Abstract
While anal sphincter neurogenic injury documented by needle electromyography (EMG) has been implicated to cause fecal incontinence (FI), most studies have been uncontrolled. Normal values and the effects of age on anal sphincter motor unit potentials (MUP) are ill defined. The functional significance of anal sphincter neurogenic injury in FI is unclear. Anal pressures and EMG were assessed in 20 asymptomatic nulliparous women (age, 38 ± 5 yr; mean ± SE) and 20 women with FI (54 ± 3 yr). A computerized program quantified MUP duration and phases. These parameters and MUP recruitment were also semiquantitatively assessed by experienced electromyographers in real time. Increasing age was associated with longer and more polyphasic MUP in nulliparous women by quantitative analysis. A higher proportion of FI patients had prolonged (1 control, 7 patients, P = 0.04) and polyphasic MUP (2 controls, 9 patients, P = 0.03) at rest but not during squeeze. Semiquantitative analyses identified neurogenic or muscle injury in the anal sphincter (11 patients) and other lumbosacral muscles (4 patients). There was substantial agreement between quantitative and semiquantitative analyses (κ statistic 0.63 ± 95% CI: 0.32–0.96). Anal resting and squeeze pressures were lower (P ≤ 0.01) in FI than controls. Anal sphincter neurogenic or muscle injury assessed by needle EMG was associated (P = 0.01) with weaker squeeze pressures (83 ± 10 mmHg vs. 154 ± 30 mmHg) and explained 19% (P = 0.01) of the variation in squeeze pressure. Anal sphincter MUP are longer and more polyphasic in older than younger nulliparous women. Women with FI have more severe neurogenic or muscle anal sphincter injury, which is associated with lower squeeze pressures.
Keywords: aging, electromyography, pudendal neuropathy
initially documented by histological evidence of sphincter denervation (27), the concept that pudendal nerve injury contributes to anal weakness in fecal incontinence (FI) gained traction when prolonged pudendal nerve terminal motor latencies were documented in FI and after obstetric anal sphincter injury (2, 22, 30). However, prolonged pudendal nerve latencies are flawed markers of pudendal nerve function (13). Hence, needle electromyography (EMG) is the only technique that can reliably identify anal sphincter neurogenic injury that can result from damage to the sacral spinal cord, cauda equina, S2–4 spinal nerves, the pudendal nerve, or its branches as they enter the anal sphincter (28, 29, 34). Anal sphincter EMG can also identify local muscle trauma (3). However, anal sphincter EMG is not widely available and infrequently used, mainly when neurogenic injury is suspected, in clinical practice. Also, based on limited data that suggest that anal sphincter denervation predicts poorer outcome after anal sphincteroplasty (10, 21), anal sphincter EMG should also be considered before surgery.
Previous studies with anal sphincter needle EMG evaluated neurological disorders, especially conus medullaris and cauda equina lesions (28, 29) after childbirth and in FI. With two exceptions, studies in FI were uncontrolled (1, 31). Together, these controlled studies enrolled 31 patients with FI and 46 controls. However, the obstetric history was unspecified, which is a limitation since even uncomplicated vaginal delivery can cause neurogenic injury to the anal sphincter and pelvic floor (18, 32). Moreover, most controls were younger than 70 yr of age. While anal resting and squeeze pressures decline considerably with age (16) the effects of age on anal sphincter innervation are poorly understood.
Most published studies have analyzed motor unit potential (MUP) characteristics by quantitative approaches (e.g., single MUP online or offline, automated quantitation, interference pattern analysis, and single fiber EMG analysis) (11, 15, 28, 29). There are few reports using semiquantitative approaches, which are generally used in clinical practice (29, 33). With semiquantitation, two to six simultaneously active MUP are recorded and displayed on a digital screen and over a loudspeaker. A machine trigger delay line isolates and superimposes recurring single (i.e., the same) MUP with an amplitude window for online review while the needle is moved to different locations in the muscle. Experienced electromyographers are able to isolate and accurately interpret MUP characteristics within a few minutes. However, semiquantitative and quantitative approaches have not been compared in the anal sphincter. Moreover, the accuracy of semiquantitative analysis for identifying anal sphincter neurogenic injury in FI is unclear.
Hence, the aims of this study were 1) to evaluate the effects of age on anal sphincter MUP characteristics in asymptomatic nulliparous women, 2) to compare anal sphincter MUP characteristics in women with and without FI, 3) to compare semiquantitative and quantitative approaches to analyzing anal sphincter EMG, and 4) to assess whether anal sphincter neurogenic injury explains anal weakness in FI.
MATERIALS AND METHODS
Experimental design.
This study was conducted in 20 healthy asymptomatic nulliparous women (age, 38 ± 5 yr; mean ± SE) (controls) and 20 women (age, 54 ± 3 yr) with FI (patients). Study assessments (anal manometry and needle EMG) were performed for research and clinical practice in controls and patients, respectively. Seventeen patients also had anal imaging with endoanal ultrasound (8 patients) or MRI (9 patients). This study was approved by the Institutional Review Board at Mayo Clinic, Rochester, MN. Some findings from asymptomatic subjects. but not patients. were published previously (12).
Participants.
All patients had FI for 6 mo or longer and were referred for anal sphincter needle EMG as part of their clinical assessment. Healthy subjects were nulliparous and did not have functional gastrointestinal disorders (as assessed by questionnaires) (4) or current or prior history of anorectal disorders or procedures. Neither healthy subjects nor patients had known neurological disorders (e.g., spinal cord injury, dementia, multiple sclerosis, Parkinson's disease, diabetes with peripheral neuropathy); history of rectal cancer, scleroderma, or inflammatory bowel disease; or prior pelvic radiation or rectal surgery (except for hemorrhoidal procedures).
