Abstract
Aims
Anti-N-methyl-d-aspartate receptor encephalitis (NMDARE) is a recently recognized form of autoimmune encephalitis that typically affects young women, often as a paraneoplastic syndrome related to ovarian teratoma. Clinical features include psychiatric and neurological disturbances, central hypoventilation, autonomic instability, and cardiac dysrhythmias. The prevalence, nature, and outcomes of cardiac dysrhythmias in patients with NMDARE have not been well described.
Methods and results
Records of 10 consecutive patients with NMDARE were reviewed to obtain clinical, laboratory, echocardiographic, electrocardiographic, and radiological data. Patients were all female with an average age of 23 ± 5.5 years. Echocardiograms revealed structurally normal hearts with the exception of mild left ventricular hypertrophy in two cases. Eight patients had inappropriate sinus tachycardia. Six patients developed significant sinus bradycardia, which included periods of sinus arrest in four cases. Five patients manifested both sinus bradycardia and tachycardia. Bradycardia was often triggered by identifiable vagal stimuli. Temporary pacing was instituted in three patients, but permanent pacing was not required in any of the patients. Magnetic resonance imaging (MRI) scans revealed mesial temporal abnormalities in nine patients. In all cases, the dysrhythmias resolved with treatment of the underlying immune disorder with immunotherapy and/or teratoma resection. There was no evidence of dysrhythmia recurrence in any patient at follow-up.
Conclusion
Anti-N-methyl-d-aspartate receptor encephalitis is a recently recognized cause of autoimmune encephalitis with a predilection to cause severe sinus node abnormalities. Temporary pacing is occasionally required, but permanent pacing appears to be unnecessary. An analysis of the clinical syndrome coupled with MRI and experimental data may offer insight into central mechanisms of heart rate regulation.
Keywords: NMDA, N-methyl-d-aspartate, Encephalitis, Autoimmune, Ovarian teratoma, Bradycardia, Asystole, Sinus arrest, Tachycardia, Autonomic dysfunction
Introduction
Anti-N-methyl-d-aspartate receptor encephalitis (NMDARE) is a recently recognized form of autoimmune encephalitis. It most often affects young women and occurs as a paraneoplastic syndrome associated with ovarian teratoma in approximately one-half of the cases.1 Patients may experience a viral-like prodrome and usually present with a characteristic, progressive neuropsychiatric syndrome that includes behaviour, memory, and psychotic changes.2 Symptoms progress to include seizures, dyskinesias, central hypoventilation, autonomic instability, and coma. Reported autonomic derangements include cardiac dysrhythmias, temperature dysregulation, hyperhydrosis, sialorrhoea, hyperpnoea, and adynamic ileus.2 Patients often require prolonged intensive care, mechanical ventilation, and parenteral nutrition. Although the disease can be fatal, it is potentially reversible with treatment.1,3
Cardiac dysrhythmias are frequent manifestations of NMDARE, but the rhythm disturbances have not been described in detail. In the largest published series in the neurological literature, cardiac dysrhythmias were reported in one-third of the 100 cases, but were described only in a qualitative manner.2 Four patients in this series required permanent pacemaker implantation, but the indications for permanent pacing were not reported. Smaller published series of patients with NMDARE also describe the occurrence of dysrhythmias.1,4–6 There are isolated case reports in the neurological literature that describe severe bradycardia with periods of sinus arrest and asystole.7,8 Despite the frequency and severe nature of the rhythm disturbances in NMDARE, the clinical features of the dysrhythmias have not been described in detail in the cardiovascular literature. Other relevant data, including baseline electrocardiograms (ECGs), presence or absence of structural heart disease, and use of chronotropic medications, are also not available. We report a series of patients with NMDARE with a specific focus on the cardiac dysrhythmias.
Methods
This study was approved by the Columbia University Institutional Review Board. A retrospective case series was constructed of consecutive patients diagnosed with anti-NMDA-receptor encephalitis at our institution between 2006 and 2009. All cases were diagnosed on the basis of a suggestive clinical presentation and a positive cerebrospinal fluid laboratory test for autoantibodies reactive to the NMDA receptor (laboratory testing was performed at the laboratory of Dr Joseph Dalmau at the University of Pennsylvania). A review of the patients' medical records was conducted to record clinical, laboratory, echocardiographic, and radiological data. Arrhythmias were identified by review of discharge summaries, inpatient or outpatient notes, vital sign recordings, ECGs, and telemetry recordings. Telemetry was standard in the intensive care unit or was started at the onset of dysrhythmias on the ward.
