Abstract
We demonstrated previously that the activation of ALK7 (activin receptor-like kinase-7), a member of the type I receptor serine/threonine kinases of the TGF-β superfamily, resulted in increased apoptosis and reduced proliferation through suppression of Akt signaling and the activation of Smad2-dependent signaling pathway in pancreatic β-cells. Here, we show that Nodal activates ALK7 signaling and regulates β-cell apoptosis. We detected Nodal expression in the clonal β-cell lines and rodent islet β-cells. Induction of β-cell apoptosis by treatment with high glucose, palmitate, or cytokines significantly increased Nodal expression in clonal INS-1 β-cells and isolated rat islets. The stimuli induced upregulation of Nodal expression levels were associated with elevation of ALK7 protein and enhanced phosphorylated Smad3 protein. Nodal treatment or overexpression of Nodal dose- or time-dependently increased active caspase-3 levels in INS-1 cells. Nodal-induced apoptosis was associated with decreased Akt phosphorylation and reduced expression level of X-linked inhibitor of apoptosis (XIAP). Remarkably, overexpression of XIAP or constitutively active Akt, or ablation of Smad2/3 activity partially blocked Nodal-induced apoptosis. Furthermore, siRNA-mediated ALK7 knockdown significantly attenuated Nodal-induced apoptosis of INS-1 cells. We suggest that Nodal-induced apoptosis in β-cells is mediated through ALK7 signaling involving the activation of Smad2/3-caspase-3 and the suppression of Akt and XIAP pathways and that Nodal may exert its biological effects on the modulation of β-cell survival and β-cell mass in an autocrine fashion.
Keywords: activin receptor-like kinase-7, X-linked inhibitor of apoptosis protein, Akt, caspase-3, insulin
the tgf-β superfamily consists of a wide range of factors including TGF-β proteins, activins, bone morphogenetic proteins, differentiation factors, and Nodal and its related proteins (4, 17, 26). Members of the TGF-β superfamily regulate diverse cellular processes including cell growth, cell differentiation, extracellular matrix modification, immunosuppression, apoptosis, and other functions (3, 39). A TGF-β protein exerts its biological function through interactions with type I and type II transmembrane serine/threonine kinase receptors on the cell surface (44). Five members of type II and seven members of type I receptors [activin receptor-like kinase (ALK)1–7] have been characterized in mammals (44). Upon ligand binding, type II receptor recruits and phosphorylates a type I receptor to form a ternary ligand-receptor complex (13) and initiates the downstream signaling cascades by phosphorylating the Smad proteins (61, 62). Typically, the phosphorylated receptor-regulated Smads (R-SMAD) can bind to the common-mediator Smad (co-SMAD) to form Smad protein complexes, which are then translocated into the nucleus to act as transcription factors to participate in the regulation of targeted gene expression(44).
ALK7, initially isolated from rat brain as an orphan receptor, is known to be expressed in several tissues including adipose tissue, gut, and pancreas (7, 9, 39, 49). Upon heterodimerization with type II (ActRII) B, ALK7 confers responsiveness to activin-A, activin-AB, and Nodal and its related proteins (47). An in vitro study has demonstrated that ALK7 is specifically expressed during the late phase of adipocyte differentiation (31). Recent studies in rodents and human tissue have shown that the expression levels of ALK7 are negatively correlated with the markers of metabolic syndrome, implying a potential role of ALK7 in the regulation of metabolism and adipose tissue function (2, 31, 34). ALK7 is specifically detected in insulin-producing β-cells (52, 64). A study by Wawenable et al. (60) suggested that Nodal and activin AB could signal through the ALK7-Smad2/3 pathway to regulate PDX-1 (pancreatic duodenal homeobox-1) and proinsulin gene activity. Mice lacking ALK7 displayed islet enlargement, enhanced capacity of insulin secretion, progressive hyperinsulinemia, and impaired glucose tolerance, suggesting that ALK7 plays a negative regulatory role in the modulation of β-cell function (7).
The homeostatic control of β-cell mass is based on the balance in survival and death of the insulin-secreting cells (28). Progressive loss of β-cell mass due to β-cell destruction is implicated in the pathogenesis of type 1 and type 2 diabetes (14, 20, 28, 36). Under pathological conditions, inflammation, lipotoxity, and glucotoxity are the three main stimuli that trigger β-cell apoptosis (28). These stimuli can potentially initiate β-cell apoptosis by activating death receptors at the cell surface, including Fas-Fas ligand interactions (37, 40). Increased expression and activation of Fas in β-cells appear to comprise a molecular event common to the pathogenesis of both type 1 and type 2 diabetes (65). Furthermore, activation of granzymes, or various proinflammatory cytokines, as well as a high concentration of glucose, induces the production of free radicals and oxidative stress, which results in the release of cytochrome c and cell death (1, 28). Islet amyloid formation also plays a role in β-cell apoptosis (11, 28, 38).
We (64) demonstrated previously that endogenous ALK7 levels were increased during the induction of β-cell apoptosis by high glucose and/or fatty acid. Elevation of ALK7 by induction with adenovirus-carrying expression vector coding for active ALK7 resulted in a remarkable inhibition of β-cell proliferation and the acceleration of β-cell apoptosis (64). In the β-cells, activation of ALK7-induced inhibition of cell proliferation and increased apoptosis appeared to be mediated by the Smad2-caspase-3 signaling pathway(s), as demonstrated in many other cell lineages (30, 41, 46, 63), and the suppression of Akt activation (64). Previous in vitro and in vivo studies showed that activins or Nodal may interact with ALK7 to exert downstream events (7, 27, 41, 47).
Here, we show that Nodal is specifically expressed in the pancreatic INS-1 β-cell line and in rodent islet β-cells. Upregulation of Nodal expression increased β-cell apoptosis, which is associated with increased ALK7 expression, the activation of Smad2/3 signaling pathways and the inhibition of Akt activation, along with reduced X-linked inhibitor of apoptosis protein (XIAP) expression. ALK7 knockdown using siRNA strategy significantly attenuated Nodal-induced apoptosis of INS-1 cells. Our results suggest that Nodal, in an autocrine fashion, downregulates β-cell function through the ALK7 and downstream signaling pathways involving the activation of Smad2/3-caspase-3 signaling and the suppression of Akt and XIAP signaling pathways.
MATERIALS AND METHODS
Plasmids, reagents, cells, islets and pancreatic tissue preparation.
The construct pcDNA3 constitutively active (CA) Akt was a gift from J. R. Woodgett (University of Toronto). XIAP was a gift of H. Hiu (University of California, Los Angeles). Expression plasmids for Nodal, wild-type (WT) ALK7, CA (threonine-194 was replaced with aspartic acid) ALK7, dominant negative (DN; lysine-222 was replaced with arginine) ALK7, DN-Smad2, and DN-Smad3 have been reported previously (41, 63). The small interfering (si)RNA reagents (siGENOME SMARTpool targeting rat and mouse ALK7, and Scrambled siRNA) were purchased from Dharmacon RNAi technologies (Chicago, IL). Recombinant mouse Nodal protein was purchased from R&D Systems (cat. no. 1315-ND-025; Minneapolis, MN). Palmitate was dissolved in serum-free RPMI 1640 (Invitrogen, Carlsbad, CA) containing 1% fatty acid-free BSA (Sigma-Aldrich, Oakville, ON, Canada). A cytokine cocktail mixture (IL-1β, 10 ng/ml; TNF-β, 50 ng/ml; and IFN-γ, 50 ng/ml; R&D Systems) was prepared in RPMI 1640. Streptozotocin (Sigma-Aldrich) was freshly prepared in RPMI 1640 prior to treatment. INS-1 and MIN6 clonal cell lines were maintained in RPMI 1640 containing 10 mmol/l HEPES, 10% fetal bovine serum, 100 U/ml penicillin G, 100 μg/ml streptomycin, 1 mol/l sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mol/l NaOH, as described previously (64).
Rat pancreatic islets were isolated from male Sprague-Dawley rats (Charles River Canada, Montreal, QC, Canada), as described previously (64). Islets were maintained in RPMI 1640 medium for 16–24 h prior to the experiments. Mouse pancreata were isolated from CD1 mouse (Charles River Canada), processed, and paraffin- embedded as described previously (50, 57). All procedures complied with guidelines approved by St. Michael's Hospital's animal care committee.
