Abstract
It is well established that long durations of bed rest, limb immobilization, or reduced activity in respiratory muscles during mechanical ventilation results in skeletal muscle atrophy in humans and other animals. The idea that mitochondrial damage/dysfunction contributes to disuse muscle atrophy originated over 40 years ago. These early studies were largely descriptive and did not provide unequivocal evidence that mitochondria play a primary role in disuse muscle atrophy. However, recent experiments have provided direct evidence connecting mitochondrial dysfunction to muscle atrophy. Numerous studies have described changes in mitochondria shape, number, and function in skeletal muscles exposed to prolonged periods of inactivity. Furthermore, recent evidence indicates that increased mitochondrial ROS production plays a key signaling role in both immobilization-induced limb muscle atrophy and diaphragmatic atrophy occurring during prolonged mechanical ventilation. Moreover, new evidence reveals that, during denervation-induced muscle atrophy, increased mitochondrial fragmentation due to fission is a required signaling event that activates the AMPK-FoxO3 signaling axis, which induces the expression of atrophy genes, protein breakdown, and ultimately muscle atrophy. Collectively, these findings highlight the importance of future research to better understand the mitochondrial signaling mechanisms that contribute to disuse muscle atrophy and to develop novel therapeutic interventions for prevention of inactivity-induced skeletal muscle atrophy.
Keywords: cell signaling, redox balance, oxidative stress, muscle wasting
prolonged skeletal muscle inactivity due to limb immobilization, bed rest, or denervation results in a loss of muscle protein and fiber atrophy. Although it is clear that inactivity-induced muscle atrophy occurs due to an imbalance in muscle protein synthesis and breakdown, complete details of the signaling events that regulate these processes remain obscure. In reference to important regulators of muscle protein breakdown, it is well established that disuse muscle atrophy is associated with mitochondrial dysfunction and increased mitochondrial production of reactive oxygen species (ROS). Nonetheless, the role that mitochondrial damage and/or increased mitochondrial ROS production play as signaling events to promote muscle atrophy has only recently received experimental attention.
The objective of this review is to provide a summary of our current understanding about the role that mitochondrial signaling events play in disuse muscle atrophy that occurs in response to both denervation and reduced contractile activity (e.g., limb immobilization). We begin with an overview of experimental models to investigate disuse muscle atrophy, which will be followed by an overview of the protein synthesis/turnover events that lead to loss of muscle protein. We will then examine the evidence that disuse muscle atrophy is regulated by mitochondrial signaling events and conclude with a discussion of unanswered questions in this field.
MODELS TO INVESTIGATE DISUSE MUSCLE ATROPHY
It is well established that long durations of bed rest, limb immobilization, denervation, or reduced activity in respiratory muscles during mechanical ventilation results in skeletal muscle atrophy in animals and humans. Because of the invasive nature of obtaining skeletal muscle samples from humans, animal models of disuse muscle atrophy are often used to explore the mechanisms responsible for inactivity-induced muscle wasting. Each of these laboratory animal models is designed to closely mimic a specific type of disuse muscle atrophy that occurs in humans. For instance, a tail suspension method to unload hindlimb muscles of rodents is commonly used to simulate the muscle atrophy that occurs during lengthy bed rest or space flight. Furthermore, a laboratory animal model of limb immobilization is commonly utilized to study the impact of muscle inactivity caused by the casting of a limb. Moreover, both denervation and spinal cord isolation are experimental manipulations that are often used to investigate the impact of neuromuscular diseases or nerve injury on skeletal muscle size and function.
A distinctive form of human skeletal muscle atrophy that has received limited experimental attention is the atrophy of respiratory muscles that occurs when patients are exposed to prolonged periods of mechanical ventilation. Mechanical ventilation is used clinically to provide respiratory support during surgery and in critically ill patients. During controlled mechanical ventilation, the ventilator delivers all of the breaths and the patients' inspiratory muscles (e.g., diaphragm) are completely inactive. A large number of both human and animal studies reveal that only 12–18 h of controlled mechanical ventilation is required to promote 15–20% atrophy of diaphragm myofibers (6, 32, 34, 38, 61, 71). Therefore, ventilator-induced diaphragmatic atrophy is a unique type of disuse muscle wasting, because a comparable level of inactivity-induced fiber atrophy in locomotor skeletal muscles would require ∼96 h (67).
DISUSE MUSCLE ATROPHY OCCURS DUE TO INCREASED PROTEIN BREAKDOWN AND DECREASED PROTEIN SYNTHESIS
Skeletal muscle mass is regulated by the balance between the rates of protein synthesis and degradation. Experiments using animal models have concluded that inactivity-induced muscle atrophy occurs due to both decreases in muscle protein synthesis and elevated rates of protein breakdown (60, 67). For example, both hindlimb unloading and prolonged mechanical ventilation studies indicate that muscle protein synthesis rates decline within the first 6 h following the onset of muscle inactivity (60, 67). This disuse-induced decline in muscle protein synthesis reaches a new and depressed steady-state level within 18–48 h (67). Animal studies also establish that prolonged periods of muscle disuse results in increased rates of muscle proteolysis (9, 61). Hence, in animals, the loss of skeletal muscle protein that occurs during inactivity is due to a decline in protein synthesis and increased protein breakdown with accelerated proteolysis playing the dominant role (24, 58, 59). In regard to proteolytic systems contributing to muscle atrophy, it is established that the calpain, caspase-3, ubiquitin-proteasome, and autophagy systems are all involved in protein breakdown during disuse muscle atrophy (24, 52, 53, 59). Although disuse muscle atrophy results in the loss of both myofibrillar and sarcoplasmic proteins, a preferential loss of myofibrillar proteins is a hallmark of inactivity-induced muscle wasting (43).
