Abstract
Aims
Remodelling of the extracellular matrix (ECM) plays an important role in the production of arrhythmogenic substrate for atrial fibrillation (AF), and is considered to be promoted by the connective tissue growth factor (CTGF). Our objective was to assess the relationship between CTGF and ECM synthesis, and the effect of olmesartan on these processes.
Methods and results
Fifteen canine AF models were produced by rapid atrial stimulation. They were divided into three groups: pacing control (n = 5): 6-week pacing, pacing + olmesartan (n = 5): pacing with olmesartan (2 mg/kg/day), and non-pacing group (n = 5). In the pacing control group, messenger ribonucleic acid expressions of CTGF and collagen types 1 and 3 were up-regulated in comparison with the non-pacing group (P < 0.05) while transforming growth factor-β (TGF-β) did not exhibit a significant difference. In the pacing + olmesartan group, these up-regulations were suppressed (P < 0.05). In fluorescent immunostaining, the expression of CTGF was localized in the cytoplasm. The protein level of collagen type 3 was increased in the pacing control and it was suppressed in the pacing + olmesartan group.
Conclusions
CTGF and associated genes were up-regulated in the atria with the appearance of fibrosis. Because this up-regulation was independent of TGF-β and suppressed by olmesartan, CTGF up-regulation was considered to be mediated by angiotensin II.
Keywords: Angiotensin receptor blocker, Atrial fibrillation, Connective tissue growth factor, Rapid pacing
Introduction
Atrial remodelling as the construction of the arrhythmogenic substrate of atrial fibrillation (AF) can be characterized electrophysiologically by the shortening of atrial refractoriness and the decrease in conduction velocity.1 Because atrial interstitial fibrosis, fibroblastic degeneration, and extracellular matrix (ECM) synthesis destroy the electrophysiological connection between atrial myocytes, they can cause conduction disturbance and may play important roles in atrial remodelling for AF.2 Interstitial changes, i.e. ECM synthesis, can be caused by various stimulations, e.g. inflammatory cytokines, angiotensin-II, oxidative stress, etc., but one of the main and important mediators is connective tissue growth factor (CTGF), regardless of basic stimulation. Connective tissue growth factor is a potent profibrotic factor and it implicates cellular adhesion as well as the promotion of ECM synthesis.3,4 Tissue fibrosis can be strongly mediated by CTGF and CTGF up-regulation has been reported in various fibrotic diseases of the lung, kidney, liver, and skin, and even in the myocardium in patients with structural heart disease.5–9 Because various stimulations are induced under AF conditions, CTGF can also be up-regulated and may play a role in atrial structural remodelling. In this study, we evaluated the expression of CTGF and atrial fibrosis, and then the effect of olmesartan, an angiotensin receptor blocker (ARB), on these factors in the canine AF model to clarify the role of CTGF and the stimulation of angiotensin in atrial remodelling for AF.
Methods
Initial surgery
Fifteen adult female beagle dogs (12.6 ± 1.2 kg) were anaesthetized with sodium pentobarbital (NEMBUTAL®, 30 mg/kg, intravenous), and followed by additional dose of 2 mg/kg at the end of each hour. Preceding the intubation, the dogs were anaesthetized with butorphanol tartrate (Butorphanol®, 0.3 mg/kg, intramuscular) as an analgesic. After endotracheal intubation, dogs were maintained on controlled ventilation with 100% oxygen using a mechanical ventilator (Model SN-480-5; Shinano Manufacturing, Tokyo, Japan)10–15 Core body temperature was maintained at normothermia (38 ± 1°C) with an electric heater (Pig-heaterdan®, Tozai Co., Ltd, Japan) and body temperature was monitored with a temperature probe in the rectum. Saline was given intravenously (250 mL/h) to replace fluid loss. To keep adequate anaesthesia level, continuous arterial blood pressure, heart rate, and end-tidal carbon dioxide were monitored during the surgical procedure. Two pairs of electrodes were sutured at the right atrial free wall to monitor the atrial electrograms. The other ends of the electrode wires were tunnelled subcutaneously and exposed at the back of the neck. For continuous rapid atrial pacing, a unipolar screw-in lead (CapSureFix 5568; Medtronic Inc., Minneapolis, MN, USA) was inserted through the right external jugular vein, and the distal end of the lead was screwed into the endocardial side of the right atrial appendage. The proximal end of the pacing lead was connected to a rapid pulse generator (Soletra®; Medtronic Inc.), which was implanted into a subcutaneous pocket at the back of the neck. Atrioventricular block was not produced in this study in order to mimic the hemodynamic situation of clinical cases of AF.10–15
All studies were performed in accordance with the guidelines specified by the Animal Experimentation and Ethics Committee of Kitasato University School of Medicine. The investigation conforms to the Guide for the Care and Use of Laboratory Animals, US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Study protocol
To obtain stable baseline conditions, each dog was allowed to recover after the initial surgical procedure for at least 1 week without pacing. Atrial rapid pacing (400 beats/min) was performed in 10 of the 15 dogs, and no pacing was carried out in the remained 5 dogs assigned as a non-pacing group. We divided the 10 dogs with atrial rapid pacing to two groups, a pacing control group (n = 5) without any oral administration, and a pacing + olmesartan group (n = 5) with oral administration of olmesartan (2 mg/kg/day) starting 2 weeks before the initiation of rapid pacing.
