Abstract
Plasma VLDL and LDL cholesterol were markedly elevated (>40-fold) in high-responding opossums, but moderately elevated (6-fold) in low-responding opossums after they had consumed a high-cholesterol and high-fat diet for 24 wk. In both high- and low-responding opossums, plasma triglycerides were slightly elevated, threefold and twofold, respectively. Dietary challenge also induced fatty livers in high responders, but not in low responders. We studied the lipid composition, histopathological features, and gene expression patterns of the fatty livers. Free cholesterol (2-fold), esterified cholesterol (11-fold), and triglycerides (2-fold) were higher in the livers of high responders than those in low responders, whereas free fatty acid levels were similar. The fatty livers of high responders showed extensive lobular disarray by histology. Inflammatory cells and ballooned hepatocytes were also present, as were perisinusoidal fibrosis and ductular proliferation. In contrast, liver histology was normal in low responders. Hepatic gene expression revealed differences associated with the development of steatohepatitis in high responders. The accumulation of hepatic cholesterol was concomitant with upregulation of the HMGCR gene and downregulation of the CYP27A1, ABCG8, and ABCB4 genes. Genes involved in inflammation (TNF, NFKB1, and COX2) and in oxidative stress (CYBA and NCF1) were upregulated. Upregulation of the growth factor genes (PDGF and TGFB1) and collagen genes (Col1A1, Col3A1, and Col4A1) was consistent with fibrosis. Some of the histological characteristics of the fatty livers of high-responding opossums imitate those in the livers of humans with nonalcoholic steatohepatitis.
Keywords: ABCB4, hypercholesterolemia, Monodelphis domestica, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
the gray short-tailed opossum (Monodelphis domestica) has been established as a laboratory animal for biomedical research (39). Inbreeding and selection for high- or low-plasma cholesterol in response to a high-cholesterol and high-fat (HCHF) diet led to the development of partially inbred strains of laboratory opossums that exhibit dramatic differences in plasma cholesterol when they are challenged with the HCHF diet. After an 8-wk challenge, the HCHF diet induces a marked increase (>10-fold) in VLDL and LDL cholesterol (V+LDLC) in high responders, but only a slight increase (<2-fold) in V+LDLC in low responders. However, plasma V+LDLC levels of high and low responders do not differ on the basal diet (17, 28).
In search of genes that mediate diet-induced hypercholesterolemia in high responders, we observed that the levels of phospholipids and cholesterol in gall bladder bile were significantly lower in high responders relative to those in low responders on the HCHF diet (4). This observation led us to focus on the ABCB4 gene, because the ABCB4 protein facilitates secretion of phospholipid and cholesterol from the liver into the bile (18, 33). Two missense mutations were identified in the ABCB4 gene of high responders, and ABCB4 allelic variants showed a strong association with variations in plasma V+LDLC concentration on the HCHF diet in genetic crosses involving high responders and low responders (4). Therefore, a defective ABCB4 gene that impairs the ability to dispose of excess cholesterol through secretion into the bile for excretion in the feces is largely responsible for elevating plasma V+LDLC in high responders fed the HCHF diet.
Impairment of biliary cholesterol excretion not only elevates plasma V+LDLC, but also leads to the development of fatty livers in high responders on the HCHF diet. Low responders do not develop fatty livers on the same diet. We also observed that plasma levels of bile acids, liver enzymes, and bilirubin were elevated in hypercholesterolemic opossums (3, 4), suggesting that they have liver injury because phospholipid-deficient bile cannot provide adequate protection of the canalicular membrane from damage by bile acids (25). Here we present a study of the fatty livers of high responders that are induced by the HCHF diet. We compared liver histology of high and low responders after a 24-wk dietary challenge. We also compared the expression of genes involved in lipid metabolism, inflammation, and fibrosis to investigate differences in gene expression that were associated with the development of steatohepatitis in high responders.
METHODS
Laboratory opossums and diet.
High- and low-responding opossums were produced and maintained at the Texas Biomedical Research Institute under laboratory conditions for this species (39). TestDiet (Richmond, IN) prepared the basal (5ATD) and HCHF (5AM9) diets. The basal diet had a cholesterol content of 0.016% and a fat content of 10%. The HCHF diet had a cholesterol content of 0.7% and a fat content of 19%. Opossums were fed the basal diet from weaning to 13–18 mo of age, when they were switched to the HCHF diet for 24 wk. The Institutional Animal Care and Use Committee of Texas Biomedical Research Institute approved the protocol.
Blood and tissue samples.
Blood was collected from opossums after an overnight fast by cardiac puncture under isoflurane anesthesia. At the conclusion of the dietary challenge, blood was collected, and the animals were euthanized. Body weights were recorded; the range for female opossums was 70–80 g and for males 96–126 g. Both males and females were included in the high- and low-responding groups. The livers and spleens were removed and weighed. One liver sample from each animal was put into 10% neutral buffered formalin for histology, and several liver samples were snap-frozen in liquid nitrogen for measuring hepatic lipids and gene expression.
Measurement of plasma cholesterol and triglycerides.
Total cholesterol, high-density lipoprotein cholesterol (HDLC), and triglycerides in plasma samples were measured on a Bayer Express Plus Analyzer (Bayer, East Walpole, MA). V+LDLC concentration was calculated as the difference between total cholesterol concentration and HDLC concentration (17).
Measurement of glucose and hepatic function markers.
Analyses of glucose, proteins, liver enzymes, and bilirubin in serum samples were performed on a Beckman Unicell DxC600 Chemistry Analyzer (Beckman Coulter, Fullerton, CA).