In contrast to the consensus view that disuse muscle atrophy in animals occurs due to both increased proteolysis and reduced protein synthesis, a contemporary review concludes that the primary factor driving disuse-induced locomotor muscle atrophy in humans is depressed protein synthesis (48). The basis for this conclusion comes from human disuse muscle studies that consistently report a decrease in muscle protein synthesis, whereas the impact of muscle inactivity on proteolysis in human muscle is less consistent (16, 18, 66). Clearly, additional well-designed experiments are needed to determine if prolonged inactivity results in a significant increase in muscle proteolysis in humans.
Although the impact of prolonged inactivity on protein breakdown in locomotor muscle is controversial, human studies involving mechanical ventilation consistently report that respiratory muscle inactivity is associated with the activation of numerous proteolytic systems, including the calpain, caspase-3, ubiquitin-proteasome, and autophagy pathways of protein breakdown (21, 23, 31, 32). Similarly, mechanical ventilation studies using animal models have consistently concluded that inactivity in inspiratory muscles (i.e., diaphragm) results in a rapid and large increase in proteolysis (25, 26, 34, 37, 50, 61, 71). Collectively, these studies demonstrate that diaphragmatic inactivity due to prolonged mechanical ventilation results in a significant increase in proteolysis in this important inspiratory muscle in both humans and animals.
DISUSE MUSCLE ATROPHY IS ASSOCIATED WITH CHANGES IN MITOCHONDRIAL STRUCTURE AND FUNCTION
Numerous studies have described changes in mitochondria shape, number, and function in skeletal muscles exposed to prolonged periods of inactivity. Collectively, these reports indicate that disuse muscle atrophy is associated with a loss of mitochondria, changes in mitochondrial morphology, increased mitochondrial reactive oxygen species (ROS) production, and impaired mitochondrial function. However, whether these alterations in muscle mitochondria structure and function are simply a consequence of fiber atrophy or whether these changes produce signals that contribute to muscle atrophy remains a relatively new area of investigation. In the next segments we will provide an overview of skeletal muscle mitochondrial structure/function and discuss the evidence linking skeletal muscle inactivity to mitochondrial damage and dysfunction.
Mitochondrial Function and Subpopulations in Skeletal Muscle
Although the major function of mitochondria is the aerobic production of ATP in cells, these organelles are also involved in many other important cellular functions. For example, in muscle fibers, mitochondria play an important role in the regulation of myonuclear apoptosis and also serve as a sink for calcium (40). Moreover, mitochondria continuously produce superoxide radicals that are dismutated into hydrogen peroxide (H2O2) (51). H2O2 is a relatively stable ROS that can exit the mitochondria and diffuse freely within the cytosol (28). This release of H2O2 from the mitochondria to the cytosol has been postulated to play an important role as a signaling molecule in the cell, affecting multiple networks that control the cell cycle, cellular stress response, energy metabolism, and the expression of numerous redox-sensitive genes (15). The role that mitochondrial ROS emission plays in disuse muscle atrophy will be discussed in detail later in this report.
Skeletal muscle is unique in that it contains two distinct subpopulations of mitochondria, with one group positioned among the myofibrils [intermyofibrillar mitochondria (IMF)], whereas the second population of mitochondria is located directly beneath the sarcolemma [subsarcolemmal mitochondria (SS)] (65). In regard to the relative abundance of these subpopulations, IMF mitochondria account for 80% of the total mitochondria in muscle fibers, with SS mitochondria accounting for the remaining 20% (20). These two mitochondrial subpopulations differ in several aspects, including a divergent protein content and function. For example, compared with SS, IMF mitochondria express a higher level of proteins associated with oxidative phosphorylation (17). This is reflected by the higher activities in complexes I, II, III, IV, and V in the electron transport chain in IMF mitochondria compared with SS (17). It follows that, compared with SS, IMF mitochondrial exhibit higher levels of state 3 oxidative phosphorylation along with higher respiratory control ratios, which is indicative of superior mitochondrial coupling (55). Collectively, these observations suggest that IMF mitochondria are highly specialized toward energy production for contractile activity and the associated calcium handling, whereas the SS mitochondria are thought to provide energy for membrane-related events (57, 69).
Numerous other important differences also exist between SS and IMF mitochondria. For example, during prolonged muscle inactivity, SS mitochondria are preferentially lost from skeletal muscle (30). It also appears that SS mitochondria produce a higher level of ROS than IMF mitochondria in muscle undergoing atrophy due to denervation (3). In contrast, compared with SS, IMF mitochondria are more susceptible to apoptotic stimuli, and this high level of apoptotic susceptibility is increased in IMF mitochondria following prolonged periods of muscle inactivity (2, 3).