Significant bradycardia was defined as a heart rate of ≤40 b.p.m. persistent for at least 10 s, and sinus arrest was defined as bradycardia in the absence of a sinus mechanism for at least 3s. Inappropriate sinus tachycardia was defined as sinus rhythm at a sustained heart rate of >100 b.p.m. for at least 24 consecutive hours in the absence of any identifiable cause. Causes that were evaluated included hypotension, fever, infection, pain, or other documented clinical cause of tachycardia. Other supraventricular and ventricular tachycardias were considered to be present if recorded for any duration. All arrhythmias were reviewed and confirmed by an electrophysiologist. The clinical context of the identified arrhythmias was examined to identify possible triggers. The patient outcomes at most recent follow-up were recorded. Age- and sex-matched patients on telemetry in the neurological intensive care unit for other neurological problems but no history of cardiac disease were analysed for comparison.
Results
We identified 10 patients diagnosed with NMDARE between 2006 and 2009 (Table 1). The patients were all female with a mean age of 23 ± 5.5 years (range 17–33 years). The median duration of follow-up was 18.5 months (range 3–36 months). All the patients presented with the characteristic neuropsychiatric signs and symptoms of NMDARE. Seven of the patients developed hypoventilation requiring mechanical ventilation. Eight patients developed autonomic instability, which included pyrexia in the absence of documented infection in eight cases. None of the patients had a history of previously diagnosed cardiovascular disease. Twelve-lead ECGs showed non-specific T-wave abnormalities, but no other significant findings. Echocardiograms were obtained in seven cases and revealed structurally normal hearts with the exception of mild left ventricular hypertrophy in two patients. Thyroid function tests were available in nine cases and were all normal.
Table 1.
Baseline characteristics and autonomic manifestations
| Patient NMDARE | Age | Tumour | Brain MRI abnormality | TSH | EKG | Echocardiogram | Other autonomic instability |
|---|---|---|---|---|---|---|---|
| 1 | 18 | B/L ovarian teratoma | R>L medial temporal, extra-temporal | Normal | NSR, NT | EF 55%, WNL | Fever, hypotension |
| 2 | 19 | R ovarian teratoma | B/L medial temporal | – | ST | EF 60%, WNL | Fever, hypertension |
| 3 | 20 | L ovarian teratoma | B/L medial temporal | Normal | NSR, NT | EF 60%, WNL | Fever, hypertension, hypotension |
| 4 | 33 | – | – | Normal | NSR | EF 60%, Mild LVH | Fever, hypertension |
| 5 | 21 | – | B/L medial temporal | Normal | ST, NT | EF 75%, WNL | Fever, hypertension |
| 6 | 29 | – | B/L medial temporal | Normal on LT3 | NSR, NT | EF 60%, WNL | Fever, hypertension, hypotension |
| 7 | 27 | – | L>R medial temporal | Normal | SB | – | Fever, hypertension |
| 8 | 20 | – | B/L medial temporal, extra-temporal | Normal | ST | EF > 55%, Mild LVH | Fever hypertension |
| 9 | 24 | – | B/L medial temporal | Normal | NSR, NT | – | – |
| 10 | 17 | R ovarian teratoma | – | Normal | NSR | – | – |
| Controls | Neurological abnormality | ||||||
| 1 | 32 | Lupus cerebritis | Inflammatory nodules | Normal | NSR | EF > 60%, WNL | na |
| 2 | 21 | Drug overdose | Subacute infarcts | – | NSR, NT | – | na |
| 3 | 32 | Status epilepticus | Mild atrophy | Normal | ST, NT | EF > 60%, WNL | na |
| 4 | 26 | Status epilepticus | Normal | Normal | NSR | EF > 60%, WNL | na |
| 5 | 25 | Benign brain tumour | Cystic mass | – | NSR | – | na |
| 6 | 32 | Dermato-myositis | Normal | – | NSR | EF > 60%, WNL | na |
| 7 | 27 | Epilepsy | Normal | – | NSR | – | na |
| 8 | 32 | Cerebral haemorrhage | Frontoparietal bleed | – | NSR | EF > 60%, WNL | na |
| 9 | 27 | Pseudotumour cerebri | Normal | Normal | NSR | – | na |
| 10 | 25 | Meningitis | Diffuse hypodensities | – | ST | EF > 60%, WNL | na |
R, right; L, left; B/L, bilateral; LT3, levothyroxine; NSR, normal sinus rhythm; ST, sinus tachycardia; SB, sinus bradycardia; NT, non-specific T-wave abnormality; EF, ejection fraction; LVH, left ventricular hypertrophy; WNL, within normal limits.
