Abstract
Gastroesophageal reflux disease (GERD) is often associated with decreased upper gastrointestinal motility, and ghrelin is an appetite-stimulating hormone known to increase gastrointestinal motility. We investigated whether ghrelin signaling is impaired in rats with GERD and studied its involvement in upper gastrointestinal motility. GERD was induced surgically in Wistar rats. Rats were injected intravenously with ghrelin (3 nmol/rat), after which gastric emptying, food intake, gastroduodenal motility, and growth hormone (GH) release were investigated. Furthermore, plasma ghrelin levels and the expression of ghrelin-related genes in the stomach and hypothalamus were examined. In addition, we administered ghrelin to GERD rats treated with rikkunshito, a Kampo medicine, and examined its effects on gastroduodenal motility. GERD rats showed a considerable decrease in gastric emptying, food intake, and antral motility. Ghrelin administration significantly increased gastric emptying, food intake, and antral and duodenal motility in sham-operated rats, but not in GERD rats. The effect of ghrelin on GH release was also attenuated in GERD rats, which had significantly increased plasma ghrelin levels and expression of orexigenic neuropeptide Y/agouti-related peptide mRNA in the hypothalamus. The number of ghrelin-positive cells in the gastric body decreased in GERD rats, but the expression of gastric preproghrelin and GH secretagogue receptor mRNA was not affected. However, when ghrelin was exogenously administered to GERD rats treated with rikkunshito, a significant increase in antral motility was observed. These results suggest that gastrointestinal dysmotility is associated with impaired ghrelin signaling in GERD rats and that rikkunshito restores gastrointestinal motility by improving the ghrelin response.
Keywords: gastric emptying, food intake, growth hormone, rikkunshito
gastroesophageal reflux disease (GERD) is a condition in which regurgitation of gastric acid and other gastric contents into the esophagus causes discomfort and complications (49). Decreased the lower esophageal sphincter function and esophageal clearance are involved in impairment of the esophageal mucosa due to gastric acid (4), and proton pump inhibitors, which suppress gastric acid secretion, have been the drugs of choice for the treatment of GERD. Delayed gastric emptying occurs frequently among patients with acid reflux (3, 31), and treatment with prokinetic agents, which are agonists of the 5-hydroxytryptamine 4 (5-HT4) receptor, partially improves symptoms in patients with GERD (16, 39). Thus it was concluded that gastric dysmotility is also a factor involved in acid reflux into the esophagus (3, 10, 29, 31). However, the reasons for gastric dysmotility remain unclear.
Ghrelin is a 28-amino-acid endogenous ligand of the growth hormone secretagogue receptor (GHS-R) and is primarily secreted from gastric endocrine cells (8, 26). In addition to its secretagogue action on growth hormone (GH), ghrelin is known to have a strong orexigenic effect (26, 34) and has been reported to enhance gastrointestinal motility (12, 20, 30). In vitro, ghrelin contracts muscle strips of rat forestomach and antrum (7, 15). Ghrelin administration induces dose-dependent phase III-like contractions in the antrum and increases the motility index (MI) in rodents (11, 45, 52) and humans (42). In addition, treatment with ghrelin and GH-releasing peptide-6, which is an agonist of ghrelin receptors, improves delayed gastric emptying in mice administered with cisplatin (28) and diabetic mice with gastroparesis (37). These effects of ghrelin are believed to act on the central nervous system via the afferent vagus nerve and promote gastrointestinal motility through an efferent pathway (1, 9, 30). However, whether impaired ghrelin signaling is involved in gastrointestinal dysmotility in GERD remains unknown.
Rikkunshito is a Kampo medicine (traditional Japanese medicine) that is widely prescribed to patients with various gastrointestinal symptoms. Clinically, it has been reported that rikkunshito improves gastrointestinal complaints related to functional dyspepsia (47, 51), and its efficacy has been proven in a multicenter double-blind study (17). Recently, it has also been reported that rikkunshito leads to a fundamental and clinical reduction in esophageal acid reflux and related symptoms in patients with GERD (21–23, 32). In addition, we and others have found that rikkunshito promotes ghrelin secretion in the stomach (13, 44) and increases ghrelin receptor sensitivity (14).
We hypothesized that impaired ghrelin signaling is involved in gastrointestinal dysmotility in GERD. To examine this hypothesis, we investigated endogenous ghrelin levels and the effects of exogenously administered ghrelin in an experimental model of GERD. Furthermore, we also examined whether rikkunshito improves ghrelin signaling in this GERD model.
MATERIALS AND METHODS
All experiments were performed in accordance with the protocols approved by the Animal Experiments Review Board of Tsumura (approved protocol No. 09-22, 10-047, 11-010).
Animals.
Eight-week-old male Wistar rats were obtained from CLEA Japan (Tokyo, Japan) and maintained under stable conditions of temperature and humidity and a 12-h:12-h dark/light cycle (7 AM to 7 PM). Rats had access to food and water ad libitum and were housed in groups of four or five. All animal experiments were performed between 9 AM and 6 PM. To avoid the influence of diurnal variations, feeding was stopped 24 h before the experiment, and blood and tissue samples were collected between 1 PM and 4 PM. During intravenous administration through the tail vein, we held the conscious rat lightly with a rat holder and warmed the tail in hot water. The test substance was administered slowly using 26-gauge needles to cause less stress. This procedure was concluded within 1 min.
Chemicals.
Rat ghrelin was obtained from the Peptide Institute (Osaka, Japan) and was dissolved in 0.9% sterilized physiological saline (Otsuka Pharmaceutical, Tokyo, Japan). Cisapride (an agonist of the 5-HT4 receptor) was obtained from Sigma-Aldrich (St. Louis, MO) and was suspended in 0.1% carboxymethyl cellulose (Maruishi Pharmaceutical, Osaka, Japan). Rikkunshito (Tsumura, Tokyo, Japan) was made from a hot-water extract of a mixture of eight varieties of crude drugs: sojutsu (Atractylodis lanceae rhizoma), ninjin (Ginseng radix), hange (Pinelliae tuber), bukuryo (Hoelen), taiso (Zizyphi fructus), chinpi (Aurantii nobilis pericarpium), kanzo (Glycyrrhizae radix), and shokyo (Zingiberis rhizoma), which was then spray dried. Rikkunshito was suspended in distilled water (DW) at a concentration of 1.2% wt/vol and ingested ad libitum by GERD rats for 10 days (32). The approximate daily intake of rikkunshito was 1,000 mg/kg.
Preparation of the GERD model.
GERD was surgically induced in rats according to the method of Omura et al. (36, 40). In brief, rats were first anesthetized with ether after 24 h of fasting, and then laparotomy was then performed at the midline. The stomach and duodenum were exposed extracorporeally, and a transitional (boundary) section from the forestomach to the glandular stomach was ligated using 1–0 silk thread (Natsume Seisakusho, Tokyo, Japan). The duodenal side of the pylorus was covered with a 2-mm-wide 18-Fr nelaton catheter (Terumo, Tokyo, Japan) that was sutured and fixed to the surface of the duodenal serous membrane using a 5–0 nylon yarn. The stomach and duodenum were returned into the abdominal cavity, which was then closed. Sham-operated rats were first laparotomized to expose their stomach and duodenum for about 1 min, after which their abdominal cavities were closed. After surgery, rats were fasted for a further 24 h (resulting in a total fasting of 48 h). Sham-operated and GERD rats were given food ad libitum from the day after surgery, and the pair-fed sham-operated rats were given the same amount of food as that consumed by GERD rats on the day before to examine the influence of nutritional decline.