In summary, two distinct subpopulations of mitochondria exist in skeletal muscle fibers that differ in both protein content and function. The more prevalent subpopulation (i.e., IMF) is highly specialized toward energy production to support muscle force production, whereas the SS mitochondria provide energy for membrane related events. Prolonged muscle inactivity is associated with numerous changes in mitochondria morphology and function and will be introduced in the next segment.
Disuse Muscle Atrophy Is Accompanied by Alterations in Mitochondrial Morphology and Function
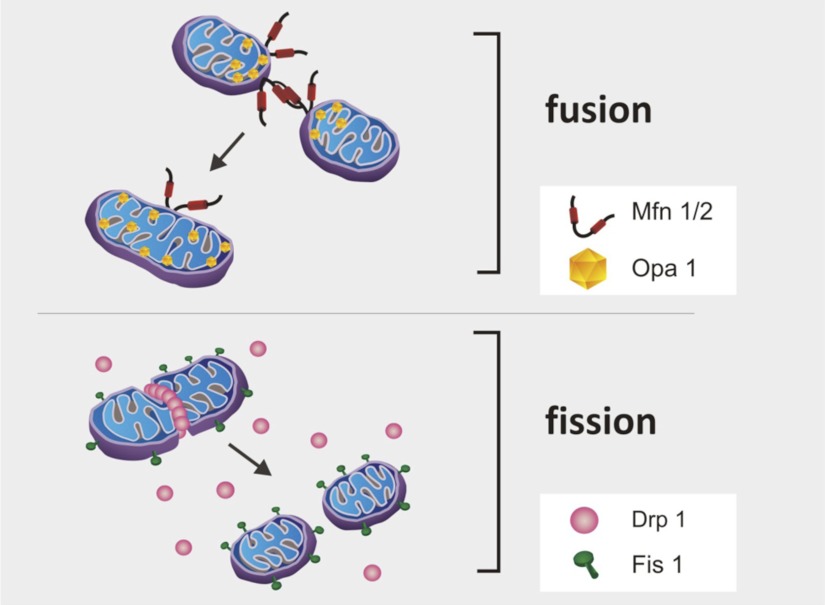
Although isolated mitochondria appear as spherical shaped individual organelles, in vivo, mitochondria form a complex interconnected network. The shape of this mitochondrial network is dynamic, because mitochondria frequently divide (i.e., mitochondrial fission) and fuse (i.e., mitochondrial fusion) (14). Indeed, the dynamic equilibrium between mitochondrial fission and fusion determines mitochondrial shape and size along with the degree of connectivity of the mitochondrial network. Mitochondrial fission and fusion events are controlled by a family of mitochondrial shaping proteins (14, 73). Key proteins that promote mitochondrial fission include Dynamin-related protein-1 (Drp1) and Fission 1 (Fis1), whereas proteins regulating mitochondrial fusion include optic atrophy 1 (Opa1) and mitofusion 1 and 2 (Mfn1/2) (14, 73) (Fig. 1). Recent evidence reveals that increased mitochondrial fission is linked to increased apoptosis in some cell types and increased atrophy in skeletal muscle fibers (56, 73). For example, inhibiting Drp1 activity can delay caspase activation and apoptotic cell death in cells exposed to apoptotic stimuli (73). Furthermore, inhibition of Fis1 can also protect against apoptosis, whereas overexpression of Fis1 promotes apoptotic cell death (73). Similarly, mice lacking Mfn1/2 function in skeletal muscle exhibit both mitochondrial dysfunction and profound muscle atrophy (12). Indeed, mitochondrial fusion is required for mitochondrial DNA stability in skeletal muscle and protection against mitochondrial DNA mutations (12). Finally, the loss of Opa1 in cells results in disorganization of the mitochondrial inner membrane, release of cytochrome c, and apoptosis (46).
Fig. 1.
Mitochondrial fission and fusion determine mitochondrial shape and size along with the degree of connectivity of the mitochondrial network. Optic atrophy 1 (Opa1) and mitofusion 1 and 2 (Mfn1/2) are involved in mitochondrial fusion, whereas dynamin-related protein-1 (Drp1) and fission 1 (Fis1) are involved in fission.
