Abstract
The ionic basis of nitrergic “slow'” inhibitory junction potential (sIJP) is not fully understood. The purpose of the present study was to determine the nature and the role of calmodulin-dependent protein kinase II (CaMKII)-dependent ion conductance in nitrergic neurotransmission at the intestinal smooth muscle neuromuscular junction. Studies were performed in guinea pig ileum. The modified Tomita bath technique was used to induce passive hyperpolarizing electrotonic potentials (ETP) and membrane potential change due to sIJP or drug treatment in the same cell. Changes in membrane potential and ETP were recorded in the same smooth muscle cell, using sharp microelectrode. Nitrergic IJP was elicited by electrical field stimulation in nonadrenergic, noncholinergic conditions and chemical block of purinergic IJP. Modification of ETP during hyperpolarization reflected active conductance change in the smooth muscle. Nitrergic IJP was associated with decreased membrane conductance. The CAMKII inhibitor KN93 but not KN92, the Cl− channel blocker niflumic acid (NFA), and the KATP-channel opener cromakalim hyperpolarized the membrane. However, KN93 and NFA were associated with decreased and cromakalim was associated with increased membrane conductance. After maximal NFA-induced hyperpolarization, hyperpolarization associated with KN93 or sIJP was not seen, suggesting a saturation block of the Cl− channel signaling. These studies suggest that inhibition of CaMKII-dependent Cl− conductance mediates nitrergic sIJP by causing maximal closure of the Cl− conductance.
Keywords: KN93, niflumic acid, cromakalim, Tomita bath, electrotonic potential, calmodulin-dependent protein kinase II
enteric motor nerves release two major inhibitory neurotransmitters: a purine and nitric oxide (NO). They produce two distinct inhibitory junction potentials (IJP), namely a prominent, large amplitude, purinergic, fast IJP (fIJP) and a subdued, low amplitude, nitrergic, slow IJP (sIJP) in the intestinal smooth muscles (4, 12, 25).
The signaling cascade of purinergic fIJP has been shown to involve the release of a purine and activation of P2Y1 receptor that stimulates Gq/11-phospholipase C to produce inositol 1,4,5-trisphosphate (IP3). IP3 causes localized Ca2+ release from IP3 receptor-operated (IP3R) stores. Localized Ca2+ release activates small conductance K+ (SK) channels (3, 35, 36).
The signaling cascade of nitrergic inhibitory junction potential-associated hyperpolarization is more complicated. Nitrergic sIJP involves stimulation of guanylate cyclase (GC) by NO˙. However, some NO donors, in addition to causing GC stimulation, may also cause direct nitrosylation and activation of SK channels (10). NO˙-GC stimulation leads to accumulation of cGMP and activation of PKG (10, 16). However, the ion channels involved in cGMP-PKG-mediated smooth muscle hyperpolarization has been controversial (18, 26, 43).
Potassium and chloride are two major conductances that exert opposing effects of smooth muscle membrane potential (39). Initial studies of the ionic basis of the prominent IJP in the guinea pig (now recognized as purinergic fIJP) were performed by investigating the behavior of the IJP during prolonged conditioning hyperpolarization and ion substitution, using the Tomita bath. These studies suggested that the IJP was mediated by opening of K+ channels (1, 34). However, it also became known that in the guinea-pig intestinal smooth muscle, in addition to the prominent purinergic fIJPs, there was also a low-amplitude, sIJP (4, 12, 25). Studies of IJPs during induced passive hyperpolarization in the smooth muscle cells in the guinea-pig ileum suggested that while the fIJP was associated with increased K+ conductance as originally proposed (34), the sIJP was associated with decreased Cl− conductance (6, 7) and a noncholinergic component of the excitatory junction potential was associated with increased Cl− conductance (7). In the opossum esophageal circular muscle, there is no purinergic IJP. The sIJP in the opossum was also reported to be due to closure of a chloride conductance (5). However, the view that the nitrergic sIJP could be due to closure of Cl− conductance was not readily accepted and some leaders in the field continued to believe that nitrergic IJP was also due an increase in K+ conductance [see Daniel et al. (8)]. The view that K+ channel opening was responsible for the nitrergic IJP was fueled by the fact that NO donors were found to stimulate K+ channels (19, 20). Over the last 30 yr, many reports regarding the involvement of K+ currents in nitrergic IJP have appeared (19, 20, 40). On the other hand, many reports supporting the role of closure of Cl− channels have also appeared (6, 10, 41, 43). However, there is still no general agreement among the investigators, whether opening of K+ or closing of a Cl− conductance underlies the nitrergic sIJP.
It is generally thought that the ion channels that are involved in IJP are activated by Ca2+ (26, 42). In our preliminary studies, we observed that calmodulin-dependent protein kinase II (CaMKII) inhibitor KN93, but not a related inactive analog, KN92, hyperpolarized the smooth muscle membrane and suppressed the nitrergic IJP. However, CaMKII may be involved in the regulation of either K+ or Cl− channels, leaving unanswered the question whether K+ or Cl− channel mediated the sIJP.
The purpose of these studies was to investigate 1) whether opening of K+ channels or closure of Cl− conductance was responsible for smooth muscle hyperpolarization associated with sIJP or CaMKII, and 2) the mechanism of block of sIJP by KN93.
MATERIALS AND METHODS
All experiments were approved by Institutional Animal Care and Use Committee at Veterans Affairs Boston HealthCare System and were performed according to the Institutional guidelines.