RT-PCR.
Total cellular RNA was extracted from rat islets, brain tissues or cultured cells using Trizol (Invitrogen Life Technologies, Carlsbad, CA). Nodal mRNA transcripts were detected using a one-step RT-PCR kit (Qiagen). Briefly, 100 ng of total RNA was used in 25-μl one-step RT-PCR reactions containing 0.4 mmol/l dNTPs and 0.6 μmol/l of each primer. The template was omitted for the negative control. Thermocycler conditions were 50°C for 30 min, 95°C for 15 min, and 35 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. This was followed by a 10-min extension at 72°C. The RT-PCR products were separated on a 1% agarose gel and visualized with ethidium bromide. The Nodal gene-specific primers used were as follows: forward 5′-CAGAAGCCAACTATGTAGGAGGGTCA-3′ and reverse 5′-CGGGAGCACA GCATGTAGAAGGAAC-3′.
Immunostaining.
Cells grown on coverslips were fixed using 4% paraformaldehyde and permeabilized using 0.1% Triton X-100 in PBS and then blocked (3% bovine serum albumin in PBS, 1 h) prior to overnight incubation with the appropriate antibodies: mouse or goat anti-Nodal IgG (1:500; R&D Systems) and guinea pig anti-insulin (1:1,000; Dako, Mississauga, ON, Canada). The corresponding FITC- or Cy3-conjugated secondary antibodies were used. Pancreatic sections (5 μm) were dual-stained for Nodal [1:100, rabbit polyclonal antibody, Millipore (cat. no. AB4334), Temecula, CA] and insulin (guinea pig anti-insulin, 1:1,000) or glucagon (rabbit anti-glucagon, 1:1,000; Dako) and detected with biotinylated secondary antibodies followed by incubation with avidin-biotin-peroxidase complex (Vector Laboratories) before chromogen staining of DAB (Sigma-Aldrich) or Fuchsin red (Dako), as described previously (50, 51, 57).
DNA transfection.
Cells were transfected with or without indicated plasmid DNA, siRNA constructs, or scrambled siRNA using lipofectamine 2000 according to the manufacturer's instructions. The transfected cells were allowed to grow in Complete medium for 24–72 h posttransfection before treatment with various stimuli as indicated.
Western blot analysis.
Cells were lysed in RIPA lysis buffer containing protease inhibitors phenylmethylsulfonylfluoride (PMSF) (1 mol/l) and EDTA (1 mol/l), Na3VO4 (1 mol/l), and NaF (1 mol/l). Pancreatic tissue (50–100 μg total wet wt) was minced and homogenized in RIPA lysis buffer; 25 μg of protein was loaded and resolved by SDS-PAGE followed by semidry transfer (Bio-Rad Laboratories) to nitrocellulose membranes. The membranes were probed with primary poly- or monoclonal anti-ALK7 antibodies (1:1,000; R&D Systems), anti-phospho-Amad3 (rabbit monoclonal, cat. no. 9520; Cell Signaling), anti-Nodal antibodies (1:500, R&D Systems), anti-XIAP (1:1,000, R&D Systems), anti-Akt (1:1,000) and anti-phospho-Akt (1:1,000; Cell Signaling), and anti-cleaved caspase-3 (1:1,000; Cell Signaling) or anti-β-actin or GAPDH (1:30,000; Abcam, Cambridge, MA) and visualized with horseradish peroxidase-conjugated secondary antibodies using ECL Plus detection (Amersham, Mississauga, ON, Canada). The protein levels were quantified by densitometry analysis using ImageJ (Research Services Branch, National Institute of Mental Health, Bethesda, MD). The graphs were made after normalization with the protein loadings.
Proliferation and cell death assays.
Cell proliferation was measured in INS-1 cells treated with or without Nodal protein using [3H]thymidine (PerkinElmer, Boston, MA) incorporation by incubating the cells with the culture medium containing [3H]methylthymidine for 4 h, as described previously (64). Cell apoptosis was measured by 4′-6-diamidino-2-phenylindole (DAPI) nuclear staining or terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) using the In Situ Cell Death Detection Kit and TMR red (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions, as described previously (59, 64).
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was done with Student's t-test or ANOVA with Tukey's post hoc test as appropriate. Significance was assumed at a P value of <0.05.
RESULTS
Nodal expression was detected in pancreatic β-cells.
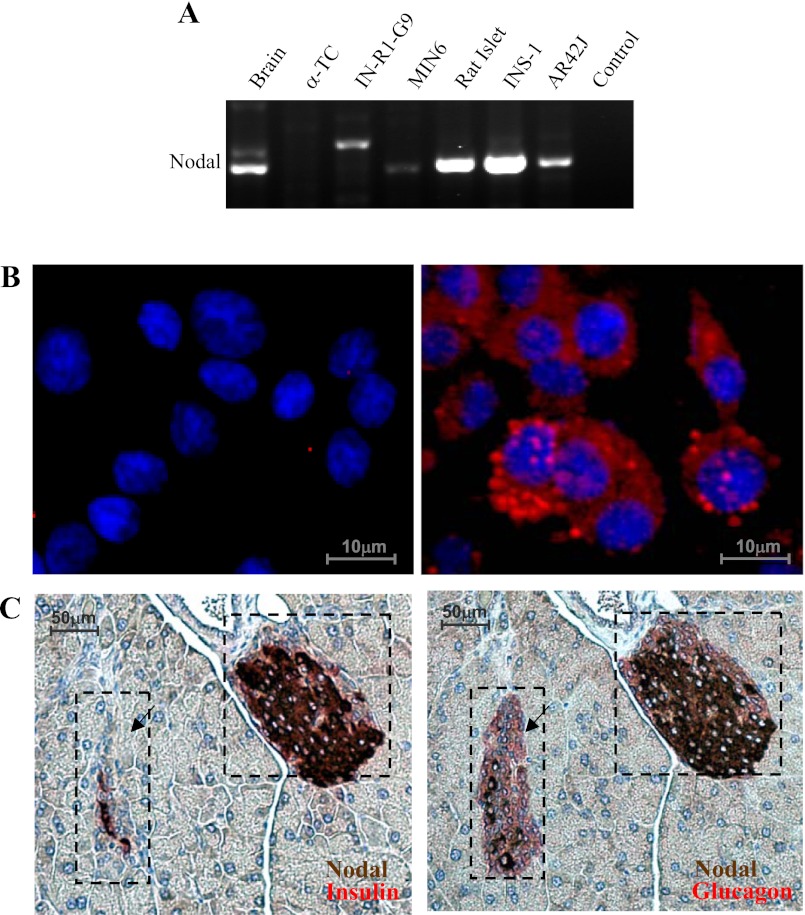
RT-PCR detected the Nodal transcripts in rat islets and insulin-secreting β-cell lines INS-1 and MIN6 (Fig. 1A). Nodal mRNA was also detected in clonal acinar AR42J cells or in the brain as control but not in clonal glucagon-secreting α-TC cells (Fig. 1A). Nodal protein was detected by immunocytochemistry in INS-1 cells (Fig. 1B). Mouse pancreatic sections were dual-stained for insulin (red) and Nodal (brown) (Fig. 1C, left), and the adjacent sections were dual-stained for glucagon (red) and Nodal (brown, Fig. 1C, right). The results showed that Nodal expression is localized mostly in the islet β-cells. In contrast, the detection of Nodal protein in the pancreatic acinar or α-cells was at background levels (Fig. 1C).
Fig. 1.
Expression of Nodal mRNA and protein in pancreatic β-cells. A: 100 ng of total RNA from various tissues or cells as indicated was used for RT-PCR. PCR products were separated on a 1% agarose gel. Control was performed by omitting the RNA. B: microscopic images of INS-1 cells stained with anti-Nodal antibody (rat anti-mouse monoclonal IgG) and detected by Cy3-conjugated secondary antibody (red). DAPI was used for nuclear staining (blue). C: 2 adjacent CD1 mouse pancreatic sections were dual-stained for Nodal (brown) and insulin (red) or glucagon (red) followed by incubation with avidin-biotin-peroxidase complex before chromogen staining of DAB (brown) or Fuchsin red (red) and subsequent hematoxylin counterstaining. Images were visualized with a Nikon fluorescence microscope (n = 5).
