Abstract
The Caco-2 cell line represents absorptive polarized intestinal epithelial cells that express multiple forms of Na+/H+ exchanger (NHE) in their plasma membranes. Caco-2 cells express the major apical NHE isoform NHE3, but low NHE3 expression together with inefficient transfection often hamper intended studies. In this study, we examined whether SK-CO15 cells could be used to study NHE3 regulation. SK-CO15 cells grown on Transwell inserts developed polarized epithelial cells with microvilli. The transfection efficiency of SK-CO15 cells was markedly higher compared with Caco-2 cells, an advantage in gene transfer and knockout. SK-CO15 cells expressed NHE1, NHE2, and NHE3. NHE3 expression was significantly greater in these cells than Caco-2, and NHE3 comprised more than half of total NHE activity. Apical expression of NHE3 in SK-CO15 cells was confirmed by confocal immunofluorescence and surface biotinylation. NHE regulatory factors NHERF1 and NHERF2, which are important for regulation of NHE3 activity, were expressed in these cells. Stimulatory response of NHE3 in SK-CO15 cells was assessed by dexamethasone and lysophosphatidic acid (LPA). Treatment with dexamethasone for 24–48 h increased NHE3 expression and activity. Similarly to Caco-2 cells, SK-CO15 cells lacked the expression of the LPA receptor LPA5, but exogenous expression of LPA5 resulted in acute stimulation of NHE3. Forskolin acutely inhibited NHE3 activity in SK-CO15 cells, further attesting the validity of these cells. We conclude that SK-CO15 cells with the amenity for transfection and high endogenous NHE3 expression are a new and better cell model for NHE3 regulatory investigation than widely used Caco-2 cells.
Keywords: intestine, epithelia
polarized intestinal epithelial cells form an interface separating the internal from external environments and maintain homeostasis between intestinal lumen and the body interior. The plasma membranes of polarized epithelial cells are divided into apical and basolateral domains with asymmetric distribution of cytoplasmic organelles by vectorial sorting mechanisms (27). Na+/H+ exchange is a major route of Na+ absorption in the small intestine and colon (9). Intestinal epithelial cells express multiple forms of Na+/H+ exchangers (NHEs; Slc9a) among which the type 3, NHE3 (Slc9a3), is the primary brush-border NHE. The functions and mechanisms of NHE3 regulation by hormones and growth factors have been investigated since its cloning in the early 1990s using several cell model systems. Among these, PS120 Chinese hamster lung fibroblasts and AP1 Chinese hamster ovarian cells provide an ideal cell system for reductionist approach to characterize NHEs (26, 28). However, the nonepithelial origins of PS120 and AP1 cells often led to question the physiological validity of the findings. Originated from human intestines, T84 and HT29 human colon carcinoma epithelial cell lines are not suitable, as these cells represent secretory crypt epithelial cells (5, 6). The Caco-2 human adenocarcinoma cell line, on the other hand, express several brush-border enzymes, such as sucrase-isomaltase, alkaline phosphatase, and aminopeptidase, characteristics of the enterocytes lining the small intestinal villi (25). Caco-2 cells build a polarized monolayer and exhibit epithelial cell characteristics, i.e., transepithelial electric resistance of ∼200 Ω·cm2, brush-border microvilli, and tight junctions (7). Because of these features, Caco-2 cells have been deployed to study NHE3 regulation; however, Caco-2 cells have several limitations, including a high degree of heterogeneity leading to the nonuniformity in NHE3 activity, low transfection efficiency, and slow rates of cell proliferation (14). A clonal variant of Caco-2 cells, Caco-2bbe, has been used, but as for Caco-2 cells, the endogenous level of NHE3 in Caco-2bbe cells is either low or undetected, and the usefulness of the Caco-2bbe clone is largely limited to the characterization of transfected NHE3 (20, 21). Alternatively, rat intestinal epithelial IEC-6 has been used, but these cells primarily express NHE1 and lack the endogenous expression of NHE3 (35). Isolation of alternative intestinal epithelial cells suitable for the characterization of NHE3 has been sluggish due to the difficulties in isolation and long-term propagation of primary intestinal epithelial cells.
The SK-CO15 cell line derived from human adenocarcinoma of the colon develops polarized epithelial cells that form numerous domes on an impermeable support (19). These cells develop high-resistance monolayers with well-defined adhesion and tight junctions (11, 19). In search of an alternative model system that is more amenable to transfection, we examined the usability of SK-CO15 cells to study NHE3 regulation. In this study, we show that NHE3 is endogenously expressed in the apical membrane of SK-CO15 cells and the activity level of NHE3 in SK-CO15 cells is significantly higher than in Caco-2 cells. The regulation of NHE3 in SK-CO15 cells by selected agonists is consistent with previous reports, demonstrating the practicability of SK-CO15 cells as a suitable epithelial model for characterization of NHE3 regulation.
MATERIALS AND METHODS
Cell culture and treatment.
SK-CO15 was a gift from Dr. Rodriguez-Boulan at Weil Medical College, Cornell University, New York, NY. SK-CO15, Caco-2, and Caco-2bbe cells were maintained in DMEM supplemented with 10% FBS, penicillin (50 mU/ml), streptomycin (50 μg/ml), 1 mM sodium pyruvate, 15 mM HEPES, and 1× nonessential amino acids. Caco-2bbe stably expressing NHE3 was previously described (20, 40). When necessary, cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Expression of lysophosphatidic acid receptor type 5 (LPA5) in Caco-2bbe cells was attained by using lentivirus harboring hemagglutinin (HA)-tagged LPA5, as previously reported (20, 40).
Transfection.
When necessary, Caco-2 or SK-CO15 cells were seeded in six-well plates at a density of 5–6×105 cells/well (5–6×104 cells/cm2) and grown overnight. Then 4 μg of plasmid DNA were mixed with 10 μl Lipofectamine 2000 in 500 μl Opti-MEM according to the manufacturer's recommendation (Invitrogen). Transfection complexes were added to the cells overlaid with 1.5 ml DMEM without antibiotics. After 4 h of incubation at 37°C, the transfection mixture was replaced with fresh DMEM containing antibiotics. For transient expression, cells were harvested after 48 h.
For an estimation of transfection efficiency using enhanced green fluorescence protein (eGFP), cells were transiently transfected with peGFP as described above. Cells expressing GFP were viewed under an Eclipse T1 inverted fluorescence microscope (Nikon, Melville, NY) and the number of GFP-positive cells was counted using NIS Elements Advanced Research image software (Nikon). The transfection efficiency was expressed as the ratio of number of cells expressing GFP to the total cell number times 100 from more than six fields of vision in each transfection.
Reagents and antibodies.
