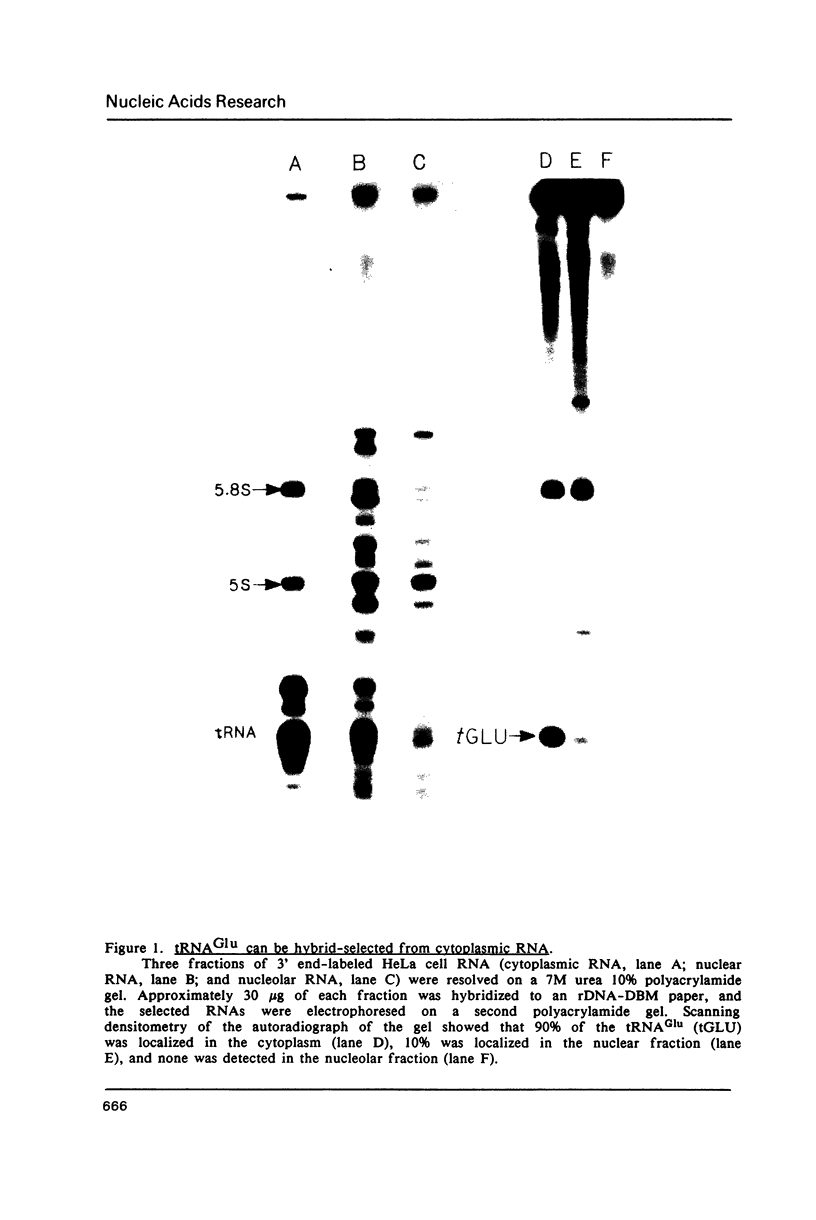
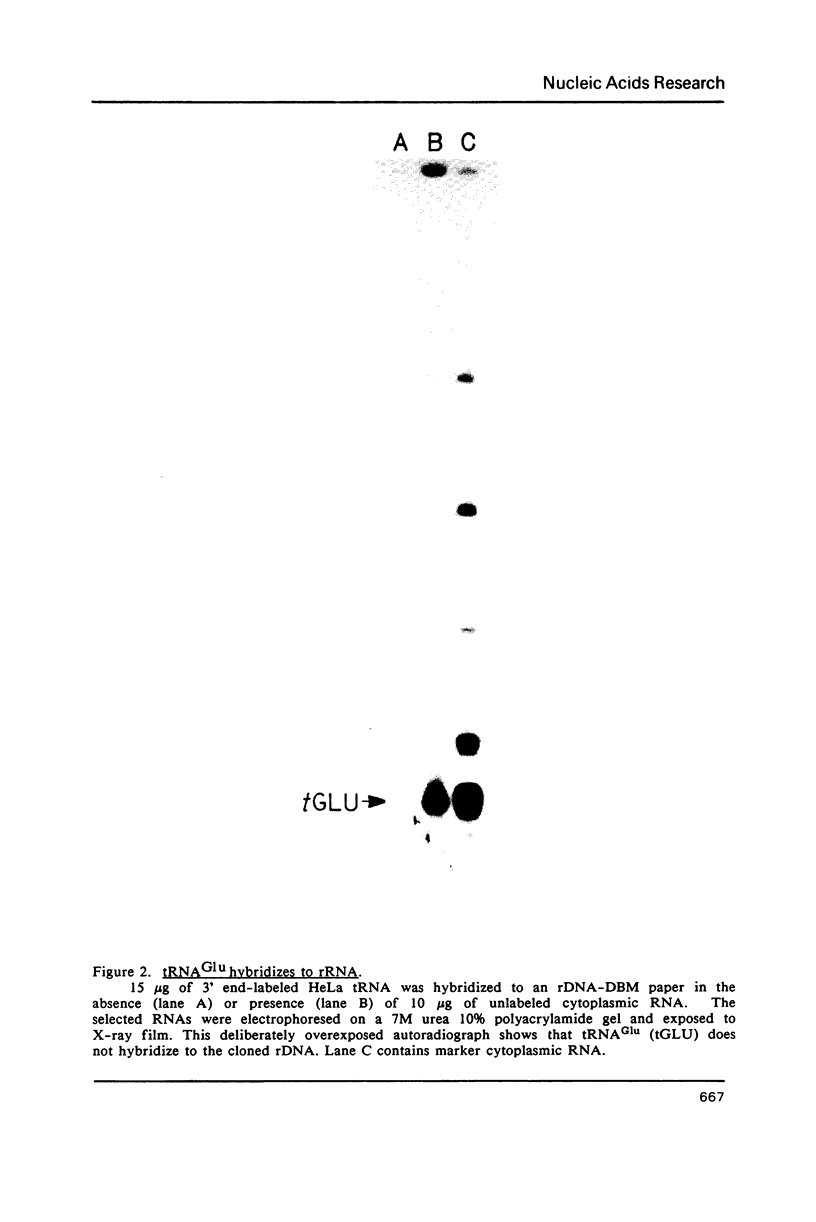
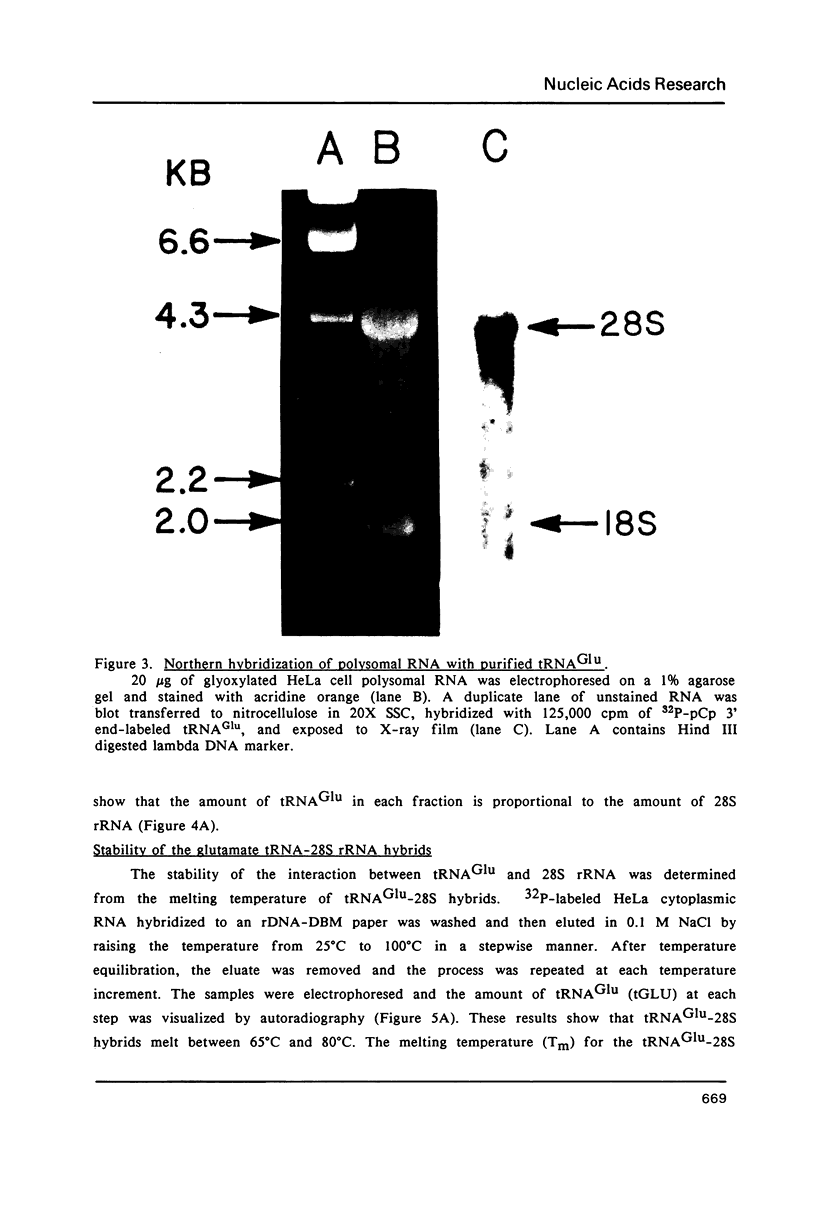
Abstract
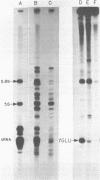
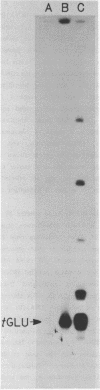
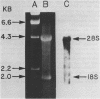
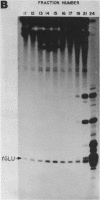
