Abstract
Endoplasmic reticulum (ER) stress has been implicated in the pathogenesis of nonalcoholic steatohepatitis. The ER stress response is activated in the livers of mice fed a methionine- and choline-deficient (MCD) diet, yet the role of ER stress in the pathogenesis of MCD diet-induced steatohepatitis is unknown. Using chemical chaperones on hepatic steatosis and markers of inflammation and fibrosis in mice fed a MCD diet, we aim to determine the effects of reducing ER stress. C57BL/6J mice were fed a MCD diet with or without the ER chemical chaperones 4-phenylbutyric acid (PBA) and tauroursodeoxycholic acid (TUDCA) for 2 wk. TUDCA and PBA effectively attenuated the ER stress response in MCD diet-fed mice, as evidenced by reduced protein levels of phosphorylated eukaryotic initiation factor 2α and phosphorylated JNK and suppression of mRNA levels of CCAAT/enhancer binding protein homologous protein, glucose-regulated protein 78 kDa, and X-box binding protein 1. However, PBA and TUDCA did not decrease MCD diet-induced hepatic steatosis. MCD diet-induced hepatic inflammation, as evidenced by increased plasma alanine aminotransferase and induction of hepatic TNFα expression, was also not reduced by PBA or TUDCA. PBA and TUDCA did not attenuate MCD diet-induced upregulation of the fibrosis-associated genes tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9. ER chemical chaperones reduce MCD diet-induced ER stress, yet they do not improve MCD diet-induced hepatic steatosis, inflammation, or activation of genes associated with fibrosis. These data suggest that although the ER stress response is activated by the MCD diet, it does not have a primary role in the pathogenesis of MCD diet-induced steatohepatitis.
Keywords: unfolded protein response, steatosis, 4-phenylbutyric acid, tauroursodeoxycholic acid, chemical chaperones
the prevalence of nonalcoholic fatty liver disease (NAFLD) is reaching epidemic proportions in the United States and is estimated to affect up to one-third of the US population (2). Cirrhosis resulting from NAFLD is expected to surpass hepatitis C as the leading indication for liver transplantation in the United States in the next 10–20 years (15). Unfortunately, there is no approved pharmacological therapy to prevent the development of hepatic steatosis or the progression to more advanced liver disease. The lack of an effective treatment is due, in part, to the poor understanding of the pathogenesis of this common disease.
Recent data indicate that endoplasmic reticulum (ER) stress may have an important role in the development and/or progression of NAFLD (7, 13, 18, 24). The ER functions to maintain protein homeostasis by regulating protein synthesis, folding, and processing. When normal ER function is disturbed, unfolded or misfolded proteins accumulate within the ER, triggering the unfolded protein response (UPR), also known as the ER stress response (29). The ER stress response is mediated through three ER transmembrane receptors, PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1α (IRE1α), and one master chaperone, glucose-regulated protein 78 kDa (GRP78/BiP). In the unstressed state, GRP78 is bound to these transmembrane receptors. However, when unfolded proteins accumulate in the ER, GRP78 preferentially binds to unfolded proteins, thereby releasing PERK, ATF6, and IRE1α. Release of GRP78 triggers a signaling cascade that initially aims to restore homeostasis and allow the cell to adapt to the cellular stressor; however, if homeostasis is not restored, pathways leading to apoptosis are initiated (10, 23).
Hepatic lipid accumulation induces ER stress, and, in turn, the ER stress response promotes hepatic lipogenesis, thus creating a positive-feedback loop, which may contribute to the development of hepatic steatosis (9, 11, 24). ER stress has been implicated not only in the development of hepatic steatosis, but also in the development of hepatocellular injury and fibrosis, which herald the progression of simple steatosis to nonalcoholic steatohepatitis (NASH). One of the commonly cited lines of evidence supporting the role of ER stress in the pathogenesis of NASH is the observation that mice fed a methionine- and choline-deficient (MCD) diet, a well-established murine model of steatohepatitis, demonstrate activation of the hepatic ER stress response. MCD diet-induced steatosis is thought to be due, in large part, to impaired hepatic triglyceride secretion (16, 28). However, the mechanisms underlying the development of steatohepatitis in MCD-fed mice remain incompletely understood. Whether ER stress promotes steatohepatitis in mice fed a MCD diet is unknown.