Measurement of gastric emptying.
This experiment was performed 10 days after GERD induction (10 AM to 3 PM). Gastric emptying was assessed according to a previously reported method (33). Sham-operated and GERD rats were orally administered 0.5 ml of the test meal prepared by mixing standard powdered chow (Oriental Yeast, Tokyo, Japan) and glass beads (0.2-mm diameter, BZ-02; AS ONE, Osaka, Japan) using Teflon tubes (internal diameter, 1.68 mm) connected to a 2.5-ml syringe with a 10-Fr nelaton catheter 24 h after fasting. The test meal was prepared by mixing 16 g of powdered chow, 20 g of glass beads, and 40 ml of DW. The glass beads were added to enhance the solidity of the meal. The rats were decapitated 2 h or immediately after administration of the test meal to measure gastric emptying. The stomach was removed after incising the abdomen along the median line and ligating the pylorus and cardia. The stomach contents were collected in a 50-ml tube with DW and then centrifuged. We measured the weight of the sediments after drying overnight at 45°C. Gastric emptying was calculated according to the following formula: gastric emptying (%) = (1 − A/B) × 100, where A represents the dry weight of the test meal recovered from the stomach 2 h after its administration and B represents the average value of the dry weight of the test meal recovered from the stomach immediately after its administration. Cisapride (20 mg/kg) or vehicle was administered orally 1 h before test meal administration to elucidate the effects of prokinetic agents in GERD rats.
To examine the effects of exogenous ghrelin on gastric emptying in sham-operated and GERD rats, rat ghrelin (3 or 10 nmol/rat) or saline was injected intravenously via the tail vein immediately after test meal administration and gastric emptying was measured 1 h later. In addition, GERD rats were administered DW containing rikkunshito (1,000 mg/kg) ad libitum for 10 days after GERD induction, whereas the control group was administered DW alone. Next, rats were intravenously administered 1 nmol/rat ghrelin or saline, and gastric emptying was measured 1 h later.
Measurement of food intake.
Rats were housed separately after GERD-inducing surgery, and 24-h cumulative food intake was measured on day 10. To examine the effects of exogenous ghrelin on food intake of sham-operated and GERD rats, rat ghrelin (3 nmol/rat) or saline was injected intravenously via the tail vein and food intake was measured 1 h later. Food intake was calculated as the difference between the preprandial and postprandial weight of the food. The experiment was conducted between 10 AM and 12 PM under fed conditions 10 days after GERD induction.
Fixation of a strain gauge force transducer.
After 24 h of fasting, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (Kyoritsu Seiyaku, Tokyo, Japan). During GERD-inducing surgery, laparotomy was performed at the midline. Thereafter, a strain gauge force transducer (F-08IS; Star Medical, Tokyo, Japan) was sutured to the surface of the antral and duodenal serous membrane to measure circular muscle contractions. In addition, a catheter filled with heparin in physiological saline was fixed to the jugular vein. After surgery, rats were housed separately and fasted for a further 24 h (resulting in a total fasting of 48 h).
Analysis of gastroduodenal motility.
After GERD induction, rikkunshito (1,000 mg/kg) was administered to rats ad libitum for 10 days. On day 10 (9 AM to 3 PM), after 24 h of fasting, gastroduodenal motility was measured in conscious, freely moving rats. The control groups of sham-operated and GERD rats were given DW only. The transducer was connected to a preamplifier (FS-04M; Star Medical) via a bridge box (FB-01; Star Medical). Data were recorded using an MP150 (Biopac Systems, Aero Camino Goleta, CA) and analyzed using AcqKnowledge (Biopac Systems). To observe phase III-like contractions, gastroduodenal motility was monitored for 2–3 h.
To examine the effects of exogenous ghrelin on gastroduodenal motility in sham-operated and GERD rats, rat ghrelin (3 nmol/rat) or saline was administered through the jugular vein catheter. The effects of the drug were evaluated by frequency of phase III-like contractions and changes in MI before and after its administration.
Phase III-like contractions were defined as the existence of at least three potent contractions (>1/3 of maximum contraction magnitude) of short duration (<5 min). MI was defined as the mean of the area under the contractility recording curve per minute. The definition was slightly modified from a previous report (46).
Measurement of plasma ghrelin and GH levels.
Ten days after GERD induction (1 PM to 4 PM), blood was taken after decapitation in a tube containing aprotinin (Wako Pure Chemical Industries, Osaka, Japan) and EDTA-2Na (Dojindo Laboratories, Kumamoto, Japan) to obtain plasma samples as reported previously (44). Blood samples were immediately centrifuged at 4°C. Plasma was acidified with 1 mol/l HCl (1/10 volume) and stored at −80°C until measurement. Plasma ghrelin levels were measured using the Active- and Desacyl-Ghrelin ELISA Kit (Mitsubishi Chemical Medience, Tokyo, Japan). Plasma GH levels were measured using the Rat/Mouse GH ELISA Kit (Millipore, Billerica, MA) using nonacidified plasma samples. To examine the effects of exogenous ghrelin on GH release in sham-operated and GERD rats, rat ghrelin (3 nmol/rat) or saline was injected intravenously via the tail vein, and plasma GH levels were measured at 0, 10, 30, and 60 min.
Immunohistochemical studies.
The stomachs of 24-h-fasted rats were excised 10 days after GERD induction (1 PM to 4 PM). A part of the gastric body was fixed for 2 days in a 10% formalin/phosphoric acid buffer solution. A 1.5-cm section was excised along the proximal to distal stomach, paraffin embedded, and immunohistochemically stained. Methanol containing 3% hydrogen peroxide was used to inhibit endogenous peroxidase activity. After wash with PBS, paraffin sections were incubated with polyclonal anti-ghrelin antibody (Trans Genic, Kumamoto, Japan) diluted to 1:500 for 90 min. The sections were then washed with PBS and incubated with the Immunohistochemical Staining Kit (MAX-PO; Nichirei Biosciences, Tokyo, Japan) for 20 min, washed again with PBS, and then incubated with a 3,3′-diaminobenzidine solution. The total number of ghrelin-positive cells in this section of the gastric body was counted and expressed as the number of ghrelin-positive cells per millimeter of mucosa in longitudinal sections.
RNA extraction, reverse transcription, and qPCR analysis.
