Abstract
Endotoxemia is a contributing cofactor to alcoholic liver disease (ALD), and alcohol-induced increased intestinal permeability is one of the mechanisms of endotoxin absorption. Probiotic bacteria have been shown to promote intestinal epithelial integrity and protect barrier function in inflammatory bowel disease (IBD) and in ALD. Although it is highly possible that some common molecules secreted by probiotics contribute to this action in IBD, the effect of probiotic culture supernatant has not yet been studied in ALD. We examined the effects of Lactobacillus rhamnosus GG culture supernatant (LGG-s) on the acute alcohol-induced intestinal integrity and liver injury in a mouse model. Mice on standard chow diet were supplemented with supernatant from LGG culture (109 colony-forming unit/mouse) for 5 days, and one dose of alcohol at 6 g/kg body wt was administered via gavage. Intestinal permeability was measured by FITC-FD-4 ex vivo. Alcohol-induced liver injury was examined by measuring the activity of alanine aminotransferase (ALT) in plasma, and liver steatosis was evaluated by triglyceride content and Oil Red O staining of the liver sections. LGG-s pretreatment restored alcohol-induced reduction in ileum mRNA levels of claudin-1, intestine trefoil factor (ITF), P-glycoprotein (P-gp), and cathelin-related antimicrobial peptide (CRAMP), which play important roles on intestinal barrier integrity. As a result, LGG-s pretreatment significantly inhibited the alcohol-induced intestinal permeability, endotoxemia and subsequently liver injury. Interestingly, LGG-s pretreatment increased ileum mRNA expression of hypoxia-inducible factor (HIF)-2α, an important transcription factor of ITF, P-gp, and CRAMP. These results suggest that LGG-s ameliorates the acute alcohol-induced liver injury by promoting HIF signaling, leading to the suppression of alcohol-induced increased intestinal permeability and endotoxemia. The use of bacteria-free LGG culture supernatant provides a novel strategy for prevention of acute alcohol-induced liver injury.
Keywords: alcoholic liver disease, hypoxia-inducible factor, gut permeability
alcohol abuse is a major cause of liver injury. It encompasses a spectrum of stages including fatty liver, inflammation, fibrosis, cirrhosis, and even malignancy (2, 30). There is a strong interaction between the gut and the liver, and it is well-known that alcohol causes changes in gut microbiota and gut permeability that further accelerate the progression of liver disease. Although alcohol and its metabolites have direct toxic effects on the liver, they can also affect the intestinal barrier in multiple ways, including altering the normal gut flora and disrupting the gut barrier function, leading to increased portal circulating endotoxin levels and hepatic exposure, thus further promoting liver inflammation and the progression to alcoholic liver disease (ALD) (38).
The intestinal epithelial barrier is a complex system composed of cellular, physical, and chemical components (44). The epithelial cells form a lining with the paracellular space sealed by tight junctions (TJ) and adherens junctions. The epithelial cells are covered by a protective mucin layer that physically blocks most particles from direct contact with the epithelial cells (56). The intestines are unique in that they function at a steep oxygen gradient (55). Oxygen is an important regulator of intestinal epithelial cells, and changes in blood volume to the gut cause drastic changes in local oxygen partial pressure. The intestinal epithelium buffers such change by shifting to increased oxygen-independent glycolysis under stress conditions (55, 56). The oxygen adaptation process is characterized by the expression of a master transcription factor, hypoxia-inducible factor (HIF) (60). HIF1 and HIF2 have been described, and many intestinal epithelial cell types express both forms (33). In contrast to TJs and adherens junctions, which provide a physical barrier, HIF triggers gene transcription to maintain epithelial tissue integrity (56). In states of inflammation-related hypoxia, HIF acts by inducing mucin production and stabilization via regulation of intestinal trefoil factor (ITF), xenobiotic clearance by P-glycoprotein (P-gp), and various other nucleotide signaling (7).
Another significant portion of an intact gut barrier is provided by the gut flora, composed of billions of bacteria that have metabolic, trophic, and immune functions that are vital to the body (1). The defensive role of the luminal bacteria includes providing a protective barrier surface on the gut lumen, releasing bacteriocins, competing with harmful bacteria for attachment sites, and limiting autoantibodies.
Because of the composite barrier effects of gut bacteria, epithelial TJs, and epithelial transcription factors, endotoxins derived from Gram-negative bacteria can only penetrate the gut epithelium in trace amounts under normal conditions (51). Changes to intestinal permeability, however, allow for the upregulation of proinflammatory mediators and increased plasma endotoxin levels. Efforts to decrease endotoxin exposure by using antibiotics to “sterilize” the gut in clinical practice and animal models have been proven effective in preventing alcohol-induced liver injury (3). Furthermore, it has been shown in human alcoholics that treatment with probiotics altered gut flora and improved liver function (26).
Probiotics are defined as live microorganisms, which, when administered in adequate amounts, confer health benefits to the host (43). Probiotics have clinical utility in many of gastrointestinal diseases including diarrhea and inflammatory bowel disease. Studies by various groups have demonstrated that the probiotic Lactobacillus rhamnosus Gorbach-Goldin (LGG) has beneficial effects on intestinal function, including stimulating development and mucosal immunity, ameliorating diarrhea, prolonging remission in ulcerative colitis and pouchitis, and maintaining and improving intestinal barrier function (6, 13, 37, 54, 59). However, adverse events noted with probiotic use include bacteremia (28), fungemia (45), and worsened outcomes in severe pancreatitis, with an increased incidence of bowel ischemia and mortality (5). In addition, probiotics may not be effective in intestinal disorders with altered epithelium, as the bacteria must colonize in the intestine to be effective. As an alternative, heat-killed bacteria and probiotic-produced nonviable soluble proteins have been demonstrated to be effective in recent studies (58). LGG culture supernatant (LGG-s) was shown to protect intestinal epithelial cells from apoptosis and promote proliferation (64), whereas our group recently showed that LGG-s attenuated alcohol-induced decrease in epithelial cell resistance and the increase in permeability in Caco-2 cell monolayers (61).
In the present study, we tested the effectiveness of LGG-s pretreatment on binge alcohol-induced liver injury. We investigated the effects of LGG-s on intestinal-mucus-layer-protective factors, TJs and gut permeability, regulation of intestinal HIF signaling, and consequent liver injury in a binge alcohol mouse model of ALD.
MATERIAL AND METHODS
Culture of LGG.
LGG was purchased from American Type Culture Collection (ATCC 53103; Rockville, MD) and was cultured in Lactobacillus De Man, Rogosa, and Sharpe broth (MRS broth; Difco; BD, Sparks, MD) at 37°C in accordance with ATCC guidelines. LGG was cultured to reach the bacterial density of 109 colony-forming units/ml. The culture suspension was then centrifuged at 5,000 g for 10 min. The supernatant was removed and filtered through 0.22-μm filters. This procedure yielded the LGG-s from the culture at a concentration of 109 colony-forming units/ml bacterial cells.
