Abstract
Nitric oxide (NO) is an established inflammatory mediator. However, it remains controversial whether NO enhances the inflammatory response in the colon or suppresses it. We investigated the epigenetic regulation of Icam-1 expression by NO following induction of colonic inflammation in rats by 2,4,6-trinitrobenzene sulfonic (TNBS) acid and obtaining colonic muscularis externae tissues 24 h later. TNBS inflammation induced intercellular adhesion molecule-1 (ICAM-1) expression by translocating NF-κB to the nucleus. The incubation of inflamed tissues with S-nitrosoglutathione (GSNO) did not affect the nuclear translocation of NF-κB; however, it suppressed the NF-κB binding to DNA. Chromatin immunoprecipitation analysis (ChIP)-qPCR assays showed that the increase in NF-κB/DNA interaction following inflammation is due to the transcriptional downregulation of global HDAC3 and a decrease in its interaction with the DNA on the Icam-1 promoter containing the binding motifs of NF-κB. The decrease in the association of histone deacetylase (HDAC) 3 with the Icam-1 promoter increased the acetylation of histone 4 lysine residue 12 (H4K12), which would favor chromatin relaxation and greater access of NF-κB to its DNA binding sites. HDAC3 dissociation from the DNA did not affect the acetylation levels of H4K8 and H4K16. The NO release by GSNO countered the upregulation of Icam-1 by increasing the transcription of global HDAC3 and its association with the Icam-1 promoter, and by suppressing H4K12 acetylation. We conclude that chromatin modification by transcriptional downregulation of HDAC3 plays a critical role in the induction of the inflammatory response. NO may serve as an anti-inflammatory mediator during the acute stage of inflammation by blunting the downregulation of global HDAC3, increasing HDAC3 interaction with the nucleosomes containing the binding moieties of NF-κB, reducing H4K12Ac to restrict the access of NF-κB to DNA, and suppressing ICAM-1 expression.
Keywords: intercellular adhesion molecule-1, inflammation
nitric oxide (NO) and inducible NO synthase (iNOS) are elevated in the inflamed tissues of patients with ulcerative colitis and Crohn's disease (9, 32, 37) as well as in the tissues of rodent models of these diseases, dextran sodium sulfate (DSS)- and trinitrobenzene sulfonic acid (TNBS)-induced inflammation, respectively (15). These findings suggest that NO may serve as an inflammatory mediator in inflammatory bowel disease. However, experiments in intact animal models have been inconclusive. Some studies found that prophylactic or therapeutic inhibition of iNOS attenuates gut inflammation and injury, implying that NO is deleterious (10, 16, 28). Other studies found that the blockade of iNOS by pharmacological inhibitors or knockout of the iNOS gene either has no effect or worsens the inflammatory response, suggesting that NO has a benign or beneficial role in inflammation (6, 11, 18, 26). The cellular mechanisms by which NO may suppress or enhance the inflammatory response remain incompletely understood.
The inflammatory response in intact animals is complex and involves numerous cell types and a multitude of pro- and anti-inflammatory mediators with varying time courses of expression and intensity of release. Most of the above studies investigated the potential role of iNOS-derived NO by administering iNOS antagonists in vivo at various acute or chronic stages of the inflammatory response (16, 18, 26, 28), which may be one of the factors in divergent outcomes. In addition, studies in intact animals restrict the investigation of cellular and molecular mechanisms of pathology. We investigated the role of NO in TNBS-induced colonic inflammation in intact rats, obtaining muscularis externae tissues 24 h later to investigate cellular, molecular, and epigenetic mechanisms of the action of NO in regulating the expression of intercellular adhesion molecule-1 (ICAM-1). ICAM-1 is a prominent mediator of inflammation, with greater expression throughout the thickness of the intestinal wall in Crohn's disease and ulcerative colitis patients (42). ICAM-1 and other adhesion molecules express early in the inflammatory response to recruit immune cells. We investigated whether NO released by NO donor S-nitrosoglutathione (GSNO) alters the expression of ICAM-1 in inflamed tissues.
We tested the hypothesis that inflammation downregulates histone deacetylase-3 (HDAC3) mRNA and protein expression and its association with DNA to upregulate the acetylation of histone protein H4 on lysine residue 12 in the region of the Icam-1 promoter containing the binding motifs of NF-κB. The resulting relaxation of the chromatin allows NF-κB greater access to its binding moieties, resulting in increased Icam-1 mRNA and protein expression. We found that NO is an anti-inflammatory mediator that counters the transcriptional downregulation of global HDAC3, increases the association of HDAC3 with the Icam-1 promoter, and decreases H4K12Ac, which condenses the chromatin to restrict the access of NF-κB to binding motifs on the Icam-1 promoter. NO does not affect the activation or translocation of NF-κB to the nucleus.
MATERIALS AND METHODS
Reagents and plasmids.
We purchased TNBS and GSNO from Sigma (St. Louis, MO), trichostatin A (TSA) from Enzo Life Sciences (Plymouth Meeting, PA), and recombinant rat IL-1β from R&D Systems (Minneapolis, MN). We generated a rat Icam-1 promoter-luciferase reporter construct by subcloning a PCR fragment of the Icam-1 promoter [nucleotides (nt) −1,758/+80] between the Kpn I and Mlu I sites of the pGL3-Basic (Promega, Madison, WI). We used GeneTailor Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA) to construct all mutant Icam-1 promoter plasmids with mutant NF-κB binding sites (site 1: −221/−209, TTTGGAAATTCCT vs. TTTGCTAATTCCT, mutant; site 2: −149/−137, CGGAAATACCGAA vs. CGCTAATACCGAA, mutant).
Animals.
We used 6-wk-old male Sprague-Dawley rats purchased from Harlan Laboratories (Indianapolis, IN). The animals were fasted for 24 h with free access to GoLYTELY (Braintree Laboratories, Braintree, MA) to cleanse the colon before induction of inflammation with 65 mg/kg TNBS dissolved in saline containing 40% ethanol (vol/vol). Rats received isoflurane anesthesia for intracolonic administration of 250 μl of TNBS/ethanol/saline solution injected 8 cm into the colon via a catheter. Holding the animals in a head-down position for 1 min prevented leakage of TNBS. Rats in the control group received saline. The rats were euthanized 24 h later to obtain tissues. The University of Texas Medical Branch Institutional Animal Care and Use Committee approved all procedures.
