Abstract
Among organ systems, skeletal muscle is perhaps the most structurally specialized. The remarkable subcellular architecture of this tissue allows it to empower movement with instructions from motor neurons. Despite this high degree of specialization, skeletal muscle also has intrinsic signaling mechanisms that allow adaptation to long-term changes in demand and regeneration after acute damage. The second messenger adenosine 3′,5′-monophosphate (cAMP) not only elicits acute changes within myofibers during exercise but also contributes to myofiber size and metabolic phenotype in the long term. Strikingly, sustained activation of cAMP signaling leads to pronounced hypertrophic responses in skeletal myofibers through largely elusive molecular mechanisms. These pathways can promote hypertrophy and combat atrophy in animal models of disorders including muscular dystrophy, age-related atrophy, denervation injury, disuse atrophy, cancer cachexia, and sepsis. cAMP also participates in muscle development and regeneration mediated by muscle precursor cells; thus, downstream signaling pathways may potentially be harnessed to promote muscle regeneration in patients with acute damage or muscular dystrophy. In this review, we summarize studies implicating cAMP signaling in skeletal muscle adaptation. We also highlight ligands that induce cAMP signaling and downstream effectors that are promising pharmacological targets.
Keywords: cyclic AMP, skeletal muscle, cell signaling, muscle regeneration, atrophy, protein kinase A
originally discovered by Sutherland and Rall in liver homogenates in 1958 (218), adenosine 3′,5′-monophosphate (cyclic AMP, or cAMP) has since been intensively studied and is one of the best-characterized signaling molecules. In skeletal muscle, acute cAMP signaling has been implicated in regulation of glycogenolysis (213), contractility (32, 83, 84, 88, 138, 234), sarcoplasmic calcium dynamics (58, 147, 184, 204), and recovery from sustained contractile activity (44, 162). The net result of acute cAMP action on the order of minutes in skeletal muscle can generally be described as increased contractile force and rapid recovery of ion balance, especially during prolonged contractions. These acute changes are most pertinent to muscle contraction and energy utilization during exercise, when epinephrine is rapidly released into the circulation (92) and cAMP accumulates in muscle (72).
Many studies have shown, however, that cAMP-inducing agents or genetic modification of proteins involved in cAMP signaling can also have adaptive effects on skeletal muscle by increasing myofiber size and promoting fiber-type transitions to glycolytic fibers (9, 18, 42, 43, 57, 63, 86, 91, 101, 121, 128, 154, 155, 163, 190, 193, 194, 215). The prohypertrophic actions of β-adrenergic receptor (β-AR) agonists and corticotropin-releasing factor receptor 2 (CRFR2) agonists have recently been harnessed to improve muscle function and ameliorate atrophy in several rodent models, including disuse (99, 101, 245), denervation (98, 100–102, 127, 154, 253), aging (195, 254), and muscular dystrophy (90, 103, 185, 254, 256). β-AR agonists have also shown some promise in promoting muscle function in patients with muscular dystrophy (64, 125, 126, 211, 235). Despite many physiological studies, the molecular mechanisms underlying these effects in skeletal muscle are still being elucidated.
In addition to functional adaptation, skeletal muscle has an extraordinary ability to repair and regenerate (93). Resident muscle precursor cells, or satellite cells, become activated in damaged muscle (93). They then proliferate, migrate, and fuse with existing myofibers and with each other to restore normal muscle structure and function. cAMP signaling participates in muscle precursor cell differentiation (39), migration (80), and fusion (48, 249). All of these cellular events are required for efficient regeneration of adult skeletal muscle (93). In adult muscle, stimulation of cAMP production can slow degeneration or promote regeneration in rodent models of necrotic muscle injury (15, 80, 196) and Duchenne's muscular dystrophy (86, 90, 103, 254).
In this review, we explore cAMP-dependent physiological adaptation in skeletal muscle fibers and muscle precursor cells. We first discuss the components of cAMP signaling machinery that function in this tissue. We then review the known molecular mechanisms by which sustained treatment with ligands capable of inducing cAMP promotes hypertrophy and alters muscle fiber types. Finally, we examine how cAMP signaling affects muscle development and how these same pathways participate in regeneration. For this discussion, we define “adaptation” as cellular events that normally occur on the order of days to weeks and that change phenotypes in individual myofibers or whole muscles.
A cAMP SIGNALING PRIMER
In the archetypic view of cAMP signaling, activation of G protein-coupled receptors (GPCRs) on the cell surface results in activation of heterotrimeric G proteins, including those containing the stimulatory subunit Gαs (Fig. 1A) (111). Gαs, in turn, stimulates membrane-bound adenylyl cyclase (AC), which converts ATP to cAMP. Production of soluble, diffusible cAMP is a means of signal amplification and was thought to activate the distant effectors, PKA (cAMP-dependent protein kinase) and Epac (exchange protein activated by cAMP) (112). We now understand that cAMP production and signaling are highly localized (53) and heterogeneous among cell types depending on the complement of GPCRs, G proteins, and AC isoforms expressed. This is particularly pertinent in skeletal muscle, which expresses many GPCRs and has intrinsic subcellular organization that not only accomplishes contraction but also provides a framework for spatially localized cell signaling (Fig. 1B).
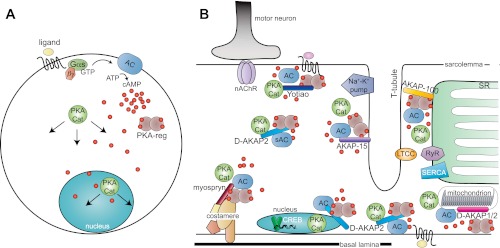
Fig. 1.
Models of cAMP signaling in muscle cells. A: classic view of cAMP production at the plasma membrane with diffusion into the cytoplasm. A ligand binds to a G protein-coupled receptor (GPCR), which activates Gαs. Gαs activates adenylyl cyclase (AC), which produces cAMP. cAMP binds to PKA regulatory subunits (PKA-reg), allowing dissociation of PKA catalytic subunits (PKA-cat), which diffuse into the cytoplasm and nucleus and phosphorylate target proteins. B: in a differentiated myofiber, cAMP production and PKA activity are localized to different subcellular compartments by anchoring proteins (AKAPs), including Yotiao (NMJ), AKAP-15 (T-tubules), AKAP-100 (SR), myospryn (costameres), D-AKAP2 (sarcoplasm, nucleus, sarcolemma, mitochondria), and D-AKAP1 (mitochondria). Protein complexes at the neuromuscular junction, on T-tubules, the SR, mitochondrion, and nucleus are shown. PKA-reg (in A, sienna) are not labeled in B. Several PKA substrates are shown including the Na+-K+ pump, L-type calcium channel (LTCC), and ryanodine receptor (RyR). PKA also modulates activity of the SERCA calcium pump and stability of nicotinic acetylcholine receptors (nAChR).
Ligand-receptor pairs in skeletal muscle cells.
Although many circulating or locally secreted peptides and molecules can elicit cAMP production in diverse cell types, we have an incomplete picture of the physiologically relevant ligands that activate cAMP signaling in skeletal muscle. A partial list of GPCRs expressed in skeletal muscle has been catalogued (114), and many more have subsequently been found in myoblasts undergoing differentiation (79, 80). It is important to note that many GPCRs in skeletal muscle signal through Gαq, which activates phospholipase C and calcium signaling pathways (reviewed in Ref. 112). Gαs and Gαi are the primary regulators of cAMP production. The ligands and receptors known to directly induce cAMP accumulation in muscle precursor cells, myocytes, or myofibers are listed in Table 1. In the text of the review, we focus on the actions of cAMP and in some cases point out the physiologically relevant receptor or ligand.
Table 1.
Gαs-coupled receptors expressed in skeletal muscle
| Receptor | Ligand | Cell Type | Effect | Citation |
|---|---|---|---|---|
| β-Adrenergic receptors (β1, β2, β3) | Epinephrine norepinephrine (clenbuterol) (fenoterol) (salmeterol) (isoproterenol) | Myofiber | Glycogen breakdown | (213) |
| Transcription | (131, 164) | |||
| Excitation-contraction coupling | (172) | |||
| Metabolic adaptation | (115) | |||
| Hypertrophy | (121, 151) | |||
| CGRP receptor | Calcitonin gene-related peptide | Myofiber | nAchR synthesis | (173, 222) |
| Recovery after strenuous contraction | (162) | |||
| CRFR1 | Corticotropin-releasing factor | Myoblast | Activate cAMP reporter gene, functional effect unknown | (133) |
| CRFR2 | Urocortin 2 | Myofiber | Inhibit insulin action | (37) |
| Urocortin 3 | Reduce atrophy | (99, 101) | ||
| (sauvagine) | Reduce degeneration (mdx) | (86, 103, 185) | ||
| (PD-873637) | ||||
| MOR23 | Unknown (lyral) | Myocyte | Adhesion, migration, fusion during myogenesis and regeneration | (80) |
| Frizzled (?) | Wnt1, Wnt3a, Wnt7a | Muscle precursor cells | Differentiation (proliferation) | (39) |
| Frizzled7 (Frz7) | Wnt7a | Myofiber | Hypertrophy | (237) |
| IL-6 receptor | IL-6 | Myofiber | Induce cAMP (indirectly?), activate AMPK | (118) |
| Adenosine | A2A, A2B | Myoblast, Myofiber | Activate CREB, protect during ischemia | (152, 258) |
| Prostaglandin E1 | EP1, EP2, EP3 | Myoblast | Promote fusion | (249) |
Known endogenous ligands (agonists), differentiation state (cell type), and a rough description of functional effects are presented. Additional citations can be found in the relevant section of the text by function. A partial list of β-adrenergic agonists is given.
Among GPCR-ligand pairs, catecholamines and β-adrenergic receptors (β-ARs) have enjoyed the vast majority of experimental attention as cAMP inducers in skeletal muscle, owing most likely to the strong activation of β-AR signaling during exercise, the dramatic inotropic effects of β-AR agonists in cardiac muscle, and the pharmacological importance of β-agonists in heart failure and asthma (reviewed in Ref. 194). Two subtypes of β-ARs are detectable in skeletal muscle (β1 and β2), although β2 is the predominant isoform expressed in muscle tissue (122, 142). β2-AR is primarily activated by the catecholamine epinephrine, which is released by the adrenal medulla (82). β-ARs signal through Gαs to activate AC and induce cAMP. In addition, after PKA phosphorylation, β2-AR switches to activate pertussis toxin-sensitive Gαi (49); β2-AR coupling to Gαi has been demonstrated to occur in soleus (76) and heart (243) but has not been reported in fast-twitch fibers or isolated differentiated myotubes. β2-AR can also activate Gβγ-dependent signaling to PI 3-kinase, Akt (also called protein kinase B, PKB) and MAP kinases (151). We will discuss how these signaling mechanisms could have profound effects on muscle phenotype in subsequent sections (see Hypertrophy). Molecular mechanisms of β-AR regulation have been thoroughly reviewed elsewhere (129, 160, 194).
The corticotropin-releasing factor receptor 2 (CRFR2) is also highly expressed in skeletal muscle (38), and activation of this receptor by a family of ligands called urocortins (Ucn1–3) stimulates cAMP production in differentiated myotubes (133) and isolated muscle tissue (99, 101). Ucn2 is also strongly expressed in skeletal muscle (36), suggesting that it can serve as an autocrine signaling molecule. Although CRFR2 is capable of stimulating cAMP production, it is important to note that CRFR2 can also activate MAP kinase signaling (38). During C2C12 cell differentiation, Crfr2 mRNA is induced via a MEF2 binding site in the proximal promoter (133). This is thought to account for the selective activity of Ucn3 on CREB (cAMP response element binding protein) reporters in differentiated myotubes (133). Ucn2 knockout mice are more insulin sensitive and protected from high-fat diet-induced obesity; this is due, at least in part, to enhanced muscle insulin sensitivity (37). Thus, Ucn2-CRFR2 signaling normally inhibits insulin action in muscle, though the molecular mechanism is unknown. These findings complicate interpretations of muscle phenotypes resulting from chronic delivery of CRFR2 agonists (86, 99–101, 103, 185) (see Hypertrophy and Injury and regeneration).
Additional ligands capable of inducing or inhibiting cAMP signaling in muscle cells include calcitonin gene-related peptide (CGRP) (173, 222, 223), corticotropin-releasing factor (CRF) (124, 179), interleukin-6 (IL-6) (118), adenosine (152), prostaglandin E1 (247), Arg8-vasopressin (AVP) (167), and numerous chemokine receptors such as CXCR4 (CXC chemokine receptor 4) (79) and Wnts (39). There are undoubtedly additional ligands that induce or repress cAMP signaling in skeletal muscle. Indeed, numerous Gαs-coupled GPCRs of the odorant family are transcriptionally regulated during myogenesis and after skeletal muscle injury (80). Intramuscular cAMP can also be stimulated by chronic stimulation of the motor nerve by an implanted nerve cuff in rabbits and rats (131, 244). This does not require β-AR activity (132) but could involve direct activation of adenylyl cyclases by Ca2+-activated PKC isoforms (5, 113, 116, 171) or liberated Gβγ subunits (41, 225) (see Muscle ACs). By contrast, in human skeletal myotubes, LPA (lysophosphatidic acid) stimulates pertussis toxin-sensitive inhibitory G proteins (Gαi), which antagonize cAMP responses induced by direct AC activators (163). Although many signaling pathways may indirectly modulate intramuscular cAMP signaling via similar pathways or indirect modulation of AC activity, we will limit our discussion to ligands that directly regulate cAMP production.
G protein subunits.
