Abstract
Increasing Na delivery to the connecting tubule (CNT) causes afferent arteriole (Af-Art) dilation, a process we call CNT glomerular feedback (CTGF). Angiotensin II (ANG II) in the CNT lumen enhances CTGF via PKC. We hypothesized that luminal ANG II stimulates CTGF via activation of protein kinase C (PKC), NADPH oxidase 2 (NOX2), and enhanced production of superoxide (O2−). Rabbit Af-Arts and adherent CNTs were microdissected and microperfused in vitro. Dilation of the Af-Art was induced by increasing luminal CNT NaCl from 0 to 5, 10, 30, 45, and 80 mM, and the concentration of NaCl that elicited a half-maximal response (EC50) was calculated. Compared with vehicle, adding ANG II (10−9 M) to the CNT lumen reduced EC50 from 37 ± 3 to 14 ± 1 mM (P < 0.001), indicating ANG II potentiates CTGF. In the presence of ANG II, the O2− scavenger tempol (10−4 M) increased EC50 from 20 ± 4 to 41 ± 3 mM (P < 0.01), the NOX inhibitor apocynin (10−5 M) increased EC50 from 17 ± 2 to 39 ± 4 mM (P < 0.01), and the specific NOX2 inhibitor gp91ds-tat (10−5 M) increased EC50 from 19 ± 2 to 34 ± 2 mM (P < 0.01). However, tempol, apocynin, and gp91ds-tat had no effect on CTGF in the absence of ANG II. Compared with vehicle, the PKC activator PMA (2 × 10−7 M) decreased EC50 from 35 ± 2 to 14 ± 1 (P < 0.001). In the presence of PMA, tempol increased EC50 from 14 ± 2 to 35 ± 2 mM (P < 0.01). We conclude the PKC/NOX2/O2− pathway mediates the enhancement of CTGF by luminal ANG II but it does not participate in CTGF in the absence of ANG II.
Keywords: superoxide, protein kinase C, NADPH oxidase
in the renal cortex, two segments of the distal nephron, the macula densa and the connecting tubule (CNT), closely contact the afferent arteriole (Af-Art) (3, 8, 11, 47). The anatomical contact between the macula densa and the Af-Art was also long known to be a functional cross talk, in that increases in NaCl in the lumen of the macula densa cause constriction of the adjacent Af-Art, a mechanism known as tubuloglomerular feedback (TGF). There was also evidence from in vivo experiments that another renal autoregulatory mechanism existed, likely in the distal nephron (13), which prompted us to test whether a functional cross talk also existed between the CNT and the Af-Art. We provided direct in vitro evidence that increases in CNT NaCl cause dilation of the adjacent Af-Art, a mechanism that we named connecting tubule glomerular feedback (CTGF) (40, 41). We also recently found that CTGF occurs in vivo and that it opposed the vasoconstrictor effect of TGF (48).
We also found that tubular angiotensin II (ANG II) enhanced CTGF (39), probably by increasing CNT Na transport. However, the signaling pathway mediating this effect is not known.
It is well-known that ANG II activates the phospholipase C signaling pathway, thereby elevating cytosolic Ca2+ and protein kinase C (PKC) (9). PKC has been shown to activate superoxide (O2−) generation by NADPH oxidases (NOX) in several types of cells, including phagocytes (10), cardiomyocytes (51), aortic endothelial cells (20, 23), and renal mesangial cells (50). On the other hand, O2− can also activate PKC (22, 25, 43). We previously reported that the enhancement of CTGF by ANG II is mediated by PKC. However, whether PKC acts upstream, downstream, or independently from O2− in the enhancement of CTGF remains unknown.
O2− is an important regulator of renal function (18, 19, 30, 31, 36, 52, 53) that has been proposed to mediate several of the actions of ANG II in the cardiovascular system (6) and kidney (30). In renal tubular cells, ANG II stimulates the formation of O2− (18) and O2− enhances tubular Na transport (26). Since the enhancement of CTGF by ANG II is mediated by the epithelial sodium channel (ENaC) (39) and because O2− increases ENaC activity (54), it is reasonable to propose that ANG II enhances CTGF via O2−.
There are many potential sources of O2− in the kidney. These include the mitochondria (27), NOX (5), chemical reactions that produce O2− as a byproduct, including catabolism of xanthine by xanthine oxidase (37), and other enzymes such as nitric oxide synthase (49) and cyclooxygenase (29). However, the primary source of ANG II-stimulated O2− in the CNT is unknown.
