Abstract
When consumed separately, whey protein (WP) is more rapidly absorbed into circulation than casein (Cas), which prompted the concept of rapid and slow dietary protein. It is unclear whether these proteins have similar metabolic fates when coingested as in milk. We determined the rate of appearance across the splanchnic bed and the rate of disappearance across the leg of phenylalanine (Phe) from coingested, intrinsically labeled WP and Cas. Either [15N]Phe or [13C-ring C6]Phe was infused in lactating cows, and the labeled WP and Cas from their milk were collected. To determine the fate of Phe derived from different protein sources, 18 healthy participants were studied after ingestion of one of the following: 1) [15N]WP, [13C]Cas, and lactose; 2) [13C]WP, [15N]Cas, and lactose; 3) lactose alone. At 80–120 min, the rates of appearance (Ra) across the splanchnic bed of Phe from WP and Cas were similar [0.068 ± 0.010 vs. 0.070 ± 0.009%/min; not significant (ns)]. At time 220–260 min, Phe appearance from WP had slowed (0.039 ± 0.008%/min, P < 0.05) whereas Phe appearance from Cas was sustained (0.068 ± 0.013%/min). Similarly, accretion rates across the leg of Phe absorbed from WP and Cas were not different at 80–120 min (0.011 ± 0.002 vs. 0.012 ± 0.003%/min; ns), but they were significantly lower for WP (0.007 ± 0.002%/min) at 220–260 min than for Cas (0.013 ± 0.002%/min) at 220–260 min. Early after meal ingestion, amino acid absorption and retention across the leg were similar for WP and Cas, but as rates for WP waned, absorption and assimilation into skeletal muscle were better retained for Cas.
Keywords: phenylalanine, splanchnic bed, muscle bed, protein turnover
body protein homeostasis does not seem to be related entirely to the absolute protein intake (23). For a given amount of protein intake, the rate of the amino acid absorption may be important when a mixed meal is consumed, because individuals who digest protein more slowly and gradually tend to have better amino acid retention than those who digest the same protein more rapidly (13). To study the metabolic effects of a more physiological protein intake by a discrete bolus, a non-steady-state technique developed originally for studies of carbohydrate metabolism (24) was adopted to allow simultaneous measurements of rates of appearance (Ra) of an essential amino acid from exogenous and endogenous sources (5). Although a free amino acid tracer may be admixed into a meal, such free amino acids are very rapidly and transiently absorbed compared with amino acids in the ingested intact protein, resulting in serious methodological problems (5). For this reason, a method utilizing intrinsically labeled protein was developed (4). Using this method, the two most abundant protein fractions in bovine milk, whey protein (WP) and casein (Cas), were shown to cause markedly different metabolic responses at the whole body level when ingested separately (3). When given separately, compared with Cas, amino acids from WP briefly and rapidly appeared systemically, stimulating a brief increase in whole body protein synthesis rates and a marked increase in amino acid oxidation and thus a less favorable amino acid balance (3, 9, 10, 18). Cas ingestion, on the other hand, induced a late and prolonged plateau of hyperaminoacidemia associated with an inhibition of protein breakdown. In analogy with rapidly and slowly absorbed dietary carbohydrates, these protein fractions can therefore be considered to be fast and slow proteins (3).
However, a human diet typically consists of a mix of several proteins as well as other macronutrients. The metabolic effects of such mixed meals containing more than one protein fraction may be different from the effects of separately ingested protein fractions. For example, it is unclear whether the markedly different metabolic effects of “slow” and “fast” protein fractions in bovine milk are also seen when such proteins are ingested simultaneously. It may be hypothesized, in light of the data cited above, that the two protein fractions would be absorbed in sequence when ingested together. Thus, a rapid and substantial influx of amino acids from WP may be expected to occur before a more prolonged appearance of amino acids from Cas. This may lead to an initial stimulation of whole body protein synthesis and a later and sustained inhibition of protein breakdown. Furthermore, it remains unclear whether what is observed at the whole body level reflects what occurs in two main body compartments, skeletal muscle and splanchnic beds, that are key players of amino acid and protein metabolism. We investigated in healthy volunteers who ingested a mixture of intrinsically labeled WP and Cas fractions the impact of these two different protein fractions in milk upon whole body and leg vs. splanchnic protein metabolism.
MATERIALS AND METHODS
Materials
[15N]phenylalanine [99 molar% excess (MPE)], [13C-ring C6]phenylalanine (99 MPE), and [15N]lysine(99 MPE) were obtained from Cambridge Isotope Laboratories (Andover, MA). Isotopic and chemical purity of the tracers were checked by gas chromatography-mass spectrometry (GC-MS). All solutions for bovine or human intravenous use were prepared in sterile conditions, tested for sterility and pyrogenicity, and filtered through 0.22-μm filters before use.
Dietary milk containing proteins intrinsically labeled with [15N]phenylalanine and [13C-ring C6]phenylalanine separately was obtained as described previously (4). Briefly, two lactating cows were intravenously infused over 36 h with large amounts of either [15N]phenylalanine or [13C-ring C6]phenylalanine. Milk was collected from each cow during and for 24 h following the infusions. The milk was heated at 40°C and transformed to skim milk before being microfiltrated on a 0.1-μM pure size diameter membrane, as described previously (4). Native micellar casein was obtained, and the resulting milk was ultrafiltrated for whey protein purification. After freeze-drying, native micellar casein powder was obtained, packed, and stored at 2°C (4). Microfiltration and ultrafiltration offered the opportunity to purify both main groups of milk proteins while preserving their native properties. Thus four batches of intrinsically labeled protein fractions were produced: two batches of WP labeled with either [15N]phenylalanine or [13C-ring C6]phenylalanine and two batches of Cas labeled with either [15N]phenylalanine or [13C-ring C6]phenylalanine. These labeled milk protein fractions were mixed with unlabeled proteins to obtain proteins of appropriate enrichment. Those mixtures will be referred to as [15N]WP, [13C]WP, [15N]Cas, and [13C]Cas. The final tracer isotopic enrichments were checked in each batch by GC-MS after hydrolysis by the method described below for plasma amino acids (14.63, 17.82, 16.57, and 19.67 MPE, respectively).
From these protein fractions and from lactose, the following isocaloric [7.4 kcal/kg fat-free mass (FFM)] experimental liquid meals were prepared: 1) 0.625 g/kg FFM each of [15N]WP and [13C]Cas and 0.9 g/kg FFM lactose ([15N]WP/[13C]Cas group), 2) 0.625 g/kg FFM each of [13C]WP and [15N]Cas and 0.9 g/kg FFM lactose ([13C]WP/[15N]Cas group), and 3) 1.85 g/kg FFM of lactose only (lactose group). Thus these first two meals contained an identical amount of protein, with different isotopomer labeling of each of the two protein fractions to exclude an isotope effect. The third meal served as an isocaloric control meal with no protein. The different isotope labels for WP and casein in the same meal would allow us to trace the fate of phenylalanine, a representative essential to amino acid derived from the two different protein sources (WP and CAS).
Each experimental meal was admixed in 5 ml/kg FFM water. After ingestion of the meal, the container used was rinsed with 100 ml of water, which was then also ingested to minimize residual proteins in the container.
By design, fractional synthesis rates of the two protein groups are expected to be the same because the protein content is the same in both groups, and therefore, kinetics across various tissue compartments are critical to evaluate the separate effects of CAS and WP from the same meal.