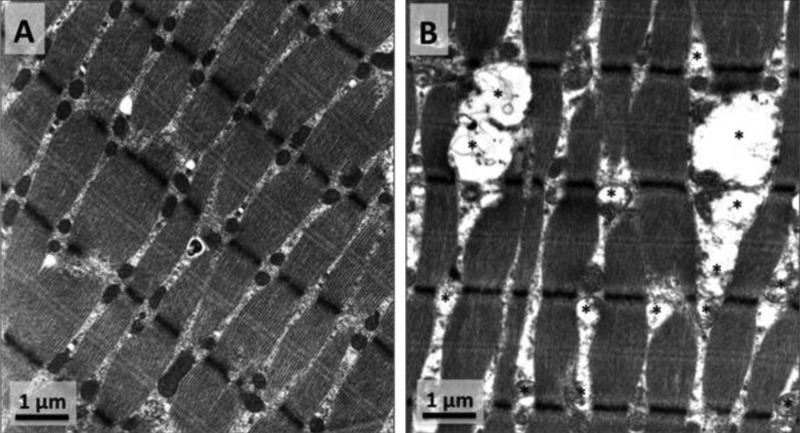
The first studies demonstrating that muscle inactivity is associated with significant changes in mitochondrial morphology and biochemical properties appeared in the literature in the 1960s. These initial studies used a variety of experimental animals (pigeon, frog, and rat), and all reports concluded that denervation-induced muscle atrophy was accompanied by reductions in mitochondrial number and changes in mitochondrial morphology (e.g., mitochondrial swelling, irregular shapes) (5, 11, 44, 45). An early study also reported a time-dependent impairment in mitochondrial coupling that began within 24 h following the beginning of muscle inactivity (36). Since these initial findings, many studies have confirmed that prolonged skeletal muscle inactivity results in altered mitochondrial morphology, decreased expression of mitochondrial proteins, mitochondrial respiratory dysfunction, and increased mitochondrial ROS production (Fig. 2) (1, 3, 26, 42, 50, 63). Moreover, denervation-induced muscle atrophy is also associated with deleterious changes in the mitochondrial protein import system along with increased mitochondrial susceptibility to apoptosis (3, 63). Collectively, these studies indicate that prolonged skeletal muscle inactivity is associated with significant changes in mitochondrial structure and bioenergetic function.
Fig. 2.
A: normal morphology of intermyofibrillar (IMF) mitochondria in rat soleus muscle. B: rat soleus muscle atrophy induced by hindlimb suspension is accompanied by changes in IMF mitochondrial morphology [e.g., mitochondrial swelling (*) and irregular shapes].
The cellular mechanism(s) responsible for the inactivity-induced dysfunction of skeletal muscle mitochondria remains an active area of investigation. In this regard, emerging evidence suggests that increased mitochondrial ROS production is a major contributor to the mitochondrial damage and dysfunction that is associated with prolonged skeletal muscle inactivity. Indeed, recent studies have demonstrated that prevention of inactivity-induced increases in mitochondrial ROS production protected skeletal muscle mitochondria against the impairment in mitochondrial respiratory function that is associated with prolonged inactivity (41, 50). Hence, it appears likely that inactivity-induced mitochondrial ROS production contributes to the mitochondrial damage associated with disuse muscle atrophy.
Why Does Muscle Inactivity Increase Mitochondrial ROS Production?
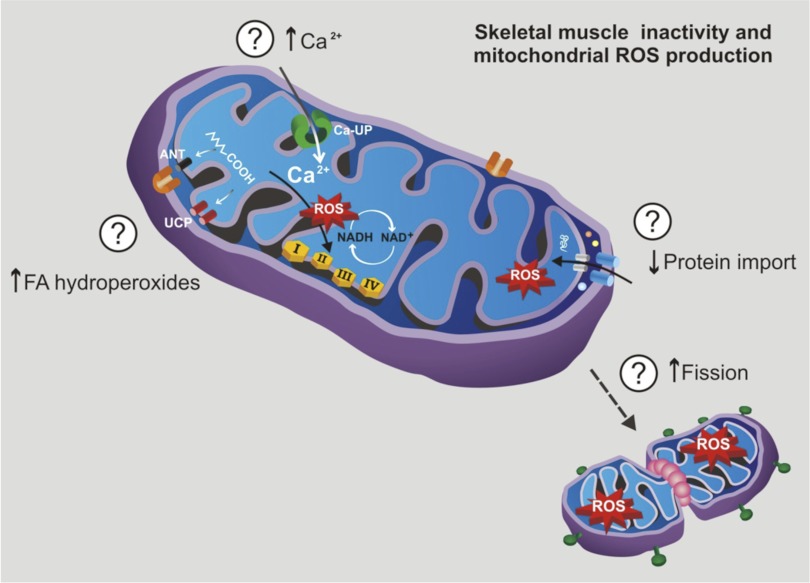
A key question remains, why does prolonged skeletal muscle inactivity result in increased mitochondrial ROS production? A definitive answer to this question is not currently available, but several possibilities exist (Fig. 3). First, evidence indicates that prolonged muscle inactivity is associated with disturbed intracellular calcium handling and increases in cyctosolic levels of calcium within the inactive muscle fibers (22, 68, 70). This increase in cytosolic levels of free calcium would likely lead to increases in mitochondrial calcium uptake via the mitochondrial calcium uniporter, which transports calcium into the matrix (28). Excessive mitochondrial calcium can promote mitochondrial ROS production by several mechanisms including activation of the citric acid cycle resulting in increased NADH formation, stimulation of nitric oxide synthase, and activation of ROS-generating enzymes (e.g., α-ketoglutarate dehydrogenase) (10, 28).
Fig. 3.
Specific mechanism(s) responsible for inactivity-induced increases in mitochondrial production of reactive oxygen species (ROS) remain unknown. However, in theory, prolonged skeletal muscle inactivity could increase mitochondrial ROS production in 4 ways: 1) mitochondrial uptake of calcium, 2) increased mitochondrial levels of fatty acid hydroperoxides, 3) depressed protein transport into the mitochondria, and/or 4) increased mitochondrial fission.