Based on questionnaire responses, the severity of FI was computed using the validated FI and Constipation Assessment (FICA) incontinence symptom severity score, which incorporates the type, frequency, and amount of FI and the presence of urgency (6–8). While most other scales for rating FI severity include the type and frequency of leakage and some also incorporate urgency, only the FICA symptom severity scale includes the amount of FI. Without the latter, the severity of FI would be similar for two subjects who were incontinent for a small stain or a large liquid bowel movement once a week. A severity score (maximum score = 13) was calculated by summing scores for the individual components. Symptom severity scores of 1–6, 7–10, and 11–13 were categorized as mild, moderate, and severe, respectively.
Needle EMG of the external anal sphincter.
Needle EMG was performed with standard concentric, 25-mm, 30-gauge, disposable, needle electrodes (Carefusion Teca Elite US53153) by two experienced electromyographers (J. Dauble and W. Litchy) who were aware of subject status (i.e., control or FI) but not aware of anal pressures or imaging findings. The electrodes were inserted into the superficial anal sphincter at the anal verge at a 15° angle to the skin surface. Short rise time MUP confirmed that the electrode tip was in muscle tissue. The electrode length and angle precluded reaching structures other than the external anal sphincter. Recordings were made from at least four sites, i.e., one site in each quadrant of the superficial anal sphincter (1:30, 4:30, 7:30, and 10:30 on the simulated clock with noon anterior). The electrode was repositioned by short, straight line movements to a location with MUP rise times of < 0.8 ms. If changes in needle movement direction were necessary, they were made only after withdrawing the needle to subcutaneous tissue. At one site in each quadrant, MUP were recorded for 1 min at rest followed by two 15-s recordings during strong, voluntary contraction. Visual and auditory displays were used to monitor the strength and duration of MUP activation. Patients were encouraged to listen to the auditory signal to ensure optimal relaxation and maximum contraction during maneuvers. Needle position was adjusted as necessary during contraction to maintain a short rise time of recorded MUPs. All recordings were stored electronically on a Viking Select EMG machine (Viasys, Nicolet).
Quantitative measurements were made of each isolated MUP recorded in the electronic buffer. Thirty to fifty MUP from each anal sphincter (four quadrants) were measured after the recording was completed (11) using the decomposition, quantitative EMG program on a Viking Select EMG machine (Viasys, Nicolet). MUP recruitment (number of MUP firing compared with firing frequency), duration, and phases were measured but amplitude was not since it is influenced by even subtle variations in needle position and does not independently predict pathogenicity (11). Normal values for MUP characteristics depend on the method of analysis (29). Hence, normal values for MUP duration were defined by 10th and 90th percentile values at each site separately at rest and during squeeze in controls. Long-duration MUP occur in diseases in which there is increased fiber density, an increased number of fibers, or a loss of synchronous firing of fibers in a motor unit, typically due to collateral sprouting and reinnervation (11). Short-duration MUP are often seen in muscle diseases in which muscle fibers are lost due to necrosis or degeneration. Consistent with other skeletal muscles, MUP with more than four phases were defined as polyphasic, which reflects asynchronous firing of muscle fibers within the MUP. Polyphasic MUP are observed in neuropathic and myopathic conditions. The proportion of abnormally polyphasic and prolonged MUP at each site was calculated at rest and during squeeze; the overall assessment was characterized as polyphasic or prolonged when > 25% of the motor units were polyphasic or prolonged, respectively, at two or more (of four) sites at rest or separately during squeeze.
In contrast to the quantitative analysis, the semiquantitative analysis entailed assessment of 1–4 MUP in each quadrant in real time (11). The electromyographer (J. Dauble or W. Litchy) graded on a scale (0 to 4+) assessment of recruitment, duration, phases, turns, and stability. Reduced recruitment was identified when the first MUP was firing at >10 Hz when the second MUP started firing. Since normal values for anal sphincter MUP duration and phase were not available prior to studies in patients, the on-line semiquantitative analysis considered MUP with ≤ 4 phases and between 4 and 11 ms in duration as normal.
Based on an integrated assessment of these parameters, findings were characterized as normal, neurogenic, muscle injury, or nonspecific. In order of specificity, the features of 1) neurogenic damage are reduced recruitment, long duration MUP, fibrillation potentials, and polyphasic potentials and 2) muscle injury are short-duration MUP, normal or rapid recruitment, polyphasic MUP and fibrillation potentials. Polyphasic MUP or fibrillation potentials do not, by themselves, identify a specific type of damage. Neurogenic MUP are more likely to be of long duration and unstable, while myopathic MUP are more likely to be of short duration and stable. Fibrillation potentials are more common immediately after nerve damage or muscle trauma and will subside with time after the immediate cause. With severe damage, they can persist after either myopathic or neuropathic damage.
Anorectal manometry.
Average anal resting and squeeze pressures were measured using the station pull-through technique with water perfused manometry and summarized by the highest pressure in the anal canal (5).
Anal imaging.
Anal imaging was performed with ultrasound or MRI using previously described techniques (3). Ultrasound imaging was performed in the left lateral decubitus position with a rotating rectal probe (B&K Medical, Gentofte, Denmark), which provides a 360-degree view with a 7 MHz or 10 MHz transducer. Endoanal MRI was performed with a disposable endorectal colon coil (MRInnervu, Medrad, Indianola, PA).
Statistical analysis.