Nine patients developed cardiac dysrhythmias, including eight with inappropriate sinus tachycardia and six with significant bradycardia (Table 2). Five patients manifested both inappropriate sinus tachycardia and significant bradycardia. In the eight patients with inappropriate sinus tachycardia, the tachycardia tended to be sustained for days and was sometimes recurrent over multiple admissions (Table 2). Six of the patients received therapy with negative chronotropic agents after persistent tachycardia. One patient had a single-documented episode of non-sustained ventricular tachycardia, but no other supraventricular or ventricular tachyarrhythmias were identified.
Table 2.
Tachycardia and bradycardia
| Patient NMDARE | IAST | Max HR (b.p.m.) | Max duration tachy (h) | Significant bradycardia | Sinus arrest | Longest sinus arrest (s) | Nadir heart rate (b.p.m.) | Precipitants of brady/assystole | Medical treatment | Cardiac pacemaker |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | 140 | 26 | Yes | Yes | 10 | Asystole | Cough, gag reflex, tracheostomy manipulation, bronchoscopy | Atropine | TVP |
| 2 | Yes | 145 | 65 | Yes | Yes | 12 | Asystole | Nasogastric tube placement | Atropine | – |
| 3 | Yes | 184 | 36 | Yes | Yes | 10 | Asystole | Cough, bowel movement | Atropine | TCP |
| 4 | Yes | 170 | 24 | Yes | Yes | 30 | Asystole | Cough, suctioning | – | TCP |
| 5 | Yes | 193 | 48 | Yes | – | – | 39 | – | – | – |
| 6 | Yes | 188 | 48 | – | – | – | – | – | – | – |
| 7 | Yes | 160 | Unknown | – | – | – | – | – | – | – |
| 8 | Yes | 165 | 66 | – | – | – | – | – | – | – |
| 9 | – | – | – | Yes | – | – | 40 | – | – | – |
| 10 | – | – | – | – | – | – | – | – | – | – |
| Controls | ||||||||||
| 1 | No | 120 | <1 | No | No | – | 78 | – | – | – |
| 2 | No | 139 | 6 | No | No | – | 72 | – | – | – |
| 3 | No | 130 | 12 | No | No | – | 54 | – | – | – |
| 4 | No | 128 | 6 | No | No | – | 68 | – | – | – |
| 5 | No | 120 | <1 | No | No | – | 56 | – | – | – |
| 6 | No | 125 | 6 | No | No | – | 88 | – | – | – |
| 7 | No | 110 | 1 | No | No | – | 78 | – | – | – |
| 8 | No | 106 | 1 | No | No | – | 68 | – | – | – |
| 9 | No | 98 | 0 | No | No | – | 65 | – | – | – |
| 10 | No | 121 | 20 | No | No | – | 91 | – | – | – |
Inappropriate sinus tachycardia (IAST) defined as HR > 100 for > 24 h.
Significant bradycardia defined as HR ≤40 b.p.m. for >10 s. Sinus arrest defined as sinus pause >3 s.
TVP, transvenous pacemaker; TCP, transcutaneous pacemaker.