We hypothesized that luminal ANG II enhances CTGF via activation of PKC and consequently of NOX-derived O2− and Na transport in the CNT. To test this hypothesis, we examined the effect of ANG II on CTGF in the absence and presence of an O2− scavenger, a NOX inhibitor, or a PKC inhibitor. We also studied the effect of a PKC activator on CTGF. Experiments were performed by simultaneously perfusing a microdissected rabbit Af-Art and its adherent CNT. This approach avoids the confounding influence of the multiple systemic factors that regulate the renal microcirculation.
MATERIALS AND METHODS
New Zealand White rabbits weighing 1.5–2 kg (Myrtle's Rabbitry) were given standard chow (Harlan Laboratories, Indianapolis, IN) and tap water ad libitum and anesthetized with ketamine (50 mg/kg im), xylazine (10 mg/kg im) followed by pentobarbital sodium (25 mg/kg iv). All protocols were approved by Henry Ford Health System's Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and APS's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.
We used rabbits because their CNTs are well-demarcated and microdissection of the CNT and attached Af-Art is easier than in rats or mice. To isolate and microperfuse the Af-Art and CNT, we used methods similar to those described previously (38, 41). The kidneys were sliced along the corticomedullary axis and slices were placed in ice-cold minimum essential medium (MEM; Invitrogen, Carlsbad, CA) containing 5% bovine serum albumin (BSA; Sigma, St. Louis, MO). With the use of fine forceps, a single superficial Af-Art with its glomerulus intact was dissected together with the adherent CNT. Using a micropipette, the microdissected complex was transferred to a temperature-regulated perfusion chamber mounted on an inverted microscope with Hoffmann modulation. Both the Af-Art and CNT were cannulated with an array of concentric glass pipettes as described previously (4, 24). This system allows us to exchange the perfusion solution in a few seconds, while keeping the holding and perfusion pipettes in place. The Af-Art was perfused with MEM containing 5% BSA gassed with room air. Intraluminal pressure was measured by Landis' technique and maintained at 60 mmHg. The CNT perfusion solution contained (in mM) 4 KHCO3, 10 HEPES, 0.5 Na acetate, 0.5 Na lactate, 0.5 K2HPO4, 1.2 MgSO4, 1 CaCO3, and 5.5 glucose, adding 1 M NaCl to achieve the desired final NaCl concentration. Tubular perfusion was controlled by use of a syringe microperfusion pump (Harvard Apparatus Inc, Holliston, MA) set to 20 nl/min (calibration checked to be ≈20 nl/min), which is within the range of physiological flow rates (15, 28). The bath was superfused with MEM containing 0.15% BSA at a rate of 1 ml/min.
Experimental protocols.
Microdissection and cannulation of the Af-Art and CNT were completed within 60 min at 4–6°C, after which the temperature was gradually raised to 37°C. A 30-min equilibration period was allowed, during which no drugs were added and the CNT was perfused with 0 NaCl. At the end of the equilibration period, Af-Art diameter was measured before and after adding norepinephrine (NE; 2–5 × 10−7 M) to the bath. NE was used to preconstrict the Af-Art because our preliminary studies showed that isolated arterioles have little or no tone and therefore, little or no vasodilation can be elicited in the basal state. Next, luminal CNT NaCl was increased from 0 to 5, 10, 30, 45, and 80 mM (first curve, represented in all figures by the open symbols); NaCl was returned to 0 and again increased to 5, 10, 30, 45, and 80 mM (second curve, represented in all figures by the closed symbols). Af-Art diameter was measured at each CNT NaCl level on images of the Af-Art acquired at 5-s intervals with a video camera. Measurements were performed with a computer equipped with Metavue image analysis system (Molecular Devices, Sunnyvale, CA). For the purpose of standardizing our measurements, each data point resulted from averaging three individual measurements taken at the site of maximum constriction and ±5 μm around it.