Animals.
Thirty-five (male or female) guinea pigs weighing between 250 and 400 g were anesthetized by means of CO2 narcosis and subsequently stunned and bled via the carotid artery.
Drugs and chemicals.
Drugs and chemicals used in this study were as follows: atropine, guanethidine, nifedipine, cromakalim (CK), apamin, substance P, niflumic acid (NFA), and Nω-nitro-l-arginine (l-NNA) and were obtained from Sigma Chemical (St. Louis, MO). KN93, KN92, calmidazolium, and W7 were obtained from Research Biochemicals (Natick, MA). Nifedipine was dissolved in 95% ethanol at 10 mM/l and stored in a light-protected container. l-NNA was dissolved in 0.01 N HCl and diluted in Kreb's solution before use. NFA, KN93, KN92, and calmidazolium were dissolved in 10% DMSO and shielded from light. The Krebs solution consisted of the following (in mM): 11.5 glucose, 21.9 bicarbonate, 1.2 phosphate, 138.5 sodium, 2.5 calcium, 1.2 magnesium, 4.6 K+, and 125 Cl−. The pH of the Krebs solution after 30 min of bubbling with 95% O2-5% CO2 ranged between 7.34 and 7.39.
Tomita bath.
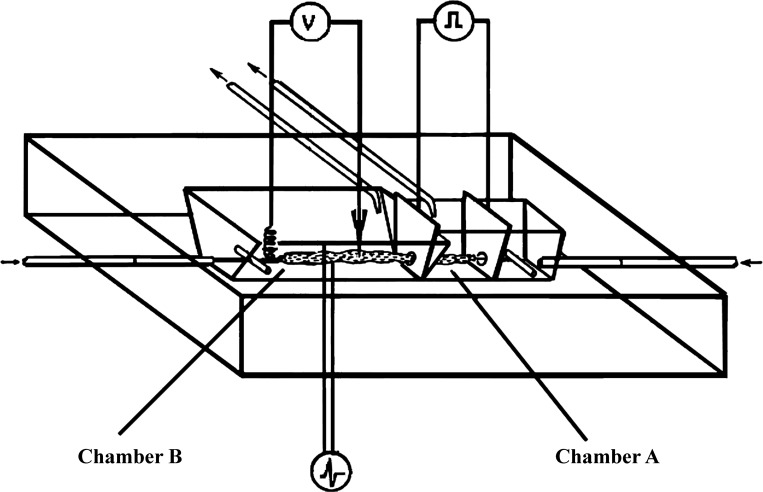
To determine whether the nerve-mediated junction potential was passively conducted from a neighboring cell or was actively generated in the same cell (generated potential), we used a modification of the technique described by Abe and Tomita (1) (Fig. 1). Other recording parameters being the same, an increase in conductance in the recording cell would be expected to be associated with a decrease in the amplitude of the electrotonic potential (ETP) and a decrease in conductance would be associated with increase in the amplitude of the ETP. This technique can similarly be used to investigate whether drug-induced hyperpolarization is conducted from a neighboring cell or generated in the same smooth muscle cell. In these studies, long-term intracellular recordings from the same smooth muscle cells were performed during the entire study to record changes in ETP with treatments in relation to the pretreatment control period.
Fig. 1.
Modified Tomita bath. Details of the bath have been described in detail elsewhere (1, 6,7, 34). Briefly, it consists of two chambers, called chamber A and chamber B, that are isolated from each other except a small hole through which a muscle strip passes from one chamber to the other. Chamber A is designed to apply a direct current that causes a passive change in the membrane potential [electrotonic potential (ETP)] of smooth muscles in the part of the muscle strip that in this chamber. ETP applied to the smooth muscles in chamber A is conducted to smooth muscles in the part of the muscle strip that in chamber B where it can be recorded. This technique was investigated to study cable properties of the smooth muscles (1). Chamber B was modified to add electrodes to provide transmural electrical stimulation and induce inhibitory junction potential that can be recorded from the same cell from which the ETP are recorded and effect of the inhibitory junction potential (IJP) on ETP investigated. Drugs and chemicals can also be perfused in chamber B to study the interaction of drug induced changes, IJP and ETP in the same smooth muscle cell.
Intracellular recording.
Intracellular recordings of membrane potential and ETP were obtained from the same smooth muscle cell using microelectrodes made from glass of 1.2-mm external diameter (Frederick Haer, Brunswick, ME) and filled with 1 M K+ methyl sulfate and 3 M KCl. The resistance of the microelectrodes was between 30 and 80 MΩ as described earlier (6, 7). All membrane potential values were determined by the difference between the stable potential recorded within the cell compared with the balanced zero potential upon withdrawal, as described earlier (6, 7).
Generation of ETP.
Direct current, hyperpolarizing potentials of 0.5 s in duration were generated by passing current between the two stimulating plates in this bath and were monitored by a constant-current monitor unit (Grass Instruments CCUI) positioned in series between the plates and the stimulator. The direct current potentials were conducted to other coupled smooth cells in the strip and produced passive ETP in the coupled cells. The ETP were recorded using an intracellular microelectrode positioned within 2 mm of the stimulating plate next to the chamber B. The effect of nitrergic IJP and various treatments on membrane potential and amplitude of the applied ETP was recorded. The details of handling of current leakage and other technical issues have been described elsewhere (6, 7).