Animal studies.
Male C57BL/6N mice (9 wk of age) were obtained from Harlan (Indianapolis, IN). They were maintained at 22°C with a 12-h:12-h light/dark cycle and had free access to normal chow diet and tap water. The LGG-s was mixed with drinking water at a ratio ensuring one mouse consumed 1 ml supernatant a day. This treatment results in a dose of LGG-s equivalent to 109 LGG bacteria, as described in our previous study (61). Mice were maintained on the treatments for a total of 5 days during the experiment (25). They were given one dose of ethanol at 6 g/kg body wt by gavage after overnight fasting but with access to drinking water containing LGG-s. At the end of the experiment, the mice were anesthetized with Avertin. Plasma and tissue samples were collected for assays. All mice were treated according to the protocols reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Liver histology.
Formalin-fixed paraffin tissue sections were processed for staining with hematoxylin and eosin and then studied by light microscopy.
Liver oil red O staining.
Frozen tissue sections were processed for staining with Oil red O and then studied by light microscopy.
Ileum permeability.
Ileum was freshly isolated and placed in modified Krebs-Henseleit bicarbonate buffer containing 8.4 mM HEPES, 119 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, and 11 mM glucose (KHBB, pH 7.4). One end of the gut segment was first ligated with suture, and 100 μl FITC-dextran (molecular weight 4,000, FD-4, 40 mg/ml) was injected into the lumen using a gavage needle to avoid mucosal injury. Then the other end of the gut segment was ligated to form an 8-cm gut sac. After being rinsed in the KHBB buffer, the gut sac was placed in 2 ml of KHBB and incubated at 37°C for 20 min. The FD-4 that penetrated from the lumen into the incubation buffer was measured spectrofluorometrically with an excitation wavelength of 485 nm and an emission wavelength of 530 nm in a microplate fluorescence reader (FLX-800 fluorescence microplate reader; Bio TEK Instruments, Winooski, VT). The FD-4 permeability was expressed as micrograms per centimeter per minute.
Biochemical assays.
Blood samples from control and alcohol-treated mice were drawn from the dorsal vena cava. Plasma was obtained by centrifuging the blood at 2,000 g for 30 min at 4°C. LPS levels were measured with Limulus Amebocyte Lysate test kit (Lonza, Walkersville, MD) according to the manufacturer's instructions. Plasma alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) were measured using an ALT and LDH Enzymatic Assay Kit (Thermo Scientific, Waltham, MA), respectively.
Liver triglyceride assay.
For liver triglyceride assay, ∼70–100 mg of liver tissue was homogenized in 1 ml of 50 mM NaCl. Homogenate (500 μl) was finally mixed with 4 ml of the extraction reagent (methanol: chloroform = 1:2) and incubated overnight at 4°C before being centrifuged at 1,800 g for 20 min at room temperature. The lower chloroform phase was collected. Chloroform (500 μl) phase was dried using a speed vacuum, and the pellets were used for triglyceride assay using the Triglyceride Kit (Thermo Scientific).
Liver TNF-α assay.
Liver tissue was homogenized (50 mg/ml) in RIPA buffer. TNF-α was measured using the Infinity Assay Kit (BD) according to the manufacturer's instructions.
Liver MPO assay.
Liver tissue was homogenized (50 mg/ml) in 0.5% hexadecyltrimethylammonium bromide in 10 mM 3-(N-morpholino) propanesulfonic acid and centrifuged at 15,000 g for 40 min. The suspension was then sonicated three times for 30 s each with an interval of 1 min. An aliquot of supernatant was mixed with a solution of 1.6 mM tetramethylbenzidine and 1 mM hydrogen peroxide. Activity was measured spectrophotometrically as the change in A650 at 37°C with a Spectramax microplate reader (Biotek; Molecular Devices, Sunnyvale, CA). Results are expressed as milliunits of myeloperoxidase (MPO) activity per milligram of protein, as determined by the Bradford assay.
ROS determination by fluorescence microscopy.
Reactive oxygen species (ROS) accumulation in the liver and ileum was examined by dihydroethidium fluorescence microscopy (62). Nonfluorescent dihydroethidium is oxidized by ROS to yield the red fluorescent product, ethidium that binds to nucleic acids and stains the nucleus with bright fluorescent red. Cryostat sections of liver or ileum were incubated with 5 μM dihydroethidium (Molecular Probes, Eugene, OR) for 30 min at 37°C in the dark. The ROS-catalyzed ethidium red fluorescence was examined under fluorescence microscopy. The relative fluorescence intensity was quantified by using Sigma Scan Pro 5 software.
Real time RT-PCR assay.
The mRNA levels were assessed by real-time RT-PCR. In brief, the total RNA was isolated with Trizol according to manufacturer's protocol (Invitrogen, Carlsbad, CA) and reverse-transcribed using GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA). The sequences of forward and reverse primers are listed in Table 1. Quantitative real-time PCR was performed on an ABI 7500 real-time PCR thermocycler, whereas SYBR green PCR Master Mix (Applied Biosystems) was used for real-time RT-PCR analysis. The relative quantities of target transcripts were calculated from duplicate samples after normalization of the data against the housekeeping gene, β-actin. Dissociation curve analysis was performed after PCR amplification to confirm the specificity of the primers. Relative mRNA expression was calculated using the ΔΔCt method.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Sequences (Forward/Reverse 5′-3′) | |
|---|---|---|
| ZO-1 | tgggaacagcacacagtgac | gctggccctccttttaacac |
| Occludin | acccgaagaaagatggatcg | catagtcagatgggggtgga |
| Claudin-1 | cgggcagatacagtgcaaag | acttcatgccaatggtggac |
| Fordrin | cgcatctttttcctcagcag | ccaggacttgctgtcgtctc |
| Cigulin | gagcaaatgcagcctctgg | cccaaccctggatggttcta |
| Symplekin | tgagggctgagaaggctgta | cagcacctctgccttgaatc |
| MDR1(P-gp) | gtgggggacagaaacagaga | gaacggtagacaagcgatgag |
| ITF | tgggatagctgcagattacg | gccacagtccactctgacat |
| CRAMP | cagccctttcggttcaagaa | cccacctttgcggagaagt |
| HIF-1α | tcatcagttgccacttccccac | ccgtcatctgttagcaccatcac |
| HIF-2α | ggagctcaaaaggtgtcagg | caggtaaggctcgaacgatg |
| β-actin | gagaccttcaacacccc | atagctcttctccagggagg |
ZO, zonula occludens; MDR1, multidrug resistance 1; P-Gp, P-glycoprotein; ITF, intestinal trefoil factor; CRAMP, cathelin-related antimicrobial peptide; HIF, hypoxia-inducible factor.