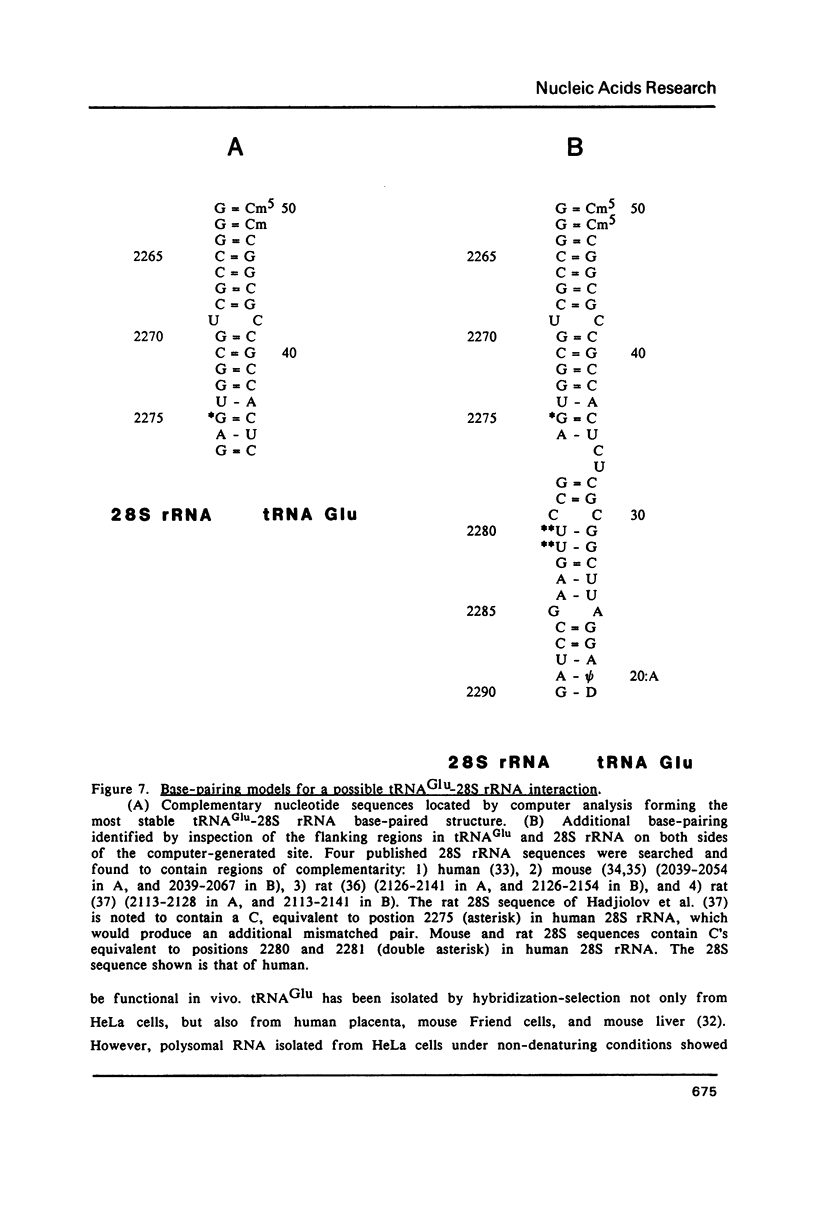
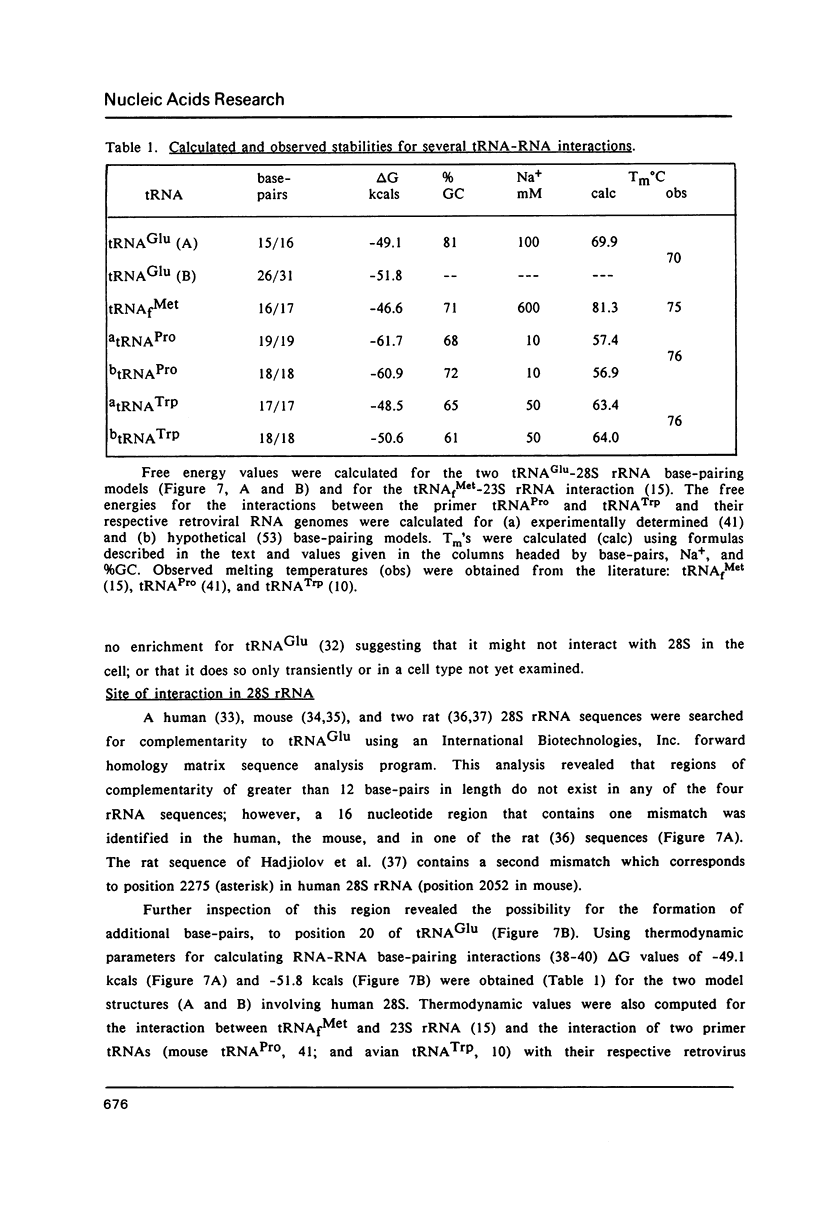
A human glutamate tRNA has been shown to form stable hybrids with 28S ribosomal RNA. This tRNA was purified from HeLa cell cytoplasmic RNA by RNA-RNA solution hybridization followed by the isolation of tRNA-28S rRNA complexes by hybridization-selection with ribosomal DNA or by recovery of the 28S peak from formamide-sucrose gradients. The single hybridizing tRNA species was identified as tRNAGluCUC by sequencing: pU-C-C-C-U-G-G-U-G-m2G-U-C-phi-A-G-U-G-G-D-phi-A-G-G-A-U-U- C-G-G-C-G-C-U-C-U-C-A-C-C-G-C-G-G-C-m5C-m5C-G-G-G-Tm-phi-C-G-A- U-U-C-C-C-G-G-U-C-A-G-G-G-A-A-C-C-AOH. Computer analysis located a nucleotide sequence near the middle of human 28S rRNA which is complementary to 15-26 nucleotides between residues 20 and 50 of this tRNA. An interaction between this tRNA and 28S rRNA suggests that tRNAGluCUC may have functions in the cell in addition to translation.