At the end of the protocol, all dogs were anaesthetized and ventilated mechanically as previously mentioned in the initial surgery to evaluate hemodynamic parameters. To exclude the hemodynamic difference caused by olmesartan administration, the hemodynamic parameters of systemic blood pressure, pulmonary arterial pressure, pulmonary arterial wedge pressure, central venous pressure, and cardiac output were evaluated by a thermo-dilution catheter in the pacing control and pacing + olmesartan groups at the end of the protocol.
After the hemodynamic evaluation, the chest was opened to expose the heart under anaesthesia and euthanasia was performed in all dogs, including the non-pacing group, by following procedure; ventricular fibrillation was induced with a small amount of electric current directly to the heart using an alkaline battery (9 V). Then small portions of the right and left atrial free walls were rapidly excised for histological and biochemical analyses. The histology of the atrial tissue was evaluated by haematoxylin and eosin (HE) staining.
Quantitative real-time reverse transcriptase–polymerase chain reaction
Small portions of both atria were sampled from the area close to the electrodes at the end of the protocol. The atrial tissues were rinsed and soaked in RNA stabilization solution (RNAlater® Ambion) immediately. Total RNA was prepared from the right and left atrial free walls using a total RNA isolation kit (SV Total RNA Isolation system; Promega, Madison, WI, USA). Complementary deoxyribonucleic acid (cDNA) was synthesized from 3 µg total RNA with reverse transcriptase (Invitrogen, San Diego, CA, USA) in a final volume of 20 µL. The messenger ribonucleic acid (mRNA) levels of the ECM-related molecules, CTGF, collagen type 1 (COL1), collagen type 3 (COL3), and fibronectin 1 (FN1), and inflammation-related molecules, transforming growth factor-β (TGF-β) and monocyte chemotactic protein-1 (MCP-1) were evaluated by quantitative real-time reverse transcriptase–polymerase chain reaction (RT–PCR). The level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also evaluated as the internal control. Real-time RT–PCR was performed with a QuantiTectTM SYBER Green PCR Master Mix (Qiagen, Valencia, CA, USA) and CFD 3240 Chromo4TM Detection System (Bio-Rad Lab, Inc., Richmond, CA, USA) using each primer pair described in Table 1. For the standard curve, the standard plasmid for each molecule was constructed as previously described.16 Each cDNA sample was diluted in 200 µL of nuclease-free water, and then 1 µL was used as the template for real-time RT–PCR. Serially diluted standard plasmids were analysed at the same time and the absolute copy numbers were calculated.
Table 1.