Generation of the fIJP and sIJP.
Two Ag-AgCl electrodes (0.26-mm diameter) positioned above and below the intestinal preparation perpendicular to its longitudinal axis and 5 mm away from the recording microelectrode were used to deliver transmural nerve stimulation. These electrodes were insulated up to 2 mm from their tips and connected to a stimulator (Grass S-88) in series with a stimulus isolation unit (Grass SIU5) and a constant-current unit (Grass CCUl). Optimal stimulus parameters (70-V, 1-ms duration square pulses at 10 Hz for 0.5–5 s) were used (12).
Atropine (1 μM) and guanethidine (5 μM) were added to create nonadrenergic, noncholinergic conditions. Nifedipine (0.1 μM) was added to the perfusing solution to suppress L-type calcium channels and reduce contraction of the muscle. To further reduce the substance P-mediated muscle excitability, substance P tachyphylaxis was used to as described earlier (6). Apamin (0.3 μM) was added to the perfusing solution to block the fIJP or l-NNA (100 μM) to mask the sIJP (6, 7).
Effect of CaM and CaMKII inhibitors on fIJP and sIJP.
Intracellular recordings of pharmacologically isolated fIJP and sIJP junction potentials were also performed without the use of Tomita bath as described earlier (4, 12). The effects of the CaM inhibitors calmidazolium and W7 as well as the CaMKII inhibitor KN93 were examined on the fIJP and the sIJP.
Application of chemicals.
The chemicals KN93, KN92, NFA, and CK were dissolved in DMSO and diluted in Kreb's solution to achieved desired concentration and perfused in the bath at a rate of 3 ml/min. Smooth muscle cell in which impalement was maintained for the entire duration of study lasting ∼45 min were included for analysis.
Statistics.
Statistical comparisons were made using standard Student's paired and unpaired t-statistics and covariance analysis. All data are expressed as means ± SD. One-way ANOVA with P < 0.05 was accepted as statistically significant.
RESULTS
ETP during sIJP.
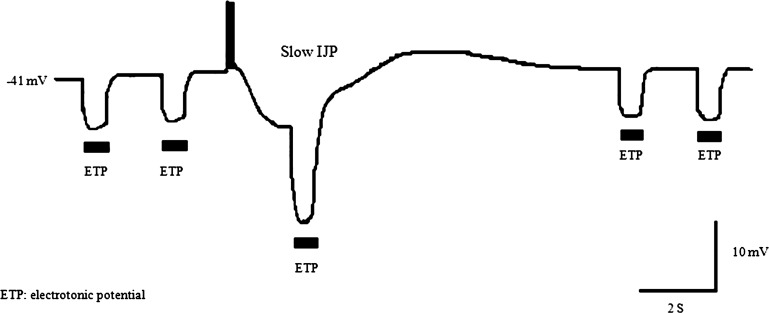
With the use of the Tomita bath setup, passive hyperpolarizing ETPs of ∼7-mV and ∼ 0.5-s duration were applied every 1–4 s, before and after, and once during the sIJP. An example of ETP during sIJP in a smooth muscle is shown in Fig. 2. Note that in this example, amplitude of the ETP was 6.6 mV during the control period and increased to 13.5 mV during the IJP-associated hyperpolarization and returned to baseline level of 6.7 mV. The ETP increased by 104% during the nitrergic IJP compared with period of baseline. In three studies in three separate animals, the means ± SD value of membrane potential was −41 ± 0.6 mV, after apamin to suppress the fIJP and before the sIJP and −50 ± 0.6 during the sIJP. Spontaneous small amplitude membrane potential fluctuations described earlier were not consistently recorded due to technical reasons (41). The mean ± SD amplitude of the sIJP was −8.3 ± 0.6 mV, and the amplitude of the ETP increased from 7 ± 0.3 to 14.3 ± 0.6 mV during the sIJP (P < 0.0001). Since increased ETP indicates reduced membrane conductance, these observations suggest that the nitrergic sIJP is associated with decrease in membrane conductance or closure of ion channels.
Fig. 2.
Effect of hyperpolarization associated with slow (s)IJP on amplitudes of hyperpolarizing ETPs in circular muscle of guinea pig ileum. The sIJP of ∼8 mV, which was followed by a small depolarization before the membrane potential, returned to the base line. Note a clear increase in the amplitude of ETP during the IJP, suggesting a marked decrease in membrane conductance during the IJP. These studies were performed using the modified Tomita bath. ETP were applied in the chamber A. sIJP was produced by electrical field stimulation of the muscle in the recording chamber, under nonadrenergic, noncholinergic conditions and addition of apamin to block the fast (f)IJP.
ETP during hyperpolarization due to various drug treatments.
During drug treatments, hyperpolarizing ETPs of durations between 0.5 and 1.5 s with an interval 1.5–3 s were continuously applied during the control period and period of drug infusion. Membrane potential and ETP were simultaneously recorded in the same smooth muscle cell for over 45 min.