Measurement of hepatic lipids.
Commercially available enzymatic reagents were used to quantify hepatic lipids; all enzymatic assays were performed in 96-well microtiter plates. For the determination of total cholesterol, free cholesterol, and triglycerides, liver samples (100 mg) were homogenized, and lipids were extracted with 2 ml of chloroform and methanol (2:1), as described by Folch et al. (8). Lipids were dissolved in 2% Triton X-100 (Sigma, St. Louis, MO), as described by Carr et al. (2). Total cholesterol was measured with reagents from Roche Diagnostics (Indianapolis, IN) using the CHOD-PAP method, and free cholesterol was measured using a Free Cholesterol E reagent kit (Wako Diagnostics, Richmond, VA). Esterified cholesterol was the difference between total and free cholesterol. Triglycerides were measured using a triglyceride reagent kit from Stanbio Laboratory (Boerne, TX). For the determination of free fatty acids, liver samples (50 mg) were homogenized, and lipids were extracted with 2 ml of ethanol. The extracts were dried under nitrogen and dissolved in 2% Triton X-100 for assay with reagents from the NEFA-HR(2) kit (Wako Diagnostics) using oleic acid as a standard.
Histopathological analyses of liver and spleen.
Portions of liver and spleen were fixed for several hours in 10% neutral buffered formalin and postfixed in 70% ethanol. After processing and paraffin embedding in the usual manner, 4-μm-thick liver sections were stained with hematoxylin-eosin and Masson trichrome. Spleen sections were stained with hematoxylin-eosin. Nonalcoholic fatty liver disease features were scored according to Kleiner et al. (16). Immunostains were performed on a Ventana XT stainer for the following antigens (clone, manufacturer): AE1/3 (PCK26, Ventana), CD 31 (1A10, CellMarque), CD68 (KP1, Dako), desmin (DE-R-11, Dako), vimentin (V9, Ventana), and smooth muscle actin (1A4, Dako).
Quantification of gene expression.
Gene expression was quantified by real-time PCR using SYBR Green chemistry on a 7900 HT fast real-time PCR system (Applied Biosystems, Foster City, CA). Primers for real-time PCR were designed based on published sequences using the Primer3 program (30). Primer sequences are available upon request. A dissociation curve analysis was performed to verify that a single product was amplified for each gene. Total RNA was isolated from frozen, pulverized tissues using the TRI Reagent (Molecular Research Center, Cincinnati, OH) and quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Total RNA was then treated with DNAse from the TURBO DNA-free kit (Applied Biosystems) and reverse-transcribed into single-stranded cDNA with random primers and reagents from the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). An aliquot of the cDNA was mixed with gene-specific primers and fast SYBR Green master mix (Applied Biosystems) for PCR amplification, according to the manufacturer's instructions. A standard curve was generated for each gene by serial dilutions of cDNAs pooled from high and low responders. Gene expression was determined from the standard curve and normalized to 18S rRNA.
Statistical analysis.
Data are expressed as means ± SE. For all experiments, N = 5 animals per group. Data were analyzed using Student's t-test. Differences between groups were considered significant at P < 0.05.
RESULTS
Differences in lipoprotein cholesterol and liver function markers in blood.
In this study, high and low responders were fed the HCHF diet for 24 wk compared with a dietary challenge of 6–8 wk in previous studies (17, 28). The high and low responders were genotyped for the two alleles of the ABCB4 gene. Allele 1 is the predominant allele in high responders, whereas allele 2 is the predominant allele in low responders (4). All of the high responders were homozygous for allele 1. Four of five low responders were homozygous for allele 2, and one had the ABCB4 1/2 genotype.
On the basal diet, high and low responders had similar concentrations of V+LDLC (Table 1). However, at 24 wk on the HCHF diet, the increase in plasma V+LDLC was more than 40-fold in high responders, but only 6-fold in low responders. There were no significant changes in plasma HDLC of high and low responders after the dietary challenge, but plasma triglycerides increased in high (3-fold) and low (2-fold) responders (Table 1).
Table 1.
Plasma levels of lipoprotein cholesterol and triglycerides in high and low responders fed the basal diet or the HCHF diet for 24 wk
| Basal Diet |
HCHF Diet |
|||
|---|---|---|---|---|
| High Responders | Low Responders | High Responders | Low Responders | |
| Total cholesterol, mg/dl | 78 ± 8† | 75 ± 7‡ | 773 ± 138* | 152 ± 24 |
| VLDL+LDL cholesterol, mg/dl | 15 ± 4† | 11 ± 2‡ | 718 ± 143* | 67 ± 19 |
| HDL cholesterol, mg/dl | 63 ± 6 | 64 ± 5 | 55 ± 17 | 85 ± 7 |
| Triglycerides, mg/dl | 17 ± 2† | 18 ± 1‡ | 51 ± 9 | 38 ± 8 |
Values are means ± SE; N = 5 opossums in each group. HCHF, high cholesterol and high fat.
Different from high responders on the HCHF diet, P < 0.01.
Different from low responders on the HCHF diet, P < 0.05.
Different from low responders on the HCHF diet, P < 0.005.
Fasting blood glucose levels of high and low responders on the basal and HCHF diets were normal (Table 2); the normal range is 47–168 mg/dl (38). A small but significant increase in total serum protein levels was detected in high and low responders fed the HCHF diet, but there were no significant changes in serum albumin levels. Also, alanine aminotransferase (normal range: 34–69 mg/dl) and aspartate aminotransferase (normal range: 25–76 mg/dl) were significantly elevated when high and low responders consumed the HCHF diet. Lastly, γ-glutamyltransferase (γ-GT) activity and bilirubin (total and direct) levels were elevated only in high responders fed the HCHF diet, and they were significantly different from those in low responders fed the same diet (Table 2).