The main EMG response variables were the number, duration, and proportion of polyphasic MUP. For squeeze maneuvers, data were averaged across both maneuvers at every site before analysis. Univariate associations of age with MUP characteristics were assessed by Spearman's correlation coefficient. The univariate association of subject status (healthy subjects vs. patients) with abnormal (i.e., excess) polyphasic and prolonged MUP, defined as > 25% polyphasic or prolonged MUP, respectively, at two or more sites was assessed by Fisher's exact test.
ANCOVA models were used to assess the association between disease status (i.e., control or FI) and anal pressures and separately between anal sphincter EMG findings and anal pressures in patients. Multiple linear regression models assessed whether anal sphincter MUP characteristics could explain intersubject variation in anal resting and squeeze pressures. All models included age and body mass index. Data are reported as means ± SE. The unweighted κ statistic assessed agreement between quantitative and semiquantitative analyses, which were categorized as normal or abnormal. This represents the proportion of agreement beyond that expected by chance alone; it is scaled to vary from −1 to 1 (24). The within-subject reproducibility of MUP duration and phases was evaluated by Lin's concordance statistic (25).
RESULTS
Clinical features.
Patients (54 ± 3 yr) were older (P = 0.04) than controls. The body mass index was also higher (P = 0.006) in patients (30.7 ± 1.6 kg/m2) than controls (25.1 ± 1.0 kg/m2) (Table 1). Of the 20 patients, 18 had FI for 1 yr or longer, while two reported symptoms for between 6–12 mo. FI symptoms were urge predominant (n = 6), passive (n = 6), or combined (i.e., passive and urge) FI (n = 8). Patients reported FI approximately once a month (n = 5), more often but not daily (n = 8 patients), or daily (n = 7). Patients were incontinent for liquid (n = 10) or solid stool (n = 3 patients) or both (n = 7). The typical amount of leakage was small (i.e., staining only, n = 5), moderate (i.e., more than staining but less than a full bowel movement, n = 12), or large (i.e., a full bowel movement, n = 3). Thus, the FICA incontinence symptom severity score suggested mild (n = 1), moderate (n = 14), or severe FI (n = 5).
Table 1.
Demographic characteristics, anal manometry, and anal sphincter electromyography (EMG) findings by quantitative analysis in patients and controls
| Comparison Between Controls and Patients |
||||
|---|---|---|---|---|
| Demographic Features | Controls | Patients | P Value Before Adjusting for Age and BMIa | P Value After Adjusting for Age and BMIb |
| Age, yr | 37.9 ± 4.9 | 54.0 ± 3.0* | NA | NA |
| BMI, kg/m2 | 25.1 ± 1.0 | 30.7 ± 1.6* | NA | NA |
| Number of vaginal deliveries | 0 | 2.4 ± 1.3 | ||
| Anal manometry | ||||
| Anal resting pressure | 92 ± 7 | 53 ± 6c | <0.001 | 0.02 |
| Anal squeeze pressure | 203 ± 12 | 108 ± 14c | <0.001 | 0.006 |
| Anal sphincter EMGd | ||||
| Rest, no. of motor units | 6.8 ± 0.6 | 5.1 ± 0.4 | 0.07 | 0.03 |
| Rest, motor unit duration, ms | 5.5 ± 0.4 | 6.6 ± 0.4 | <0.05 | 0.71 |
| Rest, polyphasic motor units, % | 14 ± 2 | 22 ± 2 | 0.07 | 0.52 |
| Squeeze, no. of motor units | 4.8 ± 0.3 | 4.2 ± 0.3 | 0.28 | 0.41 |
| Squeeze, motor unit duration, ms | 4.6 ± 0.4 | 6.5 ± 0.5 | 0.003 | 0.11 |
| Squeeze, polyphasic motor units, % | 9 ± 2 | 16 ± 3 | 0.09 | 0.44 |
Rank sum test for association of subject status with specific feature.
Multiple variable regression model to assess association of subject status with specific feature adjusting for age and body mass index (BMI).
P < 0.05 vs. controls.
Values are averaged across all sites and also across multiple maneuvers during squeeze, in each patient.
Quantitative analysis.
Seventeen of 20 patients had vaginal deliveries with forceps assistance (n = 5), perineal sutures (n = 16), and Grade III–IV injury (n = 9). Two of three remaining patients had cesarean sections. Nine patients reported one or more anorectal procedures, including anal sphincteroplasty (n = 5), rectocele repair (n = 2), rectal prolapse surgery (n = 2), and a hemorrhoidectomy (2 patients). Eight patients had a hysterectomy and two had a cholecystectomy. The type (passive vs. other types of FI) and severity of FI were not associated with EMG findings.
Anorectal manometry and imaging.
Anal resting and squeeze pressures were normal in 19 controls. Anal resting pressure was reduced in one control and 13 patients (Table 1). Anal squeeze pressure was reduced in nine patients. After adjusting for age and body mass index, anal resting pressure (P = 0.004) and the squeeze increment (P = 0.01) were reduced in FI compared with controls; age was a significant (P = 0.03) covariate for anal resting but not squeeze pressure.
Anorectal imaging revealed normal anal sphincters (4 patients), external sphincter abnormalities only (5 patients), or internal and external anal sphincter abnormalities (8 patients). External sphincter abnormalities were a focal scar or tear (7 patients), atrophy (5 patients), or a tear and atrophy in one patient. Four patients had a tear, scar, or moderate focal thinning and four patients had diffuse thinning of the internal anal sphincter. The three women who did not have anal imaging had none, one, and three vaginal deliveries. Anal imaging revealed external sphincter injury in all patients with muscle injury by EMG.
MUP number by quantitative assessment–univariate analysis.