Participants
Recruitment of study volunteers began in July 2000, and the last recruitment was in April 2001. Eighteen healthy participants were studied. Participants were studied on a single occasion in which they received one of the three experimental meals (subject characteristics; Table 1). All participants had a normal physical examination and no concurrent disease or regular medication. All participants had normal circulating concentrations of hemoglobin, electrolytes, and fasting glucose; all subjects had normal hepatic and renal function tests. Written, informed consent was obtained from each participant prior to enrollment. The study protocol was reviewed and approved by the Institutional Review Board of the Mayo Foundation.
Table 1.
Demographics of subjects ingesting isocaloric meals containing protein and lactose ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups) or lactose alone (lactose group)
| [15N]WP/[13C]Cas | [13C]WP/[15N]Cas | Lactose | P Value | |
|---|---|---|---|---|
| Sex (M/F) | 2/4 | 2/4 | 2/4 | 0.00 |
| Age, yr | 25.7 (2.2) | 21.8 (0.7) | 24.0 (1.4) | 0.26 |
| BMI, kg/m−2 | 24.2 (1.4) | 22.7 (1.1) | 23.9 (1.2) | 0.68 |
| FFM, kg | 44.0 (3.1) | 45.8 (4.4) | 50.2 (5.6) | 0.62 |
Values are nos. or means (SE); n = 6 in each group.
WP, whey protein; Cas, casein; M, male; F, female; BMI, body mass index; FFM, fat-free mass. One-way ANOVA.
Weight and height of all participants were measured, and body composition was measured by dual-energy X-ray absorptiometry (DPX-L; Lunar, Madison, WI) prior to the metabolic studies.
Experimental Protocol
A weight-maintaining diet (20% protein, 50% carbohydrate, and 30% fat) was provided by the Clinical Research Unit metabolic kitchen to all subjects for 3 days prior to the study. All study participants maintained their usual level of physical activity prior to studies. Subjects were admitted to the General Clinical Research Center at 5 PM the evening prior to the study. A meal was served at 6 PM, after which subjects fasted overnight, with the exception of a standardized snack (5.5 kcal/kg) at 10 PM and water ad libitum. Subjects rested in the supine position for the duration of the metabolic study.
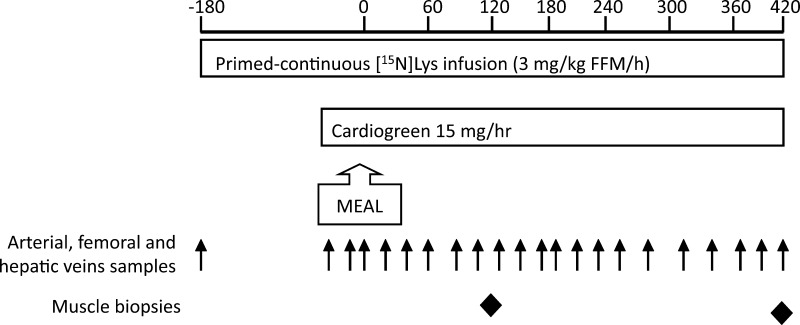
The study design is depicted in Fig. 1. At time point −180 min (∼0600, 3 h before meal) and after collection of baseline venous blood samples, a primed (3 mg/kg FFM) continuous (3 mg·kg FFM−1·h−1) infusion of l-[15N]lysine was started and maintained until +420 min. At −120 min, catheter sheaths were placed in the right femoral artery and vein. A catheter was then positioned in the hepatic vein by an interventional radiologist under fluoroscopic guidance. The correct position of the hepatic vein catheter was confirmed by nonionic contrast injection. Femoral artery and vein catheters were inserted through the catheter sheaths. A slow infusion of normal saline maintained patency of each catheter. An intravenous catheter in a superficial forearm vein was used for all tracer infusions.
Fig. 1.
Study outline. FFM, fat-free mass.
Lysine was chosen as the intravenous amino acid tracer rather than phenylalanine because a third phenylalanine tracer ([ring-2H5]phenylalanine) was given intravenously in pilot studies, but the precision of the mass spectrometry measurements was impaired significantly when three isotopomers of phenylalanine were present in significant abundances (data on file), and therefore, this third tracer was not used. Lysine is an essential amino acid that is not (or only minimally) metabolized in muscle (26), and therefore, it is especially suitable for usage in studies over the skeletal muscle bed.
The femoral artery sheath was used for continuous infusion of indocyanine green (Cardio-Green, 2.5 mg/ml and 6 ml/min, starting at time point −50 min; Beckton-Dickinson Microbiology Systems, Cockeysville, MD) to measure plasma flow over the leg and splanchnic region using the dye dilution technique.
The subjects were then assigned randomly to one of the three experimental meals, and the allotted meal was ingested at time point 0 min within 20 min.
The femoral artery and vein catheter sheaths and the hepatic vein catheter were used to sample blood for measurement of regional substrate turnover, as detailed below. Blood samples were collected simultaneously from the three vascular catheters at −20, −15, −10, and −5 min and then every 20 min from +20 to +420 min (Fig. 1). Muscle biopsies from the vastus lateralis of the quadriceps femoris muscle were obtained at +120 and +420 min under infiltration anesthesia (2% lidocaine), using a 6-mm Bergstrom needle (Popper and Sons, New Hyde Park, NY) with wall suction. One biopsy was taken from each limb.
The plasma supernatant was separated and stored at −80°C until batch analysis. Muscle tissue was immediately frozen by immersion in liquid nitrogen and then stored at −80°C until batch analysis.
Analytical Methods
Blood glucose was measured by the glucose oxidase method using a glucose analyzer (Beckman Instruments, Fullerton, CA). Plasma insulin was measured by a chemiluminescent sandwich assay (based on a kit from Sanofi Diagnostics, Chaska, MN), and glucagon was measured by a radioimmunoassay technique (based on kits from Diagnostic Products, Los Angeles, CA). Indocyanine green concentration was measured using spectrophotometry, and arterial plasma amino acid concentrations were measured using reversed-phase high-performance liquid chromatography (15). Arterial, femoral, venous, and hepatic venous plasma enrichment levels of [15N]phenylalanine, [13C-ring C6]phenylalanine, and l-[15N]lysine were determined using GC-MS. The amino acids from 100-μl plasma samples were isolated by ion exchange chromatography and dried. All standards and samples were derivatized as their N-heptafluorobutyryl methyl esters and separated on a 30 m × 0.25 mm id × 0.25 μm DB5MS column (J & W Chromatography, Folsom, CA) at a constant flow of helium (1.1 ml/min) with the following temperature programming: 120°C initially, which was then ramped to 210°C at 25°C/min, 225°C at 10°C/min, 255°C at 25°C/min, 265°C at 10°C/min, 300°C at 25°C/min, and finally 325°C at 10°C/min and held for 2 min. Under positive ion chemical ionization conditions, using ammonia as reactant gas, fragment ions were monitored at mass-to-charge ratio (m/z) of 393, 394, and 399 for phenylalanine, [15N]phenylalanine, and [13C-ring C6]phenylalanine, respectively, and m/z 570 and 571 for lysine and [15N]lysine, respectively, using an Agilent Technologies 5973N GC-MS (Agilent Technologies, Avondale, CA) to determine the isotopic enrichment. The concentrations of phenylalanine and lysine were determined from a separate aliquot of 40 μl of plasma using [U-13C9]phenylalanine and [U-13C6,15N2]lysine, respectively, as internal standards. The extraction and derivatization of the amino acids were as described above, and the extracts were analyzed on a Waters Quattro Micro GC-MS-MS (Manchester, UK) under negative ion chemical ionization conditions, using isobutane as reactant gas. The amino acids were separated on the same column as described above. Fragment ions were monitored under multiple reaction monitoring conditions following transitions at m/z 355–284 and 364–293 for phenylalanine and [U-13C9]phenylalanine, respectively, and m/z 532–512 and 540–520 for lysine and [U-13C6,15N2]lysine, respectively. Area ratios of analyte to internal standard were compared against calibration curves for the two species.