Preparation and treatment of rat colonic muscle externae tissues.
Freshly obtained, full-thickness colon tissues were immersed in carbogenated Krebs solution with 5% O2/95% CO2 mix (2). The mucosal/submucosal layers were peeled off. The remaining muscularis externae tissues were placed in high glucose DMEM (HyClone, South Logan, UT) containing 10% FBS, antibiotics, and test reagents and incubated in a water-jacketed CO2 incubator. For time course experiments, we incubated all specimens in the medium for the same duration and added the reagents at different time points. The treated tissues were snap-frozen in liquid nitrogen at the end of the whole incubation period. For example, GSNO was added 12, 6, and 1 h before harvesting for 12-, 6-, and 1-h time points, respectively. Samples at 0-h time point served as vehicle controls.
Cell culture, transient transfection, and luciferase assay.
We used primary cultures of rat colonic circular smooth muscle cells (RCCSMC) in passage 2 or 3 for reporter assays. Transient transfection of Icam-1 reporter constructs, luciferase, and β-galactosidase (β-Gal) assays were performed, as described previously (21, 22).
Isolation of nuclear extracts and Western blot.
We extracted cytoplasmic and nuclear extracts by using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Rockford, IL). We prepared whole cell lysates by using immunoprecipitation (IP) lysis buffer (22). Western blotting was performed as described previously (22). We used the following antibodies: anti-NF-κB p65 rabbit polyclonal (Cell Signaling, Danvers, MA), anti-rat ICAM-1 monoclonal (R&D Systems), anti-histone H4 polyclonal and anti-histone H4K8Ac (Millipore, Temecula, CA), anti-histone H4K12Ac, anti-histone H4K16Ac, and anti-HDAC3 rabbit polyclonal (Active Motif, Carlsbad, CA), anti-α-tubulin and anti-histone H1 (Santa Cruz, CA), and anti-β-actin mouse monoclonal (Sigma).
Oligonucleotide pulldown assay.
Wild-type oligonucleotides no. 1 (5′-TTACTTCAGTTTGGAAATTCCTGGGTCGCAG-3′) and no. 2 (5′-GTCTCCATCCGGAAATACCGAAGCCCTCATT-3′) (NF-κB binding motifs in bold), as well as mutant oligonucleotides no. 1 (5′-TTACTTCAGTTTGCTAATTCCTGGGTCGCAG-3′) and no. 2 (5′-GTCTCCATCCGCTAATACCGAAGCCCTCATT-3′) (mutated nucleotides underlined) were biotinylated and incubated with 50 μg of nuclear extracts from rat colonic muscularis externae (22). NF-κB p65, precipitated by biotin-labeled oligos, was detected by immunoblotting.
HAT and HDAC assays.
Colonic inflammation was induced by intracolonic administration of 17, 65, or 130 mg/kg TNBS dissolved in saline containing 40% ethanol (vol./vol.). Saline infusion served as vehicle control. Colonic muscle strips were collected 24 h later and snap-frozen in liquid nitrogen. Frozen tissues were smashed, and nuclear extracts were extracted by using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific). We measured HAT and HDAC activities in nuclear extracts by using the HAT Activity Colorimetric Kit and the Colorimetric HDAC Activity Assay Kit (BioVision, Mountain View, CA), respectively. Each reaction used 100 μg of nuclear extract.
Chromatin immunoprecipitation-real-time PCR assay.
We performed chromatin immunoprecipitation (ChIP) assays as described previously (22). The rat Icam-1-specific primers (forward: 5′-CTTCTCTCCCGGACTCTCCT-3′; reverse: 5′-GGAATGAGGGCTTCGGTATT-3′) covering the NF-κB binding region (nt −290/−127) of the promoter and SYBR Green Master Mix (Applied Biosystems, Foster City, CA) were used for real-time PCR. We normalized fold differences in precipitated DNA against input.
Real-time RT-PCR.
We extracted the total RNA by using RNeasy mini kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse-transcribed using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Quantification of Icam-1 and HDAC3 mRNA levels by real-time PCR was performed with a StepOnePlus Thermal Cycler and Taqman probe and primers (Applied Biosystems). 18S rRNA was quantified as an internal control for the amount and quality of cDNA. All samples were assayed in triplicate in an optical 96-well reaction plate with optical adhesive covers in a 20-μl volume containing 7 μl (2 μl for 18S rRNA) diluted cDNA (1:5 dilution in water).
Statistics.
We expressed all data as means ± SE and used two-tailed Student's t-test or one-way ANOVA followed by Fisher's post hoc analysis and considered P < 0.05 as significant.
RESULTS
GSNO suppresses inflammation-induced Icam-1 transcription in colonic muscularis externae.
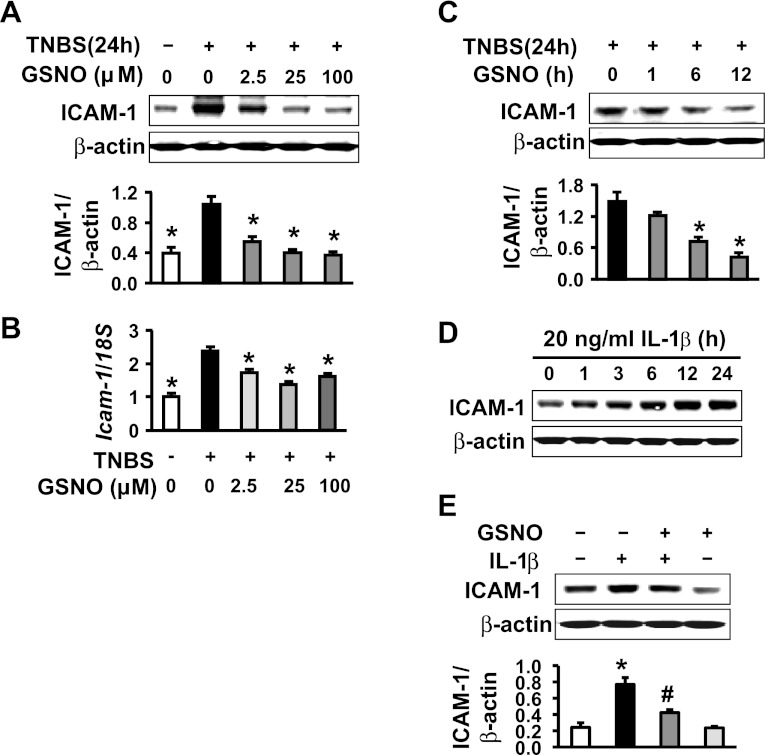
TNBS-induced inflammation significantly upregulated ICAM-1 protein and mRNA levels in the muscularis externae isolated from the rat colon at 24 h after the insult (Fig. 1, A and B). The treatment of these tissues with the NO donor GSNO suppressed the increase in ICAM-1 protein (Fig. 1A) and mRNA expression (Fig. 1B) in a concentration-dependent manner. GSNO treatment (25 μM) took <6 h to suppress significantly the ICAM-1 protein expression in the above tissues (Fig. 1C). In addition, in vitro incubation of colonic muscularis externae tissues from naive rats with 20 ng/ml IL-1β time-dependently upregulated ICAM-1 protein expression (Fig, 1D), whereas co-incubation of tissues with GSNO blunted this increase (Fig. 1E).
