Abstract
The significance of conserved cysteines in the human organic cation transporter 2 (hOCT2), namely the six cysteines in the long extracellular loop (loop cysteines) and C474 in transmembrane helix 11, was examined. Uptake of tetraethylammonium (TEA) and 1-methyl-4-phenypyridinium (MPP) into Chinese hamster ovary cells was stimulated >20-fold by hOCT2 expression. Both cell surface expression and transport activity were reduced considerably following mutation of individual loop cysteines (C51, C63, C89, C103, and C143), and the C89 and C103 mutants had reduced Michaelis constants (Kt) for MPP. The loop cysteines were refractory to interaction with thiol-reactive biotinylation reagents, except after pretreatment of intact cells with dithiothreitol or following cell membrane solubilization. Reduction of disulfide bridge(s) did not affect transport, but labeling the resulting free thiols with maleimide-PEO2-biotin did. Mutation of C474 to an alanine or phenylalanine did not affect the Kt value for MPP. In contrast, the Kt value associated with TEA transport was reduced sevenfold in the C474A mutant, and the C474F mutant failed to transport TEA. This study shows that some but not all of the six extracellular loop cysteines exist within disulfide bridge(s). Each loop cysteine is important for plasma membrane targeting, and their mutation can influence substrate binding. The effect of C474 mutation on TEA transport suggests that it contributes to a TEA binding surface. Given that TEA and MPP are competitive inhibitors, the differential effects of C474 modification on TEA and MPP binding suggest that the binding surfaces for each are distinct, but overlapping in area.
Keywords: kidney, tubular secretion, transport, structure-function, SLC22A2
proximal tubular secretion represents an important pathway in the renal excretion of a diverse array of organic compounds carrying a net positive charge at physiological pH (organic cations), including clinically important therapeutics, endobiotics, and environmental toxins (19, 22, 26). The organic cation transporter 2 (OCT2; SLC22A2) is expressed in basolateral membranes of human renal proximal tubule cells and represents the initial step (cellular uptake) in tubular organic cation secretion (14). Depending on the prevailing set of electrical and chemical gradients, OCT-mediated transport can occur either via electrogenic facilitated diffusion (i.e., driven by the negative membrane potential) or organic cation exchange (3, 4, 9). Metformin (antidiabetic), cimetidine (antihistamine), oxaliplatin (anticancer), and amantadine (antiparkisonian) are a few examples of therapeutic substrates of OCT2, and their physicochemical and structural differences highlight the multiselectivity of ligand interaction with OCT2 (11, 12, 15). Consequently, OCT2-mediated renal proximal tubular secretion is a potential site of drug-drug interactions (5). A clearer understanding of substrate binding to OCT2 and the translocation process moves toward a priori predictions of the interaction of organic cations as substrates and inhibitors of OCT2.
OCT2 belongs to a larger family of solute carriers (OCT family), which include other OCTs (OCT1 and OCT3), the “novel” organic cation transporters 1–3 (OCTN1–3), and the organic anion transporters 1–5 (OAT1–5). OCT family members have several structural features in common, including 12 putative transmembrane spanning helices (TMHs), intracellular COOH and NH2 termini, a large extracellular loop between TMHs 1 and 2, and a large intracellular loop between TMHs 6 and 7. Homology models of the three-dimensional structures of OAT1, OCT1, and OCT2 have been generated (20, 21, 27). Centrally located within these models is a large hydrophilic cleft that is formed by TMHs 1, 2, 4, 5, 7, 8, 10, and 11. The hydrophilic cleft is proposed to contain the substrate binding surface(s) since mutation of several residues lining the cleft has been shown to influence substrate selectivity (6, 7, 20, 21, 27).
Previously our laboratory tested the validity of the OCT2 model using membrane-impermeant, thiol-reactive reagents to probe accessibility of cysteine residues from the extracellular space (16, 18). The human ortholog of OCT2 (hOCT2) contains 13 cysteine residues in its sequence, and based on their relative position in the model, six occur in the long extracellular loop (“loop” cysteines), three in TMHs peripheral to the hydrophilic cleft (TMHs 3, 6, and 9), and four in TMHs that form the cleft (TMHs 10 and 11). Of the 13 cysteine residues, only C451 in TMH 10 and C474 in TMH 11 were accessible from the extracellular space, and these findings were consistent with the placement of these residues in the homology models, i.e., they are in contact with the aqueous phase of the hydrophilic cleft. The presence of substrate in the binding surface rendered C474 less accessible to thiol-reactive reagents, suggesting that it is close to or part of the substrate binding surface. Indeed, the adjacent residue D475 has been shown to be important for substrate binding (7). Additionally, the refractoriness of the six putative loop cysteines to modification by thiol-reactive probes suggested that they may form disulfide bridges (16, 18). The six cysteine residues in the extracellular loop of hOCT2 are conserved at homologous positions in all orthologs of OCTs and OCTNs, and four of the six cysteines are conserved in all orthologs of OATs, further supporting the hypothesis that they may be structurally/functionally significant. The present study sought further understanding of structure activity relationships in hOCT2 by determining 1) the influence of loop cysteines on membrane expression and transport kinetics, 2) whether disulfide bridges occur in the long extracellular loop and their functional significance, and 3) the influence of modifying C474 on transport kinetics.
MATERIALS AND METHODS
Chemicals.