Thus, each experiment consisted of two consecutive concentration-response curves generated by increasing luminal NaCl in the CNT. Each perfusion level was maintained for 5 min, making the whole experiment ∼1 h long. In preliminary studies, we found that our preparation remains stable for the duration of perfusion, as NE-induced constriction is sustained when NaCl in the CNT perfusate is maintained at zero NaCl. Pharmacological agents were added to the CNT lumen as follows: 1) time control and effect of ANG II on CTGF: 1a: time control, no drugs added; 1b: ANG II 10−9 M added to the second curve. 2) Role of O2− in ANG II-induced potentiation of CTGF: 2a: tempol (10−4 M) added to the second curve; 2b: ANG II added to the first curve, ANG II plus tempol added to the second curve. 3) Role of NOX in ANG II-induced potentiation of CTGF: 3a: apocynin (10−5 M) added to the second curve; 3b: ANG II added to the first curve, ANG II plus apocynin added to the second curve. 4) Role of NOX2 in ANG II-induced potentiation of CTGF: 4a: ANG II added to the first curve, ANG II plus scramble-gp91ds-tat (10−5 M) added to the second curve; 4b: ANG II added to the first curve, ANG II plus gp91ds-tat (10−5 M) added to the second curve. 5) Connection between PKC and O2− in ANG II-induced potentiation of CTGF: ANG II plus tempol added to the first curve, ANG II plus tempol plus staurosporine (10−8 M). 6) Role of O2− in phorbol-12-myristate-13-acetate (PMA)-induced potentiation of CTGF: 6a: PMA (2 × 10−7 M) added to the second curve; 6b: PMA added to the first curve, PMA plus tempol added to the second curve.
The specific NOX2 inhibitor peptide gp91ds-tat (RKKRRQRRRCSTRIRRQL-NH2) and its scrambled control peptide (RKKRRQRRRCLRITRQSR-NH2) were synthesized by Tufts University Core Facility. All other chemicals were purchased from Sigma.
Statistics.
Values are expressed as means ± SE. Paired t-tests were used to compare CTGF (ΔAf-Art diameter) between the control and experimental periods at each dose of NaCl. Hochberg's step-up procedure was used to adjust the P values for multiple comparisons. EC50 calculation was performed by best-fit regression analysis and four-parameter modeling using XLSTAT software. These estimates were compared using paired t-tests.
RESULTS
Effect of ANG II on CTGF.
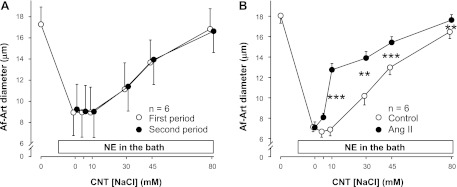
Experiments consisted of preconstriction of the Af-Art with NE, followed by two consecutive curves of progressive dilation of the Af-Art induced by increasing NaCl in the lumen of the attached CNT from 0 to 5, 10, 30, 45, and 80 mM. To show that CTGF remains stable over time, we first performed two CTGF curves with vehicle. During the first vehicle period, the concentration of NaCl in the CNT perfusate needed to achieve half of the maximal vasodilatory response in the attached preconstricted Af-Art was (EC50) was 38.3 ± 4.2 mM. During the second vehicle period, the EC50 was 37.1 ± 3.0 mM (Fig. 1A). Thus, the two curves were not significantly different, indicating that the CTGF response is stable over time.
Fig. 1.
A: increasing NaCl concentration 2 consecutive times in the connecting tubule (CNT) dilated preconstricted afferent arterioles (Af-Arts) in a similar manner, indicating that CNT glomerular feedback (CTGF) is stable and reproducible over time (n = 6). B: adding 10−9 M ANG II to the CNT perfusate enhanced CTGF (n = 6; **P < 0.01; ***P < 0.001; with vs. without ANG II).
Then we studied the effect of intratubular ANG II (10−9 M) on CTGF. The EC50 was 36.8 ± 3.3 mM during the vehicle period, and 14.4 ± 0.9 mM during the ANG II treatment period (Fig. 1B). Thus, adding ANG II to the CNT perfusate enhanced NaCl-induced dilation by shifting the curve to the left (P < 0.001), confirming our previous finding that ANG II potentiates CTGF.
Role of O2− in ANG II-induced potentiation of CTGF.