Gastric body and hypothalamus were excised 10 days after GERD induction (1 PM to 4 PM) and placed in a tube on dry ice for freezing. Once completely frozen, tubes were stored at −80°C. The tissue was homogenized, and total RNA was extracted using the RNeasy Universal Tissue Kit (Qiagen, Valencia, CA). Total RNA from each sample was diluted to 100 ng/μl, allowed to react for 5 min at 70°C, and finally cooled on ice. An aliquot of 1 μg of total RNA was reverse transcribed using the TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. qPCR analysis was performed with the PRISM 7900HT Sequence Detection System (Applied Biosystems) using the TaqMan Universal PCR Master Mix (Applied Biosystems). To compensate for the differences in the amount of total RNA added to each reaction, mRNA expression was normalized with β-actin as an endogenous control and expressed by the Δ threshold cycle (ΔCt) value: ΔCt = 2(−|A − B|), where A is the number of cycles that reached the β-actin gene threshold and B is the number of cycles that reached the target gene threshold. The set of oligonucleotide primers and fluorescent probes used in the TaqMan quantitative PCR were provide by Applied Biosystems: cytoplasmic β-actin: Rn00667869_m1; preproghrelin: Rn00572319_m1; GHS-R: Rn00821417_m1; neuropeptide Y (NPY): Rn00561681_m1; and agouti-related protein (AgRP): Rn01431703_g1.
Statistical analysis.
Statistical significance was examined using the Student's t-test or Aspin-Welch t-test after the F test or by Dunnett's analysis after one-way ANOVA. Data are expressed as the means ± SE of each group, and P < 0.05 was considered to be significant.
RESULTS
Gastric emptying was delayed and food intake was decreased in GERD rats.
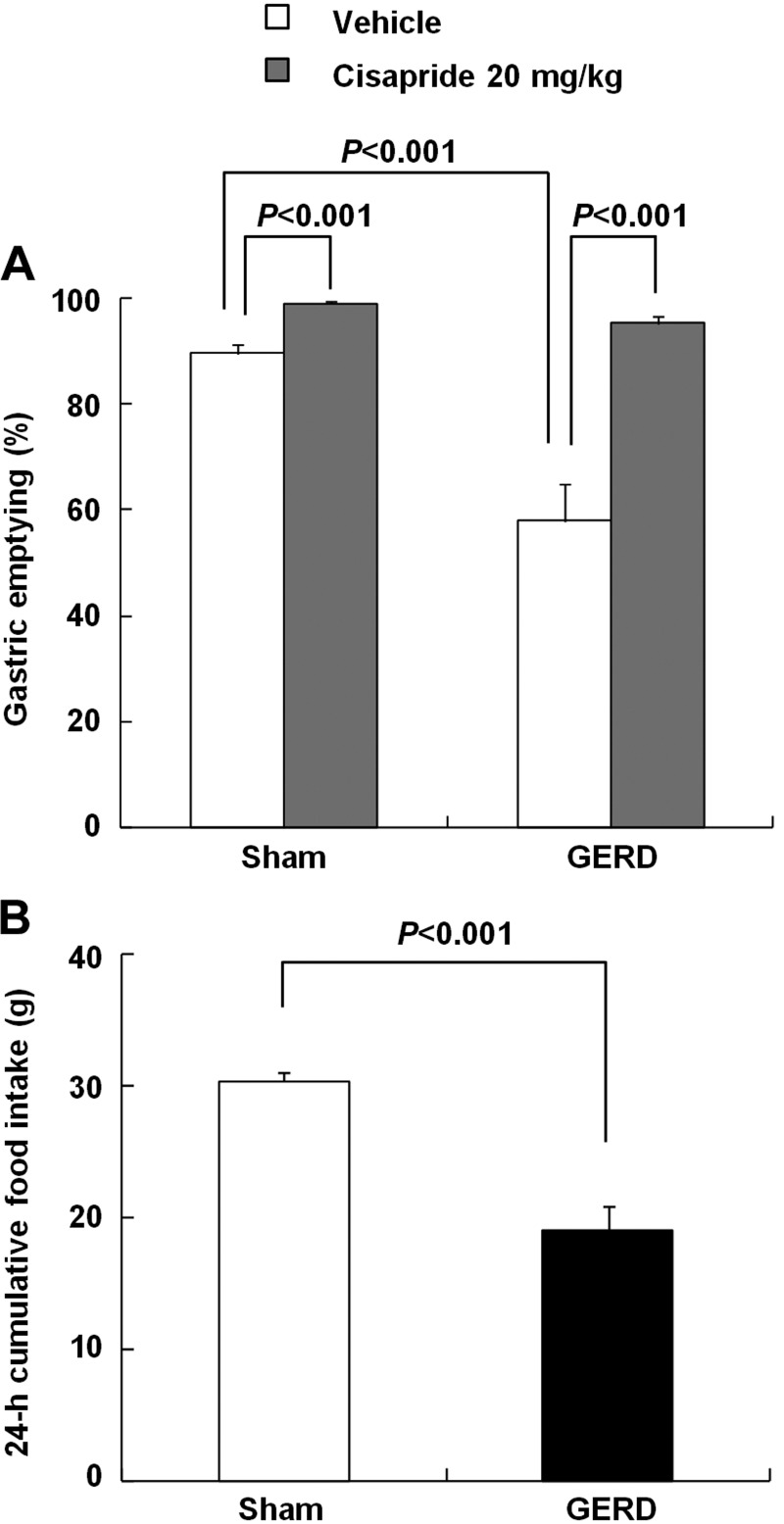
The body weight of GERD rats after 24 h of fasting decreased significantly compared with that of sham-operated rats (sham-operated, 247.3 ± 2.0 vs. GERD, 202.4 ± 4.7 g; P < 0.001). Gastric emptying in vehicle-treated GERD rats was significantly delayed compared with that in vehicle-treated sham-operated rats 2 h after test meal administration (Fig. 1A). The prokinetic agent cisapride (20 mg/kg) significantly accelerated gastric emptying in sham-operated rats. In addition, cisapride improved the delayed gastric emptying in GERD rats. Twenty-four-hour cumulative food intake of GERD rats was significantly decreased compared with sham-operated rats (Fig. 1B).
Fig. 1.
Gastric emptying and food intake in sham-operated and gastroesophageal reflux disease(GERD) rats. A: gastric emptying measured 2 h after administration of 0.5-ml test meal. Cisapride (20 mg/kg) or vehicle was administered orally 1 h before test meal administration. B: 24-h cumulative food intake under free-feeding conditions. Results are expressed as means ± SE (n = 6–16).
Interdigestive antral motility was decreased in GERD rats.
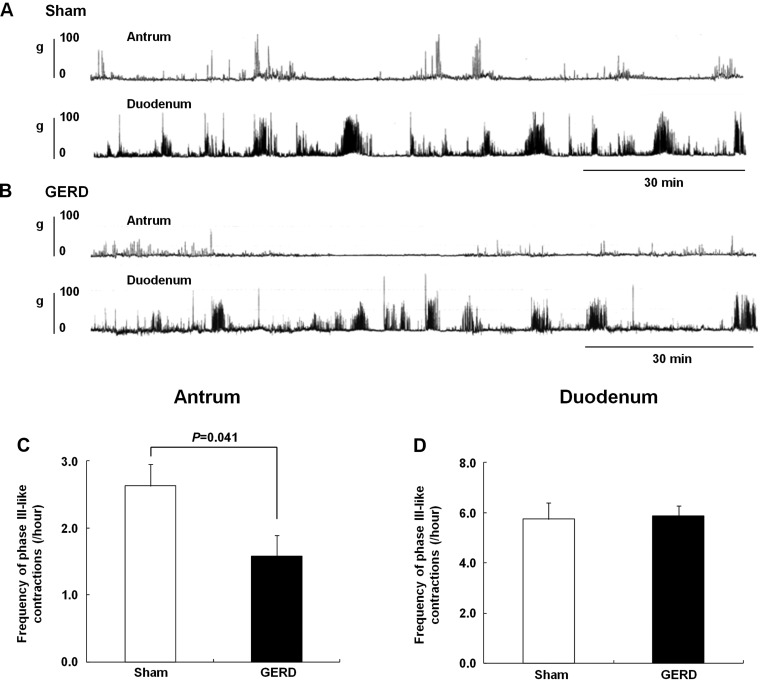
Contractions were observed in the antrum and duodenum at regular intervals in sham-operated and GERD rats (Fig. 2, A and B) although the frequency of phase III-like contractions in the antrum was significantly decreased in GERD rats compared with that in sham-operated rats (Fig. 2C). However, no difference in phase III-like contractions was observed in the duodenum between both groups (Fig. 2D).