We aim to establish the role of ER stress in the pathogenesis of MCD diet-induced steatohepatitis in mice.
Tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (PBA) are chemical chaperones that have been shown to reduce ER stress by facilitating proper protein folding and trafficking (22, 26). Inhibition of ER stress with these chemical chaperones in a murine model of hepatic steatosis has been shown to reduce hepatic steatosis, suggesting that modulating the ER stress response may serve as a therapeutic strategy for NAFLD (12). In the present study, we will determine the effects of ER chemical chaperones on the development of hepatic steatosis, inflammation, and fibrosis in mice fed a MCD diet.
MATERIALS AND METHODS
Animals and diets.
Male C57BL/6J mice (8–10 wk of age) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were fed a MCD or methionine- and choline-sufficient (MCS) diet (MP Biomedical, Solon, OH) for 14 days. The MCS diet is identical to the MCD diet, except for the content of methionine and choline. MCD and MCS diet-fed mice were treated with PBA (Sigma-Aldrich, St. Louis, MO; 200 mg·kg−1·day−1 ip), TUDCA (Calbiochem, La Jolla, CA; 500 mg·kg−1·day−1 ip), or vehicle (sterile saline ip) daily during the feeding protocol. Mice were exposed to a 14:10-h light-dark cycle and were given free access to food and water. Mice were fasted for 4 h prior to euthanasia by CO2 inhalation. Blood was collected by cardiac puncture and centrifuged to yield the plasma. The liver was rapidly excised, weighed, and flushed with ice-cold saline. An aliquot was fixed in 10% formalin for histological analysis, which was performed at the Northwestern University Pathology Core (Chicago, IL). The remainder of the liver was sectioned and snap-frozen in liquid nitrogen. All animal protocols were approved by the Northwestern University Animal Care and Use Committee.
Liver and plasma chemistries.
Liver samples were homogenized in Dulbecco's phosphate-buffered saline for hepatic lipid analysis (100 mg liver tissue/ml). Triglyceride and cholesterol levels were measured in liver homogenate and fresh plasma using an Infinity spectrophotometric assay according to the manufacturer's protocol (Thermo Electron, Melbourne, Australia). Plasma alanine aminotransferase (ALT) was measured using a spectrophotometric assay according the manufacturer's protocol (Teco Diagnostics, Anaheim, CA).
Analysis of gene expression by real-time quantitative PCR.
Total RNA from frozen liver samples was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). A qScript cDNA synthesis kit (Quanta BioSciences, Gaithersburg, MD) was used to carry out RT-PCR with 2 μg of total RNA. Real-time quantitative PCR was performed using 2 μl of cDNA from each sample in a 25-μl reaction mixture containing Quantitect SYBR Green PCR Master Mix (Qiagen, Valencia, CA) along with primers specific for the gene of interest. GAPDH was employed as a housekeeping gene. Amplification was performed on an ABI 7300 sequence detector (Applied Biosystems, Foster City, CA). Gene expression was calculated relative to respective age- and sex-matched controls with use of the comparative threshold cycle method, as described in the Applied Biosystems sequence detection systems instruction guide.
Western blot analysis of protein expression.
Liver samples were homogenized in T-Per (Thermo Scientific, Rockford, IL) containing Halt phosphatase inhibitor (Thermo Scientific) and protease cocktail inhibitor (Calbiochem). Protein concentrations of homogenates were determined by the Bradford assay using Coomassie blue reagent (Thermo Scientific) and subsequently diluted with Laemmli buffer (Bio-Rad, Hercules, CA) containing β-mercaptoethanol to a standard concentration of 2 μg/μl and heated at 95°C for 5 min. Samples containing 50 μg of protein were separated on a 12% SDS-polyacrylamide gel by electrophoresis. Protein was then transferred to a nitrocellulose membrane by electrophoresis. Protein was detected using polyclonal rabbit antibodies to total and phosphorylated eukaryotic initiation factor 2α (eIF2α) and JNK (Cell Signaling Technology, Danvers, MA). Bound antibody was detected using goat anti-rabbit polyclonal horseradish peroxidase antibody (Santa Cruz Biotechnology) and developed using enhanced chemiluminescence Western blotting substrate (Thermo Scientific). Representative Western blots of pooled samples are shown.