Muscle Protein (Mixed Proteins, Mitochondrial Proteins, and Sarcoplasmic Proteins) Amino Acid Tracer Enrichments
A 5% homogenate using 100–150 mg of muscle was prepared in an ice-cold buffer containing 0.25 M sucrose, 2 mM EDTA, and 10 mM Tris·HCl (pH 7.4) using a Potter-Elvehjem homogenizer (Wheaton, Millville, NJ). The mitochondria were isolated by centrifuging the homogenate at 600 g (4°C), followed by a 7,000-g (4°C) spin of the obtained supernatant. The sarcoplasmic protein was obtained by centrifuging the 7,000-g supernatant at 100,000 g for 60 min. The sarcoplasmic protein contained in supernatant was precipitated using 1 M perchloric acid and centrifuged at 1,500 g. The sarcoplasmic protein pellet was washed twice with perchloric acid. The pellet from the 7,000-g spin, containing the mitochondria, was washed twice using an ice-cold buffer containing 100 mM KCl, 5 mM EGTA, 5 mM MgSO4, 1 mM ATP, and 50 mM Tris·HCl (pH 7.4) and spinning at 7,000 g (4°C). The final mitochondrial pellet was suspended in the sucrose-EDTA-Tris·HCl buffer for further analyses.
Mixed muscle protein was hydrolyzed using 6 M HCl (110°C for 18 h). The amino acids in the hydrolyzates were derivatized as their N-heptafluorobutyryl methyl ester and analyzed on a Finnigan Delta-S GC-combustion-isotope ratio mass spectrometer (Finnigan-MAT, Bremen, Germany) for 13C enrichment in [13C-ring C6]phenylalanine and 15N enrichment in [15N]phenylalanine and [15N]lysine. The two labels were measured in separate runs.
Tissue fluid tracer enrichments were also measured. Tissue fluid consists of ∼85% intracellular fluid and 15% extracellular fluid. Tissue fluid amino acids were extracted from muscle tissue using perchloric acid and analyzed as their t-butyldimethylsilyl ester derivative.
Calculations
Leg and splanchnic plasma flow.
Plasma flow over the leg was calculated by the indicator dilution principle (1). Splanchnic plasma flow was calculated from the rate of splanchnic extraction of the indicator (6).
Ra of exogenous phenylalanine in the systemic circulation.
The Ra of phenylalanine from each of the two protein fractions WP and Cas could be followed semiquantitatively by the appearance of their respective intrinsic phenylalanine tracer in the hepatic vein. Because the total Ra of phenylalanine was not known (the intravenous tracer being [15N]lysine rather than a phenylalanine tracer; see above), the transposition of Steele's equation by Proietto et al. (24) could not be used to quantify the rate of appearance of exogenous phenylalanine, as was done in previous studies (3, 5). Instead, the Ra of phenylalanine from each of the two ingested protein fractions (Ra Pheexo) in the hepatic vein was calculated as the difference between the efflux from and the influx into the splanchnic region of phenylalanine originating from that protein fraction. Thus the Ra Pheexo calculated is a measurement of the Ra in the systemic circulation (hepatic vein) rather than the rate of absorption from the gastrointestinal lumen into the body. The difference between these two rates may be substantial since it has been shown that splanchnic first-pass extraction of dietary phenylalanine is ∼30% in young healthy subjects (21, 27).
The efflux from and influx into the splanchnic region of total phenylalanine was calculated in the traditional way:
where Chv and Ca are plasma phenylalanine concentrations in the hepatic vein and femoral artery (in μmol/ml), respectively, and Fspl is the measured splanchnic plasma flow (in ml/min).
The proportion of Phe efflux that originated from each of the intrinsically labeled protein fractions (pe; be it from absorption during that same transsplanchnic passage or during an earlier passage) equals the ratio of hepatic venous enrichment to the enrichment in that protein fraction:
where Ehv is the tracer enrichment (in MPE) in the hepatic venous plasma and Em is the enrichment in the labeled protein fraction. Similarly, the proportion of Phe influx into the splanchnic region (pi) that originated from each of the two protein fractions is calculated:
where Ea is the phenylalanine enrichment in the femoral artery.
The Ra of phenylalanine from a protein fraction is the difference between splanchnic region efflux and influx of phenylalanine from that protein fraction:
This calculation assumes steady-state conditions, which were not present after meal ingestion taken as a whole. Therefore, periods of apparent steady-state phenylalanine concentrations and enrichment levels were selected for analysis on the basis of a horizontal slope of these data over time. In addition, rates were calculated on the basis of the areas under the curve of plasma concentrations and enrichments during an “early” and a “late” time period. The time period of 0–80 min was selected a priori as the early time period based on the timing of the physiological effects of WP (3). The remainder of the study (100–420 min) was designated as the late time period.
Because the abundance of phenylalanine differs somewhat between WP and Cas (4.4 vs. 4.8 g/100 g protein fraction powder, respectively) (9), the Ra in the systemic circulation of phenylalanine from WP and Cas are presented as the percentage of the ingested amount of phenylalanine from that protein fraction that appeared in the systemic circulation per minute rather than the absolute amount. The phenylalanine abundance in WP and Cas quoted was obtained by a collaborative group analyzing the same batch of milk protein powder (9). Therefore, the final equation is
where Relative Ra Pheexo is the portion of phenylalanine from each protein fraction that appears in the systemic circulation (in %/min), and m is the mass of phenylalanine ingested in each protein fraction (in mg).
Rate of retention of exogenous phenylalanine across the leg.
The rate of retention across the leg of phenylalanine from each of the two ingested protein fractions (Rd Pheexo) was calculated using the principle outlined above for the splanchnic region. Thus
where Rd Pheexo is the rate of disappearance over the leg of phenylalanine originating from each of the two protein fractions (in μmol/min), Fleg is the measured plasma flow across the leg (in ml/min), Efv is the enrichment of the corresponding tracer (in MPE) in the femoral venous plasma, and Cfv is femoral vein plasma phenylalanine concentration in (in μmol/ml). Again, this parameter was calculated during the selected time periods of apparent steady-state conditions and during the early and late time periods.
Again, the Rd over the leg of phenylalanine from WP and Cas are presented as the percentage of the ingested amount of phenylalanine from that protein fraction that appeared in the systemic circulation per minute rather than the absolute amount (see above). Therefore, the final equation is
where relative Rd Pheexo is the portion of phenylalanine from each protein fraction that disappears across the leg (in %/min).
Amino acid kinetics across skeletal muscle bed.
Lysine Ra and Rd over the leg were calculated on the basis of leg plasma flow, lysine concentrations, and [15N]lysine enrichment in the artery and femoral vein, as described previously (22) during the selected time periods of apparent steady-state conditions. Protein turnover in the leg is assumed to represent mostly skeletal muscle, although it is recognized that drainage from skin, fat, and other tissues may contribute to femoral venous drainage.
