Abstract
Bariatric surgery is currently the most effective treatment for obesity. Vertical sleeve gastrectomy (VSG), a commonly applied bariatric procedure, involves surgically incising most of the volume of the stomach. In humans, partial loss of melanocortin receptor-4 (MC4R) activity is the most common monogenic correlate of obesity regardless of lifestyle. At present it is unclear whether genetic alteration of MC4R signaling modulates the beneficial effects of VSG. Following VSG, we analyzed body weight, food intake, glucose sensitivity, and macronutrient preference of wild-type and MC4R-deficient (Mc4r+/− and Mc4r−/−) rats compared with sham-operated controls. VSG reduced body weight and fat mass and improved glucose metabolism and also shifted preference toward carbohydrates and away from fat. All of this occurred independently of MC4R activity. In addition, MC4R was resequenced in 46 human subjects who underwent VSG. We observed common genetic variations in the coding sequence of MC4R in five subjects. However, none of those variations appeared to affect the outcome of VSG. Taken together, these data suggest that the beneficial effect of VSG on body weight and glucose metabolism is not mediated by alterations in MC4R activity.
Keywords: bariatric surgery, energy metabolism, food choice, gastric bypass
the currently approved antiobesity drugs have limited efficacy, making bariatric surgery an increasingly important therapeutic option (6). Vertical sleeve gastrectomy (VSG), which is rapidly becoming the surgical procedure of choice (2, 19), results in a reduction of stomach volume by ∼80% without modification of the intestines (8, 21). Roux-en-Y gastric bypass (RYGB) is another common but more extensive bariatric procedure in which the stomach lumen is reduced by ∼90% and the remaining gastric pouch is anastomosed to the jejunum, bypassing the remaining stomach, the entire duodenum, and the proximal jejunum (8, 13, 27). Although both VSG and RYGB have been reported to produce similar reductions in weight and metabolic improvements in both humans and rodent models, VSG is technically less complex due to lack of intestinal manipulation (6, 8, 9, 13, 21, 22, 27) and has minimal malabsorptive effects (21).
The melanocortin receptor-4 (MC4R) is a critical element in the central nervous system (CNS) control of energy homeostasis (20, 24). Functional loss of MC4R activity is the most common monogenetic cause of human obesity (7, 12, 16, 25). MC4R-deficient rats have phenotypes similar to those seen in MC4R-deficient mice and in humans with MC4R haploinsufficiency, including increased food intake, meal size, body weight, and white adipose tissue mass (5, 11, 15, 18). Thus, MC4R might well be important in the efficacy of bariatric surgical procedures. MC4R-deficient rats have been developed recently (18), and their relatively larger size makes the rat better suited than the mouse for mechanistic studies of bariatric surgery.
Although the impact of MC4R mutations has been studied in combination with RYGB and gastric banding [a procedure that physically limits the diameter of the upper part of the stomach to reduce food intake (3, 4, 14)], it is unknown whether VSG still has beneficial effects in humans or rodents that are partially or completely deficient for MC4R. Therefore, we used wild-type (WT), Mc4r+/−, and Mc4r−/− rats (18) to determine whether MC4R function is necessary for producing the beneficial effects of VSG with regard to body weight, glucose metabolism, and macronutrient preference. In addition, 46 obese humans who underwent VSG were screened for mutations in MC4R to determine whether genetic variations in MC4R might be associated with differential efficacy of VSG in humans.
RESEARCH DESIGN AND METHODS
Animals.
Age-matched experimental male rats (littermates) were obtained by partnering rats heterozygous (HET) for a nonfunctional mutation in Mc4r [Mc4rK314X (18)] that had been outcrossed for at least seven generations. Genotyping was performed by KBiosciences (Hoddesdon, UK) using the KASPar SNP genotyping system. Experimental rats {Mc4r+/+ (WT), Mc4r+/− (HET), or Mc4r−/− [homozygous (HOM)]}were weaned at postnatal day (PND) 21, group-housed (2–3/cage) until PND 42, and subsequently housed individually. Rats were maintained at the Metabolic Diseases Institute of the University of Cincinnati on a 12:12-h light-dark cycle (lights off at 1800) at 23°C and 50–60% humidity. All rats had ad libitum access to water and a pelleted low-fat chow diet [LFD; LM-485 no. 7012, 25% protein, 58% carbohydrate, and 17% fat, 3.1 kcal/g Atwater Fuel Energy (AFE); Harlan Teklad, Madison, WI] via an in-cage dispenser until PND 56. To increase presurgical body weight, rats were given ad libitum access to water and a pelleted 40% high-fat butter/oil-based diet (HFD; D03082706, 4.54 kcal/g AFE, 15% calories protein, 46% calories carbohydrate, and 40% calories fat; Open Source Diets, New Brunswick, NJ) via an in-cage dispenser from PND 56 onward. All rats also had access to home-cage enrichment (red rat retreat; Bioserve). The University of Cincinnati Institutional Animal Care and Use Committee approved all procedures for animal use.
Presurgical body weight and food intake.