Statistics.
All data are expressed as means ± SE. The data were analyzed by ANOVA and Newman-Keuls multiple-comparison test. Differences between groups were considered significant at P < 0.05.
RESULTS
Effects of LGG-s pretreatment on acute alcohol-induced hepatic steatosis and plasma enzyme levels.
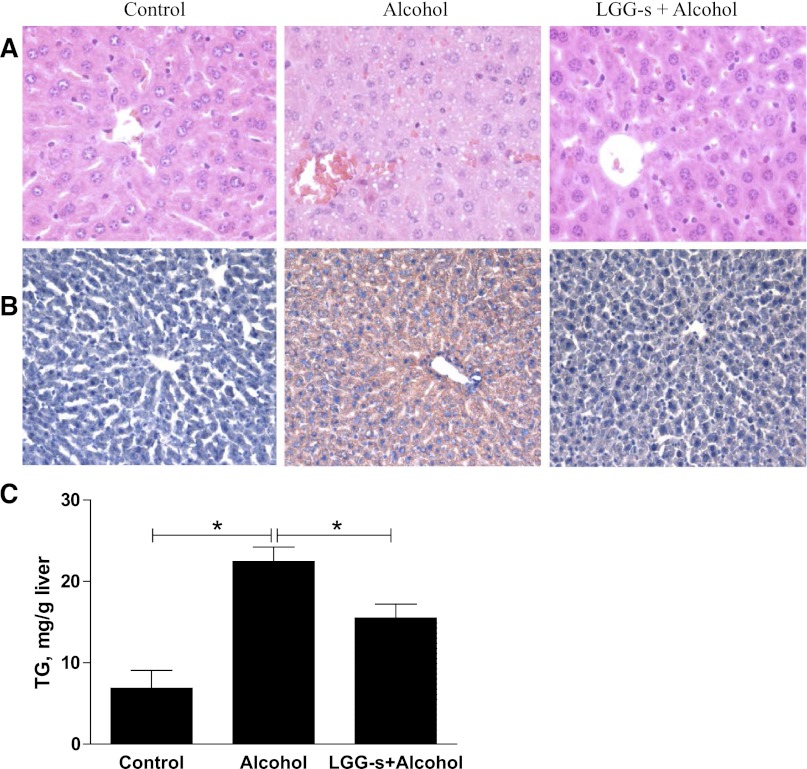
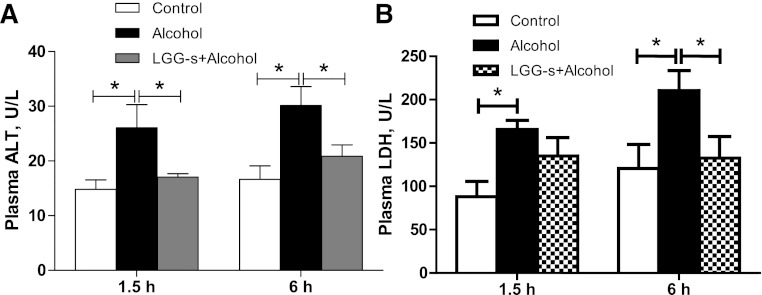
Hepatic steatosis was evaluated with histological analysis and triglyceride measurement. Mice exposed to binge alcohol treatment had significantly increased hepatic lipid accumulation compared with control mice, as evaluated by hematoxylin and eosin (Fig. 1A) and Oil Red O (Fig. 1B) staining, which has been used in our previous studies (66). However, LGG-s pretreatment significantly prevented fatty liver (Fig. 1, A and B). Confirming the histological observations, the hepatic triglyceride content was significantly higher in alcohol-exposed group compared with control group. Pretreatment with LGG-s markedly decreased the acute alcohol-induced hepatic triglyceride accumulation (Fig. 1C). To assess liver injury, plasma ALT and LDH levels were measured. Mouse plasma was collected 1.5 and 6 h after binge alcohol administration. Plasma ALT and LDH levels were significantly elevated by alcohol treatment. LGG-s pretreatment significantly prevented the elevation in plasma ALT levels (Fig. 2A) at both alcohol postadministration time points. LGG-s pretreatment also significantly reduced binge alcohol-induced plasma LDH levels at 6 h after alcohol treatment time, but not at 1.5 h (Fig. 2B). Taken together, LGG-s pretreatment attenuated binge alcohol-induced hepatic steatosis and liver injury.
Fig. 1.
Effects of Lactobacillus rhamnosus GG culture supernatant (LGG-s) pretreatment on acute alcohol-induced liver steatosis. Male C57/6N mice were fed LGG-s for 5 days. All mice were starved for 16 h, and 1 dose of ethanol was given by gastric gavage at 6 g/kg 1.5 h or 6 h before the mice were killed. Formalin-fixed paraffin tissue sections were processed for staining with hematoxylin and eosin (A). Frozen tissue sections of the mice exposed to alcohol for 6 h were processed for staining with Oil red O (B). Hepatic tissue triglyceride (TG) levels (6 h alcohol exposure) were determined (C). Results are means ± SE; *significant.
Fig. 2.
Effects of LGG-s pretreatment on acute alcohol-induced liver injury. Plasma was collected at 1.5 h and 6 h after binge ethanol administration by centrifuging the blood at 3,000 revolution/min for 30 min at 4°C. Alanine aminotransferase (ALT) (A) and lactate dehydrogenase (LDH) (B) levels were measured using an ALT and LDH Enzymatic Assay Kits. Results are means ± SE; *significant.
Effects of LGG-s pretreatment on ROS formation.
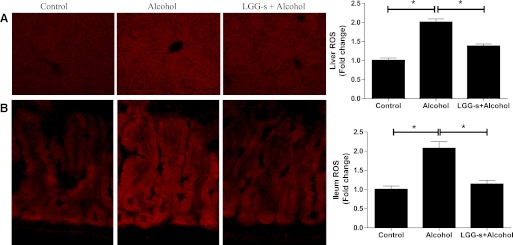
Next, we investigated whether LGG-s pretreatment could protect hepatic and ileal cells from oxidative stress. Frozen sections of liver and ileum tissues were stained with fluorescent ethidium. Binge alcohol treatment generated higher superoxide in both liver and ileum than in untreated subjects. However, the livers and ilea of the mice pretreated with LGG-s before alcohol administration displayed a reduced dihydroethidium staining (Fig. 3), indicating that LGG-s pretreated animals were more resistant compared with controls.
Fig. 3.
Effects of LGG-s pretreatment on hepatic and ileal reactive oxygen species (ROS) formation. Cryostat sections of liver (A) or ileum (B) were incubated with 5 μM dihydroethidium (Molecular Probes, Eugene, OR) for 30 min at 37°C in the dark. The superoxide anion-catalyzed ethidium red fluorescence was examined under fluorescence microscopy.