Polyclonal rabbit anti-NHE3 serum (EM450) was generated at Covance (Princeton, NJ) against a purified recombinant NHE3 protein corresponding to aa 621–702 of human NHE3. To affinity purify EM450, 1.2 mg of purified NHE3 recombinant protein (aa 621–702) was resolved by 15% SDS-PAGE, followed by transfer onto nitrocellulose membrane. NHE3 protein on the nitrocellulose membrane identified by Ponceau-S stain was excised and washed with PBS buffer containing 0.05% Tween 20 (PBST). EM450 anti-serum diluted 1:20 in 10 ml of PBST was added to the cut membrane and incubated on a shaking platform for 4 h at room temperature. After washing with PBST buffer twice, bound antibodies were eluted with 0.1 M glycine, pH 2.5, immediately neutralized with 1 M Tris·HCl, pH 8.0, and concentrated using Amicon Ultra-15 filter units (Millipore, Billerica, MA). Short-hairpin RNA (shRNA) targeting NHE3 was obtained from Sigma (St. Louis, MO). LPA was purchased from Avanti Polar Lipids (Alabaster, AL) and prepared as described (20). All the other chemicals and antibodies were obtained from Sigma or Cell Signaling (Boston, MA).
RNA extraction, RT-PCR and quantitative real-time RT-PCR.
Total RNA from SK-CO15 or Caco-2 cells was isolated using Trizol (Invitrogen), and 2 μg of RNA was subsequently used to synthesize cDNA using the SuperScript III First Strand Synthesis kit as recommended by manufacturer (Invitrogen). NHE1, NHE2, NHE3, and NHE8 were amplified by using the primer pairs for NHE1 (5′-TGGCCTCCAGCTCAGCCCAA-3′ and 5′-AGCGGCTCGATGACCCGGAT-3′), NHE2 (5′-GGCGCGATGCGTTGAGC-3′ and 5′-TGTCGCTGAGGCCGAATGCTT-3′), NHE3 (5′-AGAGCGGGGGCTTCCAGGTG-3′ and 5′-AAGACCACCCCCACCAGCGT-3′), and NHE8 (5′-GCTGGCATGAGGCCAAGGGG-3′ and 5′-GGACCCGGGGCTGTGGAGAT-3′). cDNA was amplified with SYBR Green Supermix (Bio-Rad, Hercules, CA) on Mastercycler Realplex (Eppendorf, Hauppauge, NY). The reaction mix consists of 1 μl of cDNA, 10 μl of SYBR Green Supermix, 0.5 μM of target primers in total volume of 20 μl. Amplification was carried out at 4 min at 95°C for polymerase activation, and 40 cycles of 95°C for 30 s (denaturation), 60°C for 30 s (annealing), and 72°C for 1 min (extension). The amounts of NHE1, NHE2, and NHE3 mRNA were normalized to β-actin.
NHE3 activity.
The Na+-dependent changes in intracellular pH (pHi) by NHE3 was determined using the ratio-fluorometric, pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester (BCECF-AM) as previously described (34). Briefly, cells grown on Transwells (BD Falcon, Franklin Lakes, NJ) for 7 days postconfluence were serum starved overnight, and the next morning washed in Na+ buffer [in mM: 130 NaCl, 20 HEPES, 5 KCl, 1 tetramethylammonium-PO4 (TMA-PO4), 2 CaCl2, 1 MgSO4, and 25 glucose] and then dye-loaded by incubating for 20 min with 6.5 μM BCECF-AM in the same solution. When needed, forskolin or the equal volume of DMSO was added to cells and incubated at room temperature during the dye-loading period. Cells on permeable supports were mounted on a perfusion chamber, placed on an inverted microscope, and superfused with NH4+ buffer (in mM: 50 NH4Cl, 80 TMA-Cl, 20 HEPES, 5 KCl, 1 TMA-PO4, 2 CaCl2, 1 MgSO4, and 25 glucose) and subsequently with TMA+ buffer (in mM: 130 TMA-Cl, 20 HEPES, 5 KCl, 1 TMA-PO4, 2 CaCl2, 1 MgSO4, and 25 glucose). In some experiment, TMA+ buffer contained 1 μM LPA or 0.1% BSA as a control. Hence, cells were preexposed to LPA or control BSA for 2–3 min prior to perfusion with Na+ buffer that drives Na+-dependent pH recovery. Na+ buffer was supplemented with 50 μM HOE694 to inhibit NHE1 and NHE2 activities. Calibration of the fluorescence signal was performed using the K+/H+ ionophore nigericin as described previously (34). The microfluorometry was performed on a Nikon TE200 inverted microscope with a Nikon CFI Super Fluor magnification, ×40 objective, coupled to a Lambda 10–2 filter wheel controller equipped with a multi-wavelength filter set designed for BCECF. Photometric data was acquired using the Metafluor software (Molecular Devices, Sunnyvale, CA). Na+/H+ exchange rate was described by the rate of pHi recovery, which was calculated by determining slopes along the pHi recovery by linear least-squares analysis over a minimum of 9 s.
Surface biotinylation.
Surface biotinylation of NHE3 was performed as previously described (10). Briefly, cells grown on permeable filters were rinsed twice in PBS and 10 min incubation in borate buffer composed of (in mM): 154 NaCl, 7.2 KCl, 1.8 CaCl2, and 10 H3BO3, pH 9.0. Cells were then incubated for 40 min with 0.5 mg/ml NHS-SS-biotin (Pierce, Rockford, IL) in borate buffer. Unbound NHS-SS-biotin was quenched with Tris buffer (20 mM Tris, 120 mM NaCl, pH 7.4). Cells were then rinsed with PBS, scraped, lysed in the lysis buffer described above, and sonicated for 2 × 15 s. The lysate was agitated for 30 min and spun at 14,000 g for 15 min to remove the insoluble cell debris. Protein concentration was determined and 1 mg of lysate was then incubated with streptavidin-agarose beads (Pierce) for 2 h. The streptavidin-agarose beads were washed three times in lysis buffer and twice in PBS. All of the above procedures were performed at 4°C or on ice. Biotinylated surface proteins were then eluted by boiling the beads at 95°C for 10 min. Dilutions of the total and surface NHE3 were resolved by SDS-PAGE and immunoblotted with anti-NHE3 antibody. Densitometric analysis was performed using Scion Image software (National Institutes of Health, Bethesda, MD).
Immunofluorescece confocal microscopy.
SK-CO15 cells grown on Transwells were washed twice with cold PBS, fixed with ice-cold ethanol for 10 min, permeabilized with 0.2% Triton X-100 in PBS for 10 min, and blocked with PBS containing 5% normal goat serum for 45 min at RT. Cells were then incubated with anti-NHE3 EM450, anti-vesicular stomatitis virus glycoprotein P5D4, anti-NHERF1 Ab5199, anti-NHERF2 Ab2570, or anti-occludin antibodies (a gift from Dr. Asma Nusrat, Emory University) for 2 h at room temperature. Following three washes, 10 min each, with PBS, the cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) or Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen) or Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen) for 1 h or rhodamine-conjugated phalloidin for 30 min at room temperature. After 3 × 10 min washes with PBS, the excised Transwells were mounted with ProLong Gold Antifade Reagent (Invitrogen) and observed under a Zeiss LSM510 laser confocal microscope (Zeiss Microimaging, Thornwood, NY) coupled to a Zeiss Axioplan2e with ×100 magnification Pan-Apochromat oil lenses.
Transmission electron microscopy.
SK-CO15 and Caco-2 grown on Transwell inserts were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, dehydrated, and embedded in Epon resin. Ultrathin sections from SK-CO15 cells and Caco-2 cells were examined using the JEM-1400 (JEOL, Peabody, MA) microscope at the Electron Microscopy Core at Emory University.
Alkaline phosphatase fluorometric assay.