Statistical analysis.
Values are means ± SD. Comparisons between groups were analyzed using Student's t-test.
RESULTS
Chemical chaperones inhibit the ER stress response in MCD diet-fed mice.
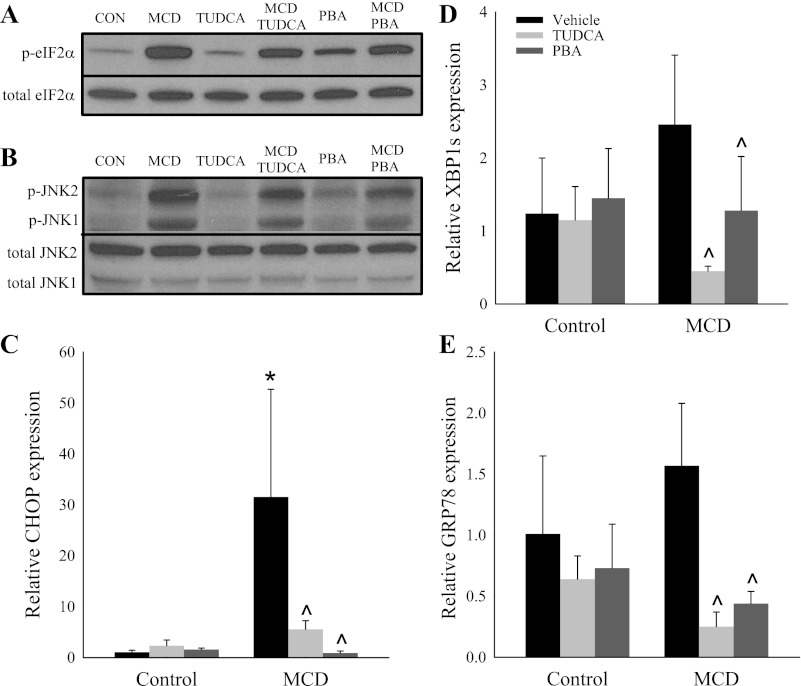
The livers of mice fed a MCD diet for 14 days showed significant activation of the UPR. Specifically, we found robust activation of the PERK targets eIF2α (Fig. 1A) and CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP) (Fig. 1C). Additionally, MCD feeding significantly activated the IRE1α target JNK (Fig. 1B). There was a trend toward increased expression of the IRE1α target spliced X-box binding protein 1 (XBP1s) and the UPR master regulator GRP78 with MCD feeding (Fig. 1, D and E). Consistent with their established role as inhibitors of the ER stress response, TUDCA and PBA suppressed the expression of phosphorylated eIF2α, phosphorylated JNK, CHOP, XBP1s, and GRP78 in MCD diet-fed mice (Fig. 1). Among MCS diet-fed mice, the chemical chaperones did not alter the expression of UPR markers, with the exception of modest activation of eIF2α in response to PBA.
Fig. 1.
Effect of chemical chaperones on markers of endoplasmic reticulum (ER) stress in mice fed a methionine- and choline-deficient (MCD) diet. A–E: hepatic protein expression of total and phosphorylated (p-) eukaryotic initiation factor 2α (eIF2α) and total and phosphorylated JNK and hepatic gene expression of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), spliced X-box binding protein 1 (XBP1s), and glucose-regulated protein 78 kDa (GRP78) in C57BL/6J mice fed a methionine- and choline-sufficient (MCS) or MCD diet with or without vehicle, 4-phenylbutyric acid (PBA), or tauroursodeoxycholic acid (TUDCA) for 14 days. Representative Western blots of pooled samples (n = 4) are shown in A and B. Gene expression is relative to MCS diet-fed mice treated with vehicle. Values are means ± SD (n = 5). *P < 0.05 vs. vehicle-injected MCS diet-fed mice. ^P < 0.05 vs. vehicle-injected MCD diet-fed mice.
Effect of chemical chaperones on phenotypic sequelae of the MCD diet.