Fractional synthesis rates in skeletal muscle.
Fractional synthesis rates of mixed muscle protein, sarcolemmal protein, and mitochondrial protein were calculated using the traditional equation adapted for the current protocol:
where FSR is fractional synthesis rate (in %/h), Et420 and Et120 are the isotopic enrichments of the intravenous tracer ([15N]lysine) in the protein at time points +420 and +120 min, respectively, Ep is the mean enrichment in the precursor pool, and the denominator 5 is the number of hours between the two muscle biopsies in the present protocol. As expected, enrichments in free tissue fluid (a typical surrogate measurement of aminoacyl-tRNA) were not stable during the study (data not shown), and therefore, the average isotopic enrichment in plasma from the femoral vein for the 5-h period was used as the precursor pool isotopic enrichment that has been shown to reasonably reflect enrichments in aminoacyl-tRNA (8) but benefited from multiple sampling times that could be averaged.
We also calculated FSR separately, using [15N]phenylalanine and [13C6]phenylalanine as tracers that allow the estimation of FSR or incorporation of phenylalanine to muscle protein.
Statistical analysis.
Results are expressed as means (SE). Differences between and within groups for single variables were evaluated by Student's t-test. Differences between and within groups for repeated measurements over time were evaluated by two-way mixed-effects analysis of variance with a level for statistical significance at P < 0.05. Post hoc testing was performed using Tukey's honestly significantly different (HSD) test where appropriate. All statistical analyses were performed using JMP 7.0 for Mac OS X (SAS Institute, Cary, NJ).
RESULTS
Arterial Concentrations of Hormones and Substrates
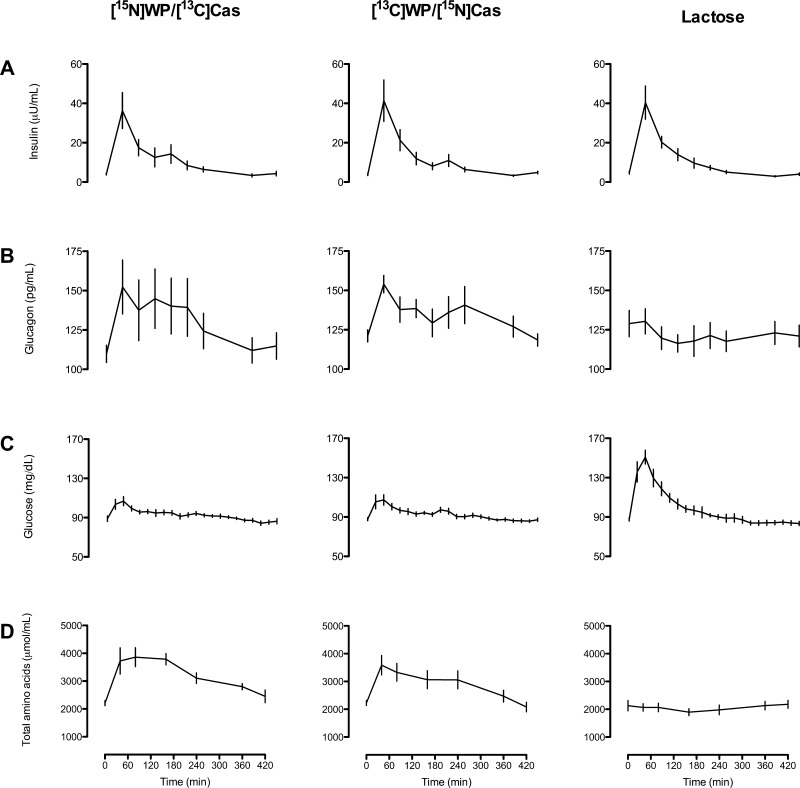
Plasma insulin concentrations increased similarly following ingestion of experimental meals in all three groups (Fig. 2A), whereas glucagon increased in the [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups only (group × time interaction, P = 0.0013; Fig. 2B).
Fig. 2.
Arterial plasma concentrations of insulin (A), glucagon (B), glucose (C), and total amino acids (D) in subjects ingesting isocaloric meals containing protein and lactose ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively) or lactose alone (lactose group; n = 6). Group × time interactions: P = 1.0 (A), P = 0.0013 (B), P < 0.0001 (C), and P < 0.0001 (D). WP, whey protein; Cas, casein.
Lactose ingestion caused a rapid and marked increase in plasma glucose concentrations, whereas the increase was moderate and brief in the [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups (group × time interaction, P < 0.0001; Fig. 2C). Conversely, plasma concentrations of total amino acids and essential amino acids (EAA) increased rapidly in the [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups, whereas lactose ingestion alone did not alter amino acid concentrations (group × time interaction, P < 0.0001; Fig. 2D). Neither total nor EAA levels reached the baseline at 420 min.
Regional Plasma Flow and Phenylalanine Concentrations
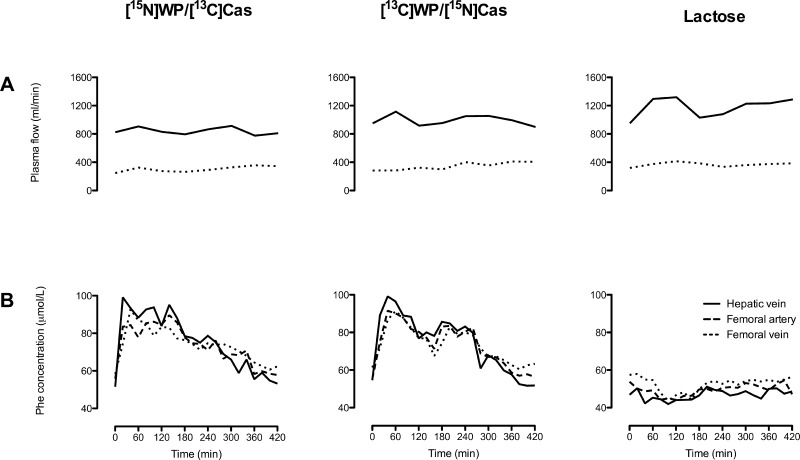
Throughout the study, the measured plasma flow rates over the splanchnic bed and leg did not differ significantly between the three groups (group × time interaction, P = 0.92 and 0.62, respectively). Neither was there any statistically significant change in plasma flow rates over time within groups (Fig. 3A).
Fig. 3.
Regional plasma flow rates (A) and phenylalanine concentrations (B) in subjects ingesting isocaloric meals containing protein and lactose ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively) or lactose alone (lactose group; n = 6). Group × time interactions: P = 0.92 for hepatic vein plasma flow and P = 0.62 for femoral vein plasma flow (A); P < 0.0001 for phenylalanine concentrations in the hepatic vein, femoral artery, and femoral vein (B).
At baseline, plasma Phe concentrations were highest in the femoral vein and lowest in the hepatic vein in all groups, reflecting a net uptake of Phe in the splanchnic region at basal and a net Phe release from the leg (Fig. 3B). In the Lactose group, there was no significant change in Phe concentrations following meal ingestion. In the [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups, concentrations in all three sampling sites increased rapidly after ingestion of the experimental meal, with the highest levels in the hepatic vein, returning to baseline by time point +420 min (group × time interaction, P < 0.0001 for all 3 sampling sites).