From PND 25 onward, body weight of WT (n = 34), HET (n = 35), and HOM (n = 36) rats was followed serially once or twice per week. Home cage food intake was measured between PND 52 and 55 (LFD) and again between PND 72 and 75 (HFD). Feed efficiency was calculated as the change in body weight (g) divided by the total amount of ingested calories (kcal).
Surgical procedures.
After 26 days on the HFD (PND 82), rats were counterbalanced on the basis of fat and lean mass and assigned to surgical groups (sham operation or VSG). Surgeries were performed as described previously (21), and the same surgeons conducted all surgeries. Briefly, the lateral 80% of the stomach was excised, leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly. The VSG sham procedure involved analogous isolation of the stomach, followed by applying pressure manually with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. The surgical cohort included sham-operated Mc4r+/+ (WT-SHAM), VSG-operated Mc4r+/+ (WT-VSG), Mc4r+/− (HET-SHAM), VSG-operated Mc4r+/− (HET-VSG), Mc4r−/− (HOM-SHAM), and VSG-operated Mc4r−/− (HOM-VSG) rats.
Pre- and postoperative care.
Rats were preexposed to a liquid diet (Osmolite OneCal) twice before surgery, fasted overnight prior to surgery, and then maintained on the liquid diet for the first 3 postoperative days (POD) when they were transitioned back to HFD. Subcutaneous injections of Metacam (0.25 mg/100 g body wt once daily for 3 days), Buprenex (0.3 ml twice/daily for 3 days), and warm saline (10 ml twice/daily, day 1, and 5 ml twice/daily, days 2 and 3) were given to all rats following surgery. A wire grate was used until POD 4 to prevent the VSG groups from ingesting their bedding.
Postsurgical body weight, food intake, and body composition.
Body weight and food intake were monitored daily for the first 2 wk following surgery. Subsequently, body weight and food intake were followed serially once or twice per week until POD 73. Body composition was assessed using an EchoMRI analyzer (Houston, TX) 3 days presurgery (PND 79), on POD 43 (PND 126), and on POD 73 (PND 156).
Intraperitoneal glucose tolerance test.
On POD 58 (PND 141), 5-h-fasted rats were given an intraperitoneal (ip) injection of 50% dextrose (1.25 g/kg) at 1300. Blood glucose was assessed at baseline (0), 15, 30, 45, 60, and 120 min via hand-held glucose analyzer (Accuchek; Roche Diagnostics, Indianapolis, IN). Glucose area under the curve (AUC) was calculated using the ΔAUC method. In addition, blood samples (100 μl/time point) were collected using heparinized Eppendorf tubes at 0, 15, 30, and 60 min for insulin determination. Blood was cold-centrifuged, and plasma was stored at −80°C. Plasma insulin was assayed in duplicate using an insulin ELISA (Crystal Chem, Downers Grove, IL), following the manufacturer's instructions.
Macronutrient selection testing.
Using a food preference paradigm (26), three pure macronutrient diets [TD.02521 (carbohydrate: 3.3 kcal/g, 0.1% calories protein, 99.9% calories carbohydrate, and 0% calories fat), TD.02522 (fat: 6.9 kcal/g, 0.1% calories protein, 1.3% calories carbohydrate, and 98.6% calories fat), and TD02523 (protein 3.2 kcal/g, 96.1% calories protein, 1.4% calories carbohydrate, and 2.6% calories fat); Harlan Teklad] were presented in separate containers simultaneously for 6 days (POD 74–80, PND 157–163). Nutrient intake was monitored daily, and data are depicted for the final 4 days.
Hemoglobin A1c assay.
On POD 98 (PND 181), rats were fasted for 4 h, and tail blood was collected in EDTA tubes and stored at 4°C. Glycated hemoglobin A1c (Hb A1c) was determined using a rat Hb A1c kit (Crystal Chem), following the manufacturer's instructions.
Human subjects.
Blood samples were collected from 46 subjects at the Imperial Weight Centre (Charing Cross Hospital, London, UK) that underwent VSG through Imperial College Healthcare National Health Service Trust (London, UK) between September 2007 and June 2011. All subjects met the National Institute for Clinical Excellence guidelines [a body mass index (BMI) of >40 kg/m2 or a BMI between 35 and 40 kg/m2 and other significant comorbidities (1)] and received preoperative psychological and medical evaluations. The London-Riverside Research Ethics Committee approved the protocols, and informed written consent was obtained from all subjects. Height, weight, and metabolic status were obtained at the initial visit and at each followup visit. Body weight is expressed as percentage of body weight in kilograms at surgery. The BMI was calculated as the weight (kg) divided by the square of the height (square meters). Subjects without a genetic variation in MC4R are indicated as noncarriers.
Sequencing.