We next assessed whether reducing ER stress in MCD diet-fed mice prevents the classic phenotypic sequelae of the MCD diet. Weight loss is a well-established consequence of MCD feeding (19). Consistent with prior studies, MCD feeding resulted in a 25% reduction in body weight during the feeding protocol (Table 1). Treatment with TUDCA or PBA did not prevent MCD diet-induced weight loss. Consistent with the induction of steatohepatitis, the MCD diet results in significant elevation in plasma ALT. We determined that MCD diet-fed mice demonstrated a >20-fold increase in plasma ALT compared with MCS diet-fed controls (259 ± 119 vs. 11 ± 6 U/l, P < 0.001; Table 1). TUDCA and PBA did not attenuate the MCD diet-induced elevation in plasma ALT (240 ± 54 and 303 ± 163 U/l in MCD + TUDCA and MCD + PBA vs. 259 ± 119 U/l in MCD, not significant). Treatment with PBA and TUDCA had no effect on plasma ALT in MCS diet-fed mice. Hypoglycemia and a reduction in plasma cholesterol level are additional known sequelae of MCD feeding (7, 17). The MCD, MCD + TUDCA, and MCD + PBA cohorts showed reductions in blood glucose levels relative to their respective MCS controls (Table 1). The MCD diet caused a reduction in plasma total cholesterol, which was not attenuated by TUDCA or PBA.
Table 1.
Body weight change, blood glucose, and plasma total cholesterol in C57BL/6J mice fed MCD or MCS diet with or without PBA or TUDCA for 14 days
| Body Weight Change, % | Blood Glucose, mg/dl | Plasma Total Cholesterol, mg/dl | Plasma ALT, U/l | |
|---|---|---|---|---|
| MCS diet | ||||
| Vehicle | −0.5 ± 5.5 | 208 ± 8 | 159 ± 27 | 11 ± 6 |
| TUDCA | −6.6 ± 7.7 | 203 ± 19 | 139 ± 16 | 13 ± 10 |
| PBA | −12.7 ± 4.7* | 173 ± 43 | 98 ± 44 | 11 ± 4 |
| MCD diet | ||||
| Vehicle | −25.3 ± 1.6* | 110 ± 31* | 73 ± 30* | 259 ± 119* |
| TUDCA | −30.9 ± 1.8*†‡ | 72 ± 14*†‡ | 73 ± 8*†‡ | 239 ± 54*†‡ |
| PBA | −27.9 ± 0.2*†‡ | 128 ± 12*†‡ | 59 ± 13*†‡ | 303 ± 63*†‡ |
Values are means ± SD (n = 5).
MCD, methionine- and choline-deficient; MCS, methionine- and choline-sufficient; PBA, 4-phenylbutyric acid; TUDCA, tauroursodeoxycholic acid; ALT, alanine aminotransferase.
P < 0.05 vs. MCS + vehicle.
P < 0.05 vs. MCD + vehicle.
P < 0.05 vs. MCS diet-fed mice with the same treatment (TUDCA or PBA).
Chemical chaperones do not reduce MCD diet-induced hepatic steatosis.
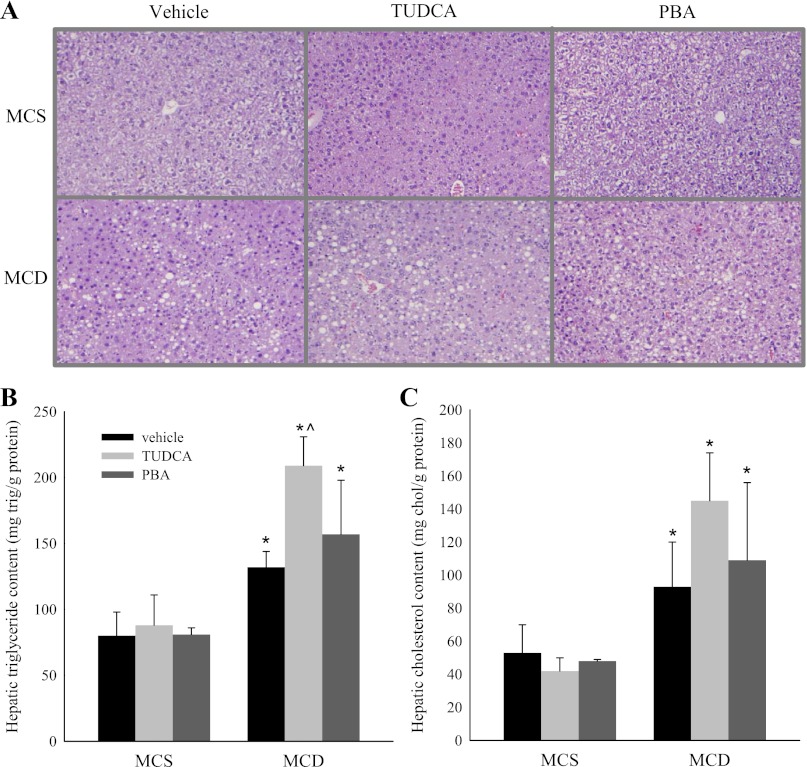