Polymerase chain reaction primers used for amplification of inflammation and fibrosis-related genes
| Sense | Antisense | |
|---|---|---|
| Connective tissue growth factor | 5′-TTTAGGAACAGTGGGAGAGC | 5′-CATGAAGAAGGCTGGAGAAC |
| Transforming growth factor-b | 5′-TATATGCCAGCGTGAAGTGC | 5′-GAAGCAGGTTGGGCATTAGT |
| Collagen type 1 | 5′-CAAGAACCCCAAGGAGAAGA | 5′-AGTGGTAGGTGATGTTCTGG |
| Collagen type 3 | 5′-TCTGTGAATCCTGCCCTACT | 5′-AGCCAGGATGACCAGATGTA |
| Glyceraldehyde-3-phosphate dehydrogenase | 5′ AAAGCTGTGGGCAAGGTCAT | 5′-TCCGATGCCTGCTTCACTAC |
| Fibronectin 1 | 5′-GAGAAGACAGGACCGATGAA | 5′-TTCTTGGAGGGCTGACATTC |
| Monocyte chemotactic protein-1 | 5′-GCCAGCTATAAAAGAGTCAC | 5′-CAGTTTGGGTTTGGCTTTTC |
Fluorescent immunostaining of connective tissue growth factor
The cardiac tissues were fixed in 4% paraformaldehyde for 7 days. Paraffin sections were prepared using a Young-type sliding microtome (Sakura Finetek Japan, Co., Ltd) and the disposable microtome blade. The sections were about 3 μm in thickness and mounted on silane-coated slides and deparaffinized with xylene followed by a stepwise change of ethyl alcohol. The slides were then exposed to 1% hydrogen peroxide/methanol for 30 min in order to block endogenous peroxidase activity, and rinsed in Tris–buffered saline (TBS). The slides were treated with 8% skimmed milk for 30 min. Anti-rabbit-polyclonal antibody to CTGF (GeneTex®, Inc., USA) in TBS was applied to the sections in a moisture chamber at 4°C for 1 h. After incubation in Alexa Fluor 488 goat anti-rabbit IgG antibody for 45 min and rinsing in TBS, the sections were counterstained with DAPI (Vectashield Mounting Medium). The stained sections were then observed with a fluorescence microscope, Olympus DP70 (Olympus, Japan).
Western blot analysis
The cardiac tissues were homogenized in ice-cold lysis buffer containing 50 mM of Tris–HCl (pH 7.5), 1 mM of ethylene glycol tetraacetic acid, 150 mM of NaCl, 0.25% SDC, 1 mM of sodium orthovanadate, 1% Triton X-100, 2 μg/mL of aprotinin, 1 mM of phenylmethane sulfonyl fluoride, and 5 μg/mL of leupeptin. For western blotting, 20 μg of total proteins were separated by 7.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electroblotted onto a polyvinylidene fluoride transfer membrane (Amersham HypondTM-P; GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK). After blocking with 5% non-fat milk, the membrane was incubated with anti-rabbit COL1 antibody (sc-8784; Santa Cruz Biotechnology, Inc., CA, USA), anti-rabbit COL3 antibody (LSL-LB-1387; Cosmo Bio Co., Ltd., Japan) or anti-mouse GAPDH antibody (GTX28245; GeneTex® Inc., USA) and subsequently with the secondary antibody (Amersham ECL Plus Western blotting reagent pack; GE Healthcare UK Ltd.). After development with the ECL detection kit (Amersham ECL PlusTM Western Blotting Detection System), the densities of each band in the digitized images were measured using the public domain NIH Image programme.
Statistical analysis
All statistical analyses were performed using JMP statistical software (SAS Institute Inc., Cary, NC, USA). The values are presented as the mean ± standard deviation. The basic comparative statistics were analysed with one-way analysis-of-variance test or the paired t-test. A P value of < 0.05 was considered significant.
Results
Histopathology
Macroscopically, no obvious difference was observed among the three groups; however, on HE staining, tissue fibrosis and ECM changes were observed. In comparison with the non-pacing group, the pacing control group exhibited partial cellular degeneration, fatty degeneration, and interstitial proliferation, but the infiltration of mononuclear cells was not observed. The degrees of these changes seemed to be more prominent in the left atrium (LA) than in the right atrium (RA). In contrast, in the pacing + olmesartan group, these changes were suppressed.
Haemodynamic parameters
Table 2 shows the haemodynamic parameters obtained in the pacing control and pacing + olmesartan groups. There were no significant differences between the two groups in these parameters.
Table 2.
Haemodynamic parameters
| Pacing control | Pacing + olmesartan | P value | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 156 ± 15 | 156 ± 4 | 0.940 |
| Diastolic blood pressure (mmHg) | 85 ± 5 | 68 ± 8 | 0.120 |
| Systolic pulmonary arterial pressure (mmHg) | 15 ± 1 | 18 ± 1 | 0.094 |
| Diastolic pulmonary arterial pressure (mmHg) | 6 ± 1 | 6 ± 1 | 1.000 |
| Pulmonary arterial wedge pressure (mmHg) | 7 ± 2 | 6 ± 1 | 0.690 |
| Central venous pressure (mmHg) | 2 ± 1 | 2 ± 1 | 0.760 |
| Cardiac output | 2.9 ± 0.4 | 4.2 ± 0.7 | 0.180 |
| Ventricular activation rate during induced atrial fibrillation (bpm) | 185 ± 5 | 183 ± 9 | 0.8353 |
Messenger ribonucleic acid expressions of extracellular matrix and inflammatory-related molecules
Figure 1 exhibits the expression levels of mRNA of ECM and inflammatory-related molecules. In the pacing control group, the mRNA expression of CTGF was up-regulated in comparison with the non-pacing group in both the LA and RA. In the pacing + olmesartan group, this up-regulation of CTGF was suppressed in both atria in comparison with the pacing control group. The mRNA expressions of COL1, COL3, and FN1, which reflect ECM synthesis, exhibited a similar pattern to CTGF, except for COL1 in the LA in which up-regulation was not suppressed in the pacing + olmesartan group in comparison with the pacing control group.