All procedures were performed using SK-CO15 and Caco-2 grown for 10 days postconfluence according to the manufacturer's protocol (Alkaline Phosphatase Fluorometric Assay kit; Abcam, Cambridge, MA). Briefly, cells were scraped in 110 μl of assay buffer, homogenized, centrifuged at 14,000 g for 3 min to remove insoluble material, and added to each well of Fluotrac 96-well plate (Sigma). Methylumbelliferyl phosphate disodium salt (Abcam) substrate was added to each well, incubated for 30 min at 37°C, and stopped by adding 20 μl of STOP solution to each well. Fluorescence intensity was measured at 360 nm for excitation and 440 nm for emission by using a fluorescence microplate reader (BioTek, Winooski, VT). Enzyme activity was calculated from the angular coefficient of the linear slope obtained from alkaline phosphatase standard (Abcam) solution, and expressed as 4-methyumbelliferon generated per volume of sample per minute (mU/ml). All experiments were independently performed three times in triplicates.
Statistics.
Results were presented as means ± SE. Statistical analyses were performed by Student's t-test for paired comparison with P < 0.05 considered significant.
RESULTS
SK-CO15 cells endogenously express NHE1, NHE2 and NHE3.
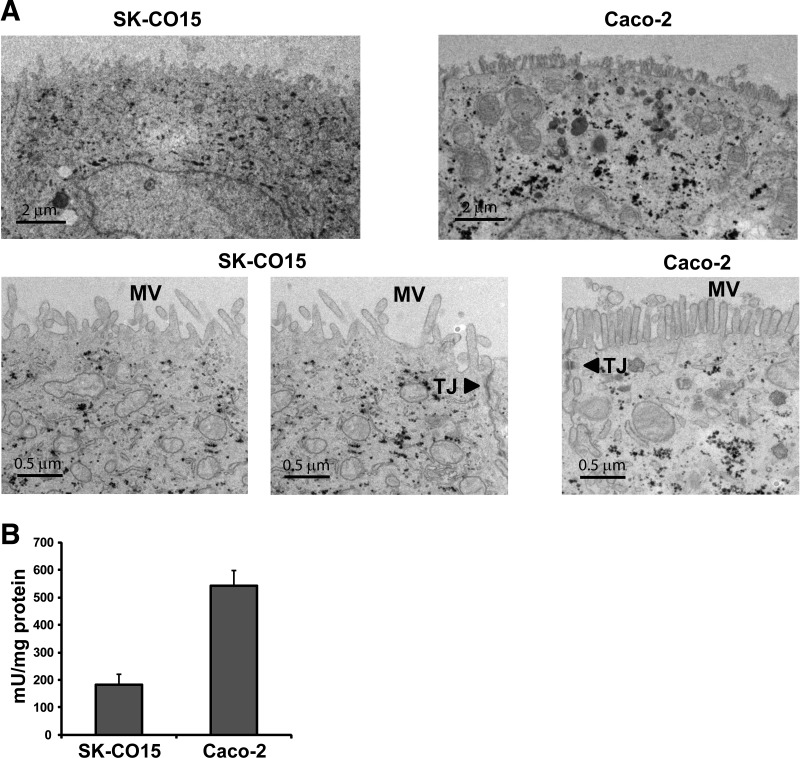
It was reported that SK-CO15 cells lack the expression of sucrase-isomaltase and express villin and alkaline phosphatase at lower levels (19). On the other hand, SK-C015 cells express well-defined apical junctional complexes and form a monolayer with transepithelial electric resistance >2,000 Ω·cm2 (19). Hence, we sought to compare the ultrastructure of SK-CO15 and Caco-2 cells. SK-CO15 and Caco-2 cells display typical polarized epithelial cell features with junctional complexes and microvilli (Fig. 1A). However, the microvilli were less abundant and disordered in SK-CO15 cells compared with Caco-2 cells. Similarly, the activity of alkaline phosphatase in SK-CO15 cells was significantly lower level (∼33%) compared with Caco-2 cells (Fig. 1B), reflecting less abundant and shortened microvilli in SK-CO15 cells shown in Fig. 1A.
Fig. 1.
The SK-CO15 cell line develops polarized epithelial cells. A: transmission electron microscopy images of SK-CO15 and Caco-2 cells showing the presence of microvilli. Cells were grown 7 days postconfluence on Transwells, and transepithelial electric resistance was determined to ensure the polarization of the cells. MV, microvilli; TJ, tight junctions. B: alkaline phosphatase activity was determined in SK-CO15 and Caco-2 cells grown on Transwells for 7 days postconfluence. Data are from 3 independent experiments, and each experiment was carried out in triplicate. Data are presented as means ± SE.
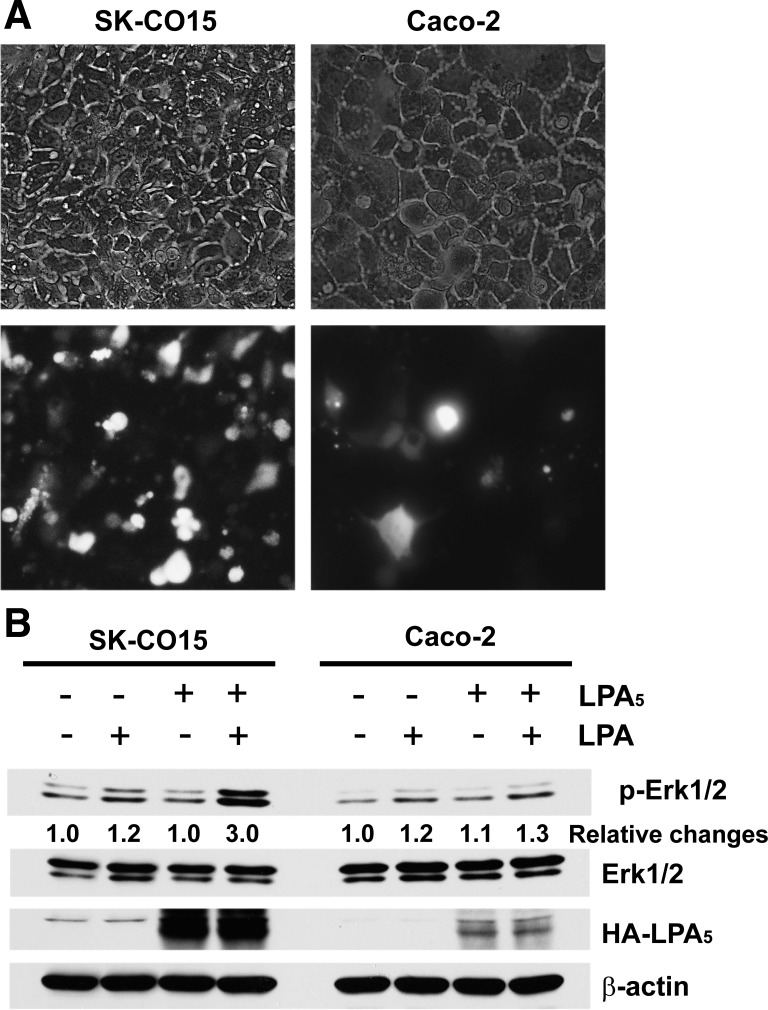
One inherent problem of Caco-2 cells is its relative resistance to transfection. We compared the transfection efficiency of Caco-2 and SK-CO15 by transfecting pEGFP. Typical determination of GFP expression after 48 h of transfection is shown in Fig. 2A where 11 out of 99 Caco-2 cells (11%) expressed GFP. In comparison, ∼71% (78/110) of SK-CO15 cells expressed GFP. To further validate the higher transfection efficiency in SK-CO15 cells, we compared the level of HA-LPA5 expression in Caco-2 and SK-CO15 cells transfected with pcDNA-3.1/HA-LPA5. The expression level of LPA5 in SK-CO15 cells was markedly greater compared with that in Caco-2 cells (Fig. 2B). We showed previously that LPA5 phosphorylates Erk1/2 via transactivation of the epidermal growth factor receptor (40). Consistent with the robust expression of HA-LPA5, LPA markedly activated Erk1/2 in SK-CO15 cells. In comparison, activation of Erk1/2 by LPA in HA-LPA5 transfected Caco-2 cells was marginally above control transfected cells. Together, these results demonstrate a significant advantage of SK-CO15 over Caco-2 cells in liposome-mediated transfection.
