Abstract
Normal pregnancy is associated with uterine relaxation to accommodate the stretch imposed by the growing fetus; however, the mechanisms underlying the relationship between pregnancy-associated uterine stretch and uterine relaxation are unclear. We hypothesized that increased uterine stretch during pregnancy is associated with upregulation of matrix metalloproteinases (MMPs), which in turn cause inhibition of myometrium contraction and promote uterine relaxation. Uteri from virgin, midpregnant (day 12), and late-pregnant rats (day 19) were isolated, and myometrium strips were prepared for measurement of isometric contraction and MMP expression and activity using RT-PCR, Western blot analysis, and gelatin zymography. Oxytocin caused concentration-dependent contraction of myometrium strips that was reduced in mid- and late-pregnant rats compared with virgin rats. Pretreatment with the MMP inhibitors SB-3CT (MMP-2/MMP-9 Inhibitor IV), BB-94 (batimastat), or Ro-28–2653 (cipemastat) enhanced contraction in myometrium of pregnant rats. RT-PCR, Western blot analysis, and gelatin zymography demonstrated increased mRNA expression, protein amount, and activity of MMP-2 and MMP-9 in myometrium of late-pregnant>midpregnant>virgin rats. Prolonged stretch of myometrium strips of virgin rats under 8 g basal tension for 18 h was associated with reduced contraction and enhanced expression and activity of MMP-2 and MMP-9, which were reversed by MMP inhibitors. Concomitant treatment of stretched myometrium of virgin rats with 17β-estradiol (E2), progesterone (P4), or E2+P4 was associated with further reduction in contraction and increased MMP expression and activity. MMP-2 and MMP-9 caused significant reduction of oxytocin-induced contraction of myometrium of virgin rat. Thus, normal pregnancy is associated with reduced myometrium contraction and increased MMPs expression and activity. The results are consistent with the possibility that myometrium stretch and concomitant increase in sex hormones during pregnancy are associated with increased expression/activity of specific MMPs, which in turn inhibit uterine contraction and promote uterine relaxation.
Keywords: estrogen, progesterone, matrix metalloproteinases, uterus
the uterus is sensitive to various mechanical and chemical stimuli, and its response varies in the nonpregnant and pregnant state and during parturition. The nonpregnant uterus contracts in response to stretch, but during pregnancy mechanisms are in play to stabilize the excitability of the myometrial muscles and maintain mechanical quiescence to allow sufficient time for the development of the fetus before it is delivered during labor (43). The uterus dramatically expands in volume during pregnancy. Hypertrophy and distension of the pregnant uterus provide sufficient space for the developing and growing fetus. At term, other changes occur to counteract the factors that maintain uterine quiescence, increase electrical excitability of myometrial muscles, and coordinate contractions between uterine smooth muscle bundles and thereby ensure successful parturition. The mechanisms that facilitate uterine quiescence during pregnancy and enhance contractility at term are not fully understood (7).
Several steroid and neurohypophysial hormonal changes occur during the menstrual cycle, pregnancy, and parturition, and could influence the uterine structure and mechanical properties. Plasma 17β-estradiol (E2) levels increase during the proliferative phase of the menstrual cycle. A decrease in plasma E2 is associated with endometrial shedding, and an increase in plasma progesterone (P4) occurs during the luteal phase of the menstrual cycle (30). Plasma E2 and P4 markedly increase during pregnancy (52) and could contribute to the pregnancy-associated uterine relaxation (15, 45). Changes in neurohypophysial hormones such as oxytocin also affect uterine contraction. Oxytocin is a nonapeptide produced largely by the hypothalamus-pituitary and other tissues including the corpus luteum (65, 66), adrenal medulla (1), and placenta (16). In the nonpregnant state, plasma levels of oxytocin are relatively low and do not appear to change during the menstrual cycle. During pregnancy, plasma oxytocin levels demonstrate progressive increases starting at week 12 of gestation (60). Oxytocin is important for cervical dilation before birth and causes uterine contractions during the second and third stages of labor.
In addition to the role of steroid and neurohypophysial hormones, uterine enzymes and proteins could modulate its structure and function. Matrix metalloproteinases (MMPs) are a group of zinc-dependent proteases that degrade the extracellular matrix (49). MMP-2 (gelatinase A), MMP-9 (gelatinase B), and MMP-7 are expressed in the uterus and could play a role in the endometrial tissue remodeling during normal estrous and menstrual cycles and pregnancy (53, 62, 70), as well as in the endometrial changes associated with menstrual disorders and endometriosis (35, 58). MMP-2 and -9 have been localized in the bovine endometrium and myometrium during pregnancy (25, 53, 62) and in uterine natural killer cells in early human pregnancy (41). Also, trophoblast- and vascular smooth muscle-derived MMP-12 mediate elastolysis and uterine spiral artery remodeling during pregnancy (22). MMP-2 and MMP-9 could also play a role in degradation of the uterine proteins and remodeling of the cervical extracellular matrix (33), and increased MMP-2 expression may precede the final ripening process and collagen denaturation of the cervix in late pregnancy (63).
MMPs are regulated by several factors including mechanical stretch and sex hormones. MMP-2 expression increases in mechanically stretched skeletal muscle fibers (39). Also, we have recently shown that protracted increases in vein wall tension are associated with increased MMP-2 and -9 expression and decreased contraction of rat inferior vena cava (48, 50, 51). Other studies have suggested that uterine tissue remodeling and endometrium shedding during menstruation involve E2-induced changes in MMPs activity (54, 56, 70). Progestins may also regulate important factors for the establishment and maintenance of endometrial lesions partly by affecting MMPs expression (40). However, the interrelationship between myometrium stretch, sex hormones, and MMPs expression in the uterine relaxation during pregnancy is unclear.
The objective of this study was to test the hypothesis that uterine stretch during pregnancy is associated with increased expression of MMPs, which in turn inhibit myometrium contraction and promote uterine relaxation. We used the rat uterus to investigate whether: 1) different stages of pregnancy are associated with decreased myometrium contraction and enhanced MMPs expression/activity, 2) prolonged myometrium stretch is associated with decreased contraction and increased MMP expression/activity, 3) sex hormones augment the effects of prolonged stretch on uterine relaxation and MMP expression, and 4) specific MMPs inhibit myometrium contraction.
MATERIALS AND METHODS
Animals and Tissue Preparation
Virgin, midpregnant (12 days of gestation, mid-Preg) and late-pregnant (19 days of gestation, late-Preg) Sprague-Dawley rats (12 wk of age, 250 to 350 g weight) were purchased from Charles River Laboratories (Wilmington, MA). The rats were housed in the animal facility and maintained on ad libitum standard rat chow and tap water in 12:12-h light-dark cycle. All experiments on virgin rats were conducted during estrus to control for reproductive cycle and endocrine confounders. The estrus cycle was determined by taking a vaginal smear with a Pasteur pipette daily in the morning (36). An estrus smear primarily consisted of anucleated cornified squamous cells (68), and this was confirmed prior to all experimentations. On the day of experiment, the rats were euthanized by inhalation of CO2. Death was judged by cessation of breathing and heart beats. The abdominal cavity was opened, and the uterus was rapidly excised, placed in Krebs solution, and carefully dissected and cleaned of connective tissue under microscopic visualization. The virgin uterus was cut open, and the pregnant uterus was cut open and the placentae and pups were removed. The uterus was then portioned along its longitudinal axis into 7-mm long, 3-mm wide strips. We did not attempt to separate the circular muscle layer from the longitudinal muscle or to remove the endometrium lining from the uterine strip. Experiments were performed on two to four uterine strips from each rat, and cumulative data from four to six rats were collected. All procedures followed the guidelines of the Institutional Animal Care and Use Committee at Harvard Medical School.
Isometric contraction. Using silk string, one tie was made 1 mm from each end of the myometrium strip, leaving a functional 5-mm-long strip between two string loops. Each myometrium strip was suspended in a water-jacketed tissue bath filled with 50 ml Krebs solution bubbled with 95% O2/5% CO2 at 37°C. One end of the uterine strip was attached to a fixed glass hook at the bottom of the tissue bath, and the other end was connected to a Grass force transducer (model FT03; Astro-Med, West Warwick, RI). Changes in isometric contraction were recorded on Grass polygraph (model 7D; Astro-Med).
To construct basal tension-contraction relationship, uterine strips of virgin rats were stretched under increasing basal tension 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 g, maintained under each basal tension for 30 min and then stimulated with 96 mM KCl. These experiments demonstrated maximal KCl contraction at 2 g basal tension and significant decrease in contraction at 8 g basal tension.
Uterine strips of virgin and pregnant rats were stretched under control of 2 g basal tension for 30 min. To determine the control contraction properties, uterine strips were stimulated twice with 96 mM KCl, and each control KCl contraction was followed by three washes in Krebs, 10 min each. The uterine strips were then stimulated with increasing concentrations of oxytocin (10−11 to 10−7 M) and the contractile response was recorded. To correct for differences in the myometrium thickness, contraction measurements were normalized to the weight of the myometrium strip and presented as gram per milligram tissue weight. The contractile response at each oxytocin concentration was also presented as percentage of maximum oxytocin contraction. The individual oxytocin concentration-response curves were analyzed using a nonlinear regression curve (best-fit sigmoidal dose-response curve; Sigmaplot), and the effective concentration that produced half of the maximal contraction was presented as pEC50 (−log M).
We have previously demonstrated that prolonged increases in rat inferior vena cava wall tension for 18 to 24 h was associated with decreased contraction (31, 50). To demonstrate the effect of prolonged stretch on myometrium contraction, uterine strips of virgin rats were subjected to either control (2 g basal tension) or high (8 g tension) for 18 h in tissue culture medium. On the next day, the bathing solution was changed to Krebs solution, and oxytocin contraction was measured. To determine the effects of sex hormones, uterine strips of virgin rats were treated with E2 (10−6 M), P4 (10−5 M), or both, and their effects on uterine contraction were measured.