Regional Phenylalanine Kinetics
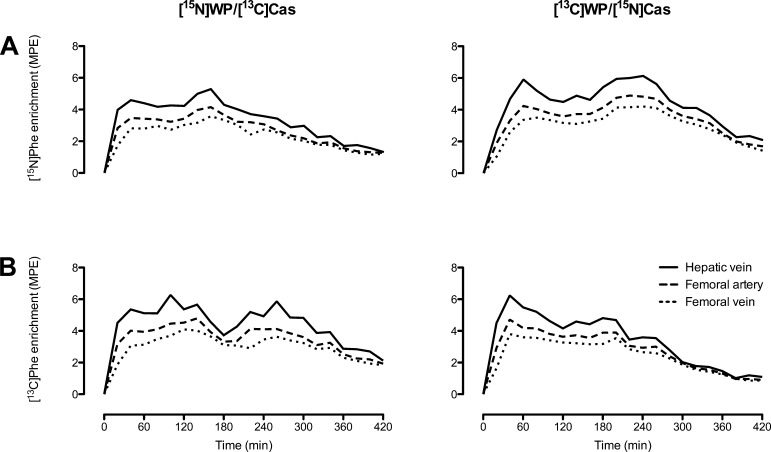
Isotopic enrichments of [15N]Phe and [13C]Phe were higher in hepatic venous than in arterial plasma following ingestion of the protein meals (Fig. 4, A and B), indicating absorption of labeled phenylalanine from the meals. The [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups differed significantly in the changes over time in [15N]Phe in the femoral artery and vein (group × time interaction, P = 0.029 and 0.043, respectively). Enrichments of [15N]Phe at the hepatic vein were not statistically different between groups (group × time interaction, P = 0.096), and neither were enrichments of [13C]Phe at the femoral artery, femoral vein, or hepatic vein (group × time interactions, P = 0.18, 0.074, and 0.062, respectively).
Fig. 4.
Regional plasma isotopic enrichment of tracers [15N]Phe (A) and [13C]Phe (B) in subjects ingesting isocaloric meals containing differentially labeled milk protein fractions ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively). Group × time interactions: for [15N]Phe enrichments, P = 0.096 at the hepatic vein, P = 0.029 at the femoral artery, and P = 0.043 at the femoral vein (A); for [13C]Phe enrichments, P = 0.062 at the hepatic vein, P = 0.18 at the femoral artery, and P = 0.074 at the femoral vein (B).
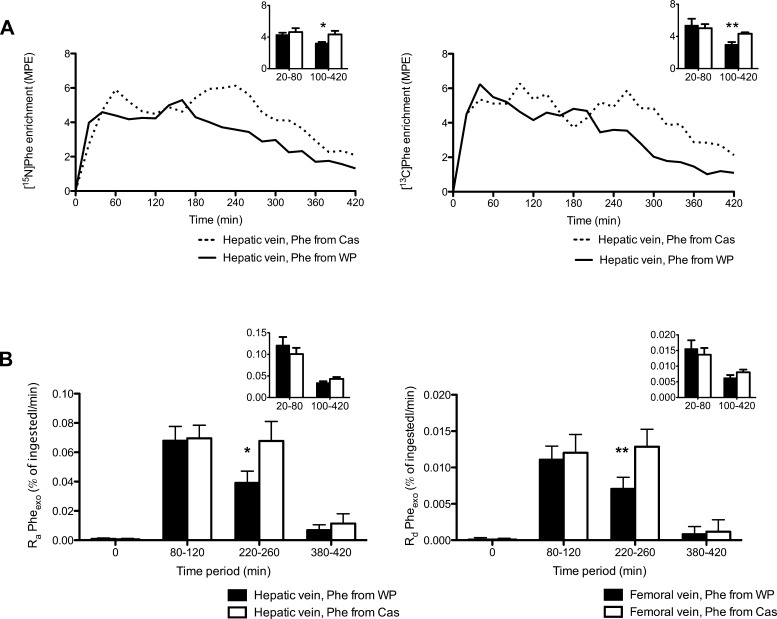
However, further comparisons of the time course of hepatic venous isotopic enrichment and the calculated Ra of phenylalanine from the two protein fractions were performed and showed differences for WP vs Cas. In Fig. 5A, isotopic enrichments of phenylalanine originating from WP and Cas are plotted. The tracer [15N]Phe originated from WP in the [15N]WP/[13C]Cas group and from Cas in the [13C]WP/[15N]Cas group (Fig. 5A, left). Conversely, [13C]Phe originated from WP in the [13C]WP/[15N]Cas group and from Cas in the [15N]WP/[13C]Cas group (Fig. 5A, right). Whereas the differences in hepatic venous [15N]Phe and [13C]Phe enrichments between groups over time failed to attain statistical significance (group × time interaction, P = 0.096 and 0.074, respectively), the areas under the curves were different between groups for both isotope enrichments (P = 0.046 and 0.014, respectively). Furthermore, average enrichments during the early (0–80 min) and late (100–420 min) phases demonstrate significant differences between groups in the late phase only (Fig. 5A, insets). These data give a qualitative indication that systemic appearance of phenylalanine from Cas was sustained for a longer time period than phenylalanine from WP.
Fig. 5.
A: comparison of hepatic venous enrichments of phenylalanine tracers from WP and Cas in subjects ingesting isocaloric meals containing differentially labeled milk protein fractions ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively). Group × time interactions: P = 0.096 for [15N]Phe (left) and P = 0.074 for [13C]Phe (right). Insets: mean enrichments during “early” (20–80 min) and “late” (100–420 min) periods. Student's t-tests for comparisons between groups of [15N]Phe enrichments: P = 0.55 during early period, *P = 0.041 during late period; for [13C]Phe enrichment, P = 0.71 during early period, **P = 0.0031 during late period. B, left: mean rates of appearance (Ra) across the splanchnic region of phenylalanine from WP and Cas in subjects ingesting isocaloric meals containing differentially labeled milk protein fractions ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively) during time periods 0, 80–120, 220–260, and 380–420 min. Student's t-test between WP and Cas: P = not significant during the 80- to 120-min period, *P = 0.0498 during 220- to 260-min period. Inset: mean rates calculated from areas under curve of raw data during early (20–80 min) and late (100–420 min) phases. No significant differences between WP and Cas; Student's t-test. B, right: mean rates of retention across the leg of phenylalanine from the 2 protein fractions during time periods 0, 80–120, 220–260, and 380–420 min. Student's t-test between WP and Cas, P = 0.6 during the 80- to 120-min period, **P = 0.002 during the 220- to 260-min period. Inset: mean rates calculated from areas under curve of raw data during “early” (20–80 min) and “late” (100–420 min) phases. No significant differences between WP and Cas; Student's t-test.
For calculation of Ra Pheexo, the following periods of apparent steady-state conditions for phenylalanine concentrations and tracer enrichments were selected: 80–120, 220–260, and 380–420 min. The slopes of femoral arterial and hepatic venous plasma concentrations and isotopic enrichments plotted against time during those periods were not significantly different from 0 (data not shown). To address the main two hypotheses of this study, Ra Pheexo for WP and Cas were compared during the time periods 80–120 and 220–260 min only.