We next sought to determine whether inhibition of ER stress translates to a reduction in MCD diet-induced hepatic steatosis. MCD diet-fed mice showed macrovesicular steatosis on hematoxylin-eosin staining of liver samples (Fig. 2A). There was no significant reduction in the degree of steatosis in the MCD + TUDCA or MCD + PBA cohort compared with MCD cohort. Accordingly, quantification of the hepatic triglyceride and cholesterol level showed that MCD diet-induced hepatic lipid accumulation was not attenuated by TUDCA or PBA treatment (Fig. 2, B and C). In fact, the increase in hepatic triglyceride content was greater in the MCD + TUDCA than in the MCD cohort. Given the increase in hepatic triglyceride content, we examined whether TUDCA regulates genes involved in hepatic triglyceride synthesis and/or degradation. Consistent with previous reports, the MCD diet caused suppression of sterol regulatory element binding protein 1c and stearoyl-coenzyme A desaturase-1, genes involved in triglyceride synthesis (4, 19) (Table 2). MCD diet-fed mice treated with TUDCA also demonstrated suppression of these genes, indicating that the increase in hepatic triglyceride content in this cohort is not due to increased triglyceride synthesis. The MCD + TUDCA cohort showed an increase in expression of the fatty acid oxidation genes fatty acyl-CoA oxidase and carnitine palmitoyltransferase-1, which may be a compensatory response to the increased hepatic triglyceride content.
Fig. 2.
Effect of chemical chaperones on hepatic lipid accumulation in MCD diet-fed mice. A–C: hematoxylin-eosin-stained liver samples, hepatic triglyceride (trig) levels, and hepatic cholesterol (chol) levels in C57BL/6J mice fed a MCS or MCD diet with or without TUDCA or PBA for 14 days. Values are means ± SD (n = 5). *P < 0.05 vs. MCS diet-fed mice treated with the same intraperitoneal injection. ^P < 0.05 vs. vehicle-injected MCD diet-fed mice.
Table 2.
Expression of genes associated with triglyceride synthesis and oxidation in C57BL/6J mice fed MCD diet with or without TUDCA for 14 days
| MCS (Control) Diet |
MCD Diet |
|||
|---|---|---|---|---|
| Vehicle | TUDCA | Vehicle | TUDCA | |
| SREBP-1c | 1.13 ± 0.63 | 0.55 ± 0.08 | 0.30 ± 0.12† | 0.26 ± 0.11† |
| SCD-1 | 1.04 ± 0.34 | 0.73 ± 0.31 | 0.07 ± 0.02† | 0.12 ± 0.06† |
| AOX | 1.01 ± 0.20 | 1.69 ± 0.45 | 0.69 ± 0.21 | 4.71 ± 2.05*† |
| CPT-1 | 1.09 ± 0.51 | 1.36 ± 0.26 | 1.11 ± 0.40 | 8.19 ± 0.71*† |
Values are means ± SD (n = 5).
SREBP-1c, sterol regulatory element binding protein-1c; SCD-1, stearoyl-CoA desaturase; AOX, fatty acyl-CoA oxidase; CPT-1, carnitine palmitoyltransferase 1.
P < 0.05 vs. vehicle-treated mice fed the same diet (MCS or MCD).
P < 0.05 vs. MCS diet-fed mice with the same treatment (TUDCA or PBA).
Effect of chemical chaperones on markers of hepatic inflammation and fibrosis.