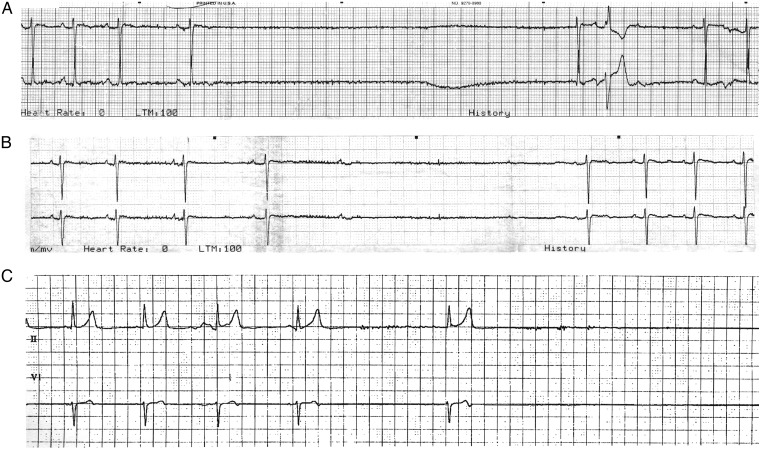
Six patients developed significant bradycardia, including four with episodes of sinus arrest persisting for a maximum duration of 10–30 s (Table 2). Analysis of the available cardiac telemetry recordings shows slowing down of the sinus rate prior to sinus arrest in many instances (Figure 1). Concomitant high-degree atrio-ventricular (AV) block was also observed in some cases, although AV block never occurred in the absence of sinus bradycardia. In contrast to sinus tachycardia, the bradycardic events tended to be transient rather than sustained, typically lasting minutes. Some patients had isolated bradycardic episodes, while others had recurrent events over the course of multiple admissions separated by months. In all cases, the bradycardia occurred in the absence of therapy with negative chronotropic agents. Many of the episodes occurred in the context of documented vagal stimuli, including defecation, cough, tracheostomy manipulation, bronchoscopy, and nasogastric tube placement. However, episodes often persisted after resolution of the inciting vagal trigger. Given the altered mental status of many of the patients, symptoms were not reported for most cases of bradycardia. Temporary cardiac pacing was instituted in three patients, but no patient required permanent pacemaker therapy.
Figure 1.
Examples of sinus bradycardia and sinus arrest in Anti-N-methyl-d-aspartate receptor encephalitis. Recordings demonstrating sinus bradycardia and sinus arrest in two patients: Examples A and B—Patient 3, C—Patient 4. In example B, sinus arrest is preceded by slowing of the sinus rate and by atrio-ventricular block. The paper speed is 25 mm/s.
Table 1 lists the characteristics of an age- and sex-matched group of patients that had no cardiac history, normal ECGs and/or echocardiograms, and had other types of neurological disorders requiring admission to the neurological intensive care unit for at least 48 h. Three of these patients required mechanical ventilation for their entire stay in the intensive care unit, and one was intubated for 2 days after neurosurgery. While these young patients also had sinus tachycardia, it was never persistent for >20 h. None of the control patients had significant bradycardia or pauses (Table 2).
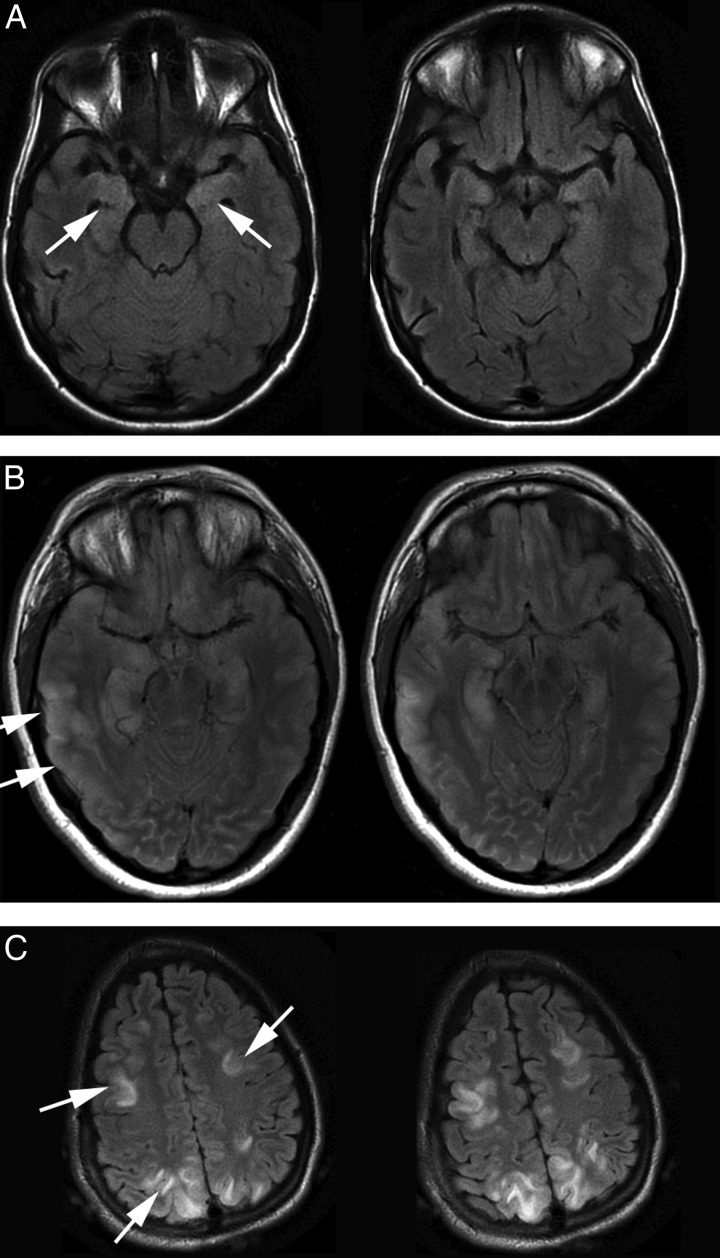