A second mechanism that can promote inactivity-induced mitochondrial ROS production is augmented mitochondrial levels of fatty acid hydroperoxides (8). Specifically, a recent report reveals that inactivity-induced increases in fatty acid hydroperoxides are important modulators of muscle mitochondrial function that increase mitochondrial matrix-directed superoxide production in skeletal muscle mitochondria in denervated muscles (7). It has been proposed that fatty acids exert these effects in three possible ways: 1) acting as protonophoric uncouplers, 2) interfering with components of the electron transport chain, and/or 3) inhibiting adenine nucleotide translocase (7).
Another mechanism that could increase mitochondrial ROS production during disuse muscle atrophy is an impairment of protein import into mitochondria resulting in mitochondria dysfunction and increased ROS production. The majority of mitochondrial proteins are nuclear encoded, and mitochondrial biogenesis requires the protein import machinery to transport these cytosolic proteins into the mitochondria. Recent experiments have carefully described a denervation-induced decline in the skeletal muscle mitochondrial protein transport system resulting in impaired organelle function (63). Those authors speculated that this deleterious change in the mitochondrial protein import system contributed to increased mitochondrial ROS production in skeletal muscle during chronic inactivity (63).
A final potential cause of the increased mitochondrial ROS production during disuse muscle atrophy is an increase in mitochondrial fission (74–76). For example, recent experiments reveal that fission-mediated fragmentation of mitochondria is causally linked with enhanced production of ROS in the mitochondria of ventricular myocytes (76). Indeed, prevention of fission or the promotion of fusion can prevent hyperglycemia-induced increases in mitochondrial ROS production in myocytes (75). This fission-mediated increase in mitochondrial ROS production is also associated with altered mitochondrial morphology, most notably swelling or the accumulation of small mitochondria in the myocytes (75). Nonetheless, definitive evidence that links increased fission to elevated mitochondrial ROS production during disuse muscle atrophy is not currently available.
In summary, there are at least four potential mechanisms that could contribute to increased mitochondrial ROS production in skeletal muscle during prolonged disuse (Fig. 3). However, which mechanism is most responsible for the increase in mitochondrial ROS production in skeletal muscle during prolonged periods of inactivity remains unknown. Regardless of the mechanism(s) responsible, the observation that mitochondrial ROS production increases in skeletal muscle fibers undergoing disuse atrophy has led several investigators to predict that inactivity-induced changes in mitochondrial function produces signals leading to disuse muscle atrophy. This important topic will be discussed in detail in the remaining segments of this report.
MITOCHONDRIAL SIGNALING AND DISUSE MUSCLE ATROPHY:THEORY
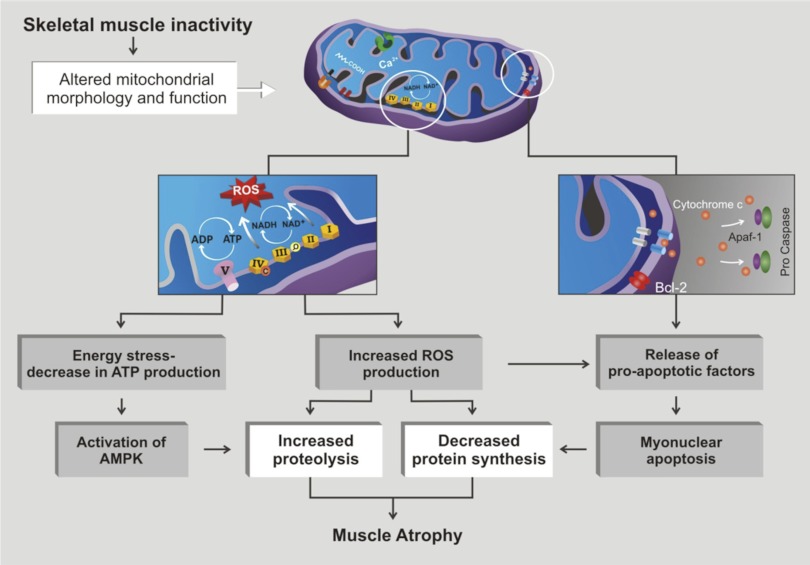
Numerous investigators have postulated that mitochondria play an important role triggering catabolic signals that contribute to disuse muscle atrophy (2, 35, 54, 56, 57, 63). In theory, mitochondrial signaling can contribute to disuse muscle atrophy in three major ways (Fig. 4): 1) increased mitochondrial ROS production, 2) mitochondrial release of proapoptotic factors, and/or 3) mitochondrial damage resulting in energy stress. A brief discussion of each of these factors follows.
Fig. 4.
Mitochondrial signaling can contribute to disuse muscle atrophy in 3 major ways: 1) mitochondrial damage resulting in energy stress, 2) increased mitochondrial ROS production, and/or 3) mitochondrial release of pro-apoptotic factors. (cf. Fig. 1 of Ref. 57).
Increased mitochondrial ROS production.
Several studies have demonstrated that prolonged periods of inactivity promotes increases in mitochondria ROS production in the inactive fibers (3, 26, 41, 42). This increased mitochondrial ROS production can promote disuse muscle atrophy by increasing proteolysis and depressing protein synthesis. Indeed, increased mitochondrial ROS production has been shown to activate both calpain and caspase-3, and oxidative stress can also increase the expression of key E3 ligases, leading to increased muscle protein degradation by the ubiquitin-proteasome system (33, 39, 50, 54, 71). Furthermore, oxidative modification of muscle proteins can expose previously shielded peptide bonds to enzymatic hydrolysis (13). This is significant because cellular proteases (i.e., calpain, caspase-3, ubiquitin-proteasome system) can degrade oxidized proteins more rapidly than normal proteins (13, 64).