Fig. 1.
S-nitrosoglutathione (GSNO) suppresses intercellular adhesion molecule-1 (ICAM-1) expression in colonic muscularis externae. A: GSNO concentration-dependently suppressed TNBS-induced ICAM-1 protein expression. B: GSNO downregulated TNBS-induced Icam-1 mRNA level (real-time RT-PCR). C: 25 nM GSNO time-dependently suppressed ICAM-1. D: IL-1β upregulated ICAM-1 protein expression in rat colonic muscularis externae. E: 25 nM GSNO abrogated ICAM-1 protein induction by 20 ng/ml of IL-1β in rat colonic muscularis externae. *Significant difference vs. values at 24 h after TNBS inflammation in A and B and vs. time 0 in C and control in E (P < 0.05). #Significant difference vs. IL-1β alone (P < 0.05).
The roles of NF-κB and HDAC inhibition in the transcription of Icam-1.
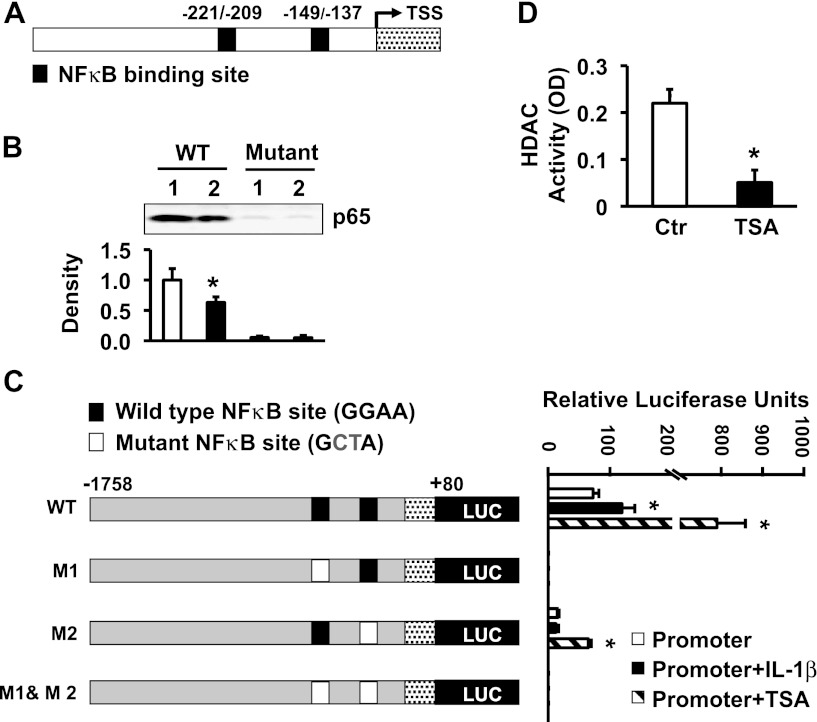
Pro-inflammatory cytokines IL-1β and TNF-α induce the expression of Icam-1 in immune and nonimmune cells by activating the transcription factor NF-κB, causing it to translocate to the nucleus (31, 36, 41, 43). MatInspector software (Genomatrix, Germany) identified two NF-κB binding motifs (−221/−209 and −149/−137) on the Icam-1 promoter, separated by 60 nucleotides (Fig. 2A). We investigated whether one or both of these cis-elements mediate the transcription of Icam-1 in response to IL-1β. We incubated nuclear extracts of the muscularis externae with biotinylated oligonucleotides containing each putative NF-κB recognition sequence. Each sequence precipitated p65 NF-κB (Fig. 2B), but the upstream binding site showed higher affinity to p65 than the downstream site. Oligonucleotides containing mutant NF-κB sites pulled down no p65.
Fig. 2.
NF-κB binding to its recognition sequences on Icam-1 promoter in response to IL-1β and trichostatin A (TSA). A: schematic presentation of the rat Icam-1 promoter. MatInspector Software identified two putative NF-κB binding motifs in the rat Icam-1 promoter. TSS, transcription start site. Numbering is relative to the TSS. B: oligonucleotides containing either NF-κB binding sequence pulled down NF-κB p65. Mutant oligonucleotides served as negative controls. *Significant difference vs. wild type NF-κB binding site 1 (P < 0.05). C: both NF-κB binding motifs are critical to the rat Icam-1 promoter activity. Wild-type and mutant Icam-1 promoter reporter constructs (left) were transfected into rat colonic circular smooth muscle cells in the presence or absence of 20 ng/ml of IL-1β or 1 μM TSA, followed by luciferase and β-galactosidase (β-Gal) assays (right). *Significant difference vs. promoter alone (P < 0.05). D: TSA suppressed HDAC activity in the nuclear extracts of rat colonic circular smooth muscle cells. *Significant difference vs. control (Ctr) (P < 0.05).