MUP characteristics assessed by quantitative analysis averaged across all sites and by region (anal quadrant) are shown in Tables 1 and 2, respectively. FI patients had fewer MUP than controls at rest and during squeeze; differences were significant at rest after adjusting for age and body mass index (Table 1). At rest, there were fewer MUP in the anterior than the posterior quadrants and fewer MUP in the left than the right anterior quadrant (P = 0.003 by univariate and 0.0002 by multiple variable analysis) (Table 2). These regional differences were more pronounced in FI, as evidenced by a significant interaction term (site*status) in patients (P = 0.001) but not controls (P = 0.08). During squeeze, regional differences were not statistically significant.
Table 2.
Regional variation of anal sphincter motor unit characteristics by quantitative analysis in health and fecal incontinence (FI)
| Needle EMG Location | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left Posterior Quadrant |
Right Posterior Quadrant |
Left Anterior Quadrant |
Right Anterior Quadrant |
|||||
| Features | Controls | FI | Controls | FI | Controls | FI | Controls | FI |
| Rest | ||||||||
| No. of needle motor units | 7.70 ± 0.98 | 6.65 ± 0.74 | 7.40 ± 0.88 | 5.35 ± 0.58 | 5.65 ± 0.64 | 3.60 ± 0.44 | 6.35 ± 0.58 | 4.52 ± 0.51 |
| Duration of needle motor units | 5.92 ± 0.45 | 7.24 ± 1.05 | 5.67 ± 0.52 | 6.53 ± 0.51 | 5.26 ± 0.49 | 6.49 ± 0.50 | 5.15 ± 0.35 | 5.94 ± 0.49 |
| SD of duration for needle motor units | 2.88 ± 0.34 | 3.22 ± 0.47 | 2.05 ± 0.26 | 3.51 ± 0.45 | 2.40 ± 0.35 | 2.64 ± 0.39 | 1.99 ± 0.29 | 2.68 ± 0.38 |
| Polyphasic motor units, % | 11 ± 0.16 | 19 ± 5 | 13 ± 3 | 21 ± 5 | 17 ± 3 | 25 ± 6 | 15 ± 4 | 25 ± 7 |
| Squeeze | ||||||||
| Number of needle motor units | 4.72 ± 0.48 | 3.97 ± 0.47 | 4.97 ± 0.59 | 4.15 ± 0.42 | 4.52 ± 0.28 | 4.32 ± 0.49 | 4.82 ± 0.44 | 4.23 ± 0.42 |
| Duration of needle motor units | 4.50 ± 0.44 | 7.05 ± 0.81 | 4.74 ± 0.34 | 5.98 ± 0.49 | 4.62 ± 0.53 | 6.49 ± 0.58 | 4.73 ± 0.46 | 6.50 ± 0.53 |
| SD of duration for needle motor units | 1.78 ± 0.25 | 2.98 ± 0.43 | 2.05 ± 0.22 | 2.54 ± 0.40 | 1.52 ± 0.20 | 2.83 ± 0.36 | 1.93 ± 0.25 | 2.84 ± 0.40 |
| Polyphasic motor units, % | 8 ± 2 | 16 ± 5 | 12 ± 0 | 17 ± 5 | 6 ± 2 | 15 ± 4 | 12 ± 3 | 19 ± 5 |
MUP duration by quantitative assessment-univariate analysis.
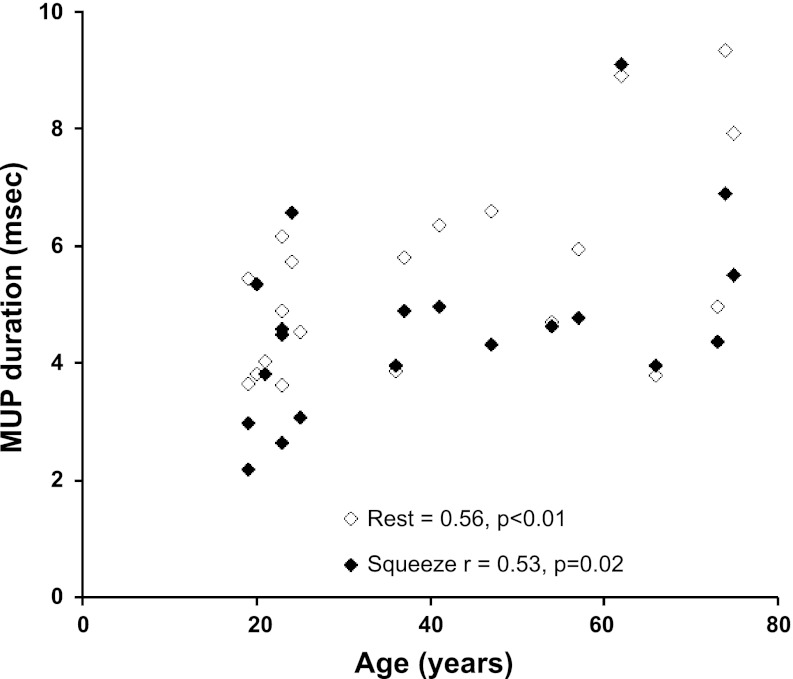
The duration of MUP in controls was 5.5 ± 0.4 ms (95% CI: 2.0, 11.6; 90% CI: 2.5, 9.3) at rest and 4.6 ± 0.4 ms (95% CI: 1.5, 10.5; 90% CI: 1.8, 7.9) during squeeze. The MUP duration was not significantly different across all sites (Table 2). MUP duration was associated with age at rest (R = 0.56, P < 0.01) and during squeeze (R = 0.53, P = 0.02) in healthy subjects but not in FI (Fig. 1).
Fig. 1.