Accumulating evidence suggests that oxidative stress can diminish protein synthesis in a variety of cell types (4, 47, 62). For example, exposure of cardiac myocytes to H2O2 can depress protein synthesis by ∼49% (47). This oxidative stress-induced depression of protein synthesis is predicted to occur at the level of mRNA translation, due, in part, to a decrease in Akt/mTOR signaling (54). Nonetheless, all of the aforementioned investigations were conducted in cell culture, and it is currently unknown whether ROS act as a regulatory signal to downregulate protein synthesis in skeletal muscle in vivo.
Mitochondrial release of proapoptotic factors.
Numerous factors, including increased mitochondrial ROS production, can promote permeabilization of the mitochondrial outer membrane resulting in the release of proapoptotic factors such apoptosis-inducing factor (AIF) and cytochrome c (2, 25). The release of cytochrome c from the mitochondria can subsequently activate caspase-3, leading to muscle protein breakdown and myonuclear apoptosis. In theory, the loss of myonuclei could reduce the ability of the muscle fibers to synthesize proteins (54). Specifically, a reduction in the number of nuclei in a skeletal muscle fiber would reduce its transcriptional capacity and therefore reduce the overall protein synthetic capabilities of the fiber. Consistent with this concept, recent experiments using caspase-3 knockout animals suggest that the absence of caspase-3 protects against denervation-induced muscle atrophy by suppressing apoptosis and protecting against the loss of myonuclei (49).
Mitochondrial damage and energy stress.
Numerous studies have demonstrated that prolonged muscle inactivity results in mitochondrial damage and an impaired ability to produce ATP that could result in cellular energy stress (e.g., low ATP levels) (26, 36, 41, 50, 56). In theory, low ATP availability could contribute to disuse muscle atrophy by both decreased protein synthesis and increased proteolysis. In theory, inactivity-related mitochondrial damage could decrease the level of cellular energy available for protein synthesis (36). Nonetheless, whether or not low energy levels are a major contributor to the depressed protein synthesis that occurs in muscle during disuse atrophy remains unknown.
It is also possible that inactivity-induced mitochondrial energy stress can promote increased proteolysis via the AMP kinase (AMPK)-FoxO3 axis (56, 57). Specifically, AMPK is a cellular sensor of energy levels, and active AMPK modulates FoxO3 action independently of Akt (19, 56). In particular, increased cellular levels of active AMPK results in activation of the transcriptional activating factor FoxO3, resulting in increased expression of atrogin-1, MuRF-1, LC3, and Bnip3 (19, 56). This is significant because atrogin-1 and MuRF-1 are important muscle-specific E3 ligases that play a major role in the degradation of muscle contractile proteins via the ubiquitin-proteasome system. Similarly, LC3 and Bnip3 are key proteins involved in autophagy. Therefore, AMPK activation of FoxO3 can accelerate muscle protein breakdown via both the ubiquitin-proteasome system and autophagy.
In summary, there are at least three mitochondrial signaling pathways that could contribute to disuse muscle atrophy. Furthermore, it appears likely that these signaling pathways are not necessarily independent. Indeed, it is feasible that increased mitochondrial ROS production could contribute to both the mitochondrial release of proapoptotic factors and mitochondrial damage resulting in energy stress (29). Direct experimental evidence connecting mitochondrial signaling to muscle atrophy is presented in the next segment.
EXPERIMENTAL EVIDENCE LINKING MITOCHONDRIAL SIGNALING TO DISUSE MUSCLE ATROPHY
The view that mitochondria contribute to disuse muscle atrophy was first proposed in the 1960s (11, 27). Since those initial reports, numerous studies have confirmed that muscle inactivity is associated with mitochondrial changes that, in theory, could contribute to disuse muscle atrophy (3, 8, 26, 42, 63, 72). Nonetheless, most published reports have been descriptive and do not provide direct evidence that mitochondrial signaling contributes to muscle fiber atrophy. Romanello et al. (56) provided the first data that establish a causal link between the mitochondrial network and denervation-induced muscle atrophy. These investigators demonstrated that the muscle mitochondrial network is altered during atrophy and that inhibition of two key fission proteins (i.e., Drp1 and Fis1) resulted in significant protection against muscle wasting. The authors concluded that denervation atrophy-related mitochondrial fragmentation results in energy stress and AMPK-induced activation of FoxO3. The activation of the AMPK-FoxO3 axis induces expression of atrophy-related genes, protein breakdown, and muscle loss. Mechanistically, this AMPK-FoxO3-mediated expression of atrophy-related genes occurs because active AMPK increases FoxO3 recruitment to target promoters, resulting in enhanced transcriptional activity (19). Romanello et al. also predicted that increased mitochondrial fission during denervation muscle atrophy would result in damaged mitochondria and promote the release of proapoptotic factors (56). These investigators also concluded that fission-mediated damage to mitochondria would increase mitochondrial ROS production, resulting in the activation of FoxO3 and NF-κB, which could sustain muscle protein breakdown during prolonged periods of disuse (56).