Magnetic resonance imaging (MRI) of the brain showed T2-weighted signal abnormalities in nine patients, including mesial temporal abnormalities in eight cases (Figure 2). Four patients were found to have ovarian teratoma and underwent tumour resection or total oophorectomy. All patients were treated with some form of immunotherapy, including combinations of corticosteroids, intravenous immunoglobulin, plasmapheresis, and rituximab. In all cases, the cardiac dysrhythmias resolved with treatment of the underlying disorder with immunotherapy and/or teratoma resection (Table 3). Patients with recurrent dysrhythmias after the initial hospitalization only had recurrences in the context of exacerbations of the underlying neurological disorder. No patient required long-term therapy with negative chronotropic agents or a permanent pacemaker, and there was no clinical evidence of sinus node dysrhythmia occurrence in any patient at the time of most recent follow-up.
Figure 2.
Brain magnetic resonance imaging findings in Anti-N-methyl-d-aspartate receptor encephalitis. T2-weighted images from three patients in this series, showing reversible magnetic resonance imaging T2 signal changes in grey matter. In Patient A, there is only an abnormal, bilateral increase in mesial temporal T2-weighted signal. Patient B had similar temporal signal changes, but also left parietal cortical abnormalities. Patient C had little temporal lobe abnormality (sections not shown), but multiple areas of frontal and parietal gyral signal change.
Table 3.
Treatment and clinical outcomes at follow-up
| Patient | Index hospitalization (months) | Follow-up (months) | Trach & PEG | Surgical treatment | Medical treatment | Neurological outcome | Autonomical instability | Chronotropic medications | Permanent pacemaker |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 19 | T&P | Bilateral ovarian cystectomy | Steroids, pheresis, IVIG, ritux | 2 | – | – | – |
| 2 | 1 | 13 | P | Right salpingo-oophorectomy | Steroids, IVIG | 2 | – | – | – |
| 3 | 4 | 19 | T&P | Left ovarian cystectomy | Pheresis, IVIG, ritux | 3 | – | – | – |
| 4 | 2 | 3a | T&P | – | a | 4–5a | – | – | – |
| 5 | 4 | 36 | P | – | Steroids, pheresis, IVIG | 3 | – | – | – |
| 6 | 4 | 36 | T&P | – | Steroids, IVIG, pheresis | 5 | – | – | – |
| 7 | 2 | 31 | T&P | – | IVIG, pheresis | 3 | – | – | – |
| 8 | 1 | 18 | P | – | Steroids, IVIG, pheresis | 1 | – | – | – |
| 9 | 1 | 14 | – | – | Steroids, IVIG | 2 | – | – | – |
| 10 | 2 | 5 | – | Right ovarian cystectomy | Steroids, IVIG, pheresis | 1 | – | – | – |
Neurological outcomes are reported by a modified ranking scale: 0—asymptomatic; 1—symptoms without disability or impairment of daily activities; 2—slight disability, unable to carry out all prior activities; 3—moderate disability, requiring assistance with daily activities; 4—moderate-severe disability, requiring assistance with walking, and bodily needs, 5—severe disability, requiring constant nursing care; 6—death.