Effects of LGG-s pretreatment on alcohol-induced endotoxemia, intestinal permeability, and TJ gene expression.
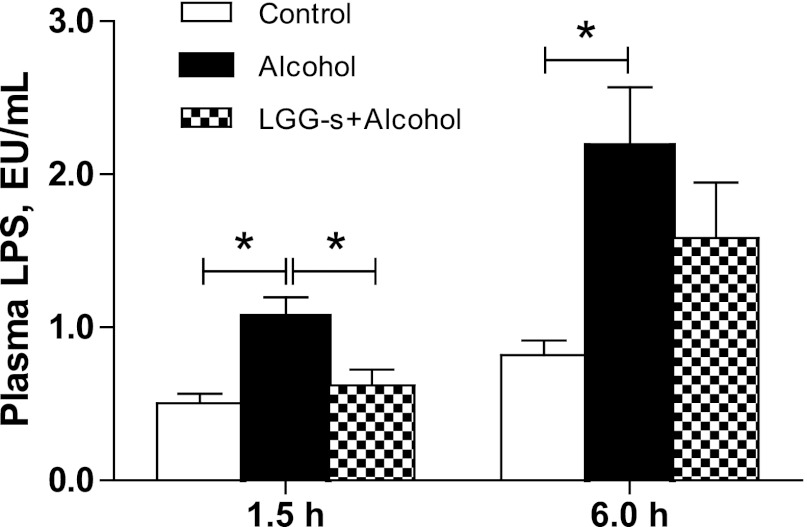
To evaluate the effects of acute alcohol administration and LGG-s pretreatment on plasma endotoxemia, plasma LPS levels were measured at 1.5 and 6 h after binge alcohol exposure.
Binge alcohol exposure significantly increased plasma LPS level at 1.5 h after binge time, which was further increased at 6 h after binge time (Fig. 4). LGG-s pretreatment significantly attenuated the rise in LPS level at the 1.5 h after binge time. At the 6 h after binge time, LPS level was also lower in LGG-s group compared with alcohol group. However, the difference was not statistically significant (Fig. 4).
Fig. 4.
Effects of LGG-s pretreatment on plasma endotoxemia. Plasma was collected at 1.5 h and 6 h after binge ethanol administration by centrifuging the blood at 3,000 revolution/min for 30 min at 4°C. LPS levels were measured with Limulus Amebocyte Lysate test kit (Lonza, Walkersville, MD) according to the manufacturer's instructions. Results are means ± SE; *significant.
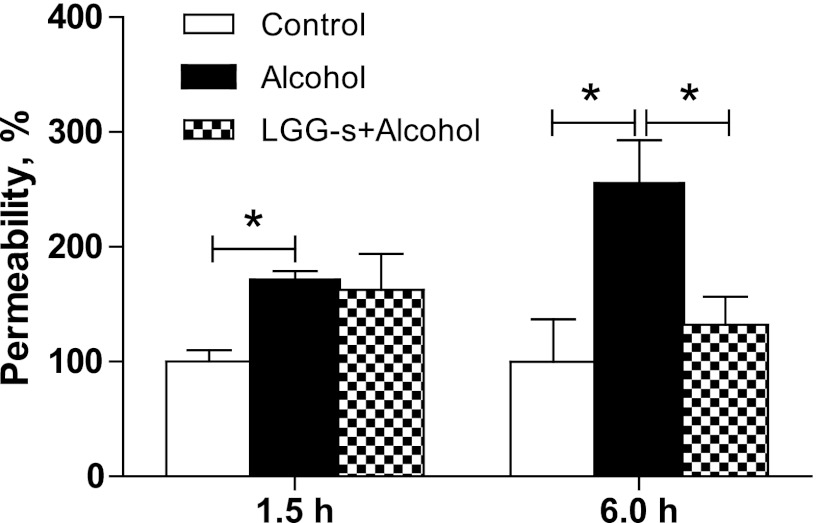
The elevated plasma LPS levels resulted from defects in intestinal barrier integrity. Therefore, we evaluated the effects of binge alcohol and LGG-s pretreatment on the intestinal permeability by an ex vivo procedure using FD-4 as a fluorescent marker. Our previous study measuring permeability showed that the ileum was the part mostly affected by chronic alcohol exposure (25); thus only the ileum permeability to FD-4 was measured in the current binge alcohol study. Significantly increased permeability was observed in mice treated with binge alcohol at 6 h after binge time, and this increase was prevented by LGG-s pretreatment (Fig. 5).
Fig. 5.
Effects of LGG-s pretreatment on ileum permeability. Ileum permeability was determined by measuring FD-4 leakiness from ileum sac ex vivo. The ileum was freshly isolated and placed in modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4). One end of the gut segment was first ligated with suture, and 100 μl FITC-dextran (molecular weight 4,000, FD-4, 40 mg/ml) was injected into the lumen using a gavage needle to avoid mucosal injury. Then the other end of the ileum segment was ligated to form an 8-cm gut sac. After being rinsed in the KHBB buffer, the gut sac was placed in 2 ml of KHBB and incubated at 37°C for 20 min. The FD-4 that penetrated from the lumen into the incubation buffer was measured spectrofluorometrically with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The FD-4 permeability was expressed as micrograms per centimeter per minute. The results were normalized to control. Results are means ± SE; *significant.
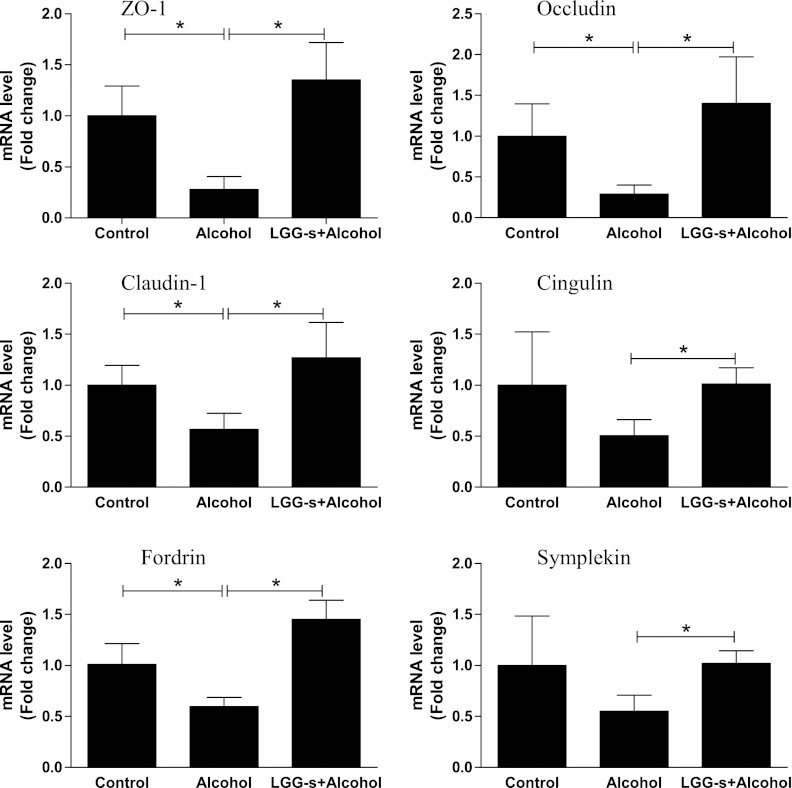
Intestinal barrier function is provided by TJ integrity. Therefore, we determined the mRNA levels of three key TJ proteins in response to alcohol (6 h exposure) and LGG-s treatment. Binge alcohol exposure significantly downregulated zonula occludens-1, occludin, and claudin-1 mRNA levels by 72%, 71%, 43.5%, respectively, and this was prevented by LGG-s pretreatment. Similarly, expressions of several TJ protein adaptors, such as fordrin, cingulin and symplekin, were significantly downregulated by binge alcohol, although the changes did not reach statistical significance for cingulin and symplekin. LGG-s pretreatment prevented these decreases as well (Fig. 6).