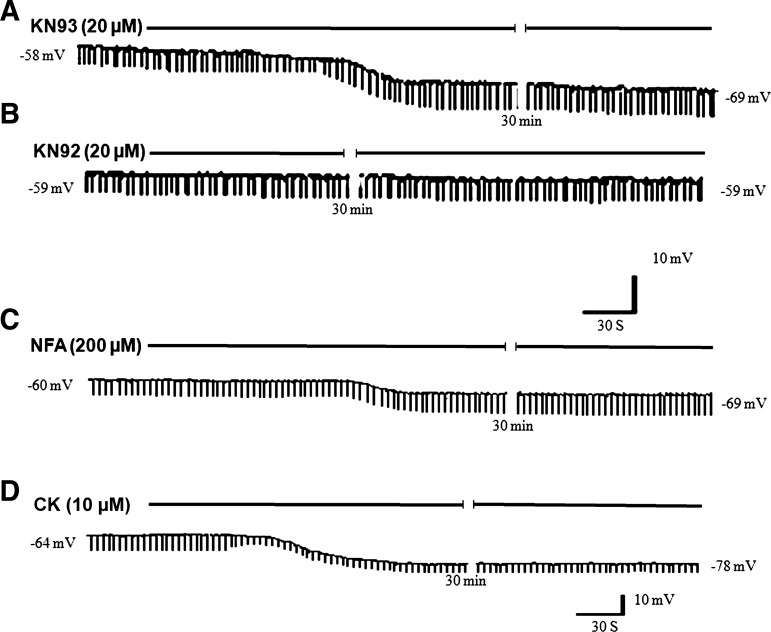
KN93 (20 μM) perfusion caused membrane hyperpolarization. Hyperpolarization started within 30 s, reached a peak in ∼2 min and persisted for ∼45 min. The steady-state hyperpolarization was 11 ± 2.1 mV (from −55 ± 4.6 to −66 ± 3.6 mV, means ± SD; Fig. 3A; Table 1). KN93-associated hyperpolarization of smooth muscles was associated with increase in the amplitude of ETPs which increased by 20.3 ± 5.9%. On the other hand, the inactive analog KN92 did not affect membrane potential or the ETP (Fig. 3B; Table. 1). These observations show that KN93 is associated with decreased membrane conductance and mimics the action of nitrergic IJP.
Fig. 3.
Examples of the effects of prolonged perfusion of KN93, KN92, niflumic acid (NFA), and cromakalim (CK) on the smooth muscle membrane potential and ETP on the same cell. A: perfusion of a calmodulin-dependent protein kinase II (CaMKII) inhibitor, KN93, hyperpolarized the smooth muscle and increased the amplitude of the ETP. Increase in the amplitude of the ETP is consistent with decrease in membrane conductance. B: perfusion of KN92, an analog of KN93 that is inactive against CaMKII, did not affect membrane potential or the ETP. C: perfusion of a known Cl− channel blocker, NFA, hyperpolarized the smooth muscle increase in the amplitude of the ETP that is consistent with decrease in membrane conductance. D: perfusion of a known KATP channel opener, CK, hyperpolarized the smooth muscle and decreased in the amplitude of the ETP that is consistent with increase in membrane conductance (membrane potential values in representative strips are indicated).
Table 1.
Effects of perfusion of KN93, NFA, and CK on MP and ETP and interaction of KN93 with NFA and CK
| Treatment | MP, mV |
ETP, mV |
||||
|---|---|---|---|---|---|---|
| Control | Treatment | Change | Control | Treatment | Change, % | |
| KN93 | −55 ± 4.6 | −66 ± 4* | −11.2 ± 2.1 | 6 ± 2 | 7.1 ± 2.1 | Up 20.3 ± 5.9* |
| KN92 | −56 ± 4 | −56 ± 4 | 0 ± 0 | 5.7 ± 0.7 | 5.8 ± 0.6 | 0 ± 0 |
| NFA | −58 ± 3.5 | −69 ± 4.3* | −10.8 ± 1.3 | 9 ± 1.3 | 11.4 ± 1.8* | Up 25 ± 2.2* |
| CK | −59 ± 6.1 | −73 ± 6.2* | −15 ± 1 | 6.3 ± 1.2 | 3.7 ± 0.3* | Down 41 ± 6* |
| KN93 after NFA | −69 ± 4.4 | −69 ± 4.3 | −0.2 ± 0.46 | 14.5 ± 1.7 | 15 ± 1.4 | Up 3.8 ± 4.4 |
| KN93 after CK | −70 ± 9.2 | −78 ± 8.6* | −8.3 ± 0.6 | 3.7 ± 0.3 | 5.3 ± 0.3* | Up 46 ± 9.8* |
Values represent means ± SD of 3–8 observations in 3 animals. MP, membrane potential; ETP, electrotonic potential; NFA, niflumic acid; CK, cromakalin.
P < 0.05, significantly different from control value.
A putative Cl− channel blocker, NFA (200 μM), perfusion hyperpolarized the smooth muscle membrane by 10.8 ± 1.3 mV (from −58 ± 3.5 to −69 ± 1.5 mV; n = 3–8 observations in 3 animals) associated with increase in the amplitude of ETPs by 25 ± 2.2% (Fig. 3C; Table 1), suggesting that NFA hyperpolarization is associated with closure of Cl− conductance and mimics the action of KN93 or nitrergic IJP.
CK is a well-known KATP channel opener. CK (10 μM) infusion strongly hyperpolarized the smooth muscle by 15 ± 1 mV (from −59 ± 6 mV to −73 ± 6.3 mV). However, the hyperpolarization was associated with decrease in the amplitude of ETP by 41 ± 10% (Fig. 3D; Table 1), suggesting that, as expected, CK hyperpolarization was associated with increase in K+ conductance.