Steroids, glucocorticoids; pheresis, plasmapheresis; IVIG, intravenous immunoglobulins; ritux, rituximab; T, tracheostomy; P, percutaneous gastrostomy.
aDiagnosed retrospectively and lost to follow-up.
Discussion
N-methyl-d-aspartate receptor encephalitis was first described in 2005 as a severe but potentially reversible form of paraneoplastic limbic encephalitis affecting young women with ovarian teratomas.9,10 The pathogenic antibodies were subsequently found to be directed against the NR1/NR2 heteromers of the NMDA receptor, which is expressed by neuronal tissue in the teratomas.1,2 Investigators soon identified additional patients without malignancies, including males.11 It is now recognized that NMDARE occurs as a paraneoplastic condition in approximately half of the cases and as an apparent autoimmune disorder in the other half.2 The characteristic, severe neuropsychiatric syndrome of NMDARE has been associated with cardiovascular abnormalities including haemodynamic instability and cardiac dysrhythmias.1–3 The latter appear to be much more common in NMDARE than in other encephalitides, for which only isolated cases of dysrhythmias are reported.12–16
Although cardiac dysrhythmias are frequent manifestations of NMDARE, the rhythm disturbances are described in only a cursory fashion in the neurological literature. In the largest published series of 100 patients, approximately two-thirds of the patients developed autonomic instability, and one-third developed cardiac dysrhythmias.2 The dysrhythmias are described in a general sense as ‘tachycardia’ (53%), ‘bradycardia’ (19%), or ‘both’ (38%). Other smaller case series or reports also describe the occurrence of dysrhythmias in NMDARE4–8 To our knowledge, the current series is the first to report in detail the nature and outcomes of cardiac dysrhythmias associated with NMDARE.
This study demonstrates that the rhythm disturbances consist primarily of sinus node dysrhythmias, which occurred in 90% of the patients. This percentage is higher than the previously reported incidence of ∼30–70%.2,4,5 This difference may reflect a surveillance bias in that our focus on cardiac dysrhythmias may have led to closer scrutiny of the medical records. Alternatively, it may be due to chance given the relatively small sample size of this study. The majority of patients in our series demonstrated inappropriate sinus tachycardia, but no other significant supraventricular or ventricular tachyarrhythmias were identified. The majority of patients also developed sinus bradycardia with periods of sinus arrest, although some episodes also involved concomitant AV block. Many bradycardic episodes occurred in the context of identifiable vagal stimuli, and telemetry recordings support a vagal aetiology with slowing down of the sinus rate prior to sinus arrest. In contrast, a control group of patients with severe neurological abnormalities and equal propensity to vagal stimuli did not develop the degree of bradycardia or duration of tachycardia seen in the NMDARE patients. For reference, the ranges of normal resting heart rates for older children and healthy adults are ∼65–85 b.p.m. and 52–76 b.p.m., respectively.17,18
Heart rate regulation can be conceived of as occurring along a neurocardiac axis consisting of the cardiac conduction system, peripheral afferents, and efferents of the autonomic nervous system, central autonomic control centres, and higher-order central systems. N-methyl-d-aspartate receptors are expressed at every level of this axis.19–22 However, evidence from clinical, pathological, and animal studies suggests that the pathogenic abnormality most likely occurs in either the brainstem centres or the higher-order systems. Magnetic resonance imaging abnormalities reported in this study and elsewhere support a role of the higher-order central systems with abnormalities primarily identified in the mesial temporal cortex, which underlies the limbic system.