Relationship between age and motor unit potentials (MUP) duration in healthy subjects. MUP duration at rest and during squeeze was longer in older asymptomatic women.
The duration of MUP at rest and during squeeze was longer (P < 0.05) in FI than in controls before but not after adjusting for age and body mass index (Table 1). Using site-specific normal values (i.e., 10–90th percentile values) in healthy subjects, only one of 20 (5%) asymptomatic controls but seven of 20 FI patients (35%, P = 0.04), of whom six were aged less than 60 yr, had ≥ 25% prolonged MUP at two or more sites at rest; corresponding differences during squeeze (5 controls, 10 patients) were not significant. The proportion of subjects with an excess number of short MUP at rest (2 controls, 3 patients) and during squeeze (3 controls, 3 patients) was not significantly associated with FI.
Polyphasic MUP by quantitative assessment-univariate analysis.
All subjects had one or more polyphasic MUP at rest as also during squeeze. In controls, MUP had 3.1 (95% CI: 2.0, 7.0; 90% CI: 2.0, 5.0) phases at rest and 2.8 (95% CI: 1.5, 9.5; 90% CI: 2.0, 4.0) phases during squeeze. Age was associated (r = 0.56, P = 0.01) with polyphasic MUP during squeeze in controls only.
The proportion of polyphasic MUP at rest and squeeze was not significantly different between controls and FI (Table 1). However, a higher (P = 0.03) proportion of patients (9 of 20 patients vs. 2 of 20 controls) had excess polyphasic MUP (i.e., ≥ 25% polyphasic MUP at 2 or more sites) at rest. Both controls and two of these nine patients were older than 70 yr. During squeeze, corresponding differences (1 control, 5 patients) were not different (P = 0.18, Fisher's exact test). Only one of 20 controls but six of 20 FI patients (P = 0.09) had polyphasic and prolonged MUP at rest or during squeeze.
Semiquantitative assessment of needle EMG and comparison with quantitative findings.
Recruitment of MUP was normal in all controls and reduced in six of 20 patients (P = 0.02). Semiquantitative analysis were summarized as normal (18 controls, 5 patients), nonspecific features that were not sufficiently severe to be characterized as neurogenic injury (1 control, 4 patients), neurogenic injury (1 control, 9 patients), or muscle injury (2 patients) (Table 4, Fig. 2). Hence, anal sphincter neurogenic or muscle injury were more common (p = 0.001) in FI than in controls. Imaging revealed external sphincter damage in both patients who had features of muscle injury by EMG.
Fig. 2.
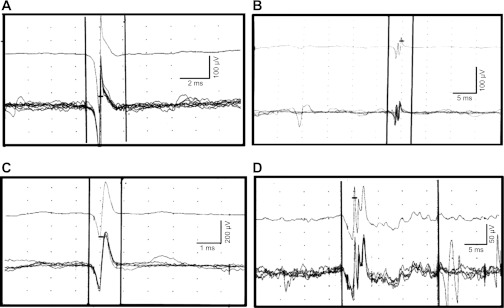
Normal (A and B) and abnormal (C and D) MUPs recorded by needle electromyography (EMG) of the anal sphincter. A and B: 5 superimposed similar MUPs (bottom) and the average of these MUP (top). These MUP have the normal number of phases (i.e., 2 , left; 4, right) and are of normal duration (i.e., 3.4 ms, left; and 4.8 ms, right). C: MUP of short duration (1.4 ms) with 2 phases suggestive of muscle injury. D: prolonged (19.7 ms) polyphasic MUP (10 phases) suggestive of neurogenic injury.
Since normal values for qualitative analysis were based on data in healthy subjects, semiquantitative and quantitative findings were compared in patients only; one patient who could only activate the anal sphincter by a Valsalva maneuver (i.e., not by squeeze) was not included in this comparison. In the remaining 19 patients, there was substantial agreement between semiquantitative and quantitative assessments characterized as normal or abnormal, as indicated by a κ statistic of 0.63 (95% CI: 0.32–0.96) (Table 3). Findings were concurrent in three of five patients who were normal, four who had nonspecific abnormalities, six of nine who had neurogenic injury, and both patients who had muscle injury by semiquantitative analysis. Two patients with neurogenic injury and excess prolonged MUP also had an excess of short MUP, which probably reflect ongoing reinnervation.
Table 3.
Comparison of semiquantitative and quantitative assessment of anal sphincter EMG in FI
| Quantitative Assessment |
||||
|---|---|---|---|---|
| Semiquantitative Assessment | No. | Normal | Excess Prolonged MUP at Rest or Squeezea | Excess Short MUP at Rest or Squeezea |
| Normal | 5 | 3b | 1 | 1 |
| Nonspecific | 4 | 4b | ||
| Neurogenic | 9 | 2 | 6b | 1 |
| Muscle injury | 2 | 2b | ||
Excess defined as ≥25% of motor unit potentials (MUP) at 2 or more sites were prolonged or short as applicable.
Values in these cells were considered as agreement.
Four of nine patients with neurogenic anal sphincter injury also had neurogenic changes in the lumbosacral paraspinal and proximal L5 and S1 muscles. MRI of the lumbosacral spine in these four patients revealed severe degenerative changes of the lumbosacral spine with spinal nerve compression in three and cauda equina involvement in one of four patients. Another patient had unilateral neurogenic changes affecting the anterior and posterior quadrants, which suggests pudendal nerve injury. The association between history of obstetric anal sphincter injury (i.e., 3rd or 4th degree tear or anal sphincteroplasty) and needle EMG findings by semiquantitative analysis was not significant.
Relationship between anal EMG, MRI, and pressures.