Next, we used mutation analysis to investigate the relative roles of the two NF-κB binding motifs in mediating transcription of Icam-1 in response to IL-1β or TSA, an established histone deacetylase (HDAC) inhibitor (29) (Fig. 2C, left). We transiently transfected the rat Icam-1 promoter (−1,758/+80)-pGL3-basic luciferase reporter constructs into primary cultures of RCCSMCs, with β-galactosidase (β-Gal) as an internal control. Twenty-four hours later, we treated the transfected cells with 20 ng/ml IL-1β or 1 μM TSA for another 24 h. TSA significantly suppressed the global HDAC activity (Fig. 2D). Luciferase and β-Gal assays showed that both IL-1β and TSA significantly increase the wild-type Icam-1 promoter activity (Fig. 2C, right). The increase in promoter activity induced by TSA was sixfold greater than that induced by IL-1β. Mutation of the upstream NF-κB recognition site completely abolished the basal Icam-1 promoter activity as well as its induction by IL-1β or TSA, indicating that this NF-κB binding domain is essential for constitutive and evoked expression of Icam-1. Mutation of the downstream NF-κB site significantly decreased the basal Icam-1 promoter activity, indicating that this binding motif also contributes to the constitutive ICAM-1 transcription. In addition, this mutation abrogated the induction of Icam-1 promoter activity by IL-1β, whereas TSA still significantly elevated promoter activity of Icam-1 TSA to a level significantly lower than that of the wild-type promoter. This observation indicates that upstream NF-κB binding motif in the rat Icam-1 promoter is functional even without the downstream binding motif. The concurrent mutations of both NF-κB binding motifs completely abolished basal and evoked Icam-1 promoter activities. These findings show that HDAC inhibition in colonic muscularis externae induces Icam-1 transcription.
NO does not affect the translocation of NF-κB to the nucleus.
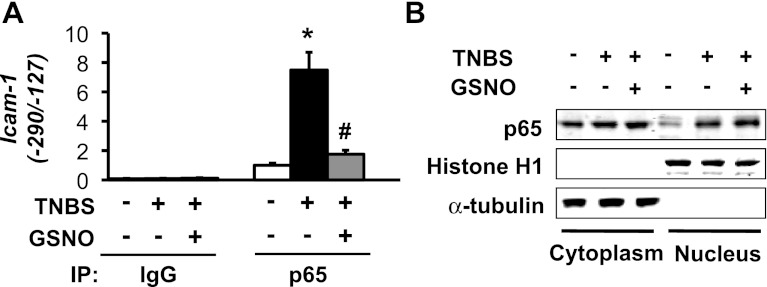
We investigated whether NO suppresses the expression of Icam-1 by blocking the translocation of NF-κB to the nucleus. ChIP-qPCR assays with anti-p65 antibody and primers specific to the rat Icam-1 promoter region containing both NF-κB binding moieties (−290/−127) showed that TNBS insult significantly increases the binding of p65 to the Icam-1 promoter at 24 h post-inflammation (Fig. 3A, second column). The incubation of inflamed muscularis externae tissues with 25 μM GSNO for 12 h significantly reduced this binding (Fig. 3A). The separation of nuclear fractions followed by Western blotting showed that inflammation significantly increased nuclear p65 (Fig. 3B); co-incubation with GSNO did not affect this increase, suggesting that NO does not affect the translocation of p65 NF-κB to the nucleus. The absence of α-tubulin, a cytoplasmic marker, and the clear presence of histone H1, a nuclear marker, established the clean separation of nuclear fractions from cytoplasmic proteins (Fig. 3B).
Fig. 3.
GSNO attenuates NF-κB p65 binding to the rat Icam-1 promoter without affecting nuclear p65 protein levels. A: TNBS-induced inflammation augmented NF-κB p65 association with the Icam-1 promoter; 25 μM GSNO abrogated the increase in binding in rat colonic muscularis externae. Chromatin immunoprecipitation (ChIP) assays followed by real-time PCR assessed p65/Icam-1 promoter interaction. Rabbit IgG was used for mock precipitation. N = 3. *Significant difference vs. Ctr (P < 0.05). #Significant difference vs. value at 24 h after TNBS insult (P < 0.05). B: GSNO had no effect on the increase of nuclear p65 by inflammation. Cytoplasmic marker α-tubulin and nuclear marker histone H1 showed clean separation of cytoplasmic and nuclear fractions.
Competitive modification of chromatin containing NF-κB binding motifs by inflammation and NO in the regulation of Icam-1 expression.
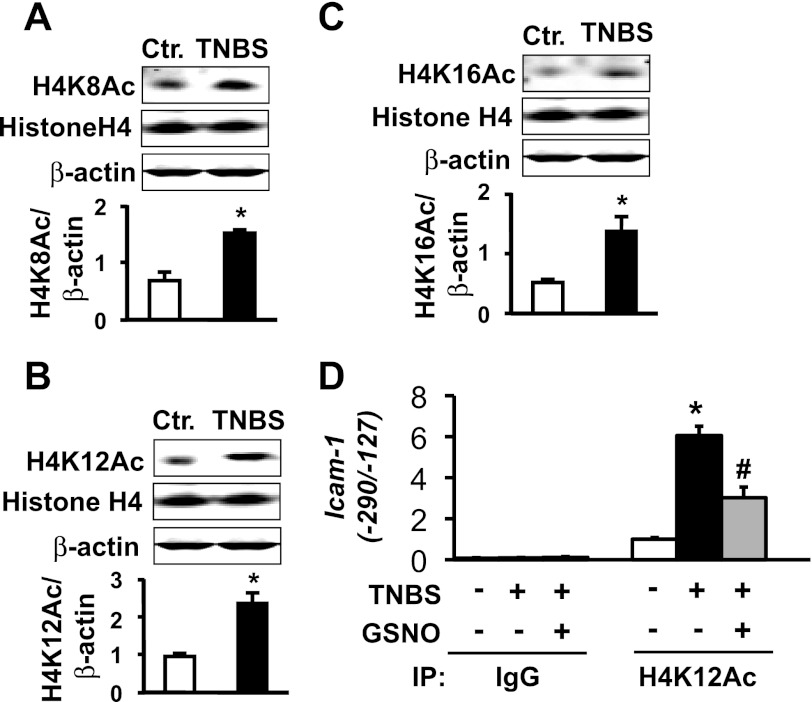
Histone acetylation and deacetylation play critical roles in regulating gene transcription (7, 23, 44). The acetylation of lysine residues at histone NH2-terminal tails relaxes the chromatin to allow transcription factors greater access to their binding motifs and to enhance transcription; deacetylation does the opposite. We investigated whether NO modulates the acetylation of H4 lysine residues 8, 12, and 16 associated with the nucleosomes containing the recognition sequences of p65 NF-κB to suppress Icam-1 transcription by TNBS inflammation. Western blotting for H4K8Ac, H4K12Ac, and H4K16Ac showed significant global increases in all three acetylated lysine residues, whereas total histone H4 protein remained unchanged (Fig. 4, A–C). The acetylation of histone proteins at the lysine residues of NH2-terminal tails is gene and sequence specific. ChIP-qPCR assays showed that TNBS inflammation significantly increases the amount of H4K12Ac in the nucleosomes containing the two NF-κB binding moieties without affecting the acetylation of H4K8 and H4K16 (data not shown). NO released by GSNO significantly suppressed the acetylation of H4K12 (Fig. 4D) but had no significant effect on the acetylation levels of H4K8 and H4K16 (data not shown).