Table 2.
Serum levels of glucose and liver function measures in high and low responders fed the basal diet or the HCHF diet for 24 wk
| Basal Diet |
HCHF Diet |
|||
|---|---|---|---|---|
| High Responders | Low Responders | High Responders | Low Responders | |
| Glucose, mg/dl | 90 ± 6 | 92 ± 12 | 118 ± 10 | 121 ± 30 |
| Total protein, g/dl | 5.2 ± 0.2† | 5.2 ± 0.1‡ | 6.5 ± 0.3 | 6.5 ± 0.2 |
| Albumin, g/dl | 2.9 ± 0.2 | 3.0 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.2 |
| ALT, U/l | 56 ± 6† | 57 ± 11‡ | 128 ± 19 | 118 ± 11 |
| AST, U/l | 33 ± 6† | 25 ± 2‡ | 92 ± 20 | 59 ± 13 |
| ALP, U/l | 98 ± 10 | 108 ± 9 | 118 ± 11 | 84 ± 11 |
| γ−GT, U/l | 5.6 ± 0.2† | 5.6 ± 0.4 | 10 ± 1* | <5 |
| Total bilirubin, mg/dl | 0.82 ± 0.21† | 0.60 ± 0.07 | 5.6 ± 1.9* | 0.66 ± 0.12 |
| Direct bilirubin, mg/dl | 0.26 ± 0.05† | 0.20 ± 0.03 | 3.3 ± 1.1* | 0.16 ± 0.02 |
Values are means ± SE; N = 5 opossums in each group. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; γ−GT, γ-glutamyltransferase.
Different from high responders on the HCHF diet, P < 0.05.
Different from low responders on the HCHF diet, P < 0.05.
Different from low responders on the HCHF diet, P < 0.05.
Elevated hepatic lipids in high responders.
We observed significantly enlarged livers and spleens of high responders fed the HCHF diet. The liver was 10% of body weight in high responders, but only 3% of body weight in low responders (Table 3). The spleen was ∼1% of body weight in high responders compared with 0.4% of body weight in low responders (Table 3).
Table 3.
Liver and spleen weights, and hepatic lipids of high and low responders on the HCHF diet for 24 wk
| High Responders | Low Responders | |
|---|---|---|
| Body weight, g | 70–107 | 74–126 |
| Liver weight, g | 5.6–12.9 | 2.2–3.9 |
| Spleen weight, g | 0.6–1.7 | 0.3–0.5 |
| Liver weight/body weight, % | 10.1 ± 0.8 | 3.1 ± 0.3* |
| Spleen weight/body weight, % | 1.1 ± 0.1 | 0.43 ± 0.06* |
| Total cholesterol, mg/g liver | 14.1 ± 0.8 | 2.9 ± 0.5* |
| Free cholesterol, mg/g liver | 4.9 ± 0.1 | 2.1 ± 0.3* |
| Esterified cholesterol, mg/g liver | 9.2 ± 0.8 | 0.8 ± 0.2* |
| Triglycerides, mg/g liver | 23 ± 3 | 10 ± 2* |
| Free fatty acids, μmol/g liver | 1.9 ± 0.1 | 2.4 ± 0.1 |
Values are means ± SE; N = 5 opossums in each group.
P < 0.01 vs. high responders.
An enormous amount of cholesterol and triglycerides accumulated in the enlarged livers of high responders. Hepatic cholesterol levels of high responders were fivefold higher than that of low responders. Free (2-fold) and esterified cholesterol (11-fold) levels were elevated in high responders. Hepatic triglyceride levels of high responders were twofold higher than those of low responders. but free fatty acid levels were similar in high and low responders.
Histopathological features of liver and spleen.
Low responders displayed no significant morphological changes in the liver after 24 wk on the HCHF diet (Table 4, Fig. 1A). Portal tracts and central veins were morphologically and architecturally appropriate in all animals in this group. Hepatocytes were small and uniform, without any evidence of steatosis. Hepatic plates were well preserved. Masson trichrome stain showed fibrous tissue to be confined to portal tracts and central veins. There were no inflammatory cell collections in the portal tracts or lobular parenchyma.
Table 4.
Nonalcoholic fatty liver disease features of liver sections from high and low responders on the HCHF diet for 24 wk
| Item | Definition | High Responders | Low Responders |
|---|---|---|---|
| Steatosis grade | <5% | 0 | 5 |
| 5–33% | 0 | 0 | |
| >33–66% | 0 | 0 | |
| >66% | 5 | 0 | |
| Liver cell injury | |||
| Ballooned cells | None | 0 | 5 |
| Few | 5 | 0 | |
| Many | 0 | 0 | |
| Acidophil bodies | None to rare | 4 | 5 |
| Many | 1 | 0 | |
| Inflammation | |||
| Lobular | No foci | 0 | 5 |
| <2 foci/200× field | 1 | 0 | |
| 2-4 foci/200× field | 2 | 0 | |
| >4 foci/200× field | 2 | 0 | |
| Portal | None to minimal | 5 | 5 |
| Greater than minimal | 0 | 0 | |
| Fibrosis stage | None | 0 | 5 |
| Perisinusoidal or periportal | 5 | 0 | |
| Perisinusoidal and portal/periportal | 0 | 0 | |
| Bridging fibrosis | 0 | 0 | |
| Cirrhosis | 0 | 0 |
N = 5 opossums in each group.