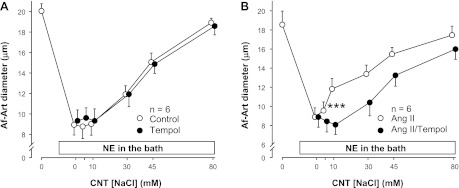
To test whether the enhancement of CTGF induced by ANG II is mediated by O2−, we used the O2− scavenger tempol (10−4 M). First, we compared the effect of tempol to vehicle on CTGF in the absence of exogenous ANG II. The EC50 was 37.0 ± 2.2 mM during the vehicle period, and 37.2 ± 3.3 mM during the tempol treatment period (Fig. 2A). Thus, the two curves were not significantly different, indicating that O2− does not mediate basal CTGF in the absence of exogenous ANG II. Then, we compared the effect of ANG II alone to that of ANG II plus tempol. The EC50 was 20.5 ± 3.7 mM during the ANG II alone period, and 41.2 ± 2.9 mM during the ANG II + tempol treatment period (Fig. 2B). Thus, adding tempol to the CNT perfusate attenuated NaCl-induced dilation by shifting the curve to the right (P < 0.01), indicating that O2− mediates ANG II-induced potentiation of CTGF.
Fig. 2.
A: adding tempol (resulting in scavenging of O2−) to the CNT lumen had no effect on the basal CTGF response induced by increasing NaCl concentration in preconstricted Af-Arts (n = 6). B: adding tempol to the CNT lumen blocked the stimulatory effect of ANG II on CTGF (n = 6; ***P < 0.001, with vs. without tempol).
Role of NOX in ANG II-induced potentiation of CTGF.
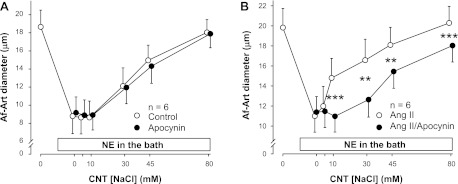
The sources of O2− in the CNT are unknown. To test whether the enhancement of CTGF induced by ANG II is mediated by NOX, we used the NOX inhibitor apocynin (10−5 M). First, we compared the effect of apocynin to vehicle on CTGF in the absence of exogenous ANG II. The EC50 was 35.4 ± 2.6 mM during the vehicle period, and 38.1 ± 0.9 mM during the apocynin treatment period (Fig. 3A). Thus, the two curves were not significantly different, indicating that NOX does not mediate basal CTGF in the absence of exogenous ANG II. Then, we compared the effect of ANG II alone to that of ANG II plus apocynin. The EC50 was 17.0 ± 2.4 mM during the ANG II alone period, and 39.5 ± 4.1 mM during the ANG II + apocynin treatment period (Fig. 3B). Thus, adding apocynin to the CNT perfusate attenuated NaCl-induced dilation by shifting the curve to the right (P < 0.01), indicating that O2− derived from NOX mediates ANG II-induced potentiation of CTGF.
Fig. 3.
A: adding the NADPH oxidase (NOX) inhibitor apocynin to the CNT lumen had no effect on the basal CTGF response induced by increasing NaCl concentration (n = 6). B: adding apocynin to the CNT lumen blocked the stimulatory effect of ANG II on CTGF (n = 6; **P < 0.01; ***P < 0.001, with vs. without apocynin).
Role of NOX2 in ANG II-induced potentiation of CTGF.
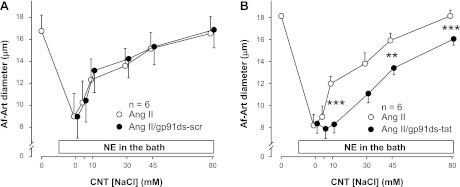
The NOX isoform responsible for ANG II-induced O2− production in the CNT is unknown. To test whether the enhancement of CTGF induced by ANG II is mediated by NOX2 we used the NOX2 inhibitor gp91ds-tat (10−5 M). First, we compared the effect of ANG II alone to that of ANG II plus a scramble-gp91ds-tat on CTGF. The EC50 was 15.2 ± 1.2 mM during the ANG II alone period, and 12.8 ± 1.1 mM during the ANG II plus a scramble-gp91ds-tat treatment period (Fig. 4A). Thus, the two curves were not significantly different, indicating that scrambled-gp91ds-tat does not affect ANG II-stimulated CTGF. Then, we compared the effect of ANG II alone to that of ANG II plus the gp91ds-tat. The EC50 was 18.8 ± 1.7 mM during the ANG II alone period, and 34.4 ± 2.2 mM during the ANG II + gp91ds-tat treatment period (Fig. 4B). Thus, adding gp91ds-tat to the CNT perfusate attenuated NaCl-induced dilation by shifting the curve to the right (P < 0.01), indicating that O2− derived from NOX2 mediates ANG II-induced potentiation of CTGF.