Fig. 4.
GSNO treatment suppresses histone H4K12 acetylation elevated by TNBS inflammation. Histone H4 acetylation at lysine residues 8 (A), 12 (B), and 16 (C) increased significantly in colonic muscularis externae of TNBS rats. N = 4. *Significantly different vs. Ctr (P < 0.05). D: inflammation increased histone H4K12 acetylation at the NF-κB binding region of rat Icam-1 promoter and GSNO significantly suppressed it. We quantitated Icam-1 promoter precipitation by anti-acetylated H4K12 using real-time PCR and normalized to input. Rabbit IgG served as negative control. Values are means ± SE; n = 3. *Significantly different vs. Ctr. (P < 0.05). #Significantly different vs. TNBS (P < 0.05).
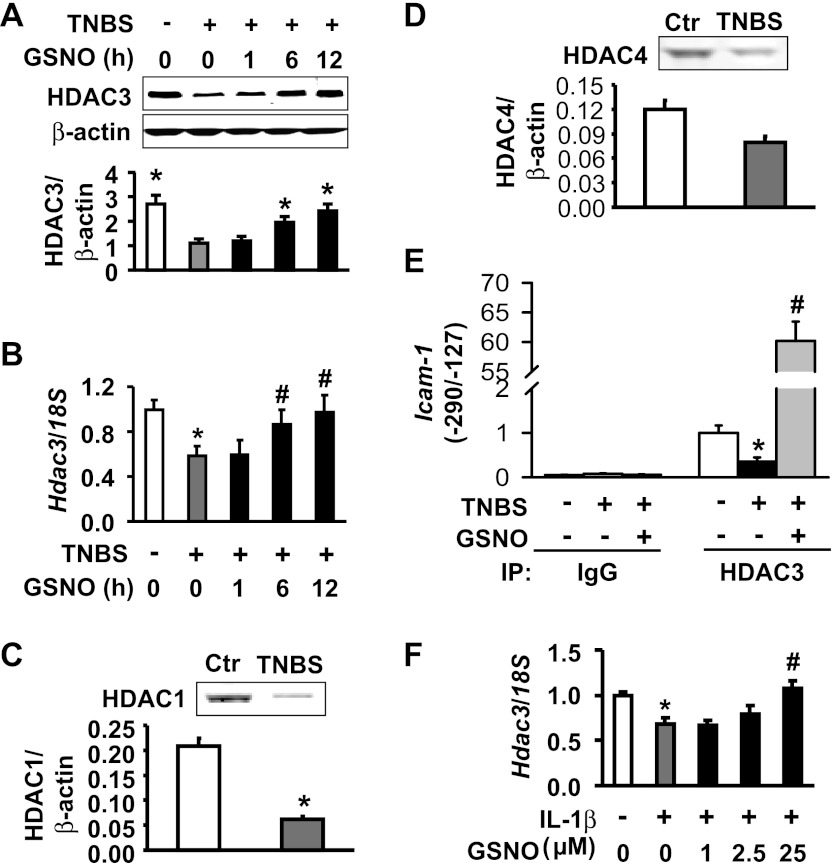
Inflammation suppresses and NO enhances HDAC3 transcription to regulate acetylation of H4K12 and Icam-1 transcription.
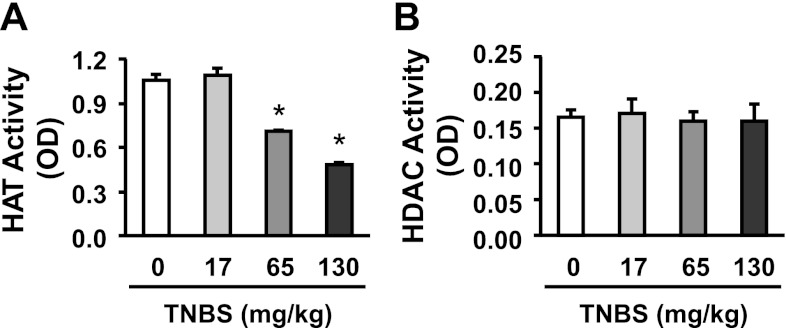
HAT and HDAC assays showed that TNBS inflammation reduces the total activity of HATs in a dose-dependent manner (Fig. 5A) but has no effect on global HDAC activity (Fig. 5B) in nuclear extracts of colonic muscularis externae at 24 h after insult. These data suggest that the increase in acetylation of lysine residues on histone H4 may be due to suppression of specific HDACs. HAT activity did not increase; therefore, it is unlikely to contribute to the increase in acetylation of lysine residues. We found that the colonic muscularis externae tissues express HDAC3 (Fig. 6A) and low levels of HDAC4 and HDAC1 (Fig. 6, C and D). Therefore, we investigated whether inflammation and NO both target HDAC3 in regulating ICAM-1 expression. Western blots and real-time RT-PCR showed that TNBS inflammation significantly suppressed both HDAC3 protein and mRNA (Fig. 6, A and B, respectively). HDAC1 also decreased significantly, but the decrease in HDAC4 was not significant (Fig. 6, C and D). The treatment of colonic muscularis externae tissues from TNBS rats with GSNO significantly reversed the suppression of HDAC3 by TNBS inflammation in under 6 h (Fig. 6B) but had no effect on HDAC1 and HDAC4 (data not shown). Earlier, we showed in Fig. 1B that GSNO treatment in the inflamed tissues suppressed the expression of ICAM-1, also within 6 h.
Fig. 5.
TNBS inflammation concentration-dependently suppressed global HAT activity (A) but did not affect global HDAC activity (B) in nuclear extracts of colonic muscularis externae at 24 h after insult. *Significantly different vs. Ctr (saline) (P < 0.05). Colonic inflammation was induced by intracolonic administration of 17, 65, or 130 mg/kg TNBS dissolved in saline containing 40% ethanol (vol./vol.). Saline administration served as vehicle control.
Fig. 6.