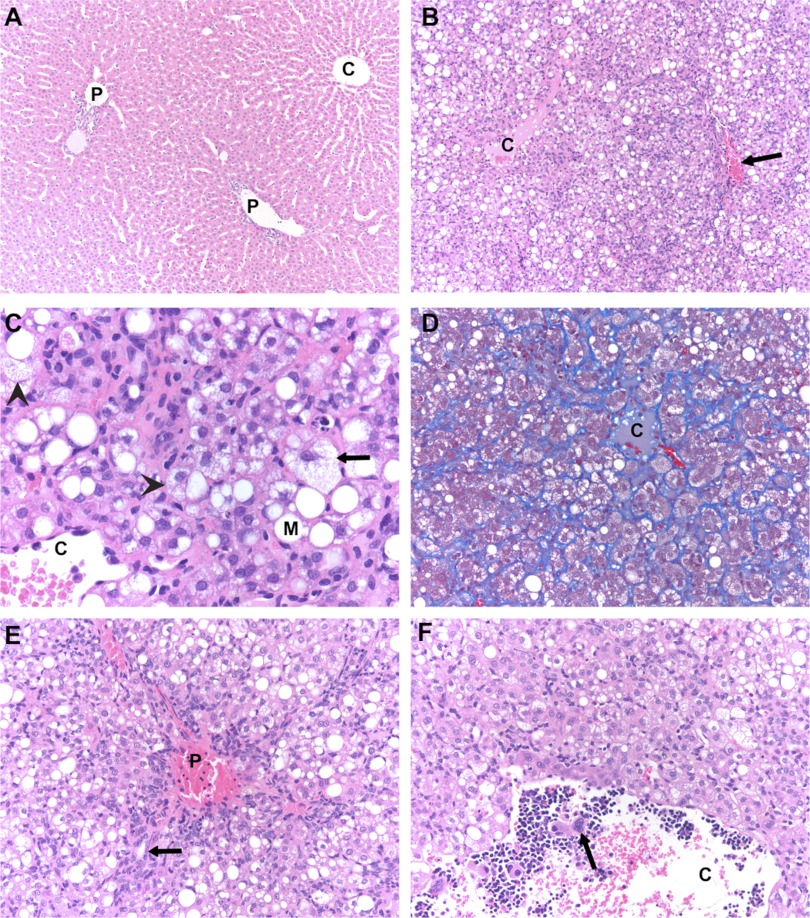
Fig. 1.
Photomicrographs of liver morphology of high and low responders fed the high-cholesterol and high-fat diet. A: low responder. Normal architecture is illustrated [hematoxylin-eosin (H&E); ×100]. C, central vein; P, portal vein. B: high responder. Steatosis and lobular disarray is shown (H&E; ×100). Arrow, portal vein. C: high responder. Steatosis with hepatocyte ballooning is shown (H&E; ×400). Arrow, ballooned hepatocyte; arrowheads, microvesicular steatosis; M, macrovesicular steatosis. D: high responder. Delicate intralobular/perisinusoidal fibrosis is shown (Masson trichrome; ×200). E: high responder. Expansion of portal tract due to ductular proliferation is shown (H&E; ×200). Arrow, ductule extending into lobular parenchyma. F: high responder. Extramedullary hematopoiesis lining the inside of a central vein is shown (H&E; ×200). Arrow indicates a megakaryocyte.
In contrast, the enlarged livers of high responders displayed histopathological features consistent with nonalcoholic fatty liver disease features in several categories (Table 4). The most prominent morphological findings in the livers of high responders were the presence of steatosis and ballooning of hepatocytes, lobular disarray, hyperplasia of perisinusoidal cells and cholangiole-like cells, intralobular perisinusoidal fibrosis, expansion of portal tracts, and extramedullary hematopoiesis. However, there was no evidence of platelet thrombi.
The extent of lobular disarray was a striking finding (Fig. 1B), but was not accompanied by loss of overall hepatic architecture; i.e., the relationship of portal tracts to central veins was preserved. There was complete loss of hepatic plates, and hepatocytes were jumbled together in a haphazard manner. Cytokeratin AE1/3 immunostain identified strings of cholangiole-like cells extending out from portal tracts between clusters of hepatocytes. Other perisinusoidal cells were difficult to characterize as either Kupffer cells or hepatic stellate (Ito) cells, but the presence of small cytoplasmic vacuoles in many suggested that the latter were predominant; immunostain for vimentin was positive in these cells and highlighted the intracytoplasmic vacuoles, while stains for desmin, smooth muscle actin, CD68, and CD31 were negative. Small collections of mononuclear inflammatory cells were also common in the lobular parenchyma, although sometimes it was difficult to distinguish these from the extramedullary hematopoiesis.
The hepatocyte steatosis was of the mixed macro- and microvesicular type and was accompanied by hepatocyte ballooning (Fig. 1C). The relative proportions of steatosis types varied among the high responders. The extent of hepatocyte ballooning was often quite marked (Fig. 1C), but did not correlate with the amount or predominant type of steatosis. Steatosis was present throughout the lobule, but did not affect every hepatocyte. Acidophil bodies were infrequent. Multinucleated giant cells could also sometimes be observed in the lobular parenchyma.
Trichrome stain showed intralobular perisinusoidal fibrosis (Fig. 1D); it was typically not confined to zone 3, but was instead scattered throughout the lobule. Neither portal fibrosis nor fibrous bridging was observed.
Portal tracts were expanded by variable amounts of duct proliferation and extramedullary hematopoiesis, and they were often stellate rather than rounded in appearance (Fig. 1E). It was common for “naked” bile ducts to appear out in the lobular parenchyma without accompanying blood vessels, but the apparent discontinuity with regular portal tracts may have been an artifact of two-dimensional observation. Inflammation was infrequent in the portal tracts; when present, it was composed of lymphocytes, but never accompanied by interface hepatitis.