Fig. 2.
SK-CO15 cells have higher transfection efficiency than Caco-2 cells. A: SK-CO15 and Caco-2 cells transiently transfected with peGFP (green fluorescence protein) are shown. Top and bottom: brightfield and fluorescence images, respectively. Representative images are shown from 3 independent transfection. B: expression levels of hemagglutinin-tagged lysophosphatidic acid receptor type 5 (HA-LPA5) in SK-CO15 and Caco-2 cells were compared by Western blot analysis. The expression levels of HA-LPA5 relative to β-actin are shown at bottom. LPA-mediated activation of Erk1/2 was determined by Western blot analysis, and the relative levels of Erk1/2 activation are indicated. pErk1/2, phosphorylated Erk1/2. Representative images are shown from 3 independent experiments.
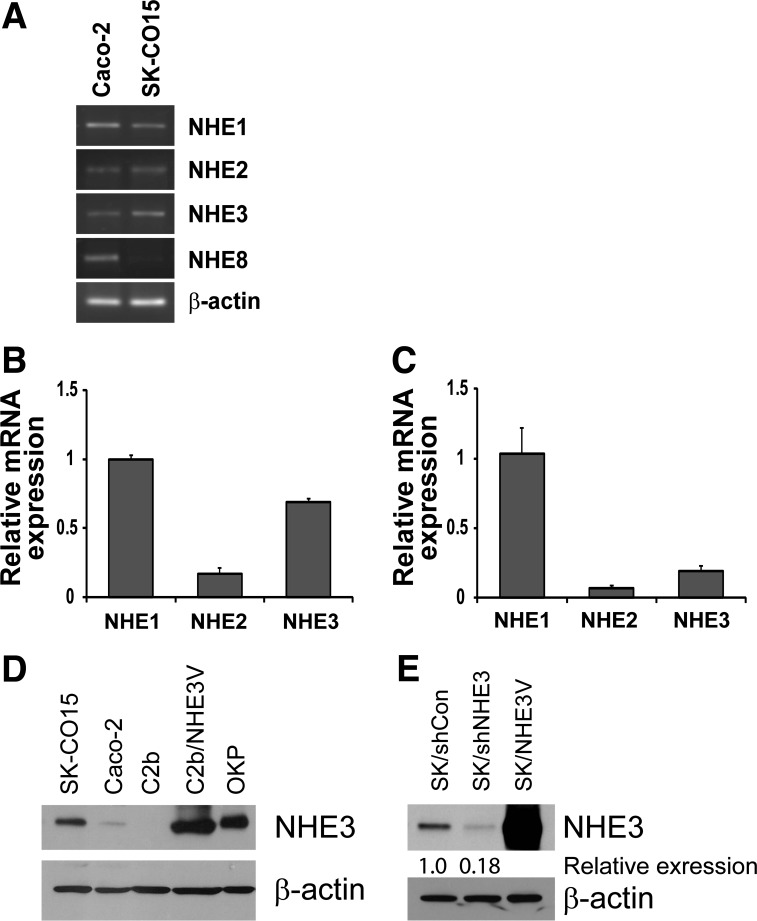
We next examined expression of NHEs in SK-CO15 cells by RT-PCR. Figure 3A shows the presence of amplicons corresponding to NHE1–3 with predicted sizes (915 bp for NHE1; 669 bp for NHE2; 691 bp for NHE3) in both Caco-2 and SK-CO15 cells, but NHE8 (515 bp) was expressed in Caco-2 cells and not in SK-CO15 cells. qRT-PCR showed that the expression level of NHE3 mRNA was significantly higher in SK-CO15 cells when compared with NHE1 mRNA levels (Fig. 3, B and C). NHE3 has a molecular mass of 75–80 kDa and a single band of ∼75 kDa was detected in SK-CO15 cell lysates by a polyclonal antibody raised against the COOH terminus of human NHE3, EM450 (Fig. 3D). The expression level of NHE3 was markedly higher in SK-CO15 cells than in Caco-2 cells. NHE3 protein in Caco-2bbe cells was not detected, consistent with our previous report that Caco-2bbe cells lack NHE3 expression (20). Lysates from Caco-2bbe cells transfected with NHE3 and opossum kidney (OK) cells, which were used as controls, showed the presence of NHE3 with a similar molecular mass. To confirm the specificity of the 75 kDa band as NHE3 protein, we transiently knocked down NHE3 expression in SK-CO15 cells by NHE3-specific shRNA. Knockdown of NHE3 expression by shRNA decreased the protein band intensity by ∼80%, confirming the specificity of the anti-NHE3 antibody and the expression of NHE3 in SK-CO15 cells (Fig. 3E). The transfection efficiency in SK-CO15 cells and the specificity of our anti-NHE3 antibody were further demonstrated by transient transfection of SK-CO15 cells with pcDNA3.1/NHE3V that resulted in a robust increase in NHE3 expression (Fig. 3E).
Fig. 3.
SK-CO15 cells express Na+/H+ exchanger 3 (NHE3). A: expression of NHE1, NHE2, NHE3, and NHE8 in Caco-2 and SK-CO15 cells were determined by RT-PCR. A representative figure of 3 independent experiments is shown. Quantitative real-time RT-PCR of SK-CO15 (B) and Caco-2 (C) was performed to determine relative levels of NHE1, NHE2, and NHE3 transcripts. Threshold cycle values of NHE1–3 were normalized to that of β-actin. The relative abundance of NHEs was then calculated by normalizing to the expression level of NHE1 mRNA. D: NHE3 protein expression was determined using anti-NHE3 antibody EM450 in SK-CO15, Caco-2, Caco-2bbe (C2b), C2b/NHE3V, and OKP cells. β-actin was used as a loading control. E: SK-CO15 cells were transiently transfected with control shRNA (shCon) or NHE3-specific shRNA (shNHE3). The specificity of EM450 was determined by Western blot analysis, which showed a decrease in NHE3 protein band intensity in cells transfected with shNHE3. Cells transfected with pcDNA3.1/NHE3V were used as a control. β-actin was used as a loading control (n = 3).