Figure 1.
Messenger ribonucleic acid expressions of extracellular matrix and inflammation-related molecules. In the pacing control group, mRNA expressions of extracellular matrix-related genes (connective tissue growth factor, collagen type 1 and 3, and fibronectin 1) were up-regulated, especially in the right atrium in comparison with the non-pacing group, and this up-regulation was suppressed in the pacing + olmesartan group. In contrast, messenger ribonucleic acid expression of transforming growth factor-β did not differ among the three groups. See text for details. RA, right atrium; LA, left atrium; CTGF, connective tissue growth factor; COL1, collagen type 1; COL3, collagen type 3; FN1, fibronectin 1; TGF-β, transforming growth factor-β; MCP-1, monocyte chemotactic protein-1. *P< 0.05 vs. non-pacing group; †P< 0.05 vs. pacing control group.
Regarding inflammation-related molecules, the mRNA expression of TGF-β did not exhibit any difference among the three groups in both atria. The mRNA expression of MCP-1 did not differ among the three groups in the RA, but significant up-regulation in the pacing control group, and suppression of the up-regulation in the pacing + olmesartan groups were observed in the LA.
Fluorescent immunostaining of connective tissue growth factor
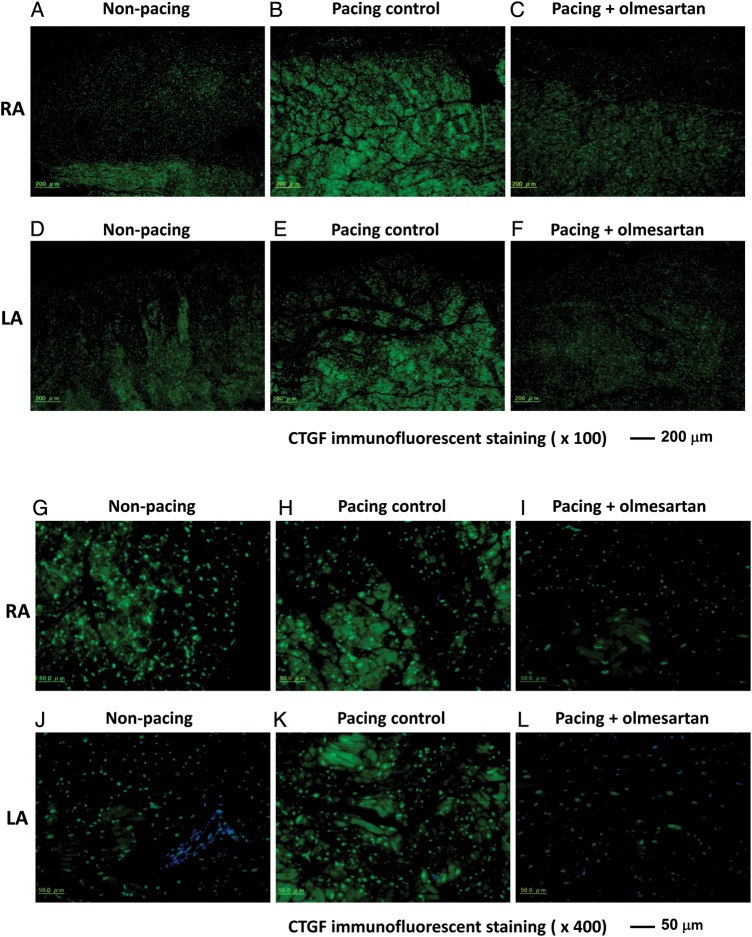
Figure 2 shows representative examples of the fluorescent immunostaining of CTGF in the atrial tissues of the three groups. The upper six panels show low magnification (×100) and the lower panels show high magnification (×400). Green indicates green fluorescence bound to CTGF protein, and blue shows nuclei. In comparison with the non-pacing group (Figure 2A, D, G, J), the pacing control group exhibited enhanced expression of CTGF (Figure 2B, E, H, K). In contrast, in the pacing + olmesartan group, the enhancement of CTGF expression was suppressed (Figure 2C, F, I, L). In low-magnification images, the enhancement of CTGF expression seemed to be more prominent in the RA than LA (Figure 2B and E). In the high-magnification images, the enhancement of CTGF expression was localized in the cytosol of the myocardium.