To determine whether the changes in uterine contraction associated with pregnancy or prolonged stretch involve changes in MMP activity, contraction experiments were repeated in the presence of the MMP inhibitors SB-3CT (MMP-2/MMP-9 inhibitor IV), BB-94 (batimastat), or Ro-28–2653 (cipemastat). SB-3CT is a potent, selective, slow-binding and mechanism-based inhibitor of the gelatinases MMP-2 (Ki ∼ 13.9 nM) and MMP-9 (Ki ∼ 600 nM) (5). The interaction of SB-3CT with MMP-2 and -9 follows a slow-binding inhibition similar to that observed with TIMP-1 and TIMP-2, and ultimately results in covalent modification of the enzyme in the active site (42). SB-3CT directly binds to the catalytic zinc ion of MMP-2 and changes the conformational environment around the active site zinc ion back to that of the proenzyme (26). BB-94 is a potent broad spectrum MMP inhibitor (IC50 = 3, 4, 4, 6, and 20 nM for MMP-1, -2, -9, -7 and -3, respectively) (13, 64). Other studies have reported BB-94 IC50 values of 25, 32, 67, 27, 23, 19, and 29 nM for MMP-1, -2, -3, -8, -9, -14 (membrane-type 1-MMP, MT-1-MMP), and -16 (MT-3-MMP), respectively (21). Ro-28–2653 {5-biphenyl-4-yl-5-[4-(4-nitro-phenyl)-piperazin-1-yl]-pyrimidine-2,4,6-trione} is a new zinc chelator of MMPs, with higher selectivity for MMP-2, -9, -14, and -8 (IC50 = 10, 12, 10, and 12 nM, respectively), compared with MMP-3 and -1 (IC50 = 1200 and 16000 nM, respectively) (21, 28, 34). We have previously shown that these MMP inhibitors at 10−6 M concentration reverse the inhibitory effects of MMP-2 on vein contraction (48). Therefore, the present experiments were conducted using these inhibitors at 10−6 M concentration. In the initial contraction experiments, SB-3CT was more effective than BB-94 and Ro-28–2653 in causing contraction in uterine segments of pregnant rats. Therefore, further experiments to study the effects of sex hormones and stretch on uterine contraction and MMP expression/activity were conducted using only two MMP inhibitors: SB-3CT as a potent inhibitor and Ro-28–2653 for comparison.
Also, to test the direct effects of MMPs, uterine strips of virgin rats were precontracted with oxytocin (10−7 M), MMP-2 or MMP-9 (1 μg/ml) was added, and the effect on uterine contraction was measured.
Real-time RT-PCR.
RNA was isolated from the uterine strips using RNeasy Fibrous Tissue Mini Kit (QIAGEN, Valencia, CA). Total RNA (1 μg) was used for RT to synthesize single-strand complimentary DNA (cDNA) in 15- to 33-μl reaction mixture following the instructions for the First-Strand cDNA Synthesis Kit (Amersham Biosciences, Pittsburgh, PA). Next, 2 μl of the cDNA dilution (1:5 for MMP-2, MMP-7, and MMP-9 and 1:25 for α-actin) of the RT product was applied to 20 μl of RT-PCR reaction mixture. Quantification of gene expression was performed using a real-time RT-PCR machine (model Mx4000; Multiplex Quantitative PCR System, Stratagene, La Jolla, CA), published oligonucleotide primers for MMP-2, MMP-7, and MMP-9 (Integrated DNA Technologies, Coralville, Iowa), and iQSYBR-Green Supermix, which employs the fluorescein compound SYBR-Green for amplicon detection (Bio-Rad, Hercules, CA). α-Actin primer was included in the RT-PCR reaction as internal standard to normalize the results (31).
The following primers were used. MMP-2: forward, 5′-CATCGCTGCACCATCGCCCATCATC-3′; reverse, 5′-CCCAGGGTCCACAGCTCATCATCATCAAAG-3′. MMP-7: forward, 5′-TGGGTCTGGGTCACTCTTCT-3′; reverse, 5′-CACAGCTTGTTCCTCTTTCC-3′. MMP-9: forward, 5′-GAAGACTTGCCGCGAGACCTGATCGATG-3′; reverse, 5′-GCACCAGCGATAACCATCCGAGCGAC-3′. α-Actin: forward, 5′-GACACCAGGGAGTGATGGTT-3′; reverse, 5′-GTTAGCAAGGTCGGATGCTC-3′.
PCR was carried out with one cycle for 10 min at 95°C and then 40 cycles of 30 s of denaturation at 95°C, 45 s of annealing at 59°C, and 30 s of extension at 72°C, followed by 1 min of final extension at 95°C. The number of PCR cycles varied according to the expression level of the target gene. An appropriate primer concentration and number of cycles was determined to ensure that the PCR is taking place in the linear range and thereby guarantees a proportional relationship between input RNA and the cycles readout. The relative gene expression was calculated by comparison of cycle thresholds with the housekeeping gene α-actin.
Western blot analysis.
The uterus was homogenized in a buffer containing 20 mM 3-[N-morpholino] propane sulfonic acid, 4% SDS, 10% glycerol, 2.3 mg dithiothreitol, 1.2 mM EDTA, 0.02% BSA, 5.5 M leupeptin, 5.5 M pepstatin, 2.15 M aprotinin and 20 M 4-(2-aminoethyl)-benzenesulfonyl fluoride, using a 2-ml tight-fitting homogenizer (Kontes Glass). The homogenate was centrifuged at 10,000 g for 2 min. The supernatant was collected, and protein concentration was determined using a protein assay kit (Bio-Rad). Tissue homogenate was subjected to electrophoresis on 8% SDS polyacrylamide gel and then transferred electrophoretically to nitrocellulose membranes (Bio-Rad). The membranes were incubated in 5% dried nonfat milk in PBS-Tween buffer for 1 h, and then in the antibody solution containing MMP-2, MMP-7, or MMP-9 (1:500) rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 24 h. α-Actin was used as an internal control and detected by a monoclonal anti-actin antibody (1:500,000; Sigma). The nitrocellulose membranes were washed in PBS-Tween five times for 15 min each and then incubated in horseradish peroxidase-conjugated secondary antibody (1:1,000) for 1.5 h. The membrane blots were washed with PBS-Tween and visualized with enhanced chemiluminescence (ECL) Western blotting detection reagent (GE Healthcare Bio-Sciences, Piscataway, NJ), and the reactive bands corresponding to MMP-2, MMP-7, and MMP-9 were analyzed by optical densitometry and ImageJ software (National Institutes of Health, Bethesda, MD). The densitometry values represented the pixel intensity normalized to α-actin to correct for loading.
Gelatin zymography.
The uterus homogenate (without dithiothreitol) was subjected to electrophoresis on 8% SDS polyacrylamide gel containing 0.1% gelatin (Sigma). The gel was then incubated in a zymogram renaturing buffer containing 2.5% Triton X-100 (Sigma) with gentle agitation for 30 min at room temperature. The gel was then equilibrated in a zymogram developing buffer containing 50 mM Tris-base, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij35 (Fisher Scientific), and 1 μM ZnCl2 (Sigma) for 30 min at room temperature and then incubated in the zymogram developing buffer at 37°C for 24 h. The gel was stained with 0.5% coomassie blue R-250 (Sigma) for 30 min and then destained with an appropriate coomassie R-250 destaining solution (methanol/acetic acid/water = 50:10:40). Areas corresponding to MMP-2 and MMP-9 activity appeared as clear bands against a dark blue background. The clear bands were analyzed by optical densitometry and ImageJ software, and the integrated protease activity density was recorded (pixel intensity × mm2) and then normalized to actin intensity.
Solutions and drugs.
Normal Krebs solution contained (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose (Fisher Scientific, Fair Lawn, NJ), 2.5 CaCl2 (BDH Laboratory Supplies, Poole, England), 1.2 MgCl2 (Sigma). Krebs solution was bubbled with 95% O2/5% CO2 for 30 min at an adjusted pH 7.4. High KCl solution (96 mM) was prepared as normal Krebs but with equimolar substitution of NaCl with KCl. Stock solution of oxytocin (10−3 M; Sigma) was prepared in deionized water. Stock solution (10−2 M) of MMP-2/MMP-9 inhibitor IV (SB-3CT; EMD Millipore, Billerica, MA), Ro-28–2653 {5-biphenyl-4-yl-5-[4-(-nitro-phenyl)-piperazin-1-yl]-pyrimidine-2,4,6-trione; Roche Diagnostics, Pharma Research. Penzberg, Germany}, and BB-94 (batimastat; Tocris Bioscience, Minneapolis, MN) was prepared in DMSO. The final concentration of DMSO in the experimental solution was < 0.1%. The tissue culture medium used to incubate the uterus overnight was composed of MEM supplemented with penicillin, streptomycin, and amphotericin B (Gibco/Invitrogen, Grand Island, NY). All other chemicals were of reagent grade or better.
Statistical analysis.
Cumulative data from uterine strips of 4 to 6 rats were presented as means ± SE. The data were first analyzed using ANOVA. When a statistical difference was observed, the data were further analyzed using Student-Newman-Keuls post hoc test for multiple comparisons. Student's t-test for paired and unpaired data was used for comparison of two means. Differences were considered statistically significant at P < 0.05.
RESULTS
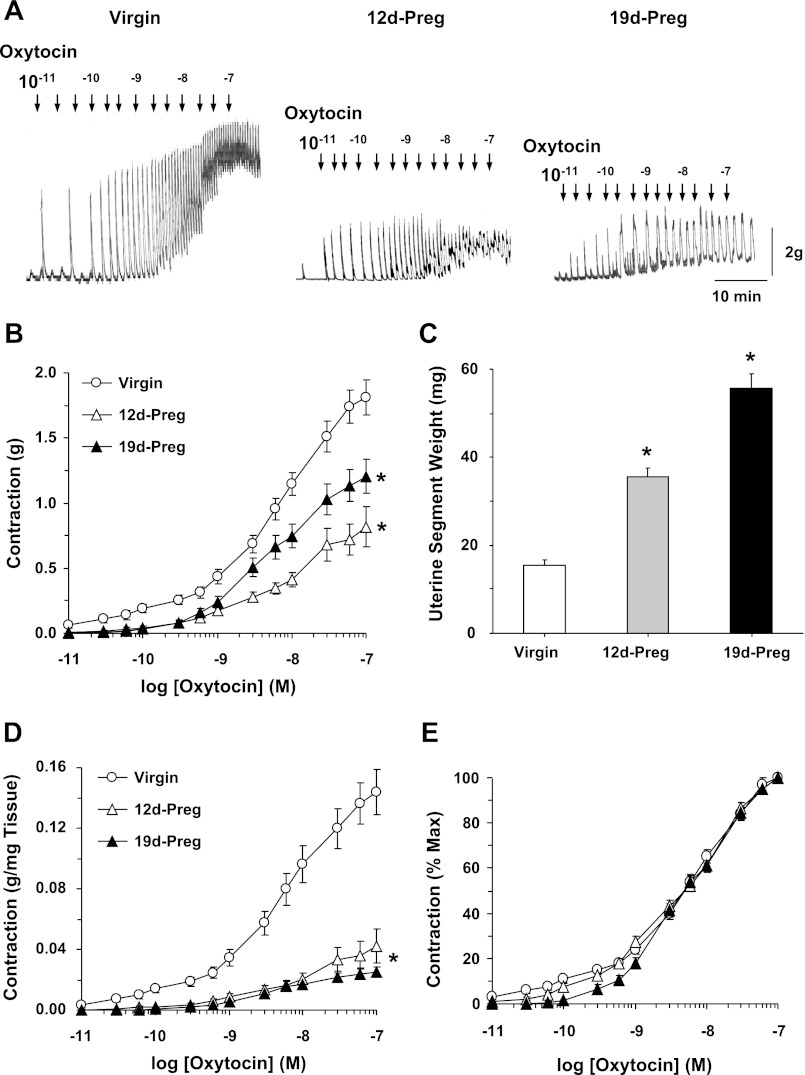
In uterine strips of virgin rats, oxytocin caused a contractile response that had two components: 1) a steady increase in contraction and 2) an additional variable spontaneous phasic contractile response (Fig. 1A). For consistency, we focused on the steady component of oxytocin-induced contraction. Oxytocin caused concentration-dependent contraction that reached a maximum of 1.81 ± 0.13 g at 10−7 M. The oxytocin-induced contraction was significantly reduced in uterine strips of mid-Preg rats (maximum, 0.82 ± 0.15 g) and late-Preg rats (maximum, 1.20 ± 0.13 g) compared with virgin rats (Fig. 1, A and B). Measuring the weight of the uterine strips at the end of the contraction experiments demonstrated that the uterine strip weight was in late-Preg > mid-Preg > virgin rats (Fig. 1C). When the oxytocin response was normalized to the uterine strip weight, the oxytocin-induced contraction was reduced in uterine strips of mid-Preg (maximum, 0.04 ± 0.01 g/mg tissue wt) and further reduced in late-Preg rats (maximum, 0.03 ± 0.00 g/mg) compared with virgin uterus (maximum, 0.14 ± 0.01 g/mg) (Fig. 1D). When the oxytocin response was measured as percentage of maximum contraction and the EC50 was calculated, oxytocin was equally potent in uterine strips of virgin (pEC50, 6.36 ± 0.12), mid-Preg (6.4 ± 0.1), and late-Preg rats (6.34 ± 0.09) (Fig. 1E).