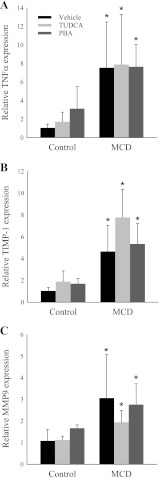
Hepatic inflammation and fibrosis are features that distinguish simple steatosis from steatohepatitis. TNFα is a proinflammatory cytokine that is critically important in the pathogenesis of numerous inflammatory liver diseases, including NASH (25, 27). We found that TNFα mRNA expression was increased nearly eightfold by MCD feeding. Chemical chaperones did not attenuate the expression of TNFα in MCD diet-fed mice (Fig. 3A).
Fig. 3.
Effect of chemical chaperones on hepatic expression of genes associated with inflammation and fibrosis in MCD diet-fed mice. A–C: quantitative PCR analysis of TNFα, tissue inhibitor of metalloproteinase-1 (TIMP-1), and matrix metalloproteinase-9 (MMP-9) in C57BL/6J mice fed a MCS or MCD diet with or without TUDCA or PBA for 14 days. Expression is relative to vehicle-injected MCS diet-fed mice. Values are means ± SD (n = 5). *P < 0.05 vs. MCS diet-fed mice treated with the same intraperitoneal injection.
The MCD diet induces significant hepatic fibrosis by 4–8 wk of feeding depending on the murine strain (8, 21). As expected, we did not find significant fibrosis histologically at 2 wk; however, even by 2 wk, we found that the MCD diet induced expression of genes associated with the development of fibrosis, including tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9. TUDCA and PBA did not attenuate the MCD diet-induced upregulation of these fibrosis-associated genes (Fig. 3, B and C).
DISCUSSION
ER stress may have an essential role in the pathogenesis of NAFLD. One of the frequently cited lines of evidence supporting this assertion is the observation that MCD feeding in rodents is associated with activation of the ER stress response (6, 7, 14). However, it has not been proven that ER stress promotes MCD diet-induced steatohepatitis. We now show that reducing ER stress in MCD diet-fed mice does not result in a reduction in hepatic steatosis, inflammation, or fibrosis. The present work indicates that although components of the UPR are upregulated by MCD feeding, ER stress may not play a primary role in the pathogenesis of MCD diet-induced steatohepatitis.
It must be considered, however, that chemical chaperones ameliorate, but do not completely prevent, ER stress. Therefore, we cannot exclude the possibility that low levels of ER stress, as observed in MCD-fed mice treated with chemical chaperones, are sufficient to induce steatohepatitis. Arguing against this hypothesis, however, is the observation that some of the negative sequelae of the MCD diet were actually exacerbated by the introduction of chemical chaperones. Most notably, worsening of MCD diet-induced hepatic triglyceride accumulation by the administration of TUDCA could not be attributed to enhanced hepatic lipogenesis or suppressed fatty acid oxidation. This may suggest that the ER stress response has a protective role in the setting of methionine and choline deficiency. Alternatively, TUDCA and PBA do not function exclusively as inhibitors of the ER stress response, and it is possible that modulation of other physiological processes may be causing these untoward effects.
The accumulation of saturated fat and cholesterol in the liver is an established trigger of ER stress (3, 5, 24). It is, therefore, plausible that MCD diet-induced ER stress may be a consequence, rather than a cause, of the hepatic lipid accumulation in this dietary model. Furthermore, it has been shown that the ER stress response induced by saturated fatty acids is characterized by a preferential induction of PERK signaling (3). We found that the MCD diet most robustly activated eIF2α and CHOP, which are components of the PERK signaling pathway. As such, if induction of hepatic ER stress by a MCD diet is due to lipid accumulation in the liver, this may explain the disproportionate induction of PERK signaling.
However, eIF2α is activated not only by the PERK arm of the UPR, but also by general control nonderepressible 2 (GCN2), a component of an “integrated stress response” (1, 20). It has previously been shown that MCD feeding activates PERK and GCN2 (14, 20), and the relative importance of ER stress vs. an integrated stress response in MCD diet-related eIF2α signaling is unclear. The fact that ER chemical chaperones attenuate MCD diet-induced activation of eIF2α, as shown in this study, highlights the importance of ER perturbation in MCD diet-induced eIF2α phosphorylation.