Full text
PDF





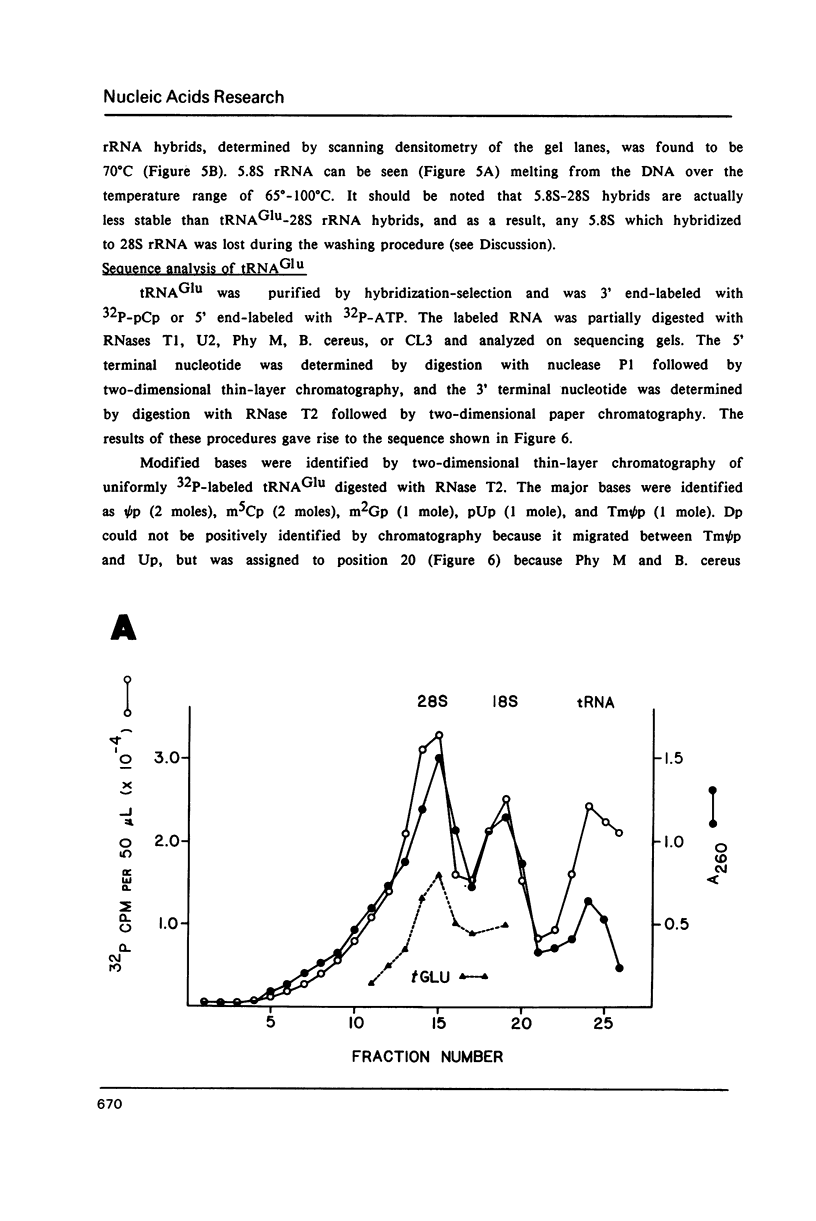
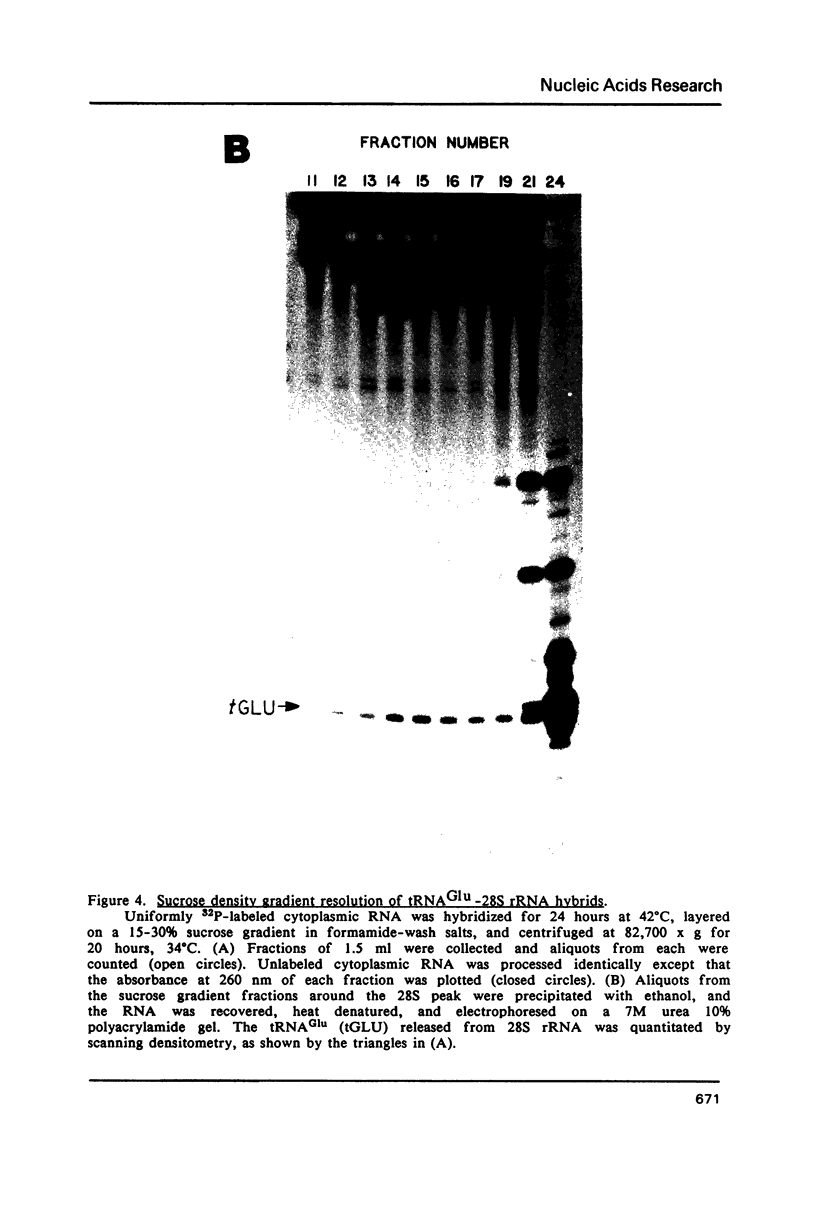
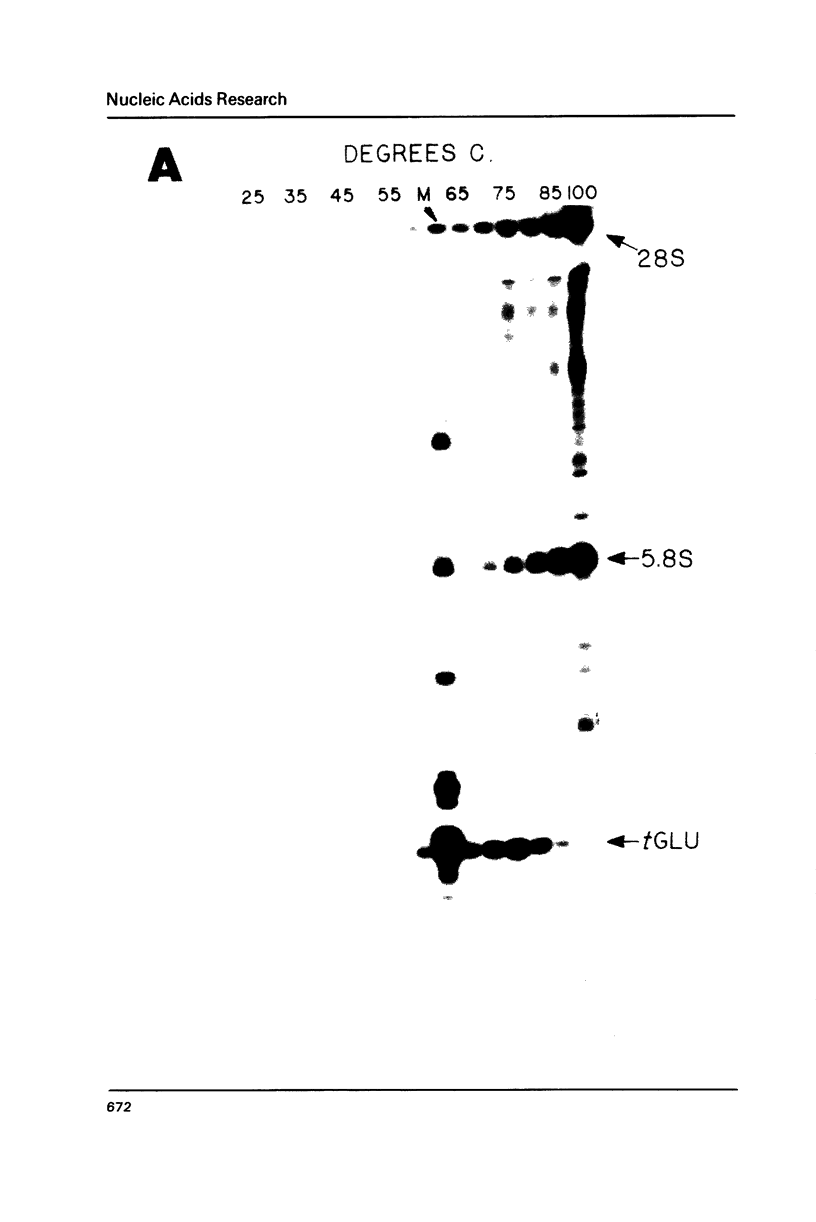
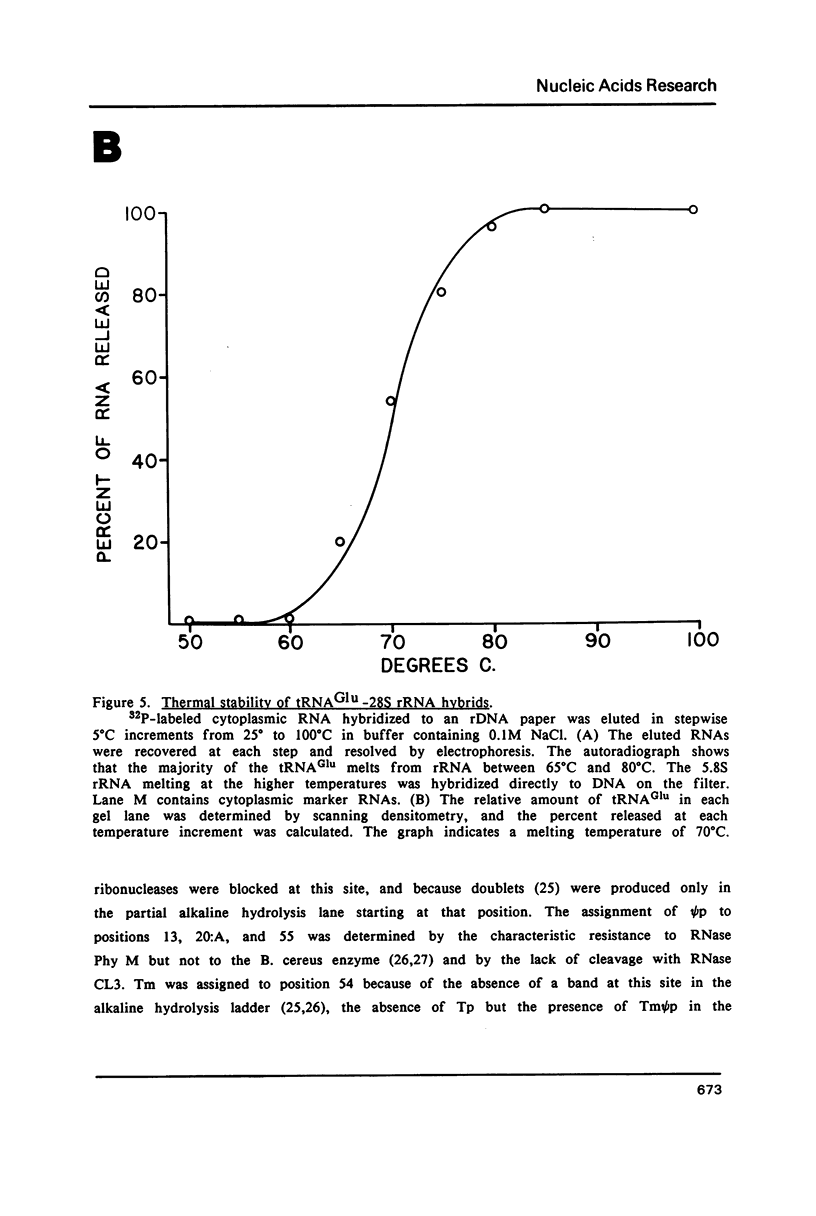






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Aspects of biochemical catalysis. Cell. 1984 Feb;36(2):237–239. doi: 10.1016/0092-8674(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Bernstein L. B., Mount S. M., Weiner A. M. Pseudogenes for human small nuclear RNA U3 appear to arise by integration of self-primed reverse transcripts of the RNA into new chromosomal sites. Cell. 1983 Feb;32(2):461–472. doi: 10.1016/0092-8674(83)90466-x. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Chan J. C., Yang J. A., Dunn M. J., Agris P. F., Wong T. W. The nucleotide sequence of a glutamate tRNA from rat liver. Nucleic Acids Res. 1982 Aug 11;10(15):4605–4608. doi: 10.1093/nar/10.15.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Wolin S. L., Steitz J. A., Lodish H. F. Transfer RNA is an essential component of the ubiquitin- and ATP-dependent proteolytic system. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1341–1345. doi: 10.1073/pnas.82.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs D. L., Gillam I. C., Tener G. M. The structure of tRNA 5 Lys from Drosophila melanogaster. Nucleic Acids Res. 1982 Oct 25;10(20):6393–6399. doi: 10.1093/nar/10.20.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Kintner C., Lund E. Specific binding of tRNAMet to 23S rRNA of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1071–1075. doi: 10.1073/pnas.75.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Georgiev O. I., Nosikov V. V., Yavachev L. P. Primary and secondary structure of rat 28 S ribosomal RNA. Nucleic Acids Res. 1984 Apr 25;12(8):3677–3693. doi: 10.1093/nar/12.8.