The Ra in the hepatic vein of phenylalanine from WP and Cas are shown in Fig. 5B, left. During the period of 80–120 min, the Ra in the hepatic vein of phenylalanine from WP (Ra PheWP) were similar to Ra PheCas [0.068 (0.010) vs. 0.070 (0.009) %ingested amount/min, not significant (NS)]. In contrast, during the later period of 220–260 min, Ra PheWP was approximately half of Ra PheCas [0.039 (0.008) vs. 0.068 (0.013) %ingested amount/min, P = 0.0498]. Comparing the average rates based on the areas under curve of the raw data during the entire early (20–80 min) and late (100–420 min) phases did not demonstrate significant differences between the two protein fractions but did display an apparent trend (P = 0.14 and 0.06, respectively; Fig. 5B, left inset).
In similarity to their appearance in the hepatic vein, the rates of retention across the leg of phenylalanine from WP (Rd PheWP) were similar to Rd PheCas during the period of 80–120 min [0.011 (0.002) vs. 0.012 (0.003), P = 0.60] but approximately half of Rd PheCas during the period of 220–260 min [0.007 (0.002) vs. 0.013 (0.002), P = 0.049; Fig. 5B, right]. The rates calculated from the areas under curve of the raw data no longer revealed differences between the protein fractions when considered in the context of early (P = 0.17) or late phases (P = 0.09) (Fig. 5B, right inset).
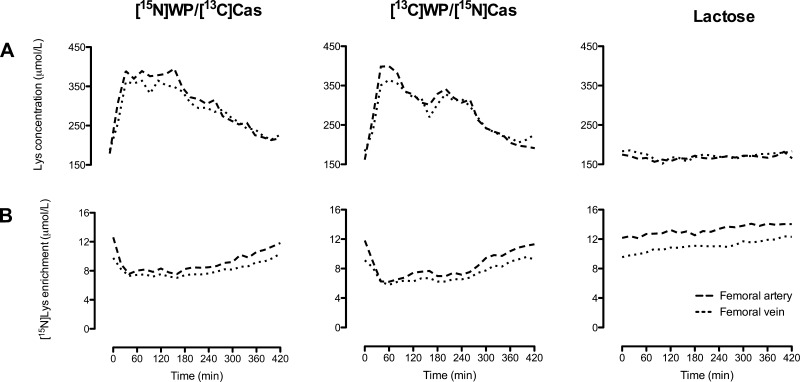
Lysine Kinetics Across the Leg
Ingestion of the protein/lactose meals more than doubled arterial plasma lysine concentrations at time point +40 min, after which they slowly returned toward baseline, whereas lactose ingestion had no effect on lysine concentrations (group × time interaction for arterial and venous concentrations, P < 0.001; Fig. 6A). Plasma [15N]lysine enrichments decreased at time point +20 min after protein meal ingestion, remaining depressed until 280 min, whereas again lactose had no effect on arterial and venous enrichments (group × time interaction, P < 0.001; Fig. 6B).
Fig. 6.
Plasma concentrations of lysine (A) and isotopic enrichment of [15N]lysine (B) at the femoral artery and vein in subjects ingesting isocaloric meals containing protein and lactose ([15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups; n = 6 and 6, respectively) or lactose alone (lactose group; n = 6). Group × time interactions: P < 0.001 for arterial and venous plasma concentrations of lysine, P < 0.001 for arterial and venous isotopic enrichment of [15N]lysine.
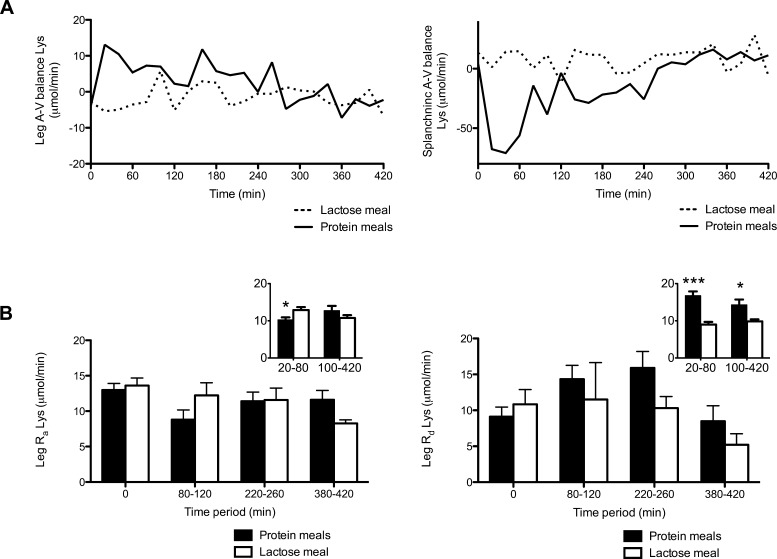
For calculation of regional arteriovenous (A-V) lysine balances and lysine kinetics across the leg, as well as FSR in muscle, the [15N]WP/[13C]Cas and [13C]WP/[15N]Cas groups were pooled into a protein group since no differences in raw data between these two groups were seen. The A-V balance of lysine across the leg was significantly different between the protein and the lactose groups (group × time interaction, P = 0.0057; Fig. 7A, left), with a significantly higher level in the protein group very soon after ingestion of the experimental meals, indicating net lysine uptake across the leg (Tukey's HSD test, P < 0.05 at 20 min). Conversely, A-V balance across the splanchnic region was significantly lower in the protein group soon after meal ingestion, indicating net lysine release from the splanchnic region (group × time interaction, P < 0.0001; Tukey's HSD, P < 0.05 at 20–60 min; Fig. 7A, right).
Fig. 7.
A: arteriovenous balances of lysine across leg (left) and splanchnic region (right) in subjects ingesting isocaloric meals containing milk protein and lactose (protein meals; n = 12) or lactose alone (lactose meals; n = 6). Group × time interactions: P = 0.0057 across splanchnic region, P < 0.0001 across leg. B, left: rates of appearance of lysine (Ra Lys) across leg in subjects ingesting isocaloric meals containing milk protein and lactose (protein group; n = 12) or lactose alone (lactose group; n = 6). Student's t-tests between groups, P = 0.16 during 80- to 120-min period, P = 0.94 during 220- to 260-min period. Inset: rates calculated on the areas under curve of the raw data during early (20–80 min) and late (100–420 min) periods. Student's t-test, protein vs. lactose groups; *P = 0.025. B, right: rates of disappearance of lysine (Rd Lys) across leg. Student's t-test between groups; P = 0.62 during 80- to 120-min period, P = 0.063 during 220- to 260-min period. Inset: rates calculated on the areas under curve of the raw data during early (20–80 min) and late (100–420 min) phases. Student's t-test, protein vs. lactose groups; ***P < 0.0001, *P = 0.011.
The rates of disappearance of lysine (Rd Lys) across the leg did not differ between the protein and the lactose groups during the 80- to 120- or the 220- to 260-min periods of pseudo-steady state (P = 0.62 and 0.063, respectively; Fig. 7B, right). However, the protein group had significantly higher Rd Lys across the leg calculated from the areas under curve of raw data during both the early and the late phase (P < 0.0001 and 0.011, respectively; Fig. 7B, right inset).
The rates of appearance of lysine across the leg were also similar during the 80- to 120- and the 220- to 260-min periods of pseudo-steady state (P = 0.16 and 0.94, respectively; Fig. 7B, right). The protein group had significantly lower rates (i.e., suppression of proteolysis) during the early phase compared with the lactose group, whereas the rates during the late phase were similar (P = 0.025 and 0.19, respectively; Fig. 7B, right inset).