The effect of a MCD diet on the IRE1α pathway of the UPR is controversial. In the present study, we found an insignificant increase in the spliced transcript of XBP1 with MCD feeding, indicating minimal activation of the IRE1α pathway. Others have shown transient activation of hepatic XBP1 early in the course of MCD feeding followed by sustained suppression (20). In our prior work, we showed a twofold elevation in hepatic XBP1s in female FVB mice fed a MCD diet (7). However, MCD feeding in female FVB mice also induced a more severe degree of steatohepatitis than in male C57BL/6J mice in the current study. Rinella et al. (18) recently showed that, after 4 wk of MCD feeding, db/db mice showed no induction of hepatic XBP1s, whereas db/m controls showed a more than twofold induction of XBP1s mRNA. We can conclude that induction of the IRE1α pathway in the MCD model is highly dependent on the length of feeding and mouse strain.
The present work challenges the notion that ER stress promotes the development of MCD diet-induced steatohepatitis. However, our observations do not challenge the assertion that ER stress plays a role in the pathogenesis of human NAFLD. Although the MCD diet produces histological findings similar to human NASH, this model is not associated with the classic systemic manifestations associated with human NAFLD, most notably obesity and insulin resistance. Instead, MCD diet-fed mice lose significant amounts of weight and become more insulin-sensitive. It would not be surprising if the pathogenesis of MCD diet-induced steatohepatitis does not exactly parallel the mechanisms governing the progression of human NAFLD. It has been previously shown that human subjects with NAFLD demonstrate activation of the UPR in the liver, and the significance of this finding warrants further investigation to establish whether there is a causal relationship (13).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-080810 and F32 DK-076342 and an American Gastroenterological Association Research Scholar Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.H. and R.M.G. are responsible for conception and design of the research; A.S.H., A.M.D., K.A.A., and S.O. performed the experiments; A.S.H., A.M.D., K.A.A., and S.O. analyzed the data; A.S.H. and R.M.G. interpreted the results of the experiments; A.S.H. prepared the figures; A.S.H. drafted the manuscript; A.S.H. and R.M.G. edited and revised the manuscript; A.S.H., A.M.D., K.A.A., S.O., and R.M.G. approved the final version of the manuscript.
REFERENCES
- 1. Berlanga JJ, Santoyo J, De Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur J Biochem 265: 754–762, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang Q, Chen J, Cheng WH, Shen W, Yang ZX. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem 364: 115–129, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Da Silva Morais A, Lebrun V, Abarca-Quinones J, Brichard S, Hue L, Guigas B, Viollet B, Leclercq IA. Prevention of steatohepatitis by pioglitazone: implication of adiponectin-dependent inhibition of SREBP-1c and inflammation. J Hepatol 50: 489–500, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Greene MW, Burrington CM, Ruhoff MS, Johnson AK, Chongkrairatanakul T, Kangwanpornsiri A. PKCδ is activated in a dietary model of steatohepatitis and regulates endoplasmic reticulum stress and cell death. J Biol Chem 285: 42115–42129, ???? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henkel AS, Elias MS, Green RM. Homocysteine supplementation attenuates the unfolded protein response in a murine nutritional model of steatohepatitis. J Biol Chem 284: 31807–31816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol 41: 592–598, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 86: 1133–1149, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118: 316–332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 45: 1108–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Rinella ME. Will the increased prevalence of nonalcoholic steatohepatitis (NASH) in the age of better hepatitis C virus therapy make NASH the deadlier disease? Hepatology 54: 1118–1120, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res 49: 1068–1076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 40: 47–51, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Rinella ME, Siddiqui MS, Gardikiotes K, Gottstein J, Elias M, Green RM. Dysregulation of the unfolded protein response in db/db mice with diet-induced steatohepatitis. Hepatology 54: 1600–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res 47: 2280–2290, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Soon RK, Jr, Yan JS, Grenert JP, Maher JJ. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology 139: 1730–1739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Starkel P, Sempoux C, Leclercq I, Herin M, Deby C, Desager JP, Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-β up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol 39: 538–546, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum stress-mediated apoptosis. Surgery 138: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147: 943–951, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-κB: effects on liver homeostasis and beyond. Endocr Rev 28: 365–386, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592–601, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA 94: 2557–2562, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 263: 2998–3004, 1988 [PubMed] [Google Scholar]
- 29. Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279: 25935–25938, 2004 [DOI] [PubMed] [Google Scholar]