The extramedullary hematopoiesis was predominantly of the myelocytic cell series, although megakaryocytes could sometimes be observed. It variably expanded portal tracts, occurred occasionally as small foci in the lobular parenchyma, and occurred frequently lining the inside of dilated central veins (Fig. 1F).
As mentioned above, the spleens of high responders were at least twice as large as those of low responders. Splenic parenchyma from high and low responders was microscopically indistinguishable and displayed a combination of trilineage extramedullary hematopoiesis and lymphocytic follicular hyperplasia.
Differences in expression of cholesterol metabolism genes.
Cholesterol in the liver is determined by the balance of de novo cholesterol synthesis, uptake of cholesterol from circulating lipoproteins, conversion of cholesterol into bile acids, and secretion of cholesterol into the bile. We studied some of the genes that play a role in these processes to investigate whether their expression differs between high and low responders (Table 5).
Table 5.
Hepatic gene expression levels of high and low responders on the HCHF diet for 24 wk
| Group and Gene Symbol | High Responders | Low Responders | P Value |
|---|---|---|---|
| Cholesterol metabolism | |||
| HMGCR | 101 ± 20 | 41 ± 5 | 0.02* |
| LDLR | 43 ± 7 | 102 ± 24 | 0.04* |
| SRBI | 77 ± 4 | 88 ± 14 | 0.43 |
| CYP7A1 | 33 ± 6 | 78 ± 22 | 0.09 |
| CYP27A1 | 31 ± 2 | 90 ± 8 | <0.001* |
| Bile secretion | |||
| ABCG5 | 78 ± 5 | 85 ± 11 | 0.54 |
| ABCG8 | 37 ± 4 | 103 ± 9 | <0.001* |
| NPC1L1 | 34 ± 4 | 85 ± 11 | 0.002* |
| ABCB11 | 85 ± 8 | 123 ± 31 | 0.27 |
| ABCB4 | 64 ± 3 | 105 ± 14 | 0.02* |
| Transcription factors | |||
| SREBP2 | 43 ± 2 | 121 ± 14 | <0.001* |
| FXR | 66 ± 6 | 70 ± 9 | 0.76 |
| LXRA | 69 ± 7 | 56 ± 3 | 0.11 |
| Fatty acid metabolism | |||
| FASN | 65 ± 4 | 86 ± 41 | 0.63 |
| CPT1 | 20 ± 2 | 25 ± 6 | 0.40 |
| PPARA | 22 ± 3 | 32 ± 6 | 0.19 |
| CD36 | 58 ± 7 | 85 ± 10 | 0.06 |
| Inflammation | |||
| TNF | 69 ± 7 | 10 ± 3 | <0.001* |
| NFKB1 | 18 ± 1 | 6 ± 1 | <0.001* |
| COX2 | 67 ± 13 | 21 ± 11 | 0.026* |
| Oxidative stress | |||
| CYBA | 21 ± 3 | 2.5 ± 0.4 | <0.001* |
| NCF1 | 63 ± 5 | 13 ± 4 | <0.001* |
| Collagen synthesis | |||
| TGFB1 | 61 ± 7 | 13 ± 2 | <0.001* |
| PDGFA | 64 ± 9 | 22 ± 11 | 0.02* |
| PDGFB | 61 ± 7 | 7 ± 1 | <0.001* |
| Col1A1 | 18 ± 1 | 0.15 ± 0.07 | <0.001* |
| Col3A1 | 18 ± 3 | 0.65 ± 0.16 | <0.001* |
| Col4A1 | 19 ± 2 | 1.8 ± 0.3 | <0.001* |
Values are means ± SE, N = 5 opossums in each group. Gene expression was measured by real-time PCR.
Significant difference between high and low responders.
The HMGCR gene encodes 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the major rate-limiting enzyme in cholesterol synthesis (32). HMGCR mRNA expression in high responders was 2.5-fold higher than that in low responders, despite hepatic cholesterol being markedly elevated in the livers of high responders (Table 3).
The LDL receptor (LDLR) mRNA levels were 2.4-fold lower in high responders relative to those in low responders, suggesting that the uptake of cholesterol from circulating LDL into the livers of high responders is reduced (32). The SRBI gene encodes a receptor for uptake of cholesterol from circulating HDL (36). The mRNA levels of SRBI were similar in high and low responders, suggesting that HDLC uptake mediated by SRBI does not influence hepatic cholesterol in high responders.
The CYP7A1 gene encodes cholesterol 7α-hydroxylase, the rate-limiting enzyme in the major pathway of bile acid synthesis (31). The difference in CYP7A1 mRNA levels between high and low responders was statistically not significant due to variable CYP7A1 expression in low responders. The CYP27A1 gene encodes sterol 27-hydroxylase, an enzyme that catalyzes the first reaction in the alternate pathway of bile acid synthesis, and also catalyzes several reactions in later steps of the major and alternate bile acid synthesis pathways (31). The CYP27A1 gene was downregulated threefold in high responders, suggesting slower bile acid synthesis.
Differences in expression of biliary cholesterol secretion genes.