Heterotrimeric G proteins consist of a GTP-binding α-subunit (Gα) and a heterodimer of β- and γ-subunits (Gβγ). Of the four classes of Gα subunits (αs, αi, αq, and α12/13), only Gαs and Gαi signal directly to ACs (95). Upon activation of a GPCR, Gα subunits bind GTP. This releases bound Gβγ subunits and renders Gα competent to interact with downstream effectors. Gαs-GTP stimulates ACs, whereas Gαi inhibits these enzymes (95). Developing mouse muscle expresses Gαs and two Gαi isoforms (Gαi2 and Gαi3); all are downregulated after birth but strongly upregulated in denervated gastrocnemius muscle (220). In skeletal muscle, pertussis toxin-sensitive G proteins containing Gαi signal via PKC-dependent pathways (135, 163) as well as through released Gβγ in differentiating skeletal muscle cells (61). A very exciting recent study showed that expression of Gαi2 is sufficient to promote myofiber hypertrophy, oxidative fiber-type switching, myogenic differentiation, and muscle regeneration in mice (163). Although all of the effector pathways are not yet elucidated, PKC mediates Gαi2-induced hypertrophy and myoblast fusion. It is still unclear how Gαi2 promotes regeneration of skeletal muscle, but the authors presented evidence of enhanced satellite cell activation and differentiation (163). Gαi also participates in synapse elimination in neonatal rat muscle by an undefined signaling mechanism that could involve PKC (135). Gαi2 does not seem to act primarily by modulating cAMP production in muscle, as phenotypes observed after Gαi2 overexpression do not precisely phenocopy Gαs deletion. For example, myofiber size is smaller in Gαs knockout mice than in littermate controls (42); if Gαi2 promoted hypertrophy by antagonizing Gαs signaling, then Gαs knockout mice would be expected to have larger myofibers. On the other hand, Gαs knockout mice do have more slow-twitch fibers than controls (42). This is consistent with enhanced slow fiber phenotypes after Gαi2 overexpression (163). However, there is currently no experimental evidence that Gαi2 induces slow fiber transitions by inhibiting Gαs. Thus, it will be important to fully delineate the rest of the downstream signaling pathways underlying the Gαi2-induced phenotypes and to use additional methods to directly increase or reduce intracellular cAMP in myofibers to determine which observed ligand-induced phenotypes truly involve modulation of intracellular cAMP.
Muscle ACs.
Mammalian genomes have a large repertoire of transmembrane ACs (AC1–9) and a soluble AC (sAC), which catalyze the conversion of ATP to cAMP (reviewed in Ref. 198). The most abundant forms in adult skeletal muscle are AC2, AC7, and AC9 (87, 220, 232), at least in rodents and rabbits (Table 2). sAC is also detectable (70). AC2 and AC9 are reportedly enriched in fast-twitch myofibers in model organisms (87, 232), and AC2 expression becomes downregulated with chronic motor nerve stimulation in rabbits, coincident with adaptation to a slow-twitch phenotype (232). In addition, AC6 and AC7 are expressed in developing mouse skeletal muscle, although AC6 expression declines dramatically in the postnatal period (220). There is surprisingly little known about AC regulation specifically in skeletal myofibers. Experiments in other cell types and in vitro show that AC isoforms exhibit unique responses to upstream activators and phosphorylation (Table 2). For example, although AC2, AC6, and AC7 are strongly activated by the direct cyclase agonist forskolin or by Gαs (87), AC9 is comparatively poorly sensitive to forskolin (85, 182). In addition to forskolin, PKC (113, 116, 171) and Gβγ (41, 225) can activate AC2 and AC7. Thus, other signaling pathways or GPCRs can potentially activate intracellular cAMP production by indirect mechanisms. AC6, on the other hand, is inhibited by PKC or PKA phosphorylation (198). Mouse strains with targeted deletion of AC6 (224), AC7 (97), AC9 (139), and sAC (59) have been generated, and none were reported to have obvious skeletal muscle phenotypes, although histological and functional characterizations were not shown.
Table 2.
Expression and regulation of adenylyl cyclase isoforms in skeletal muscle
| Property | AC2 | AC6 | AC7 | AC9 | sAC |
|---|---|---|---|---|---|
| Detectable adult expression | Yes (87, 220, 232) | No | Yes (87, 220, 232) | Yes (87, 220, 232) | Yes (70) |
| Developmental expression (220) | − | ++++ | ++++ | ++ | ND |
| Relative adult expression (220) | + | − | ++ | ++++ | ND |
| Expression after denervation (220) | Decrease | Increase | Dramatic increase | Decrease | ND |
| FSK activated | Yes (87) | Yes (87) | Yes (87) | No (85) | No (87) |
| Weak (182) | |||||
| Ca2+ or PKC sensitive | Activated (113) | Inhibited (134) | Activated (171) | No (85, 182) | Activated (146) |
| Inhibited (6, 7) | |||||
| Gβγ sensitive | Activated (41) | Inhibited (13) | Activated (246) | No (182) | Not reported |
| Activated (68) |
Activation properties noted. In rows 2–4, expressions of AC2, AC6, AC7, and AC9 were compared in the same samples (220), and expression is relative between them. ND, not determined.
cAMP effectors.
The major effector of cAMP signaling in skeletal muscle is PKA, which was identified biochemically in rabbit skeletal muscle extracts (52). PKA was later shown to mediate the effects of cAMP on muscle glycogen breakdown by both direct inactivation of glycogen synthetase and indirect stimulation of glycogen phosphorylase (213). Inactive PKA is a tetramer of two dimers comprised of regulatory (R) and catalytic (C) subunits (reviewed in Ref. 24) (Fig. 1A). cAMP binding to PKA regulatory subunits permits release of catalytic subunits, which are then free to catalyze phosphorylation of target proteins. There are multiple isoforms of regulatory (RIα, RIβ, RIIα, and RIIβ) and catalytic (Cα, Cβ, and Cγ) subunits. The α-isoforms are ubiquitously expressed, but β- and γ-isoforms are restricted to brain, testis, and adipose (24). PKA catalyzes phosphorylation on serine and threonine residues in numerous proteins. In skeletal muscle, these include metabolic enzymes (213), ion channels (204, 205), transcription factors (74), and structural proteins (187) (Fig. 2).
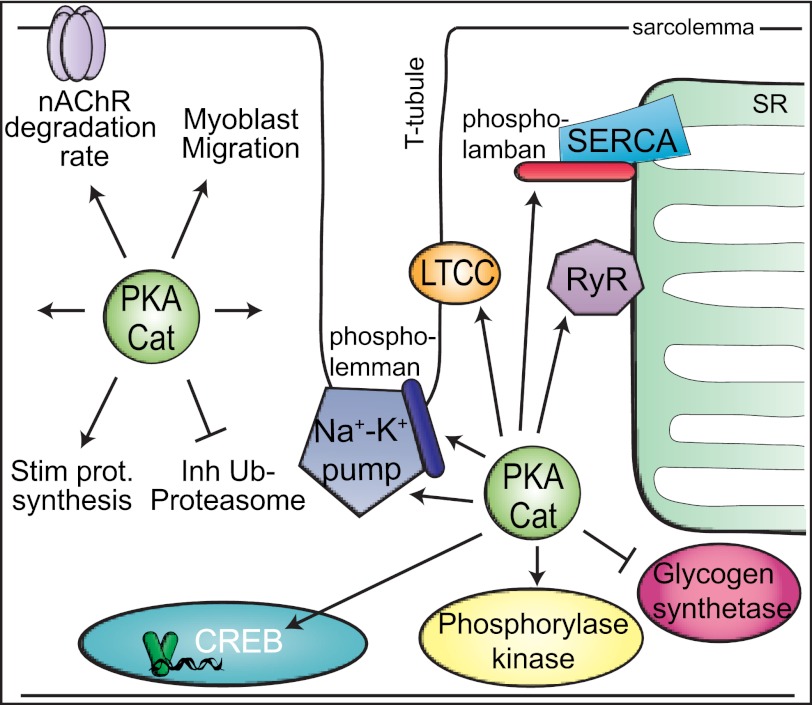
Fig. 2.
PKA targets in myofibers. Some specific substrates include the Na+-K+ pump, phospholemman, LTCC, RyR1, phospholamban (slow-twitch fibers only), glycogen synthetase, phosphorylase kinase, and CREB. Other general cellular processes for which specific targets are not clearly identified are also depicted: nAChR degradation, protein synthesis, the ubiquitin-proteasome pathway, and migration (undifferentiated myoblasts only). This is a partial depiction of PKA substrates.
In isolated primary myoblasts, PKA activity increases prior to fusion (189), after which it declines, in part due to stabilization of RI-α subunits (148), which can act as a sink for extra catalytic subunits synthesized during differentiation (149). In concert, intracellular cAMP levels also peak prior to myoblast fusion (250) and are highly regulated during limb muscle development (251) (see cAMP dynamics during myogenesis). In mouse embryonic skeletal muscle, both RIα and Cα are highly expressed, with strong mRNA and protein localization to neuromuscular junctions (NMJ) (110), where cAMP signaling is thought to control nicotinic acetylcholine receptor (nAChR) subunit expression during development and after denervation (104, 110) (see Injury and regeneration). In keeping with this notion, RIα mRNA is localized to the NMJ from late embryonic development through adulthood and is strongly induced after denervation injury, particularly in slow-twitch soleus muscle (104). In later fetal development, Cα mRNA becomes uniformly distributed throughout the muscle tissue, and both RIα and Cα proteins are abundant and uniformly distributed in the myofibers, at least when viewed in cross-sections (110).
No skeletal muscle phenotypes have been reported in any of the PKA knockout models to date, although some of these mutations result in embryonic or perinatal lethality (4, 30, 108, 210). In skeletal muscle of PKA-RIIα-deficient mice, analysis of L-type calcium channel (LTCC) regulation suggests that PKA-RIα can functionally compensate for loss of PKA-RIIα (30). This functional compensation has been overcome by overexpression of a dominant-negative RIα mutant incapable of binding cAMP (39, 241), which traps PKA catalytic subunits in inactive complexes. This mutant blocks somitogenesis in developing mouse embryos (39). Tissue-specific expression of dominant-negative RIα in mouse embryonic fibroblasts or in liver tissue is sufficient to reduce PKA activity and block activation of a CREB reporter (241), suggesting that this new animal model will be a useful genetic tool for studying cAMP-PKA signaling in skeletal muscle.
Epac1 and Epac2 are also directly activated by cAMP, whereupon they catalyze guanine nucleotide exchange and activate small Ras-like GTPases Rap1 and Rap2 (51). Of the two isoforms, Epac1 is modestly or poorly expressed in muscle tissue (51, 117), whereas Epac2 is restricted to brain and kidney (50, 51). The physiological effects of Epac1 in skeletal muscle have recently been studied using the Epac-selective agonist 8-pCPT-2′-O-Me-cAMP (12, 26). These two studies indicate that Epac may mediate cross-talk between β-ARs and the PI 3-kinase-Akt pathway, but no genetic loss-of-function studies have tested Epac function in skeletal muscle or myocytes. Additionally, skeletal muscle phenotypes were not discussed in the recent report of Epac1 knockout mice (219), so it is unknown whether this protein plays a key role in muscle in vivo. Given the paucity of data regarding Epac action in skeletal muscle, we will focus on cAMP effects mediated by PKA.
Restricting cAMP action: PDEs and AKAPs.
Although initially envisioned as an event that occurs in diffuse regions of the cytosol and in the nucleus (Fig. 1A), cAMP-PKA signaling is spatially restricted by A-kinase anchoring proteins (AKAPs), especially in highly structured cell types like skeletal myofibers (53) (Fig. 1B). AKAPs organize PKA and its substrates into macromolecular complexes at specific subcellular locales (Fig. 1B). The prominent AKAP isoforms expressed in skeletal muscle include AKAP15 (AKAP7) (78), AKAP-100/mAKAP (AKAP6) (159), Yotiao (AKAP9) (144), D-AKAP1 (AKAP1) (107) and D-AKAP2 (AKAP10) (29, 106), and myospryn (CMYA5) (187), which localize PKA to sites of excitation-contraction coupling, the SR, the NMJ, mitochondria, the nucleus, and costameres, respectively (Table 3). Of the AKAPs listed above, only mAkap (161), D-Akap1 (Akap1) (174) and D-Akap2 (Akap10) (230) have been deleted in mice, but skeletal muscle phenotypes were not reported in any of these knockout models. It is notable that cAMP microdomains are disrupted (188) and PKA activity is reduced (186) in dystrophic skeletal muscle from a mouse model of Duchenne's muscular dystrophy (mdx). This could partly result from disrupted expression and localization of myospryn, which interacts with dystrophin in costameres in normal muscle (186). Myospryn misregulation correlates with reduced PKA activity and RIIα mislocalization in dystrophic myofibers (186), although it is not known whether altered PKA signaling contributes to the dystrophic phenotype. For more discussion of AKAP-PKA complexes, the reader is referred to an excellent recent review (53).
Table 3.
AKAPs expressed in skeletal muscle and localization
Termination of cAMP signals is accomplished by cAMP phosphodiesterases (PDEs), which have been purified from skeletal muscle (218). Subsequent to the initial discovery, no less than eleven different PDE classes, many with multiple isoforms encoded by distinct genes, have been identified. Among cAMP-specific PDEs, PDE4 isoforms are highly expressed in skeletal muscle (8, 207) and account for the majority of PDE activity in mouse limb muscle (21). PDE7B and PDE11A are also expressed in skeletal muscle (60, 69, 96, 202). Pharmacological inhibition of PDEs reduces proteolysis and atrophy in rodent muscle ex vivo (170) and in vivo after denervation or casting (98) or in rodent models of diabetes (11) and sepsis (145). These data suggest that selective upregulation of cAMP signaling in skeletal muscle may be a strategy to prevent muscle atrophy in patients with aging-related atrophy, disuse atrophy, cancer cachexia, or sepsis. Interestingly, elevated PDE activity is observed in skeletal muscle of dystrophic mice (20, 21, 143). Although these findings do not imply that reduced cAMP abundance in dystrophic muscle causes the dystrophic phenotype, it would be interesting to test whether chronic PDE inhibition can improve muscle regeneration or function in dystrophic muscle in a manner analogous to β2-AR and CRFR2 agonists (90, 103, 185, 254, 256). Studies with prolonged PDE inhibitor treatment should be evaluated with caution, as mice lacking Pde4d display exercise intolerance and evidence of myofiber damage after downhill running (17). As no thorough histological and functional investigation of skeletal muscle phenotypes in any of the PDE knockout lines has been reported, we have little additional genetic information about the functions of specific PDE isoforms in skeletal muscle in vivo.
cAMP IN FUNCTIONAL ADAPTATION OF SKELETAL MUSCLE
Adaptive functions of sustained GPCR signaling have been identified using chronic treatment with agonists and antagonists in humans and model organisms (9, 18, 43, 57, 63, 86, 91, 101, 115, 121, 154, 155, 197) as well as by targeted genetic approaches in mice (42, 163, 164). These and other studies have revealed striking myofiber hypertrophy and fiber-type transitions to faster fiber types with prolonged activation of β-AR signaling in particular. Although there is evidence supporting cAMP-dependent signaling as a mediator of long-term adaptive responses to β-AR agonists and possibly other GPCR ligands (42, 98), additional downstream effector pathways mediated by Gαi/PKC (163) and Akt (127) are also clearly involved. These alternate pathways may or may not respond to initial or sustained cAMP production. In this section, we provide an overview of the effects of sustained activation of cAMP-coupled GPCRs in skeletal muscle, focusing on myofiber hypertrophy and fiber-type transitions. For an additional discussion, the reader is referred to a comprehensive review by Lynch and Ryall (151).