Genomic DNA was extracted from white blood cells using standard methods. Eight primers, MC4R-F1 (5′-GCAAC GCTCA GGCTG GAAAC AG-3′), MC4R-R1 (5′-AGAGG TGCAG AGAAG TGTG-3′), MC4R-F2 (5′-CCCAG GAGGT TAAAT CAATT CA-3′), MC4R-R2 (5′-GCAAG CTGCC CAGAT ACAAC T-3′), MC4R-F3 (5′-CTGTA GCTCC TTGCT TGCATC C-3′), MC4R-R3 (5′-CCAGC AGACA ACAAA GACGC-3′), MC4R-F4 (5′-TTGCT GTCCT CCCCG GCACT-3′), and MC4R-R4 (5′-CCAGT ACCCT ACACG GAAGA-3′), were used in PCR to amplify four amplicons covering most of the 5′-untranslated region (UTR), the entire coding region, and the 3′-UTR of MC4R. The sequencing reaction was performed with the BigDye terminator kit (Applied Biosystems, Foster City, CA), using the manufacturer's conditions. Both strands of each amplicon were sequenced on an ABI PRISM 3700 automated DNA sequencer (Applied Biosystems).
Statistics.
All data are presented as means ± SE. Data were analyzed using Statistica 10 (StatSoft, Tulsa, OK). Specifically, data were analyzed analysis of variance (ANOVA) and ANOVA for repeated measurements where necessary and were followed by Fisher's least significant difference test post hoc when appropriate to examine significant overall interactions. Values regarding the statistical analyses are presented in Table 1. The null hypothesis was rejected at the 0.05 level.
Table 1.
Statistical information
| Measurement | Graph | Interaction | F | P |
|---|---|---|---|---|
| Presurgery body weight | Fig. 1A | time × genotype | F(22, 1,122) = 180.943 | <0.001 |
| Presurgery food intake on chow diet (PND 52–55) | Fig. 1B | food intake × genotype | F(2, 102) = 116.579 | <0.001 |
| Presurgery food intake on HFD (PND 72–75) | Fig. 1B | food intake × genotype | F(2, 102) = 188.092 | <0.001 |
| Presurgery feed efficiency on chow diet (PND 52–55) | Fig. 1C | feed efficiency × genotype | F(2, 102) = 15.813 | <0.001 |
| Presurgery feed efficiency on HFD diet (PND 72–75) | Fig. 1C | feed efficiency × genotype | F(2, 102) = 21.799 | <0.001 |
| Postsurgery body weight | Fig. 2A | time × genotype × surgery | F(52, 1,196) = 5.340 | <0.001 |
| Postsurgery food intake | Fig. 2B | genotype × surgery | F(2, 46) = 3.306 | <0.05 |
| Pre- and postsurgery lean mass | Fig. 2D | time × genotype × surgery | F(4, 92) = 0.850 | 0.50 |
| Pre- and postsurgery lean mass | Fig. 2D | main effect: genotype | F(2, 46) = 28.615 | <0.001 |
| Pre- and postsurgery lean mass | Fig. 2D | main effect: time | F(2, 92) = 46.355 | <0.001 |
| Pre- and postsurgery fat mass | Fig. 2E | time × genotype × surgery | F(42, 92) = 2.738 | <0.05 |
| Fasting blood glucose | Fig. 3A | surgery × genotype | F(2, 42) = 3.380 | <0.05 |
| Blood glucose during IPGTT | Fig. 3B | time × genotype × surgery | F(10, 210) = 1.269 | 0.25 |
| Blood glucose during IPGTT | Fig. 3B | time × genotype | F(10, 210) = 11.647 | <0.001 |
| Blood glucose during IPGTT | Fig. 3B | time × surgery | F(5, 210) = 4.715 | <0.001 |
| Glucose AUC | Fig. 3C | surgery × genotype | F(2, 42) = 0.483 | 0.62 |
| Glucose AUC | Fig. 3C | main effect: surgery | F(1, 42) = 19.560 | <0.001 |
| Glucose AUC | Fig. 3C | main effect: genotype | F(2, 42) = 40.779 | <0.001 |
| Fasting plasma insulin | Fig. 3D | surgery × genotype | F(2, 42) = 6.909 | <0.01 |
| Plasma insulin during IPGTT | Fig. 3E | time × genotype × surgery | F(6, 126) =1.501 | 0.18 |
| Plasma insulin during IPGTT | Fig. 3E | time × genotype | F(6, 126) =3.632 | <0.01 |
| Plasma insulin during IPGTT | Fig. 3E | main effect: surgery | F(1, 42) =13.164 | <0.001 |
| Rat blood Hb A1c | Fig. 3F | surgery × genotype | F(2, 44) =0.098 | 0.91 |
| Rat blood Hb A1c | Fig. 3F | main effect: surgery | F(1, 44) =11.412 | <0.01 |
| Total caloric intake | Fig. 4A | surgery × genotype | F(2, 35) =4.015 | <0.05 |
| Percentage macronutrient intake | Fig. 4B | surgery × genotype × macronutrient | F(4, 70) =0.885 | 0.48 |
| Percentage macronutrient intake | Fig. 4B | surgery × macronutrient | F(2, 70) =13.017 | <0.001 |
PND, postnatal day; HFD, high-fat diet; IPGTT, intraperitoneal glucose tolerance test; AUC, area under the curve.
RESULTS
Loss of MC4R signaling in the rat induces hyperphagia and obesity.