Stimuli-induced apoptosis was associated with elevated levels of nodal expression in the clonal β-cells and isolated rat islets.
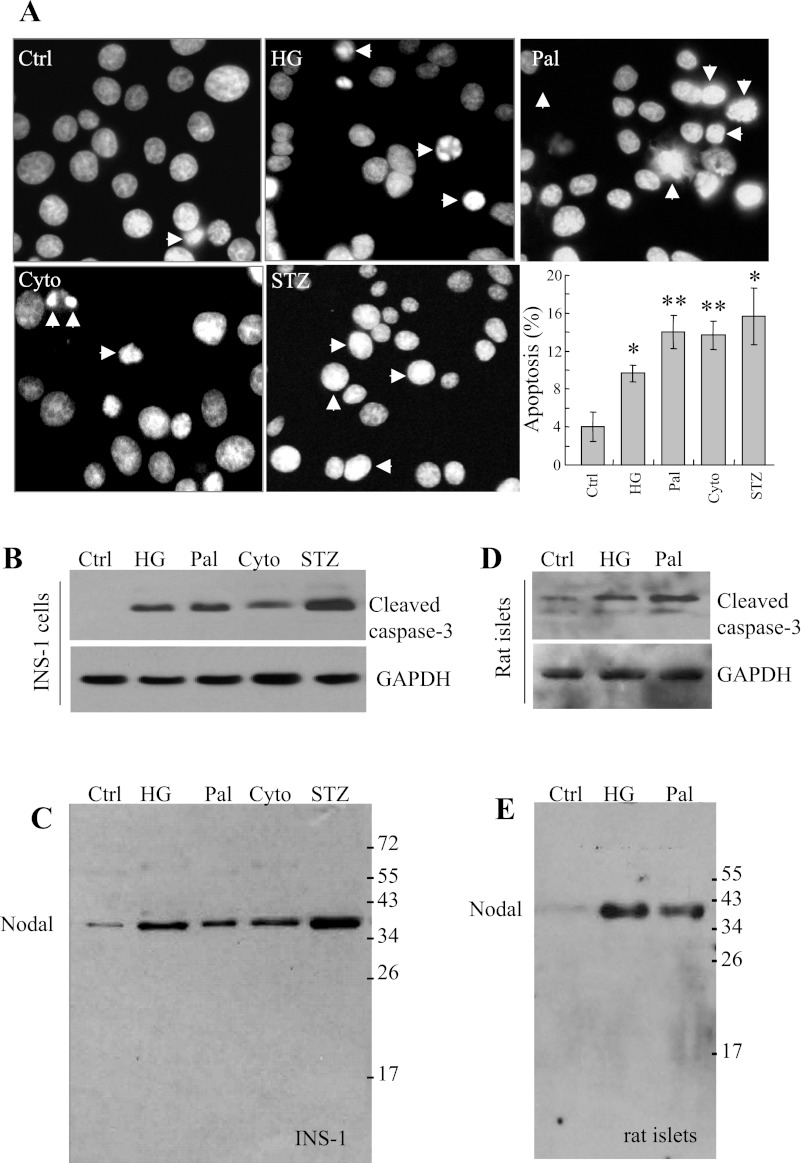
Various stimuli were used for the induction of β-cell apoptosis, including high glucose (30 mM), palmitate (0.4 mM), cytotoxic cytokines (IL-1β 10 ng/ml, TNF-α 50 ng/ml, and IFN-γ 50 ng/ml) and streptozotocin (0.5 mg/ml). Incubation of the INS-1 cells with the stimuli for 24 h increased apoptosis as determined by nuclear staining, with characteristic condensed and fragmented nuclei (Fig. 2A) or by Western blot analysis using pro-caspase-3 antibody (Fig. 2B) or Nodal antibody (Fig. 2C). The results showed that treatment with the stimuli induced upregulation of Nodal protein expression and apoptosis of INS-1 cells. Similar results were obtained in MIN6 cells (not shown) or in isolated rat islets (Fig. 2, D and E). These observations suggest that stimuli-induced apoptosis is associated with elevated Nodal expression in pancreatic β-cells.
Fig. 2.
Pathophysiological stimuli increase Nodal expression, associated with elevated β-cell apoptosis. INS-1 cells were incubated with medium alone or with 30 mM glucose (HG) or 0.4 mM palmitate (Pal) or a cytokine cocktail (Cyto; IL-1β 10 ng/ml, TNF-α 50 ng/ml, and IFN-γ 50 ng/ml), or streptozotocin (STZ; 0.5 mg/ml) for 24 h. A: representative images show DAPI nuclear staining. Arrowheads indicate typical condensed and fragmented nuclei of apoptotic cells; bar graphs represent means ± SE (n = 3). B: cell lysates (25 μg protein) were subjected to Western blotting using active caspase-3 antibody and GAPDH antibody as loading control, or using Nodal antibody (C). Isolated rat islets (30 islets) were treated with medium alone or with HG or Pal for 24 h, and cell lysates were subjected to Western blotting using active caspase-3 antibody and GAPDH antibody as loading control (D), or Nodal antibody (E). Data shown represent 3–5 separate experiments.
Stimuli-induced apoptosis was associated with upregulation of nodal expression and activation of the ALK7-Smad3 signaling pathway.
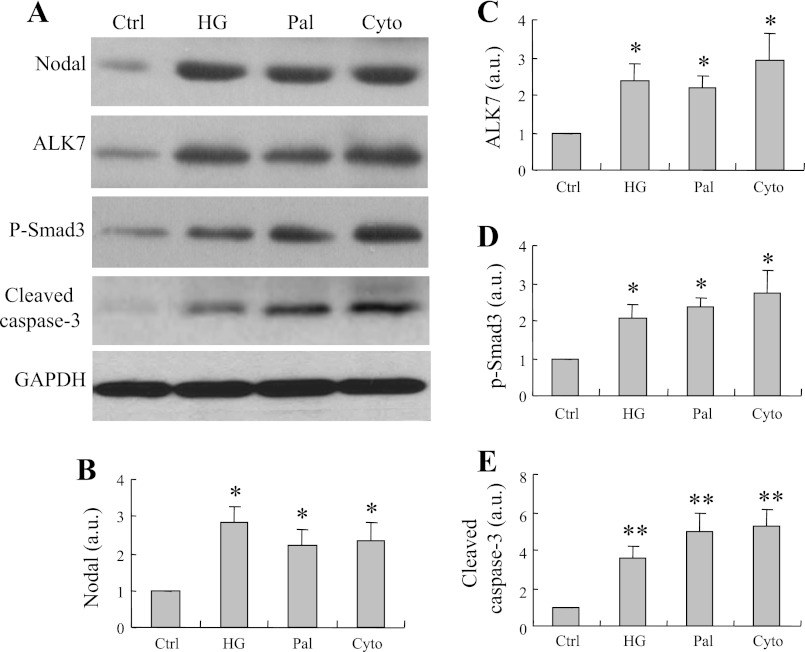
We previously demonstrated that elevation of ALK7 induces β-cell apoptosis (64). To gain insight into the functional roles of Nodal in β-cells, we examined the potential downstream mechanisms involved in the stimuli-induced activation of Nodal signaling. Western blot analysis showed that high glucose-, palmitate-, or proinflammatory cytokine-induced INS-1 cell apoptosis (Fig. 3A) is associated with enhanced Nodal (Fig. 3B), ALK7 (Fig. 3C), and phsopho-Smad3 (Fig. 3D) protein expression, along with increased pro-caspase-3 protein expression (Fig. 3E), suggesting the activation of the Nodal-ALK7-Smad3 pathway during the process of pancreatic β-cell apoptosis.
Fig. 3.