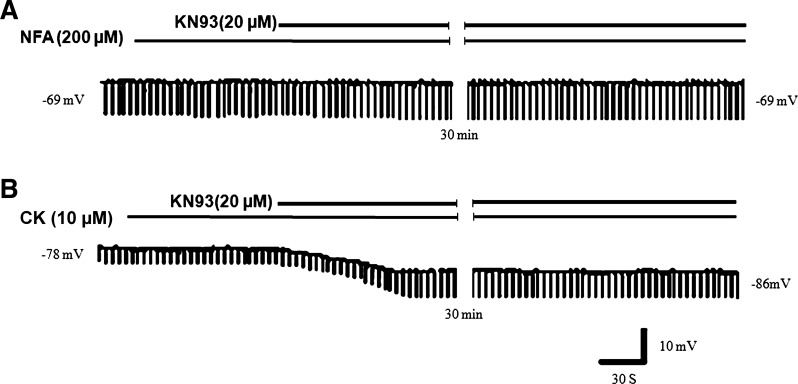
Effect of NFA and cromakalim on responses to KN93.
We also examined the effect of KN93 on membrane potential and ETP after treatment with NFA or CK. The membrane potential after NFA treatment was −69 ± 4.4 mV. During NFA hyperpolarization, KN93 hyperpolarization was obliterated (being only 0.25 ± 0.46 mV, from −69 ± 4.4 to −69.3 ± 4.3 mV) and there was little change (+3.80 ± 2%) in the amplitude of ETP (Fig. 4A; Table. 1). These observations show that NFA antagonizes the action of KN93. However, the hyperpolarizing action of KN93 was not affected by CK. CK perfusion hyperpolarized the smooth muscle membrane to −70 ± 9.2 mV. During CK-induced hyperpolarization, KN93 caused further hyperpolarization of 8.3 ± 0.6 mV from −70 ± 5 to −78 ± 8.6 mV and an increase in the amplitude of ETPs by 46 ± 9.8% (Fig. 4B; Table. 1). These observations show that KN93, by closing Cl− conductance, can cause additional hyperpolarization on top of CK-induced hyperpolarization associated with closure of membrane conductance. These observations are consistent with the fact that KN93 and CK cause membrane hyperpolarization via effects of different ion conductances.
Fig. 4.
Examples of effect of perfusion of NFA and CK on the action of KN93 on the smooth muscle membrane potential and ETP on the same cell. A: during perfusion of NFA, smooth muscle had membrane potential of −69 mV and ETP of 14.5 mV. Addition of KN93 did not change either the membrane potential or the ETP, suggesting that NFA blocked the action of KN93 by saturation block of Cl− channels. B: after perfusion of cromakalim, the smooth muscle was hyperpolarized (membrane potential: −78 mV) and had small amplitude ETP of 3.7 mV. Addition of KN93 further hyperpolarized the membrane by 8 to −86 mV, and increased the amplitude of the ETP, suggesting that CK hyperpolarization does not block the action of KN93, which can still act to close Cl− channels (membrane potential values in representative strips are indicated).
Effect of CaMKII inhibitors on fIJP and sIJP.
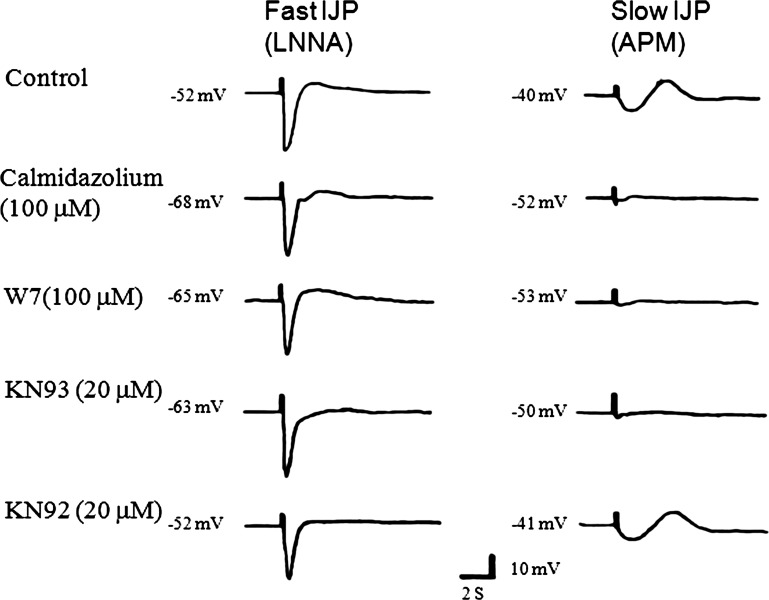
In a separate study, the effect of CaMKII inhibitors was investigated on the fIJP and the sIJP. The fIJP was elicited after suppression of nitrergic IJP with l-NNA (100 μM). In the control studies, the membrane potential after l-NNA treatment was −52 ± 2.2 mV and the amplitude of fIJP was 28 ± 2.5 mV. The CaM inhibitors calmidazolium and W7 as well as the CaMKII inhibitor KN93 hyperpolarized the membrane from −52 ± 2.2 to −68 ± 2.7, −65 ± 5.9, and −63 ± 3.4 mV, respectively. However, these treatments did not affect the amplitude of fIJP (Fig. 5; Table 2).
Fig. 5.