In the rat model, NMDA receptors have been identified in the nodose ganglion which contains the cell bodies of vagal afferents, as well as in the nucleus tractus solitarii (NTS) in vagal afferent neuron axons terminals, the dendrites that they contact, and cell bodies.19,22 Injection of NMDA into the NTS has been demonstrated to cause both vasodepressor and bradycardic effects.23 Injection of NMDA antagonists into the rostral ventrolateral medulla attenuated the bradycardic and depressor effects of clonidine.24 Animal experiments have also implicated NMDA receptor-mediated signalling in the mechanism of heart rate regulation by higher-order CNS centres.20,25,26 The clinical manifestations of NMDARE are believed to be due to antibody-mediated down-regulation of NMDA receptors in the central nervous system. Dalmau et al.2 have experimentally shown that the antibodies from affected patients' serum cause a reversible decrease in neuronal cell-surface NMDA receptors and post-synaptic dendrite NMDA receptors. Pharmacological antagonists of NMDA receptors in the central nervous system, including ketamine, phencyclidine (PCP), and MK801, cause a syndrome with features similar to NMDARE, including autonomic instability and cardiac dysrhythmias.2
A theoretical model for the mechanism of cardiac dysrhythmias in NMDARE can be proposed based on these experimental findings, coupled with the aforementioned clinical and radiological data. N-methyl-d-aspartate receptor-mediated signalling in the higher-order central systems has an excitatory effect on vagal, parasympathetic outflow. This exerts tonic control of heart rate through direct effects on the sinoatrial and atrioventricular nodes. In this model, antibody-mediated loss of NMDA receptor function would cause a loss of tonic vagal signalling, leading to inappropriate sinus tachycardia. The sustained loss of functioning NMDA receptors may cause an up-regulation of alternative receptors or pathways that respond to afferent vagal signals. This would be expected to lead to increased sensitivity to afferent signals that could trigger a profound, prolonged response to vagal stimuli. This model would explain the observation of prolonged sinus tachycardia with superimposed, episodic sinus bradycardia in patients with NMDARE. With treatment of the underlying autoimmune processes, NMDA-receptor-mediated signalling in the limbic cortex would return to baseline, leading to resolution of the cardiac dysrhythmias.
Two recent studies suggest that NMDARE may be more common than previously recognized. In one study investigators were able to identify antibodies directed against the NMDA receptor in 6 of 31 patients (19%) with unexplained encephalitis.27 Another group similarly diagnosed NMDARE in 5 of 19 (26%) young, female patients with new onset, idiopathic epilepsy.28 It is therefore important that the diagnosis of NMDARE be entertained in any patient with unexplained encephalitis and cardiac dysrhythmias. The syndrome, despite its severity, is often reversible with tumour resection, immunotherapy, or supportive management.1–3,6
Limitations
This study is limited by its small sample size; however, this condition is uncommon and our series is comparable in number to other previously reported single institution series. Like any retrospective analysis, this study is limited by the completeness of available data, but our review was exhaustive in terms of cardiac arrhythmias, and patients were monitored extensively during hospitalization. Outpatient follow-up data, however, were limited to clinical notes and ECGs, and so asymptomatic outpatient dysrhythmias cannot be ruled out.
Conclusion
Anti-NMDA receptor encephalitis is a recently recognized cause of autoimmune encephalitis that is likely to increasingly come to the attention of clinical cardiologists. Patients present with a characteristic neuropsychiatric syndrome that is frequently associated with derangements of sinus node function. The dysrhythmias appear to resolve with treatment of the underlying autoimmune disorder. Analysis of the neurological processes underlying cardiac dysrhythmias in this syndrome may shed light on the central mechanisms of heart rate regulation.
Conflict of interest: none declared.
Funding
This work was supported by the National Institute on Aging/National Institutes of Health (P50AG008702 to L.S.H.).