Pathophysiological stimulus-induced apoptosis is associated with upregulation of Nodal expression and activation of activin receptor-like kinase-7 (ALK7)-Smad3 signaling pathway in INS-1 cells. A: Western blotting using protein extracts (25 μg) from INS-1 cells treated with 30 mM HG or 0.4 mM Pal or Cyto cocktail (IL-1β 10 ng/ml, TNF-α 50 ng/ml, and IFN-γ 50 ng/ml) or STZ (0.5 mg/ml) for 24 h and probed for Nodal, ALK7, phosphorylated Smad3, active caspase-3, or GAPDH as loading control. B–E: bar graphs represent densitometry analysis. Data are means ± SE; n = 3–5. *P < 0.05, **P < 0.01.
Nodal induced apoptosis and suppressed proliferation of INS-1 cells.
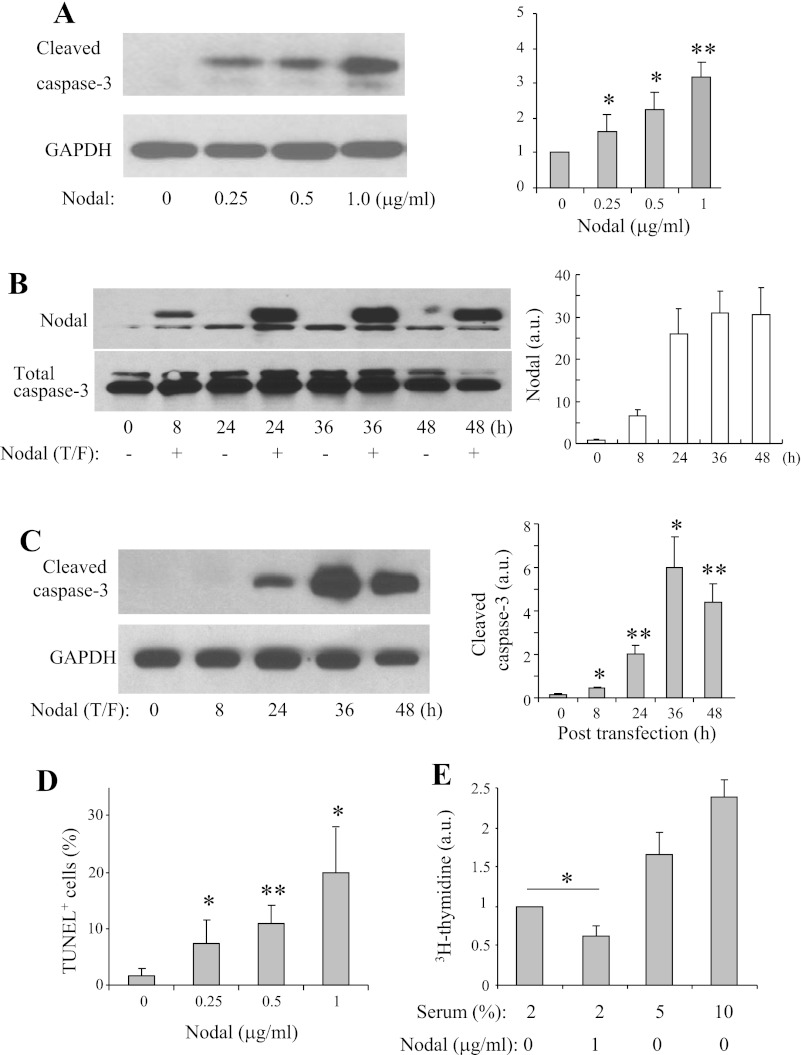
To determine whether Nodal exerts direct apoptotic effects on β-cells, we performed Western blotting in INS-1 cells treated with increased concentrations of Nodal (16 h). As shown in Fig. 4A, Nodal significantly and dose-dependently increased active caspase-3 expression in the INS-1 cells. Consistently, elevation of Nodal protein levels by plasmid transfection resulted in significant and time-dependent increases in pro-caspase-3 levels in INS-1 cells (Fig. 4B). Nodal-induced INS-1 β-cell apoptosis was also verified by the TUNEL-labeling method (Fig. 4C). Furthermore, the effects of Nodal on β-cell proliferation were evaluated by the [3H]thymidine incorporation assay. Nodal treatment (1 μg/ml) significantly inhibited the proliferation of INS-1 cells (Fig. 4D). We therefore suggest that Nodal induces β-cell apoptosis and inhibits β-cell proliferation.
Fig. 4.
Nodal induces apoptosis and suppresses proliferation in INS-1 cells. A: Western blot analysis was performed in INS-1 cells treated with medium alone or with Nodal as indicated for 16 h. B: Western blotting detection of Nodal expression in INS-1 cells transfected with Nodal cDNA for indicated time. C: Western blot analysis was performed in INS-1 cells transfected with Nodal cDNA for indicated time. D: INS-1 cells were incubated with medium alone or with Nodal as indicated for 16 h, and apoptosis was quantified by TUNEL labeling. E: [3H]thymidine incorporation assay performed in INS-1 cells treated with or without Nodal (1 μg/ml) in the presence of 2% fetal bovine serum for 24 h (5 and 10% serum were used as positive controls). Data represent means ± SE; n = 3–5. *P < 0.05, **P < 0.01.
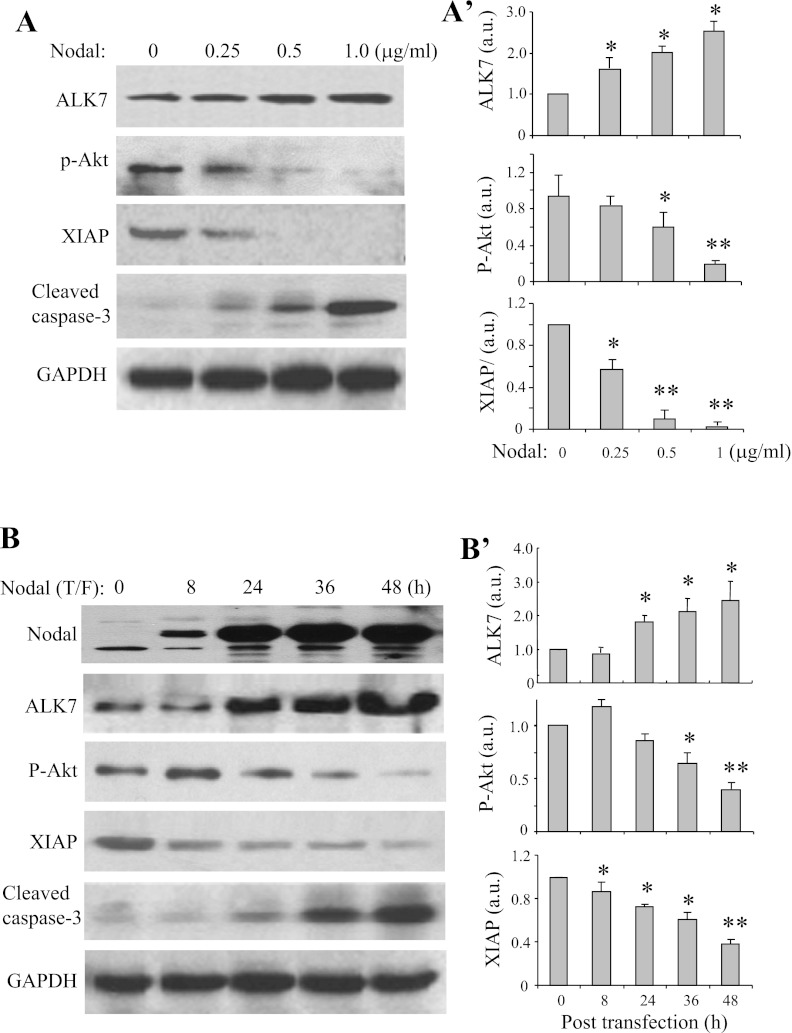
Nodal upregulated ALK7, suppressed akt activity, and reduced XIAP expression.