The body eliminates cholesterol by secreting it from the liver into the bile, either as free cholesterol or bile acids. Secretion of cholesterol is regulated by two ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, and the Niemann-Pick C1-like 1 protein (NPC1L1). The ABCG5 and ABCG8 transporters efflux cholesterol from the liver into the bile (42), whereas the NPC1L1 protein transports cholesterol in the opposite direction, from the bile to the liver (34). ABCG5 mRNA expression was similar in high and low responders, but ABCG8 mRNA expression was 2.8-fold lower in high responders (Table 5). ABCG5 and ABCG8 function as obligate heterodimers (11); therefore, lower expression of either gene could reduce biliary cholesterol secretion in high responders. NPC1L1 expression was downregulated 2.5-fold in high responders (Table 5), which would favor a reduction in hepatic cholesterol.
Cholesterol secreted into the bile is solubilized by bile salt mixed micelles, which are composed of bile acids and phospholipids (25). Secretion of bile acids is mediated by the bile salt export pump, which is encoded by the ABCB11 gene (9). Secretion of phospholipids is mediated by ABCB4 (33). There was no significant difference in ABCB11 mRNA levels between high and low responders, but ABCB4 was slightly (1.6-fold) downregulated (Table 5).
Differences in expression of transcription factor genes.
Sterol-responsive element-binding protein-2 (SREBP2) activates the transcription of the LDLR gene and all of the genes that encode cholesterol synthesis enzymes (13). In addition, the NPC1L1 gene has been shown to be activated by SREBP2 (26). Higher levels of SREBP2 mRNA (2.8-fold) in low responders were associated with higher levels of LDLR and NPC1L1 mRNAs, except HMGCR mRNA, in low responders.
The farnesoid X receptor (FXR) forms a heterodimer with retinoid X receptor and regulates gene expression when activated by bile acids (19). FXR is a positive transcription factor for the ABCB4 gene (14). FXR mRNA levels did not differ between high and low responders. The difference in ABCB4 mRNA expression could be explained by a weaker stimulation of ABCB4 gene expression by activated FXR in high responders because of lower production of bile acids, as suggested by lower CYP27A1 expression.
The liver X receptor (LXRα) also forms a heterodimer with retinoid X receptor and stimulates gene expression when activated by oxysterols (15). High and low responders had similar levels of LXRα mRNA levels. ABCG8 is a target gene of LXR (29), and its expression was downregulated in high responders. One of the oxysterols that activates LXRα is 27-hydroxycholesterol, which is synthesized by CYP27A1. Since CYP27A1 expression was downregulated in high responders, it would reduce the cellular concentration of 27-hydroxycholesterol; therefore, the induction of ABCG8 expression in high responders was weaker than that in low responders.
Differences in expression of fatty acid metabolism genes.
We measured the expression of several genes that play a role in fatty acid synthesis and metabolism (Table 5). The FASN gene encodes fatty acid synthase, an enzyme that catalyzes the last step in the biosynthesis of fatty acids (23). There was no difference in FASN expression between high and low responders. The CPT1 gene encodes carnitine O-palmitoyltransferase, an enzyme that participates in the mitochondrial β-oxidation of fatty acids (23). The PPARA gene encodes peroxisome proliferator-activated receptor-α, a transcription factor that regulates oxidation of fatty acids (23). Similarly, CPT1 and PPARα mRNA levels in high responders did not differ from those in low responders. The CD36 gene encodes fatty acid translocase, a protein that transports fatty acids into the liver (23). High and low responders expressed CD36 mRNA at similar levels. The expression patterns of these genes could not explain the difference in hepatic triglyceride between high and low responders.
Differences in expression of inflammation genes.
Tumor necrosis factor (TNF) is a proinflammatory cytokine. The binding of TNF to its receptor leads to activation of nuclear factor-κB (NF-κB) (12). NF-κB is a transcription factor that regulates the expression of other proinflammatory cytokines, such as interleukin-6 and interleukin-1β (12). Cyclooxygenase-2 (COX-2) catalyzes the synthesis of prostaglandins from arachidonic acid, and its expression has been shown to be associated with NF-κB expression and inflammation in rodent models of nonalcoholic steatohepatitis (NASH) (5, 41). The TNF (6.9-fold), NFKB1 (3-fold), and COX2 (3.2-fold) genes were all upregulated in high responders (Table 5).
Differences in expression of oxidative stress genes.
Excessive production of reactive oxygen species by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex causes liver injury and subsequent fibrosis in repairing damaged tissues. Hepatocytes, Kupffer cells, and hepatic stellate cells express NADPH oxidase and are capable of producing reactive oxygen species (6). The CYBA gene encodes p22phox, which is one of the membrane-bound components of the NADPH complex. The NCF1 gene encodes p47phox, which is one of the cytosolic components of the NADPH complex. CYBA and NCF1 mRNA levels were upregulated 8.4-fold and 4.8-fold, respectively, in high responders, reflecting the increase in inflammatory and hepatic stellate cells, as noted histologically.
Differences in expression of fibrogenesis genes.
Hepatic stellate cells are the major fibrogenic cells in the liver (12). Transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) activated hepatic stellate cells to produce liver fibrosis during dietary challenge in high responders, as indicated by higher levels of TGFB1 (4.7-fold), PDGFA (2.9-fold), and PDGFB (8.7-fold) mRNAs (Table 5). The contribution of three collagen synthesis genes to liver fibrosis was evaluated (24). Collagen gene expression was upregulated in coordination with TGFB1 and PDGF gene expression. Expression of the Col1A1 (120-fold), Col3A1 (28-fold), and Col4A1 (10-fold) genes was highly elevated in high responders, the Col1A1 gene in particular; extremely low levels of Col1A1 mRNA were present in low responders (Table 5).