GSNO abrogates HDAC3 downregulation in response to TNBS inflammation. A: GSNO time-dependently reversed the suppression of HDAC3 protein expression by TNBS. N = 3. *Signficantly different vs. TNBS alone (P < 0.05). B: GSNO time-dependently reversed HDAC3 mRNA expression suppressed by TNBS inflammation. *Significantly differenct vs. naïve control (P < 0.05; n = 3). #Significantly different vs. TNBS alone (P < 0.05). C: TNBS inflammation significantly suppressed HDAC1 in colonic muscularis externae. *Signficantly different vs. Ctr (P < 0.05). D: TNBS treatment slightly decreased HDAC4. E: inflammation suppressed and GSNO reversed the suppression of HDAC3 association with the NF-κB binding region of the rat Icam-1 promoter (ChIP-qPCR). N = 3. *Signficantly different vs. Ctr (P < 0.05). #Significantly different vs. TNBS alone (P < 0.05). F: GSNO abrogated IL-1β-induced downregulation of HDAC3 mRNA in muscularis externae tissues from naive rats. Inflamed colonic muscularis externae tissues were treated with GSNO for 12 h or indicated durations. N = 3. *Significantly different vs. Ctr (P < 0.05). #Significantly different vs. IL-1β alone (P < 0.05).
ChIP-qPCR assays showed that TNBS inflammation significantly suppressed the association of HDAC3 with the Icam-1 promoter region containing the NF-κB binding moieties; GSNO treatment reversed this suppression (Fig. 6E). Together, these findings suggest that the suppression of HDAC3 may play a key role in the TNBS-induced increase in the expression of ICAM-1 in colonic inflammation; NO counters this increase by upregulating the expression of HDAC3. In vitro experiments also showed that HDAC3 suppression occurs via transcriptional downregulation or increased mRNA turnover. The in vitro incubation of naive muscularis externae tissues with IL-1β significantly suppressed HDAC3 mRNA; treatment with GSNO concentration-dependently reversed this suppression (Fig. 6F).
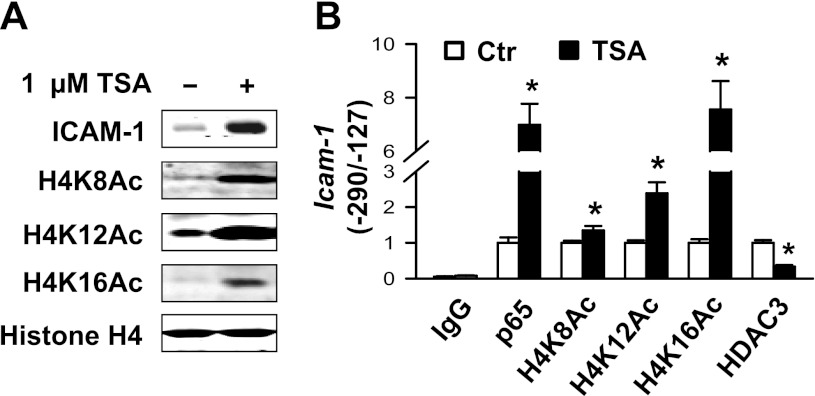
We confirmed the role of increased acetylation of H4K12 in enhancing the binding of p65 NF-κB to DNA by treating muscularis externae tissues from naive rats with 1 μM TSA. TSA markedly upregulated ICAM-1 protein expression and the global acetylation of H4K8, H4K12, and H4K16 (Fig. 7A). ChIP-qPCR assays showed that TSA significantly reduced the association of HDAC3 with the −290/−127 region of Icam-1 promoter containing the binding moieties of NF-κB, which enhanced the acetylation of H4K8, H4K12, and H4k16 in this segment of the promoter (Fig. 7B). TSA also enhanced the binding of p65 NF-κB to its binding moieties (Fig. 7B), indicating that histone H4 hyperacetylation relaxes the nucleosomes containing NF-κB binding domains and hence increases the accessibility of NF-κB to the rat Icam-1 promoter. Note that inflammation increased H4K12Ac only, whereas nonselective HDAC inhibitor increased H4K8Ac and H4K16Ac as well, which explains the greater increase in Icam-1 promoter-reporter activity initiated by TSA over that of IL-1β (Fig. 2C).
Fig. 7.
Trichostatin A (TSA) dramatically elevates ICAM-1 protein expression and histone H4 acetylation. Colonic muscularis externae from naive adult rats were incubated with 1 μM TSA for 24 h and snap-frozen in liquid nitrogen. Whole cell lysates were prepared for immunoblotting. ChIP-qPCR assays were performed with indicated antibodies, SYBR Green Master Mix (Applied Biosystems), and primers specific to the NF-κB binding area (−290/−127) of the rat Icam-1 promoter. Rabbit IgG was used for mock precipitation. A: TSA markedly increased ICAM-1 protein expression and global H4K8Ac, H4K12Ac, and H4K16Ac in rat colonic muscularis externae. B: TSA enhanced p65 binding to the Icam-1 promoter. It also increased histone H4K8Ac, H4K12Ac, and H4K16Ac associated with the Icam-1 promoter. In addition, TSA significantly attenuated HDAC3 association with the Icam-1 promoter. N = 3. *Significantly different vs. Ctr (P < 0.05).
DISCUSSION
The transcription factor NF-κB is a key regulator of inflammation. In response to the activation of membrane receptors of proinflammatory mediators, NF-κB translocates to the nucleus. However, nuclear translocation by itself does not induce transcription of target genes. Chromatin modification is the next level that regulates the access of the NF-κB complex to its cognate sequences on the promoters of genes involved in the inflammatory response (19, 33). The addition/removal of acetyl groups to lysine residues on the NH2-terminal tails of histone proteins is the most understood mechanism of regulation of DNA/histone interaction. Our findings show that colonic inflammation has no effect on global HDAC activity, but it significantly downregulates HDAC3 protein expression in colonic muscularis externae. By contrast, global HDAC activity increases in the tissues of several types of cancers (1), decreasing the transcription rates of multiple susceptible genes, specifically tumor suppressor genes promoting apoptosis. HDAC inhibitors are in clinical trials or are approved by FDA for treatment of cancer (25). Treating colonic inflammation with HDAC inhibitors would be counterproductive because HDAC3 is already suppressed.