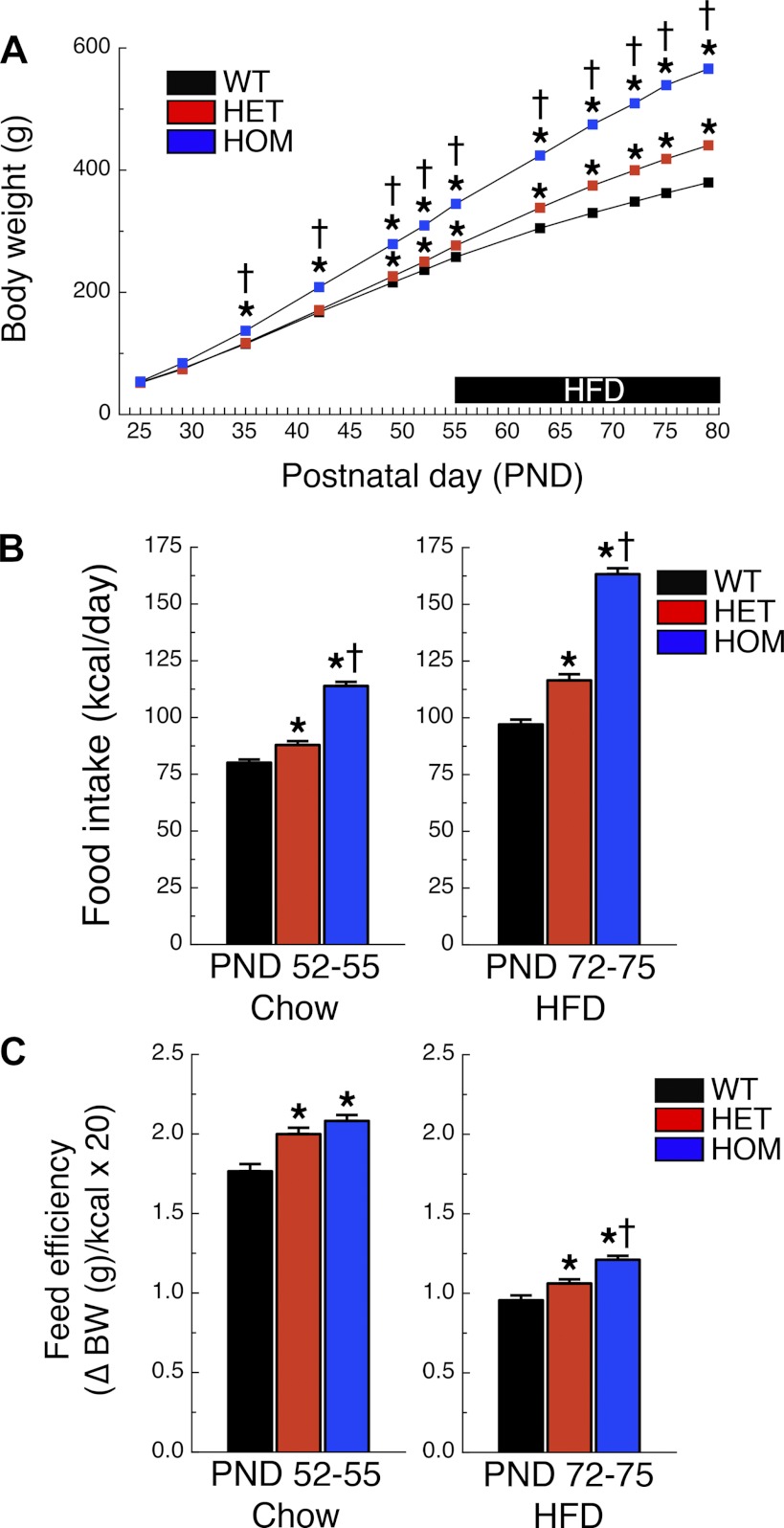
Compared with that of WT rats, body weight of HET rats was higher from PND 52 onward (P < 0.05), and body weight of HOM rats was higher from PND 35 onward (P < 0.05) (Fig. 1A and Table 1). Food intake was higher in HOM rats when the rats were maintained on low-fat chow (PND 52–55) and on the HFD (PND 72–75) relative to WT or HET rats (Ps < 0.001; Fig. 1B and Table 1). Food intake of HET rats was higher than that of WT rats on both LFD (P < 0.005) and HFD (P < 0.001; Fig. 1B). Feed efficiency was higher in HOM rats compared with WT rats on LFD or HFD (P < 0.001) and higher in HOM rats compared with HET rats on HFD only (P < 0.001; Fig. 1C and Table 1). Feed efficiency of HET rats was higher compared with WT rats on LFD (P < 0.001) and HFD (P < 0.01; Fig. 1C).
Fig. 1.
Melanocortin receptor-4 (MC4R) deficiency increases body weight and food intake in the rat. Body weight (A) of wild-type (WT) (n = 34; black squares), heterozygous (HET) (n = 35; red squares), and homozygous (HOM) (n = 36; blue squares) rats during development. From postnatal day (PND) 56 until surgery, rats were fed high-fat diet (HFD; black bar). Food intake (B) and feed efficiency (C) during PND 52–55 (chow) or PND 72–75 (HFD). *P < 0.05 vs. WT; †P < 0.05 vs. HET.
VSG decreases body weight independent of MC4R function.