Figure 2.
Fluorescent immunostaining of connective tissue growth factor. These pictures show fluorescent immunostaining of connective tissue growth factor. The expression of connective tissue growth factor was localized in cytoplasm of the atrium. The expression level of CTGF was increased in the pacing control group in comparison with the non-pacing group, whereas this increase was suppressed in the pacing + olmesartan group. (A) and (G) Samples from RA in Non-pacing group; (B) and (H) RA in Pacing control; (C) and (I) RA in Pacing + olmesartan; (D) and (J) LA in Non-pacing; (E) and (K) LA in Pacing control; (F) and (L) LA in Pacing + olmesartan.
Western blot analysis of collagen type 1 and 3
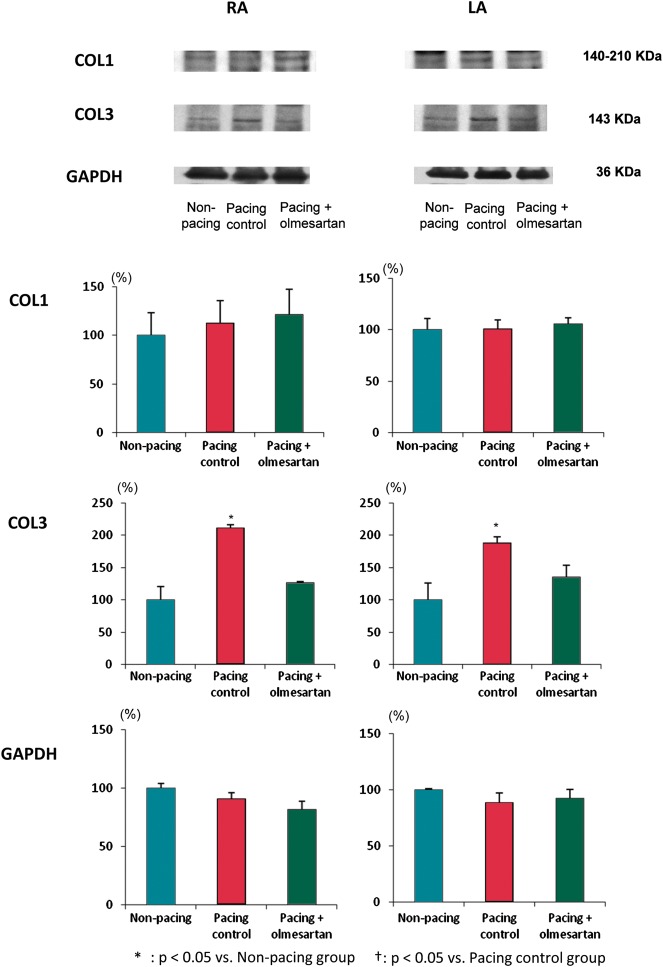
Figure 3 shows the results of the western blot analysis of COL1 and 3 and GAPDH protein levels. Figure 3A shows representative examples of the bands and Figure 3B shows a summary of all samples. GAPDH protein level was measured as an internal control and did not show any difference among the three groups. The protein level of COL1 did not show any difference among the three groups; however, the protein level of COL3 was up-regulated in the pacing control group in comparison with the non-pacing group in both the LA and RA, and this up-regulation was suppressed in the pacing + olmesartan group, almost completely in the RA and partially in the LA.
Figure 3.
Western blot analysis of protein levels of collagens. (A) Representative examples of the bands and (B) a summary of all samples. Glyceraldehyde-3-phosphate dehydrogenase protein level was measured as an internal control. The protein level of collagen type 1 did not show any difference, but collagen type 3 was up-regulated in the pacing control group in comparison with the non-pacing group, and this up-regulation was suppressed in the pacing + olmesartan group. See text for details. RA, right atrium; LA, left atrium; COL1, collagen type 1; COL3, collagen type 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P< 0.05 vs. non-pacing group; †P< 0.05 vs. pacing control group.