Fig. 1.
Oxytocin-induced contraction. Uterine strips from virgin, mid-Preg (12d-Preg), and late-Preg rats (19d-Preg) were incubated in normal Krebs solution. The uterine strips were stimulated with oxytocin (10−11 to 10−7 M) and the contractile response was recorded (A). At the end of the experiment, the uterine tissue weight was recorded (C). Cumulative contraction data from uterine strips of different rats were presented in grams (B), grams/milligram tissue weight (D) and % of maximal contraction (E). Preg, pregnant; 12d, 12 days. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05).
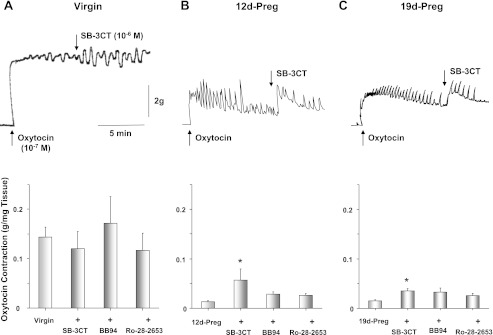
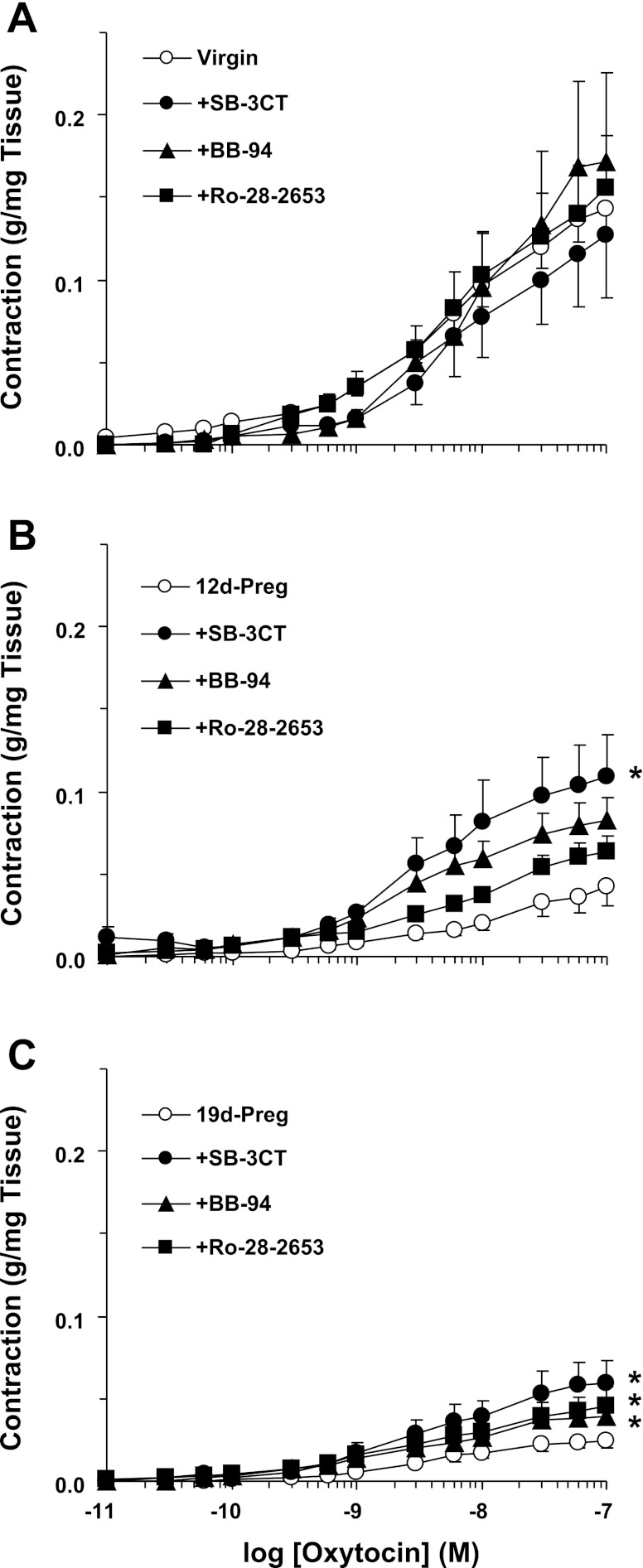
In uterine strips of virgin rats precontracted with oxytocin, addition of MMP-2/MMP-9 inhibitor IV (SB-3CT, 10−6 M), BB-94 (10−6 M), or Ro-28–2653 (10−6 M) did not cause any significant change in contraction. In contrast, in uterine strips of mid-Preg and late-Preg rats, addition of the MMP inhibitors, particularly the MMP-2/MMP-9 inhibitor IV (SB-3CT), enhanced uterine contraction (Fig. 2). Similarly, pretreatment of uterine strips of virgin rats with SB-3CT, BB-94, or Ro-28–2653 for 30 min did not significantly change oxytocin contraction. In contrast, pretreatment with MMP inhibitors, particularly SB-3CT, enhanced oxytocin contraction in uterine strips of mid-Preg rats. Also, pretreatment with SB-3CT, BB-94, or Ro-28–2653 significantly enhanced oxytocin contraction in uterine strips of late-Preg rats (Fig. 3).
Fig. 2.
Effect of matrix metalloproteinase (MMP) inhibitors on oxytocin-induced contraction. Uterine strips of virgin (A), mid-Preg (B), and late-Preg rats (C) were precontracted with oxytocin 10−7 M. Once oxytocin contraction reached steady state the tissues were treated with SB-3CT (MMP-2/MMP-9 inhibitor IV), BB-94, or Ro-28–2653 (10−6 M) or the vehicle (DMSO), and the effect on oxytocin contraction was observed. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05).
Fig. 3.
Effect of MMP inhibitors on oxytocin-induced contraction. Uterine strips of virgin (A), mid-Preg (B), and late-Preg rats (C) were pretreated with SB-3CT, BB-94, or Ro-28–2653 (10−6 M) for 30 min. The uterine strips were stimulated with oxytocin (10−11 to 10−7 M), and the contractile response was recorded and presented (in g/mg tissue wt). Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05).
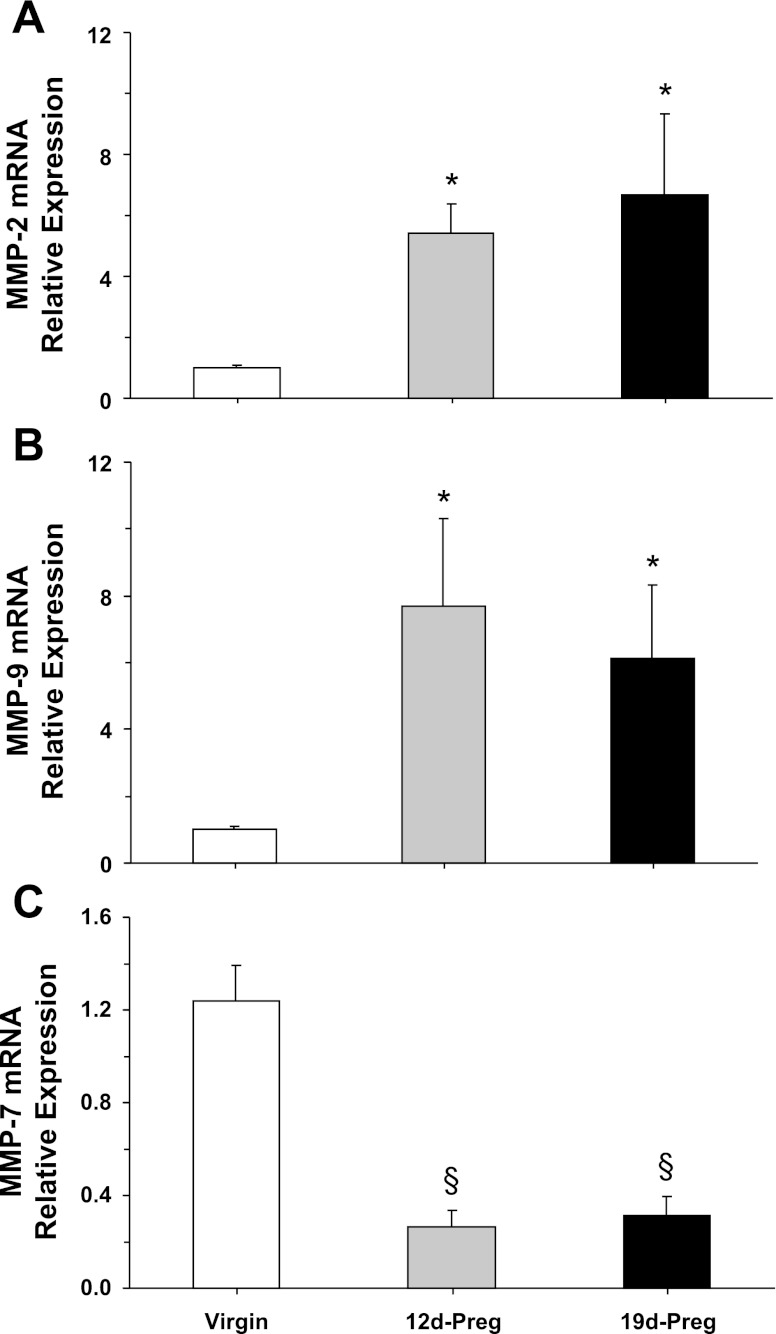
RT-PCR analysis revealed expression of MMP-2, MMP-9, and MMP-7 mRNA in uterine strips of virgin rats (Fig. 4). MMP-2 and -9 mRNA expression was significantly enhanced in uterine strips of mid-Preg and late-Preg rats compared with virgin rats (Fig. 4, A and B). In contrast, MMP-7 mRNA expression was significantly reduced in mid-Preg and late-Preg rats compared with virgin rats (Fig. 4C).