In healthy subjects, anal sphincter MUP characteristics during squeeze explained 39% (P = 0.04) of the variation in anal squeeze pressure and MUP duration explained 20% (P = 0.06) of this total variation. In contrast, anal sphincter MUP characteristics at rest did not explain the variation in resting pressure in healthy subjects.
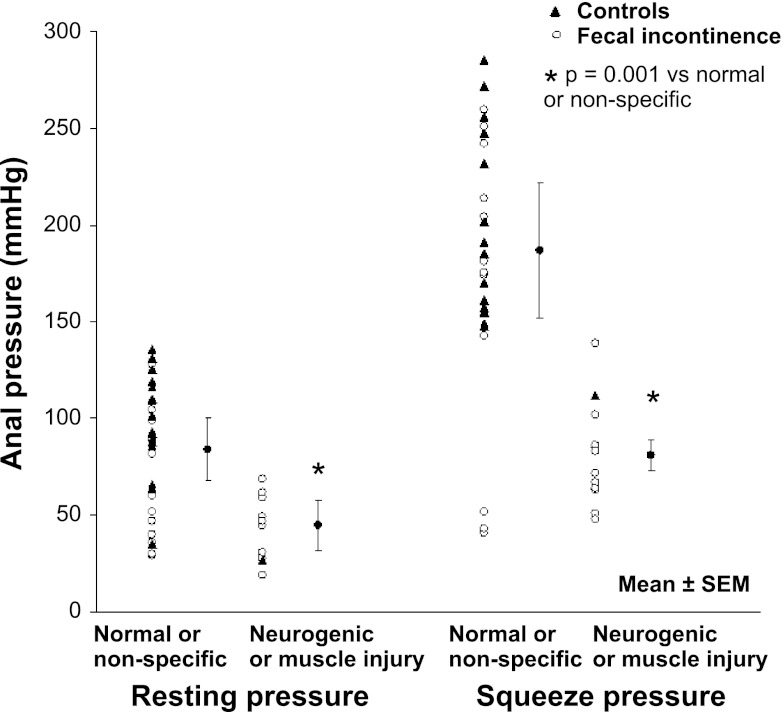
Anal pressures were univariately associated with injury status at rest (P < 0.001) and during squeeze (P < 0.001). In particular, anal resting pressure was lower (P < 0.001 by rank sum test) in subjects with neurogenic or myogenic injury (45 ± 13 mmHg) than with normal or nonspecific anal sphincter EMG findings (84 ± 16 mmHg). Anal squeeze pressures were also lower (P < 0.001) in subjects with neurogenic or myogenic injury (81 ± 8 mmHg) than with normal or nonspecific anal sphincter EMG findings (187 ± 35 mmHg) (Fig. 3). Neurogenic or myogenic injury at rest explained 8% (P = 0.10) of the variation in resting pressure while neurogenic or myogenic injury during squeeze explained 19% (P = 0.01) of the variation in squeeze pressure.
Fig. 3.
Anal resting and squeeze pressures were lower in women with neurogenic or muscle injury than with normal or nonspecific features by EMG. *P = 0.001 for anal resting and (separately) squeeze pressures for normal pattern or nonspecific injury vs. neurogenic or myogenic injury.
Finally, anal pressures were normal (resting pressure, 61 ± 12 mmHg; squeeze pressure, 144 ± 26 mmHg) in nine patients with normal or nonspecific changes by semiquantitative EMG; pressures were comparable in patients with normal (rest pressure, 47 ± 3 mmHg; squeeze pressure, 151 ± 43 mmHg) or nonspecific anal sphincter EMG (rest pressure, 65 ± 17 mmHg; squeeze pressure, 135 ± 32 mmHg) patterns.
DISCUSSION
There are three main observations in this study. First, quantitative analysis revealed that age was associated with longer and more polyphasic MUP even in asymptomatic nulliparous women. Second, semiquantitative analysis documented anal sphincter neurogenic and/or muscle injury more frequently in FI than in controls; neurogenic and/or muscle injury was associated with impaired anal pressures. Third, there was substantial agreement between semiquantitative and quantitative analysis of anal sphincter needle EMG. Together, these findings advance our understanding of neurogenic injury and its functional significance in health and FI.
While the external anal sphincter is not frequently evaluated in most clinical neurophysiology laboratories, the basic techniques of the needle examinations are similar to any other muscle. EMG evaluation of the anal sphincter muscles, as reported in this study, is performed in the Mayo clinical neurophysiology labs by most clinical electromyographers. The normal values for anal sphincter MUP duration observed in this study differ slightly from other muscles and may enable anal sphincter EMG to be performed more widely. The automated MUP measurement is a clinically accepted, commercially available program for isolating up to six distinct MUP from multiple, simultaneous MUP. While electromyographers were not blinded to subject status, the number of sites examined and the technique was identical in all subjects. Moreover, the large number of MUP examined from each site helps to minimize potential examiner bias.
Needle EMG data were interpreted in real time by semiquantitative and later, by quantitative analysis. While semiquantitative analysis was based on a priori estimated normal values, the quantitative analysis used observed normal values in asymptomatic subjects. The a priori normal range for phase duration (i.e., 4–11 ms) was different from observed normal values in 20 asymptomatic controls (i.e., rest, 2.5–9.3 ms; squeeze, 1.8–7.9 ms). For the number of phases, a priori and observed normal values were comparable. Nonetheless, the results of quantitative and semiquantitative analyses were in substantial agreement. When expertise is available, the advantages of the semiquantitative analysis are that it can be performed in real time, takes less time, provides auditory input, and also evaluates turns, stability, and recruitment, which can be particularly useful for distinguishing nonspecific features from neurogenic injury.