To determine whether Nodal affects ALK7 expression, Western blot analysis was performed in INS-1 cells treated with medium alone or with Nodal protein at indicated concentrations for 24 h (Fig. 5A) or in INS-1 cells transfected with Nodal cDNA for the indicated time periods (Fig. 5B). As shown, Nodal treatment or transfection of Nodal-encoding plasmid dose- or time-dependently increased ALK7 expression levels (Fig. 5, A and B). Given that elevation of ALK7, while activating Smad2 signaling significantly suppresses Akt activities (64), Western blotting was also performed to probe for Akt and its potential downstream molecule XIAP (15). The results showed that Nodal treatment or transfection of Nodal-encoding plasmid dose- or time-dependently reduced phospho-Akt levels (Fig. 5, A and B), whereas total Akt protein levels remained unchanged (not shown). Concomitantly, the treatment significantly reduced XIAP expression in the INS-1 β-cells (Fig. 5, A and B). These results suggest that Nodal-induced apoptosis is involved with the elevation of ALK7, suppression of Akt activity, and reduced XIAP expression in pancreatic β-cells.
Fig. 5.
Nodal treatment upregulates ALK7 and decreases active Akt and X-linked inhibitor of apoptosis (XIAP) expression in INS-1 cells. Western blotting used protein extracts (25 μg) from INS-1 cells treated with Nodal protein as indicated for 24 h (A) or from INS-1 cells transfected with Nodal cDNA for indicated time period (B), using relevant antibodies as indicated and GAPDH as loading controls. Bar graphs show densitometry analysis. Data represent means ± SE; n = 3–5. *P < 0.05, **P < 0.01.
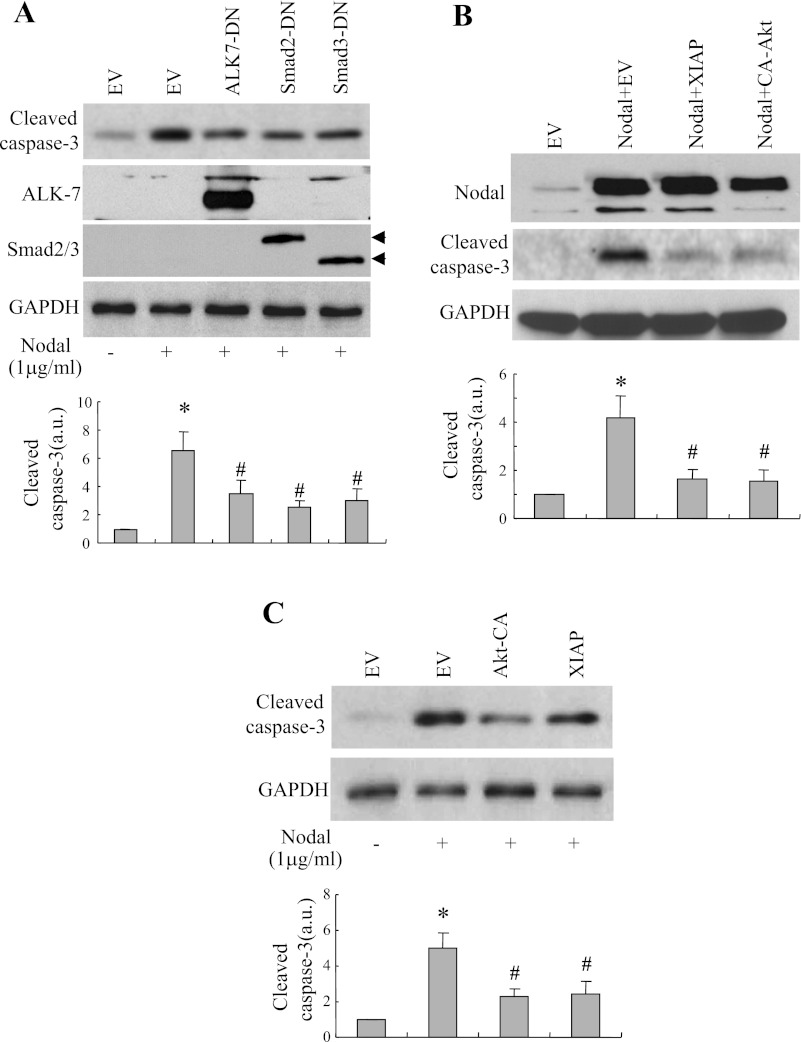
Elevation of Akt/XIAP or suppression of ALK7/Smad2 attenuated Nodal-induced β-cell apoptosis.
Experiments involving cotransfection combined with Western blotting were conducted to examine the role of ALK7/Smad2/3 and of Akt/XIAP in mediating Nodal-induced apoptosis in INS-1 cells. As shown, the β-cell apoptosis determined by pro-caspase-3 levels was consistently increased in the INS-1 cells cotransfected with Nodal and empty vector (Fig. 6A). Cotransfection of Nodal with DN-ALK7, DN-Smad2, or DN-Smad3 significantly reduced active caspase-3 protein levels in these cotransfected INS-1 cells (Fig. 6A). Furthermore, Nodal-induced apoptosis either by transfection of Nodal cDNA (Fig. 6B) or Nodal protein treatment (Fig. 6C) was dramatically reduced in INS-1 cells cotransfected with expression vectors coding for XIAP or the active form of Akt (Akt-CA). These results collectively suggest that pancreatic β-cell apoptosis induced by Nodal occurs via two distinct signaling pathways: activation of the Smad2/3-caspase-3 cascade and suppression of Akt/XIAP signaling.
Fig. 6.
Nodal-induced apoptosis is modulated by various signaling molecules. A: Western blotting was performed using protein extracts (25 μg) from INS-1 cells transfected with expression vectors coding for Nodal and either empty vector (EV), or dominant negative (DN)-ALK7, DN-Smad3, or DN-Smad2 for 36 h or from INS-1 cells cotransfected with Nodal cDNA and EV or Nodal cDNA with constitutively active (CA)-Akt or with XIAP (B) or transfected with or without CA-Akt or XIAP and exposed to Nodal protein (1 μg/ml) for 24 h using anti-cleaved caspase-3, anti-Akt, or anti-GAPDH antibodies (C). Data represent ≥3–4 experiments.
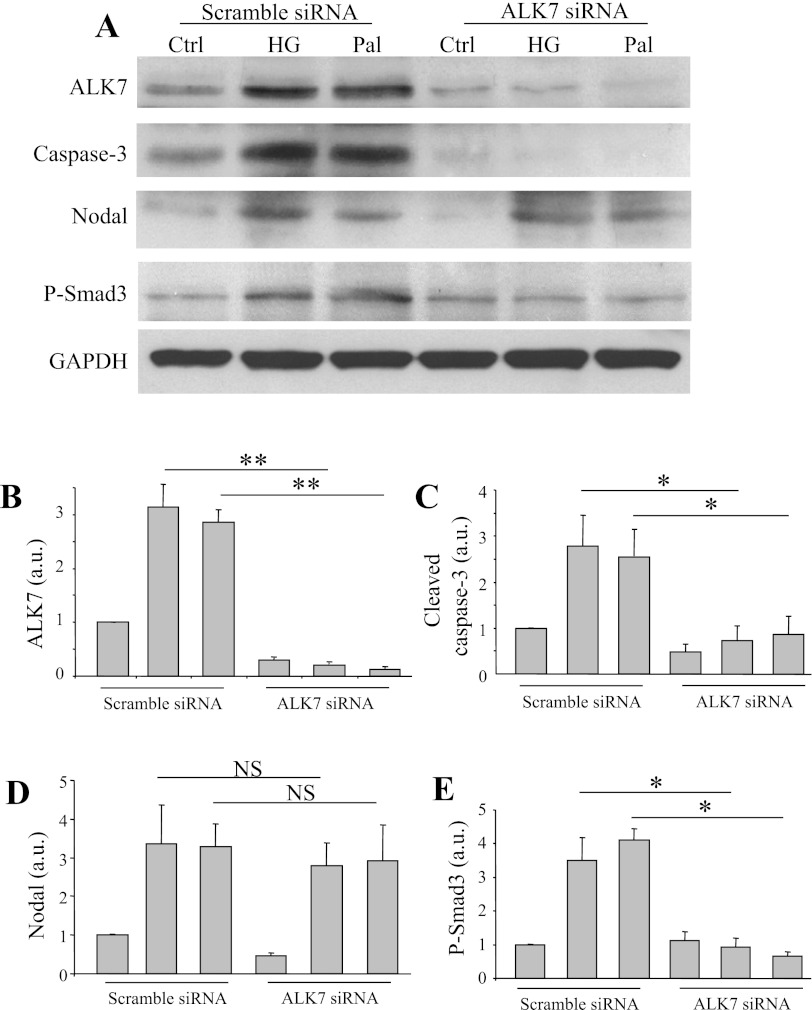
Ablation of ALK7 diminished stimulus-induced β-cell apoptosis.