Discussion
In this study, we have demonstrated several interesting findings. First, 6-week rapid atrial pacing caused ECM synthesis in the atrial tissue as well as interstitial fibrosis in our canine AF model. Second, CTGF, a strong mediator of ECM synthesis, was up-regulated with the proliferation of collagen fibres. Third, TGF-β, a major mediator of CTGF, was not up-regulated in the phase of CTGF up-regulation in our AF model. Finally, olmesartan, an ARB, suppressed the up-regulation of CTGF, COL3, and atrial ECM remodelling at least in the sub-acute phase, i.e. 6-week continuous pacing, of our canine model of AF.
Role of connective tissue growth factor and extracellular matrix synthesis in atrial remodelling of atrial fibrillation
In the preceding publications, atrial interstitial fibrosis was considered to be involved as the mechanism for constructing the arrhythmogenic substrate for AF because atrial fibrosis may destroy cell-to-cell electrophysiological coupling.2 As a result, the conduction velocity in atrial tissue would be decreased and then AF inducibility would be increased due to shortening of the wavelength.1 In this mechanism, the role of extracellular signal-regulated kinase (ERK) has been emphasized because ERK would be activated by stimulation of angiotensin-receptor 1 and would cause cellular hypertrophy, degeneration, and apoptosis, resulting in tissue fibrosis.17 In this study, we did not examine the expression levels of phospho-Erk2, but several additional mediators may contribute to tissue fibrosis in the situation of AF. Connective tissue growth factor is a potent profibrotic factor and is a most common and important mediator of ECM synthesis and tissue fibrosis.3,4 The up-regulation of CTGF has been reported in various fibrotic diseases in many organs, including the lung, kidney, liver, skin, and even in fibrosis of the myocardium.5–9 In accordance with a report by Tsai et al.,18 tachycardia of atrial myocytes induced paracrine section of angiotensin II and reactive oxygen species, which in turn induced expression of CTGF and procollagen in cocultured atrial fibrosis. In this study, we have documented the up-regulation of CTGF in atrial tissue with enhanced expressions of collagen in the canine AF model. These results suggest that CTGF works as a mediator of the induction of tissue fibrosis even in AF. In contrast, Garrett et al.19 reported that CTGF alone is not sufficient to induce myofibroblast differentiation and collagen matrix contraction. On the contrary, Panek et al.20 demonstrated that cardiomyocyte-specific overexpression of CTGF does not prompt the development of fibrosis, and CTGF transgenic mice were protected from the AngII-dependent induction of fibrosis and showed preserved cardiac function under these conditions. In support of a ‘protective role’ of CTGF, Abreu et al.21 reported that CTGF via its CR–domain bind to and, thus, antagonize BMP-4 and TGF-β1. There is no evidence to prove causality between CTGF and atrial fibrosis. However, CTGF is one of the most important factors for tissue fibrosis and its role in atrial fibrosis and AF should be investigated in the future study.
Type of collagen in atrial fibrosis in the atrial fibrillation model
Xu et al.22 demonstrated that the volume fraction of COL1 was increased in atrial tissue in patients with persistent AF in comparison with that without AF in analysis of an extracted human heart, whereas no difference was observed in the volume fraction of COL3. This suggests that the main component of proliferated ECM protein in atrial tissue in patients with AF is COL1 instead of COL3; however, in this study, the result indicated that the predominant ECM protein was not COL1 but COL3, at least in the 6-week rapid atrial pacing of the canine AF model. This difference might be explained by the phase of myocardial remodelling. Human samples of atrial tissues were extracted from patients who underwent heart transplantation because of dilated cardiomyopathy and end-stage heart failure, so the myocardium is considered to suffer from long-term myocardial remodelling. In contrast, myocardial tissues in the canine heart should be basically normal and just suffer from relatively short-term, remodelling without heart failure. This speculation is supported by the following reports. In the early phase of established left ventricular hypertrophy, dominant up-regulation of COL3 and a decreased COL1/COL3 ratio were observed in ventricular tissues in an experimental hypertensive canine model, but COL3 did not increase in the late phase.23 Ventricular myocardial biopsies revealed a significantly higher proportion of COL3 in patients with diabetes in comparison with those without diabetes, whereas COL1 did not exhibit any difference;24 therefore, predominant proliferation of COL3 instead of COL1 is considered to appear in a relatively earlier phase of myocardial remodelling. In this study, COL1 and COL3 were up-regulated at the mRNA level, but only COL3 was increased at the protein level. The reason is not clear; however, we speculate that COL1 and COL3 protein levels depend on AF duration. Further examinations in the longer- or shorter-term rapid-pacing model should be performed.