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Peters G. G., Dahlberg J. E. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979 Nov 10;254(21):10979–10985. [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hassouna N., Michot B., Bachellerie J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984 Apr 25;12(8):3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgcoth C., Hayenga K., Harrison M., Ortwerth B. J. Lysine tRNAs from rat liver: lysine tRNA sequences are highly conserved. Nucleic Acids Res. 1984 Mar 12;12(5):2535–2541. doi: 10.1093/nar/12.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Mayrand S., Pederson T. Sequence complexity of nuclear and messenger RNA in HeLa cells. J Mol Biol. 1980 Apr 25;138(4):755–778. doi: 10.1016/0022-2836(80)90064-9. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzara G. P., Seidman J. G., McClain W. H., Yesian H., Abelson J., Guthrie C. Nucleotide sequence of an arginine transfer ribonucleic acid from bacteriophage T4. J Biol Chem. 1977 Nov 25;252(22):8245–8253. [PubMed] [Google Scholar]
- Michot B., Hassouna N., Bachellerie J. P. Secondary structure of mouse 28S rRNA and general model for the folding of the large rRNA in eukaryotes. Nucleic Acids Res. 1984 May 25;12(10):4259–4279. doi: 10.1093/nar/12.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A., Higashinakagawa T., Nakamura T., Kondo S., Saito H., Yoshimatsu H., Suzuki H., Munakata Y., Tsuchiya M. RNA tumor virus-like particles in human fetal thymus cells stimulated by human B-cells. Gan. 1983 Dec;74(6):802–805. [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Dahlberg J. E. RNA-directed DNA synthesis in Moloney murine leukemia virus: interaction between the primer tRNA and the genome RNA. J Virol. 1979 Aug;31(2):398–407. doi: 10.1128/jvi.31.2.398-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Glover C. tRNA's and priming of RNA-directed DNA synthesis in mouse mammary tumor virus. J Virol. 