Fig. 4.
MMPs mRNA expression. Uterine tissue homogenate from virgin, mid-Preg, and late-Preg rats were prepared for real-time RT-PCR. The mRNA expression of MMP-2 (A), MMP-9 (B), and MMP-7 (C) was measured and normalized to the housekeeping gene for actin. Data represent means ± SE, n = 4 to 6. *Significantly greater (P < 0.05) than corresponding measurement in virgin rats. §Significantly less (P < 0.05) than corresponding measurements in virgin rats.
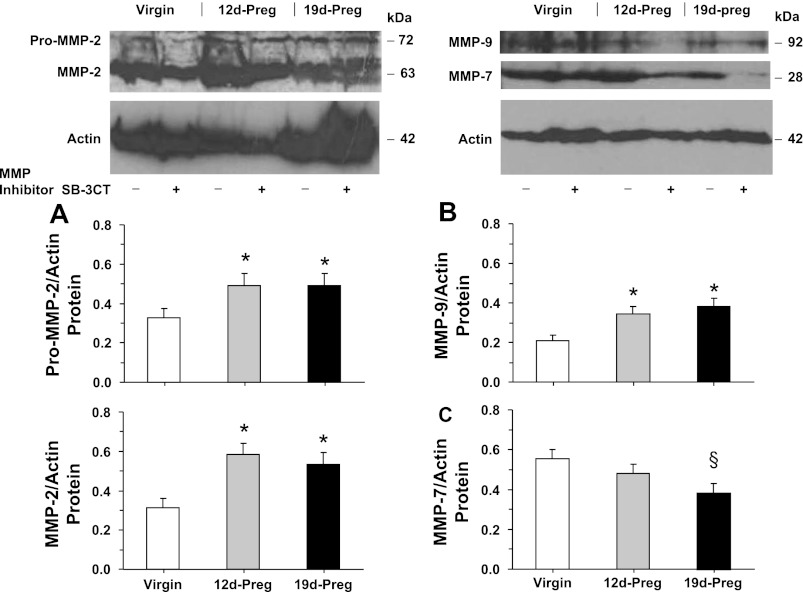
Western blot analysis revealed prominent bands corresponding to pro-MMP-2 (72 kDa), MMP-2 (63 kDa), MMP-9 (92 kDa), and MMP-7 (28 kDa) in uterine strips of virgin rats (Fig. 5). The protein amount of MMP-2 and MMP-9 was significantly enhanced in mid-Preg and late-Preg rats compared with virgin rats (Fig. 5, A and B). In contrast, MMP-7 protein was slightly reduced in mid-Preg rats and significantly reduced in late-Preg rats compared with virgin rats (Fig. 5C). In parallel Western blot experiments, the effect of pretreating the uterine strips of virgin, mid-Preg, and late-Preg rats with the MMP inhibitor SB-3CT (10−6 M) on MMPs protein amount was tested (Fig. 5C). Western blot analysis demonstrated that the optical density of the pro-MMP-2, MMP-2, MMP-9, and MMP-7 immunoreactive bands in uterine strips of virgin, mid-Preg, and late-Preg rats were not significantly different in uterine strips nontreated or concomitantly pretreated with the MMP inhibitor SB-3CT.
Fig. 5.
MMPs protein amount. Uterine strips from virgin, mid-Preg, and late-Preg rats, were either nontreated (−) or pretreated (+) with the MMP inhibitor SB-3CT (10−6 M) for 30 min and then homogenized in preparation for Western blot analysis. MMPs were detected using antibodies to MMP-2 (1:500; A), MMP-9 (1:500; B), and MMP-7 (1:500; C). The intensity of the immunoreactive bands was analyzed using optical densitometry and normalized to the housekeeping protein actin. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurement in virgin rats. §Significantly less (P < 0.05) than corresponding measurements in virgin rats.
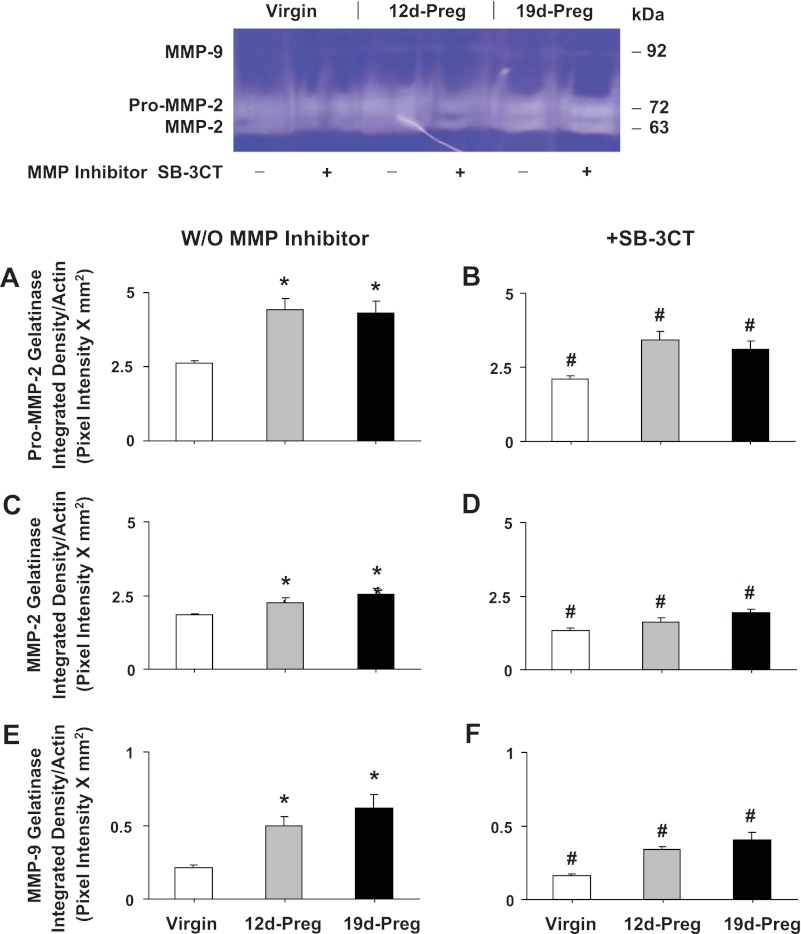
Gelatin zymography analysis revealed prominent digested gelatin bands corresponding to pro-MMP-2 and MMP-2, and a less prominent band corresponding to MMP-9 in uterine tissue homogenate from virgin rats (Fig. 6). The intensity of pro-MMP2, MMP-2, and MMP-9 digested gelatin bands were significantly enhanced in uterine strips of mid-Preg and late-Preg rats compared with virgin rats (Fig. 6, A, C, and E). The intensity of the pro-MMP-2, MMP-2, and MMP-9 digested gelatin bands was significantly reduced in uterine strips treated with the MMP inhibitor SB-3CT (Fig. 6, B, D, and F) compared with control nontreated uterine strips of virgin, mid-Preg, and late-Preg rats.
Fig. 6.
MMPs activity. Uterine strips isolated from virgin, mid-Preg, and late-Preg rats were either nontreated (A, C, and E) or pretreated with MMP-2/MMP-9 inhibitor IV (SB-3CT, 10−6 M) (B, D, and F). The uterine tissue was then homogenized and prepared for gelatin zymography analysis. Digested bands corresponding to pro-MMP-2 (A and B), MMP-2 (C and D), and MMP-9 (E and F) were measured and presented (pixel intensity × mm2 normalized to actin intensity). W/O, without. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurement in virgin rats. #Significantly different (P < 0.05) from corresponding measurements in the absence of MMP inhibitor.
To determine whether the observed changes in oxytocin contraction and MMP expression/activity in the pregnant uterus are related to uterine tissue stretch, experiments were conducted to determine the effects of uterine tissue stretch on uterine contraction and MMP expression/activity. Our working hypothesis predicts that the decreased uterine contraction during pregnancy are related to increased MMPs expression partly due to prolonged uterine stretch caused by the developing fetus and partly by the pregnancy-associated increase in sex hormones. To dissect these components, we tested the effects of prolonged stretch alone and sex hormones alone compared with combined stretch and sex hormones in virgin uterus, which would roughly mimic the conditions during pregnancy. Our rationale for not testing the effects of stretch on the pregnant uterus is that the pregnant myometrium has already been stretched and MMPs have already been overexpressed in vivo. If this is the case, then one would predict that stretching the pregnant uterus should not cause any further increases in MMPs expression/activity or reduction in contraction, and this should be further examined in future experiments.
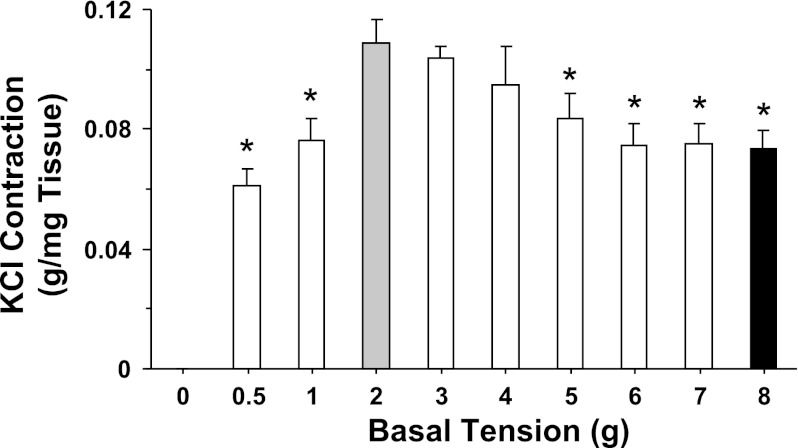
In uterine strips of virgin rat, the magnitude of KCl (96 mM)-induced contraction, dependent on the initial basal tension applied to the tissue, showed progressive increases with increasing basal tension and reached a maximum at 2 g basal tension (Fig. 7). The KCl-induced contraction remained steady at 3 and 4 g basal tension, significantly decreased at 5 and 6 g basal tension, and remained at reduced levels at 7 and 8 g basal tension (Fig. 7).