NHE3 is the major NHE in SK-CO15 cells.
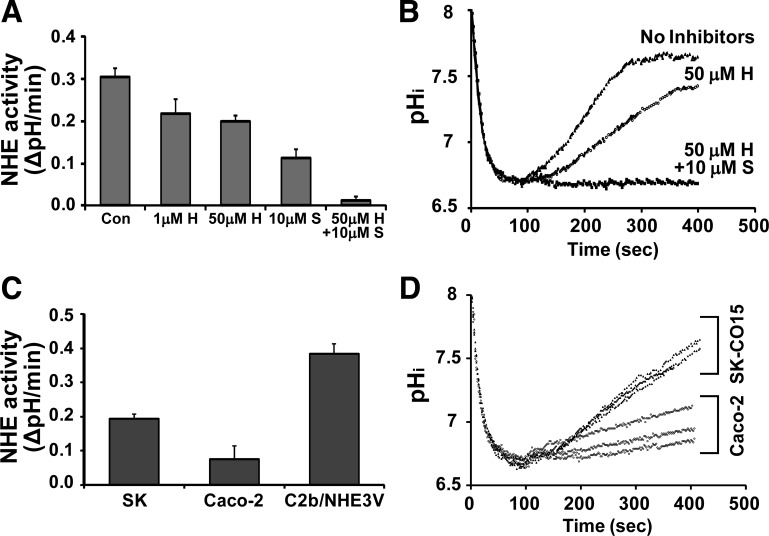
We next sought to determine the level of Na+/H+ exchange activity by NHE3 in these cells. NHEs exhibit differential sensitivities to several inhibitors, including amiloride and HOE694, as such different NHEs can be selectively inhibited using these inhibitors (4). Figure 4A shows that 1 μM HOE694 (H), which blocks NHE1 activity (4), decreased the rate of Na+-dependent pH recovery from 0.31 ± 0.02 pH U/min to 0.21 ± 0.03 pH U/min, indicating a significant contribution by NHE1. Increasing HOE694 to 50 μM to block both NHE1 and NHE2 resulted in a small decrease compared with 1 μM HOE694, suggesting that NHE2 is a minor NHE in these cells. Na+/H+ exchange activity was almost completely ablated (0.02 pH U/min) in the presence of 50 μM HOE694 and the NHE3 inhibitor S3226 (S) (Fig. 3, A and B). The complete ablation of Na+/H+ exchange in the presence of HOE694 and S3226 indicates that SK-CO15 cells lack other HOE694-insensitive NHEs than NHE3. NHE8 is reported to be relatively sensitive to HOE694 (38), and it is either absent or expressed at a level undetectable by RT-PCR in SK-CO15 cells (Fig. 3A). Consistently, S3226 alone decreased the NHE activity by > 50%, confirming that NHE3 is a major active NHE in SK-CO15 cells. Since Caco-2 cells have been used as a model intestinal epithelial cell system for NHE3 regulation, we compared the levels of NHE3 activity in Caco-2 and SK-CO15 cells. Figure 4C showed that endogenous NHE3 activity in SK-CO15 cells was more than twofold greater than Caco-2 cells, although overexpression of NHE3 in Caco-2bbe cells resulted in even greater activity. Moreover, SK-CO15 cells showed markedly more uniform NHE3 activities than Caco-2 cells (Fig. 4D). Together, these studies suggest that SK-CO15 cells represent a cell model that has greater and more consistent NHE3 activity compared with Caco-2 cells, with an advantage of higher transfection efficiency.
Fig. 4.
SK-CO15 cells show robust NHE3 activity. A: Na+-dependent intracellular pH (pHi) recovery was measured to determine the rate of Na+/H+ exchange in SK-CO15 (SK) cells. HOE694 was used to selectively inhibit NHE1 (1 μM) or NHE1 and 2 (or 50 μM). The 10 μM S3226 was used to inhibit NHE3. Results are presented as means ± SE (n = 9). Con, control; H, HOE694; S, S3226. B: representative traces of Na+-dependent intracellular pH recovery in SK-CO15 cells are shown. C: NHE3 activities in SK-CO15, Caco-2, and C2b/NHE3V cells were compared. Results are presented as means ± SE (n = 9). D: representative traces of Na+-dependent pH recovery in SK-CO15 cells and Caco-2 cells in the presence of 50 μM HOE694 are shown. The rates of pHi recovery were much more consistent in SK-CO15 compared with Caco-2 cells.
NHE3 is localized on the apical membrane in SK-CO15 cells.
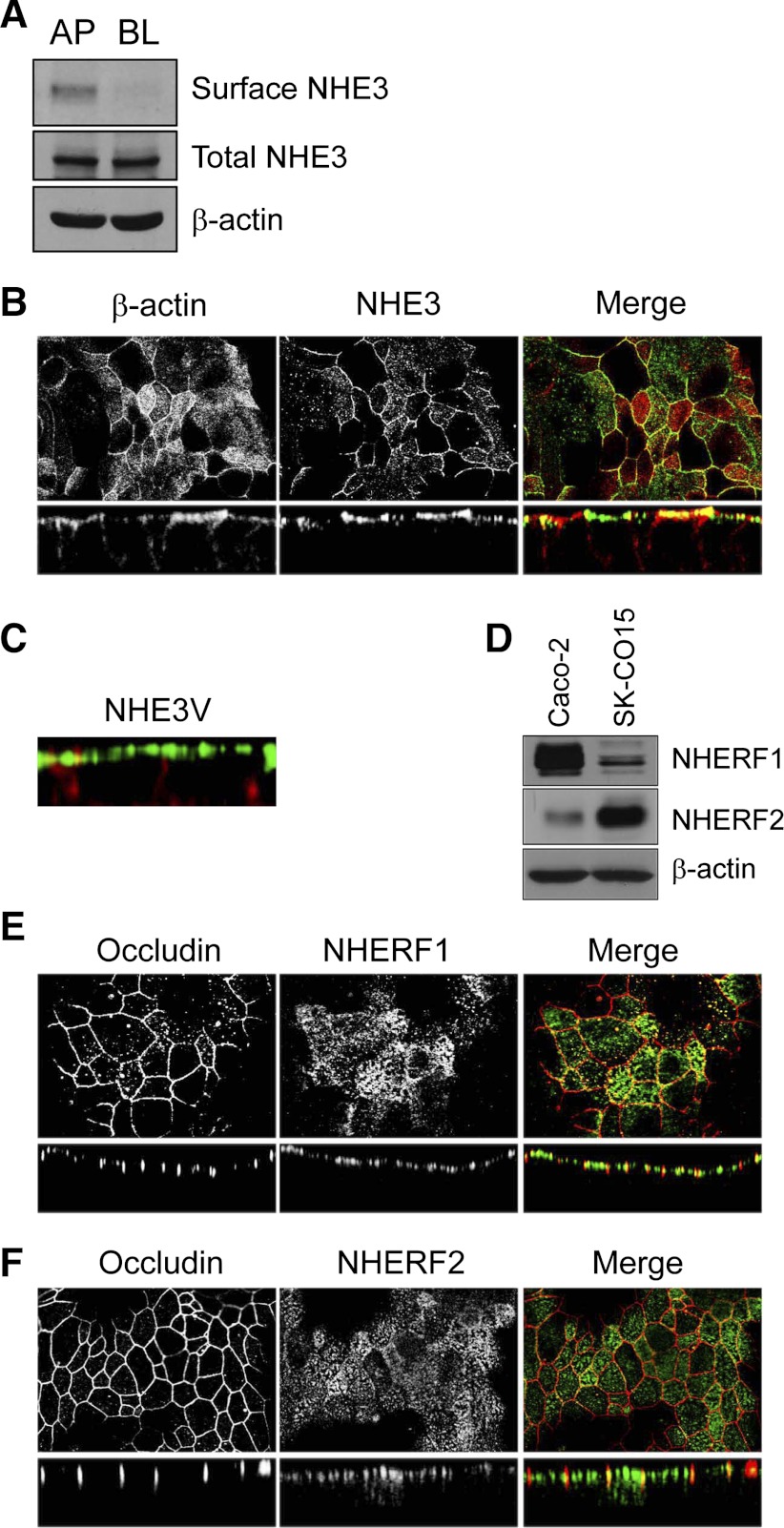
NHE3 is an apical NHE of intestinal epithelial cells. To determine the cellular localization of NHE3, we performed surface biotinylation of SK-CO15 cells. NHE3 was exclusively detected in the apical surface fraction, but not in the basolateral membrane fraction (Fig. 5A). To corroborate the apical expression, polarized SK-CO15 cells were stained with EM450 (Fig. 5B, middle) and phalloidin (Fig. 5B, left). Figure 5B shows localization of NHE3 on the apical membrane with low NHE3 signal in subapical regions. To determine whether exogenously expressed NHE3 is targeted properly to the apical membrane, we transfected SK-CO15 cells with epitope-tagged NHE3V. Consistent with the cellular expression of endogenous NHE3 shown earlier, NHE3V was localized to the apical membrane without any detectable signal in the basolateral membrane (Fig. 5C).