Examples of effects of various inhibitors of CaMKII on the fIJP and the sIJP. IJPs were elicited by electrical field stimulation. fIJP was isolated by blocking the sIJP by Nω-nitro-l-arginine (l-NNA) and sIJP was isolated by blocking the fIJP by apamin (APM). Note that the fIJP has a large amplitude and is not suppressed by CaM inhibitors calmidazolium or W7 that indirectly inhibit CaMKII and KN93 that directly inhibit CaMKII. However, calmidazolium, W7, and KN93 strongly suppressed the sIJP.
Table 2.
Effect of CaM and CaMKII inhibitors and NFA and CK on purinergic and nitrergic IJP
| Purinergic IJP |
Nitrergic IJP, mV |
|||||
|---|---|---|---|---|---|---|
| Treatment | MP After l-NNA | Before treatment | After Treatment | MP After Apamin | Before treatment | After treatment |
| Control | −52 ± 2.2 | 28 ± 2.5 | — | −40 ± 1.6 | 6.6 ± 0.9 | — |
| Calmidazolium | −68 ± 2.7* | 27 ± 0.8 | 27 ± 0.4 | −52 ± 1.9* | 6.6 ± 0.3 | 0.5 ± 0.4* |
| W7 | −65 ± 5.9* | 27.8 ± 0.9 | 27 ± 0.5 | −53 ± 2.4* | 7.1 ± 0.3 | 0.2 ± 0.2* |
| KN93 | −63 ± 3.4* | 28 ± 2.2 | 28 ± 0.4 | −50 ± 2.4* | 6.7 ± 0.1 | 0.2 ± 0.2* |
| KN92 | −52 ± 2.6 | 27.5 ± 1.8 | 27.5 ± 0.5 | −41 ± 1.6 | 6.7 ± 0.1 | 6.7 ± 0.3 |
Values represent means ± SD of 4–17 observations in 3–6 animals.
CaMKII, calmodulin (CaM)-dependent protein kinase II; l-NNA, Nω-nitro-l-arginine; IJP, inhibitory junction potential.
P < 0.001, significantly different from control.
Nitrergic sIJP was isolated by blocking the purinergic IJP with apamin. Apamin (0.3 μM) treatment depolarized smooth muscle from −52 ± 2.2 to −40 ± 1.6 mV. electrical field stimulation elicited nitrergic sIJP of 6.6 ± 0.9 mV. KN93 hyperpolarized the membrane potential to −50 ± 2.4 mV and suppressed the sIJP to −0.2 ± 0.2 mV (P < 0.01; Fig. 5; Table 2). In contrast, KN92 was without effect on membrane potential or the sIJP. CaM inhibitors, calmidazolium, and W7, which also indirectly inhibit CaMKII also hyperpolarized the smooth muscle and suppressed the sIJP (Fig. 5; Table 2).
DISCUSSION
In our preliminary studies, we observed that the CaMKII inhibitor KN93, which inhibits all of its isoforms (32), caused suppression of the nitrergic IJP without affecting the purinergic IJP. The nitrergic IJP is due to production and release of NO from the nitrergic nerve terminals (31). In the prejunctional nerve terminals, NO production from neuronal nitric oxide synthase-α is known to be regulated by CaMKII that acts to phosphorylate neuronal nitric oxide synthase-α and suppress its action and CaMKII inhibitors should counteract this inhibition and may enhance NO production and the nitrergic IJP (22). Therefore, the inhibitory action of KN93 on the nitrergic IJP appeared paradoxical and was puzzling.
The present studies show that 1) the nitrergic IJP is associated with decrease in membrane conductance; 2) the CaMKII inhibitor KN93 and the calcium-activated chloride channels (CaCC) blocker NFA mimicked the action of nitrergic IJP and caused smooth muscle membrane hyperpolarization associated with a decrease in membrane conductance; 3) hyperpolarization associated with NFA but not K+ channel opener CK blocked the action of KN93 by a “maximal” block of CaCC; and 4) the nitrergic IJP, but not the purinergic IJP, was blocked by CaM or CaMKII inhibitors and by NFA. These results suggest that nitrergic IJP, KN93, and NFA act by closing CaMKII-activated CaCC in postjunctional smooth muscle cells.
The Tomita bath technique has been used to investigate the junction potentials during prolonged ETP. Such studies had originally suggested that the fIJP was due to opening of K+ channels and the sIJP may be due to closure of Cl− conductance (5–7, 34). In this study, we directly investigated membrane conductance during the nitrergic IJP. Because of the brief time period, membrane conductance in the fIJP could not be investigated. However, the amplitude of the ETP was significantly increased during the sIJP, indicating that the sIJP is due to decrease in membrane conductance.
The CaMKII inhibitor KN93 is selective inhibitor of CaMKII, and its structural analog KN92 is inactive on CaMKII (30, 33). KN93 also have other effects: it suppresses L-type Ca2+ channels (9). Moreover, KN93 and its analog KN92 both have been reported to block K+ channel opening (27). However, these nonselective effects do not explain the hyperpolarizing action of KN93 and suggest that KN93-produced hyperpolarization of the smooth muscle was due to modulation of a CaMKII-dependent signaling. In smooth muscle cells, the Cl− levels are higher than those predicted by Cl− equilibrium potential. Therefore, resting Cl− conductance is responsible for depolarized state of smooth muscle. Closure of Cl− conductance by putative Cl− channel blockers has been shown to cause membrane hyperpolarization of intestinal smooth muscles (6, 7). Many Ca2+-activated CaCC have been described in smooth muscles (11, 17), including those in the gut (2, 37, 43). Moreover, calcium-activated Cl− channels have been described in the interstitial cells of Cajal that may possible mediate sIJP (15, 44). However, only the CaMKII-regulated CaCC may explain the action of CaMKII inhibitor. CaMKII by its property of autophosphorylation can convert transient Ca2+ releases into sustained CaMKII-mediated activation of the Cl− channels that maintain resting Cl− conductance.