Fig. 7.
Basal tension-contraction relationship in the rat uterus. Uterine strips of virgin rats were stretched under increasing basal tension 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 g for 30 min, stimulated with 96 mM KCl, and the contractile response was then recorded and presented (in g/mg tissue wt). Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurement under 2 g basal tension.
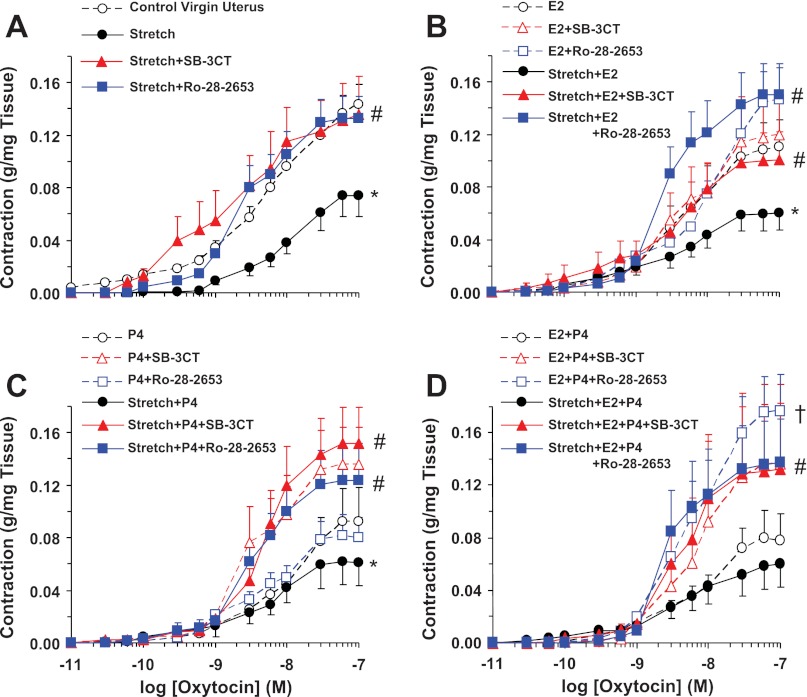
We tested the effect of uterine tissue stretch on oxytocin contraction. In uterine strips of virgin rats stretched under 8 g basal tension for 30 min, oxytocin contraction was significantly reduced to a maximum of 0.04 ± 0.02 g/mg tissue compared with the contraction of control uterine strips under 2 g basal tension (0.14 ± 0.01 g/mg tissue). In virgin uterine strips subjected to prolonged 18-h stretch under 8 g basal tension, the oxytocin contraction was significantly reduced (Fig. 8A). In uterine strips of virgin rats stretched under 8 g basal tension for 18 h and treated with the MMP inhibitor SB-3CT or Ro-28–2653, the oxytocin contraction was significantly enhanced to levels similar to oxytocin contraction in uterine strips under control 2 g basal tension (Fig. 8A).
Fig. 8.
Effect of prolonged stretch and sex hormones on uterine contraction. Uterine strips from virgin rats were stretched under control (2 g) or high (8 g) basal tension for 18 h in the absence or presence of the MMP inhibitor MMP-2/MMP-9 inhibitor IV (SB-3CT) or Ro-28–2653 (A). In other experiments, uterine strips under control (2 g) or high (8 g) tension were concomitantly treated with 17β-estradiol (E2; 10−6 M) (B), progesterone (P4; 10−5 M) (C), or E2+P4 (D) and either in the absence or presence of MMP inhibitors. The uterine strips were stimulated with oxytocin (10−11 to 10−7 M), and the contractile response was recorded and presented (in g/mg tissue wt). Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurement in uterine strips under control basal tension. #Significantly different (P < 0.05) from corresponding measurements in the absence of MMP inhibitor. †Response to Ro-28-2653 was not significantly different from that of SB-3CT.
To test the effects of sex hormones on uterine contraction, uterine strips of virgin rats were incubated under 2 g tension for 18 h in the presence of E2 (10−6 M), P4 (10−5 M), or E2+P4. Oxytocin contraction was reduced in virgin uterus treated with E2 (Fig. 8B), P4 (Fig. 8C), or E2+P4 (Fig. 8D) compared with virgin uterus without hormone treatment (Fig. 8A). In uterine strips under 2 g tension and treated with E2, SB-3CT caused no effect and Ro-28–2653 caused insignificant enhancement of contraction (Fig. 8B). Also, in uterine strips under 2 g tension and treated with P4, SB-3CT caused no effect and Ro-28–2653 caused slight enhancement of contraction that was not statistically different from that in uterine strips treated with P4 alone or with P4+SB-3CT (Fig. 8C). In uterine strips under 2 g tension and treated with E2+P4, both SB-3CT and Ro-28–2653 caused significant enhancement of contraction and the Ro-28–2653 response was not significantly different from that of SB-3CT (Fig. 8D). In virgin uterus stretched under 8 g basal tension and treated with E2, P4, or E2+P4 for 18 h, the oxytocin contraction was further reduced. In virgin uterus exposed to prolonged 18-h stretch under 8 g basal tension and treated with E2, P4, or E2+P4, concomitant treatment with the MMP inhibitor SB-3CT or Ro-28–2653 significantly enhanced oxytocin-induced contraction to levels approaching those observed in control virgin uterus (Fig. 8).
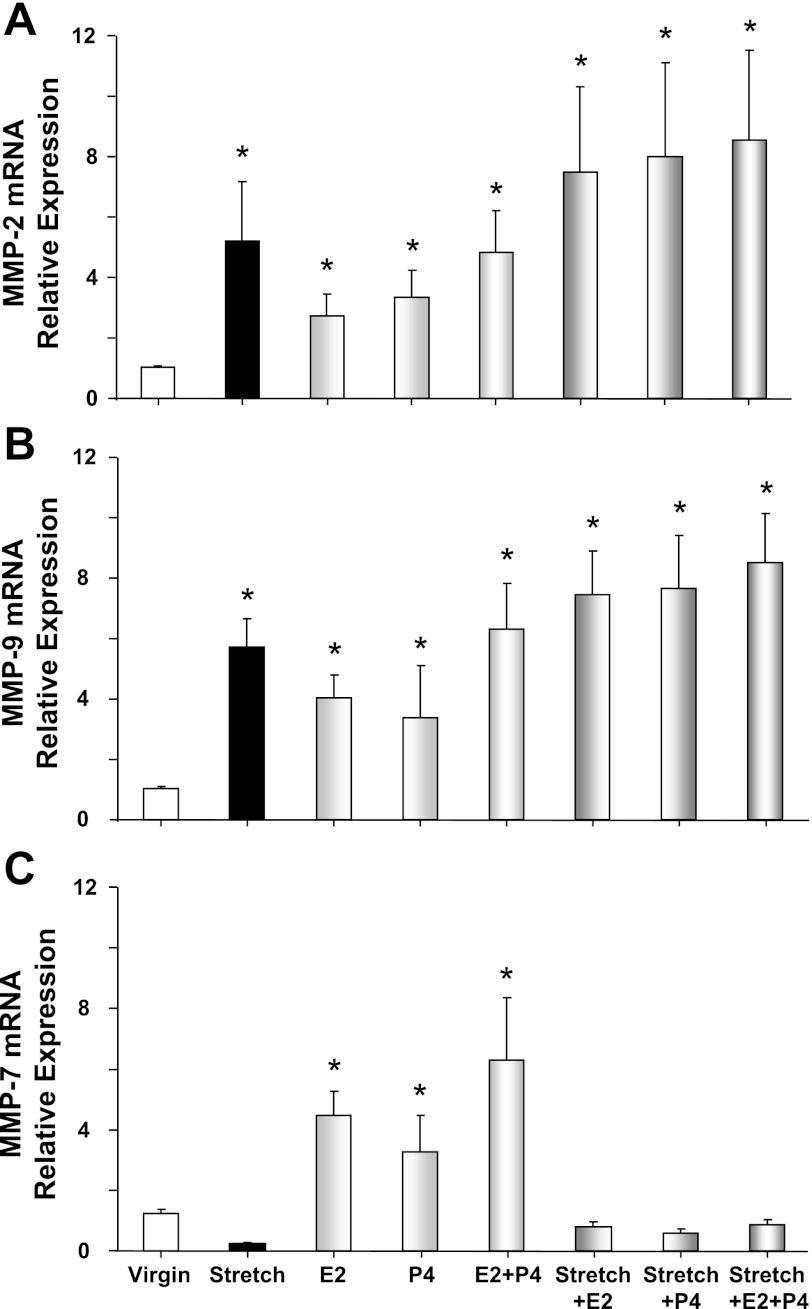
RT-PCR analysis revealed an increase in MMP-2 and MMP-9 mRNA expression in virgin uterus stretched under 8 g basal tension for 18 h compared with control uterine strips under 2 g basal tension (Fig. 9, A and B). MMP-2 and MMP-9 mRNA expression was slightly enhanced in virgin uterus treated with E2, P4, or E2+P4, and further enhanced in virgin uterus stretched under 8 g for 18 h and treated with E2, P4, or E2+P4 (Fig. 9, A and B). In contrast, MMP-7 mRNA was reduced in virgin uterus exposed to prolonged stretch (Fig. 9C). In uterine strips under 2 g basal tension, prolonged treatment with E2, P4, or E2+P4 caused an enhancement of MMP-7 expression (Fig. 9C). However, in uterine strips exposed to prolonged 18-h stretch under 8 g basal tension, the enhancing effects of E2, P4, or E2+P4 appear to be prevented, and the sex hormones did not cause any significant change in MMP-7 mRNA expression (Fig. 9C).
Fig. 9.
Effect of prolonged stretch and sex hormones on uterine MMP mRNA expression. Uterine strips from virgin rats were stretched under control (2 g) or high (8 g) basal tension for 18 h, concomitantly treated with E2, P4, or E2+P4, and the uterine tissue homogenate was prepared for real-time RT-PCR. The mRNA expression of MMP-2 (A), MMP-9 (B), and MMP-7 (C) was measured and normalized to the housekeeping gene for actin. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurements in uterine strips under control basal tension.