In regard to mitochondrial ROS production and disuse muscle atrophy, two recent studies reveal that increases in mitochondrial ROS production plays an important role in inactivity-induced skeletal muscle atrophy (41, 50). Both of these studies used a mitochondria-targeted antioxidant to prevent inactivity-induced increases in mitochondrial ROS production in muscles and concluded that increased mitochondrial ROS emission is a required signal to promote protease activation and atrophy in both respiratory muscles (i.e., diaphragm) and locomotor muscles in rodents. Indeed, prevention of increased mitochondrial ROS production in the diaphragm during mechanical ventilation protected diaphragmatic mitochondria from damage and respiratory dysfunction (50). Moreover, avoidance of ventilator-induced mitochondrial ROS production was successful in preventing the expression of both atrogin-1 and MuRF-1 and also prevented activation of calpain and caspase-3 in the diaphragm (50). Importantly, the prevention of increased mitochondrial ROS production successfully prevented ventilator-induced diaphragmatic atrophy. Similar results were obtained in the hindlimb muscles of animals exposed to 14 days of immobilization (41).
While the release of ROS (e.g., H2O2) from the mitochondria could be an independent signal to promote muscle atrophy, it is also feasible that increased mitochondrial ROS production can promote mitochondrial damage resulting in energy stress-induced activation of AMPK and the release of proapoptotic factors (29). Hence, it is possible that increased mitochondrial ROS production is a required upstream signal to promote both signaling via the mitochondrial damage/energy stress pathway and signaling in the form of proapoptotic factors.
Collectively, these three independent experiments demonstrate that mitochondrial signaling plays an important role in disuse skeletal muscle atrophy (41, 50, 56). During ventilator-induced diaphragm inactivity- and immobilization-induced limb muscle disuse, increased mitochondrial ROS production appears to be a key signal to activate proteolytic pathways and promote fiber atrophy. Because denervation-induced muscle atrophy is associated with increased mitochondrial ROS production (3, 42, 63), it is tempting to speculate that increased mitochondrial ROS production could also play an important signaling role in denervation-induced muscle atrophy. Nonetheless, additional experiments are required to verify this prediction.
CONCLUSIONS AND UNANSWERED QUESTIONS
The proposal that mitochondrial dysfunction is a contributory factor to disuse muscle atrophy was first presented over 40 years ago. However, direct evidence linking mitochondrial signaling to muscle atrophy was lacking until recent years. On the basis of the latest studies, it appears that mitochondrial signaling is an important contributory factor to disuse muscle atrophy induced by denervation, limb immobilization, and diaphragmatic inactivity induced by prolonged mechanical ventilation. Indeed, it has been shown that increased mitochondrial ROS production plays a key signaling role in both limb immobilization-induced locomotor muscle atrophy and diaphragmatic inactivity occurring during mechanical ventilation. The causal link between ROS and these forms of disuse muscle atrophy appears to be that increased mitochondrial ROS emission promotes increased muscle protein breakdown. During denervation-induced muscle atrophy, increased mitochondrial fragmentation due to fission appears to be a required signaling event that activates the AMPK-FoxO3 signaling axis, which induces the expression of atrophy genes, protein breakdown, and, ultimately, muscle atrophy.
Although recent studies provide direct evidence to link mitochondrial signaling to disuse muscle atrophy, several unanswered questions remain. First, it is unknown whether SS and IMF mitochondria are equal contributors to the mitochondrial signaling that promotes disuse muscle atrophy. Second, it is unclear whether increased mitochondrial ROS production is the initial signal that damages mitochondria and promotes mitochondrial signaling or whether mitochondrial damage occurs via a separate event. Moreover, the specific mechanism(s) responsible for the increase in mitochondrial ROS production remains unknown. Finally, it is unclear whether increased mitochondrial ROS production is a contributory factor to mitochondrial fission in skeletal muscle during disuse muscle atrophy. Studies that address these important questions could lead to the identification of new biological targets for drug development and the prevention of disuse muscle atrophy.
GRANTS
This work was supported from a grant from the National Institutes of Health (National Heart, Lung, and Blood Institute Grant R01 HL-087839) awarded to S. K. Powers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.K.P., M.P.W., J.D., A.M.Z., and H.A.D. prepared figures; S.K.P., M.P.W., J.D., A.M.Z., and H.A.D. drafted manuscript; S.K.P., M.P.W., J.D., A.M.Z., and H.A.D. edited and revised manuscript; S.K.P., M.P.W., J.D., A.M.Z., and H.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Pinar Arpinar Avsar for preparation of the graphics included in this report.