Fig. 6.
Effects of LGG-s pretreatment on mRNA levels of ileum tight junction proteins and adaptors. The total ileum RNA was isolated with Trizol according to manufacturer's protocol (Invitrogen, Carlsbad, CA) and reverse-transcribed using GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed on an ABI 7500 real-time PCR thermocycler, whereas SYBR green PCR Master Mix (Applied Biosystems) was used for real-time RT-PCR analysis. The relative quantities of target transcripts were calculated from duplicate samples after normalization of the data against the housekeeping gene, β-actin. Dissociation curve analysis was performed after PCR amplification to confirm the specificity of the primers. Relative mRNA expression was calculated using the Ct method. Results are means ± SE; *significant. ZO, zonula occludens.
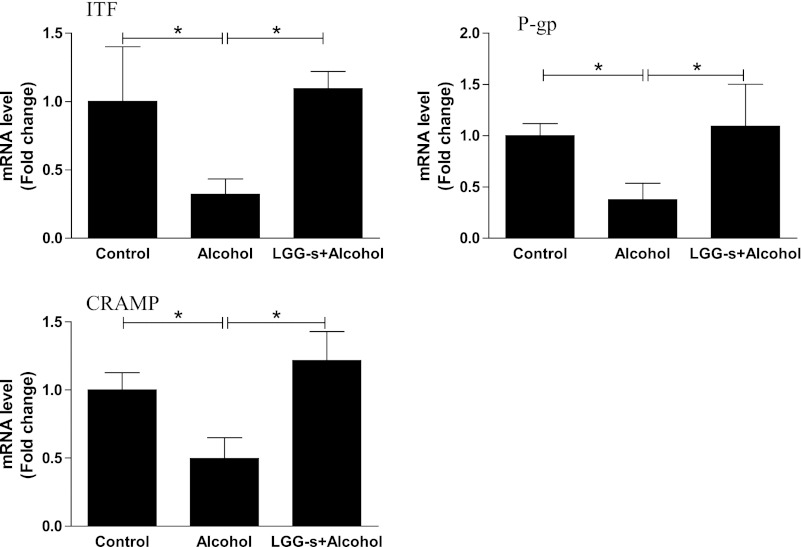
Effects of LGG-s pretreatment mRNA levels of ileum barrier-protecting proteins.
Intestinal barrier integrity is also provided by the mucus layer. ITF is a small peptide secreted by goblet cells in the intestine and plays a critical role in the formation and stabilization of mucus layer (27). Analysis of ITF mRNA levels in the ileum revealed a decrease by binge alcohol treatment, which was normalized by LGG-s pretreatment (Fig. 7). Another intestinal epithelium protein, P-gp, was similarly regulated by binge alcohol exposure and LGG-s pretreatment (Fig. 7). P-gp, encoded by the multidrug resistance 1 (MDR1) or ABCB1 gene, is a 170-kDa transmembrane protein that is abundantly expressed on the apical surface of intestinal epithelial cells (9). Increasing evidence suggests that P-gp plays a critical role in protection of the intestinal epithelia by mediating the efflux of drugs/xenobiotics and bacterial toxins from the intestinal mucosa into the gut lumen (20).
Fig. 7.
Effects of LGG-s pretreatment on mRNA levels of ileum mucus protecting factors. Results are means ± SE; *significant. ITF, intestine trefoil factor; P-gp, P-glycoprotein; CRAMP, cathelin-related antimicrobial peptide.
Cathelin-related antimicrobial peptide (CRAMP) is a member of antimicrobial peptides and protein-effector molecules that function to protect epithelial surfaces by killing invading microbes and working in synergy as a barrier against bacterial/endotoxin invasion (53). Similar to ITF and P-gp, mRNA levels of CRAMP were significantly reduced by alcohol exposure and normalized by LGG-s pretreatment (Fig. 7).
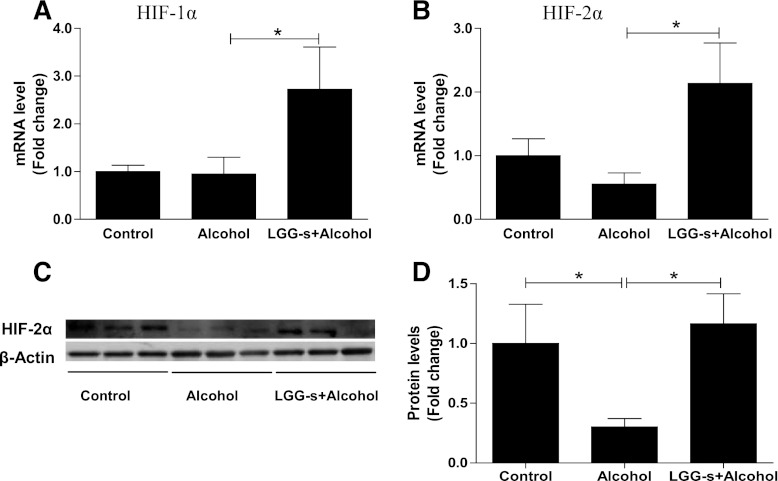
Effects of LGG-s pretreatment on intestine HIF-1α/2α levels.