While MUP in patients with neurogenic and muscle injury share certain features (e.g., fibrillation potentials, polyphasic MUP), they can readily be distinguished because MUP are prolonged in neurogenic and short in myogenic injury. Moreover, recruitment is often reduced in neurogenic and normal or rapid in muscle injury. Findings indicative of neurogenic or muscle injury were more common in FI than in controls. Indeed, 18 of 20 controls were normal by semiquantitative analysis; one control each had nonspecific abnormalities and features of neurogenic injury. None of the four patients who had nonspecific changes by semiquantitative analysis had reduced recruitment, which is the most specific feature of neurogenic injury, and has been associated with lower anal squeeze pressures previously (14, 20, 26).
Because MUP duration was not significantly different among sphincter quadrants, examination of both anterior quadrants should suffice in most patients. However, when MUP in both anterior quadrants are abnormal or when there is concern that the posterior quadrants are affected (e.g., in patients with iatrogenic anal sphincter injury), the posterior quadrants should also be examined. The paraspinal muscles should also be examined by needle EMG in patients with anal sphincter neurogenic injury. When the paraspinal examination does not suggest involvement of the sacral spinal cord or roots, examination of other muscles innervated by the pudendal nerve (e.g., ischiocavernosus) may be useful to distinguish between a pudendal neuropathy and local (i.e., anal sphincteric) nerve injury (3).
Neurogenic or muscle injury were associated with lower anal squeeze pressures. However, three patients with normal or nonspecific features also had reduced squeeze pressure perhaps due to the descending perineum syndrome (1 patient) or external sphincter damage as documented by MRI (2 patients). Although the sample size was small, anal pressures were comparable in patients with normal and nonspecific abnormalities. The nonspecific category is useful for categorizing abnormalities of uncertain clinical significance, which is akin to the concept that since no single cut-off diagnostic criterion has satisfactory sensitivity and specificity, it is necessary to characterize findings into possible, probable, and definite neuropathic abnormalities (28).
The semiquantitative analysis revealed neurogenic or muscle injury in 11 of 20 FI patients (i.e., 55%), which is comparable to the prevalence (40–65%) reported in previous studies. (23, 26, 34) In the quantitative analysis, prolonged and polyphasic MUP at rest were also individually associated with FI, extending previous findings (1). Polyphasic potentials are correlated with increased fiber density (15), which also occurs in FI. In contrast, prolonged and polyphasic MUP during squeeze were not associated with FI probably because overlapping MUP limit an accurate assessment of MUP onset and end times during strong voluntary contractions. Auditory input is better than an automated program for identifying polyphasic MUP intermingled with many normal MUP.
Vaginal delivery and perhaps even cesarean section are associated, typically with mild, anal sphincter and pelvic floor neurogenic injury in asymptomatic subjects (19, 32). However, these findings demonstrate for the first time that age is associated with longer and more polyphasic MUP by quantitative analysis and that anal sphincter MUP characteristics explained 39% of the variation in anal squeeze pressures even in asymptomatic nulliparous women. They partly explain the apparent discrepancy between low anal squeeze pressures and anal sphincter injury documented by imaging in asymptomatic older women (17) and emphasize the need to consider neurogenic injury as an explanation for anal weakness, particularly prior to anal sphincteroplasty. Increasing age was also associated with longer MUP in distal lower extremity muscles (9). While healthy individuals loose, on average, ∼25% of motor neurons in the lumbo spinal segments (L1–S3) from the second to tenth decade of life, several subjects older than 60 yr of age have only 50% of those in early adult or middle age (35). As motor neurons die, muscle fibers are innervated by healthy motor neurons. Hence, each motor unit innervates a larger number of muscle fibers. MUP amplitude and duration are determined by the number of muscle fibers and their synchrony of firing. Hence, the duration of MUP is longer when there are more muscle fibers in each motor unit.
While these studies were conducted with robust techniques, the findings were derived from a relatively small group of Caucasian subjects. Patients were older, had a higher body mass index, and a higher prevalence of obstetric anal sphincter injury than controls. While a history of obstetric anal sphincter injury was not associated with anal sphincter neurogenic or myogenic injury in this study, larger studies in which the age distribution is carefully matched between cases and controls and including a control group of asymptomatic parous women are necessary to refine our understanding of the contribution of age to anal sphincter neurogenic injury in FI.
GRANTS
This work was supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-78924.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.E.B., J.D., W.L., J.T., P.E., and A.R.Z. conception and design of research; A.E.B., J.D., W.L., J.T., J.E., and A.R.Z. analyzed data; A.E.B., J.D., and W.L. interpreted results of experiments; A.E.B. and J.E. prepared figures; A.E.B. drafted manuscript; A.E.B., J.D., W.L., J.T., J.E., P.E., and A.R.Z. edited and revised manuscript; A.E.B., J.D., W.L., J.T., J.E., P.E., and A.R.Z. approved final version of manuscript; J.D., W.L., and J.T. performed experiments. All authors contributed to revising the article critically for important intellectual content and final approval. In addition A.E.B., J.D., W.L., J.T., and P.E. substantially contributed to conception and design; J.D., W.L., and J.T. to data acquisition, and J.E. and A.R.Z. also contributed to analysis.
ACKNOWLEDGMENTS
This work was presented at the 16th Neurogastroenterology and Motility Scientific Meeting, St. Louis, MO, September 16–18, 2011.