Hypertrophy.
Myofiber hypertrophy is a form of muscle adaptation that occurs in response to resistance exercise (226). There is great interest in understanding molecular mechanisms underlying this adaptive response, because skeletal muscle atrophy accompanies and exacerbates many pathological states, including disuse, denervation injury, cancer, AIDS, and aging (reviewed in Ref. 71). Increased muscle mass and myofiber cross-sectional area in adult skeletal muscles are brought about by shifts in the relative rates of protein synthesis and degradation; the net result is enhanced muscle strength (71). It has been appreciated for many years that systemic treatment with β2-AR agonists such as clenbuterol (Table 1) induces skeletal muscle hypertrophy in rodents, large mammals, and humans (reviewed in Ref. 151) (Fig. 3). Additionally, β-adrenergic agonists blunt muscle atrophy after denervation (253), muscular dystrophy (192), or nutritional deficiency (73). At the cellular level, clenbuterol has been shown to stimulate total protein synthesis (57, 154) and translational efficiency (155) as well as reduce proteolysis in rabbit, rat, and chick skeletal muscles (18, 63, 190). These effects are probably mediated by cAMP, as PDE inhibitors reduce atrophy in skeletal muscle (98) and a cell-permeable cAMP analog (db-cAMP) or PDE inhibitors reduce proteolysis in muscle studied ex vivo (11, 170). Moreover, in cell culture models of hyperthermia-induced proteolysis, formoterol (a β2-AR agonist) reduced proteolysis by a mechanism dependent on AC activity (3). In addition, activation of the GPCR Fzd7 (Frizzled 7) by Wnt7a was also shown to induce hypertrophy in rodent myotubes via a pathway involving Gαs (237). On the contrary, mice lacking Gαs, which stimulates cAMP production, have smaller myofibers than littermate controls (42).
Fig. 3.
GPCRs that induce myofiber hypertrophy. Four GPCRs (Fzd7, β2-AR, CRFR2, and LPA receptor) have been shown to stimulate hypertrophy in myotubes and/or myofibers. Cognate ligands are italicized. A partial view of the known signaling mediators is shown. CRFR2 stimulates myofiber growth by an uncharacterized effector pathway. β2-AR also induces fiber type transitions to fast-twitch fibers (not shown).
In pathological settings, ligands for two GPCRs, β2-AR and CRFR2, reduce atrophy or help sustain muscle function and strength in rodent models after denervation (100, 101, 123, 127, 154, 253, 255), unloading (99, 101, 123, 127) or aging (35). β2-AR agonists have also been shown to reduce muscle atrophy in animals with cancer cachexia (31, 33, 46). In addition, agonists for β2-AR or CRF receptors improve muscle function in dystrophin-deficient mdx (86, 90, 103, 185, 192, 254, 256) and laminin-deficient dy/dy (94) mice. However, efficacy of β2-AR agonists on muscle size and force generation vary with the type and dose of agonist, as well as the muscle studied. Small scale clinical trials in muscular dystrophy patients revealed limited promise of the β2-AR agonist albuterol (64, 125, 126, 235), which appeared to improve muscle function with varying effects on muscle strength (211). These studies have been extensively analyzed by Lynch and Ryall (151).
Although genetic and pharmacological studies confirmed that the anabolic effects of clenbuterol in rodents are mediated by β2-ARs (43, 102), the precise molecular mechanisms by which β2-AR signaling induces hypertrophy are still not fully elucidated. Both β2-AR and CRFR2 are expressed in muscle cells and are capable of stimulating cAMP production (54, 124, 179). The phenotype of smaller myofibers in Gαs knockout mice provides genetic evidence that cAMP signaling does have a role in myofiber hypertrophy (42) (Fig. 3). Moreover, activation of a noncanonical signaling pathway by Wnt7a-Fzd7 is also sufficient to drive muscle fiber hypertrophy in vitro (237). The authors found that Fzd7 physically interacts with Gαs, and Gαs is required for Fzd7-induced PI 3-kinase activation and hypertrophy (237). However, the recent study by Minetti et al. (163) demonstrates that the inhibitory G protein Gαi2 is also sufficient to promote myofiber hypertrophy in vitro and in vivo (Fig. 3). In other cell types, β2-AR and CRFR2 have been shown to activate additional effector pathways such as Gαq-PLC, Gαi-PKC, Gβγ, or β-arrestin-Akt (25, 76, 77, 89, 243), which could mediate anabolic effects of these receptors (Fig. 4). An additional possibility is that after initial activation of Gαs, PKA phosphorylation of the β2-AR causes it to couple to Gαi. This mechanism occurs in cardiac muscle (243) and soleus (76), and it will be interesting to determine whether this regulatory mechanism is a general feature of all skeletal muscle types. Nonetheless, dynamic coupling to G proteins by chronically stimulated receptors could account for observations that inhibition of Gαs or activation of Gαi leads to opposite changes in myofiber size.
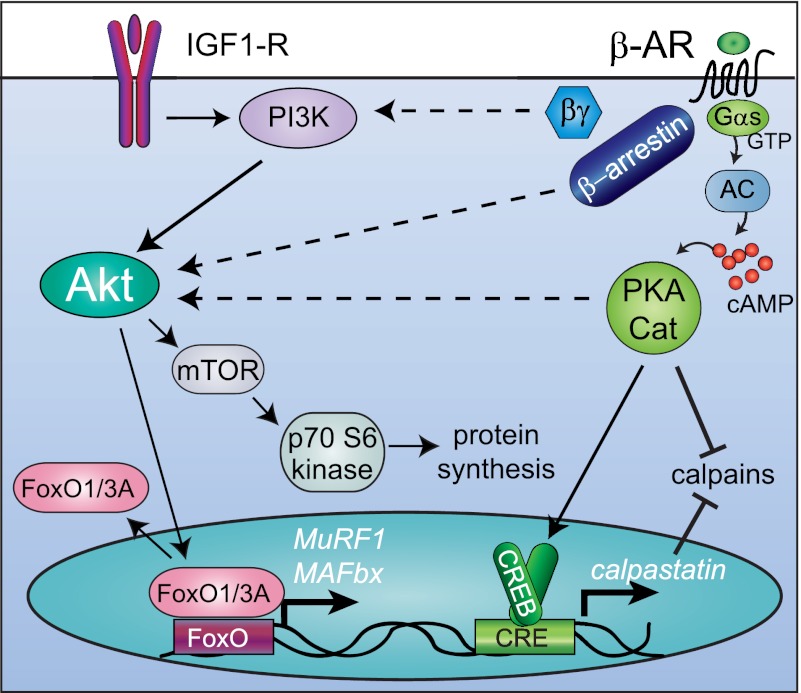
Fig. 4.
Possible mechanisms of Akt activation by β-AR signaling. IGF-I activates Akt, which stimulates protein synthesis via activating mTOR and inhibits muscle-specific ubiquitin ligase expression via repressive phosphorylation of FoxO transcription factors. β2-AR signaling leads to muscle hypertrophy, which is accompanied by activation of Akt, activation of protein synthesis, and inhibition of proteolysis. PKA signaling induces calpastatin transcription and inhibits calpains by an unknown mechanism. β2-AR signaling also activates Akt by an unknown mechanism, possibly mediated by Gβγ subunits, β-arrestin, or PKA (dashed arrows). Not shown: Wnt7a-Fzd7-Gαs activates PI 3-kinase directly.
Many studies have established that IGF-I strongly activates muscle hypertrophy by stimulating the PI 3-kinase-Akt pathway (10, 166) (Fig. 4). Akt, in turn, activates the downstream kinase mTOR, which stimulates p70 S6 kinase and other effectors, ultimately culminating in enhanced protein synthesis (23, 191). In addition, Akt represses FoxO (F box, class O) transcription factors, which drive expression of muscle-specific ring finger E3 ubiquitin ligases MuRF1 (Muscle RING Finger 1) and MAFbx (Muscle Atrophy F-box, also called Atrogin-1) when Akt signaling tone is reduced (22, 201, 217). The potent effects of IGF-I-Akt signaling on muscle hypertrophy prompted several investigators to address the role of Akt signaling in muscle responses to clenbuterol (Figs. 3 and 4). Kline et al. (127) showed that clenbuterol activates Akt in skeletal muscle, and the mTOR inhibitor rapamycin partly blocks anabolic effects of clenbuterol. In agreement with these findings, β2-AR agonists promoted muscle protein synthesis after 7 days with concomitant activation of Akt (130) (Fig. 4). Clenbuterol treatment has also been associated with a transient increase of activated p70 S6 kinase (212). Signaling from β2-AR to Akt appears to be direct and not mediated by an autocrine pathway, as adrenergic signaling does not induce IGF-I protein expression in skeletal muscle (127, 245). It has been proposed that, similar to cardiac muscle, the signaling mechanism from β2-AR to Akt could be mediated by Gβγ (127). Consistent with this model, myotube hypertrophy induced by ectopically expressed Gαi2 was blocked by rapamycin or PKC inhibitors, but not by PI 3-kinase inhibitors, suggesting that G protein signaling to Akt is direct (163) (Fig. 4). Alternatively, the recent data from von Maltzahn et al. (237) clearly implicate direct activation of PI 3-kinase by Gαs or released Gβγ as a mechanism for Akt activation by Fzd7, a Gαs-coupled receptor. Another intriguing possibility is that Akt could be activated by β-arrestin upon β2-AR activation, as recently observed in fibroblasts (89). This hypothesis has not been tested in skeletal muscle. The same signaling mechanism may apply to other GPCRs. However, it is important to note that, although CRFR2 agonists induce muscle hypertrophy and blunt muscle atrophy (99–101), they actually inhibit insulin-Akt signaling (37). Therefore, multiple molecular mechanisms may mediate anti-atrophic effects of GPCRs in skeletal muscle. Ultimate determination of how Gαs-coupled receptors activate Akt will provide mechanistic insight into the commonly observed effects of β2-AR agonists and other GPCR ligands on muscle hypertrophy and may uncover new targets for therapeutic promotion of muscle growth.
In addition to promoting muscle growth and protein synthesis, cAMP signaling inhibits proteolysis by both calpains and the ubiquitin proteasome system. Calpains are calcium-activated proteases, which are inhibited by the protein calpastatin (reviewed in Ref. 168). β-AR agonists reduce calpain activity in skeletal muscle tissue in animal models (9, 63, 169). This occurs in part by cAMP-induced expression of the inhibitor calpastatin (Fig. 4) via CREB binding sites in the bovine (45), porcine (206), and human (257) calpastatin gene promoters. cAMP might also regulate calpastatin expression by posttranslational mechanisms, as CREB binding sites have not been annotated in the rodent promoters (257). In addition, calpastatin activity was elevated in porcine and rat skeletal muscle after continuous infusion with β-AR agonists even though the calpastatin mRNA was not uniformly induced (130, 176). Animal models support modulation of the calpain system as a useful therapeutic strategy: mdx mice have aberrantly high levels of calpain activity (228), whereas muscle-specific overexpression of calpastatin reduces atrophy in disuse models (199, 229) and reduces necrosis in mdx muscle (214), but perhaps does not improve overall regeneration (27).
The other major proteolytic system in skeletal muscle is the ubiquitin-proteasome system. Two E3 ubiquitin ligases, MuRF1 and MAFbx/Atrogin-1, undergo massive transcriptional induction in skeletal muscle after denervation injury (22). Mice lacking MuRF1 or MAFbx/Atrogin-1 are resistant to muscle atrophy, demonstrating the crucial role of the ubiquitin-proteasome pathway in this pathology (22). MuRF1 and MAFbx/Atrogin-1 mRNAs are induced by FoxO transcription factors FoxO1 and FoxO3a, which are normally sequestered in the cytoplasm by Akt phosphorylation (201, 217) (Fig. 4). The β2-AR agonist clenbuterol reduces amounts of high-molecular-weight ubiquitin conjugates and proteasome activity in fast-twitch skeletal muscles undergoing atrophy from hindlimb unloading (245), suggesting the possibility that β-AR activation also suppresses FoxO activity. Consistently, clenbuterol potently suppresses both MuRF1 and MAFbx/Atrogin-1 transcription in normal muscle and attenuates expression of these genes in atrophying muscle in rats (127). In addition, cAMP signaling activates Akt and represses ubiquitin-proteasome pathway activity in C2C12 cells and rat skeletal muscle (73). It is tempting to speculate that FoxO transcription factors might mediate the activity of β-AR on Murf1 and Mafbx/Atrogin-1; indeed, FoxO3A phosphorylation correlates with clenbuterol-induced Akt phosphorylation in vivo in muscle from fasted animals (73). FoxO1 phosphorylation has not been explored in this model, and much uncertainty remains regarding the mechanisms by which β2-AR agonists modulate ubiquitin-mediated protein degradation in skeletal muscle.