Fig. 5.
NHE3 is localized on the apical membrane in SK-CO15 cells. A: surface biotinylation was performed to determine the polarity of endogenous NHE3 expression in SK-CO15 cells as described in Surface biotinylation. Western blot analysis shows protein expression in the apical (AP) and basolateral (BL) fractions. β-actin was used as a loading control (n = 3). B: cells were fixed with ice-cold ethanol, permeabilized, and incubated with affinity-purified anti-NHE3 antibody EM450 followed by incubation with FITC-conjugated goat anti-rabbit secondary antibody. Rhodamine-conjugated phalloidin was used to label F-actin. Top and bottom: focal and cross-sectional views, respectively. C: cells transfected with pcDNA3.1/NHE3V were labeled with anti-vesicular stomatitis virus glycoprotein antibody P5D4 (green) and phalloidin (red). A cross-sectional view is shown. D: presence of NHE regulatory factor 1 (NHERF1) and NHERF2 in SK-CO15 cells was determined by Western blot analysis using the anti-NHERF1 antibody Ab5199 and anti-NHERF2 antibody Ab2570. β-Actin was used as a loading control. NHERF1 (E, green), NHERF2 (F, green), and occludin (E–F, red) were labeled with anti-NHERF1, anti-NHERF2, and anti-occludin antibodies, respectively. Top and bottom: show focal and cross-sectional views, respectively.
NHE regulating factor (NHERF) proteins play pivotal roles in the regulation of NHE3 (9, 17). Of four NHERF isoforms characterized to date, NHERF1 is required for cAMP-dependent inhibition of NHE3 in the kidney although it is not obligatory in the intestine (23). NHERF2, on the other hand, is necessary for glucocorticoid- and LPA-induced stimulation of NHE3 (8, 20). Hence, we determined the expression of NHERF1 and NHERF2 in SK-CO15 cells. Figure 5D shows that SK-CO15 cells expressed both NHERF1 and NHERF2. Interestingly, the expression levels of these NHERF proteins in SK-CO15 and Caco-2 appeared a mirror image, with SK-CO15 cells expressing more NHERF2 but less NHERF1 than Caco-2 cells. Figure 5E, left and 5F, left show the expression of tight junction protein occludin in SK-CO15 cells revealing well-defined tight junctions. Confocal immunofluorescence images show that both NHERF1 (Fig. 5E) and NHERF2 (Fig. 5F) were primarily expressed on or near the apical membrane, although NHERF2 exhibited diffused intracellular staining.
Regulation of NHE3 in SK-CO15 cells.
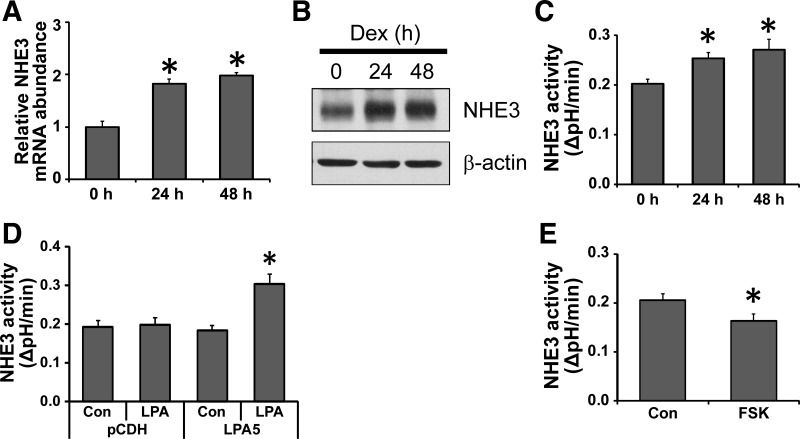
Glucocorticoids stimulate NHE3 activity via genomic as well as nongenomic mechanisms (41, 42). To further extend the utility of SK-CO15 cells as an intestinal epithelial cell model for NHE3 study, we determined the chronic effect of a synthetic glucocorticoid, dexamethasone (Dex). Incubation of SK-CO15 cells with 1 μM Dex for 24 or 48 h significantly increased the mRNA levels of NHE3 (Fig. 6A). The changes in NHE3 mRNA expression were paralleled by increased NHE3 protein expression (Fig. 6B) and NHE3 activity (Fig. 6C), consistent with previous studies using Caco-2 or rabbit intestinal brush-border membrane (35, 42).
Fig. 6.
Regulation of NHE3 in SK-CO15 cells. SK-CO15 cells were treated with 1 μM dexamethasone (Dex) for 24 or 48 h, and the expression levels of NHE3 mRNA (A) and protein (B) were determined by qRT-PCR and Western blot analysis, respectively (n = 3; *P < 0.05). C: NHE3 activity in SK-CO15 cells treated with 1 μM Dex was determined. Results are presented as means ± SE (n = 9, *P < 0.05 compared with control). D: SK-CO15 cells transfected with pCDH or pCDH-LPA5 were treated with 1 μM LPA for 5 min and NHE3 activity was determined as described earlier (n = 9; *P < 0.05 compared with control). E: cells were treated with 10 μM forskolin (FSK) for 30 min prior to NHE3 activity measurement (n = 9; *P < 0.05).