ITF, P-gp, and CRAMP are transcription targets of HIF, which plays a critical role in adaptation to intestinal hypoxic stress. Therefore, we evaluated the mRNA and protein levels of HIF-1α and HIF-2α in the ilea of mice in response to binge alcohol and LGG-s treatment. Binge alcohol exposure did not alter the HIF-1α and HIF-2α mRNA levels, but LGG-s pretreatment significantly increased their expression (Fig. 8, A and B). Immunoblotting revealed that binge alcohol treatment significantly decreased HIF-2α protein levels, and this decrease was prevented by LGG-s pretreatment (Fig. 8, C and D).
Fig. 8.
Effects of LGG-s pretreatment on hypoxia-inducible factor (HIF)-1α/2α expression. A: mRNA levels of HIF-1α. B: mRNA levels of HIF-2α. C: protein levels of HIF-2α analyzed by Western blot (as described in Ref. 61). D: quantitative analysis of the protein levels of HIF-2α. Results are means ± SE; *significant.
Effects of LGG-s pretreatment on alcohol-induced inflammation.
Next, we determined the effects of binge alcohol and LGG-s pretreatment on markers of hepatic inflammation and oxidative stress. Expression of TNF-α, a marker of acute phase reaction and macrophage infiltration, was significantly upregulated in response to binge alcohol exposure. LGG-s pretreatment prevented this increase (Fig. 9A). Similarly, the activity of MPO, a well-known marker for inflammation, was significantly increased by binge alcohol exposure and decreased by LGG-s pretreatment (Fig. 9B), suggesting a role of LGG-s in anti-inflammatory activity in response to binge alcohol exposure.
Fig. 9.
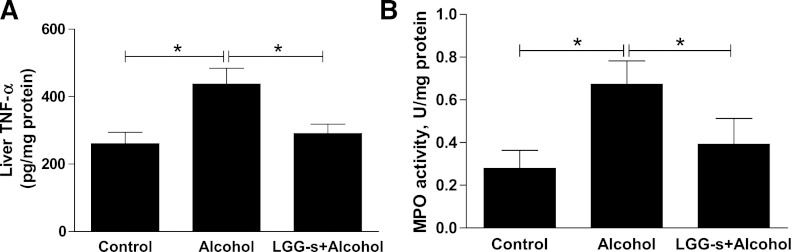
Effects of LGG-s pretreatment on hepatic TNF-α expression and myeloperoxidase (MPO) activity. TNF-α (A) was measured using the Infinity Assay kit (BD, Sparks, MD) according to the manufacturer's instructions. For MPO activity (B) assay, the liver tissue was homogenized (50 mg/ml) in 0.5% hexadecyltrimethylammonium bromide in 10 mM 3-(N-morpholino) propanesulfonic acid and centrifuged at 15,000 g for 40 min. The suspension was then sonicated 3 times for 30 s each. An aliquot of supernatant was mixed with a solution of 1.6 mM tetramethylbenzidine and 1 mM hydrogen peroxide. Activity was measured spectrophotometrically as the change in A650 at 37°C. Results are expressed as milliunits of MPO activity per milligram of protein, as determined by the Bradford assay. Results are means ± SE; *significant.
DISCUSSION
The present study investigated the effects of LGG-s on binge alcohol-induced intestinal epithelial cell permeability, endotoxemia, and liver injury. We demonstrated that the pretreatment with LGG-s, also known as bacterial conditioned media, prevents binge alcohol-induced liver injury through maintaining intestinal barrier function.
Increasing evidence demonstrates the protective effect of probiotics on multiple pathological disorders, such as obesity (11), nonalcoholic liver disease (21), cardiovascular disease (46), and ALD (17, 26, 40). Probiotics exert their beneficial activities on the host through multiple mechanisms, including prevention of pathogenic bacterial growth, blocking the pathogen from contacting mucosal surfaces, stimulating the trophic effects on the intestinal epithelial cells, sustaining intestinal barrier integrity, maintaining mucosal immune homeostasis, and participating in the xenobiotic metabolism system (41, 57). However, these treatments are not always effective because, to confer their beneficial activity, live bacteria need to be colonized to maintain their activity under various lumen conditions. Disease conditions including alcohol exposure vary from patient to patient due to the augmentation of pathogenic bacteria. In addition, drugs used by patients may be harmful to probiotics. This causes an unstable effect of probiotic treatment with live bacteria. Moreover, the clinically recommended dose of probiotics usually consists of billions of live bacteria. Generally, probiotics are considered safe, but several reports have raised safety concerns about ingesting such large amounts of bacteria, especially when the intestinal function and the patient's immune response are compromised (5, 10, 15).
Several studies have suggested that the secreted factors from probiotic bacterial growth are likely to be major contributors to the beneficial effects of probiotics, and some active ingredients in probiotic culture supernatant have been identified, including conjugated linoleic acids (14), short-chain fatty acids (35), polyamines (34), peptides (18), proteins (64), polyphosphate (48). These active ingredients have been demonstrated to be effective in the treatment of several intestinal disorders and liver disease through stimulating Gram-positive bacterial growth, changing the intestinal pH, promoting immune function, and inhibiting intestinal barrier dysfunction. However, the effect of the probiotics culture supernatant on ALD has never been examined. Our current study provides the first evidence showing the beneficial effects of LGG-s in binge alcohol-induced liver injury, which is demonstrated by reduced ileum permeability and decreased plasma endotoxin (LPS), ALT and LDH levels, and histological alterations.
LPS, a Gram-negative bacteria-derived endotoxin, is a major factor in alcoholic liver disease. Alcohol exposure stimulates LPS production and primes its binding to Toll-like receptor 4 on the surface of hepatic Kupffer cells, leading to the activation of NF-κB-mediated TNF-α signaling, resulting in hepatic steatosis and inflammation. Although intestinal barrier dysfunction-mediated endotoxemia is widely considered a major cause of liver injury due to alcohol exposure, the exact mechanisms by which alcohol and probiotics contribute to the altered intestinal permeability and endotoxemia are not fully understood. Previous studies showed that alcohol and its metabolite, acetaldehyde, induce increased intestinal permeability by disrupting intestinal epithelial cell TJs (42, 49, 61, 65). Decreased expression of several TJ proteins was found in the intestine in an experimental mouse model of ALD (25) and in human colon biopsies of alcoholics (52). Oxidative stress generated by alcohol exposure is believed to be a major contributor to the disruption of intestinal barrier via decreasing TJ. Our current study further demonstrates that binge alcohol exposure decreases intestinal TJ expression (zonula occludens-1, claudin, and occludin), and LGG-s pretreatment clearly normalizes these changes. We also observed that the mRNA levels of some TJ protein adaptors, including fordrin, cingulin, and symplekin, were decreased by binge alcohol exposure and restored by LGG-s pretreatment.