HDACs, expressed in all mammalian tissues, play critical roles in differentiation, growth, and homeostasis (46). Our understanding of HDACs is still evolving. According to a recent classification, mammalian HDACs fall into one of four classes depending on their structure and amino acid sequence (5, 8, 46). Class I (HDAC1–3 and 8) HDACs are TSA sensitive and reside predominantly in the nucleus; mammalian tissues express these ubiquitously. Class II (HDAC4–7, and 9) and class IV (HDAC10) HDACs are tissue specific, TSA sensitive, and may be found in both the cytoplasm and the nucleus; they undergo stimulus-dependent nucleo-cytoplasmic shuttling. Class III (SIRT1–7) HDACs belong to the sirtuins family; they are insensitive to TSA and can be found in the nucleus, cytoplasm, and mitochondria (27). We found that the muscularis externae tissues express HDAC3 in both the cytoplasm and the nucleus. TNBS inflammation reduced the expression of HDAC3 and the association of HDAC3 with the nucleosomes containing the NK-κB binding sequences on the Icam-1 promoter. The HDACs do not have an intrinsic affinity for DNA; multi-protein complexes comprised of the corepressors silencing mediator of retinoic and thyroid receptors (SMRT) and nuclear receptor corepressor (N-CoR) recruit them to the DNA (20). However, the mechanisms of action of NO on HDACs depend on their cellular location and on the intracellular concentration of NO. In human umbilical vein endothelial cells, NO inhibits serum-induced histone acetylation by enhancing the activity of cytoplasmic class II HDACs 5 and 6 and shuttling them to the nucleus (12). Class I HDACs are not involved in this effect. In vitro findings in cultures of rat cortical neurons showed that brain-derived neurotrophic factor (BDNF)-dependent generation of low nanomolar concentration of NO (catalyzed by neuronal NOS) S-nitrosylates HDAC2 at residues Cys 262 and Cys 274 dissociate it from the chromatin and facilitate the acetylation of histones H3 and H4 (30). Our findings show that high micromolar concentration of NO, catalyzed by iNOS, in TNBS inflammation upregulates the transcription of HDAC3 to increase its association with the Icam-1 promoter and suppress the transcription of Icam-1.
Our findings suggest that NO release at the onset of inflammatory response, when the immune and nonimmune cells express ICAM-1 to recruit leukocytes, serves as an early anti-inflammatory mediator; the incubation of inflamed tissues with GSNO, 24 h after inflammatory insult, suppressed ICAM-1 expression. However, it is noteworthy that ICAM-1 is only one of the several adhesion molecules that together regulate the trafficking of leukocytes. The epigenetic regulation is gene specific. We did not investigate whether NO also blocks the expression of other adhesion molecules or other inflammatory mediators. The inhibition of ICAM-1 by itself may have a limited effect on the overall inflammatory response (45). In addition, ICAM-1 inhibition has little effect on inflammation once it is past the initial stage of leukocyte recruitment. This may explain the lack of effect of iNOS inhibitors administered during the chronic stage of inflammation (11, 34). Perhaps for these reasons, the neutralization of ICAM-1 or inhibition of its synthesis in established Crohn's disease, rheumatoid arthritis, acute kidney allograft, and cerebral stroke patients has not yielded promising outcomes (14, 24, 35, 45).
In vitro studies using cell cultures of human vascular endothelial cells reported that NO inhibits the proinflammatory cytokine-induced translocation of p65 NF-κB to the nucleus by stabilizing IκB-α in the cytoplasm (4, 38). Our findings in intact ex vivo muscularis externae tissues show that NO does not affect the translocation of NF-κB to the nucleus in TNBS-induced inflammation. It enhances the transcription of HDAC3 and its association with the nucleosomes containing the NF-κB binding moieties, which reduces the acetylation level of H4K12 to suppress the expression of Icam-1. The biological effects of NO appear to be tissue and environment specific.
HDAC3 causes strong deacetylation of H2A, H4K5, and H4K12 but only partial deacetylation of H3, H2B, H4K8Ac, and H4K16Ac (13). The acetylated lysine 12 of histone H4 is one of the active marks of euchromatin. The bromodomain protein Brd2 selectively interacts with H4K12Ac during mitosis and leads to transcriptional activation in culture cells (17, 40). We found that increased transcription of HDAC3 by NO strongly suppresses H4K12Ac, while showing much weaker effects on H4K8Ac and H4K16Ac. Likewise, the decrease in the expression of HDAC3 in response to inflammation or IL-1β increased H4K12Ac but not H4K8Ac and H4K16Ac in the same segment of chromatin. RNAi-induced reduction of HDAC3 in SW480 colorectal cancer cells also leads to increased histone H4K12 acetylation but not acetylation of K5, K8, or K16 (39). Taken together, these results indicate that H4K12 appears to be a strong substrate of HDAC3 in colonic muscularis externae. However, chromatin modification is stimulus, cell, and gene segment specific.
In conclusion, chromatin modification plays a critical role in the induction of the inflammatory response in the muscularis externae of the colon. During the acute stage of inflammation, the proinflammatory mediators activate and translocate NF-κB to the nucleus. At the same time, these mediators transcriptionally downregulate the expression of HDAC3, which leads to its dissociation from the nucleosomes containing the binding moieties of NF-κB on the Icam-1 promoter. The decrease in HDAC3 was accompanied by increase in the acetylation of H4K12 followed by increase in the binding of nuclear NF-κB to its DNA recognition sequences on the Icam-1 promoter, which may reflect the known role of H4K12 acetylation in chromatin relaxation (3). Our data show that NO acts as an anti-inflammatory mediator; it upregulates the transcription of HDAC3 to suppress the acetylation of H4K12, which results in chromatin compaction, a decrease in the binding of NK-κB to the Icam-1 promoter, and suppression of ICAM-1.
GRANTS
This study is partly supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-032346 (S. K. Sarna).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.L. performed experiments; Q.L. analyzed data; Q.L. and S.K.S. interpreted results of experiments; Q.L. prepared figures; Q.L. drafted the manuscript; S.K.S. conception and design of research; S.K.S. edited and revised the manuscript; S.K.S. approved the final version of the manuscript.