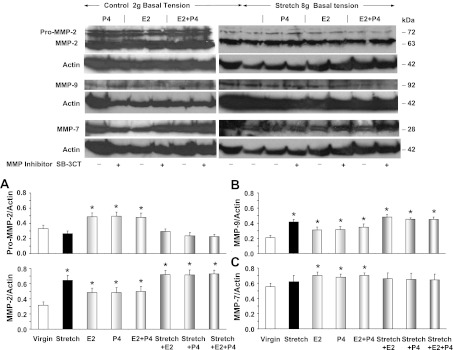
Western blot analysis revealed that the protein amount of pro-MMP-2 was not significantly changed in virgin uterus under 8 g of stretch for 18 h compared with control uterine strips under 2 g basal tension (Fig. 10A). However, the protein amount of both MMP-2 and MMP-9 was significantly enhanced in virgin uterus under prolonged stretch compared with control uterine strips (Fig. 10, A and B). The protein amount of pro-MMP-2, MMP-2, and MMP-9 was slightly but significantly enhanced in virgin uterus under 2 g basal tension and treated with E2, P4, or E2+P4 (Fig. 10, A and B). MMP-2 and MMP-9 protein was further enhanced in virgin uterine strips stretched under 8 g for 18 h and concomitantly treated with E2, P4, or E2+P4 (Fig. 10, A and B). The protein amount of MMP-7 was not significantly different in virgin uterus under prolonged stretch compared with control uterine strips (Fig. 10C). In uterine strips under 2 g basal tension, prolonged treatment with E2, P4, or E2+P4 caused some enhancement of MMP-7 protein (Fig. 10C). However, in uterine strips exposed to prolonged 18-h stretch under 8 g basal tension, the enhancing effects of E2, P4, or E2+P4 were prevented, and sex hormones did not cause significant change in the protein amount of MMP-7 (Fig. 10C). In parallel Western blot analysis, the effect of pretreating the uterine strips with the MMP inhibitor SB-3CT (10−6 M) on MMPs protein amount was tested (Fig. 10). Western blot analysis demonstrated that the optical density of the pro-MMP-2, MMP-2, MMP-9, and MMP-7 immunoreactive bands in uterine strips exposed to prolonged stretch with or without sex hormones were not significantly different in uterine strips nontreated or concomitantly pretreated with the MMP inhibitor SB-3CT.
Fig. 10.
Effect of prolonged stretch and sex hormones on uterine MMPs protein amount. Uterine strips from virgin rats were stretched under control (2 g) or high (8 g) basal tension for 18 h and concomitantly treated with E2, P4, or E2+P4, in the absence (−) or presence (+) of MMP-2/MMP-9 inhibitor IV (SB-3CT, 10−6 M), and the uterine tissue homogenate was prepared for Western blot analysis. MMPs were detected using antibodies to MMP-2 (1:500; A), MMP-9 (1:500; B), and MMP-7 (1:500; C). Representative MMP-2 and MMP-7 blots on the right side have the same actin because the membrane was reacted first with MMP-2 antibody, stripped, reacted with MMP-7 antibody, and then stripped and reacted with anti-actin antibody. The intensity of the immunoreactive bands was analyzed using optical densitometry and normalized to the housekeeping protein actin. Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurement in uterine strips under control basal tension.
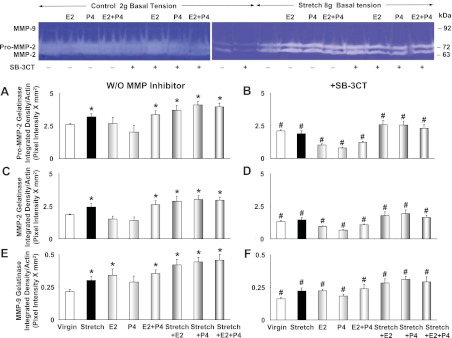
Gelatin zymography analysis revealed that pro-MMP-2, MMP-2, and MMP-9 protease activity was significantly enhanced in virgin uterine strips under high 8 g tension for 18 h compared with uterine strips under 2 g basal tension (Fig. 11, A, C, and E). Pro-MMP-2, MMP-2, and MMP-9 activity was significantly enhanced in virgin uterus under 2 g basal tension and treated with combined E2+P4, and further enhanced in uterine strips stretched under 8 g for 18 h and treated with either E2, P4, or E2+P4 (Fig. 11, A, C, and E). The increased pro-MMP-2, MMP-2, and MMP-9 gelatinase activity in uterine strips exposed to prolonged stretch and treated with sex hormones was reversed in tissues concomitantly treated with the MMP inhibitor SB-3CT (Fig. 11, B, D, and F).
Fig. 11.
Effect of prolonged stretch and sex hormones on uterine MMPs activity. Uterine strips from virgin rats were stretched under control (2 g) or high (8 g) basal tension for 18 h and concomitantly treated with E2, P4, or E2+P4, in the absence (A, C, and E) or presence of MMP-2/MMP-9 inhibitor IV (SB-3CT, 10−6 M; B, D, and F), and the uterine tissue homogenate was prepared for gelatin zymography analysis. Digested bands corresponding to pro-MMP-2 (A and B), MMP-2 (C and D), and MMP-9 (E and F) were measured and presented (as pixel intensity × mm2 normalized to actin intensity). Data represent means ± SE, n = 4 to 6. *Significantly different (P < 0.05) from corresponding measurements in uterine strips under control basal tension. #Significantly different (P < 0.05) from corresponding measurements in the absence of MMP inhibitor.
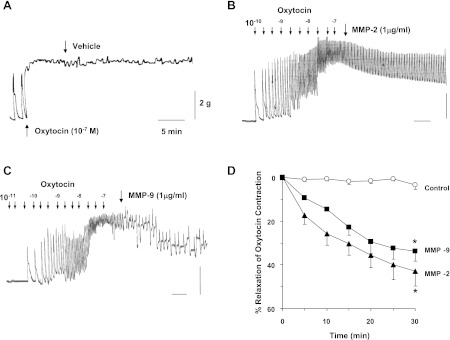
The direct effects of MMPs on oxytocin contraction were also tested. In uterine strips of virgin rats, oxytocin (10−7 M) caused rapid contraction that was maintained at steady state for at least 30 min (Fig. 12A). In uterine strips of virgin rats precontracted with oxytocin (10−7 M), MMP-2 (1 μg/ml) caused slow but progressive inhibition of contraction that reached 42.9 ± 6.8% in 30 min (Fig. 12, B and D). Similarly, MMP-9 (1 μg/ml) caused slow and progressive relaxation of oxytocin contraction that reached 33.8 ± 4.6% in 30 min (Fig. 12, C and D).
Fig. 12.
Effects of MMPs on uterine contraction. Uterine strips of virgin rats were precontracted with oxytocin (10−7 M), and the steady-state contraction was recorded. Either the vehicle H2O (A), MMP-2 (B), or MMP-9 (1 μg/ml; C) was added, and the changes in oxytocin-induced contraction were measured. Cumulative data from uterine strips of different rats were presented as %relaxation of oxytocin contraction (D). Data represent means ± SE, n = 6. *Significantly different (P < 0.05).
DISCUSSION
The main findings of the present study are 1) normal pregnancy is associated with decreased myometrium contraction and enhanced MMP-2 and MMP-9 expression and activity, 2) prolonged increases in myometrium basal tension are associated with decreased myometrium contraction and increased MMP expression/activity, 3) E2 and P4 cause further reduction in contraction and enhancement of MMPs expression/activity in uterine strips exposed to prolonged stretch, and 4) MMP-2 and MMP-9 cause inhibition of myometrium contraction.
During normal pregnancy, the balance between uterine contraction and relaxation is tightly regulated to maintain healthy and full-term pregnancy. The mechanisms responsible for maintaining the uterus in a quiescent relaxed state are important, as changes in these mechanisms could cause preterm uterine contraction with untoward outcome to the pregnancy and premature newborn. Consistent with the concept of uterine quiescence during the course of pregnancy, we found that the oxytocin contraction was reduced in mid-Preg and late-Preg rat compared with virgin rats. Some studies have shown that oxytocin contraction of midterm and late-Preg uterus is greater than that in nonpregnant uterus (2), which is opposite from the present findings. This is likely because during late pregnancy the uterus is bigger and often thicker, and studies often measure uterine contraction in absolute grams. In effect, when we measured the uterine contraction in grams, the contraction in late-Preg uterus was greater than that in mid-Preg uterus, but less than that in virgin uterus. On the other hand, when the uterine contraction was normalized to the uterine tissue weight, the contraction in late-Preg uterus was smaller than that in mid-Preg uterus and far less than that in the virgin uterus. These observations are consistent with the concept that uterine contraction is reduced during pregnancy.
Previous studies have shown that the sensitivity to oxytocin was reduced in rat mid-Preg uterus compared with nonpregnant uterus (2). This is different from the present observation that oxytocin EC50 was not different in mid-Preg and late-Preg rats compared with virgin rats. The causes of the differences in the oxytocin response is not clear, but could be related to the source of oxytocin or the orientation of the longitudinal and circular muscle in the uterine strip. Nevertheless, the present observations suggest that the decreased uterine contraction during pregnancy may not be due to reduction in the sensitivity to oxytocin, but rather due to potential changes in postreceptor signaling pathways and uterine smooth muscle contraction mechanisms.
We tested whether the pregnancy-associated reduction in uterine contraction reflect changes in MMP expression/activity. MMPs is a family of more than 28 proteases including collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs with different tissue expression, distribution, and substrate specificity (49). Previous studies have shown that during normal gestation MMP-1, -2, -3, -7, and -9 are found in the amniotic fluid and fetal membranes. MMP-2 and MMP-3 are expressed constitutively, while MMP-9 is barely detectable until labor. At labor, MMP-9 is the major MMP responsible for gelatinolytic activity in the membranes, while MMP-2 is dominant in the decidua. We have previously shown that prolonged vein wall stretch is associated with decreased venous contraction and increased expression of MMP-2 and MMP-9 (50). Therefore, the present study focused on examining the pregnancy-associated changes in the expression and effects of MMP-2 and MMP-9. In search for the potential mechanisms involved in the changes in uterine contraction during pregnancy, we observed that the reduced contraction in the mid-Preg and late-Preg rat uterus was reversed in the presence of MMP inhibitors, supporting a potential role of MMPs in the pregnancy-associated reduction in uterine contraction. The role of MMPs in the reduced uterine contraction during mid- and late gestation is further supported by the observation that the mRNA expression and protein amount of MMP-2 and MMP-9 were significantly enhanced in mid-Preg and late-Preg rat uterus. Also, MMP-2 and MMP-9 activity, as measured by gelatin zymography, was enhanced in mid-Preg and late-Preg rat uterus compared with virgin rat uterus. We should note that in mid-Preg uterus, only SB-3CT, but not BB-94 or Ro-28–2653, enhanced oxytocin contraction (Fig. 3B), whereas all three inhibitors enhanced contraction in late-Preg uterus (Fig. 3C). This can be explained by the changes in uterine MMP activity during the course of pregnancy. In mid-Preg uterus moderate increases in MMP expression/activity occur, and therefore the potent inhibitor SB-3CT potentiates the contraction. Further increases in MMP expression/activity occur on late-Preg, and therefore all inhibitors with different potencies show measurable increases in uterine contraction. However, as control experiments, it is important to determine whether the pregnancy-associated changes in MMPs expression/activity are specific to MMP-2 and -9, or involve other MMPs. Our rationale to examine the expression of MMP-7 (matrilysin-1) was that MMP-7 is known to be expressed in the uterus and is often termed uterine MMP (49). Also, MMP-7 changes during gestation, but does not appear to play a major role in labor (67). Importantly, MMP-7 expression did not increase during pregnancy, supporting that the increased MMPs expression is specific to MMP-2 and -9. Instead, MMP-7 showed a pregnancy-associated decrease in expression. Although the causes of this decrease are unclear at the present time, because MMPs may have common gene transcription pathways it is possible that the increase in MMP-2 and -9 expression will take away from the common MMPs transcription pool and lead to a decrease in MMP-7 expression. The decrease in MMP-7 expression also suggests that MMP-7 may function via a mechanism different from that induced by MMP-2 and -9. In this respect, whether MMP-7 or MMP-7 inhibitors affect uterine contraction should be examined in future studies.