1980 Jul;35(1):31–40. doi: 10.1128/jvi.35.1.31-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Agne K. M., Wu R. Isolation and structure of a rat cytochrome c gene. J Biol Chem. 1981 Jun 25;256(12):6480–6486. [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster containing the genes for tRNAAsp, tRNAGly and tRNAGlu, and pseudogenes. Nucleic Acids Res. 1982 Jul 24;10(14):4441–4448. doi: 10.1093/nar/10.14.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Singhal R. P., Fallis P. A. Structure, function, and evolution of transfer RNAs (with appendix giving complete sequences of 178 tRNAs). Prog Nucleic Acid Res Mol Biol. 1979;23:227–290. doi: 10.1016/s0079-6603(08)60135-x. [DOI] [PubMed] [Google Scholar]
- Soidla T. R., Golovanov E. I. Vozmozhnaia rol' tRNK1Lys v uznavanii uchastkov pre-mRNK, imeiushchikh reguliatornoe znachenie dlia splaisinga. Mol Biol (Mosk) 1984 Jan-Feb;18(1):277–285. [PubMed] [Google Scholar]
- Soidla T. R. Vozmozhnaia rol' tRNK1Lys v splaisinge mitochondrial'nykh transkriptov u drozhzhei. Mol Biol (Mosk) 1983 Nov-Dec;17(6):1154–1161. [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1984;12 (Suppl):r1–57. [PMC free article] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cywinski A. A defective retrovirus particle (SE21Q1b) packages and reverse transcribes cellular RNA, utilizing tRNA-like primers. J Virol. 1984 Aug;51(2):267–271. doi: 10.1128/jvi.51.2.267-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vakharia V. N., Singhal R. P. The structure of aspartate transfer RNA from rabbit liver. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1072–1081. doi: 10.1016/0006-291x(82)91079-8. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Walker T. A., Endo Y., Wheat W. H., Wool I. G., Pace N. R. Location of 5.8 S rRNA contact sites in 28 S rRNA and the effect of alpha-sarcin on the association of 5.8 S rRNA with 28 S rRNA. J Biol Chem. 1983 Jan 10;258(1):333–338. [PubMed] [Google Scholar]