To verify the role of Nodal/ALK7 in mediating stimulus-induced β-cell apoptosis, we examined the effect of ALK7 knockdown on high-glucose- or palmitate-induced apoptosis in INS-1 cells by using the siRNA strategy. As shown, transfection of INS-1 cells with ALK7 siRNA resulted in a reduction of 75–80% of ALK7 compared with transfection with scrambled siRNA (Fig. 7, A and B). Induction of apoptosis by 30 mM glucose or 0.4 mM palmitate, which occurred in the control INS-1 cells as determined by active caspase-3 protein, was significantly attenuated in the INS-1 cells with ALK7 knockdown (Fig. 7C). The high-glucose or palmitate treatment enhanced phospho-Smad3 in the control cells but not in the cells transfected with ALK7 siRNA. Interestingly, both high glucose and palmitate enhanced Nodal expression levels in the INS-1 cells with either the scramble siRNA or the ALK7 siRNA transfections (Fig. 7E).
Fig. 7.
Ablation of ALK7 diminished apoptosis induced by pathophysiological stimuli in INS-1 cells. A: INS-1 cells transfected with scramble siRNA or with ALK7 siRNA constructs were treated with medium alone or with 30 mM glucose or 0.4 mM palmitate for 24 h. Cell lysates were subjected to Western blot analysis using relevant antibodies as indicated. Densitometry analysis for ALK7 (B), active caspase-3 (C), Nodal (D), or phospho-Smad3 (E) is shown. Data represent means ± SE; n = 3. *P < 0.05, **P < 0.01.
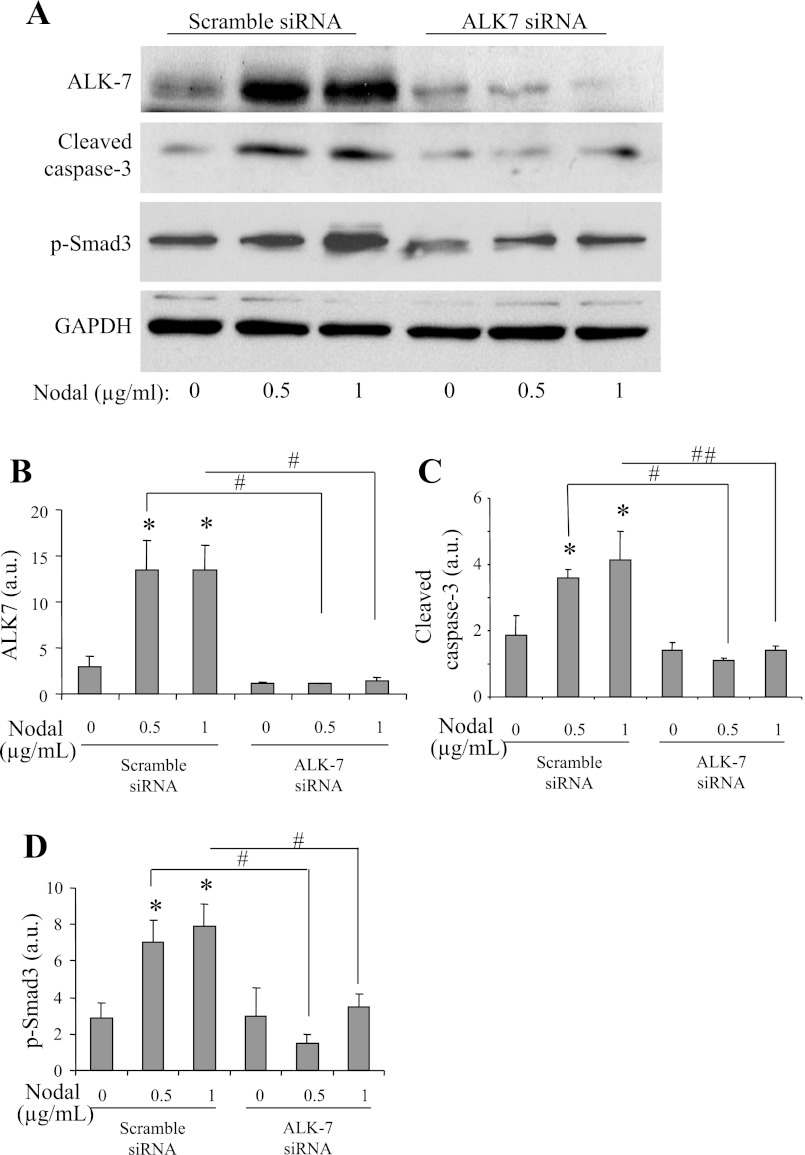
To further verify the role of ALK7 in mediating Nodal-induced β-cell apoptosis, we conducted ALK7 knockdown in INS-1 cells treated without or with Nodal. As shown in Fig. 8, Nodal-induced β-cell apoptosis, which occurred in the INS-1 cells transfected with the scramble siRNA, was significantly attenuated in the β-cells transfected with the specific ALK7 siRNAs. Of note, ALK7 knockdown also diminished Smad2/3 activation in the presence of Nodal, indicating that Nodal-induced apoptosis in INS-1 cells is partially via activation of the ALK7-Smad2/3 signaling pathway. Collectively, these data suggest that Nodal modulates cell apoptosis via the activation of ALK7 signaling pathway(s) in INS-1 cells.
Fig. 8.
Ablation of ALK7-diminished apoptosis induced by Nodal in INS-1 cells. A: Western blot analysis of INS-1 cells transfected with scramble siRNA or with ALK7 siRNA constructs and treated without or with Nodal at indicated concentrations for 24 h. Densitometry analysis for ALK7 (B), active caspase-3 (C), Nodal (D), or phospho-Samd3 (E) is shown. Data represent means ± SE; n = 3. *P < 0.05 vs. control; #P < 0.05 ALK7 siRNA vs. scramble.
DISCUSSION
In this study, we demonstrate that Nodal is expressed in pancreatic cell lines and rodent islets. Immunohistochemistry using mouse pancreatic section localized Nodal protein predominantly in the islet β-cells but not in the islet α-cells or acinar cells. We found that, in a process of β-cell apoptosis induced by stimuli including high glucose, palmitate, and cytotoxic cytokines, the expression levels of Nodal were significantly increased in clonal β-cells and isolated rat islets. Nodal could induce β-cell apoptosis, exemplified by the treatment of INS-1 cells with Nodal protein or Nodal-encoding plasmid transfection. Nodal-mediated β-cell apoptosis was associated with the elevation of ALK7 and phospho-Smad2/3, as well as the reduction in Akt activity and XIAP expression. We previously demonstrated that ALK7 mediated β-cell apoptosis through suppression of Akt and activation of Smad2 signaling pathways (64). Our observations from this study showed that Nodal-induced apoptosis was attenuated in the β-cells expressing dominant negative forms of ALK7 or Smad2/3 or expressing active Akt or XIAP, suggesting that Nodal uses ALK7 signaling to execute apoptosis in the pancreatic β-cells. Our knockdown studies confirmed the role of ALK7 in mediating apoptotic effects of Nodal in the β-cells. These findings also imply a pathophysiological role of Nodal-ALK7 signaling in gluco- and lipotoxicity-induced pancreatic β-cell apoptosis.
To our knowledge, this is the first study in which Nodal protein has been demonstrated to play a role in the modulation of β-cell survival. Although Nodal has been known to be critical in early embryonic development (10, 42), its biological function in adult tissue remains largely unknown. The observations from the present study demonstrate that Nodal indeed inhibits β-cell proliferation and induces apoptosis in pancreatic β-cells, consistent with previous findings that Nodal exerts proapoptotic or growth-inhibitory effects in various cell lineages (25, 41, 48, 54, 55), including the clonal pancreatic acinar cells AR42J (67). In ovary and placenta, through the activation of ALK7 (47), Nodal has been reported to induced apoptosis in these reproductive adult tissues (56).