REFERENCES
- 1. Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM, Tarnopolsky M. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 4: e6518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289: C994– C1001, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102: 1143– 1151, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Alirezaei M, Marin P, Nairn AC, Glowinski J, Premont J. Inhibition of protein synthesis in cortical neurons during exposure to hydrogen peroxide. J Neurochem 76: 1080– 1088, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Aloisi M, Azzone GF, Carafoli E. [Lesions of pigeon muscle mitochondria in avitaminosis B1 and denervation atrophy]. Arch De Vecchi Anat Patol 32: 33– 83, 1960 [PubMed] [Google Scholar]
- 6. Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med 170: 1179– 1184, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya A, Lustgarten M, Shi Y, Liu Y, Jang YC, Pulliam D, Jernigan AL, Van Remmen H. Increased mitochondrial matrix-directed superoxide production by fatty acid hydroperoxides in skeletal muscle mitochondria. Free Radic Biol Med 50: 592– 601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhattacharya A, Muller FL, Liu Y, Sabia M, Liang H, Song W, Jang YC, Ran Q, Van Remmen H. Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria. J Biol Chem 284: 46– 55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704– 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817– C833, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Carafoli E, Margreth A, Buffa P. Early biochemical changes in mitochondria from denervated muscle and their relation to the onset of atrophy. Exp Mol Pathol 34: 171– 181, 1964 [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280– 289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies KJ. Protein damage and degradation by oxygen radicals. I general aspects J Biol Chem 262: 9895– 9901, 1987 [PubMed] [Google Scholar]
- 14. Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 21: 233– 241, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47– 95, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627– E633, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA, Amado F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10: 3142– 3154, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 35: 125– 133, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282: 30107– 30119, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7: 187– 204, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 182: 1377– 1386, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol 87: 386– 390, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183: 364– 371, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834– C843, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928– H935, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 46: 842– 850, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohn RR. Denervation muscle atrophy: an autolytic system in vitro. Am J Pathol 47: 315– 323, 1965 [PMC free article] [PubMed] [Google Scholar]
- 28. Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333– 343, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26: 463– 471, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol 48: 23– 28, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Levine S, Biswas C, Dierov J, Barsotti R, Shrager JB, Nguyen T, Sonnad S, Kucharchzuk JC, Kaiser LR, Singhal S, Budak MT. Increased proteolysis, myosin depletion and atrophic AKT-FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med 183: 483– 490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327– 1335, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806– C812, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 175: 1134– 1138, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 1800: 235– 244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Max SR. Disuse atrophy of skeletal muscle: loss of functional activity of mitochondria. Biochem Biophys Res Commun 46: 1394– 1398, 1972 [DOI] [PubMed] [Google Scholar]
- 37. McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med 175: 150– 159, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 37: 1373– 1379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol 294: R1608– R1617, 2008 [DOI] [PubMed] [Google Scholar]
- 40. McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391– 425, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect the skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol 111: 1459– 1466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293: R1159– R1168, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Munoz KA, Satarug S, Tischler ME. Time course of the response of myofibrillar and sarcoplasmic protein metabolism to unweighting of the soleus muscle. Metabolism 42: 1006– 1012, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Muscatello U, Margreth A, Aloisi M. On the differential response of sarcoplasm and myoplasm to denervation in frog muscle. J Cell Biol 27: 1– 24, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muscatello U, Patriarca PL. Denervation and disuse atrophy in pigeon breast muscle. An electron microscopic and biochemical study. Am J Pathol 52: 1169– 1189, 1968 [PMC free article] [PubMed] [Google Scholar]
- 46. Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278: 7743– 7746, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Pham FH, Sugden PH, Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res 86: 1252– 1258, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol 107: 645– 654, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Plant PJ, Bain JR, Correa JE, Woo M, Batt J. Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J Appl Physiol 107: 224– 234, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749– 1759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243– 1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337– R344, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol 102: 2389– 2397, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15: 2519– 2528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H868– H872, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774– 1785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep 12: 433– 439, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Sandri M. Autophagy in skeletal muscle. FEBS Lett 584: 1411– 1416, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23: 160– 170, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 170: 994– 999, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369– 1374, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, Ashe MP, Grant CM. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem 281: 29011– 29021, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Singh K, Hood DA. Effect of denervation-induced muscle disuse on mitochondrial protein import. Am J Physiol Cell Physiol 300: C138– C145, 2011 [DOI] [PubMed] [Google Scholar]
- 64. Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med 49: 1152– 1160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takahashi M, Hood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions. J Biol Chem 271: 27285– 27291, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 105: 902– 906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thomason DB, Biggs RB, Booth FW. Protein metabolism and β-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol 257: R300– R305, 1989 [DOI] [PubMed] [Google Scholar]
- 68. Tischler ME, Rosenberg S, Satarug S, Henriksen EJ, Kirby CR, Tome M, Chase P. Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 39: 756– 763, 1990 [DOI] [PubMed] [Google Scholar]
- 69. Walsh B, Hooks RB, Hornyak JE, Koch LG, Britton SL, Hogan MC. Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol 100: 1765– 1769, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Weiss N, Andrianjafiniony T, Dupre-Aucouturier S, Pouvreau S, Desplanches D, Jacquemond V. Altered myoplasmic Ca(2+) handling in rat fast-twitch skeletal muscle fibres during disuse atrophy. Pflügers Arch 459: 631– 644, 2010 [DOI] [PubMed] [Google Scholar]
- 71. Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol 108: 1376– 1382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu X, Chen CN, Arriaga EA, Thompson LV. Asymmetric superoxide release inside and outside the mitochondria in skeletal muscle under conditions of aging and disuse. J Appl Physiol 109: 1133– 1139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6: 657– 663, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 14: 425– 437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653– 2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341– 351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]