REFERENCES
- 1. Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer 49: 145–154, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol 296: G632–G642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christensen ME, Rattner JB, Dixon GH. Hyperacetylation of histone H4 promotes chromatin decondensation prior to histone replacement by protamines during spermatogenesis in rainbow trout. Nucleic Acids Res 12: 4575–4592, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dikopoulos N, Nussler AK, Liptay S, Bachem M, Reinshagen M, Stiegler M, Schmid RM, Adler G, Weidenbach H. Inhibition of nitric oxide synthesis by aminoguanidine increases intestinal damage in the acute phase of rat TNB-colitis. Eur J Clin Invest 31: 234–239, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Falbo KB, Shen X. Histone modifications during DNA replication. Mol Cells 28: 149–154, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris ML, Schiller HJ, Reilly PM, Donowitz M, Grisham MB, Bulkley GB. Free radicals and other reactive oxygen metabolites in inflammatory bowel disease: cause, consequence or epiphenomenon? Pharmacol Ther 53: 375–408, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Hokari R, Kato S, Matsuzaki K, Kuroki M, Iwai A, Kawaguchi A, Nagao S, Miyahara T, Itoh K, Sekizuka E, Nagata H, Ishii H, Miura S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic Biol Med 31: 153–163, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Hosoi T, Goto H, Arisawa T, Niwa Y, Okada N, Ohmiya N, Hayakawa T. Role of nitric oxide synthase inhibitor in experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid in rats. Clin Exp Pharmacol Physiol 28: 9–12, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Illi B, Dello Russo C, Colussi C, Rosati J, Pallaoro M, Spallotta F, Rotili D, Valente S, Ragone G, Martelli F, Biglioli P, Steinkuhler C, Gallinari P, Mai A, Capogrossi MC, Gaetano C. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res 102: 51–58, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Johnson CA, White DA, Lavender JS, O'Neill LP, Turner BM. Human class I histone deacetylase complexes show enhanced catalytic activity in the presence of ATP and co-immunoprecipitate with the ATP-dependent chaperone protein Hsp70. J Biol Chem 277: 9590–9597, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kahan BD, Stepkowski S, Kilic M, Katz SM, Van Buren CT, Welsh MS, Tami JA, Shanahan WR., Jr Phase I and phase II safety and efficacy trial of intercellular adhesion molecule-1 antisense oligodeoxynucleotide (ISIS 2302) for the prevention of acute allograft rejection. Transplantation 78: 858–863, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kankuri E, Asmawi MZ, Korpela R, Vapaatalo H, Moilanen E. Induction of iNOS in a rat model of acute colitis. Inflammation 23: 141–152, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Kankuri E, Vaali K, Knowles RG, Lahde M, Korpela R, Vapaatalo H, Moilanen E. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J Pharmacol Exp Ther 298: 1128–1132, 2001 [PubMed] [Google Scholar]
- 17. Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell 13: 33–43, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Kawachi S, Cockrell A, Laroux FS, Gray L, Granger DN, van der Heyde HC, Grisham MB. Role of inducible nitric oxide synthase in the regulation of VCAM-1 expression in gut inflammation. Am J Physiol Gastrointest Liver Physiol 277: G572–G576, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Keskin D, Kalluri R. NF-κB-induced chromatin remodeling regulates angiogenesis. Blood 116: 312–313, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Li J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19: 4342–4350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Q, Dashwood WM, Zhong X, Al-Fageeh M, Dashwood RH. Cloning of the rat beta-catenin gene (Ctnnb1) promoter and its functional analysis compared with the Catnb and CTNNB1 promoters. Genomics 83: 231–242, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Sarna SK. Nuclear myosin II regulates the assembly of preinitiation complex for ICAM-1 gene transcription. Gastroenterology 137: 1051–1060, 1060e1–1060e3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lusser A. Acetylated, methylated, remodeled: chromatin states for gene regulation. Curr Opin Plant Biol 5: 437–443, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Maksymowych WP, Blackburn WD, Jr, Tami JA, Shanahan WR., Jr A randomized, placebo controlled trial of an antisense oligodeoxynucleotide to intercellular adhesion molecule-1 in the treatment of severe rheumatoid arthritis. J Rheumatol 29: 447–453, 2002 [PubMed] [Google Scholar]
- 25. Marks PA, Xu WS. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107: 600–608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCafferty DM, Miampamba M, Sihota E, Sharkey KA, Kubes P. Role of inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut 45: 864–873, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller MJ, Sadowska-Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther 264: 11–16, 1993 [PubMed] [Google Scholar]
- 29. Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res 64: 5767–5774, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455: 411–415, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Pazdrak K, Shi XZ, Sarna SK. TNF-alpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology 127: 1096–1109, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut 36: 718–723, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-κB1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol 298: L197–L209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ribbons KA, Currie MG, Connor JR, Manning PT, Allen PC, Didier P, Ratterree MS, Clark DA, Miller MJ. The effect of inhibitors of inducible nitric oxide synthase on chronic colitis in the rhesus monkey. J Pharmacol Exp Ther 280: 1008–1015, 1997 [PubMed] [Google Scholar]
- 35. Schreiber S, Nikolaus S, Malchow H, Kruis W, Lochs H, Raedler A, Hahn EG, Krummenerl T, Steinmann G. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn's disease. Gastroenterology 120: 1339–1346, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G274–G284, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111: 871–885, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Spiecker M, Darius H, Kaboth K, Hubner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol 63: 732–739, 1998 [PubMed] [Google Scholar]
- 39. Spurling CC, Godman CA, Noonan EJ, Rasmussen TP, Rosenberg DW, Giardina C. HDAC3 overexpression and colon cancer cell proliferation and differentiation. Mol Carcinog 47: 137–147, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Umehara T, Nakamura Y, Jang MK, Nakano K, Tanaka A, Ozato K, Padmanabhan B, Yokoyama S. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J Biol Chem 285: 7610–7618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Umland O, Heine H, Miehe M, Marienfeld K, Staubach KH, Ulmer AJ. Induction of various immune modulatory molecules in CD34(+) hematopoietic cells. J Leukoc Biol 75: 671–679, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Van Assche G, Rutgeerts P. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases. I. Immunology and therapeutic potential of antiadhesion molecule therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 288: G169–G174, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wong BL, Zhu SL, Huang XR, Ma J, Xia HH, Bucala R, Wong BC, Lan HY. Essential role for macrophage migration inhibitory factor in gastritis induced by Helicobacter pylori. Am J Pathol 174: 1319–1328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin “prepattern” and histone modifiers in a fate choice for liver and pancreas. Science 332: 963–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yacyshyn BR, Bowen-Yacyshyn MB, Jewell L, Tami JA, Bennett CF, Kisner DL, Shanahan WR., Jr A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology 114: 1133–1142, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9: 206–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]