Fig. 4.
A: scrambled gp91ds-tat had no effect on ANG II-enhanced CTGF (n = 6). B: NOX2 inhibitor gp91ds-tat blocked the stimulatory effect of ANG II on CTGF (n = 6; **P < 0.01; ***P < 0.001, with vs. without gp91ds-tat).
Connection between PKC and O2− in ANG II-induced potentiation of CTGF.
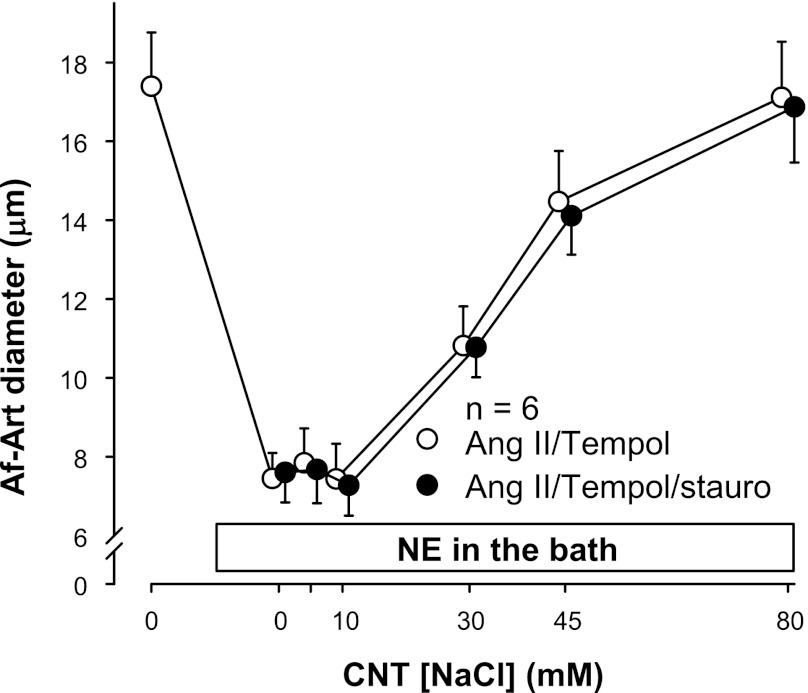
Because we previously showed that the PKC inhibitor staurosporine (10−8 M) can block the stimulatory effect of ANG II on CTGF (39), we tested whether PKC and O2− act independently of each other to stimulate CTGF in response to ANG II. We compared the effect of ANG II plus tempol to that of ANG II plus tempol plus staurosporine. The EC50 was 32.2 ± 3.1 mM during the ANG II plus tempol period, and 34.7 ± 2.3 mM during the ANG II plus tempol plus staurosporine treatment period (Fig. 5). Thus, the two curves were not significantly different, indicating that PKC and O2− share a common pathway in the enhancement of CTGF by ANG II.
Fig. 5.
Adding the protein kinase C (PKC) inhibitor staurosporine (stauro) to the CNT lumen had no effect in the presence of ANG II plus tempol (n = 6).
Role of O2− in PMA-induced potentiation of CTGF.
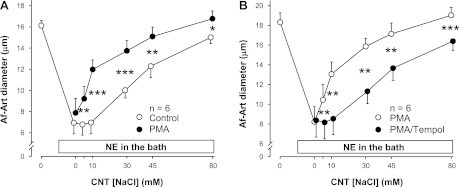
To determine whether O2− acts downstream from PKC in the enhancement of CTGF, we conducted two experiments using the PKC activator PMA (2 × 10−7 M) in the absence of exogenous ANG II. First, we compared the effect of PMA with vehicle on CTGF in the absence of exogenous ANG II. The EC50 was 34.6 ± 1.9 mM during the vehicle period, and 14.1 ± 1.3 mM during the PMA treatment period (Fig. 6A). Thus, adding PMA to the CNT perfusate enhanced NaCl-induced dilation by shifting the curve to the left (P < 0.001), indicating that PKC activation mimics the effect of ANG II on CTGF. Then, we compared the effect of PMA alone to that of PMA plus tempol. The EC50 was 13.6 ± 1.9 mM during the PMA alone period, and 34.8 ± 2.3 mM during the PMA + tempol treatment period (Fig. 6B). Thus, adding tempol to the CNT perfusate attenuated NaCl-induced dilation by shifting the curve to the right (P < 0.001), indicating that O2− mediates PMA-induced potentiation of CTGF.