DISCUSSION
A diet enriched with cholesterol and fat induces hypercholesterolemia in high-responding opossums, but not in low-responding opossums. We reported that the two ABCB4 alleles of high and low responders are distinguished by two single nucleotide polymorphisms in the coding region. The predominant allele in high responders is allele “1”, which is associated with higher concentrations of plasma V+LDLC (4). The ABCB4 protein secretes phospholipids into the bile for the formation of phospholipid-bile salt mixed micelles. The bile salt mixed micelles solubilize cholesterol secreted from the liver more efficiently than simple bile salt micelles. Hence, ABCB4 is an important factor in biliary cholesterol secretion (25). The missense mutations in the ABCB4 gene impair phospholipid secretion and consequently biliary cholesterol excretion, leading to an accumulation of blood cholesterol when high responders consume the HCHF diet. Another function of phospholipid secretion by ABCB4 is to protect the canalicular membranes of hepatocytes and bile duct epithelial cells from damage by bile acids, because incorporation of phospholipids in the bile salt mixed micelles decreases the detergent action of bile acids to attack cell membranes (25). High responders appeared to have liver damage when fed cholesterol-enriched diets (3, 4), so we examined the diet-induced fatty livers of high responders in this study.
After 24 wk of dietary challenge, high responders had a marked increase (>40-fold) in plasma V+LDLC, whereas low responders had a moderate increase (6-fold). There was a small increase (threefold or less) in plasma triglycerides in both groups of animals. Serum aspartate aminotransferase and alanine aminotransferase levels of high and low responders were similar, but serum bilirubin levels were elevated in high responders. High responders had a jaundiced appearance characterized by yellow eyes, yellow tongues and gums, and yellow skin on their paws and ears. Serum γ-GT activity was also elevated in high responders. High serum γ-GT activity is a characteristic of humans with ABCB4 mutations who develop progressive familial intrahepatic cholestasis type 3 liver disease (10).
Hepatomegaly was observed in high responders after the dietary challenge, which was largely due to the accumulation of an enormous amount of free and esterified cholesterol. The defective ABCB4 gene plays a role in cholesterol accumulation by impairing biliary cholesterol secretion. Gene expression studies revealed the ABCB4 and ABCG8 genes were downregulated, which could exacerbate the reduction in biliary cholesterol secretion from the structural defect in the ABCB4 gene.
Histology revealed normal liver architecture and the absence of steatosis, inflammation, and fibrosis in low responders fed the HCHF diet. In contrast, the hepatocytes of high responders fed the HCHF diet were filled with macrovesicular and microvesicular lipid droplets. Ballooned hepatocytes, inflammation, fibrosis, and ductular proliferation were evident in liver sections from high responders. These pathological features are probably caused by accumulation of cholesterol in the liver and phospholipid-deficient bile.
Cholesterol in the liver, specifically free cholesterol, has been linked to hepatocellular damage in patients with NASH (1, 27) and in rodent models of NASH (20, 37). Accumulation of free cholesterol in the mitochondria of hepatocytes was shown to lead to depletion of glutathione. The cholesterol-loaded and glutathione-depleted hepatocytes became susceptible to TNF-mediated liver injury (20). Recently, Teratani et al. (35) reported the effects of a high-cholesterol diet on liver fibrosis in mice with liver injury induced by bile duct ligation. They observed Toll-like receptor 4 expression increased as a consequence of free cholesterol accumulation in hepatic stellate cells, which in turn increased TGF-β signaling and liver fibrosis.
Two factors influence the phospholipid content of the bile of high responders. One is the genetic defect in ABCB4 as discussed earlier, and the other is VLDL production. Secretion of VLDL particles from the liver requires phospholipids (40). VLDL secretion was highly elevated in high responders fed the HCHF diet, as indicated by their plasma V+LDLC levels, which could result in less phospholipids available for secretion into bile, and the phospholipid-deficient bile could cause liver injury.
Liver damage by free cholesterol and phospholipid-deficient bile in high responders triggers an inflammatory response and oxidative stress. mRNA expression of genes (TNF, NFKB1, and COX2) representative of inflammation was upregulated, as was mRNA expression of genes (CYBA and NCF1) representative of oxidative stress. The response to repair tissue damage, as shown by coordinated upregulation of the growth factor genes (TGFB1, PDGFA, and PDGFB) and fibrogenic genes (Col1A1, Col3A1, and Co41A1), resulted in histologically detectable liver fibrosis.
The Abcb4 (also known as Mdr2 in rodents) knockout mice are extreme variants whose bile is devoid of phospholipids. They develop a hepatobiliary disease due to leakage of cytotoxic bile salts from the bile ducts into portal tracts (7). At a few weeks after birth, electron microscopy reveals dilated canaliculi with relatively few microvilli, and light microscopy reveals the presence of portal tract inflammation and bile duct proliferation. As the Abcb4−/− mice develop, their livers become enlarged, and nodular outgrowths are visible at 4–6 mo of age. These nodules eventually develop into metastatic tumors in older mice (21). Furthermore, Abcb4−/− mice exhibit low levels of serum HDLC and hepatic triglycerides. Dietary supplementation of fatty acids not only increases serum HDLC and hepatic triglycerides, but it also decreases fibrosis in 2-mo-old Abcb4−/− mice (22). By comparison to Abcb4−/− mice, the ABCB4 mutations of high-responding opossums produce a less severe phenotype. The residual ABCB4 activity is manifested as a deficiency only when high-responding opossums are challenged with the HCHF diet.