Previously, we showed that LPA acutely stimulates NHE3 activity in Caco-2 cells when LPA5 is coexpressed with NHERF2 (20). Similarly to Caco-2 cells (20), SK-CO15 cells expressed LPA5 at a low level (data not shown); as such LPA showed no effect on NHE3 activity (Fig. 6D). On the other hand, overexpression of LPA5 in SK-CO15 cells resulted in a marked stimulation of NHE3 activity in response to LPA, consistent with our previous studies (20, 40).
Unlike Dex and LPA, forskolin that activates adenylate cyclase acutely inhibits NHE3 activity via a mechanism involving NHE3 phosphorylation and NHERF proteins (16, 43). Next, we examined the inhibitory effect of forskolin on NHE3 in SK-CO15 cells. Figure 6E shows that forskolin acutely inhibited NHE3 activity by 24%, indicating the presence of NHE3 inhibitory signaling pathways in SK-CO15 cells. Together our results demonstrate that NHE3 in SK-CO15 cells respond to several agonists and antagonists in manners similar to previous studies using other cell lines of intestinal and nonintestinal origins.
DISCUSSION
The characterization of NHE3 has utilized several cell lines, including fibroblasts, OK, Caco-2, and, to a lesser extent, IEC-6 cells (16, 18, 35, 40, 43). Of these, the OK cell line shows robust expression of NHE3, but its renal proximal tubule origin limits its application in the study of intestinal NHEs. Caco-2 and its clonal Caco-2bbe cells have been used to represent absorptive intestinal epithelial cells. Certainly, these cells are highly polarized and exhibit many characteristics of small intestinal epithelial cells. Caco-2 cells endogenously express NHE1, 2, and 3, but NHE3 expression is relatively low. Consequently, it is often challenging to identify endogenous NHE3 proteins in these cells by immunoblot or immunofluorescence. The Caco-2bbe cell line is reported to have endogenous NHE3 expression and activity (21), but the clonal cell line in our possession does not express NHE3 in a measurable quantity (20). Searching for an alternative cell line has been largely unsuccessful; several immortalized intestinal cell lines, including IEC-6, MSIE (mouse small intestine epithelium), and YAMC (young adult mouse colon), either lack or express NHE3 at a low level (Ref. 37; B. K. Yoo and C. C. Yun, unpublished data). The difficulty in long-term propagation of primary cultures of intestinal epithelial cells further hinders isolation of absorptive epithelial cell cultures. The recent identification of intestinal stem cells and the establishment of long-term culture of intestinal crypt-villus organoids are promising (29), but the development of normal absorptive epithelial cell lines from the intestinal mucosa that are ideal for Na+/H+ exchange and other ion and nutrient transport processes occurring at the brush-border membrane is yet to be achieved.
Herein, we examined whether SK-CO15 cells can be used to study the regulation of intestinal NHE3. SK-CO15 cells have been recently used to investigate cell migration (1), epithelial cyst formation (12), epithelial barrier function (30), and apical junctional proteins (24). Several lines of evidence showed that SK-CO15 cells form a differentiated epithelial cell layer: expression of several junctional proteins including ZO-1, junctional adhesion molecule (JAM)-A, and occudin in SK-CO15 cells have been reported previously (13, 31); SK-CO15 cells rapidly form high-resistance monolayers with well-defined adhesion and tight junctions that regulate the integrity of differentiated epithelial layers (24, 33); the organization and integrity of apical junctional complexes in SK-CO15 cells are shown to be regulated by nonmuscle myosin II and adducin (24, 33); and SK-CO15 cells, like Caco-2 cells, form spherical cysts when imbedded in Matrigel (24, 33).
In this study we showed that 1) SK-CO15 cells form a polarized epithelial monolayer with microvilli formation and alkaline phosphatase activity; 2) SK-CO15 cells endogenously express NHE3 at a level significantly greater than in Caco-2 cells; 3) these cells express NHERF1 and NHERF2; 4) SK-CO15 cells are amenable to transfection; and 5) regulation of NHE3 is identical to previous studies on isolated intestinal tissues and Caco-2 cells.
We showed that SK-CO15 cells express the transcripts of NHE1–3 and that, unlike Caco-2 and Caco-2bbe cells, NHE3 protein expression in SK-CO15 cells was easily detected by immunoblotting. Na+/H+ exchange activity measurement in the presence of selective NHE inhibitors showed that Na+/H+ exchange activity by NHE3 in SK-CO15 cells constituted more than 50% of total NHE activity. A caveat of our study is that although polarized cells grown on permeable filters were used, the inhibitors were added from the apical side such that inhibition of NHE1 in the basolateral membrane might have been less efficient than if the inhibitors were added on the basolateral side. However, the combination of 50 μM HOE694 and 10 μM S3226 completely obliterated NHE activity, suggesting that apical HOE694 somehow is able to access NHE1 on the opposite side of the cells. Importantly, NHE3 activity in SK-CO15 cells was significantly higher (>2-fold) than in Caco-2 cells and the rates of Na+-dependent pH recovery were markedly more consistent in SK-CO15 cells. It is noteworthy that SK-CO15 cells do not express NHE8 (Fig. 3A). Similar to NHE3, NHE8 is localized at the apical membrane (39), but its expression is more abundantly expressed in young animals during the development and evidence supports its role in electrolyte absorption during early postnatal development (2). In human, NHE8 is ubiquitously expressed but more abundant in the duodenum and ascending colon (38). Caco-2 cells despite its colon carcinoma origin resemble the enterocytes lining the small intestine. On the contrary, the low alkaline phosphatase activity, the formation of tight epithelial layer, and low or absence of NHE8 suggest that SK-CO15 cells exhibits characteristic features of colonocytes. NHE8 is more sensitive to HOE694 than NHE3, inhibited by 10 μM HOE694 but not by 1 μM HOE694 (38). Conversely, NHE8 is less sensitive to S3226 than NHE3. The absence of NHE8 transcript suggests that SK-CO15 cells, in addition to NHE3 studies, could be used as an intestinal epithelial model to characterize acute regulation of NHE8 taking an advantage of differential sensitivity of NHE8 to the NHE specific inhibitors.
In using Caco-2 and Caco-2bbe cells to investigate cellular processes at the molecular levels one major hindrance is the low efficiency of transfection with CaPO4 or liposomes. To circumvent this limitation, viral transduction or usage of a specialized transfection tool, such as the Nucleofector (Lonza, Allendale, NJ) or Neon (Invitrogen) system, has been introduced. We estimated the transfection efficiency of SK-CO15 cells using liposomes (e.g., lipofectamine 2000) to be about 71% compared with 11% in Caco-2 cells, which eliminates the need for a specialized electroporator. The transfection efficacy of SK-CO15 cells was further demonstrated by knockdown and overexpression of NHE3 expression. Hence, SK-CO15 cells provide a major improvement in transfection over Caco-2 cells.