- Walker T. A., Pace N. R. 5.8S ribosomal RNA. Cell. 1983 Jun;33(2):320–322. doi: 10.1016/0092-8674(83)90413-0. [DOI] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Burke J. R., Stulberg M. P. Studies on the function of the non-primer tRNAs associated with the 70 S RNA of avian myeloblastosis virus. Biochim Biophys Acta. 1982 Feb 26;696(2):201–207. doi: 10.1016/0167-4781(82)90029-x. [DOI] [PubMed] [Google Scholar]
- Waters L. C. Chromatographic evidence that the AAA-coding isoacceptor of lysine tRNA primes DNA synthesis in murine mammary tumor virus. Virology. 1981 Jul 30;112(2):766–769. doi: 10.1016/0042-6822(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Ruyechan W. T., Douthart R. J. Denaturation and renaturation of Penicillium chrysogenum mycophage double-stranded ribonucleic acid in tetraalkylammonium salt solutions. Biochemistry. 1981 May 26;20(11):2999–3002. doi: 10.1021/bi00514a002. [DOI] [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage lambda and 186 DNA. J Mol Biol. 1970 Aug;51(3):501–521. doi: 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Wurst R. M., Vournakis J. N., Maxam A. M. Structure mapping of 5'-32P-labeled RNA with S1 nuclease. Biochemistry. 1978 Oct 17;17(21):4493–4499. doi: 10.1021/bi00614a021. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Hwang D. L., Kiggans J. O., Jr, Yang D. M., Stringer C. D., Moore D. J., Hartman F. C. In vitro association of selective +RNA species with 28S RNA of mouse cells. Biochem Biophys Res Commun. 1978 Jan 30;80(2):443–450. doi: 10.1016/0006-291x(78)90697-6. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]