The protective effects of LGG-s against the increase in acute alcohol exposure-induced intestinal permeability and endotoxin levels are time dependent. The increased circulating endotoxin results from alcohol-induced endotoxin-production by Gram-negative bacterial overgrowth and intestinal barrier dysfunction and from other systematic factors such as organ LPS utilization and excretion. LGG-s pretreatment did not protect the short-term alcohol exposure (1.5 h)-induced increase in intestinal permeability but decreased plasma endotoxin level. When alcohol exposure time extends (6 h), the changes in permeability and endotoxin are more pronounced, and LGG-s pretreatment protects alcohol-induced permeability increase but not significantly decreases plasma endotoxin. This dissociation is likely due to endotoxin systematic regulation and needs further investigation.
Intestinal barrier function is also provided by the mucus layer. Goblet cells in both the small and the large intestine express mucins, the major mucus components, and several mucin stabilizers and modifiers (24). ITF, a member of trefoil factor family, is expressed abundantly in ileum and colon and plays a critical role in intestinal barrier function under multiple pathogenic conditions (27). Lack of ITF resulted in an exaggerated intestinal disorder (32), whereas recombinant ITF administration attenuated dextran sulfate sodium (DSS)-induced intestinal barrier dysfunction (31). Our previous studies demonstrated that chronic alcohol exposure decreases ileum ITF mRNA and protein expressions, which are normalized by LGG culture suspension (including LGG bacterial cells and culture supernatant, Ref. 61). In this study, we observed a decrease in mRNA levels of ITF by binge alcohol exposure, and this reduction was prevented by LGG-s pretreatment. The increased ITF expression in intestine by LGG or its culture supernatant in both chronic and acute alcohol-exposed mice suggests a mucus layer repair mechanism at the site of injury.
The beneficial effects of probiotics on intestinal barrier protection seem largely to be mediated by an overall mucosal protection, ranging from xenobiotic clearance to nucleotide metabolism. P-gp, encoded by MDR1 or ABCB1 gene, is a 170-kDa transmembrane protein that is abundantly expressed on the apical surface of intestinal epithelial cells (9). Increasing evidence suggests that P-gp plays a critical role in protection of the intestinal epithelia by mediating the efflux of drugs/xenobiotics and bacterial toxins from the intestinal mucosa into the gut lumen (20). Dysregulated P-gp has been associated with the pathogenesis of several intestinal disorders, including IBD (16), ulcerative colitis and Crohn's disease, and experimental animal models of colitis (8, 63). Upregulation of P-gp by two probiotics strains, Lactobacillus acidophilus and rhamnosus, has been demonstrated in the DSS-induced colitis mouse model and in Caco-2 cells (47). In addition, CRAMP, an antimicrobial peptide secreted by Paneth cells in the intestinal epithelium, provides protection from intestinal infection and maintains enteric homeostasis. The dysregulation of P-gp and CRAMP by alcohol exposure in intestine has not yet been tested. Our results demonstrate that binge alcohol exposure significantly reduced mRNA levels of both P-gp and CRAMP. Importantly, the decreases were normalized by LGG-s pretreatment. Therefore, this protective effect of LGG-s against binge alcohol-induced intestinal barrier dysfunction is likely achieved through a combinatorial regulation of intestinal mucin function, xenobiotic/toxin clearance, and immune response.
One possible mechanism of LGG-s protection against binge alcohol exposure-induced intestinal dysfunction is the potentiation of HIF signaling. Our previous study showed that intestinal HIF-2α was downregulated in protein levels by chronic alcohol exposure and normalized by LGG treatment (61). The mRNA levels of HIF-1α and HIF-2α were not changed by binge alcohol exposure, but they were markedly increased by LGG-s treatment. The intestine functions at a uniquely steep physiological oxygen gradient (7, 55). Under pathological conditions, increased tissue metabolism causes inflammation and worsens epithelial hypoxia. As a compensatory mechanism, the transcription factor HIF is activated. Studies have shown that animals that have genetic deletions of HIF show worsened colitis compared with wild-type animals (23). Interestingly, some kinds of mucins (4, 29), ITF (19), P-gp (9), and CRAMP (39) are transcriptional targets of HIF. HIF is a transcription factor comprised of HIF-α and HIF-β subunits, and its activity is mainly regulated in posttranslational level. Normoxic conditions decrease the activity of HIF prolyl hydroxylases, which hydrolyze two proline residues of HIF-α, leading to the ubiquitin-mediated degradation. Under hypoxic conditions, or by hypoxia mimics, HIF prolyl hydroxylases remain in their inactivated steady states, and HIF-α survives and translocates into the nucleus and starts target gene transcription. Alcohol did not cause changes in HIF-1α and HIF-2α mRNA levels in the current study, indicating that binge alcohol exposure-caused HIF-α dysfunction is likely regulated at protein levels. Indeed, binge alcohol administration decreased HIF-2α protein level. Importantly, LGG-s pretreatment increasing HIF-1α and HIF-2α mRNA levels and HIF-2α protein level implies a role of LGG-s in the regulation of HIF function at gene expression level. Although experimental evidence has shown the functional difference between HIF-1α and HIF-2α in other organs and cell types, including cardiomyocytes (22) and hepatocytes (36), the exact role of both subunits of HIF in intestinal homeostasis in response to alcohol and probiotics requires further investigation.
Studies have shown conflicting results on the beneficial properties of killed bacteria and bacterial supernatant, which, in part, may be because protective effects are strain specific. For example, supernatant of L. casei culture failed to provide improved barrier function against cytokine-induced inflammation (12). In contrast, a study by Ueno et al. (58) in murine models of colitis demonstrated that heat-killed Lactobacillus brevis SBC8803 was effective in reducing colon inflammation and the mRNA expression of proinflammatory cytokines. Additionally, Polk et al. (50, 64) showed that specific proteins produced by LGG regulate intestinal epithelial cell survival and growth. Specifically, purified proteins p75 and p40 protect intestinal cells from death and promoted proliferation (64). Several active components in probiotic culture supernatant have also been identified. Polyphosphate in Lactobacillus brevis SBC8803 culture supernatant induces cytoprotective heat shock proteins and plays an important role in the molecule responsiveness for maintaining intestinal barrier actions, which are mediated through the intestinal integrin β1-p38 MAPK in an experimental colitis model (48). The data presented here and the results from previous studies demonstrate the protective effect of LGG-s in alcohol-induced barrier dysfunction in vivo and in vitro. To our knowledge, this is the first study testing the effects of probiotic supernatant in ALD. Identification of the full spectrum of active ingredients in LGG culture supernatant in response to alcohol exposure warrants further investigative attention.
One limitation to our study is that we did not include a group using probiotic cultural media as a control. However, we recently used MRS (the cultural media) as a placebo in chronic alcohol mouse model. The preliminary results showed that there are no differences in intestinal permeability and plasma endotoxin levels between alcohol-treated and MRS-alcohol-treated mice (unpublished data), indicating that the constituents of MRS have no effect on chronic alcohol-induced intestinal barrier dysfunction.