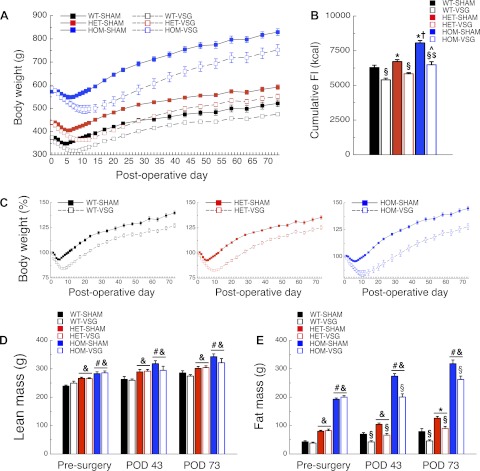
At the time of surgery, body weight of HET and HOM rats was increased relative to that of WT rats (P < 0.001), and body weight of HOM rats was higher than that of HET rats (P < 0.001; Fig. 2A and Table 1). These same relative trends persisted after surgery; i.e., rats in all three groups lost 16–17% of their presurgical weight following VSG (Fig. 2, A and C). WT-SHAM, HET-SHAM, and HOM-SHAM rats lost 7, 7, and 4% of their initial weight, respectively (Fig. 2, A and C).
Fig. 2.
Vertical sleeve gastrectomy (VSG) has beneficial effects on body weight in MC4R-deficient rats. Body weight (A) and total food intake (FI) during postoperative days (POD) 4–73 (B), %body weight relative to the day of surgery (C) following surgery, and lean (D) or fat mass (E) presurgery and on POD 43 and 73 of WT-SHAM (n = 8), WT-VSG (n = 7), HET-SHAM (n = 10), HET-VSG (n = 10), HOM-SHAM (n = 10), and HOM-VSG (n = 7) rats. *P < 0.05 vs. WT-SHAM; †P < 0.05 vs. HET-SHAM; $P < 0.05 vs. WT-VSG; ^P < 0.05 vs. HET-VSG; §P < 0.05 vs. SHAM within genotype. Genotype effects: &P < 0.05 vs. WT; #P < 0.05 vs. HET.
HOM-SHAM rats had higher total food intake following surgery (POD 4–73) than WT-SHAM rats (P < 0.001; Fig. 2B), and HET-SHAM rats were intermediate (P < 0.001 vs. HOM-SHAM, P < 0.05 vs. WT-SHAM; Fig. 2B and Table 1). WT-VSG, HET-VSG, and HOM-VSG had lower total food intake than WT-SHAM, HET-SHAM, and HOM-SHAM rats, respectively, (P < 0.001; Fig. 2B). HOM-VSG rats had higher total food intake than WT-VSG rats (P < 0.001; Fig. 2B), whereas HET-VSG rats had an intermediate phenotype (P < 0.01 vs. HOM-VSG, P = 0.06 vs. WT-VSG; Fig. 2B).
Before and following surgery (POD 43 and 73), lean mass of HOM rats was higher compared with WT rats, whereas HET rats demonstrated an intermediate phenotype (P < 0.001; Fig. 2D and Table 1). Presurgery, fat mass did not differ significantly between sham and VSG rats within genotype groups (Fig. 2E and Table 1). However, presurgery fat of HOM rats was higher than that of WT rats (P < 0.001; Fig. 2E), whereas HET rats demonstrated an intermediate phenotype (P < 0.001; Fig. 2E). On both POD 43 and 73, WT-VSG, HET-VSG, and HOM-VSG had lower fat mass compared with WT-SHAM (P < 0.05), HET-SHAM (P < 0.001), and HOM-SHAM (P < 0.001) rats, respectively (Fig. 2E).
VSG improves glucose metabolism independent of genotype.
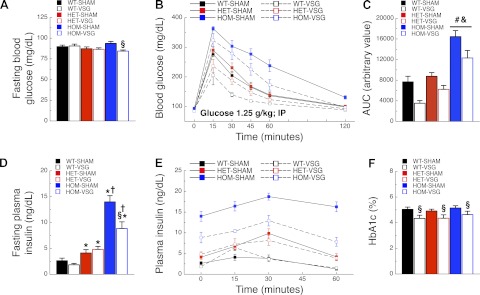
Fasting blood glucose of HOM-SHAM rats was higher than that of HOM-VSG rats (P < 0.01; Fig. 3A and Table 1). During the intraperitoneal glucose tolerance test (IPGTT), HOM rats had higher blood glucose levels than WT and HET rats 15, 30, 45, and 60 min following glucose administration (P < 0.001; Fig. 3B and Table 1). HET rats had higher blood glucose than WT rats 30 min following glucose injection (P < 0.05; Fig. 3B). HOM rats had higher glucose AUC values compared with WT and HET rats (P < 0.001; Fig. 3C and Table 1). Regardless of genotype, VSG rats had lower blood glucose levels than sham rats 15, 30, 45, and 60 min following glucose injection (P < 0.001; Fig. 3B). Fasting plasma insulin of HOM-VSG rats was lower than that of HOM-SHAM rats (P < 0.001), whereas fasting plasma insulin of both HOM-SHAM and HOM-VSG rats was higher compared with all other groups (P < 0.001; Fig. 3D and Table 1). Fasting plasma insulin of HET rats was higher compared with WT rats (P < 0.01; Fig. 3D), and in WT and HET rats we observed no effect of surgery on fasting insulin levels (Fig. 3D). HOM rats had higher plasma insulin levels during the IPGTT than WT and HET rats at 0, 15, 30, and 60 min following glucose injection (P < 0.001; Fig. 3E and Table 1). Independent of surgery, HET rats had higher plasma insulin levels than WT rats at 0, 15, 30, and 60 min following glucose injection (P < 0.05; Fig. 3E). Surgery also had an independent effect on fasting insulin, with VSG rats having lower plasma insulin levels than sham rats (P < 0.001; Fig. 3E and Table 1). Blood Hb A1c levels of VSG rats were lower than those of sham rats (P < 0.01; Fig. 3F and Table 1).