As different stages of pregnancy are associated with different degrees of uterine stretch, the present observations suggested a relationship between uterine stretch, MMP expression, and decreased contraction. To support this concept it is important to demonstrate that uterine stretch leads to increased MMP expression/activity and that MMPs cause reduction in uterine contraction.
Previous studies have demonstrated that mechanical stretch of skeletal muscle and microvessels is associated with decreased contraction and enhanced MMP expression (39). Also, we have previously shown that prolonged stretch of rat inferior vena cava is associated with decreased vein contraction and increased MMP-2 and MMP-9 expression (50). In the present study, we found that prolonged stretch of virgin uterus was associated with decreased contraction. The decreased contraction in virgin uterus exposed to prolonged stretch appeared to involve upregulation of MMP expression/activity because the decreased uterine contraction in stretched virgin uterus was reversed in tissues treated with MMP inhibitors. Also, the decreased uterine contraction in stretched virgin uterus was associated with increased mRNA expression, protein amount and enzymatic activity of MMP-2 and MMP-9. Importantly, the stretch-induced changes in MMPs were specific to MMP-2 and MMP-9, and were not observed with other MMPs such as MMP-7. The present observations in rat uterus are consistent with previous reports in skeletal muscle and blood vessels (39, 50) and support the relationship among mechanical stretch, MMP tissue expression, and decreased tissue contraction.
During pregnancy the uterus is exposed to not only mechanical stretch caused by progressive fetal growth, but also hormonal changes that could influence the myometrium structure and mechanical properties. Pregnancy is associated with increased plasma levels of E2 and P4 (52). Endometrial cellular concentrations of both E2 and P4 are positively correlated with the plasma levels of E2 during the proliferative phase of the menstrual cycle, and a large concentration of P4 receptors characterizes the early pregnancy endometrium (30). Studies have shown that E2 causes relaxation of smooth muscle of blood vessels, including rat aorta and uterine artery (11, 55), as well as the uterus (45). Studies have also shown that P4 inhibits contraction of rat blood vessels and human uterus (11, 15). Consistent with previous reports, we found that E2 and P4 decreased contraction of uterine strips of virgin rats. Also, in uterine strips exposed to prolonged stretch, sex hormones further reduced uterine contraction.
MMP-9 is one of the major MMPs in the uterus that modulates uterine biology during various reproductive processes. In mouse uterus, gelatin zymography revealed that E2 alone or in combination with P4 increased MMP-9 activation, whereas Northern blot analysis showed that E2 decreased MMP-9 steady-state mRNA expression. In contrast, uterine MMP-2 expression and activity were not affected by steroidal treatment. Collectively, these data suggest that E2 and P4 regulate uterine MMPs expression and activity via a complex mechanism and may play a role in uterine tissue remodeling (70). However, little is known on whether the effects of E2 and P4 on uterine relaxation involve MMPs. The present study demonstrates that concomitant treatment of stretched virgin uterus with E2, P4, or E2+P4 caused greater reduction of uterine contraction. The reduced contraction in the sex hormone-treated uterus was reversed in the presence of MMP inhibitors. Also, E2 and P4 treatment of stretched virgin uterus was associated with greater increases in MMP-2 and MMP-9 mRNA expression, protein amount, and enzymatic activity. Together, these observations suggest a synergistic relationship between mechanical stretch and sex hormones in the reduced uterine contraction and MMP expression/activity. The mechanisms via which E2 enhances uterine MMP expression/activity are unclear, but E2 has been shown to stimulate MMP production and activity via ER-mediated MEK/ERK (MAPK) (23). Also, while some studies suggest anti-inflammatory effects of E2 (14, 37, 61), other studies suggest E2-mediated increase in inflammatory cytokines such as TNF-α and IL-6, which could in turn increase MMPs expression/activity (3, 10, 23, 29, 70).
The questions remain of how mechanical stretch causes increases in MMPs expression and how MMPs cause uterine smooth muscle relaxation. Hypoxia-inducible factors (HIFs) are nuclear transcriptional factors that regulate genes involved in oxygen homeostasis (57). In addition to the regulation of HIF by oxygen, hormones, cytokines, metallic ions, and mechanical stretch may induce HIF expression (20, 57). HIF-1α and HIF-2α mRNA and protein are increased in skeletal muscle fibers exposed to stretch (38, 39). Also, HIF-1α is overexpressed in rat cardiac and aortic vascular smooth muscle cells exposed to mechanical stretch (8, 24). The mechanism of HIF regulation by mechanical stretch is unclear but may involve phosphatidylinositol 3-kinase and MAPK (8, 20, 38). Studies have shown that the expression and activity of MMP-2 and MMP-9 are regulated by HIF (19, 27). Also, mechanical stretch is associated with increased HIF-1α expression, and HIF could increase MMP expression (31). Studies have shown that HIF expression may increase during pregnancy (44) and that estrogen stimulates uterine HIF-2α, while P4 primarily upregulates uterine HIF-1α expression (12). Whether HIF represents a link between myometrial stretch and MMPs expression remains to be examined.
The effects of MMPs are generally thought to involve degradation of the extracellular matrix components and extensive tissue remodeling. The relatively rapid effects of MMP inhibitors and MMPs on uterine contraction suggest that these effects may not be solely due to tissue remodeling. We have previously shown that MMP-2 and -9 could have additional inhibitory effects on vascular smooth muscle contraction in the absence of detectable tissue degradation (9, 48). Also, our previous experiments on rat inferior vena cava have suggested that MMP-2 may cause membrane hyperpolarization and reduction in Ca2+ influx (48, 51). In the present study, treatment of uterine strips with MMP-2 or MMP-9 caused significant relaxation of oxytocin-induced contraction. These observations are consistent with our previous reports in vascular tissues (9, 48, 51) and point to inhibitory effects of MMPs on the mechanisms of uterine smooth muscle contraction. Whether MMPs affect the plasma membrane channels or other mechanisms of uterine smooth muscle contraction remains to be examined in future studies.
Consistent with previous reports in the rat uterus (2, 47), the present study demonstrates that oxytocin causes a steady increase in contraction and an additional spontaneous phasic contractile response. The frequency and amplitude of oxytocin-induced phasic contraction appear to be different among virgin uteri and in pregnant vs. virgin uteri. Although the present study focused on the mechanisms underlying the reduction in the steady component of oxytocin contraction during pregnancy, that should not minimize the importance of examining the causes of the different frequency and amplitude of oxytocin-induced phasic contraction among virgin uteri and the mechanisms of the pregnancy-associated changes in the phasic uterine contraction. Studies in human myometrial cells have shown that oxytocin causes an initial [Ca2+]i transient followed by uniform relatively low-frequency [Ca2+]i oscillations (4, 6, 69). The oxytocin-induced [Ca2+]i oscillations in myometrial cells are attenuated by caffeine and the voltage-dependent Ca2+ channel antagonist verapamil and blocked by the inorganic Ca2+ antagonist La3+ and the Ca2+-ATPase inhibitor 2.5-di-tert-butylhydroquinone (18). Similarly, in rat uterine segments, oxytocin induced simultaneous [Ca2+]i oscillations and phasic contractions that were inhibited by the Ca2+ channel blocker nifedipine (47). Collectively, these studies suggest that the oxytocin-induced [Ca2+]i oscillations and phasic uterine contraction are mediated by intracellular Ca2+ release from inositol 1,4,5-trisphosphate-sensitive Ca2+ stores combined with voltage-dependent and capacitative Ca2+ influx. Interestingly, we have previously reported that MMP-2 may cause hyperpolarization and reduction in Ca2+ influx in rat inferior vena cava (48, 51), a smooth muscle preparation that also demonstrates phasic contraction. These observations make it important to investigate the pregnancy-associated changes and the effects of MMPs on the frequency and amplitude of phasic uterine contractions and whether the changes in uterine contraction involve parallel changes in [Ca2+]i and Ca2+ regulatory mechanisms. These questions should be examined in future experiments.
One of the limitations of the present study is that uterine contraction and MMP expression were measured at only two time points of pregnancy, namely day 12 and day 19 of gestations, and the progressive changes during the course of pregnancy and reversal of these uterine changes in the postpartum period need to be examined. Likewise, the stretch experiments were limited to 8 g basal tension for 18 h, and the progressive effects of different levels of basal tension for more extended time periods would be more analogous to the progressive uterine stretch imposed by the growing fetus over the course of pregnancy. The present study also tested the effects of oxytocin and whether the uterine response to other contractile agonists, such as prostaglandins, are affected during pregnancy and whether uterine stretch should be examined. Also, while the present study suggests a role of MMP-2 and MMP-9 in the observed changes in uterine contraction during pregnancy and uterine tissue stretch, the results should not minimize the possibility of involvement of other MMPs.
Perspectives
Myometrium activity is tightly regulated during pregnancy. At the first and midtrimester, myometrium relaxation is needed to accommodate fetal growth. As fetal growth nears its completion during late pregnancy the uterine activity may be stabilized before the activity starts to increase in preparation for delivery. However, little is known regarding the mechanisms controlling the balance between myometrium contraction and relaxation during pregnancy. The present study demonstrates a relationship between uterine stretch, MMPs expression, and uterine relaxation during gestation. The study also demonstrates a role of sex hormones in promoting the effects of uterine stretch on MMPs expression and uterine relaxation. These findings may have clinical relevance as a disturbance in the balance of MMPs, and the endogenous tissue inhibitors of MMPs (TIMPs) could disturb uterine activity and lead to premature labor. The imbalance between MMPs and TIMPs may be further aggravated by changes in the sex hormone levels or their uterine receptors.