In summary, our data demonstrate that probiotics LGG-s pretreatment has a protective role against the deleterious effects of binge alcohol exposure on HIF adaptation signaling, mucus protective gene regulation associated with the expression of TJ proteins and adaptors, blood LPS, and eventually alcoholic liver injury. Further characterization of the LGG-s active components will enhance our understanding of the protective effect of probiotics in ALD and advance the development of new therapeutic strategy for ALD.
GRANTS
This work was supported in part by NIH grants (P01AA017103, P30AA019360, R01AA015970, R01AA018016, R01AA018869, R37AA010762, RC2AA019385 and R01DK071765 to C. McClain), a grant from the VA (to C. McClain), a grant from ADA (1-12-BS-47 to W. Feng), and grants from NSFC (31101252, 81170203).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., Y.L., and Z.M. performed experiments; Y.W. and W.F. analyzed data; Y.W. prepared figures; Y.W., A.S., and W.F. drafted manuscript; C.J.M. and W.F. conception and design of research; C.J.M. and W.F. interpreted results of experiments; C.J.M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Marion McClain for manuscript proofreading. C. McClain is a Distinguished University Scholar of the University of Louisville.
REFERENCES
- 1. Abt MC, Artis D. The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol 25: 496– 502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis 23: 255– 263, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218– 224, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Aubert S, Fauquette V, Hemon B, Lepoivre R, Briez N, Bernard D, Van Seuningen I, Leroy X, Perrais M. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res 69: 5707– 5715, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371: 651– 659, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther 20: 813– 819, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281– 287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collett A, Higgs NB, Gironella M, Zeef LA, Hayes A, Salmo E, Haboubi N, Iovanna JL, Carlson GL, Warhurst G. Early molecular and functional changes in colonic epithelium that precede increased gut permeability during colitis development in mdr1a(−/−) mice. Inflamm Bowel Dis 14: 620– 631, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62: 3387– 3394, 2002 [PubMed] [Google Scholar]
- 10. De Groote D, Van Doorn LJ, Van den Bulck K, Vandamme P, Vieth M, Stolte M, Debongnie JC, Burette A, Haesebrouck F, Ducatelle R. Detection of nonpylori Helicobacter species in “Helicobacter heilmannii”-infected humans. Helicobacter 10: 398– 406, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7: 639– 646, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Eun CS, Kim YS, Han DS, Choi JH, Lee AR, Park YK. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS 119: 49– 56, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol 12: 5941– 5950, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr 136: 1483– 1487, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Faraonio R, Moffatt P, Larochelle O, Schipper HM, S-Arnaud R, Séguin C. Characterization of cis-acting elements in the promoter of the mouse metallothionein-3 gene. Activation of gene expression during neuronal differentiation of P19 embryonal carcinoma cells. Eur J Biochem 267: 1743– 1753, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O'Toole D, Mahmud N, Keeling PW, Weir DG, Kelleher D. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology 118: 279– 288, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43: 163– 172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1: 299– 308, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027– 1034, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut 52: 759– 766, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iacono A, Raso GM, Canani RB, Calignano A, Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 22: 699– 711, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M, Philipp S, Griethe W, Maxwell PH, Frei U, Bachmann S, Willenbrock R, Eckardt KU. Persistent induction of HIF-1α and -2α in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J 18: 1415– 1417, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098– 1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12: 319– 330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic Toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res 35: 835– 846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42: 675– 682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci 66: 1350– 1369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115: 178– 181, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616– 1627, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health 27: 209– 219, 2003 [PMC free article] [PubMed] [Google Scholar]
- 31. Marchbank T, Cox HM, Goodlad RA, Giraud AS, Moss SF, Poulsom R, Wright NA, Jankowski J, Playford RJ. Effect of ectopic expression of rat trefoil factor family 3 (intestinal trefoil factor) in the jejunum of transgenic mice. J Biol Chem 276: 24088– 24096, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274: 262– 265, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119: 1159– 1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One 6: e23652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meimandipour A, Shuhaimi M, Soleimani AF, Azhar K, Hair-Bejo M, Kabeir BM, Javanmard A, Muhammad Anas O, Yazid AM. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus Poult Sci 89: 470– 476, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Menrad H, Werno C, Schmid T, Copanaki E, Deller T, Dehne N, Brune B. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology 51: 2183– 2192, 2010 [DOI] [PubMed] [Google Scholar]
- 37. O'Hara AM, Shanahan F. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol 5: 274– 284, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742– 747, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115: 1806– 1815, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42: 349– 361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rabot S, Rafter J, Rijkers GT, Watzl B, Antoine JM. Guidance for substantiating the evidence for beneficial effects of probiotics: impact of probiotics on digestive system metabolism. J Nutr 140: 677S– 689S, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 447: 171– 183, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Reid G. The importance of guidelines in the development and application of probiotics. Curr Pharm Des 11: 11– 16, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol 32: 256– 264, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Riquelme AJ, Calvo MA, Guzman AM, Depix MS, Garcia P, Perez C, Arrese M, Labarca JA. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol 36: 41– 43, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Saini R, Saini S, Sharma S. Potential of probiotics in controlling cardiovascular diseases. J Cardiovasc Dis Res 1: 213– 214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saksena S, Goyal S, Raheja G, Singh V, Akhtar M, Nazir TM, Alrefai WA, Gill RK, Dudeja PK. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol 300: G1115– G1123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, Kohgo Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One 6: e23278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287: G510– G517, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 294: G1060– G1069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 16: 1321– 1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res 32: 355– 364, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Tani K, Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae infection induces a neutrophil-derived antimicrobial peptide, cathelin-related antimicrobial peptide. Microbiol Immunol 55: 582– 588, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018– C1030, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295– 1300, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799– 809, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Turpin W, Humblot C, Thomas M, Guyot JP. Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms. Int J Food Microbiol 143: 87– 102, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Ueno N, Fujiya M, Segawa S, Nata T, Moriichi K, Tanabe H, Mizukami Y, Kobayashi N, Ito K, Kohgo Y. Heat-killed body of lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis 17: 2235– 2250, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Versalovic J. Probiotics: intestinal gatekeeping, immunomodulation, and hepatic injury. Hepatology 46: 618– 621, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510– 5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol 179: 2866– 2875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xue W, Liu Q, Cai L, Wang Z, Feng W. Stable overexpression of human metallothionein-IIA in a heart-derived cell line confers oxidative protection. Toxicol Lett 188: 70– 76, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Yacyshyn B, Maksymowych W, Bowen-Yacyshyn MB. Differences in P-glycoprotein-170 expression and activity between Crohn's disease and ulcerative colitis. Hum Immunol 60: 677– 687, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562– 575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 298: G625– G633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol 163: 1137– 1146, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]