Modulation of β-cell survival is a critical process in maintaining the homeostasis of β-cell mass. The serine/threonine kinase Akt has been implicated in the proliferation and survival of pancreatic β-cells (23, 24). We previously showed that the active Akt content in islet β-cells was significantly reduced during the progression of diabetes in a rat model (68) and that ALK7-induced β-cell apoptosis and suppression of β-cell growth was involved in the suppression of Akt kinase activity (64). We sought to determine whether Nodal-induced apoptosis affects the signaling pathway involving Akt and its potential downstream target XIAP (15). Our results showed that treatment of INS-1 cells with Nodal or Nodal-encoding plasmid transfection dose- and time-dependently reduced levels of phosphorylated/active Akt and XIAP expression, consistent with previous findings in other cell lineages that TGF-β mediated the regulation of apoptosis involved in the inhibition of the Akt-XIAP survival pathway (12). These data indicate that Nodal downregulates β-cell growth and survival signaling pathways through the suppression of Akt and XIAP.
Our cotransfection studies further characterized the role of the Nodal-ALK7 pathway in β-cells. We observed that elevation of Nodal by transfection or Nodal protein treatment induced apoptosis in INS-1 cells, which was attenuated in the β-cells coexpressing the active Akt or XIAP protein. The diminished apoptosis was also observed in the β-cells cotransfected with dominant negative Smad2 or dominant negative Smad3. These findings suggest that Nodal-induced apoptosis and suppression of proliferation in the β-cells may use intracellular machinery that involves the activation of Smad2/3 and the suppression of Akt and XIAP signaling pathways.
Akt plays an important role in the promotion of cell survival and the inhibition of apoptosis induced by apoptotic stimuli (19). Modulation of the PI3K/Akt survival pathway by TGF-β superfamily protein has been demonstrated (12). Furthermore, it has been shown that activation of the TGF-β heteromeric receptor complexes at the cell surface could initiate the Smad-independent PI3K/Akt signaling cascade (5, 17) and the Smad-dependent pathway through direct interaction between Smad molecule with Akt (12). Taking into account that upregulation of Akt activity could prevent Nodal- (this study) and ALK7-induced β-cells death in the absence of ligand through overexpression of constitutively active ALK7, as we reported previously (64), it could not, however, attenuate ALK7-induced Smad2 phosphorylation (64). It is possible that Nodal/ALK7-Akt signaling is distinct from the ALK7-Smad2/3 pathway.
The observation that the elevation of Akt or XIAP expression attenuates Nodal-induced apoptosis in pancreatic β-cells is consistent with previous findings by other groups, which demonstrates that upregulation of XIAP by induction with adenovirus-carried vector coding for XIAP improved β-cell survival against cytokine attacks in isolated human islets and partially restored insulin secretion in murine islet allografts (29, 45). It has been presumed that, while antiapoptotic effects of Akt occur through inactivating various proapoptotic factors (19), the antiapoptotic effects of XIAP may occur through direct binding and inhibition of caspase activity (18). Furthermore, it has been previously shown that XIAP could also act as a cofactor in the TGF-β signaling pathway (8). Indeed, the interaction between Akt and XIAP has also been suggested by recent studies in various cell lineages (12). Particularly, it has been shown that Akt could directly interact with and phosphorylate XIAP (16), and the phosphorylation of XIAP by Akt could prevent XIAP degradation and thus confer resistance to caspase-3 activation and apoptosis (16). On the other hand, in vitro and in vivo studies by Van Themsche et al. have recently suggested that XIAP can promote Akt activity through the downregulation of PTEN (53), a dual-specificity phosphatase that negatively regulates Akt activity (12, 29). Moreover, our data suggest an important role of Akt-XIAP in modulating β-cell apoptosis mediated by Nodal-ALK7 activation. Further studies are warranted to investigate mechanistically how Nodal/ALK7 signaling modulates the function of Akt and XIAP in β-cells.
The biological relevance of Nodal-ALK7 signaling in β-cells is largely unknown. Identification of Nodal in adult rodent islet β-cells but not in the α-cells suggests an autocrine role of Nodal in the regulation β-cell function. Within the pancreatic islets, insulin is an important autocrine regulator of β-cell growth and survival (6, 32, 35, 58). Such autocrine regulation appears to be critical in regulating the functional plasticity of pancreatic β-cell mass, such as the promotion of islet β-cell growth and the compensation for peripheral insulin resistance (33, 43). We recently demonstrated that the β-cell autocrine trophic effects converged on Akt to regulate β-cell mass (51). It is probable that Nodal in the islet β-cells acts as an antagonistic factor to the β-cell growth signaling pathway. The notion that Nodal is a negative modulator in the regulation of islet β-cell mass is in part supported by the recent findings in mice lacking ALK7, which displayed enlargement of islet β-cells, hyperinsulinemia, reduced insulin sensitivity, and impaired glucose tolerance (7). A recent study demonstrated that Nodal exerted inhibitory effects in the process of pancreatic growth and in a murine model of islet regeneration (67). Consistent with this notion, our unpublished data (not shown) showed that the islet Nodal content was remarkably reduced in the pancreatic sections from mice which had undergone pancreatectomy.
Within an islet, the negative short-loop insulin-β-cell feedback mechanism appears to be important for maintaining insulin secretion at appropriate levels (66), since inadequate feedback suppression is found in obese subjects and may partly account for their prevailing hyperinsulinemia (22). It is possible, although highly speculative, that Nodal acts as a negative regulator to induce β-cell apoptosis whenever it is necessary, for example, deleting those β-cells that are injured or with misplaced functions to permit a process of islet β-cell self-duplication (21). Nevertheless, further study is needed to investigate whether or not the dysregulation of Nodal/ALK7 signaling that leads to excessive loss of islet β-cells, contributing to the onset of diabetic hyperglycemia, is warranted.
GRANTS
The majority of this work was supported by an operating grant from the Canadian Diabetes Association to Q. Wang. Other contributions: Canadian Institute of Health Research (CIHR) Canada-China Initiative Operating Grant to Q. Wang (Co-PI: R. Hu), CIHR Grant MOP-81370 to C. Peng, CIHR Grant MOP-89987 to T. J. Q. Wang is presently supported by the New Investigator Program from CIHR. C. Peng was a recipient of a mid-career award from OWHC/CIHR. F. Huang was supported by the Chinese Overseas Scholarship Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.Z., F.H., M.T., X.L., N.Z., and A.A. performed experiments; F.Z., F.H., M.T., X.L., N.Z., A.A., C.P., and Q.W. analyzed data; F.Z., F.H., X.L., C.P., and Q.W. interpreted results of experiments; F.Z., F.H., M.T., X.L., N.Z., and A.A. prepared figures; F.Z., F.H., Y.L., R.H., T.J., C.P., and Q.W. edited and revised manuscript; F.Z., F.H., M.T., X.L., N.Z., A.A., Y.L., R.H., T.J., C.P., and Q.W. approved final version of manuscript; Q.W. conception and design of research; Q.W. drafted manuscript.
ACKNOWLEDGMENTS
Dr. F. Huang's present address: School of Life Science and Technology, China Pharmaceutical University, Nanjing, People's Republic of China.