Fig. 2.
Fasted gastroduodenal motility in sham-operated and GERD rats. A and B: antral and duodenal motor patterns detected by a strain gauge force transducer in sham-operated and GERD rats, respectively. C and D: frequency of phase III-like contractions in the antrum and duodenum. Results are expressed as means ± SE (n = 6–8).
Plasma ghrelin levels increased and the number of gastric ghrelin-positive cells decreased in GERD rats.
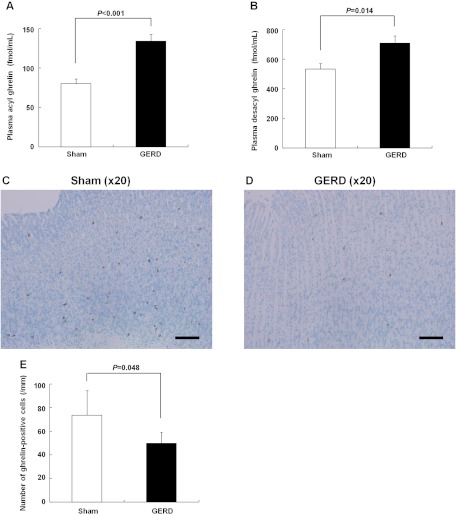
Plasma acyl and desacyl ghrelin levels significantly increased in GERD rats compared with those in sham-operated rats (Fig. 3, A and B). We examined the influence of nutritional decline on plasma ghrelin levels. Although the body weight was approximately the same (P = 0.19) between GERD (202.4 ± 4.7 g) and pair-fed sham-operated rats (209.6 ± 2.1 g), plasma ghrelin levels in GERD rats (acyl ghrelin, 133.8 ± 9.0 fmol/ml; desacyl ghrelin, 709.0 ± 49.5 fmol/ml) increased significantly compared with those in pair-fed sham-operated rats (acyl ghrelin, 86.5 ± 7.7 fmol/ml; P = 0.001; desacyl ghrelin, 423.5 ± 40.7 fmol/ml, P < 0.001). No difference in plasma GH levels was observed (sham-operated, 2.4 ± 1.2 vs. GERD, 4.7 ± 2.8 ng/ml; P = 0.54). The number of ghrelin-positive cells in the gastric body in GERD rats significantly decreased compared with that in sham-operated rats (Fig. 3, C–E).
Fig. 3.
Plasma ghrelin levels and number of gastric ghrelin-positive cells in sham-operated and GERD rats. A and B: plasma acyl and desacyl ghrelin levels. C and D: ghrelin-positive cells in the gastric body of sham-operated and GERD rats (scale bars = 100 μm). E: number of ghrelin-positive cells per millimeter of mucosa in longitudinal sections. Results are expressed as means ± SE (n = 5–8).
Expression of orexigenic genes in the hypothalamus increased in GERD rats.
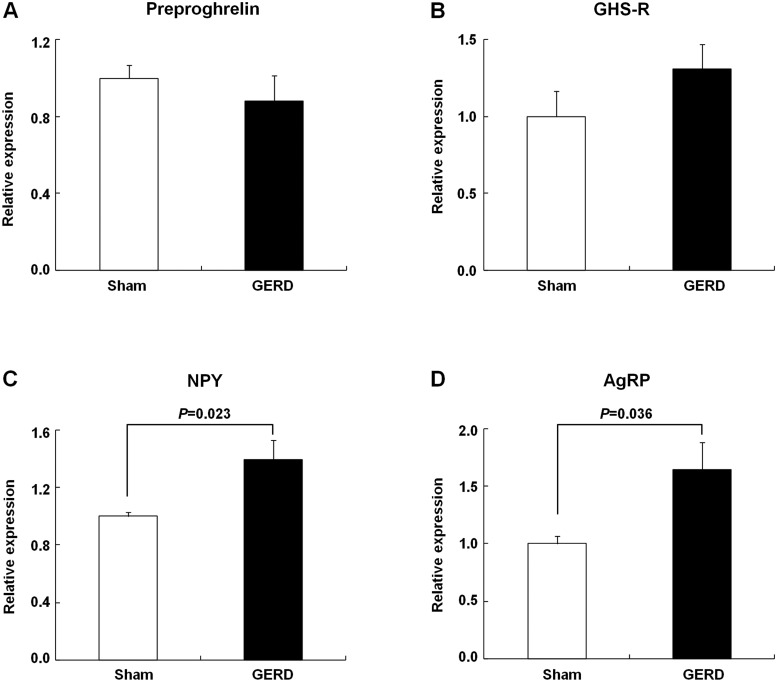
The expression of preproghrelin and GHS-R genes in the stomach of GERD rats did not differ from that of sham-operated rats (Fig. 4, A and B). In contrast, the expression of NPY and AgRP genes, which are known to be orexigenic in the hypothalamus, significantly increased in GERD rats compared with that in sham-operated rats (Fig. 4, C and D).
Fig. 4.
Gastric and hypothalamic gene expression in sham-operated and GERD rats. A and B: expression of gastric preproghrelin and growth hormone secretagogue receptor (GHS-R) mRNA. C and D: expression of hypothalamic neuropeptide Y (NPY) and agouti-related protein (AgRP) mRNA. Results are expressed as means ± SE (n = 4–8).
Ghrelin administration did not improve gastric emptying, food intake, and GH release in GERD rats.