Fig. 6.
A: adding the PKC activator phorbol-12-myristate-13-acetate (PMA; 2 × 10−7 M) to the CNT perfusate enhanced CTGF (n = 6; *P < 0.05; **P < 0.01; ***P < 0.001, with vs. without PMA). B: adding tempol to the CNT lumen blocked the stimulatory effect of PMA on CTGF (n = 6; **P < 0.01; ***P < 0.001, with vs. without tempol).
DISCUSSION
Although we reported that tubular ANG II enhances CTGF via the AT1 receptor, the mechanism involved is unknown. ANG II may act via the release of O2−, since there is evidence that it stimulates formation of O2− in tubular cells (18). In the present study, we found that the stimulatory effect of ANG II on CTGF is mediated by O2− generated primarily by NOX, since such stimulation is suppressed when O2− concentration is reduced either by 1) decreasing O2− concentration by adding the O2− scavenger tempol or 2) decreasing O2− generation using either a NOX inhibitor without isoform specificity or a selective NOX inhibitor. We also found that the action of PKC involves activation of O2− on CTGF, since a PKC activator (PMA) directly augments CTGF and tempol completely abolished the effect of PMA.
We previously showed that both basal CTGF and its enhancement by ANG II are ultimately mediated by ENaC (39). Yu et al. (54) showed that in distal nephron cells, increasing O2− by either inhibiting endogenous superoxide dismutase or by adding exogenous xanthine oxidase and hypoxanthine increases ENaC open probability and that scavenging O2− with tempol decreases it. Taken together with our present study, these data suggest that ANG II-induced O2− enhances CTGF by activating ENaC. Furthermore, Yu et al. (54) also showed that the effect of O2− on ENaC activity is mediated by a decrease in NO; this is also consistent with our data, as we previously showed that inhibition of NO synthesis potentiates CTGF (41).
O2− is formed by several oxidases and oxygenases and by incomplete mitochondrial oxidative phosphorylation. NOX isoforms are the major source of O2− that impacts the vasculature and the nephron. To clarify the source(s) of O2−, we used the NOX inhibitor apocynin. We found that ANG II-stimulated CTGF is blunted by apocynin, indicating that a NOX isoform mediates the effect of ANG II on CTGF. It has been reported that apocynin acts as scavenger of reactive oxygen species (ROS) at high concentrations (over 1 mM) (21); however, this is not likely to be the case at the concentrations we used (10 μM). Rather, the effect of apocynin we observed is likely due to NOX inhibition.
NOXs are a family of enzymes that specifically produce O2−. They are primarily composed of six subunits designated p22phox, gp91phox, p47phox, p67phox, p40, and the small GTPase Rac (16, 45). There are at least seven family members: NOX1, NOX2, NOX3, NOX4, NOX5, Duox1, and Duox2, named for the isoform of the major catalytic subunit, gp91phox (16, 32). At least three different NOX isoforms are expressed in the renal cortex: NOX1 (5), NOX2 (1, 5, 46), and NOX4 (5, 14). The primary source of ANG II-induced O2− production in the macula densa is NOX2 (13); however, whether this is also true for the CNT remains unknown. In the present study, we attempted to answer this question by using gp91ds-tat, a cell-permeant peptide that mimics a sequence of NOX2 thought to be necessary for interaction with p47phox (42). This peptide was recently shown to inhibit NOX2 specifically, without inhibiting NOX1 or NOX4 (7). We found that adding gp91ds-tat to the CNT perfusate blocked the stimulatory effect of ANG II but did not alter basal CTGF. A scrambled gp91ds-tat had no effect on ANG II enhancement of CTGF. Although we did not measure O2− in the CNT, we believe our data are sufficient to conclude that ANG II enhancement of CTGF is mediated by O2− via NOX and primarily involves activation of NOX2.