Fig. 3.
VSG has beneficial effects on glucose metabolism in MC4R-deficient rats. Fasting blood glucose levels (A), blood glucose (B), blood glucose as area under the curve (AUC; C), fasting plasma insulin (D), plasma insulin (E), and %blood glycated hemoglobin (Hb A1c; F) of WT-SHAM (n = 8), WT-VSG (n = 6), HET-SHAM (n = 9), HET-VSG (n = 10), HOM-SHAM (n = 8), and HOM-VSG (n = 7) rats before (0 and fasting conditions) and following (15, 30, 45, and 60 min) an intraperitoneal injection of glucose on POD 58 (PND 141). §P < 0.05 vs. SHAM within genotype; *P < 0.05 vs. WT-SHAM or WT-VSG; †P < 0.05 vs. HET-SHAM or HET-VSG. Genotype effects: &P < 0.05 vs. WT; #P < 0.05 vs. HET.
VSG affects macronutrient preference independent of MC4R.
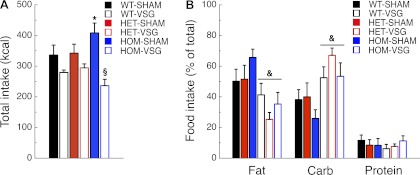
During the macronutrient selection paradigm, total caloric intake was lower in HOM-VSG than in HOM-SHAM rats (P < 0.001; Fig. 4A and Table 1). Total caloric intake was higher in HOM-SHAM than WT-SHAM rats (P < 0.05) but did not differ from HET-SHAM rats (P = 0.07; Fig. 4A). The percentages of each macronutrient consumed are depicted in Fig. 4B. Independently of genotype, VSG rats had decreased preference for fat (P < 0.001), increased preference for carbohydrates (P < 0.001), and no changed preference for proteins (P = 0.82) compared with SHAM rats (Fig. 4B and Table 1). Previously, it has been shown that MC4R-deficient rats have a preference for fat (18). In our current study, sham-operated MC4R-deficient rats showed only an increased trend to prefer fat compared with WT rats. This is likely the result of differences in the testing paradigm used here to assess primarily the effect of the surgery rather than the lack of MC4R signaling.
Fig. 4.
MC4R deficiency and VSG alter macronutrient preference in the rat. Total food intake (A) and %food intake of each macronutrient (B) of WT-SHAM (n = 7), WT-VSG (n = 7), HET-SHAM (n = 7), HET-VSG (n = 7), HOM-SHAM (n = 6), and HOM-VSG (n = 7) rats during POD 76–80 (PND 159–163). *P < 0.05 vs. WT-SHAM; §P < 0.05 vs. SHAM within genotype. Surgery effect: &P < 0.05 vs. SHAM.
Genetic variation in MC4R in a human VSG cohort.
We sequenced the coding region and part of the 3′-UTR of MC4R in 46 subjects who had undergone VSG. This cohort was 74% female, with a mean age of 48.3 ± 1.6 yr and mean BMI of 50.8 ± 1.1 kg/m2 prior to surgery. Five subjects carried common variants in the MC4R coding region. Three subjects were heterozygous, and one subject was homozygous for the common variant rs34114122 (10). Another subject was heterozygous for the common Ile251Leu (I251L; rs52820871) variant (17, 23). It is hypothesized that these common variants do not ablate but may modulate MC4R function (10, 17, 23).
Common genetic variations in MC4R do not affect the outcome of VSG in humans.
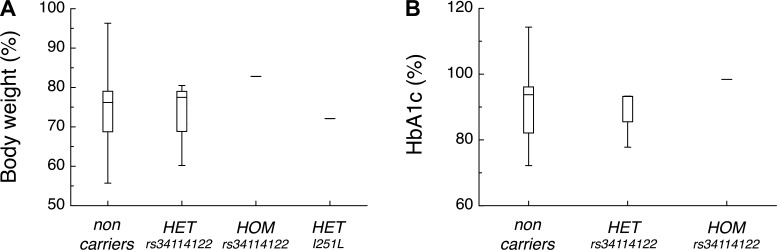
BMI at the time of surgery (data not shown) and weight loss of subjects carrying the rs34114122 or I251L variants did not differ significantly from those of noncarriers (Fig. 5A). Improvements in Hb A1c levels 12 mo following surgery did not differ significantly among carriers of common variants and noncarriers (Fig. 5B).