CaMKII regulation of CaCC is somewhat complicated [Hartzell et al. (11); Leblanc (24)]. In arterial and tracheal smooth muscles, CaMKII inhibits ICl-Ca2+ that is stimulated by CaMKII inhibitors that cause membrane depolarization (24). These CaCC would not explain the KN93-induced hyperpolarization observed in this study. However, CaMKII-stimulated CaCC have been recognized in many cell types including smooth muscle (13). The molecular identity of CaMKII-activated CaCC has recently been identified as a member of the ClC-3 subfamily. ClC-3 was originally described as intracellular channel that is present endosomes and synaptic vesicles but is now known to be widely expressed also on the cell surface. Recent studies (28) have shown that ClC-3 exists in at least three splice variants. They have different tissue distribution and are regulated by different signaling molecules. Only one of these isoforms (containing 818 amino acids) is directly regulated by CaMKII (14, 28). Our studies provide strong functional support for suppression of the CaMKII-activated Cl− channel in nitrergic IJP. We speculate that the functional current described here may be due to CaMKII binding, CLC3A (28). Further studies are needed to test this hypothesis.
NFA is a nonsteroidal anti-inflammatory agent with multiple other effects including complex effects on Cl− channels. However, it has been extensively used as a relatively selective blocker of Cl− channels (38). Because KN93 and NFA blocked the sIJP, we investigated the actions of KN93 and NFA and their interactions on the smooth muscle membrane potential and conductance to understand the mechanism of their inhibitory action. The effect of the CaMKII inhibitor KN93 was similar to that of NFA, a putative CaCC channel blocker. NFA also hyperpolarized smooth muscle membrane by ∼10 mV and decreased membrane conductance. Thus CaMKII inhibitor closely mimicked the action of NFA, suggesting that they both may act to suppress a resting Cl− conductance. Moreover, hyperpolarizing response of nitrergic IJP was associated with decrease in membrane conductance and mimicked the actions of KN93 or NFA, suggesting that nitrergic IJP, KN93, and NFA acted on a common ion channel to produce hyperpolarization of the smooth muscles.
This study shows that the CaCC blocker NFA may suppress the action of KN93 by saturation block of the Cl− channels. Thus, after maximal closure of Cl− channels by NFA, KN93 causes no additional closure of CaCC. Therefore, any hyperpolarization by KN93 is blocked. Block of nitrergic IJP by KN93 or NFA can be explained in a similar way.
Recently, CaMKII-regulated potassium channels have been described that may account for the effect of KN93 on the sIJP (26, 29). It has been shown that in murine colon myocytes, CaMKII increases the open probability of some isoforms of SK channels such as SK2 channels (23, 35). Therefore, it is possible that PKG-stimulated CaMKII may also lead to enhanced opening of SK channels and associated hyperpolarization. If so, KN93 and apamin would be expected to depolarize the membrane and suppress both the purinergic and the nitrergic IJPs. However, we found that while apamin depolarized, KN93 hyperpolarized the membrane, and whereas apamin blocked the fIJP without affecting the sIJP, KN93 did not affect the fIJP but blocked the sIJP. These observations do not support the possibility that nitrergic IJP may involve activation of CaMKII-dependent SK channels.
Recently, K+ channels of Kv4 family and Kv1.4 have also been shown to be regulated by CaMKII. Kv4.3 is molecular marker of the A current in smooth muscles (21). CaMKII has been shown to slow inactivation and accelerate rate of recovery from inactivation of Kv4.3. Basal CaMKII activity is thought to allow these K+ channels to moderate depolarization at resting potentials, and KN93 may shift this moderation towards more depolarized states (21). This effect would not explain the hyperpolarizing action of KN93 on the smooth muscles.
One of the prerequisites of hyperpolarization due to opening of channel, such as K+ channel, is that it is associated with increased ion conductance. The present study shows that a well-known KATP channel opener, CK, hyperpolarized smooth muscle membrane associated with an increase in membrane. However, KN93-associated hyperpolarization was not associated with increased membrane conductance. These observations argue against the possibility that the effect of KN93 or the nitrergic IJP is due to opening of any outward conductance.
In conclusion, these studies show that purinergic fIJP and nitrergic sIJP are due to distinct K+ and Cl− conductance changes, respectively. They also provide strong functional evidence to support the view that the nitrergic IJP is due to active closure of a Cl− conductance in the smooth muscle cell and is not due to passive transfer of the potential generated in the neighboring ICC. These studies also show that the suppression of the nitrergic IJP by CaMKII inhibition and niflumic acid is due to “maximal” block of the Cl− channels. Further studies are needed to establish molecular identity of the functionally defined CaMKII-activated Cl− channels. These findings may enhance our understanding of pathophysiology and rational treatment of neuromuscular disorders of the gut.