We previously reported that the PKC inhibitor staurosporine blocks the enhancement of CTGF induced by ANG II. Here, we showed that staurosporine was devoid of effect in the presence of the O2− scavenger tempol, indicating that PKC and O2− are part of the same pathway. This suggests two distinct possibilities: 1) O2− could activate PKC or 2) PKC could activate NOX and O2− production; in fact, there is literature to support both possibilities. On one hand, PKC-dependent, NOX-mediated oxidative bursts have been characterized in phagocytes (10), in cardiomyocytes (51), as well as in oxidative stress associated with diabetes and hypertension in the vasculature (20, 23). In cell-free systems, many PKC isoforms phosphorylate p47phox, a regulatory subunit of NOX (12). On the other hand, Silva et al. (43) reported that O2− increased NaCl absorption by the thick ascending limb via activation of PKC, and blocking PKC with calphostin C or staurosporine inhibited O2−-stimulated NaCl absorption. To clarify this, we used the PKC activator PMA, which enhanced CTGF similarly to ANG II. Interestingly, tempol blocked the effect of PMA, indicating that O2− acts downstream from PKC in the enhancement of CTGF.
In general, enhanced generation of ROS by NOX enzymes is thought to contribute to nephrotoxicity (17, 33). There are indications that NOX inhibitors may prevent renal damage in diabetic animals (2); however, the pathogenesis of hypertensive nephrosclerosis is complex, and we are not sure whether NOX-derived ROS via CTGF contributes to glomerular hypertension. It may be that increased CTGF induces glomerular hypertension by dilating the Af-Art, causing glomerulosclerosis, proteinuria, and progressive renal failure, particularly in hypertensive individuals with high ANG II and in salt-sensitive hypertension. Thus, given the pathophysiological implications of Af-Art resistance in the control of renal function and development of glomerulosclerosis, understanding the mechanisms that control Af-Art tone is of great physiological and pathological significance.
The source of ANG II in the CNT is not known. The possibility that ANG II is released locally in the CNT is supported by the presence of the various renin-angiotensin system components within the nephron, including renin expression in the CNT (34). However, ANG II does not need to be released locally to act on CTGF, it may well be carried downstream from an earlier nephron segment, as the proximal tubular fluid ANG II levels are at least 10 times higher than those we used in the present study (35). We also acknowledge that because the technique of double perfusion of CNT and Af-Art has not been adapted to mice, we have not employed genetical knockout techniques to test our hypotheses, and our conclusions rely on the use of pharmacological agents. Nevertheless, we have used several drugs acting on the same pathway to strengthen our conclusions.
Our findings suggest that intratubular ANG II, by potentiating CTGF, may act to buffer systemic ANG II-induced Af-Art constriction. Interestingly, this may provide an explanation for the long known observation that ANG II causes relatively less constriction of the afferent compared with the efferent arteriole, thus relatively preserving glomerular filtration rate in the presence of a decrease in renal blood flow. Furthermore, our findings may provide an explanation for the recent report by Singh et al. (44) showing that in subtotal nephrectomy performed by renal ischemia (a model of high ANG II), a mechanism that controls the renal microcirculation is recruited that dilates the Af-Art in response to increases in distal tubular flow and that such mechanism can be blocked by an AT1 antagonist. Of note, both the increase in distal delivery of NaCl seen in subtotal nephrectomy and the increase in ANG II would be expected to increase CTGF.
In summary, using an isolated perfused CNT and Af-Art preparation we found that adding the O2− scavenger tempol to the CNT lumen completely blocked the effect of ANG II, whereas tempol alone did not alter CTGF. The NADPH inhibitor apocynin and the selective NOX2 inhibitor gp91ds-tat also blocked the effect of ANG II on CTGF. The PKC activator PMA enhanced CTGF but its effect was prevented by tempol. These findings suggest that the effect of ANG II on CTGF is mediated by PKC, NOX2, and O2−.
GRANTS
This study was supported by National Institutes of Health Program Project Grants HL028982 and HL088036.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.R. performed experiments; Y.R., M.A.D., and E.L.P. analyzed data; Y.R. and M.A.D. drafted manuscript; Y.R., M.A.D., H.W., E.L.P., J.L.G., and O.A.C. approved final version of manuscript; M.A.D., H.W., J.L.G., and O.A.C. interpreted results of experiments; M.A.D. prepared figures; J.L.G. and O.A.C. conception and design of research; J.L.G. and O.A.C. edited and revised manuscript.