Effect of angiotensin receptor blocker on extracellular matrix synthesis and the role of inflammation
In the preceding studies, the suppressive effect of ARB on atrial remodelling in the animal AF model was documented, which can be characterized by the suppression of interstitial fibrosis.25,26 This effect was partly explained by the suppression of ERK in intracellular signal transduction; however, to the best of our knowledge, this study is the first report to focus on the effect of ARB on CTGF under the condition of atrial remodelling for AF.
In this study, we demonstrated whether olmesartan, a strong ARB, suppressed the up-regulation of CTGF and atrial ECM proliferation as well as the progression of electrical remodelling25,26 in a canine AF model. This result may indicate a role of CTGF in atrial remodelling of the AF substrate, which was strongly suppressed by olmesartan. Connective tissue growth factor is a common mediator of tissue fibrosis and can be activated by various stimulations, such as mechanical stretch, inflammation, hyperoxidative stress, enhanced activation of the renin–angiotensin system, etc.3,6,8,27 The pathological condition of AF includes all such stimulations in the environment of atrial tissue, so CTGF can be activated by any of those factors. Atrial fibrillation is frequently associated with inflammatory conditions, such as cardiac surgery, myocarditis, and pericarditis,28 and Chung et al.29 have reported the elevation of C-reactive protein even in non-post-operative AF patients. By considering these reports and that inflammatory stimulation is the most common inducer of CTGF, it was expected that the main mechanism of CTGF up-regulation would be based on inflammatory stress in our experimental model of AF; however, in this study, the inflammatory condition was not obvious on histology and the expression of TGF-β was not increased even under AF conditions. In addition, the suppressive effect of olmesartan on the proliferation of collagen was parallel to its effect on CTGF but not on TGF-β. This clearly indicates that the suppressive effect of olmesartan on ECM proliferation appeared independently of its effect on inflammation.
Some studies reported increased tissue levels of TGF-β1 during AF or rapid pacing.18,30 Verheule et al.31 and Nakajima et al.32 have reported AF inducibility is increased in MHC-TGFcys33ser transgenic mice, which have increased fibrosis in the atrium caused by overexpression of TGF-β1. In contrast, our canine atrial rapid-pacing model showed no significant alteration of TGF-β expression. In accordance with a report by Adam et al.7, transgenic mice with overexpression of constitutively active V12Rac1, which AF develops at old age, showed no significant alteration of TGF-β expression. And they also reported that angiotensin II induces CTGF through activation of the small G protein Rac1 GTPase and nicotinamide adenine dinucleotide phosphate oxidase activity, and activated CTGF by Rac1 stimulation leads to interstitial fibrosis, contributing to the signal transduction of atrial structural remodelling.7 From the results of this study, the activation of angiotensin II type 1 receptor was considered to be the main mechanism of ECM synthesis and interstitial fibrosis in the sub-acute phase of atrial remodelling of AF.
Differences between right and left atrium
The effects of olmesartan on the canine model are different between RA and LA. Collagen type 1 expression was suppressed by olmesartan in RA but not in LA. In addition, the effect of olmesartan on CTGF, COL3, and FN1 expression was stronger in RA than that in LA. We previously reported atrial effective refractory period shortening in LA was greater than that in RA, and AF inducibility by burst pacing was higher in LA than that in RA in the same canine AF model.26 In addition, olmesartan suppressed increased AF inducibility especially in RA.26 We speculate that these different results for LA and RA relate to what AF normally originates in and depends on the LA.
Conclusions
CTGF and associated genes were markedly up-regulated in the atria with the appearance of fibrosis in this AF model. Because this up-regulation was independent of TGF-β gene expression and was suppressed by olmesartan, CTGF up-regulation was considered to be mediated by angiotensin II itself or via other factors, such as oxidative stress, in the sub-acute phase of this AF model.
Funding
Funding to pay the Open Access publication charges was provided by the first author.
Acknowledgement
The authors thank Tomonichi Kanabayashi (Biopathology Institute Co., Ltd) for excellent technical assistance with fluorescent immunostaining.
Conflict of interest: none declared.