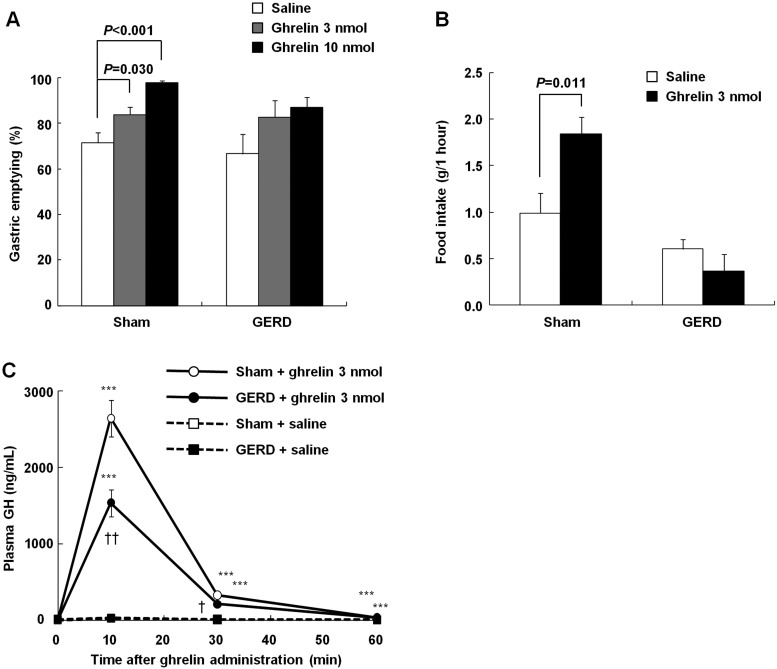
Ghrelin administration significantly accelerated gastric emptying in sham-operated rats 1 h after test meal administration in a dose-dependent manner (Fig. 5A). Gastric emptying in GERD rats 1 h after test meal administration showed a trend toward acceleration following administration of 3 or 10 nmol/rat ghrelin. Food intake in sham-operated rats after ghrelin administration (3 nmol/rat) was significantly higher than that after saline administration, but no significant difference was observed between GERD rats administered saline and ghrelin (3 nmol/rat) (Fig. 5B). In addition, plasma GH levels reached a peak 10 min after ghrelin administration (3 nmol/rat) in both sham-operated and GERD rats, but the peak level of GH in GERD rats was significantly lower than that in sham-operated rats (Fig. 5C).
Fig. 5.
The effects of exogenous ghrelin on gastric emptying, food intake, and GH release in sham-operated and GERD rats. A: effects of exogenous ghrelin administration (3 or 10 nmol/rat, intravenously) on gastric emptying 1 h after test meal administration. B: effects of exogenous ghrelin administration (3 nmol/rat, intravenously) on food intake under free-feeding conditions. C: effects of exogenous ghrelin administration (3 nmol/rat, intravenously) on GH release. Results are expressed as means ± SE (n = 5–8). ***P < 0.001 vs. each group of rats administered saline. †,††P < 0.05, 0.01 vs. sham-operated rats administered ghrelin.
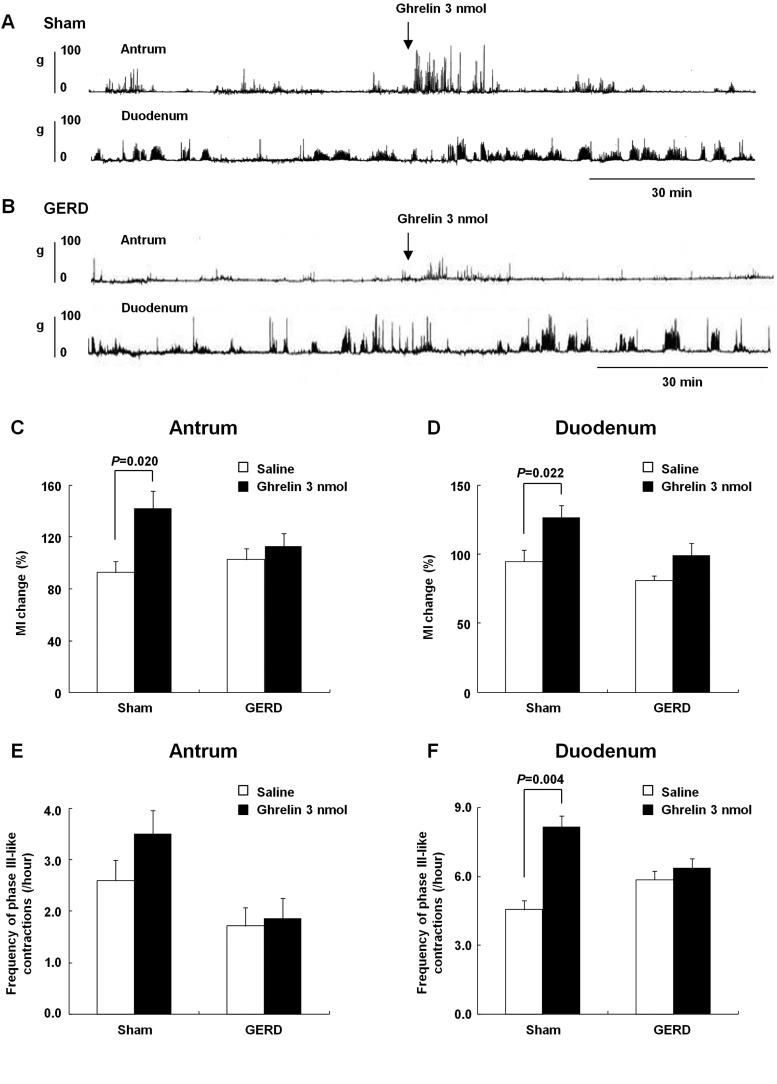
Ghrelin administration did not affect gastroduodenal motility in GERD rats.
Figure 6, A and B, shows gastroduodenal motility patterns in sham-operated and GERD rats administered ghrelin (3 nmol/rat). In sham-operated rats, the change in MI in both the antrum and duodenum significantly increased after ghrelin administration (Fig. 6, C and D). However, in GERD rats, the change in MI in the antrum and duodenum showed no difference after ghrelin and saline administration. In the antrum, the frequency of phase III-like contractions did not differ between sham-operated and GERD rats (Fig. 6E). In the duodenum, the frequency of phase III-like contractions significantly increased after ghrelin administration in sham-operated rats, but not in GERD rats (Fig. 6F).
Fig. 6.
Effects of exogenous ghrelin on fasted gastroduodenal motility in sham-operated and GERD rats. A and B: antral and duodenal motor patterns detected by a strain gauge force transducer in sham-operated and GERD rats. C and D: effects of exogenous ghrelin administration (3 nmol/rat, intravenously) on the change in motility index (MI) in the antrum and duodenum. E and F: effects of exogenous ghrelin administration (3 nmol/rat, intravenously) on the frequency of phase III-like contractions in the antrum and duodenum. Results are expressed as means ± SE (n = 5–10).