Four GPCRs, β2-AR, CRFR2, the LPA receptor, and Fzd7, have been shown to induce hypertrophy in skeletal muscle or isolated myotubes. The field is closer to understanding mechanisms underlying GPCR-induced hypertrophy, and it will be of great scientific and clinical interest to determine whether these receptors utilize common effector pathways. It is clear that CRFR2 signaling can block pathological muscle atrophy. However, genetic and pharmacological studies indicate that Ucn2 inhibits Akt signaling (37); so alternate downstream effector pathways likely mediate the anabolic effects of this ligand-receptor pair. Given the promising anabolic effects of CRFR2 agonists on mouse models, it will be important for future studies to rigorously interrogate the signaling pathway mediating these effects. It will also be exciting to determine whether chronic stimulation of other GPCRs expressed in skeletal muscle have the same hypertrophic effects in normal muscle and anti-atrophic effects in aging or diseased muscle.
Metabolic adaptation.
In the postnatal period, skeletal myofibers develop distinct phenotypic characteristics that enable classification into one of several fiber types, including slow oxidative (type 1), fast oxidative (type IIa), and fast glycolytic (type IIb or type IIx) (reviewed in Ref 203). Despite this initial specialization, phenotypes of individual muscle fibers are plastic in adult mammals. Cross-innervation studies in model organisms demonstrated that the pattern of motor neuron firing (tonic or phasic) dictates muscle fiber phenotypes and that fast and slow fibers can be interconverted by altering the pattern of innervation by denervation or by endurance exercise (203). At the molecular level, changes to a slow fiber phenotype are mediated by several signal transduction pathways, which drive mitochondrial proliferation and activate transcription of slow myosin isoforms, myoglobin, and enzymes involved in oxidative metabolism (203). Schiaffino et al. (203) provide a comprehensive discussion of muscle fiber type plasticity.
Several observations associate cAMP signaling with oxidative adaptation to exercise. First, epinephrine is released into circulation during exercise (67), and intramuscular cAMP increases within minutes of treadmill running (72). Second, β-AR density is highest in oxidative muscles and correlates with oxidative capacity in mixed or intermediate fiber types (62, 156, 240); AC activity is also increased by endurance training in rats (28). Third, in cultured muscle cells, agents that induce intracellular cAMP activate oxidative enzymes, some at the level of expression (66, 136). Additionally, β-AR signaling or cAMP-sensitive transcription factors have been reported to regulate genes involved in oxidative metabolism, including Pgc1α (PPARγ coactivator 1α) (2, 164, 242), cytochrome c (65), CoxIV (cytochrome c oxidase IV) (75), and orphan nuclear receptors Nor1 (neuron-derived orphan receptor 1, or Nr4a3) and Nur77 (nuclear receptor family member 77, or Nr4a1) (157, 178). Overexpression of cAMP-activated CREB coactivators is also sufficient to induce mitochondrial proliferation in cultured myotubes (242). In rats, the β-adrenergic antagonist propranolol blunted activation of mitochondrial enzyme activity after endurance treadmill training (115), but a histological examination of fiber types was not shown in that study. In additional studies, propranolol substantially reduced normal increases in mitochondrial enzyme activity of rat muscles (91), although the effects varied among fiber types (227). In addition, Kraus et al. (131) showed that chronic electrical stimulation of fast-twitch muscles elicited an increase in cAMP with concomitant induction of mRNAs encoding myoglobin and proteins involved in oxidative metabolism. These correlations could imply that β-AR-cAMP signaling during exercise participates in oxidative metabolic adaptation of skeletal muscle.
However, compelling genetic and pharmacological data from mice and humans suggest that this is not likely the case. In humans, mitochondrial enzyme activity increases with endurance exercise despite treatment with the beta-blocker propranolol, and no significant change in fiber-type percentage or cross-sectional area was reported in this study (221). Moreover, Pgc1α expression was still induced, albeit to a lesser extent, by treadmill training in mice lacking all β-ARs (164). It is also notable that, although the CREB binding site is required for Pgc1α promoter activation in response to electrical stimulation (2), the CREB family protein ATF2 binds this site and mediates Pgc1α transcription in this setting (1). Additionally, mice with muscle-specific deletion of Gαs or overexpression of Gαi2 (both leading to reduced cAMP accumulation in muscle) exhibit fiber-type switching toward a higher proportion of oxidative fibers (42, 163). Genetic inhibition of CREB activity in differentiated muscle leads to defects in myofiber survival but not alterations in muscle fiber type (19). Finally, a large body of data from humans and model organisms shows that chronic stimulation of β2-AR signaling causes shifts toward faster, not slower, myofiber phenotypes (14, 47, 55, 150, 180, 183, 252).
The molecular mechanism by which β2-AR agonists induce fast fiber-type transitions is not known. Shi et al. (208) found that isoproterenol treatment of C2C12 myoblasts stimulates promoters of MyHC-IIb (myosin heavy chain IIb) and Serca1 (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) genes, which are expressed in fast myofibers. In addition, clenbuterol activated a MyHC-IIb reporter in mouse gastrocnemius muscle (208). As ERK1/2 (extracellular signal-regulated protein kinase) activation by clenbuterol was greater in fast muscles than in slow muscles, the authors propose that ERK signaling downstream of the β2-AR mediates fast fiber-type transitions in clenbuterol-treated animals (208). It will be interesting to further test this mechanism using additional loss-of-function approaches. Lynch and Ryall (151) noted that overexpression of Eya1/Six1 induces expression of fast-type MyHC-IIb myosin in soleus (81) and proposed this as a possible unexplored mechanism to explain the fiber-type transitions observed with sustained clenbuterol treatment. The observations that mice with targeted deletion of Gαs (42) or overexpression of Gαi2 (163) have more slow-twitch fibers are consistent with promotion of fast fiber-type switching by cAMP effectors. Interestingly, both AC2 and AC7 mRNAs are enriched in fast myofibers (87, 232), so it is possible that these fibers have more sustained cAMP production in a chronic treatment setting. However, in rats, AC and PKA activity are both higher in slow-twitch soleus than in fast-twitch extensor digitorum longus muscles (28, 104, 240), and AC activity increases more after exercise training in oxidative muscles (28). Finally, although CREB stimulates genes involved in oxidative metabolism (242), it is possible that cAMP-induced transcriptional responses are dampened by autoregulatory feedback loops after prolonged β-AR activation (236). This notion is supported by microarray data showing that acute β-AR agonist treatment induces genes involved in oxidative metabolism, whereas prolonged treatment is associated with repression of genes involved in myostatin signaling (177).
In summary, ligands capable of inducing cAMP signaling exert hypertrophic effects on skeletal muscle and, in some cases, induce fast fiber-type transitions. Receptor and AC isoform expression is not well correlated with measured AC and PKA activities among fiber types. Thus, expression patterns do not simply account for the observed changes to faster fiber types with prolonged adrenergic agonist treatment. The recent finding that inhibitory Gαi2 induces hypertrophy as well as oxidative fiber-type transitions (163) underscores the importance of further investigation into whether and how different signaling mediators, possibly independently of cAMP, contribute to the phenotypes observed with prolonged activation of GPCRs in skeletal muscle.
cAMP IN MUSCLE DEVELOPMENT AND REGENERATION
cAMP signaling is dynamically regulated during embryonic muscle development, ex vivo myogenesis, and muscle regeneration (16, 34, 39, 120, 137, 251). In adult skeletal muscle, resident muscle stem cells, or satellite cells, become activated after acute injury or in response to hypertrophic stimuli (93). These cells then proliferate, differentiate, and fuse with each other and existing fibers. Satellite cells are absolutely crucial for skeletal muscle regeneration and are thought to contribute to ongoing productive hypertrophy (239), although this is still under debate (158). Regeneration is an adaptive response of muscle, so understanding of cAMP signaling pathways in satellite cells and their progeny is important to understanding the overall adaptive capacity of skeletal muscle. Several proteins involved in cAMP signaling, including receptors, G proteins, and cAMP-activated transcription factors, have been found to promote muscle regeneration (80, 163, 216) and functional recovery after injury (15, 196). Ligands for these GPCRs also improve muscle function and slow disease progression in dystrophic mdx mice (86, 90, 103, 185, 192, 254, 256). In conjunction with the anti-atrophy effects described in the preceding section (see Hypertrophy), GPCR signaling in muscle precursor cells is a potential area for development of novel therapeutic agents that could promote regeneration and limit atrophy in patients with muscular dystrophy, denervation atrophy, age-related sarcopenia, or muscle wasting due to cancer. In the final section of this review, we will trace the actions of cAMP in myogenesis and explore the recent studies on cAMP signaling during satellite cell-mediated skeletal muscle regeneration.
cAMP dynamics during myogenesis.
Skeletal muscle formation during embryogenesis, or myogenesis, is a complex process involving proliferation and determination of precursor cells, myoblast migration, cell-cell fusion, and myogenic differentiation to ultimately form multinucleated, contractile myotubes (93). Numerous cell surface receptors and interwoven transcriptional networks act cooperatively during myogenesis. Early work implicated cAMP signaling in myogenesis, as AC activity, cAMP, and PKA activity all increase at specific times during embryonic muscle development and differentiation of myoblasts in culture (137, 153, 189, 238, 250, 251). Moreover, transient treatment with catecholamines (48) or prostaglandin E1 (249), which stimulate intracellular cAMP production, enhances fusion of primary chick myoblasts. However, this increase in cAMP production must be tightly regulated. Intracellular cAMP and PKA activity normally decline after myoblast fusion (137, 238, 251), and sustained cAMP signaling achieved by pharmacologic methods or by ectopic expression of Gαs markedly inhibits myoblast fusion and differentiation (105, 119, 140, 233, 248). Thus, therapeutic approaches to stimulate myoblast fusion must be designed with caution to allow dynamic cAMP regulation in vivo.
Migration and fusion.
It is not known why sustained cAMP-PKA signaling inhibits myoblast fusion, nor is it understood how transient cAMP signaling exerts a priming effect on this process (249). However, recent groundbreaking work by Pavlath and colleagues provided clues that the odorant family GPCR MOR23 stimulates cAMP signaling and regulates myoblast cell migration, adhesion, and fusion during myogenesis via cAMP-mediated pathways (80). In vivo, silencing MOR23 blunted muscle regeneration after myotoxic injury and left many branched, unfused myofibers (80). Dynamic cytoskeletal rearrangements are required for cell migration and fusion (40), suggesting that unrecognized PKA effectors regulate the cytoskeleton in myoblasts. This idea is supported by findings that cAMP signaling promotes fusion in a mouse satellite cell-derived line and that a fraction of PKA-RII subunits localizes to lamellipodia of bipolar myotubes (165). Interestingly, excessive PKA signaling in mouse embryos lacking RIα also results in defective cell migration (4). Together, these findings support a model in which dynamic PKA activity at the leading edge of migrating cells is important for migration and fusion. In fibroblasts, PKA phosphorylates the small GTPase RhoA, blocking interaction between RhoA and the Rho guanine dissociation inhibitor (RhoGDI) protein, rendering RhoA active (231). Additional experimental effort will be required to determine whether PKA regulation of Rho or other cytoskeletal modulators regulates myoblast migration and fusion.
Myogenic differentiation.
In addition to blocking myoblast fusion, sustained cAMP signaling potently inhibits myogenic differentiation, in part by inhibiting myogenic transcription factors (56, 105, 140, 200). However, cAMP-PKA dynamically regulates CREB-dependent transcription during vertebrate muscle development, during which phosphorylated CREB is localized to differentiating regions of the dermomyotome in mouse (39) and Xenopus (120). Although additional PKA effectors likely participate in myogenesis, loss-of-function studies have established a key role for CREB in myogenic differentiation and myofiber survival (Fig. 5). Through an elegant series of experiments, Chen et al. (39) showed that mouse embryos lacking Creb exhibit smaller somites and drastically reduced expression of the myogenic determination factors Pax3 and Myf5. The small somite size in Creb mutant embryos resulted from reduced proliferation, although the CREB transcriptional targets mediating muscle precursor cell proliferation are unknown. Using embryo explants, the authors discovered a noncanonical Wnt signaling pathway, mediated by cAMP and PKA, which drives CREB phosphorylation on Ser133 and is necessary for myogenesis, possibly via direct transcriptional induction of Pax3 or Myf5 (39) (Fig. 5). Consistently, we found that myoblasts from knock-in mice expressing a CREB gain-of-function mutant (CREB-Y134F) exhibit enhanced proliferation in culture. Furthermore, differentiating CREB-YF myocytes expressed more Myf5 protein than wild-type controls (216). Although the Myf5 promoter contains several conserved CREB binding sites (39), chromatin immunoprecipitation assays have not been performed to demonstrate that CREB directly transactivates this gene. CREB phosphorylation is also induced during early stages of differentiation in cultured C1C12 cells (153) but declines during late differentiation (209), when it induces RB and follistatin mRNAs in conjunction with MyoD (109, 153). CREB has furthermore been implicated in transcription of the mRNA encoding the mitochondrial protein cytochrome c in differentiating myotubes (65).
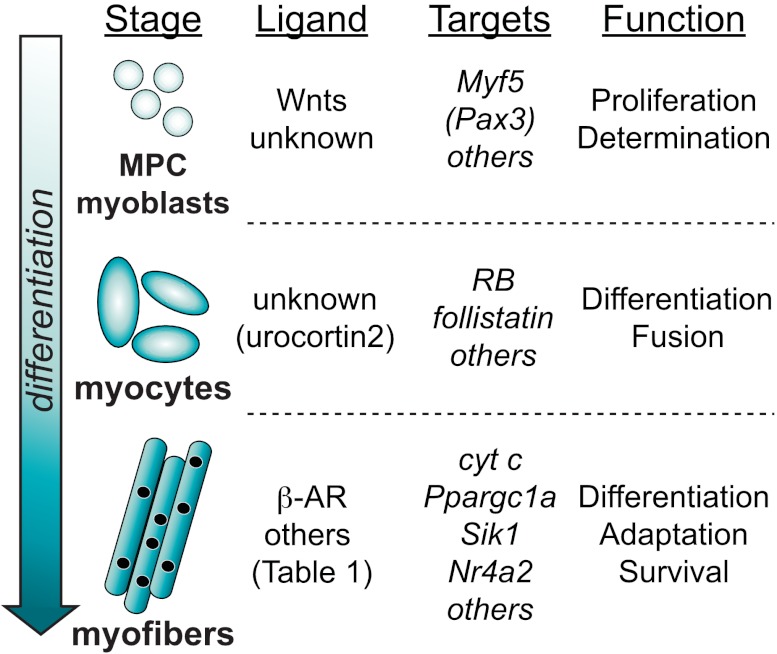
Fig. 5.