REFERENCES
- 1. Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem 16: 705–713, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci USA 105: 7252–7256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol 12: 235–243, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab 295: E751–E761, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren PO, Ibanez CF. Activin B receptor ALK7 is a negative regulator of pancreatic beta-cell function. Proc Natl Acad Sci USA 105: 7246–7251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birkey RS, Wurthner JU, Parks WT, Roberts AB, Duckett CS. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signaling. J Biol Chem 276: 26542–26549, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bondestam J, Huotari MA, Moren A, Ustinov J, Kaivo-Oja N, Kallio J, Horelli-Kuitunen N, Aaltonen J, Fujii M, Moustakas A, Ten DP, Otonkoski T, Ritvos O. cDNA cloning, expression studies and chromosome mapping of human type I serine/threonine kinase receptor ALK7 (ACVR1C). Cytogenet Cell Genet 95: 157–162, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev 16: 2339–2344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52: 2304–2314, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Caron PL, Frechette-Frigon G, Shooner C, Leblanc V, Asselin E. Transforming growth factor beta isoforms regulation of Akt activity and XIAP levels in rat endometrium during estrous cycle, in a model of pseudopregnancy and in cultured decidual cells. Reprod Biol Endocrinol 7: 80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massague J. Determinants of specificity in TGF-beta signal transduction. Genes Dev 12: 2144–2152, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54, Suppl 2: S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279: 5405–5412, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279: 5405–5412, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388: 300–304, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Dickson LM, Rhodes CJ. Pancreatic β-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab 287: E192–E198, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 48: 738–744, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Elahi D, Nagulesparan M, Hershcopf RJ, Muller DC, Tobin JD, Blix PM, Rubenstein AH, Unger RH, Andres R. Feedback inhibition of insulin secretion by insulin: relation to the hyperinsulinemia of obesity. N Engl J Med 306: 1196–1202, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab 20: 243–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 55: 318–325, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Fu G, Peng C. Nodal enhances the activity of FoxO3a and its synergistic interaction with Smads to regulate cyclin G2 transcription in ovarian cancer cells. Oncogene 30: 3953–3966, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Graham H, Peng C. Activin receptor-like kinases: structure, function and clinical implications. Endocr Metab Immune Disord Drug Targets 6: 45–58, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Ho DM, Chan J, Bayliss P, Whitman M. Inhibitor-resistant type I receptors reveal specific requirements for TGF-beta signaling in vivo. Dev Biol 295: 730–742, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Hui H, Dotta F, Di Mario U, Perfetti R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol 200: 177–200, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hui H, Khoury N, Zhao X, Balkir L, D'Amico E, Bullotta A, Nguyen ED, Gambotto A, Perfetti R. Adenovirus-mediated XIAP gene transfer reverses the negative effects of immunosuppressive drugs on insulin secretion and cell viability of isolated human islets. Diabetes 54: 424–433, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Kim BC, van Gelder H, Kim TA, Lee HJ, Baik KG, Chun HH, Lee DA, Choi KS, Kim SJ. Activin receptor-like kinase-7 induces apoptosis through activation of MAPKs in a Smad3-dependent mechanism in hepatoma cells. J Biol Chem 279: 28458–28465, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Kogame M, Matsuo S, Nakatani M, Kurisaki A, Nishitani H, Tsuchida K, Sugino H. ALK7 is a novel marker for adipocyte differentiation. J Med Invest 53: 238–245, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Kulkarni RN. New insights into the roles of insulin/IGF-I in the development and maintenance of beta-cell mass. Rev Endocr Metab Disord 6: 199–210, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kulkarni RN, Winnay JN, Daniels M, Bruning JC, Flier SN, Hanahan D, Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest 104: R69–R75, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lage K, Hansen NT, Karlberg EO, Eklund AC, Roque FS, Donahoe PK, Szallasi Z, Jensen TS, Brunak S. A large-scale analysis of tissue-specific pathology and gene expression of human disease genes and complexes. Proc Natl Acad Sci USA 105: 20870–20875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leibiger IB, Leibiger B, Berggren PO. Insulin signaling in the pancreatic beta-cell. Annu Rev Nutr 28: 233–251, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Leibowitz G, Yuli M, Donath MY, Nesher R, Melloul D, Cerasi E, Gross DJ, Kaiser N. Beta-cell glucotoxicity in the Psammomys obesus model of type 2 diabetes. Diabetes 50, Suppl 1: S113–S117, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, Halban PA, Donath MY. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci USA 99: 8236–8241, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes 55: 2192–2201, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moriwaki M, Itoh N, Miyagawa J, Yamamoto K, Imagawa A, Yamagata K, Iwahashi H, Nakajima H, Namba M, Nagata S, Hanafusa T, Matsuzawa Y. Fas and Fas ligand expression in inflamed islets in pancreas sections of patients with recent-onset Type I diabetes mellitus. Diabetologia 42: 1332–1340, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem 279: 31277–31286, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418: 96–99, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104: 8977–8982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng C, Mukai ST. Activins and their receptors in female reproduction. Biochem Cell Biol 78: 261–279, 2000 [PubMed] [Google Scholar]
- 45. Plesner A, Liston P, Tan R, Korneluk RG, Verchere CB. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes 54: 2533–2540, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Ragazzon B, Cazabat L, Rizk-Rabin M, Assie G, Groussin L, Fierrard H, Perlemoine K, Martinez A, Bertherat J. Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells. Cancer Res 69: 7278–7284, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev 15: 2010–2022, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roberts HJ, Hu S, Qiu Q, Leung PC, Caniggia I, Gruslin A, Tsang B, Peng C. Identification of novel isoforms of activin receptor-like kinase 7 (ALK7) generated by alternative splicing and expression of ALK7 and its ligand, Nodal, in human placenta. Biol Reprod 68: 1719–1726, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Ryden M, Imamura T, Jornvall H, Belluardo N, Neveu I, Trupp M, Okadome T, Ten DP, Ibanez CF. A novel type I receptor serine-threonine kinase predominantly expressed in the adult central nervous system. J Biol Chem 271: 30603–30609, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Soltani N, Kumar M, Glinka Y, Prud'homme GJ, Wang Q. In vivo expression of GLP-1/IgG-Fc fusion protein enhances beta-cell mass and protects against streptozotocin-induced diabetes. Gene Ther 14: 981–988, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud'Homme GJ, Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 108: 11692–11697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol 220: 59–65, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Van TC, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem 284: 20462–20466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang H, Jiang JY, Zhu C, Peng C, Tsang BK. Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol 20: 2469–2482, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Wang H, Tsang BK. Nodal signalling and apoptosis. Reproduction 133: 847–853, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Wang H, Tsang BK. Nodal signalling and apoptosis. Reproduction 133: 847–853, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45: 1263–1273, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Wang Q, Jin T. The role of insulin signaling in the development of beta-cell dysfunction and diabetes. Islets 1: 95–101, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 47: 478–487, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Watanabe R, Shen ZP, Tsuda K, Yamada Y. Insulin gene is a target in activin receptor-like kinase 7 signaling pathway in pancreatic beta-cells. Biochem Biophys Res Commun 377: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Watanabe R, Yamada Y, Ihara Y, Someya Y, Kubota A, Kagimoto S, Kuroe A, Iwakura T, Shen ZP, Inada A, Adachi T, Ban N, Miyawaki K, Sunaga Y, Tsuda K, Seino Y. The MH1 domains of smad2 and smad3 are involved in the regulation of the ALK7 signals. Biochem Biophys Res Commun 254: 707–712, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature 370: 341–347, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Xu G, Zhou H, Wang Q, Auersperg N, Peng C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol Cancer Res 4: 235–246, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Zhang N, Kumar M, Xu G, Ju W, Yoon T, Xu E, Huang X, Gaisano H, Peng C, Wang Q. Activin receptor-like kinase 7 induces apoptosis of pancreatic beta cells and beta cell lines. Diabetologia 49: 506–518, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Zhang S, Liu H, Yu H, Cooper GJ. Fas-associated death receptor signaling evoked by human amylin in islet beta-cells. Diabetes 57: 348–356, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Liu Y, Qu J, Hardy A, Zhang N, Diao J, Strijbos PJ, Tsushima R, Robinson RB, Gaisano HY, Wang Q, Wheeler MB. Functional characterization of hyperpolarization-activated cyclic nucleotide-gated channels in rat pancreatic beta cells. J Endocrinol 203: 45–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang YQ, Sterling L, Stotland A, Hua H, Kritzik M, Sarvetnick N. Nodal and lefty signaling regulates the growth of pancreatic cells. Dev Dyn 237: 1255–1267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao J, Zhang N, He M, Yang Z, Tong W, Wang Q, Hu R. Increased beta-cell apoptosis and impaired insulin signaling pathway contributes to the onset of diabetes in OLETF rats. Cell Physiol Biochem 21: 445–454, 2008 [DOI] [PubMed] [Google Scholar]