GRANTS
Support for this study was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-062867.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.-D.H. performed experiments; X.-D.H. and R.K.G. analyzed data; X.-D.H. and R.K.G. prepared figures; X.-D.H. and R.K.G. drafted manuscript; X.-D.H. and R.K.G. approved final version of manuscript; R.K.G. conception and design of research; R.K.G. interpreted results of experiments; R.K.G. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Arun Chaudhury for critical comments and suggestions.
REFERENCES
- 1. Abe Y, Tomita T. Cable properties of smooth muscle. J Physiol 196: 87–100, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akbarali HI, Giles WR. Ca2+ and Ca2+-activated Cl− currents in rabbit oesophageal smooth muscle. J Physiol 460: 117–133, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol 279: C126–C135, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol 447: 119–131, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 261: G752–G762, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Crist JR, He XD, Goyal RK. Chloride-mediated junction potentials in circular muscle of the guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 261: G742–G751, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Crist JR, He XD, Goyal RK. The nature of noncholinergic membrane potential responses to transmural stimulation in guinea pig ileum. Gastroenterology 100: 1006–1015, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Daniel EE, Jury J, Christinck F, Cayabyab F. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 263: G135–G138, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun 345: 1606–1610, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Goyal RK, He XD. Evidence for NO˙ redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. Am J Physiol Gastrointest Liver Physiol 275: G1185–G1192, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005 [DOI] [PubMed] [Google Scholar]
- 12. He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol 461: 485–499, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho MW, Kaetzel MA, Armstrong DL, Shears SB. Regulation of a human chloride channel a paradigm for integrating input from calcium, type ii calmodulin-dependent protein kinase, and inositol 3,4,5,6-tetrakisphosphate. J Biol Chem 276: 18673–18680, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 276: 20093–20100, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology 123: 1627–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther 237: 893–900, 1986 [PubMed] [Google Scholar]
- 17. Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503–568, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med 12: 2165–2180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol 489: 735–743, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, Horowitz B. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem 276: 44338–44346, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Koh SD, Perrino BA, Hatton WJ, Kenyon JL, Sanders KM. Novel regulation of the A-type K+ current in murine proximal colon by calcium-calmodulin-dependent protein kinase II. J Physiol 517: 75–84, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komeima K, Hayashi Y, Naito Y, Watanabe Y. Inhibition of neuronal nitric-oxide synthase by calcium/calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108–15 neuronal cells. J Biol Chem 275: 28139–28143, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Kong ID, Koh SD, Bayguinov O, Sanders KM. Small conductance Ca2+-activated K+ channels are regulated by Ca2+-calmodulin-dependent protein kinase II in murine colonic myocytes. J Physiol 524: 331–337, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O'Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol 83: 541–556, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Niel JP, Bywater RA, Taylor GS. Apamin-resistant post-stimulus hyperpolarization in the circular muscle of the guinea-pig ileum. J Auton Nerv Syst 9: 565–569, 1983 [DOI] [PubMed] [Google Scholar]
- 26. Perrino BA. Regulation of gastrointestinal motility by Ca2+/calmodulin-stimulated protein kinase II. Arch Biochem Biophys 510: 174–181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rezazadeh S, Claydon TW, Fedida D. KN-93 {2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N -methylbenzylamine}, a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther 317: 292–299, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol 556: 353–368, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sergeant GP, Ohya S, Reihill JA, Perrino BA, Amberg GC, Imaizumi Y, Horowitz B, Sanders KM, Koh SD.Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol 288: C304–C313, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181: 968–975, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Thatte HS, He XD, Goyal RK. Imaging of nitric oxide in nitrergic neuromuscular neurotransmission in the gut. PLos One 4: e4990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264: 17907–17912, 1989 [PubMed] [Google Scholar]
- 33. Tombes RM, Grant S, Westin EH, Krystal G. G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase). Cell Growth Differ 6: 1063–1070, 1995 [PubMed] [Google Scholar]
- 34. Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol 225: 693–703, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vogalis F, Goyal RK. Activation of small conductance Ca2+-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J Physiol 502: 497–508, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vogalis F, Zhang Y, Goyal RK. An intermediate conductance K+ channel in the cell membrane of mouse intestinal smooth muscle. Biochim Biophys Acta 1371: 309–316, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Wang Q, Akbarali HI, Hatakeyama N, Goyal RK. Caffeine- and carbachol-induced Cl− and cation currents in single opossum esophageal circular muscle cells. Am J Physiol Cell Physiol 271: C1725–C1734, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Wiwchar M, Ayon R, Greenwood IA, Leblanc N. Phosphorylation alters the pharmacology of Ca(2+)-activated Cl channels in rabbit pulmonary arterial smooth muscle cells. Br J Pharmacol 158: 1356–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Miller DV, Paterson WG. Opposing roles of K+ and Cl− channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol Gastrointest Liver Physiol 279: G1226–G1234, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Miller DV, Paterson WG. TREK-1 channels do not mediate nitrergic neurotransmission in circular smooth muscle from the lower oesophageal sphincter. Br J Pharmacol 159: 362–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Paterson WG. Role of Ca2+-activated Cl− channels and MLCK in slow IJP in opossum esophageal smooth muscle. Am J Physiol Gastrointest Liver Physiol 283: G104–G114, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Paterson WG. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol 140: 1097–1107, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Vogalis F, Goyal RK. Nitric oxide suppresses a Ca2+-stimulated Cl− current in smooth muscle cells of opossum esophagus. Am J Physiol Gastrointest Liver Physiol 274: G886–G890, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]