P, Kedersha N. RNA granules. J Cell Biol 172: 803– 808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 10: 430– 436, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci 33: 141– 150, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Anderson P, Kedersha N. Stressful initiations. J Cell Sci 115: 3227– 3234, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Anderson P, Kedersha N. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7: 213– 221, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 10: 1324– 1332, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318– 1321, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6: 373– 390, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Celano P, Baylin SB, Casero RA., Jr Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem 264: 8922– 8927, 1989 [PubMed] [Google Scholar]
- 11. Eisinger-Mathason TS, Andrade J, Groehler AL, Clark DE, Muratore-Schroeder TL, Pasic L, Smith JA, Shabanowitz J, Hunt DF, Macara IG, Lannigan DA. Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol Cell 31: 722– 736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci USA 104: 9041– 9046, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4: 781– 792, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Ghoda L, van Daalen Wetters T, Macrae M, Ascherman D, Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science 243: 1493– 1495, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Harter JL. Critical values for Duncan's new multiple range test. Biometrics 16: 671– 685, 1960 [Google Scholar]
- 16. Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 169: 871– 884, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JE, Ryu I, Kim WJ, Song OK, Ryu J, Kwon MY, Kim JH, Jang SK. Proline-rich transcript in brain protein induces stress granule formation. Mol Cell Biol 28: 803– 813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS One 5: e9942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Li J, Rao JN, Li M, Bass BL, Wang JY. Inhibition of polyamine synthesis induces p53 gene expression but not apoptosis. Am J Physiol Cell Physiol 276: C946– C954, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Li L, Rao JN, Bass BL, Wang JY. NF-κB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 280: G992– G1004, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Li L, Rao JN, Guo X, Liu L, Santora R, Bass BL, Wang JY. Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 281: C941– C953, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Guo X, Rao JN, Zou T, Marasa BS, Chen J, Greenspon J, Casero RA, Jr, Wang JY. Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem J 398: 257– 267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu L, Li L, Rao JN, Zou T, Zhang HM, Boneva D, Bernard MS, Wang JY. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 288: C89– C99, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Rao JN, Zou T, Xiao L, Wang PY, Turner DJ, Gorospe M, Wang JY. Polyamines regulate c-Myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell 20: 4885– 4898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazroui R, Sukarieh R, Bordeleau ME, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, Pelletier J. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2α phosphorylation. Mol Biol Cell 17: 4212– 4219, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol 10: 1224– 1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel AR, Li J, Bass BL, Wang JY. Expression of the transforming growth factor-β gene during growth inhibition following polyamine depletion. Am J Physiol Cell Physiol 275: C590– C598, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Patel AR, Wang JY. Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am J Physiol Cell Physiol 273: C1020– C1029, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Pfeffer LM, Yang CH, Murti A, McCormack SA, Viar MJ, Ray RM, Johnson LR. Polyamine depletion induces rapid NF-kappa B activation in IEC-6 cells. J Biol Chem 276: 45909– 45913, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol Gastrointest Liver Physiol 273: G253– G257, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248– 265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richman RA, Underwood LE, Van Wyk JJ, Voina SJ. Synergistic effect of cortisol and growth hormone on hepatic ornithine decarboxylase activity. Proc Soc Exp Biol Med 138: 880– 884, 1971 [DOI] [PubMed] [Google Scholar]
- 33. Seiler N, Raul F. Polyamines and the intestinal tract. Crit Rev Clin Lab Sci 44: 365– 411, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell 40: 228– 237, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Uniacke J, Zerges W. Stress induces the assembly of RNA granules in the chloroplast of Chlamydomonas reinhardtii. J Cell Biol 182: 641– 646, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang JY. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33: 241– 252, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Wang JY, Johnson LR. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 266: G878– G886, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Wang PY, Rao JN, Zou T, Liu L, Xiao L, Yu TX, Turner DJ, Gorospe M, Wang JY. Post-transcriptional regulation of MEK-1 by polyamines through the RNA-binding protein HuR modulating intestinal epithelial apoptosis. Biochem J 426: 293– 306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiao L, Cui YH, Rao JN, Zou T, Liu L, Smith A, Turner DJ, Gorospe M, Wang JY. Regulation of cyclin-dependent kinase 4 translation through CUG-binding protein 1 and microRNA-222 by polyamines. Mol Biol Cell 22: 3055– 3069, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Zhou H, Gorospe M, Wang JY. Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol Biol Cell 18: 4579– 4590, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Curr Opin Cell Biol 20: 222– 226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan Q, Ray RM, Johnson LR. Polyamine depletion prevents camptothecin-induced apoptosis by inhibiting the release of cytochrome c. Am J Physiol Cell Physiol 282: C1290– C1297, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem 279: 22539– 22547, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387– 19394, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Zou T, Rao JN, Liu L, Marasa BS, Keledjian KM, Zhang AH, Xiao L, Bass BL, Wang JY. Polyamine depletion induces nucleophosmin modulating stability and transcriptional activity of p53 in intestinal epithelial cells. Am J Physiol Cell Physiol 289: C686– C696, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Zou T, Rao JN, Liu L, Xiao L, Yu TX, Jiang P, Gorospe M, Wang JY. Polyamines regulate the stability of JunD mRNA by modulating the competitive binding of its 3′ untranslated region to HuR and AUF1. Mol Cell Biol 30: 5021– 5032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]