References
- 1.Dalmau J, Tuzun E, Wu H, Masjuan J, Rossi J, Voloschin A, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman A, Hughes E, Rossi J, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishiura H, Matsuda S, Higashihara M, Hasegawa M, Hida A, Hanajima R, et al. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71:1921–3. doi: 10.1212/01.wnl.0000336648.43562.59. [DOI] [PubMed] [Google Scholar]
- 4.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti–N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–8. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421–9. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–11. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka H, Dalmau J, Ueno S. Paraneoplastic encephalitis associated with ovarian teratoma and N-methyl-d-aspartate receptor antibodies. Eur J Neurol. 2008;15:e5–6. doi: 10.1111/j.1468-1331.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- 8.Sansing L, Tuzun E, Ko M, Baccon J, Lynch D, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–6. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128(Pt 8):1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitaliani R, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novillo-Lopez ME, Rossi JE, Dalmau J, Masjuan J. Treatment-responsive subacute limbic encephalitis and NMDA receptor antibodies in a man. Neurology. 2008;70:728–9. doi: 10.1212/01.wnl.0000305981.53537.d9. [DOI] [PubMed] [Google Scholar]
- 12.Alehan D, Ceviz N, Celiker A. Torsades des Pointes associated with encephalitis. Turk J Pediatr. 1999;41:395–8. [PubMed] [Google Scholar]
- 13.DeKeyser J, DeBoel S, Ceulemans L, Ebinger G. Torsades des Pointes as a complication of brainstem encephalitis. Intensive Care Med. 1987;13:76–7. doi: 10.1007/BF00263564. [DOI] [PubMed] [Google Scholar]
- 14.Pollack S, Reid H, Klapper P, Metcalfe RA, Ahmed N. Herpes simplex encephalitis presenting as the sick sinus syndrome. J Neurol Neurosurg Psychiatry. 1986;49:331–2. doi: 10.1136/jnnp.49.3.331-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa S, Aoki H, Akahane C, Shimada H, Takei Y, Ichinose Y, et al. Hypothalamic encephalitis with bradycardia. Intern Med. 2001;40:805–7. doi: 10.2169/internalmedicine.40.805. [DOI] [PubMed] [Google Scholar]
- 16.Smith BK, Cook MJ, Prior DL. Sinus node arrest secondary to HSV encephalitis. J Clin Neurosci. 2008;15:1053–6. doi: 10.1016/j.jocn.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddermann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunan D, Sandercock GRH, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. PACE. 2010;33:1407–17. doi: 10.1111/j.1540-8159.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- 19.Aicher SA, Sharma S, Pickel VM. N-methyl-d-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience. 1999;91:119–32. doi: 10.1016/s0306-4522(98)00530-2. [DOI] [PubMed] [Google Scholar]
- 20.Alves FHF, Crestani CC, Resstel LBM, Correa FMA. N-methyl-d-aspartate receptors in the insular cortex modulate baroreflex in unanesthetized rats. Auton Neurosci. 2009;147:56–63. doi: 10.1016/j.autneu.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Xu X, Pang J, Zhang C, Ding JM, Peng X, et al. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res. 2007;56:559–69. doi: 10.33549/physiolres.931053. [DOI] [PubMed] [Google Scholar]
- 22.Lin LH. Glutamatergic neurons say NO in the nucleus tractus solitarii. J Chem Neuroanat. 2009;38:154–65. doi: 10.1016/j.jchemneu.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo I, Hirooka Y, Hironaga K, Eshima K, Shigematsu H, Shihara M, et al. Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1285–91. doi: 10.1152/ajpregu.2001.280.5.R1285. [DOI] [PubMed] [Google Scholar]
- 24.Wanga WZ, Yuana WJ, Su DF. Blockade of N-methyl-d-aspartate receptors within the rostral ventrolateral medulla antagonizes clonidine-induced cardiovascular effects. Auton Neurosci. 2003;109:21–8. doi: 10.1016/j.autneu.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: role of sympathetic and vagal efferents. Hypertension. 2009;54:1369–75. doi: 10.1161/HYPERTENSIONAHA.109.140715. [DOI] [PubMed] [Google Scholar]
- 26.Resstel LBM, Correa FMA. Medial prefrontal cortex NMDA receptors and nitric oxide modulate the parasympathetic component of the baroreflex. Eur J Neurosci. 2006;23:481–8. doi: 10.1111/j.1460-9568.2005.04566.x. [DOI] [PubMed] [Google Scholar]
- 27.Davies G, Irani SR, Coltart C, Ingle G, Amin Y, Taylor C, et al. Anti-N-methyl-d-aspartate receptor antibodies: a potentially treatable cause of encephalitis in the intensive care unit. Crit Care Med. 2010;38:679–82. doi: 10.1097/CCM.0b013e3181cb0968. [DOI] [PubMed] [Google Scholar]
- 28.Niehusmann P, Dalmau J, Rudlowski C, Vincent A, Elger CE, Rossi JE, et al. Diagnostic value of N-methyl-d-aspartate receptor antibodies in women with new-onset epilepsy. Arch Neurol. 2009;66:458–64. doi: 10.1001/archneurol.2009.5. [DOI] [PubMed] [Google Scholar]