Preterm delivery complicates 10% to 15% of all pregnancies and is a leading cause of perinatal morbidity and death (17), but the mechanisms involved are not completely understood. Importantly, studies on samples of amniochorion and amniotic fluid collected from women undergoing cesarean delivery before term, with either premature rupture of membranes or with preterm labor with no rupture of membranes, demonstrated alterations in mRNA expression and bioactivity of MMP-2, MMP-9, MT1-MMP, and TIMP-2 in prematurely ruptured membranes compared with preterm labor membranes. Also, enzyme-linked immunosorbent assays demonstrated alterations in the amniotic fluid concentrations of immunoreactive and bioactive MMP-2 and MMP-9, as well as TIMP-2 in fluids obtained from the premature rupture of membranes group compared with the preterm labor group (17). Other studies have shown that plasma P4 levels are lower in some preterm delivery patients compared with normal pregnant women. For example, P4 concentration was ∼30% lower at 28 to 34 wk gestation in women who delivered prematurely than in women who delivered at term (59). Studies have also suggested that progestin supplementation may be a promising approach to both preventing initiation of preterm labor and treating it once it is already established (32). Whether changes in MMPs and sex hormone levels/activity could interfere with uterine relaxation and trigger uterus contraction and preterm delivery needs to be further examined. Also, whether the potential beneficial effects of P4 in preterm labor are mediated via modulation of MMP expression/activity warrant further investigation.
GRANTS
R. A. Khalil was partly supported by National Heart, Lung, and Blood Institute Grants HL-65998, HL-98724 and The Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD-60702. Z. Yin was a visiting scholar from Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei Province, P. R. China, and a recipient of a scholarship from the China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.Y., O.M.R., and R.A.K. conception and design of research; Z.Y., A.A.S., O.M.R., and N.N. performed experiments; Z.Y., A.A.S., N.N., and R.A.K. analyzed data; Z.Y. and R.A.K. interpreted results of experiments; Z.Y. and R.A.K. prepared figures; Z.Y., A.A.S., and R.A.K. edited and revised manuscript; Z.Y. and R.A.K. approved final version of manuscript; R.A.K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Apoorva Kumar and Yuanyuan Zhang for their assistance in the analysis of the Western blots and contraction data.
REFERENCES
- 1. Ang VT, Jenkins JS. Neurohypophysial hormones in the adrenal medulla. J Clin Endocrinol Metab 58: 688–691, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Arthur P, Taggart MJ, Zielnik B, Wong S, Mitchell BF. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J Physiol 586: 6063–6076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am J Physiol Cell Physiol 298: C679–C692, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Barhoumi R, Awooda I, Mouneimne Y, Safe S, Burghardt RC. Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol Lett 165: 133–141, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brown S, Margarida B, Li Z, Korta LP, Tanaka Y, Fridman R, Mobashery S. Potent and selective mechanism-based Inhibition of gelatinases. J Am Chem Soc 122: 6799–6800, 2000 [Google Scholar]
- 6. Burghardt RC, Barhoumi R, Sanborn BM, Andersen J. Oxytocin-induced Ca2+ responses in human myometrial cells. Biol Reprod 60: 777–782, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21: 514–550, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chang H, Shyu KG, Wang BW, Kuan P. Regulation of hypoxia-inducible factor-1α by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 105: 447–456, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg 40: 1001–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest 93: 17–25, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol 26: 707–715, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem 278: 7683–7691, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res 53: 2087–2091, 1993 [PubMed] [Google Scholar]
- 14. Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas 61: 330–333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledee N, Frydman R. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril 75: 1136–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Fields PA, Eldridge RK, Fuchs AR, Roberts RF, Fields MJ. Human placental and bovine corpora luteal oxytocin. Endocrinology 112: 1544–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol 184: 1399–1405, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Fu X, Liu YJ, Ciray N, Olovsson M, Ulmsten U, Gylfe E. Oxytocin-induced oscillations of cytoplasmic Ca2+ in human myometrial cells. Acta Obstet Gynecol Scand 79: 174–179, 2000 [PubMed] [Google Scholar]
- 19. Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S, Ohnishi T. Silencing hypoxia-inducible factor-1α inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol 30: 793–802, 2007 [PubMed] [Google Scholar]
- 20. Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis 64: 971–980, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grams F, Brandstetter H, D'Alo S, Geppert D, Krell HW, Leinert H, Livi V, Menta E, Oliva A, Zimmermann G, Gram F, Livi VE. Pyrimidine-2,4,6-triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol Chem 382: 1277–1285, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knofler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol 177: 2103–2115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He YY, Cai B, Yang YX, Liu XL, Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci 100: 1051–1061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1α in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res 90: E25–E33, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Kizaki K, Ushizawa K, Takahashi T, Yamada O, Todoroki J, Sato T, Ito A, Hashizume K. Gelatinase (MMP-2 and -9) expression profiles during gestation in the bovine endometrium. Reprod Biol Endocrinol 6: 66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleifeld O, Kotra LP, Gervasi DC, Brown S, Bernardo MM, Fridman R, Mobashery S, Sagi I. X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. Comparison with the latent and active forms of the enzyme. J Biol Chem 276: 17125–17131, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1α in the internal spermatic vein of patients with varicocele. J Urol 175: 1045–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Lein M, Jung K, Ortel B, Stephan C, Rothaug W, Juchem R, Johannsen M, Deger S, Schnorr D, Loening S, Krell HW. The new synthetic matrix metalloproteinase inhibitor (Roche 28–2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene 21: 2089–2096, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Lekontseva O, Jiang Y, Davidge ST. Estrogen replacement increases matrix metalloproteinase contribution to vasoconstriction in a rat model of menopause. J Hypertens 27: 1602–1608, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Levy C, Robel P, Gautray JP, De Brux J, Verma U, Descomps B, Baulieu EE. Estradiol and progesterone receptors in human endometrium: normal and abnormal menstrual cycles and early pregnancy. Am J Obstet Gynecol 136: 646–651, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg 53: 764–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucovnik M, Kuon RJ, Chambliss LR, Maner WL, Shi SQ, Shi L, Balducci J, Garfield RE. Progestin treatment for the prevention of preterm birth. Acta Obstet Gynecol Scand 90: 1057–1069, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyons CA, Beharry KD, Nishihara KC, Akmal Y, Ren ZY, Chang E, Nageotte MP. Regulation of matrix metalloproteinases (type IV collagenases) and their inhibitors in the virgin, timed pregnant, and postpartum rat uterus and cervix by prostaglandin E2-cyclic adenosine monophosphate. Am J Obstet Gynecol 187: 202–208, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Maquoi E, Sounni NE, Devy L, Olivier F, Frankenne F, Krell HW, Grams F, Foidart JM, Noel A. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res 10: 4038–4047, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Matsuzaki S, Maleysson E, Darcha C. Analysis of matrix metalloproteinase-7 expression in eutopic and ectopic endometrium samples from patients with different forms of endometriosis. Hum Reprod 25: 742–750, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol 588: 1997–2010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1α and HIF-2α play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol 583: 753–766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1α and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol Heart Circ Physiol 289: H1315–H1320, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Monckedieck V, Sannecke C, Husen B, Kumbartski M, Kimmig R, Totsch M, Winterhager E, Grummer R. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol Hum Reprod 15: 633–643, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Naruse K, Lash GE, Innes BA, Otun HA, Searle RF, Robson SC, Bulmer JN. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod 24: 553–561, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem 272: 29975–29983, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Parkington HC, Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clin Exp Pharmacol Physiol 15: 657–665, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta 31: 951–957, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Perusquia M, Navarrete E. Evidence that 17α-estradiol is biologically active in the uterine tissue: antiuterotonic and antiuterotrophic action. Reprod Biol Endocrinol 3: 30, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 α (HIF-1α) in tendon fibroblasts. J Orthop Res 22: 847–853, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Phillippe M, Basa A. Effects of sodium and calcium channel blockade on cytosolic calcium oscillations and phasic contractions of myometrial tissue. J Soc Gynecol Investig 4: 72–77, 1997 [PubMed] [Google Scholar]
- 48. Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res 159: 755–764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: potential implications in varicose veins. J Vasc Surg 48: 447–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg 45: 373–380, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand 88: 639–646, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Roy SC, Ghosh J. Dynamic in vivo changes in the activities of gelatinases, matrix metalloproteinases (MMPs), and tissue inhibitor of metalloproteinases (TIMPs) in buffalo (Bubalus bubalis) uterine luminal fluid during estrous cycle and early pregnancy. Mol Reprod Dev 77: 944–953, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Russo LA, Peano BJ, Trivedi SP, Cavalcanto TD, Olenchock BA, Caruso JA, Smolock AR, Vishnevsky O, Gardner RM. Regulated expression of matrix metalloproteinases, inflammatory mediators, and endometrial matrix remodeling by 17β-estradiol in the immature rat uterus. Reprod Biol Endocrinol 7: 124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17β-estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol 293: H3713–H3719, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Selvais C, Gaide Chevronnay HP, Lemoine P, Dedieu S, Henriet P, Courtoy PJ, Marbaix E, Emonard H. Metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 ectodomain decreases endocytic clearance of endometrial matrix metalloproteinase-2 and -9 at menstruation. Endocrinology 150: 3792–3799, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol 139: 226–232, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Stamatelou F, Deligeoroglou E, Farmakides G, Creatsas G. Abnormal progesterone and corticotropin releasing hormone levels are associated with preterm labour. Ann Acad Med Singapore 38: 1011–1016, 2009 [PubMed] [Google Scholar]
- 60. Stock S, Bremme K, Uvnas-Moberg K. Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum Reprod 6: 1056–1062, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol 292: H2333–H2340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ulbrich SE, Meyer SU, Zitta K, Hiendleder S, Sinowatz F, Bauersachs S, Buttner M, Frohlich T, Arnold GJ, Reichenbach HD, Wolf E, Meyer HH. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol Cell Endocrinol 332: 48–57, 2011 [DOI] [PubMed] [Google Scholar]
- 63. van Engelen E, Breeveld-Dwarkasing VN, Taverne MA, Everts ME, van der Weijden GC, Rutten VP. MMP-2 expression precedes the final ripening process of the bovine cervix. Mol Reprod Dev 75: 1669–1677, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res 54: 4726–4728, 1994 [PubMed] [Google Scholar]
- 65. Wathes DC, Swann RW. Is oxytocin an ovarian hormone? Nature 297: 225–227, 1982 [DOI] [PubMed] [Google Scholar]
- 66. Wathes DC, Swann RW, Pickering BT, Porter DG, Hull MG, Drife JO. Neurohypophysial hormones in the human ovary. Lancet 2: 410–412, 1982 [DOI] [PubMed] [Google Scholar]
- 67. Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci 12: 649–659, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Yener T, Turkkani Tunc A, Aslan H, Aytan H, Cantug Caliskan A. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat Histol Embryol 36: 75–77, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Yue C, Ku CY, Liu M, Simon MI, Sanborn BM. Molecular mechanism of the inhibition of phospholipase C-β3 by protein kinase C. J Biol Chem 275: 30220–30225, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Zhang X, Christenson LK, Nothnick WB. Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol Reprod Dev 74: 321–331, 2007 [DOI] [PubMed] [Google Scholar]