Fig. 5.
Genetic variations in MC4R do not differentially improve body weight or Hb A1c levels in human subjects following VSG. Box and whisker plots of both %body weight relative to body weight at surgery (A) and %Hb A1c level relative to Hb A1c level at surgery (B) 12 mo following VSG of noncarriers (n = 42), HET carriers of the rs34114122 variant (n = 3), a HOM carrier of the rs34114122 variant (n = 1), and a HET carrier of the I251L variant (n = 1; no Hb A1c data available). The lines within the boxes are the medians. The top and bottom of the boxes are the upper and lower quartiles, respectively. The whiskers are drawn at the maximum and minimum values.
DISCUSSION
Our data indicate that the beneficial effects of VSG on body weight and glucose metabolism in the rat are independent of MC4R function. VSG also affects macronutrient preference in the rat (the present study and Ref. 26), and these effects are independent of MC4R function as well. Finally, in this small-scale human study, the improvements in body weight and Hb A1c levels after VSG in human subjects with the common variants rs34114122 or I251L are not different from those observed in noncarriers.
In this study, we replicated as well as expanded on key elements of the obesity phenotype observed in MC4R-deficient rats (18). In particular, we found that MC4R deficiency in the rat increases lean mass in addition to fat mass and induces glucose intolerance. Together with previous observations (18), our data confirm that the MC4R-deficient rat is a useful animal model to study the components of MC4R signaling that regulate feeding, body weight, and glucose regulation. We also confirmed our previous data indicating that VSG results in sustained body weight loss, lowers cumulative food intake, has little to no effect on lean tissue mass, decreases fat mass, and substantially alters macronutrient choice, increasing preference for carbohydrates and decreasing preference for fat in rats (8, 21, 26). Importantly, each of these potent effects of VSG surgery does not require MC4R signaling.
We reported previously that VSG improves glucose tolerance during an IPGTT (8). In rodents, dynamics of glucose regulation are often assessed via intraperitoneal or gavaged glucose administration, neither of which reflects the natural means of providing a glucose load in humans. However, Hb A1c levels, reflecting the glycosylation of erythrocytes, reflect long-term glucose control. Using this assessment, we observed that Hb A1c levels are improved after VSG regardless of genotype. One interesting finding is that, if anything, the improvements in body weight and glucose homeostasis were slightly larger in HOM rats. This may be a result of the greater initial body weight and body fat in HOM rats at the time of surgery.
The CNS melanocortin system is key for normal energy and glucose homeostasis. This is underscored by the extent of obesity seen with MC4R-deficient individuals, including MC4R-deficient rats. We observed previously that VSG has no weight loss-independent effects on leptin sensitivity or on the expression of neuropeptide-Y, proopiomelanocortin, agouti-related peptide, or Mc4r in the mediobasal hypothalamus (21). Since several hormonal and nutrient signals converge on the CNS melanocortin system, and since gene expression does not necessarily reflect neuronal activity, MC4R deficiency provides a more persuasive probe of the role of the melanocortin system. Consistent with our previous findings, the present data indicate that, in the rat, MC4R function is not crucial for the changes in body weight, glucose metabolism, or macronutrient preference following VSG.
These data highlight the advantages of exploring the mechanisms that underlie the beneficial impact of bariatric procedures in rodent models since it allows for testing pointed hypotheses of the role of specific molecular pathways to mediate the effects of the surgery. It also allows for comparison of data among procedures. To that end, recent data made use of a mouse model of MC4R deficiency to test whether MC4R signaling is required for the effects of RYGB (14). Although the studies by Hatoum et al. (14) differ in use of species, surgical procedure, and postoperative protocol, they found that, similar to our studies with VSG, Mc4r+/− mice benefit from RYGB. Unlike the present data, these authors conclude that Mc4r−/− mice do not benefit from the surgery to the same degree as Mc4r+/− or WT mice. At face value, these data point to the hypothesis that different surgical procedures may engage the MC4R system differently to exert their beneficial effects. However, this conclusion is tempered by the differences in species and surgical approach necessitated by doing these surgeries in the mouse. The potential differences in surgical procedure are highlighted by the results from Hatoum et al. (14), where they combined data from two different institutions that had each used a different approach to the difficult task of performing RYGB in a mouse. Two of the smaller cohorts (n = 3 Mc4r−/−) showed a relatively large impact of genotype to reduce the effect of RYGB on body weight. However, the third and larger cohort (n = 7 Mc4r−/−) done with a different surgical approach showed a more modest effect of genotype. Such differences point to the possibility that surgical approach or other unknown factors could contribute to interactions between genotype and bariatric surgery outcome. Consequently, further research exploring the differences between these two studies is warranted.