Tracer Incorporation Into Skeletal Muscle Protein
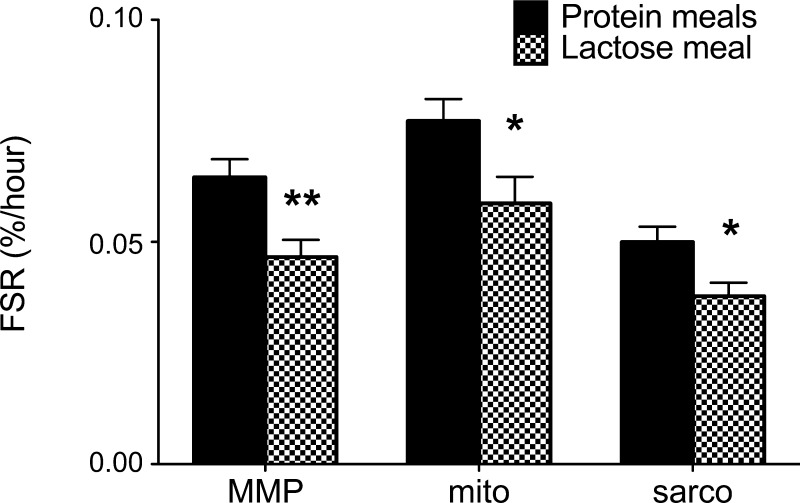
The protein group had a significantly higher FSR (based on [15N]lysine) in mixed muscle protein, mitochondrial protein, and sarcoplasmic protein during the period between the two biopsies (120 and 420 min) compared with the lactose group (P = 0.0078, 0.034, and 0.017, respectively; Fig. 8).
Fig. 8.
Fractional synthesis rates (FSR) in mixed muscle protein (MMP), mitochondrial protein (mito), and sarcoplasmic protein (sarco) during the period between the 2 biopsies (120 and 420 min) in subjects ingesting isocaloric meals containing milk protein and lactose (protein groups; n = 12) or lactose alone (lactose groups; n = 6). *P < 0.05 and **P < 0.01, Student's t-test between groups.
As expected, FSR calculation based on the tracers derived from Cas ([15N] and [13C6]phenylalanine) and WP ([15N] and [13C6]phenylalanine) showed no differences (mixed muscle protein FSR-based casein-derived labels = 0.084 ± 0.006% and WP-derived labels = 0.081 ± 0.006%). Also, no differences were noted for FSR values calculated similarly for mitochondrial protein and sarcoplasmic proteins (data not shown). FSR values are estimated from the rate of incorporation of labeled amino acid from plasma to muscle proteins. Therefore, irrespective of the source of plasma phenylalanine or lysine (intravenous or derived from the orally ingested protein), the estimated FSR values should show similar results.
DISCUSSION
The present study demonstrates, for the first time, a differential time course of systemic appearance of amino acids from two coingested milk protein fractions in different body compartments. Furthermore, whereas prior work comparing WP and Cas separately (3, 9, 10, 18) showed differences at the whole body retention level, using arteriovenous sampling we now show that those differences, which are maintained when coingested, are localized to skeletal muscle. Whereas phenylalanine derived from ingested WP and Cas appeared equally rapidly in the hepatic vein at 2 h following protein ingestion, at 4 h appearance of phenylalanine from WP was approximately half that of Cas. Furthermore, regional disposition of amino acids from coingested protein fractions was studied for the first time. Whereas there were no differences early after protein ingestion, phenylalanine from Cas was retained in the leg at double the rate of that from WP at 4 h, mirroring the hepatic vein appearance data. Thus, Cas supports muscle protein synthesis for a longer period than does WP.
The rate of appearance in the systemic circulation of amino acids from intrinsically labeled, and separately ingested, WP and Cas has been reported previously in humans (3, 9, 10, 18). In the first report on the topic of which we are aware (3), the investigators administered intrinsically labeled WP and Cas separately and showed that whole body rate of appearance of leucine from WP and Cas differed. Leucine from ingested WP appeared in arterialized venous blood rapidly but briefly, and this was associated with an increase in whole body protein synthesis rates during the first 180 min, with no significant effect seen on whole body protein breakdown. In contrast, Cas ingestion resulted in a slower leucine appearance sustained over several hours, with a smaller increase in whole body protein synthesis but a significant and sustained inhibition of breakdown. The net whole body postprandial leucine balance was more positive after ingestion of Cas than WP, and others subsequently corroborated this apparent superiority of Cas (9, 18, 26). Here, with proteins coingested, we show that phenylalanine appearance into the hepatic vein (and therefore, systemic circulation very soon after) is similar between proteins initially rather than higher, as expected from the work in which proteins were ingested separately. However, importantly, consistent with the previous whole body study, the Cas phenylalanine outflow into circulation was sustained, whereas WP phenylalanine outflow from the liver moved back toward baseline sooner. Therefore, the present study demonstrates that Cas leads to more sustained systemic amino acid delivery and, therefore, more sustained delivery to the skeletal muscle. In the present study, we administered an average of 58 g of combined Cas and WP, which is ∼65% of the daily protein intake of Americans in the age group that we studied (∼1.92 g/kg FFM). This represents a commonly ingested protein load, assuming that >60% of protein is consumed during the main meal of the day (usually dinner).
From previous work comparing WP and Cas (3, 9, 10, 18), it appeared that the amino acid absorption rate was the determinant of the partitioning between oxidation and retention of dietary protein. Previous studies have shown that protein hydrolysate is accompanied by accelerated in vivo digestion and absorption (17). Thus, gastric emptying rates could ultimately determine protein metabolism. In rats (11) and humans (19, 20), it was shown that gastric emptying is slower for Cas than for WP. The difference may be related to Cas having opioid-like activity in the gut (11) or to the Cas clotting in the stomach and thus being handled as a solid rather than liquid (19, 20). Our results here show that Cas is digested more gradually than WP even when coingested with WP. If Cas were acting as an opioid and slowing the gut, this would likely affect the absorption of all food in the stomach, not just selectively Cas. Therefore, with regard to the theories that could explain why Cas is absorbed slowly, that regarding clotting seems more likely than that in which the entire gastric emptying slows due to a receptor-mediated signaling effect such as that associated with opioid receptors.
The results from the present study administering the mixture of Cas and WP are consistent with the previous whole body study results showing an early increase in appearance rate of amino acid from WP but not sustained like Cas (18). Even when given as a mixture, Cas exhibits a more sustained effect on plasma amino acid levels and muscle protein accretion compared with WP. Moreover, the rates of appearance of amino acids from Cas and WP were not different at the early phase following ingestion. It appears that, at least acutely, Cas, isolated or as the main protein of unfractionated total milk protein, is superior for nitrogen retention compared with WP. Our results demonstrate that absorption profile differences between WP and Cas are retained even when the proteins are mixed together. It is important to know that adding WP to Cas neither mitigates the desirable sustained slow absorption of the Cas nor completely slows down the absorption of WP.