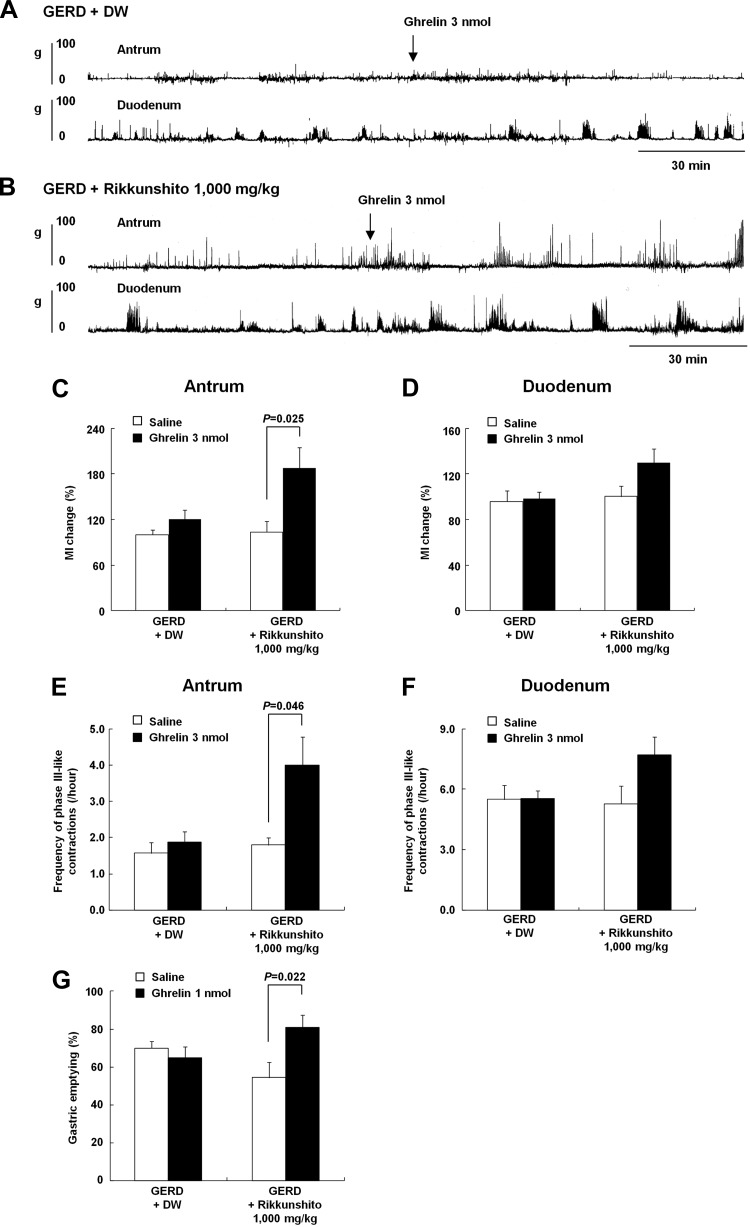
Rikkunshito administration restored impaired ghrelin response on gastroduodenal motility in GERD rats.
Figure 7, A and B show gastroduodenal motility patterns in GERD rats administered DW or rikkunshito for 10 days after GERD induction and intravenous injection of ghrelin via the jugular vein catheter (3 nmol/rat). Before ghrelin administration, the frequency of phase III-like contractions in the antrum of GERD rats administered rikkunshito significantly increased compared with that in the antrum of GERD rats administered DW (DW administration, 1.6 ± 0.1 vs. rikkunshito administration, 2.1 ± 0.2 count/h; P = 0.035). There was no difference in the frequency in the duodenum between both groups (DW administration, 5.4 ± 0.5 vs. rikkunshito administration, 5.7 ± 1.0 count/h; P = 0.78). After ghrelin administration, there was no change in MI in the antrum and duodenum of GERD rats administered DW. However, a significant increase in the change in MI in the antrum (Fig. 7C) and an increasing trend in the same in the duodenum of GERD rats administered rikkunshito (P = 0.074 vs. saline administration; Fig. 7D). Similarly, there was a significant increase in the frequency of phase III-like contractions in the antrum (Fig. 7E) and an increasing trend in the same in the duodenum of GERD rats administered rikkunshito (P = 0.074 vs. saline administration; Fig. 7F).
Fig. 7.
The effects of exogenous ghrelin on fasted gastroduodenal motility in GERD rats administered distilled water (DW) or rikkunshito (1,000 mg/kg) for 10 days. A and B: antral and duodenal motor patterns detected by a strain gauge force transducer in GERD rats administered DW or rikkunshito. C and D: combined effects of exogenous ghrelin administration (3 nmol/rat, intravenously) and rikkunshito on the change in MI in the antrum and duodenum. E and F: combined effects of exogenous ghrelin administration (3 nmol/rat, intravenously) and rikkunshito on the frequency of phase III-like contractions in the antrum and duodenum. G: combined effects of exogenous ghrelin administration (1 nmol/rat, intravenously) and rikkunshito on gastric emptying. Results are expressed as means ± SE (n = 5–12).
The food intake of GERD rats administered rikkunshito did not differ from that of GERD rats administered DW (9 days after GERD induction: DW administration, 19.9 ± 1.2 vs. rikkunshito administration, 17.9 ± 0.9 g/day; P = 0.24). Ten days after GERD induction, the effect of ghrelin administration on gastric emptying in GERD rats administered rikkunshito was examined. In GERD rats administered DW, ghrelin administration had no effect on gastric emptying (Fig. 7G). However, ghrelin administration significantly increased gastric emptying compared with saline administration in GERD rats administered rikkunshito.
DISCUSSION
In this study, we focused on the association between peripheral ghrelin and GERD. We demonstrated that 1) GERD rats showed decreased gastric emptying, food intake, and antral motility and increased peripheral ghrelin levels and 2) ghrelin administration to GERD rats did not improve decreased gastric emptying, food intake, and antral motility, whereas rikkunshito administration improved decreased antral motility.
Although decreased gastric emptying has already been reported in patients with GERD (3, 31), its mechanism remains unclear. However, treatment with prokinetic agents has been shown to improve several symptoms in patients with GERD (16, 39), and we have recently demonstrated that cisapride improves the motility proximal to the lower esophageal sphincter in GERD rats (40). These findings suggest that gastrointestinal dysmotility is involved in GERD progression. In the present study, cisapride administration had a significant inhibitory effect on delayed gastric emptying in GERD rats, suggesting that motility in the upper gastrointestinal tract in such rats is regulated by 5-HT4 signaling. At present, no study on changes in antral motility of GERD rats has been reported. We found that antral motility significantly decreased in GERD rats; however, no difference was observed in duodenal motility. These results suggest that decreased antral motility is one of the causes of delayed gastric emptying in GERD rats.
Interdigestive contractions in the antrum are regulated in part by peripheral acyl ghrelin (13). Increased circulating acyl ghrelin levels have been shown to induce phase III-like contractions in the antrum. In the present study, plasma acyl and desacyl ghrelin levels significantly increased in GERD rats; however, the number of ghrelin-positive cells in the stomach significantly decreased. Moreover, the expression of the preproghrelin gene in the stomach of GERD rats was not affected. These results suggest that higher plasma ghrelin levels observed in GERD rats is not due to an increase in the synthesis of ghrelin in the stomach but due to enhanced secretion of ghrelin into the circulation from the stomach, resulting in a decrease in stored ghrelin in X/A-like cells, which is consistent with our observation that the number of ghrelin-positive cells decreased in GERD rats.
In rats, serum ghrelin levels increased with fasting and decreased with refeeding and administration of glucose (48). Furthermore, serum or plasma ghrelin levels are inversely proportional to body mass index in humans (19, 35). In this study, the body weight of GERD rats significantly decreased compared with that of sham-operated rats. Therefore, it was suggested that nutritional status may have influenced plasma ghrelin levels. However, the pair-fed sham-operated rats that were controlled in order that they would consume the same amount of food as that consumed by GERD rats showed plasma ghrelin levels similar to those in sham-operated rats. It has been reported that plasma ghrelin levels in rats with food intake restricted by 25% and 50% that were measured 15 days after the initiation of restriction did not differ from rats with ad libitum food intake (27). Therefore, it has been suggested that the increased plasma ghrelin levels in GERD rats may have not resulted solely from reduced food intake.