In our human cohort that underwent VSG, we observed common genetic variations in MC4R in five of the subjects. Although the precise effect of the rs34114122 variant on MC4R function is not known, it is associated with elevated BMI, fat mass, plasma ghrelin levels, energy intake, and carbohydrate intake (10). Another subject carried the I251L variant, which is also common and appears to be protective against obesity, being associated with greater weight loss after RYGB (17). We observed no difference in weight loss or Hb A1c levels up to 12 mo following VSG between carriers of the common variants and noncarriers. Although the surgical procedures and mutational effects differ, our data align with published reports that heterozygous loss-of-function mutations in MC4R do not affect the outcome of RYGB (3). Since we did not detect a loss-of-function mutation in our pilot VSG cohort, a larger study would be needed to confirm that heterozygous loss-of-function mutations in MC4R do not affect the outcome of VSG. Finally, there was only one carrier of the I251L variant such that we were unable to confirm whether the I251L variant might improve the beneficial outcome of VSG even further, as has been observed for RYGB (17). Although these data suggest that VSG is an appropriate choice for individuals where altered MC4R signaling may contribute to obesity, the data should be considered preliminary. The small number of patients available to us precludes a complete analysis of potential differences among multiple variants, and our data cannot preclude the possibility of smaller effects that cannot be detected in our restricted data set. Nevertheless, these data are consistent with available data from humans with MC4R variants undergoing RYGB, where the conclusion has been that such genetic variants do not alter the response to RYGB (3, 14).
Taken together, our data suggest that MC4R signaling is not necessary for the compelling effects of VSG on food intake, food choice, body weight, or glucose homeostasis. So although it is clear that MC4R signaling is a crucial component of the complex system that regulates energy homeostasis, alterations in MC4R signaling are not an important mechanism underlying the biological impact of VSG surgery. This work points to the viability of using rodent genetic models to identify the molecular pathways that mediate the effects of bariatric surgery and how they may be different for different procedures.
GRANTS
This work was supported by Ethicon Endo-Surgery and grants from the National Institute of Diabetes and Digestive and Kidney Diseases to R. J. Seeley (DK-0938+48). D. P. Begg was supported by an National Health and Medical Research Council Early Career Fellowship (1013264). The human study was supported by grants from the Biomedical Research Centre (Imperial College Academic Health Sciences Centre) and Diabetes UK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.M. and R.J.S. did the conception and design of the research; J.D.M., D.P.B., S.I.A., G.v.H., and K.J.D. performed the experiments; J.D.M., S.I.A., G.v.H., and K.J.D. analyzed the data; J.D.M. interpreted the results of the experiments; J.D.M. prepared the figures; J.D.M. and D.P.B. drafted the manuscript; J.D.M., D.P.B., S.I.A., G.v.H., D.A.D., C.W.L.R., S.C.W., D.A.S., A.I.F.B., E.C., M.M.v.H., and R.J.S. edited and revised the manuscript; J.D.M., D.P.B., S.I.A., G.v.H., K.J.D., D.A.D., C.W.L.R., S.C.W., D.A.S., A.I.F.B., E.C., M.M.v.H., and R.J.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are indebted to all of the subjects who participated in these studies. We thank Kathleen Smith, Kenneth Parks, Mouhamadoul Toure, Jose Berger, and Alfor Lewis for their surgical expertise in conducting the VSG and sham surgeries and all members of the Seeley/Woods laboatory for helpful discussion, comments, and help in performing the IPGTT.
REFERENCES
- 1.National Institute for Health and Clinical Excellence NICE issues guidance on surgery for morbid obesity (Online). http://www.nice.org.uk/guidance/index.jsp?action=article&o=32423; [14 March 2011]. http://www.nice.org.uk/guidance/index.jsp?action=article&o=32423
- 2.Abu-Jaish W, Rosenthal RJ. Sleeve gastrectomy: a new surgical approach for morbid obesity. Expert Rev Gastroenterol Hepatol 4: 101–119, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 21: 930–934, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 35: 457–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 348: 1096–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, Bromberg Y, Pennacchio LA, Dent R, McPherson R, Ahituv N, Vaisse C. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Hum Mol Genet 18: 1140–1147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde S, Sorrell JE, Toure M, Berger J, D'Alessio DA, Sandoval DA, Seeley RJ, Woods SC. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 105: 120–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole SA, Butte NF, Voruganti VS, Cai G, Haack K, Kent JW, Jr, Blangero J, Comuzzie AG, McPherson JD, Gibbs RA. Evidence that multiple genetic variants of MC4R play a functional role in the regulation of energy expenditure and appetite in Hispanic children. Am J Clin Nutr 91: 191–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348: 1085–1095, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106: 271–279, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiy S, Brookes PS, Niijima A, Inui A, Meguid MM. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Physiol Regul Integr Comp Physiol 293: R1474–R1489, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL, Ma LL, Blaszczyk K, Keogh JM, Cone RD, Farooqi IS, Kaplan LM. Melanocortin-4 Receptor Signaling Is Required for Weight Loss after Gastric Bypass Surgery. J Clin Endocrinol Metab. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, Bertrais S, Hercberg S, Basdevant A, Clement K, Vaisse C. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 91: 1811–1818, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab 96: E2088–E2096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, Garner KM, Adan RA, Cuppen E. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 20: 612–621, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 294: 1909–1917, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–2436, 2436.e1–2436.e3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141: 939–949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougneres P, Froguel P, Meyre D. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet 16: 1837–1844, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31: 506–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106: 253–262, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond). In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]