It appears from the present study that the appearance of amino acids into systemic circulation could determine muscle protein synthesis. In the present study, we combined differentially labeled milk protein fractions that allowed us to trace the systemic appearance and retention across the leg of phenylalanine from Cas and WP when both were administered simultaneously. Cas, which was absorbed more slowly, appears to have been less extracted by the splanchnic bed in the present study, thus becoming more available to the peripheral tissues. The results demonstrated clearly that both Cas and WP increase muscle protein accretion, but the effect is more pronounced with Cas. This sustained effect of Cas is related to the sustained increase in amino acids derived from Cas. Although it has been reported that the effects of amino acid supply upon muscle protein synthesis wane fairly quickly (2), the present study demonstrated sustained retention (likely protein synthesis) of circulating amino acids into the limb from Cas still at 4 h after meal ingestion. The differences may be related to methodological differences, including the use of an amino acid mixture (2) that is likely to be absorbed rapidly, as opposed to Cas, which releases amino acids in a more sustained manner. We also infused [15N]lysine intravenously as an independent free amino acid tracer to determine the effect of the protein- and lactose-only meals on muscle protein synthesis and breakdown across the leg. The protein meals suppressed leg protein breakdown soon after the meal (20–80 min) and stimulated synthesis in this early time period and continued it at 100–420 min after ingestion, effects not seen with the ingestion of an isocaloric amount of lactose alone. Regional phenylalanine and lysine kinetics were quantified only at specific time points, selected on the basis of their apparent steady-state conditions, required for regional kinetic calculations: 80–120, 220–260, and 380–420 min. It is acknowledged that, although the slopes over time of concentrations and enrichments were not different from zero during the selected and brief time periods, this method provides data that can be seen as semiquantitative rather than an absolute measurement of an exact rate. The need to have a steady-state situation to perform calculations of amino acid kinetics across regions did not offer the opportunity to measure phenylalanine kinetics across leg and splanchnic beds. We calculated regional phenylalanine and lysine kinetics on the basis of areas under curve of the raw data (concentrations, enrichments, and plasma flow) during the entire early (20–80 min) and late (100–420 min) phases. However, coupled with the raw data shown in detail, the differing time courses of hepatic vein appearance as well as disappearance of phenylalanine from the respective protein fractions across the leg, it is clear that WP and Cas, when coingested, have differing effects on amino acid appearance in the systemic circulation and protein accretion.
The present study did not address whether administering these two proteins at a lower amount will have a similar effect as reported here, in which we administered almost 65% of daily protein intake as a single meal, nor did it address how results might differ from cases in which WP and casein are given separately. An additional point to consider is how to interpret our results in the context of how dietary selections affect physiology; WP is only 20% of the protein in milk (7, 12), and the independent effects of casein and WP at a similar composition as in milk were not tested in the present study. Some recent discussions of milk proteins in the context of their ability to improve muscle mass or mitigate muscle loss in disease states have focused upon the benefits of whey (14, 16, 25). However, we propose that greater attention should be placed upon combined administration of both Cas and WP. Although whey may be a higher-quality protein as assessed by the amino acid composition, such as branched chain amino acid content, the time course of absorption should actually be considered, and Cas may offer some advantages in that respect. These results provide a framework for the next pursuit, which may be to determine what the best formulation of dietary proteins will be for preventing catabolism and promoting anabolism for various age groups.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-41793, R01-AG-09531, and UL1-RR-024150 and a David Murdock-Dole Professorship (K. S. Nair); a research grant and Hirsch fellowship from the Karolinska Institutet and a grant from Swedish Society of Medicine (M. Soop); and Grant T32-DK-07352 (G. C. Henderson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., V.N., and K.S.N. did the conception and design of the research; M.S., G.C.H., Y.B., and G.C.F. performed the experiments; M.S., V.N., G.C.H., Y.B., G.C.F., and K.S.N. analyzed the data; M.S., V.N., G.C.H., G.C.F., and K.S.N. interpreted the results of the experiments; M.S. prepared the figures; M.S., G.C.H., G.C.F., and K.S.N. drafted the manuscript; M.S., V.N., G.C.H., Y.B., G.C.F., and K.S.N. edited and revised the manuscript; M.S., V.N., G.C.H., Y.B., G.C.F., and K.S.N. approved the final version of the manuscript.
REFERENCES
- 1.Andres R, Zierler KL, Anderson HM, Stainsby WN, Cader G, Ghrayyib AS, Lilienthal JL., Jr Measurement of blood flow and volume in the forearm of man; with notes on the theory of indicator-dilution and on production of turbulence, hemolysis, and vasodilatation by intra-vascular injection. J Clin Invest 33: 482–504, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94: 14930–14935, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boirie Y, Fauquant J, Rulquin H, Maubois JL, Beaufrère B. Production of large amounts of [13C]leucine-enriched milk proteins by lactating cows. J Nutr 125: 92–98, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrère B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol Endocrinol Metab 271: E1083–E1091, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bradley SE, Ingelfinger FJ, Bradley GP, Curry JJ. The estimation of hepatic blood flow in man. J Clin Invest 24: 890–897, 1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerbulis J, Farrell HM., Jr Composition of milks of dairy cattle. I. Protein, lactose, and fat contents and distribution of protein fraction. J Dairy Sci 58: 817–827, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin's anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 291: E729–E736, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballèvre O, Beaufrère B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280: E340–E348, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O, Beaufrère B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 549: 635–644, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J Nutr 120: 252–257, 1990 [DOI] [PubMed] [Google Scholar]
- 12.DePeters EJ, Taylor SJ, Baldwin RL. Effect of dietary fat in isocaloric rations on the nitrogen content of milk from Holstein cows. J Dairy Sci 72: 2949–2957, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Fereday A, Gibson NR, Cox M, Pacy PJ, Millward DJ. Variation in the apparent sensitivity of the insulin-mediated inhibition of proteolysis to amino acid supply determines the efficiency of protein utilization Clin Sci (Lond) 95: 725–733, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care 11: 40–44, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hill DW, Walter FH, Wilson TD, Stuart JD. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem 51: 1338–1341, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Hoppe C, Andersen GS, Jacobsen S, Mølgaard C, Friis H, Sangild PT, Michaelsen KF. The use of whey or skimmed milk powder in fortified blended foods for vulnerable groups. J Nutr 138: 145S–161S, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 90: 106–115, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lacroix M, Bos C, Leonil J, Airinei G, Luengo C, Dare S, Benamouzig R, Fouillet H, Fauquant J, Tome D, Gaudichon C. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am J Clin Nutr 84: 1070–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Mahé S, Benamouzig R, Gaudichon C, Huneau JF, De Cruz I, Rautureau J, Tomé D. Nitrogen movements in the upper jejunum lumen in humans fed low amounts of casein or beta-lactoglobulin. Gastroenterol Clin Biol 19: 20–26, 1995 [PubMed] [Google Scholar]
- 20.Mahé S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, Gaussergès N, Rautureau J, Tomé D. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein. Am J Clin Nutr 63: 546–552, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol Endocrinol Metab 264: E109–E118, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Nair KS, Schwartz RG, Welle SL. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol Endocrinol Metab 263: E928–E934, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Pacy PJ, Price GM, Halliday D, Quevedo MR, Millward DJ. Nitrogen homeostasis in man: the diurnal responses of protein synthesis and degradation and amino acid oxidation to diets with increasing protein intakes. Clin Sci (Lond) 86: 103–116, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Proietto J, Rohner-Jeanrenaud F, Ionescu E, Terrettaz J, Sauter JF, Jeanrenaud B. Non-steady-state measurement of glucose turnover in rats by using a one-compartment model. Am J Physiol Endocrinol Metab 252: E77–E84, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 12: 66–71, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Van Hall G, Saltin B, Wagenmakers AJ. Muscle protein degradation and amino acid metabolism during prolonged knee-extensor exercise in humans. Clin Sci 97: 557–567, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999 [DOI] [PubMed] [Google Scholar]