Roles of CREB in muscle cells at different stages of differentiation. During development or after injury, myogenic precursor cells (MPCs) differentiate into myoblasts, myocytes, and myofibers. The known ligands that stimulate CREB, known CREB target genes, and CREB functions at each developmental stage are listed.
cAMP signaling during myogenesis is dynamic, and ectopically sustained cAMP signaling inhibits bHLH myogenic transcription factors and MEF2D (56, 105, 140, 200). If high amounts of cAMP are needed to activate CREB but also inhibit myogenic differentiation, it seems paradoxical that CREB could participate in differentiation. A possible model to rectify these results holds that cAMP-CREB transcription exerts a priming effect on the myogenic program by induction of Myf5 and probably other early target genes (Fig. 5). Additionally, cAMP levels decline in myocytes during later stages of differentiation, when CREB participates in regulation of cytochrome c (65), so it is also possible that during differentiation CREB is regulated by a cAMP-independent kinase such as p38 MAP kinase, which activates CREB during mesoderm patterning in Xenopus embryos (120). Finally, many CREB-regulated promoters are subject to combinatorial control; CREB interacts with MyoD on both the RB and follistatin promoters (109, 153), suggesting that CREB promoter occupancy may be permissive for these genes, not limiting. Nonetheless, CREB activity is required for myogenic differentiation in vitro (153) and in vivo (39), so a thorough understanding of the upstream activating signals and the mechanisms by which sets of target genes are selected at different stages of differentiation will contribute to the mechanistic understanding of this complex process.
Injury and regeneration.
Satellite cell-mediated regeneration is studied in model organisms by injection of toxins or the anesthetic bupivacaine, which cause myofiber necrosis, or by studying regenerative capacity in genetic models of muscular dystrophy, particularly the mdx mouse (93). Modulation of cAMP signaling using pharmacological agents (86, 90, 103, 196, 254) or genetic means (80, 163, 216) can improve muscle regeneration in these models. β-AR density increases threefold after necrotizing muscle injury, with concomitant increases in intramuscular cAMP relative to normal muscle (16). Other GPCRs are also upregulated after muscle damage, including the odorant receptor MOR23, which contributes to myogenesis and fusion during regeneration (80). Similarly to β-AR, MOR23 is coupled to Gαs, suggesting that one or both of these receptors stimulates cAMP production in cells in the injured region. CRFR2 agonists also not only limit atrophy but also appear to modulate degeneration, inflammation, and fibrosis in mdx muscle by unknown mechanisms (103). Paradoxically, intramuscular injection of forskolin or a PDE inhibitor after myotoxic injury reduces expression of a MEF2 reporter during regeneration, probably due to direct inhibition of MEF2D by PKA (56). This finding indicates that, as in development, dynamic cAMP signaling is required during regeneration.
In addition to its actions during muscle development, CREB also exerts positive effects on muscle survival and regeneration in mouse skeletal muscle. We previously showed that mice expressing dominant-negative CREB (A-CREB) in skeletal myofibers exhibit muscle degeneration, due at least in part to reduced expression of salt-inducible kinase-1 (Sik1) (19). SIK1 phosphorylates and inhibits HDAC5, thereby activating MEF2 (19). In muscle A-CREB mice, compromised sarcolemmal integrity is probably secondary to reduced expression of desmin (a MEF2 target gene) or M-line structural abnormalities due to reduced Mef2c expression; mutations in either desmin or Mef2c result in similar myopathic phenotypes (141, 181). On the other hand, knock-in mice expressing activated CREB(Y134F) have enhanced proliferation after muscle injury and improved regeneration on an mdx background (216). It is not known which transcriptional targets of CREB mediate its effects on muscle repair. We favor a model in which, after injury, CREB induces genes in muscle precursor cells to drive proliferation and subsequently contributes to myoblast fusion via follistatin (109). Finally, CREB may further promote differentiation via transcriptional induction of Myf5 (39) and RB (153) (Fig. 5). In support of this model, amounts of phospho-CREB are elevated in both Pax7-positive satellite cells and nascent myofibers within regenerating mdx muscle (216). However, the endogenous ligands that activate CREB in myocytes and regenerating myofibers are poorly characterized. In the embryo, Wnt1, Wnt3a, or Wnt7a is capable of activating CREB via a noncanonical cAMP pathway (39). Wnt1 and Wnt3a mRNAs have also been detected in mature myofibers (175). Wnt7a was recently demonstrated to activate Fzd7 and Gαs-mediated signaling in differentiated myotubes (237). It is therefore tempting to speculate that local Wnt signaling activates CREB in regenerating areas. Indeed, adult muscle crushed ex vivo releases ligands that induce cAMP signaling (80) and activate CREB (216) in isolated muscle precursor cells. It is not known whether Wnts or other cAMP-inducing ligands, such as Ucn2 (36), are responsible for this activity.
It will be exciting to determine how cAMP-PKA signaling contributes to normal muscle regeneration. This likely involves promotion of muscle precursor cell proliferation (39, 216), migration and fusion (80, 249), and possibly terminal steps in differentiation (65, 153, 216). Detailed investigation of signaling pathways downstream from PKA in regenerating muscle may uncover novel, skeletal muscle-specific regulatory mechanisms that could be targeted specifically to promote muscle regeneration.
CONCLUDING REMARKS
In the five decades since cAMP was discovered, its numerous roles in skeletal myofibers have been steadily identified. Although well studied and promising for treatment of muscle atrophy in patients, the potential detrimental effects of long-term β-AR agonist treatment on the cardiovascular system must be taken into account for human therapeutic use (194). It will therefore be important in the future to further study physiological effects and downstream signaling pathways elicited by other, less-studied ligands that induce cAMP in skeletal muscle and myoblasts. At least one of these, Ucn2, has similar beneficial effects to clenbuterol on denervated and dystrophic skeletal muscle. It remains to be seen how other ligand-receptor systems may be exploited for therapeutic benefit in this tissue. A potentially more promising avenue is identification of skeletal muscle-specific cAMP effector pathways that can be selectively targeted to promote hypertrophy and muscle regeneration after injury. Such targets could be used in patients with a wide variety of pathological conditions, such as muscular dystrophy or atrophy due to aging, immobilization, cachexia, and nerve or muscle damage.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR-059847).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B. prepared figures; R.B. and R.S. drafted manuscript; R.B. and R.S. edited and revised manuscript; R.B. and R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christopher Robb for help with editing the manuscript.
Glossary
- Akt
Protein kinase B, PKB
- AKAP
A-kinase anchoring protein
- AVP
Arg8-vasopressin
- β-AR
β-Adrenergic receptor
- cAMP
Adenosine 3′,5′-monophosphate
- CGRP
Calcitonin gene-related peptide
- COXIV
Cytochrome c oxidase, subunit IV
- CREB
cAMP response element-binding protein
- CRF
Corticotropin-releasing factor
- CRFR2
Corticotropin-releasing factor receptor 2
- CXCR4
CXC chemokine receptor 4
- dy/dy
Laminin-deficient mouse with muscular dystrophy
- Epac
Exchange protein activated by cAMP
- ERK1/2
Extracellular signal-regulated kinases 1 and 2
- Forskolin
Direct AC agonist
- FoxO
Forkhead box transcription factors, class O
- Fzd7
Frizzled 7
- GPCR
G protein-coupled receptor
- IGF-I
Insulin-like growth factor I
- IL-6
Interleukin-6
- LTCC
L-type calcium channel
- MAFbx
Muscle atrophy F-box, Atrogin 1
- mdx
Dystrophin-deficient mouse strain
- MuRF1
Muscle RING finger 1
- MyHC
Myosin heavy chain
- nAChR
Nicotinic acetylcholine receptor
- NMJ
Neuromuscular junction
- Nor1
Neuron-derived orphan receptor 1, also Nr4a3
- Nur77
Nuclear receptor family member 77, also Nr4a1
- PI 3-kinase
Phosphatidylinositol 3-kinase
- MAP kinase
Mitogen-activated protein kinase
- PGC1-α
PPARγ coactivator 1α
- PKA
cAMP-dependent protein kinase, protein kinase A
- PKC
Protein kinase C
- RhoGDI
RhoGTPase guanine nucleotide dissociation inhibitor
- RyR
Ryanodine receptor
- SR
Sarcoplasmic reticulum
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase
REFERENCES
- 1. Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587– 19593, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-γ coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol 287: C790– C796, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ametller E, Busquets S, Fuster G, Figueras MT, De Oliveira CC, Toledo M, Korzeniewska K, Argiles JM, Lopez-Soriano FJ. Effects of formoterol on protein metabolism in myotubes during hyperthermia. Muscle Nerve 43: 268– 273, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, Idzerda RL, McKnight GS. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem 277: 27294– 27304, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Antipenko A, Frias JA, Parra J, Cadefau JA, Cusso R. Effect of chronic electrostimulation of rabbit skeletal muscle on calmodulin level and protein kinase activity. Int J Biochem Cell Biol 31: 303– 310, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Antoni FA, Barnard RJ, Shipston MJ, Smith SM, Simpson J, Paterson JM. Calcineurin feedback inhibition of agonist-evoked cAMP formation. J Biol Chem 270: 28055– 28061, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Antoni FA, Palkovits M, Simpson J, Smith SM, Leitch AL, Rosie R, Fink G, Paterson JM. Ca2+/calcineurin-inhibited adenylyl cyclase, highly abundant in forebrain regions, is important for learning and memory. J Neurosci 18: 9650– 9661, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baecker PA, Obernolte R, Bach C, Yee C, Shelton ER. Isolation of a cDNA encoding a human rolipram-sensitive cyclic AMP phosphodiesterase (PDE IVD). Gene 138: 253– 256, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Bardsley RG, Allcock SM, Dawson JM, Dumelow NW, Higgins JA, Lasslett YV, Lockley AK, Parr T, Buttery PJ. Effect of beta-agonists on expression of calpain and calpastatin activity in skeletal muscle. Biochimie 74: 267– 273, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603– 15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baviera AM, Zanon NM, Carvalho Navegantes LC, Migliorini RH, do Carmo Kettelhut I. Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. Am J Physiol Endocrinol Metab 292: E702– E708, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Baviera AM, Zanon NM, Navegantes LC, Kettelhut IC. Involvement of cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Mol Cell Endocrinol 315: 104– 112, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z. Inhibition of adenylyl cyclase isoforms V and VI by various Gbetagamma subunits. FASEB J 12: 1019– 1025, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Beermann DH, Butler WR, Hogue DE, Fishell VK, Dalrymple RH, Ricks CA, Scanes CG. Cimaterol-induced muscle hypertrophy and altered endocrine status in lambs. J Anim Sci 65: 1514– 1524, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Beitzel F, Gregorevic P, Ryall JG, Plant DR, Sillence MN, Lynch GS. Beta2-adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J Appl Physiol 96: 1385– 1392, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Beitzel F, Sillence MN, Lynch GS. β-Adrenoceptor signaling in regenerating skeletal muscle after β-agonist administration. Am J Physiol Endocrinol Metab 293: E932– E940, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA 105: 2198– 2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benson DW, Foley-Nelson T, Chance WT, Zhang FS, James JH, Fischer JE. Decreased myofibrillar protein breakdown following treatment with clenbuterol. J Surg Res 50: 1– 5, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Berdeaux R, Goebel N, Banaszynski L, Takemori H, Wandless T, Shelton GD, Montminy M. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med 13: 597– 603, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Bloom TJ. Age-related alterations in cyclic nucleotide phosphodiesterase activity in dystrophic mouse leg muscle. Can J Physiol Pharmacol 83: 1055– 1060, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Bloom TJ. Cyclic nucleotide phosphodiesterase isozymes expressed in mouse skeletal muscle. Can J Physiol Pharmacol 80: 1132– 1135, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704– 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014– 1019, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol 7: 397– 403, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology 145: 1718– 1729, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal 17: 1551– 1559, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Briguet A, Erb M, Courdier-Fruh I, Barzaghi P, Santos G, Herzner H, Lescop C, Siendt H, Henneboehle M, Weyermann P, Magyar JP, Dubach-Powell J, Metz G, Meier T. Effect of calpain and proteasome inhibition on Ca2+-dependent proteolysis and muscle histopathology in the mdx mouse. FASEB J 22: 4190– 4200, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Buckenmeyer PJ, Goldfarb AH, Partilla JS, Pineyro MA, Dax EM. Endurance training, not acute exercise, differentially alters β-receptors and cyclase in skeletal fiber types. Am J Physiol Endocrinol Metab 258: E71– E77, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Burns-Hamuro LL, Barraclough DM, Taylor SS. Identification and functional analysis of dual-specific A kinase-anchoring protein-2. Methods in enzymology 390: 354– 374, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Burton KA, Johnson BD, Hausken ZE, Westenbroek RE, Idzerda RL, Scheuer T, Scott JD, Catterall WA, McKnight GS. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proc Natl Acad Sci USA 94: 11067– 11072, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busquets S, Figueras MT, Fuster G, Almendro V, Moore-Carrasco R, Ametller E, Argiles JM, Lopez-Soriano FJ. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res 64: 6725– 6731, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Cairns SP, Dulhunty AF. Beta-adrenergic potentiation of E-C coupling increases force in rat skeletal muscle. Muscle Nerve 16: 1317– 1325, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Carbo N, Lopez-Soriano J, Tarrago T, Gonzalez O, Llovera M, Lopez-Soriano FJ, Argiles JM. Comparative effects of beta2-adrenergic agonists on muscle waste associated with tumour growth. Cancer Lett 115: 113– 118, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Carlsen RC. The possible role of cyclic AMP in the neurotrophic control of skeletal muscle. J Physiol 247: 343– 361, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carter WJ, Lynch ME. Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats. Metabolism 43: 1119– 1125, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR)1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology 145: 2445– 2457, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, Li C, Nagy TR, Shulman GI, Lee KF, Vale W. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 103: 16580– 16585, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol 19: 441– 458, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature 433: 317– 322, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett 581: 2181– 2193, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty DJ, Blank JL, Exton JH, Stoffel RH. A region of adenylyl cyclase 2 critical for regulation by G protein beta gamma subunits. Science 268: 1166– 1169, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Chen M, Feng HZ, Gupta D, Kelleher J, Dickerson KE, Wang J, Hunt D, Jou W, Gavrilova O, Jin JP, Weinstein LS. Gsα deficiency in skeletal muscle leads to reduced muscle mass, fiber-type switching, and glucose intolerance without insulin resistance or deficiency. Am J Physiol Cell Physiol 296: C930– C940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol on skeletal muscle are mediated by β2-adrenoceptor activation. Am J Physiol Endocrinol Metab 263: E50– E56, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269– 1324, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Cong M, Goll DE, Antin PB. cAMP responsiveness of the bovine calpastatin gene promoter. Biochim Biophys Acta 1443: 186– 192, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Costelli P, Garcia-Martinez C, Llovera M, Carbo N, Lopez-Soriano FJ, Agell N, Tessitore L, Baccino FM, Argiles JM. Muscle protein waste in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J Clin Invest 95: 2367– 2372, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Criswell DS, Powers SK, Herb RA. Clenbuterol-induced fiber type transition in the soleus of adult rats. Eur J Appl Physiol Occup Physiol 74: 391– 396, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Curtis DH, Zalin RJ. Regulation of muscle differentiation: stimulation of myoblast fusion in vitro by catecholamines. Science 214: 1355– 1357, 1981 [DOI] [PubMed] [Google Scholar]
- 49. Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88– 91, 1997 [DOI] [PubMed] [Google Scholar]
- 50. de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275: 20829– 20836, 2000 [DOI] [PubMed] [Google Scholar]
- 51. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474– 477, 1998 [DOI] [PubMed] [Google Scholar]
- 52. DeLange RJ, Kemp RG, Riley WD, Cooper RA, Krebs EG. Activation of skeletal muscle phosphorylase kinase by adenosine triphosphate and adenosine 3′,5′-monophosphate. J Biol Chem 243: 2200– 2208, 1968 [PubMed] [Google Scholar]
- 53. Dessauer CW. Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76: 935– 941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dietz MR, Chiasson JL, Soderling TR, Exton JH. Epinephrine regulation of skeletal muscle glycogen metabolism. Studies utilizing the perfused rat hindlimb preparation. J Biol Chem 255: 2301– 2307, 1980 [PubMed] [Google Scholar]
- 55. Dodd SL, Powers SK, Vrabas IS, Criswell D, Stetson S, Hussain R. Effects of clenbuterol on contractile and biochemical properties of skeletal muscle. Med Sci Sports Exerc 28: 669– 676, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Du M, Perry RL, Nowacki NB, Gordon JW, Salma J, Zhao J, Aziz A, Chan J, Siu KW, McDermott JC. Protein kinase A represses skeletal myogenesis by targeting myocyte enhancer factor 2D. Mol Cell Biol 28: 2952– 2970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Emery PW, Rothwell NJ, Stock MJ, Winter PD. Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep 4: 83– 91, 1984 [DOI] [PubMed] [Google Scholar]
- 58. Emrick MA, Sadilek M, Konoki K, Catterall WA. Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1. 1 channel in the fight-or-flight response Proc Natl Acad Sci USA 107: 18712– 18717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 101: 2993– 2998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA 97: 3702– 3707, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fedorov YV, Jones NC, Olwin BB. Regulation of myogenesis by fibroblast growth factors requires beta-gamma subunits of pertussis toxin-sensitive G proteins. Mol Cell Biol 18: 5780– 5787, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fell RD, Lizzo FH, Cervoni P, Crandall DL. Effect of contractile activity on rat skeletal muscle beta-adrenoceptor properties. Proc Soc Exp Biol Med 180: 527– 532, 1985 [DOI] [PubMed] [Google Scholar]
- 63. Forsberg NE, Ilian MA, Ali-Bar A, Cheeke PR, Wehr NB. Effects of cimaterol on rabbit growth and myofibrillar protein degradation and on calcium-dependent proteinase and calpastatin activities in skeletal muscle. J Anim Sci 67: 3313– 3321, 1989 [DOI] [PubMed] [Google Scholar]
- 64. Fowler EG, Graves MC, Wetzel GT, Spencer MJ. Pilot trial of albuterol in Duchenne and Becker muscular dystrophy. Neurology 62: 1006– 1008, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Franko A, Mayer S, Thiel G, Mercy L, Arnould T, Hornig-Do HT, Wiesner RJ, Goffart S. CREB-1alpha is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol Cell Biol 28: 2446– 2459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Freerksen DL, Schroedl NA, Johnson GV, Hartzell CR. Increased aerobic glucose oxidation by cAMP in cultured regenerated skeletal myotubes. Am J Physiol Cell Physiol 250: C713– C719, 1986 [DOI] [PubMed] [Google Scholar]
- 67. Galbo H, Richter EA, Hilsted J, Holst JJ, Christensen NJ, Henriksson J. Hormonal regulation during prolonged exercise. Ann NY Acad Sci 301: 72– 80, 1977 [DOI] [PubMed] [Google Scholar]
- 68. Gao X, Sadana R, Dessauer CW, Patel TB. Conditional stimulation of type V and VI adenylyl cyclases by G protein betagamma subunits. J Biol Chem 282: 294– 302, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Gardner C, Robas N, Cawkill D, Fidock M. Cloning and characterization of the human and mouse PDE7B, a novel cAMP-specific cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun 272: 186– 192, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol 288: C1305– C1316, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5: 87– 90, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Goldfarb AH, Bruno JF, Buckenmeyer PJ. Intensity and duration of exercise effects on skeletal muscle cAMP, phosphorylase, and glycogen. J Appl Physiol 66: 190– 194, 1989 [DOI] [PubMed] [Google Scholar]
- 73. Goncalves DA, Lira EC, Baviera AM, Cao P, Zanon NM, Arany Z, Bedard N, Tanksale P, Wing SS, Lecker SH, Kettelhut IC, Navegantes LC. Mechanisms involved in 3′,5′-cyclic adenosine monophosphate-mediated inhibition of the ubiquitin-proteasome system in skeletal muscle. Endocrinology 150: 5395– 5404, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675– 680, 1989 [DOI] [PubMed] [Google Scholar]
- 75. Gopalakrishnan L, Scarpulla RC. Differential regulation of respiratory chain subunits by a CREB-dependent signal transduction pathway. Role of cyclic AMP in cytochrome c and COXIV gene expression. J Biol Chem 269: 105– 113, 1994 [PubMed] [Google Scholar]
- 76. Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for β-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol 283: C1025– C1032, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol 14: 2076– 2091, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem 272: 6297– 6302, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Griffin CA, Apponi LH, Long KK, Pavlath GK. Chemokine expression and control of muscle cell migration during myogenesis. J Cell Sci 123: 3052– 3060, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 17: 649– 661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol 24: 6253– 6267, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guyton AC. Textbook of Medical Physiology. Philadelphia, PA: W. B. Saunders, 1991, p. 1014 [Google Scholar]
- 83. Ha TN, Fryer MW. Inhibitory effects of (+/−)-propranolol on excitation-contraction coupling in isolated soleus muscles of the rat. Br J Pharmacol 122: 463– 468, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ha TN, Posterino GS, Fryer MW. Effects of terbutaline on force and intracellular calcium in slow-twitch skeletal muscle fibres of the rat. Br J Pharmacol 126: 1717– 1724, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hacker BM, Tomlinson JE, Wayman GA, Sultana R, Chan G, Villacres E, Disteche C, Storm DR. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9). Genomics 50: 97– 104, 1998 [DOI] [PubMed] [Google Scholar]
- 86. Hall JE, Kaczor JJ, Hettinga BP, Isfort RJ, Tarnopolsky MA. Effects of a CRF2R agonist and exercise on mdx and wildtype skeletal muscle. Muscle Nerve 36: 336– 341, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41: 145– 174, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Hansen AK, Clausen T, Nielsen OB. Effects of lactic acid and catecholamines on contractility in fast-twitch muscles exposed to hyperkalemia. Am J Physiol Cell Physiol 289: C104– C112, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, Shenoy SK, Gregory SG, Ahn S, Duckett DR, Lefkowitz RJ. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477: 349– 353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul Disord 17: 47– 55, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Harri MN. Physical training under the influence of beta-blockade in rats. III. Effects on muscular metabolism. Eur J Appl Physiol Occup Physiol 45: 25– 31, 1980 [DOI] [PubMed] [Google Scholar]
- 92. Hartley LH, Mason JW, Hogan RP, Jones LG, Kotchen TA, Mougey EH, Wherry FE, Pennington LL, Ricketts PT. Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol 33: 602– 606, 1972 [DOI] [PubMed] [Google Scholar]
- 93. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534– 551, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Hayes A, Williams DA. Examining potential drug therapies for muscular dystrophy utilising the dy/dy mouse: I. Clenbuterol. J Neurol Sci 157: 122– 128, 1998 [DOI] [PubMed] [Google Scholar]
- 95. Hepler JR, Gilman AG. G proteins. Trends Biochem Sci 17: 383– 387, 1992 [DOI] [PubMed] [Google Scholar]
- 96. Hetman JM, Soderling SH, Glavas NA, Beavo JA. Cloning and characterization of PDE7B, a cAMP-specific phosphodiesterase. Proc Natl Acad Sci USA 97: 472– 476, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hines LM, Hoffman PL, Bhave S, Saba L, Kaiser A, Snell L, Goncharov I, LeGault L, Dongier M, Grant B, Pronko S, Martinez L, Yoshimura M, Tabakoff B. A sex-specific role of type VII adenylyl cyclase in depression. J Neurosci 26: 12609– 12619, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hinkle RT, Dolan E, Cody DB, Bauer MB, Isfort RJ. Phosphodiesterase 4 inhibition reduces skeletal muscle atrophy. Muscle Nerve 32: 775– 781, 2005 [DOI] [PubMed] [Google Scholar]
- 99. Hinkle RT, Donnelly E, Cody DB, Bauer MB, Isfort RJ. Urocortin II treatment reduces skeletal muscle mass and function loss during atrophy and increases nonatrophying skeletal muscle mass and function. Endocrinology 144: 4939– 4946, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Hinkle RT, Donnelly E, Cody DB, Bauer MB, Sheldon RJ, Isfort RJ. Corticotropin releasing factor 2 receptor agonists reduce the denervation-induced loss of rat skeletal muscle mass and force and increase non-atrophying skeletal muscle mass and force. J Muscle Res Cell Motil 25: 539– 547, 2004 [DOI] [PubMed] [Google Scholar]
- 101. Hinkle RT, Donnelly E, Cody DB, Samuelsson S, Lange JS, Bauer MB, Tarnopolsky M, Sheldon RJ, Coste SC, Tobar E, Stenzel-Poore MP, Isfort RJ. Activation of the CRF 2 receptor modulates skeletal muscle mass under physiological and pathological conditions. Am J Physiol Endocrinol Metab 285: E889– E898, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Hinkle RT, Hodge KM, Cody DB, Sheldon RJ, Kobilka BK, Isfort RJ. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve 25: 729– 734, 2002 [DOI] [PubMed] [Google Scholar]
- 103. Hinkle RT, Lefever FR, Dolan ET, Reichart DL, Dietrich JA, Gropp KE, Thacker RI, Demuth JP, Stevens PJ, Qu XA, Varbanov AR, Wang F, Isfort RJ. Corticortophin releasing factor 2 receptor agonist treatment significantly slows disease progression in mdx mice. BMC Med 5: 18, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hoover F, Mathiesen I, Skalhegg BS, Lomo T, Tasken K. Differential expression and regulation of the PKA signalling pathway in fast and slow skeletal muscle. Anat Embryol (Berl) 203: 193– 201, 2001 [DOI] [PubMed] [Google Scholar]
- 105. Hu JS, Olson EN. Regulation of differentiation of the BC3H1 muscle cell line through cAMP-dependent and -independent pathways. J Biol Chem 263: 19670– 19677, 1988 [PubMed] [Google Scholar]
- 106. Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. D-AKAP2, a novel protein kinase A anchoring protein with a putative RGS domain. Proc Natl Acad Sci USA 94: 11184– 11189, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem 272: 8057– 8064, 1997 [DOI] [PubMed] [Google Scholar]