REFERENCES
- 1. Bartolo DC, Jarratt JA, Read NW. The use of conventional electromyography to assess external sphincter neuropathy in man. J Neurol Neurosurg Psychiatry 46: 1115–1118, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beersiek F, Parks AG, Swash M. Pathogenesis of ano-rectal incontinence. A histometric study of the anal sphincter musculature. J Neurolog Sci 42: 111–127, 1979 [DOI] [PubMed] [Google Scholar]
- 3. Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, Stevens C, Seide B, Riederer SJ, Zinsmeister AR. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut 54: 546–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A new questionnaire for constipation and fecal incontinence. Aliment Pharmacol Ther 20: 355–364, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bharucha AE, Seide B, Fox JC, Zinsmeister AR. Day-to-day reproducibility of anorectal sensorimotor assessments in healthy subjects. Neurogastroenterol Motil 16: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bharucha AE, Seide BM, Zinsmeister AR. The effects of clonidine on symptoms and anorectal sensorimotor function in women with faecal incontinence. Aliment Pharmacol Ther 32: 681–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol 4: 1004–1009, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bharucha AE, Zinsmeister AR, Locke GR, Seide B, McKeon K, Schleck CD, Melton LJI. Prevalence and burden of fecal incontinence: a population based study in women. Gastroenterology 129: 42–49, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Buchthal F, Rosenfalck P. Action potential parameters in different human muscles. Acta Psychiatrica et Neurologica Scandinavica 30: 125–131, 1955 [DOI] [PubMed] [Google Scholar]
- 10. Cheong DM, Vaccaro CA, Salanga VD, Wexner SD, Phillips RC, Hanson MR, Waxner SD. Electrodiagnostic evaluation of fecal incontinence. Muscle Nerve 18: 612–619, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Daube JR, Rubin DI. Needle electromyography. Muscle Nerve 39: 244–270, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Daube JR, Traue J, Litchy W, Mowzoon N, Bharucha AE. Anal sphincter motor unit potential (MUP) differences between sides, between rest and voluntary contraction, and between MUP analysis programs in normal subjects. Suppl Clin Neurophysiol 60: 287–292, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Diamant NE, Kamm MA, Wald A, Whitehead WE. American gastroenterological association medical position statement on anorectal testing techniques. Gastroenterology 116: 732–760, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Felt-Bersma RJ, Strijers RL, Janssen JJ, Visser SL, Meuwissen SG. The external anal sphincter. Relationship between anal manometry and anal electromyography and its clinical relevance. Dis Colon Rectum 32: 112–116, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Fowler AL, Mills A, Durdey P, Thomas MG. Single-fiber electromyography correlates more closely with incontinence scores than pudendal nerve terminal motor latency. Dis Colon Rectum 48: 2309–2312, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum 49: 1726–1735, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum 49: 1726–1735, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gregory WT, Lou JS, Simmons K, Clark AL. Quantitative anal sphincter electromyography in primiparous women with anal incontinence. Am J Obstet Gynecol 198: e551–e556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregory WT, Lou JS, Stuyvesant A, Clark AL. Quantitative electromyography of the anal sphincter after uncomplicated vaginal delivery. Obstet Gynecol 104: 327–335, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Infantino A, Melega E, Negrin P, Masin A, Carnio S, Lise M. Striated anal sphincter electromyography in idiopathic fecal incontinence. Dis Colon Rectum 38: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Jacobs PP, Scheuer M, Kuijpers JH, Vingerhoets MH. Obstetric fecal incontinence. Role of pelvic floor denervation and results of delayed sphincter repair. Dis Colon Rectum 33: 494–497, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Kiff ES, Swash M. Slowed conduction in the pudendal nerves in idiopathic (neurogenic) faecal incontinence. Br J Surg 71: 614–616, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Lacima G, Pera M, Valls-Sole J, Gonzalez-Argente X, Puig-Clota M, Espuna M. Electrophysiologic studies and clinical findings in females with combined fecal and urinary incontinence: a prospective study. Dis Colon Rectum 49: 353–359, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 33: 159–174, 1977 [PubMed] [Google Scholar]
- 25. Lin LIK. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45: 255–268, 1989 [PubMed] [Google Scholar]
- 26. Osterberg A, Graf W, Edebol Eeg-Olofsson K, Hynninen P, Pahlman L. Results of neurophysiologic evaluation in fecal incontinence. Dis Colon Rectum 43: 1256–1261, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Parks AG, Swash M, Urich H. Sphincter denervation in anorectal incontinence and rectal prolapse. Gut 18: 656–665, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Podnar S. Criteria for neuropathic abnormality in quantitative anal sphincter electromyography. Muscle Nerve 30: 596–601, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Podnar S, Vodusek DB, Stalberg E. Comparison of quantitative techniques in anal sphincter electromyography. Muscle Nerve 25: 83–92, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Snooks SJ, Henry MM, Swash M. Faecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. Br J Obstet Gynaecol 92: 824–828, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Sorensen M, Tetzschner T, Rasmussen OO, Christiansen J. Relation between electromyography and anal manometry of the external anal sphincter. Gut 32: 1031–1034, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. South MMT, Stinnett SS, Sanders DB, Weidner AC. Levator ani denervation and reinnervation 6 months after childbirth. Am J Obstet Gynecol 200: 519. e511–e517, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Thomas C, Etienney I, Atienza P. Evaluation of the role of the puborectal part of the levator ani muscle in anal incontinence: a prospective study of 78 female patients with anal incontinence. Dis Colon Rectum 54: 1129–1133, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Thomas C, Lefaucheur JP, Galula G, de Parades V, Bourguignon J, Atienza P. Respective value of pudendal nerve terminal motor latency and anal sphincter electromyography in neurogenic fecal incontinence. Neurophysiol Clin 32: 85–90, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurolog Sci 34: 213–219, 1977 [DOI] [PubMed] [Google Scholar]