References
- 1.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Rupérez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Perbal B. CCN proteins: multifunctional signaling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu BC, Sun J, Chen Q, Ma KL, Ruan XZ, Phillips AO. Role of connective tissue growth factor in mediating hypertrophy of human proximal tubular cells induced by angiotensin II. Am J Nephrol. 2003;23:429–37. doi: 10.1159/000074534. [DOI] [PubMed] [Google Scholar]
- 7.Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S, et al. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol. 2010;55:469–82. doi: 10.1016/j.jacc.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 8.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–56. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Yamashita T, Sekiguchi A, Tsuneda T, Sagara K, Takamura M, et al. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol. 2008;19:415–20. doi: 10.1111/j.1540-8167.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 10.Niwano S, Kojima J, Fukaya H, Sato D, Moriguchi M, Niwano H, et al. Arrhythmogenic difference between the left and right atria during rapid atrial activation in a canine model of atrial fibrillation. Circ J. 2007;71:1629–35. doi: 10.1253/circj.71.1629. [DOI] [PubMed] [Google Scholar]
- 11.Niwano S, Ortiz J, Abe H, Gonzalez X, Rudy Y, Waldo AL. Characterization of the excitable gap in a functionally determined reentrant circuit. Studies in the sterile pericarditis model of atrial flutter. Circulation. 1994;90:1997–2014. doi: 10.1161/01.cir.90.4.1997. [DOI] [PubMed] [Google Scholar]
- 12.Moriguchi M, Niwano S, Yoshizawa N, Kitano Y, Kojima J, Inuo K, et al. Inhomogeneity in the appearance of electrical remodeling during chronic rapid atrial pacing: evaluation of the dispersion of atrial effective refractoriness. Jpn Circ J. 2001;65:335–40. doi: 10.1253/jcj.65.335. [DOI] [PubMed] [Google Scholar]
- 13.Kojima J, Niwano S, Moriguchi M, Ikeda K, Inuo K, Saito J, et al. Effect of pilsicainide on atrial electrophysiologic properties in the canine rapid atrial stimulation model. Circ J. 2003;67:340–6. doi: 10.1253/circj.67.340. [DOI] [PubMed] [Google Scholar]
- 14.Moriguchi M, Niwano S, Yoshizawa N, Kojima J, Inuo K, Izumi T. Verapamil suppresses the inhomogeneity of electrical remodeling in a canine long-term rapid atrial stimulation model. Pacing Clin Electrophysiol. 2003;26:2072–82. doi: 10.1046/j.1460-9592.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 15.Fukaya H, Niwano S, Satoh D, Masaki Y, Niwano H, Kojima J, et al. Inhomogenic effect of bepridil on atrial electrical remodeling in a canine rapid atrial stimulation model. Circ J. 2008;72:318–26. doi: 10.1253/circj.72.318. [DOI] [PubMed] [Google Scholar]
- 16.Wakisaka Y, Niwano S, Niwano H, Saito J, Yoshida T, Hirasawa S, et al. Structural and electrical ventricular remodeling in rat acute myocarditis and subsequent heart failure. Cardiovasc Res. 2004;63:689–99. doi: 10.1016/j.cardiores.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–77. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CT, Tseng CD, Hwang JJ, Wu CK, Yu CC, Wang YC, et al. Tachycardia of atrial myocytes induces collagen expression in atrial fibroblasts through transforming growth factor β1. Cardiovasc Res. 2011;89:805–15. doi: 10.1093/cvr/cvq322. [DOI] [PubMed] [Google Scholar]
- 19.Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–16. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- 20.Panek AN, Posch MG, Alenina N, Ghadge SK, Erdmann B, Popova E, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One. 2009;4:e6743. doi: 10.1371/journal.pone.0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–8. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 23.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circulation Res. 1988;62:757–65. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–6. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 26.Fukaya H, Niwano S, Niwano H, Masaki Y, Kiryu M, Hirasawa S, et al. Combined effects of up- and downstream therapies on atrial fibrillation in a canine rapid stimulation model. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.12.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–84. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 28.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 29.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias. Inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 30.On YK, Jeon ES, Lee SY, Shin DH, Choi JO, Sung J, et al. Plasma transforming growth factor-β1 as a biochemical marker to predict the persistence of atrial fibrillation after the surgical maze procedure. J Thorac Cardiovasc Surg. 2009;137:1515–20. doi: 10.1016/j.jtcvs.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Verheule S, Sato T, Everett T, 4th, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima H, Nakajima HO, Salcher O, Dittiè AS, Dembowsky K, Jing S, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–9. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]