REFERENCES
- 1. Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67: 1890–1898, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Barajas L, Powers K, Carretero O, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int 29: 965–970, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Burg MB. Perfusion of isolated renal tubules. Yale J Biol Med 45: 321–326, 1972 [PMC free article] [PubMed] [Google Scholar]
- 5. Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67phox and gp91phox in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol 279: H2234–H2240, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Csányi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egaña L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorup J, Morsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Invest 67: 761–769, 1992 [PubMed] [Google Scholar]
- 9. Douglas JG, Romero M, Hopfer U. Signaling mechanisms coupled to the angiotensin receptor of proximal tubular epithelium. Kidney Int Suppl 30: S43–S47, 1990 [PubMed] [Google Scholar]
- 10. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med 41: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faarup P. On the morphology of the juxtaglomerular apparatus. Acta Anat (Basel) 60: 20–38, 1965 [DOI] [PubMed] [Google Scholar]
- 12. Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of Renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386: 401–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han HJ, Lee YJ, Park SH, Lee JH, Taub M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol 288: F988–F996, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hannken T, Schroeder R, Stahl RAK, Wolf G. Angiotensin II-mediated expression of p27Kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int 54: 1923–1933, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43: 335–340, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RAK, Macharzina R, Bräsen JH, Meinertz T, Münzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int 56: 252–260, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hua H, Munk S, Goldberg H, Fantus IG, Whiteside CI. High glucose-suppressed endothelin-1 Ca2+ signaling via NADPH oxidase and diacylglycerol-sensitive protein kinase C isozymes in mesangial cells. J Biol Chem 278: 33951–33962, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–S232, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ito S, Carretero OA. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int 38: 1206–1210, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Jin N, Packer CS, Rhoades RA. Reactive oxygen-mediated contraction in pulmonary arterial smooth muscle: cellular mechanisms. Can J Physiol Pharmacol 69: 383–388, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS. Mitochondrial superoxide production during oxalate-mediated oxidative stress in renal epithelial cells. Free Radic Biol Med 32: 1339–1350, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975 [DOI] [PubMed] [Google Scholar]
- 29. Kontos HA, Wei EP, Ellis EF, Jenkins LW, Povlishock JT, Rowe GT, Hess ML. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res 57: 142–151, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis 57: S28–S29, 2004 [PubMed] [Google Scholar]
- 33. Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol 14: S221–S226, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol 5: 1153–1158, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Pawelczyk T, Bizon D, Angielski S. The distribution of enzymes involved in purine metabolism in rat kidney. Biochim Biophys Acta 1116: 309–314, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Ren Y, Carretero OA, Garvin JL. Mechanism by which superoxide potentiates tubuloglomerular feedback. Hypertension 39: 624–628, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Ren Y, D'Ambrosio MA, Garvin JL, Carretero OA. Angiotensin II enhances connecting tubule glomerular feedback. Hypertension 56: 636–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ren Y, D'Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension 53: 319–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ren Y, Garvin JL, Liu R, Carretero OA. Cross talk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Singh P, Deng A, Blantz RC, Thomson SC. Unexpected effect of angiotensin AT1 receptor blockade on tubuloglomerular feedback in early subtotal nephrectomy. Am J Physiol Renal Physiol 296: F1158–F1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeya R, Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol Cells 16: 271–277, 2003 [PubMed] [Google Scholar]
- 46. Vaziri ND, Dicus M, Ho N, Boroujerdi-Rad L, Sindhu RK. Oxidative stress and dysregulation of superoxide dismutase and NAD(P)H oxidase in renal insufficiency. Kidney Int 63: 179–185, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Vio CP, Figueroa CD, Caorsi I. Anatomical relationship between kallikrein-containing tubules and the juxtaglomerular apparatus in the human kidney. Am J Hypertens 1: 269–271, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Garvin JL, D'Ambrosio MA, Ren Y, Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 299: F1374–F1378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang W, Wang S, Yan L, Madara P, Cintron AD, Wesley RA, Danner RL. Superoxide production and reactive oxygen species signaling by endothelial nitric oxide synthase. J Biol Chem 275: 16899–16903, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Wei XF, Zhou QG, Hou FF, Liu BY, Liang M. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol 296: F427–F437, 2009 [DOI] [PubMed] [Google Scholar]
- 51. White CN, Figtree GA, Liu CC, Garcia A, Hamilton EJ, Chia KK, Rasmussen HH. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am J Physiol Cell Physiol 296: C693–C700, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Wilcox CS, Welch WJ. Interaction between nitric oxide and oxygen radicals in regulation of tubuloglomerular feedback. Acta Physiol Scand 168: 119–124, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]