- 108. Huang Y, Roelink H, McKnight GS. Protein kinase A deficiency causes axially localized neural tube defects in mice. J Biol Chem 277: 19889– 19896, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, Sartorelli V. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell 6: 673– 684, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Imaizumi-Scherrer T, Faust DM, Benichou JC, Hellio R, Weiss MC. Accumulation in fetal muscle and localization to the neuromuscular junction of cAMP-dependent protein kinase A regulatory and catalytic subunits RI alpha and C alpha. J Cell Biol 134: 1241– 1254, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Iyengar R, Hildebrandt JD. G Protein Pathways: Part B, G Proteins and Their Regulators. San Diego, CA: Academic, 2002 [Google Scholar]
- 112. Iyengar R, Hildebrandt JD. G Protein Pathways: Part C, Effector Mechanisms. San Diego, CA: Academic, 2002 [Google Scholar]
- 113. Jacobowitz O, Iyengar R. Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc Natl Acad Sci USA 91: 10630– 10634, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jean-Baptiste G, Yang Z, Khoury C, Gaudio S, Greenwood MT. Peptide and non-peptide G-protein coupled receptors (GPCRs) in skeletal muscle. Peptides 26: 1528– 1536, 2005 [DOI] [PubMed] [Google Scholar]
- 115. Ji LL, Lennon DL, Kochan RG, Nagle FJ, Lardy HA. Enzymatic adaptation to physical training under beta-blockade in the rat. Evidence of a beta 2-adrenergic mechanism in skeletal muscle. J Clin Invest 78: 771– 778, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem 269: 16554– 16558, 1994 [PubMed] [Google Scholar]
- 117. Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275– 2279, 1998 [DOI] [PubMed] [Google Scholar]
- 118. Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58: 1953– 1960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kelvin DJ, Simard G, Tai HH, Yamaguchi TP, Connolly JA. Growth factors, signaling pathways, and the regulation of proliferation and differentiation in BC3H1 muscle cells. I. A pertussis toxin-sensitive pathway is involved. J Cell Biol 108: 159– 167, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Keren A, Keren-Politansky A, Bengal E. A p38 MAPK-CREB pathway functions to pattern mesoderm in Xenopus. Dev Biol 322: 86– 94, 2008 [DOI] [PubMed] [Google Scholar]
- 121. Kim YS, Sainz RD. Beta-adrenergic agonists and hypertrophy of skeletal muscles. Life Sci 50: 397– 407, 1992 [DOI] [PubMed] [Google Scholar]
- 122. Kim YS, Sainz RD, Molenaar P, Summers RJ. Characterization of beta 1- and beta 2-adrenoceptors in rat skeletal muscles. Biochem Pharmacol 42: 1783– 1789, 1991 [DOI] [PubMed] [Google Scholar]
- 123. Kirby CR, Woodman CR, Woolridge D, Tischler ME. Cyclic adenosine monophosphate accumulation and beta-adrenergic binding in unweighted and denervated rat soleus muscle. Metabolism 41: 793– 799, 1992 [DOI] [PubMed] [Google Scholar]
- 124. Kishimoto T, Pearse RV, 2nd, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA 92: 1108– 1112, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kissel JT, McDermott MP, Mendell JR, King WM, Pandya S, Griggs RC, Tawil R. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology 57: 1434– 1440, 2001 [DOI] [PubMed] [Google Scholar]
- 126. Kissel JT, McDermott MP, Natarajan R, Mendell JR, Pandya S, King WM, Griggs RC, Tawil R. Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. FSH-DY Group. Neurology 50: 1402– 1406, 1998 [DOI] [PubMed] [Google Scholar]
- 127. Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol 102: 740– 747, 2007 [DOI] [PubMed] [Google Scholar]
- 128. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 288: 351– 357, 2002 [DOI] [PubMed] [Google Scholar]
- 129. Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397– 406, 2007 [DOI] [PubMed] [Google Scholar]
- 130. Koopman R, Gehrig SM, Leger B, Trieu J, Walrand S, Murphy KT, Lynch GS. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic (beta)-adrenoceptor stimulation in mice. J Physiol 588: 4811– 4823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kraus WE, Bernard TS, Williams RS. Interactions between sustained contractile activity and β-adrenergic receptors in regulation of gene expression in skeletal muscles. Am J Physiol Cell Physiol 256: C506– C514, 1989 [DOI] [PubMed] [Google Scholar]
- 132. Kraus WE, Longabaugh JP, Liggett SB. Electrical pacing induces adenylyl cyclase in skeletal muscle independent of the β-adrenergic receptor. Am J Physiol Endocrinol Metab 263: E226– E230, 1992 [DOI] [PubMed] [Google Scholar]
- 133. Kuperman Y, Issler O, Vaughan J, Bilezikjian L, Vale W, Chen A. Expression and regulation of corticotropin-releasing factor receptor type 2beta in developing and mature mouse skeletal muscle. Mol Endocrinol 25: 157– 169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lai HL, Yang TH, Messing RO, Ching YH, Lin SC, Chern Y. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J Biol Chem 272: 4970– 4977, 1997 [DOI] [PubMed] [Google Scholar]
- 135. Lanuza MA, Garcia N, Santafe M, Nelson PG, Fenoll-Brunet MR, Tomas J. Pertussis toxin-sensitive G-protein and protein kinase C activity are involved in normal synapse elimination in the neonatal rat muscle. J Neurosci Res 63: 330– 340, 2001 [DOI] [PubMed] [Google Scholar]
- 136. Lawrence JC, Jr, Salsgiver WJ. Evidence that levels of malate dehydrogenase and fumarase are increased by cAMP in rat myotubes. Am J Physiol Cell Physiol 247: C33– C38, 1984 [DOI] [PubMed] [Google Scholar]
- 137. Le Peuch CJ, Ferraz C, Walsh MP, Demaille JG, Fischer EH. Calcium and cyclic nucleotide dependent regulatory mechanisms during development of chick embryo skeletal muscle. Biochemistry 18: 5267– 5273, 1979 [DOI] [PubMed] [Google Scholar]
- 138. Letts LG, Richardson DP, Temple DM, Williams LR. The selectivity of beta-adrenoceptor antagonists on isoprenaline-induced changes in heart rate, blood pressure, soleus muscle contractility and airways function in anaesthetized cats. Br J Pharmacol 80: 323– 334, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lexicon Genetics I. NIH initiative supporting placement of Lexicon Genetics, Inc. mice into public repositories. In: MGI Direct Data Submission to Mouse Genome Database (MGD) 2005 [Google Scholar]
- 140. Li L, Heller-Harrison R, Czech M, Olson EN. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol 12: 4478– 4485, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol 175: 362– 366, 1996 [DOI] [PubMed] [Google Scholar]
- 142. Liggett SB, Shah SD, Cryer PE. Characterization of β-adrenergic receptors of human skeletal muscle obtained by needle biopsy. Am J Physiol Endocrinol Metab 254: E795– E798, 1988 [DOI] [PubMed] [Google Scholar]
- 143. Lin CH, Hudson AJ, Strickland KP. Adenyl cyclase and cyclic nucleotide phosphodiesterase activities in murine muscular dystrophy. Enzyme 21: 85– 95, 1976 [DOI] [PubMed] [Google Scholar]
- 144. Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci 18: 2017– 2027, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lira EC, Graca FA, Goncalves DA, Zanon NM, Baviera AM, Strindberg L, Lonnroth P, Migliorini RH, Kettelhut IC, Navegantes LC. Cyclic adenosine monophosphate-phosphodiesterase inhibitors reduce skeletal muscle protein catabolism in septic rats. Shock 27: 687– 694, 2007 [DOI] [PubMed] [Google Scholar]
- 146. Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem 278: 15922– 15926, 2003 [DOI] [PubMed] [Google Scholar]
- 147. Liu Y, Kranias EG, Schneider MF. Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am J Physiol Cell Physiol 273: C1915– C1924, 1997 [DOI] [PubMed] [Google Scholar]
- 148. Lorimer IA, Mason ME, Sanwal BD. Levels of type I cAMP-dependent protein kinase regulatory subunit are regulated by changes in turnover rate during skeletal myogenesis. J Biol Chem 262: 17200– 17205, 1987 [PubMed] [Google Scholar]
- 149. Lorimer IA, Sanwal BD. Regulation of cyclic AMP-dependent protein kinase levels during skeletal myogenesis. Biochem J 264: 305– 308, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lynch GS, Hayes A, Campbell SP, Williams DA. Effects of β2-agonist administration and exercise on contractile activation of skeletal muscle fibers. J Appl Physiol 81: 1610– 1618, 1996 [DOI] [PubMed] [Google Scholar]
- 151. Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729– 767, 2008 [DOI] [PubMed] [Google Scholar]
- 152. Lynge J, Schulte G, Nordsborg N, Fredholm BB, Hellsten Y. Adenosine A 2B receptors modulate cAMP levels and induce CREB but not ERK1/2 and p38 phosphorylation in rat skeletal muscle cells. Biochem Biophys Res Commun 307: 180– 187, 2003 [DOI] [PubMed] [Google Scholar]
- 153. Magenta A, Cenciarelli C, De Santa F, Fuschi P, Martelli F, Caruso M, Felsani A. MyoD stimulates RB promoter activity via the CREB/p300 nuclear transduction pathway. Mol Cell Biol 23: 2893– 2906, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Maltin CA, Hay SM, Delday MI, Lobley GE, Reeds PJ. The action of the beta-agonist clenbuterol on protein metabolism in innervated and denervated phasic muscles. Biochem J 261: 965– 971, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Maltin CA, Hay SM, McMillan DN, Delday MI. Tissue specific responses to clenbuterol; temporal changes in protein metabolism of striated muscle and visceral tissues from rats. Growth Regul 2: 161– 166, 1992 [PubMed] [Google Scholar]
- 156. Martin WH, 3rd, Coggan AR, Spina RJ, Saffitz JE. Effects of fiber type and training on β-adrenoceptor density in human skeletal muscle. Am J Physiol Endocrinol Metab 257: E736– E742, 1989 [DOI] [PubMed] [Google Scholar]
- 157. Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem 280: 12573– 12584, 2005 [DOI] [PubMed] [Google Scholar]
- 158. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657– 3666, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. McCartney S, Little BM, Langeberg LK, Scott JD. Cloning and characterization of A-kinase anchor protein 100 (AKAP100). A protein that targets A-kinase to the sarcoplasmic reticulum. J Biol Chem 270: 9327– 9333, 1995 [DOI] [PubMed] [Google Scholar]
- 160. McGraw DW, Liggett SB. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc Am Thorac Soc 2: 292– 296; discussion 311–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Michel JJ, Townley IK, Dodge-Kafka KL, Zhang F, Kapiloff MS, Scott JD. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol Cell 20: 661– 672, 2005 [DOI] [PubMed] [Google Scholar]
- 162. Mikkelsen UR, Gissel H, Fredsted A, Clausen T. Excitation-induced cell damage and β2-adrenoceptor agonist stimulated force recovery in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290: R265– R272, 2006 [DOI] [PubMed] [Google Scholar]
- 163. Minetti GC, Feige JN, Rosenstiel A, Bombard F, Meier V, Werner A, Bassilana F, Sailer AW, Kahle P, Lambert C, Glass DJ, Fornaro M. Galphai2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci Signal 4: ra80, 2011 [DOI] [PubMed] [Google Scholar]
- 164. Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 148: 3441– 3448, 2007 [DOI] [PubMed] [Google Scholar]
- 165. Mukai A, Hashimoto N. Localized cyclic AMP-dependent protein kinase activity is required for myogenic cell fusion. Exp Cell Res 314: 387– 397, 2008 [DOI] [PubMed] [Google Scholar]
- 166. Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195– 200, 2001 [DOI] [PubMed] [Google Scholar]
- 167. Naro F, De Arcangelis V, Sette C, Ambrosio C, Komati H, Molinaro M, Adamo S, Nemoz G. A bimodal modulation of the cAMP pathway is involved in the control of myogenic differentiation in l6 cells. J Biol Chem 278: 49308– 49315, 2003 [DOI] [PubMed] [Google Scholar]
- 168. Navegantes LC, Baviera AM, Kettelhut IC. The inhibitory role of sympathetic nervous system in the Ca2+-dependent proteolysis of skeletal muscle. Braz J Med Biol Res 42: 21– 28, 2009 [DOI] [PubMed] [Google Scholar]
- 169. Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Catecholamines inhibit Ca2+-dependent proteolysis in rat skeletal muscle through β2-adrenoceptors and cAMP. Am J Physiol Endocrinol Metab 281: E449– E454, 2001 [DOI] [PubMed] [Google Scholar]
- 170. Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Role of adrenoceptors and cAMP on the catecholamine-induced inhibition of proteolysis in rat skeletal muscle. Am J Physiol Endocrinol Metab 279: E663– E668, 2000 [DOI] [PubMed] [Google Scholar]
- 171. Nelson EJ, Hellevuo K, Yoshimura M, Tabakoff B. Ethanol-induced phosphorylation and potentiation of the activity of type 7 adenylyl cyclase. Involvement of protein kinase C delta. J Biol Chem 278: 4552– 4560, 2003 [DOI] [PubMed] [Google Scholar]
- 172. Nesher R, Karl IE, Kipnis Epitrochlearis muscle DM. II. Metabolic effects of contraction and catecholamines. Am J Physiol Endocrinol Metab 239: E461– E467, 1980 [DOI] [PubMed] [Google Scholar]
- 173. New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature 323: 809– 811, 1986 [DOI] [PubMed] [Google Scholar]
- 174. Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol 16: 321– 327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 121: 2939– 2950, 2008 [DOI] [PubMed] [Google Scholar]
- 176. Parr T, Sensky PL, Arnold MK, Bardsley RG, Buttery PJ. Effects of epinephrine infusion on expression of calpastatin in porcine cardiac and skeletal muscle. Arch Biochem Biophys 374: 299– 305, 2000 [DOI] [PubMed] [Google Scholar]
- 177. Pearen MA, Ryall JG, Lynch GS, Muscat GE. Expression profiling of skeletal muscle following acute and chronic beta2-adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics 10: 448, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, is a target of beta-adrenergic signaling in skeletal muscle. Endocrinology 147: 5217– 5227, 2006 [DOI] [PubMed] [Google Scholar]
- 179. Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 92: 2969– 2973, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Polla B, Cappelli V, Morello F, Pellegrino MA, Boschi F, Pastoris O, Reggiani C. Effects of the β2-agonist clenbuterol on respiratory and limb muscles of weaning rats. Am J Physiol Regul Integr Comp Physiol 280: R862– R869, 2001 [DOI] [PubMed] [Google Scholar]
- 181. Potthoff MJ, Arnold MA, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol Cell Biol 27: 8143– 8151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, Hanoune J. Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem 271: 13900– 13907, 1996 [DOI] [PubMed] [Google Scholar]