In the present study, although plasma ghrelin levels increased in GERD rats, decreased phase III-like contractions were observed in the antrum due of delayed gastric emptying and decreased food intake. Ghrelin administration increased gastric emptying, food intake, and the frequency of phase III-like contractions in the duodenum in sham-operated rats but did not improve delayed gastric emptying and decreased food intake in GERD rats. In this study, sham-operated and GERD rats were exogenously administered 3 nmol/rat ghrelin. Gastric emptying in the vehicle group of GERD rats varied significantly. The number of animals in each group was seven. Variations in the pathological conditions may have had an effect on gastric emptying. Although GERD rats were administered a higher dosage of ghrelin than sham-operated rats when converted into kilogram of body weigh (sham-operated, 12.1 nmol/kg; GERD, 14.8 nmol/kg), no significant improvements were observed in gastric emptying. In addition, although the frequency of phase III-like contractions in the duodenum of GERD rats was similar to that in the duodenum of sham-operated rats, ghrelin administration did not increase their frequency in the duodenum. These results suggest that ghrelin response is attenuated in GERD rats.
The expression of genes that encode the orexigenic hypothalamic neuropeptides NPY and AgRP significantly increased in GERD rats than that in sham-operated rats, whereas that of GHS-R in the stomach remained unchanged. Peripheral ghrelin is known to transmit signals by binding to receptors on the vagus nerve that are axonally transported to the gastric mucosa and activate the NPY/AgRP neurons of the arcuate nucleus of the hypothalamus, and thus increase feeding behavior (1, 34) and gastrointestinal motility (12, 20). However, upregulation of these orexigenic neuropeptides did not increase appetite in GERD rats, suggesting that their downstream orexigenic signaling was impaired. NPY and AgRP synthesis or release in the hypothalamus may have been involved, as demonstrated by Scarlett et al. (41). In rat models of colitis and obstructive cholestasis, decreased feeding response to orexigenic peptides was observed even though NPY release was normal (2, 38). These reports indicate that the orexigenic neuronal pathway may have been impaired or that anorexigenic factors, such as interleukin-1β, may have been involved, but further investigations are needed.
In the present study, GH release was attenuated after ghrelin administration in GERD rats. In ghrelin transgenic mice, which exhibit constant high peripheral ghrelin levels, and in patients with anorexia nervosa, who exhibit high plasma ghrelin levels, ghrelin had a decreased effect on GH release (5, 50). The precise mechanism of the decreased response to ghrelin caused by hyperghrelinemia is unknown. Recently, it has been reported that stress responses inhibit the effects of ghrelin (6). We measured plasma corticosterone levels as an indicator of stress (data not shown) and found no difference between sham-operated and GERD rats. Therefore, ghrelin function may have been attenuated in GERD rats not because of stress hormones (e.g., corticotropin-releasing factor, urocortin). Although the attenuation of ghrelin function needs to be studied further, we suggest that it may be due to the desensitization of ghrelin receptors.
In GERD rats administered rikkunshito for 10 days, a significant increase in MI and frequency of phase III-like contractions in the antrum was observed compared with that in GERD rats administered DW. Rikkunshito has been shown to increase esophageal clearance in patients with GERD and improve their symptoms (22). It also significantly mitigates decreased voluntary movement, which is an index of pain, in GERD rats (32). In an in vitro study using GHS-R1a-overexpressing cells or NPY neurons, rikkunshito was reported to increase [I125]ghrelin binding and elevate the influx of Ca2+ continuously after ghrelin addition (14). From these findings, it appears that rikkunshito may improve endogenous ghrelin signal transduction in GERD rats.
In this study, rikkunshito treatment enhanced the decreased response to ghrelin in MI and frequency of phase III-like contractions in the antrum of GERD rats. Furthermore, although rikkunshito alone had no effect on decreased food intake and gastric emptying, delayed gastric emptying in GERD rats was significantly improved with the combined administration of rikkunshito and ghrelin (1 nmol/rat). These results suggest that rikkunshito may improve defective signaling of ghrelin and suppress the delay in gastric emptying.
It is known that ghrelin transmits signals to the afferent vagus nerve and passes through the central nervous system to induce gastrointestinal contractions through the efferent vagus nerve. In GERD rats, we speculate that the vagus nerves are impaired and may lead to decreased ghrelin response. Therefore, to determine whether the decreased response to ghrelin in GERD rats is due to decreased functionality of the ghrelin receptors, we investigated the effects of concomitant administration of rikkunshito and ghrelin on the antral and duodenal motility and gastric emptying. Ghrelin receptors are G protein-coupled receptors and mediate Ca2+ influx through the phospholipase C/protein kinase C/inositol triphosphate pathway (18) and adenylate cyclase/cyclic AMP/protein kinase A pathway (25). In AgRP/NPY neurons in the arcuate nucleus, ghrelin signaling is blocked by the action of phosphodiesterase type III and phosphatidylinositol 3-kinase, which decrease cyclic AMP (24). Rikkunshito contains heptamethoxyflavone and nobiletin, which have been shown to inhibit phosphodiesterase type III activity in vitro (43). From those findings, it was speculated that the increase in the frequency of spontaneous phase III-like contraction in the antrum and ghrelin response in GERD rats administered rikkunshito was due to the stimulation of ghrelin signaling by protein kinase A because of the prevention of cyclic AMP decomposition by rikkunshito, which inhibits phosphodiesterase type III activity. Therefore, it is possible that factors that decrease cyclic AMP may increase in the vagal nerve endings with ghrelin receptors in our GERD model.
In conclusion, impaired ghrelin signaling is involved in gastrointestinal dysmotility in GERD rats. Moreover, rikkunshito improves gastrointestinal motility by enhancing the decreased response to ghrelin. Improvement of ghrelin signaling may provide a new therapeutic approach for GERD.
GRANTS
This study was funded by Tsumura (H. Takeda).
DISCLOSURES
H. T. received grant support from Tsumura. M. N., C. S., Y. S., and T. H. are employed by Tsumura. S. M., N. O., S. O., K. N., and M. A. have nothing to declare.
AUTHOR CONTRIBUTIONS
Author contributions: M.N., S.M., S.O., K.N., C.S., Y.S., and T.H. performed experiments; M.N., S.M., S.O., K.N., C.S., Y.S., and T.H. analyzed data; M.N., S.M., S.O., K.N., C.S., Y.S., and T.H. interpreted results of experiments; M.N. prepared figures; M.N., S.O., C.S., Y.S., and T.H. drafted manuscript; M.N., S.M., N.O., S.O., K.N., C.S., Y.S., T.H., M.A., and H.T. approved final version of manuscript; N.O., M.A., and H.T. conception and design of research; H.T. edited and revised manuscript.