In conclusion, we have established a new animal model to study the pathogenesis of NASH. A diet enriched in cholesterol and fat, together with genetic predisposition, is involved in and essential for the development of NASH with fibrosis in opossums. The mechanism that drives fatty liver formation in high responders during dietary challenge is blockage of cholesterol secretion into the bile as a consequence of mutations in ABCB4, resulting in disruption of cholesterol homeostasis and accumulation of cholesterol in the liver. The opossum model exhibits ductular proliferation, a feature that is consistent with liver pathology in Abcb4−/− mice, as well as steatosis, inflammation, ballooned hepatocytes, and fibrosis, which are histological features that characterize NASH in humans. In addition, the model supports the cholesterol hypothesis for NASH pathogenesis.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK065058, and by a grant from the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C., F.E.S., and J.L.V. conception and design of research; J.C., F.E.S., and J.F.V. performed experiments; J.C., F.E.S., and J.F.V. analyzed data; J.C., F.E.S., R.S.K., and J.L.V. interpreted results of experiments; J.C. and F.E.S. prepared figures; J.C. and F.E.S. drafted manuscript; J.C., F.E.S., R.S.K., J.F.V., and J.L.V. edited and revised manuscript; J.C., F.E.S., R.S.K., J.F.V., and J.L.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Janice MacRossin and Cynthia Mermeia for technical assistance.
REFERENCES
- 1. Caballero F, Fernandez A, De Lacy AM, Fernandez-Checa JC, Caballeria J, Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol 50: 789–796, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem 26: 39–42, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Chan J, Donalson LM, Kushwaha RS, Ferdinandusse S, VandeBerg JF, VandeBerg JL. Differential expression of hepatic genes involved in cholesterol homeostasis in high- and low-responding strains of laboratory opossums. Metabolism 57: 718–724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan J, Mahaney MC, Kushwaha RS, VandeBerg JF, VandeBerg JL. ABCB4 mediates diet-induced hypercholesterolemia in laboratory opossums. J Lipid Res 51: 2922–2928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Liu D, Bai Q, Song J, Guan J, Gao J, Liu B, Ma X, Du Y. Celecoxib attenuates liver steatosis and inflammation in non-alcoholic steatohepatitis induced by high-fat diet in rats. Mol Med Report 4: 811–816, 2011 [DOI] [PubMed] [Google Scholar]
- 6. De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology 131: 272–275, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127: 261–274, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 9. Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273: 10046–10050, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Gonzales E, Davit-Spraul A, Baussan C, Buffet C, Maurice M, Jacquemin E. Liver diseases related to MDR3 (ABCB4) gene deficiency. Front Biosci 14: 4242–4256, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem 278: 48275–48282, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 6: 425–456, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 67: 491–498, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Royo I, Blevins RA, Pelaez F, Wright SD, Cui J. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem 278: 51085–51090, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kushwaha RS, VandeBerg JF, VandeBerg JL. Effect of dietary cholesterol with or without saturated fat on plasma lipoprotein cholesterol levels in the laboratory opossum (Monodelphis domestica) model for diet-induced hyperlipidaemia. Br J Nutr 92: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Langheim S, Yu L, von Bergmann K, Lütjohann D, Xu F, Hobbs HH, Cohen JC. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J Lipid Res 46: 1732–1738, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab 4: 185–198, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 145: 1237–1245, 1994 [PMC free article] [PubMed] [Google Scholar]
- 22. Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, Kratky D, Sattler W, Reicher H, Sinner F, Gumhold J, Silbert D, Fauler G, Hofler G, Lass A, Zechner R, Trauner M. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 142: 140–151, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res 48: 1–26, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Nakken KE, Nygard S, Haaland T, Berge KE, Arnkvaern K, Odegaard A, Labori KJ, Raeder MG. Multiple inflammatory-, tissue remodelling- and fibrosis genes are differentially transcribed in the livers of Abcb4 (−/−) mice harbouring chronic cholangitis. Scand J Gastroenterol 42: 1245–1255, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflügers Arch 453: 601–610, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, Han TQ, Eriksson M, Parini P. HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res 51: 1354–1362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Rainwater DL, VandeBerg JL. Dramatic differences in lipoprotein composition among gray short-tailed opossums (Monodelphis domestica) fed a high cholesterol/saturated fat diet. Biochim Biophys Acta 1126: 159–166, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem 277: 18793–18800, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Sato R, Takano T. Regulation of intracellular cholesterol metabolism. Cell Struct Funct 20: 421–427, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, van der Valk MA, Offerhaus GJ, Berns AJ, Borst P. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75: 451–462, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest 117: 1968–1978, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, Hiroi S, Irie R, Okada Y, Kurihara C, Ebinuma H, Saito H, Hokari R, Sugiyama K, Kanai T, Miura S, Hibi T. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 142: 152–164, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol 23: 1732–1738, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, Teoh NC, Farrell GC. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology 141: 1393–1403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. VandeBerg JL, Cothran EG, Kelly CA. Dietary effects on hematologic and serum chemical values in gray short-tailed opossums (Monodelphis domestica). Lab Anim Sci 36: 32–36, 1986 [PubMed] [Google Scholar]
- 39. VandeBerg JL, Williams-Blangero S. The laboratory opossum. In: The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals (8th Ed), edited by Hubrecht R, Kirkwood J. West Sussex, UK: Wiley-Blackwell, 2010, p. 246–261 [Google Scholar]
- 40. Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 263: 2998–3004, 1988 [PubMed] [Google Scholar]
- 41. Yu J, Ip E, Dela Pena A, Hou JY, Sesha J, Pera N, Hall P, Kirsch R, Leclercq I, Farrell GC. COX-2 induction in mice with experimental nutritional steatohepatitis: role as pro-inflammatory mediator. Hepatology 43: 826–836, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 110: 671–680, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]