- 183. Rajab P, Fox J, Riaz S, Tomlinson D, Ball D, Greenhaff PL. Skeletal muscle myosin heavy chain isoforms and energy metabolism after clenbuterol treatment in the rat. Am J Physiol Regul Integr Comp Physiol 279: R1076– R1081, 2000 [DOI] [PubMed] [Google Scholar]
- 184. Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160: 919– 928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Reutenauer-Patte J, Boittin FX, Patthey-Vuadens O, Ruegg UT, Dorchies OM. Urocortins improve dystrophic skeletal muscle structure and function through both PKA- and Epac-dependent pathways. Am J Pathol 180: 749– 762, 2011 [DOI] [PubMed] [Google Scholar]
- 186. Reynolds JG, McCalmon SA, Donaghey JA, Naya FJ. Deregulated protein kinase A signaling and myospryn expression in muscular dystrophy. J Biol Chem 283: 8070– 8074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Reynolds JG, McCalmon SA, Tomczyk T, Naya FJ. Identification and mapping of protein kinase A binding sites in the costameric protein myospryn. Biochim Biophys Acta 1773: 891– 902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Roder IV, Lissandron V, Martin J, Petersen Y, Di Benedetto G, Zaccolo M, Rudolf R. PKA microdomain organisation and cAMP handling in healthy and dystrophic muscle in vivo. Cell Signal 21: 819– 826, 2009 [DOI] [PubMed] [Google Scholar]
- 189. Rogers JE, Narindrasorasak S, Cates GA, Sanwal BD. Regulation of protein kinase and its regulatory subunits during skeletal myogenesis. J Biol Chem 260: 8002– 8007, 1985 [PubMed] [Google Scholar]
- 190. Rogers KL, Fagan JM. Effect of beta agonists on protein turnover in isolated chick skeletal and atrial muscle. Proc Soc Exp Biol Med 197: 482– 485, 1991 [DOI] [PubMed] [Google Scholar]
- 191. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009– 1013, 2001 [DOI] [PubMed] [Google Scholar]
- 192. Rothwell NJ, Stock MJ. Modification of body composition by clenbuterol in normal and dystrophic (mdx) mice. Biosci Rep 5: 755– 760, 1985 [DOI] [PubMed] [Google Scholar]
- 193. Rothwell NJ, Stock MJ, Sudera DK. Changes in tissue blood flow and beta-receptor density of skeletal muscle in rats treated with the beta2-adrenoceptor agonist clenbuterol. Br J Pharmacol 90: 601– 607, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ryall JG, Lynch GS. The potential and the pitfalls of beta-adrenoceptor agonists for the management of skeletal muscle wasting. Pharmacol Ther 120: 219– 232, 2008 [DOI] [PubMed] [Google Scholar]
- 195. Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol 555: 175– 188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ryall JG, Schertzer JD, Alabakis TM, Gehrig SM, Plant DR, Lynch GS. Intramuscular beta2-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. J Appl Physiol 105: 165– 172, 2008 [DOI] [PubMed] [Google Scholar]
- 197. Ryall JG, Sillence MN, Lynch GS. Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br J Pharmacol 147: 587– 595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17: 5– 22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Salazar JJ, Michele DE, Brooks SV. Inhibition of calpain prevents muscle weakness and disruption of sarcomere structure during hindlimb suspension. J Appl Physiol 108: 120– 127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Salminen A, Braun T, Buchberger A, Jurs S, Winter B, Arnold HH. Transcription of the muscle regulatory gene Myf4 is regulated by serum components, peptide growth factors and signaling pathways involving G proteins. J Cell Biol 115: 905– 917, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399– 412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sasaki T, Kotera J, Yuasa K, Omori K. Identification of human PDE7B, a cAMP-specific phosphodiesterase. Biochem Biophys Res Commun 271: 575– 583, 2000 [DOI] [PubMed] [Google Scholar]
- 203. Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 22: 269– 278, 2007 [DOI] [PubMed] [Google Scholar]
- 204. Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364: 240– 243, 1993 [DOI] [PubMed] [Google Scholar]
- 205. Seiler S, Wegener AD, Whang DD, Hathaway DR, Jones LR. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease. J Biol Chem 259: 8550– 8557, 1984 [PubMed] [Google Scholar]
- 206. Sensky PL, Jewell KK, Ryan KJ, Parr T, Bardsley RG, Buttery PJ. Effect of anabolic agents on calpastatin promoters in porcine skeletal muscle and their responsiveness to cyclic adenosine monophosphate- and calcium-related stimuli. J Anim Sci 84: 2973– 2982, 2006 [DOI] [PubMed] [Google Scholar]
- 207. Shepherd M, McSorley T, Olsen AE, Johnston LA, Thomson NC, Baillie GS, Houslay MD, Bolger GB. Molecular cloning and subcellular distribution of the novel PDE4B4 cAMP-specific phosphodiesterase isoform. Biochem J 370: 429– 438, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Shi H, Zeng C, Ricome A, Hannon KM, Grant AL, Gerrard DE. Extracellular signal-regulated kinase pathway is differentially involved in β-agonist-induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol 292: C1681– C1689, 2007 [DOI] [PubMed] [Google Scholar]
- 209. Siow NL, Choi RC, Cheng AW, Jiang JX, Wan DC, Zhu SQ, Tsim KW. A cyclic AMP-dependent pathway regulates the expression of acetylcholinesterase during myogenic differentiation of C2C12 cells. J Biol Chem 277: 36129– 36136, 2002 [DOI] [PubMed] [Google Scholar]
- 210. Skalhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, Burton KA. Mutation of the Calpha subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol 16: 630– 639, 2002 [DOI] [PubMed] [Google Scholar]
- 211. Skura CL, Fowler EG, Wetzel GT, Graves M, Spencer MJ. Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology 70: 137– 143, 2008 [DOI] [PubMed] [Google Scholar]
- 212. Sneddon AA, Delday MI, Steven J, Maltin CA. Elevated IGF-II mRNA and phosphorylation of 4E-BP1 and p70S6k in muscle showing clenbuterol-induced anabolism. Am J Physiol Endocrinol Metab 281: E676– E682, 2001 [DOI] [PubMed] [Google Scholar]
- 213. Soderling TR, Hickenbottom JP, Reimann EM, Hunkeler FL, Walsh DA, Krebs EG. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J Biol Chem 245: 6317– 6328, 1970 [PubMed] [Google Scholar]
- 214. Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet 11: 2645– 2655, 2002 [DOI] [PubMed] [Google Scholar]
- 215. Spurlock DM, McDaneld TG, McIntyre LM. Changes in skeletal muscle gene expression following clenbuterol administration. BMC Genomics 7: 320, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Stewart R, Flechner L, Montminy M, Berdeaux R. CREB is activated by muscle injury and promotes muscle regeneration. PLoS One 6: e24714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395– 403, 2004 [DOI] [PubMed] [Google Scholar]
- 218. Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077– 1091, 1958 [PubMed] [Google Scholar]
- 219. Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem 285: 24248– 24259, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Suzuki Y, Shen T, Poyard M, Best-Belpomme M, Hanoune J, Defer N. Expression of adenylyl cyclase mRNAs in the denervated and in the developing mouse skeletal muscle. Am J Physiol Cell Physiol 274: C1674– C1685, 1998 [DOI] [PubMed] [Google Scholar]
- 221. Svedenhag J, Henriksson J, Juhlin-Dannfelt A. β-Adrenergic blockade and training in human subjects: effects on muscle metabolic capacity. Am J Physiol Endocrinol Metab 247: E305– E311, 1984 [DOI] [PubMed] [Google Scholar]
- 222. Takami K, Hashimoto K, Uchida S, Tohyama M, Yoshida H. Effect of calcitonin gene-related peptide on the cyclic AMP level of isolated mouse diaphragm. Jpn J Pharmacol 42: 345– 350, 1986 [DOI] [PubMed] [Google Scholar]
- 223. Takamori M, Yoshikawa H. Effect of calcitonin gene-related peptide on skeletal muscle via specific binding site and G protein. J Neurol Sci 90: 99– 109, 1989 [DOI] [PubMed] [Google Scholar]
- 224. Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117: 61– 69, 2008 [DOI] [PubMed] [Google Scholar]
- 225. Tang WJ, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 254: 1500– 1503, 1991 [DOI] [PubMed] [Google Scholar]
- 226. Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc 20: S132– S134, 1988 [DOI] [PubMed] [Google Scholar]
- 227. Thomas DP, Jenkins Effects of β1- vs RR. β1-β2-Blockade on training adaptations in rat skeletal muscle. J Appl Physiol 60: 1722– 1726, 1986 [DOI] [PubMed] [Google Scholar]
- 228. Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int J Biochem Cell Biol 32: 1– 5, 2000 [DOI] [PubMed] [Google Scholar]
- 229. Tidball JG, Spencer MJ. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 545: 819– 828, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, Young SG, Vranizan K, Kwok PY, Whooley MA, Conklin BR. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proc Natl Acad Sci USA 104: 8461– 8466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Tkachenko E, Sabouri-Ghomi M, Pertz O, Kim C, Gutierrez E, Machacek M, Groisman A, Danuser G, Ginsberg MH. Protein kinase A governs a RhoA-RhoGDI protrusion-retraction pacemaker in migrating cells. Nat Cell Biol 13: 660– 667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Torgan CE, Kraus WE. Regulation of type II adenylyl cyclase mRNA in rabbit skeletal muscle by chronic motor nerve pacing. Am J Physiol Endocrinol Metab 271: E253– E260, 1996 [DOI] [PubMed] [Google Scholar]
- 233. Tsai CC, Saffitz JE, Billadello JJ. Expression of the Gs protein alpha-subunit disrupts the normal program of differentiation in cultured murine myogenic cells. J Clin Invest 99: 67– 76, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Van Der Heijden HF, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Salbutamol enhances isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 85: 525– 529, 1998 [DOI] [PubMed] [Google Scholar]
- 235. van der Kooi EL, Vogels OJ, van Asseldonk RJ, Lindeman E, Hendriks JC, Wohlgemuth M, van der Maarel SM, Padberg GW. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 63: 702– 708, 2004 [DOI] [PubMed] [Google Scholar]
- 236. Viguerie N, Clement K, Barbe P, Courtine M, Benis A, Larrouy D, Hanczar B, Pelloux V, Poitou C, Khalfallah Y, Barsh GS, Thalamas C, Zucker JD, Langin D. In vivo epinephrine-mediated regulation of gene expression in human skeletal muscle. J Clin Endocrinol Metab 89: 2000– 2014, 2004 [DOI] [PubMed] [Google Scholar]
- 237. von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol 14: 186– 191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wahrmann JP, Luzzati D, Winand R. Changes in adenyl cyclase specific activity during differentiation on an established myogenic cell line. Biochem Biophys Res Commun 52: 576– 581, 1973 [DOI] [PubMed] [Google Scholar]
- 239. Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 13: 127– 133, 2011 [DOI] [PubMed] [Google Scholar]
- 240. Williams RS, Caron MG, Daniel K. Skeletal muscle β-adrenergic receptors: variations due to fiber type and training. Am J Physiol Endocrinol Metab 246: E160– E167, 1984 [DOI] [PubMed] [Google Scholar]
- 241. Willis BS, Niswender CM, Su T, Amieux PS, McKnight GS. Cell-type specific expression of a dominant negative PKA mutation in mice. PLoS One 6: e18772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wu Z, Huang X, Feng Y, Handschin C, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA 103: 14379– 14384, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Science STKE: re15, 2001 [DOI] [PubMed] [Google Scholar]
- 244. Yaspelkis BB, 3rd, Castle AL, Farrar RP, Ivy JL. Contraction-induced intracellular signals and their relationship to muscle GLUT-4 concentration. Am J Physiol Endocrinol Metab 272: E118– E125, 1997 [DOI] [PubMed] [Google Scholar]
- 245. Yimlamai T, Dodd SL, Borst SE, Park S. Clenbuterol induces muscle-specific attenuation of atrophy through effects on the ubiquitin-proteasome pathway. J Appl Physiol 99: 71– 80, 2005 [DOI] [PubMed] [Google Scholar]
- 246. Yoshimura M, Ikeda H, Tabakoff B. mu-Opioid receptors inhibit dopamine-stimulated activity of type V adenylyl cyclase but enhance dopamine-stimulated activity of type VII adenylyl cyclase. Mol Pharmacol 50: 43– 51, 1996 [PubMed] [Google Scholar]
- 247. Zalin RJ. Prostaglandins and myoblast fusion. Dev Biol 59: 241– 248, 1977 [DOI] [PubMed] [Google Scholar]
- 248. Zalin RJ. The relationship of the level of cyclic amp to differentiation in primary cultures of chick muscle cells. Exp Cell Res 78: 152– 158, 1973 [DOI] [PubMed] [Google Scholar]
- 249. Zalin RJ, Leaver R. The effect of a transient increase in intracellular cyclic AMP upon muscle cell fusion. FEBS Lett 53: 33– 36, 1975 [DOI] [PubMed] [Google Scholar]
- 250. Zalin RJ, Montague W. Changes in adenylate cyclase, cyclic AMP, and protein kinase levels in chick myoblasts, and their relationship to differentiation. Cell 2: 103– 108, 1974 [DOI] [PubMed] [Google Scholar]
- 251. Zalin RJ, Montague W. Changes in cyclic AMP, adenylate cyclase and protein kinase levels during the development of embryonic chick skeletal muscle. Exp Cell Res 93: 55– 62, 1975 [DOI] [PubMed] [Google Scholar]
- 252. Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a β2-receptor agonist. Am J Physiol Endocrinol Metab 254: E726– E732, 1988 [DOI] [PubMed] [Google Scholar]
- 253. Zeman RJ, Ludemann R, Etlinger JD. Clenbuterol, a β2-agonist, retards atrophy in denervated muscles. Am J Physiol Endocrinol Metab 252: E152– E155, 1987 [DOI] [PubMed] [Google Scholar]
- 254. Zeman RJ, Peng H, Danon MJ, Etlinger JD. Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve 23: 521– 528, 2000 [DOI] [PubMed] [Google Scholar]
- 255. Zeman RJ, Peng H, Etlinger JD. Clenbuterol retards loss of motor function in motor neuron degeneration mice. Exp Neurol 187: 460– 467, 2004 [DOI] [PubMed] [Google Scholar]
- 256. Zeman RJ, Zhang Y, Etlinger JD. Clenbuterol, a β2-agonist, retards wasting and loss of contractility in irradiated dystrophic mdx muscle. Am J Physiol Cell Physiol 267: C865– C868, 1994 [DOI] [PubMed] [Google Scholar]
- 257. Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA 102: 4459– 4464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. Protective roles of adenosine A1, A2A, and A3 receptors in skeletal muscle ischemia and reperfusion injury. Am J Physiol Heart Circ Physiol 293: H3685– H3691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]