REFERENCES
- 1. Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120: 337–345, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Ballinger AB, Williams G, Corder R, El-Haj T, Farthing MJ. Role of hypothalamic neuropeptide Y and orexigenic peptides in anorexia associated with experimental colitis in the rat. Clin Sci (Lond) 100: 221–229, 2001 [PubMed] [Google Scholar]
- 3. Benini L, Sembenini C, Castellani G, Caliari S, Fioretta A, Vantini I. Gastric emptying and dyspeptic symptoms in patients with gastroesophageal reflux. Am J Gastroenterol 91: 1351–1354, 1996 [PubMed] [Google Scholar]
- 4. Boeckxstaens GE. Review article: the pathophysiology of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 26: 149–160, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Broglio F, Gianotti L, Destefanis S, Fassino S, Abbate Daga G, Mondelli V, Lanfranco F, Gottero C, Gauna C, Hofland L, Van der Lely AJ, Ghigo E. The endocrine response to acute ghrelin administration is blunted in patients with anorexia nervosa, a ghrelin hypersecretory state. Clin Endocrinol (Oxf) 60: 592–599, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Currie PJ, Coiro CD, Duenas R, Guss JL, Mirza A, Tal N. Urocortin I inhibits the effects of ghrelin and neuropeptide Y on feeding and energy substrate utilization. Brain Res 1385: 127–134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dass NB, Munonyara M, Bassil AK, Hervieu GJ, Osbourne S, Corcoran S, Morgan M, Sanger GJ. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience 120: 443–453, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120–1128, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Emerenziani S, Sifrim D. Gastroesophageal reflux and gastric emptying, revisited. Curr Gastroenterol Rep 7: 190–195, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fujimiya M, Asakawa A, Ataka K, Kato I, Inui A. Different effects of ghrelin, des-acyl ghrelin and obestatin on gastroduodenal motility in conscious rats. World J Gastroenterol 14: 6318–6326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 550: 227–240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, Fujimiya M, Inui A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry 65: 748–759, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, Yada T, Maejima Y, Sedbazar U, Sakai T, Hattori T, Kase Y, Inui A. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry 1: e23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, Omagari K, Taniyama K, Kohno S. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol 39: 1209–1214, 2004 [PubMed] [Google Scholar]
- 16. Gardner JD, Rodriguez-Stanley S, Robinson M, Miner PB., Jr Cisapride inhibits meal-stimulated gastric acid secretion and postprandial gastric acidity in subjects with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 16: 1819–1829, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hattori T, Fujitsuka N, Asakawa A, Inui A. A new strategy using Rikkunshito (Liu-Jun-Zi-Tang), a Japanese traditional medicine, to treat gastrointestinal disease. In: Basics of Evidences-Based Herbal Medicine, edited by Satoh H. Kerala: Research Signpost, 2010, p. 149–160 [Google Scholar]
- 18. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273: 974–977, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I, Benjamin EJ, Keaney JF, Jr, Vasan RS. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metab 93: 3149–3157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 18: 439–456, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Johnson DA, Levy BH., 3rd Evolving drugs in gastroesophageal reflux disease: pharmacologic treatment beyond proton pump inhibitors. Expert Opin Pharmacother 11: 1541–1548, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Kawahara H, Kubota A, Hasegawa T, Okuyama H, Ueno T, Ida S, Fukuzawa M. Effects of rikkunshito on the clinical symptoms and esophageal acid exposure in children with symptomatic gastroesophageal reflux. Pediatr Surg Int 23: 1001–1005, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kawahara H, Mitani Y, Nomura M, Nose K, Yoneda A, Hasegawa T, Kubota A, Fukuzawa M. Impact of rikkunshito, an herbal medicine, on delayed gastric emptying in profoundly handicapped patients. Pediatr Surg Int 25: 987–990, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology 148: 2251–2263, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun 366: 388–392, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Ling PR, Bistrian BR. Comparison of the effects of food versus protein restriction on selected nutritional and inflammatory markers in rats. Metabolism 58: 835–842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu YL, Malik NM, Sanger GJ, Andrews PL. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol 58: 326–333, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Maddern GJ, Chatterton BE, Collins PJ, Horowitz M, Shearman DJ, Jamieson GG. Solid and liquid gastric emptying in patients with gastro-oesophageal reflux. Br J Surg 72: 344–347, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276: 905–908, 2000 [DOI] [PubMed] [Google Scholar]
- 31. McCallum RW, Berkowitz DM, Lerner E. Gastric emptying in patients with gastroesophageal reflux. Gastroenterology 80: 285–291, 1981 [PubMed] [Google Scholar]
- 32. Miwa H, Koseki J, Oshima T, Kondo T, Tomita T, Watari J, Matsumoto T, Hattori T, Kubota K, Iizuka S. Rikkunshito, a traditional Japanese medicine, may relieve abdominal symptoms in rats with experimental esophagitis by improving the barrier function of epithelial cells in esophageal mucosa. J Gastroenterol 45: 478–487, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Mogami S, Suzuki H, Fukuhara S, Matsuzaki J, Kangawa K, Hibi T. Reduced ghrelin production induced anorexia after rat gastric ischemia and reperfusion. Am J Physiol Gastrointest Liver Physiol 302: G359–G364, 2012 [DOI] [PubMed] [Google Scholar]
- 34. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Nanjo Y, Adachi H, Hirai Y, Enomoto M, Fukami A, Otsuka M, Yoshikawa K, Yokoi K, Ogata K, Tsukagawa E, Kasahara A, Murayama K, Yasukawa H, Kojima M, Imaizumi T. Factors associated with plasma ghrelin level in Japanese general population. Clin Endocrinol (Oxf) 74: 453–458, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Omura N, Kashiwagi H, Chen G, Suzuki Y, Yano F, Aoki T. Establishment of surgically induced chronic acid reflux esophagitis in rats. Scand J Gastroenterol 34: 948–953, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Qiu WC, Wang ZG, Wang WG, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J Gastroenterol 14: 1419–1424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rioux KP, Le T, Swain MG. Decreased orexigenic response to neuropeptide Y in rats with obstructive cholestasis. Am J Physiol Gastrointest Liver Physiol 280: G449–G456, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Ruth M, Hamelin B, Rohss K, Lundell L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 12: 35–40, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Saegusa Y, Takeda H, Muto S, Oridate N, Nakagawa K, Sadakane C, Nahata M, Harada Y, Iizuka M, Hattori T, Asaka M. Decreased motility of the lower esophageal sphincter in a rat model of gastroesophageal reflux disease may be mediated by reductions of serotonin and acetylcholine signaling. Biol Pharm Bull 34: 704–711, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, Grant WF, Meguid MM, Cowley MA, Marks DL. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology 149: 4837–4845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 55: 327–333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takeda H, Muto S, Hattori T, Sadakane C, Tsuchiya K, Katsurada T, Ohkawara T, Oridate N, Asaka M. Rikkunshito ameliorates the aging-associated decrease in ghrelin receptor reactivity via phosphodiesterase III inhibition. Endocrinology 151: 244–252, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134: 2004–2013, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol 14: 6299–6302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol 285: R862–R872, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Tatsuta M, Iishi H. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther 7: 459–462, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101: 1900–1920; quiz 1943, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Wei W, Qi X, Reed J, Ceci J, Wang HQ, Wang G, Englander EW, Greeley GH., Jr Effect of chronic hyperghrelinemia on ingestive action of ghrelin. Am J Physiol Regul Integr Comp Physiol 290: R803–R808, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Yagi M, Homma S, Kubota M, Iinuma Y, Kanada S, Kinoshita Y, Ohtaki M, Yamazaki S, Murata H. The herbal medicine Rikkunshi-to stimulates and coordinates the gastric myoelectric activity in post-operative dyspeptic children after gastrointestinal surgery. Pediatr Surg Int 19: 760–765, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil 21: 78–84, 2009 [DOI] [PubMed] [Google Scholar]