NHE3 activity is modulated by extrinsic factors via multiple mechanisms that include phosphorylation, trafficking, and interaction with regulatory proteins, which are often inter-dependent (9). We assessed the capacity of SK-CO15 cells as a model cell line for NHE3 regulation by examining the expression of NHERF proteins and regulation of NHE3 by selected agonists. NHERF1 and NHERF2 are two major regulatory proteins interacting with NHE3 and other signaling molecules (9, 17). Indeed, SK-CO15 cells express both NHERF1 and NHERF2, although we did not determine the presence of NHERF3 (PDZK1) in these cells. Evidence emerging from in vitro studies using heterologous expression and in vivo studies using mice deficient in NHERF2 expression has shown that NHERF2 is essential for regulation of NHE3 as well as CFTR by LPA (20, 32). NHERF2 interacts with the LPA5 receptor and LPA cannot induce Na+-dependent fluid absorption in mouse intestine lacking NHERF2 (20, 40). We showed previously that LPA5 is abundantly expressed in mouse enterocytes and colonocytes, but its expression is low in Caco-2 cells (20). We found that LPA5 expression was low in SK-CO15 cells and LPA failed to regulate NHE3 in SK-CO15 cells unless LPA5 was overexpressed. The low expression levels of LPA5 in Caco-2 and SK-CO15 cells is interesting, and whether the low expression is related to the cancer cell origin of these cell lines is yet to be determined.
Glucocorticoids are a well-known agonist of NHE3 that stimulates NHE3 activity via multiple mechanisms. The effects of 24–48 h treatment of Dex that increased NHE3 mRNA and protein levels in SK-CO15 cells are consistent with the effects on Caco-2 and rabbit intestine (35, 41, 42). In addition to the genomic effects, we have shown previously that the effect of glucocorticoids is also dependent on serum- and glucocorticoid-induced kinase 1 (SGK1) that interacts with NHERF2 such that the extent of NHE3 stimulation is significantly blunted in the absence of SGK1 or NHERF2 (8, 41). Although we did not examine the expression of SGK1 in SK-CO15 cells, we assumed SK-CO15 cells to express SGK1 based on the robust effect of Dex on NHE3 activity (2-fold), which would be expected to be markedly diminished in the absence of SGK1.
Inhibition of NHE3 by forskolin or protein kinase A-dependent mechanisms is another example of well-known regulation of NHE3 (16, 43, 44). Forskolin, which activates adenylate cyclase, inhibits NHE3 activity via multiple pathways involving NHE3 phosphorylation, NHERF proteins, and endocytosis. The absence of NHERF1 ablated cAMP-dependent inhibition of NHE3 in the kidney but not in the intestine (23, 36). Similarly, the absence of NHERF2 did not affect cAMP-dependent inhibition of NHE3 in the intestine (3), although Murtazina et al. (22) reported the NHERF2-dependence of NHE3 inhibition by cAMP in mouse intestine. We found that SK-CO15 cells express NHERF2 at a much higher level but less NHERF1 than Caco-2 cells. Nonetheless, NHE3 in SK-CO15 cells was acutely inhibited by forskolin, further demonstrating the usefulness of these cells in probing the regulatory mechanisms of NHE3.
In summary, we report here that SK-CO15 cells express NHE3 whose regulation is consistent with previous physiological studies. With the high transfection efficiency and the polarized morphology, our studies demonstrate that SK-CO15 cells are an ideal cell model to study NHE3 regulation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-061418 and DK-061418S1 (to C. C. Yun), T32-DK-007771 (to B. K. Yoo), and Digestive Disease Research Development Center National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-064399.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.K.Y. and C.C.Y. conception and design of research; B.K.Y., M.K.Y., and Y.R.N. performed experiments; B.K.Y. and C.C.Y. analyzed data; B.K.Y. and C.C.Y. interpreted results of experiments; B.K.Y. prepared figures; B.K.Y. drafted manuscript; B.K.Y. and C.C.Y. edited and revised manuscript; B.K.Y. and C.C.Y. approved final version of manuscript.
REFERENCES
- 1. Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, Nusrat A. Annexin I regulates SK-CO15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 281: 19588–19599, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baum M, Martin MG, Booth IW, Holmberg C, Twombley K, Zhang Q, Gattineni J, Moe O. Nucleotide sequence of the Na+/H+ exchanger-8 in patients with congenital sodium diarrhea. J Pediatr Gastroenterol Nutr 53: 474–477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen M, Sultan A, Cinar A, Yeruva S, Riederer B, Singh AK, Li J, Bonhagen J, Chen G, Yun C, Donowitz M, Hogema B, de Jonge H, Seidler U. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J Physiol 588: 5049–5063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol 44: 1041–1045, 1993 [PubMed] [Google Scholar]
- 5. Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204–G208, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Franklin CC, Turner JT, Kim HD. Regulation of Na+/K+/Cl− cotransport and [3H]bumetanide binding site density by phorbol esters in HT29 cells. J Biol Chem 264: 6667–6673, 1989 [PubMed] [Google Scholar]
- 7. Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol Cell Physiol 247: C260–C267, 1984 [DOI] [PubMed] [Google Scholar]
- 8. He P, Lee SJ, Lin S, Seidler U, Lang F, Fejes-Toth G, Naray-Fejes-Toth A, Yun CC. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol Biol Cell 22: 3812–3825, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 238080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2: e658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ivanov AI, Hopkins AM, Brown GT, Gerner-Smidt K, Babbin BA, Parkos CA, Nusrat A. Myosin II regulates the shape of three-dimensional intestinal epithelial cysts. J Cell Sci 121: 1803–1814, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol 176: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janecki AJ, Montrose MH, Tse CM, de Medina FS, Zweibaum A, Donowitz M. Development of an endogenous epithelial Na+/H+ exchanger (NHE3) in three clones of Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 277: G292–G305, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem 271: 9919–9927, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Lamprecht G, Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol 291: G766–G777, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA 86: 9313–9317, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology 138: 649–658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McSwine RL, Musch MW, Bookstein C, Xie Y, Rao M, Chang EB. Regulation of apical membrane Na+/H+ exchangers NHE2 and NHE3 in intestinal epithelial cell line C2/bbe. Am J Physiol Cell Physiol 275: C693–C701, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol 301: C126–C136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na+/H+ exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell 21: 3506–3517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47: 323–330, 1983 [Google Scholar]
- 26. Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci USA 81: 4833–4837, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powell SK, Cunningham BA, Edelman GM, Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature 353: 76–77, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Rotin D, Grinstein S. Impaired cell volume regulation in Na+/H+ exchange-deficient mutants. Am J Physiol Cell Physiol 257: C1158–C1165, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Severson EA, Kwon M, Hilgarth RS, Parkos CA, Nusrat A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem Biophys Res Commun 397: 592–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell 20: 1916–1925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285–293, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D, Zhang H, Lang F, Yun CC. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am J Physiol Cell Physiol 292: C396–C404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinman EJ, Steplock D, Shenolikar S. NHERF-1 uniquely transduces the cAMP signals that inhibit sodium-hydrogen exchange in mouse renal apical membranes. FEBS Lett 536: 141–144, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921–G927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Yun CH, Gurubhagavatula S, Levine SA, Montgomery JL, Brant SR, Cohen ME, Cragoe EJ, Jr, Pouyssegur J, Tse CM, Donowitz M. Glucocorticoid stimulation of ileal Na+ absorptive cell brush border Na+/H+ exchange and association with an increase in message for NHE3, an epithelial Na+/H+ exchanger isoform. J Biol Chem 268: 206–211, 1993 [PubMed] [Google Scholar]
- 43. Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na+/H+ exchanger NHE3 by cAMP. Role of protein kinase a and NHE3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999 [DOI] [PubMed] [Google Scholar]