[3H]Tetraethylammonium ([3H]TEA; 54 Ci/mmol) was synthesized by Amersham Biosciences. [3H]1-Methyl-4-phenylpyridinium ([3H]MPP; 85 Ci/mmol) and [14C]TEA (50 mCi/mmol) were from American Radiochemicals. (+)-Biotinyl-3-maleimidopropionamidyl-3,6-dioxaoctanediamine (maleimide-PEO2-biotin) was from Pierce Biotechnology. N-biotinylaminoethyl methanethiosulfonate (MTSEA-biotin) was from Toronto Research Chemical. TEA, MPP, dithiothreitol (DTT), and Ham's F12 Kaighn's modification medium were from Sigma. Platinum High Fidelity DNA polymerase, Zeocin, Hygromycin B, Flp recombinase expression plasmid (pOG44), and the mammalian expression vector pcDNA5/FRT/V5-His TOPO were from Invitrogen.
TOPO cloning of hOCT2 and site-directed mutagenesis.
The open reading frame of hOCT2 (contained in pcDNA3.1) containing a COOH-terminal V5 epitope tag (amino acid sequence, GKPIPNPLLGLDST) was amplified using Platinum High Fidelity DNA polymerase and sequence-specific primers with the following PCR conditions: 35 cycles of 94°C for 1 min, 54°C for 1 min, and 72°C for 3.5 min. A final elongation step of 7 min was included after the last cycle. The PCR product was gel purified and cloned into the pcDNA5/FRT/V5-His TOPO mammalian expression vector. Mutations to the V5-tagged hOCT2 sequence were introduced by site-directed mutagenesis using the Quick Change system following the manufacturer's instructions (Stratagene, La Jolla, CA). Ten different mutant constructs of hOCT2 were used in these experiments. Five of the mutant constructs each had one of the loop cysteines substituted with either an alanine or serine (C51A, C63A, C89A, C103A, and C143S). The “quadruple” mutant had the four cysteines in TMH 10 and TMH 11 converted to alanines (C437A/C451A/C470A/C474A). The “septuplet” mutant had all seven cysteines in the TMHs mutated to alanines (C179A/C282A/C418A/C437A/C451A/C470A/C474A), leaving only the loop cysteines. Another mutant had an alanine converted to a cysteine at amino acid position 144 on the septuplet background (septuplet/A144C). The final two mutants of hOCT2 had C474 in TMH 10 converted to either an alanine (C474A) or phenylalanine (C474F). Plasmid DNA was prepared using standard methods (Genesee Scientific, San Diego, CA), and sequences were confirmed with an Applied Biosystems 3730xl DNA analyzer at the University of Arizona sequencing facility.
Cell culture and stable expression of hOCT2.
Chinese hamster ovary (CHO) cells containing a single integrated Flp Recombination Target (FRT) site were acquired from Invitrogen and were used for stable expression of wild-type hOCT2 and the mutant constructs. Before transfection, CHO cells were grown in Ham's F12 Kaighn's modification medium supplemented with 10% fetal calf serum and zeocin (100 μg/ml). Cultures were split every 3 days. Cells (5 × 106) were transfected by electroporation (BTX ECM 630, San Diego, CA; 260 V and time constant of ∼25 ms) with 10 μg of salmon sperm, 18 μg of pOG44, and 2 μg of pcDNA5/FRT/V5-His TOPO containing wild-type hOCT2 or the mutant constructs. Cells were seeded in a T-75 flask following transfection and maintained under selection pressure with hygromycin B (200 μg/ml). Cells were used for experiments ∼21 days after electroporation.
Cell surface biotinylation.
The method described here has been described previously (1, 16, 18). All solutions were kept ice-cold throughout the procedure, and long incubations were conducted on ice with gentle shaking. Cells plated to confluence in a 12-well plate were initially washed three times with 2 ml of PBS solution containing calcium and magnesium (PBS/CM; containing in mM: 137 NaCl, 2.7 KCl, 8 Na2HPO4, 1.5 KH2PO4, 0.1 CaCl2, and 1 MgCl2, pH 7.0 with HCl) followed by a single 20-min incubation in either maleimide-PEO2-biotin (1 mM) or MTSEA-biotin (1 mM) diluted in PBS/CM. These concentrations were chosen since they resulted in a maximum level of hOCT2 precipitation in dose-response studies (data not shown). To probe for disulfide linkages, the cells were treated with DTT (10 mM for 20 min at room temperature) and rinsed quickly (<30-s total) with three changes (2 ml each) of PBS/CM before biotinylation, as stated above. After biotinylation, the cells were rinsed twice briefly with 3 ml of PBS/CM followed by a 20-min incubation in the same solution. The cells were solubulized in 1 ml of solubilization buffer (150 mM NaCl, 10 mM Tris·HCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, pH 7.4) containing protease inhibitors [in μM: 200 4-(2-aminoethyl)-bezenesulfonyl-fluoride, 0.16 aprotinin, 4 leupeptin, 8 bestatin, 3 pepstatin A, 2.8 E-64; Sigma] for 1 h and centrifuged at 15,800 g (4°C) for 30 min to remove insoluble material. Streptavidin-agarose beads (50 μl; Pierce Biotechnology) were added to the lysates and incubated overnight at 4°C with constant mixing. After being extensively washed with the above solubilization buffer, 50 μl of Laemmli sample buffer containing 5% 2-mercaptoethanol were added, and the proteins were eluted from the beads at 100°C for 5 min.
Biotinylation of solubilized proteins.
In some experiments, cells were treated with MTSEA-biotin after membrane solubilization. Briefly, the cells were solubilized on ice for 1 h with gentle shaking in 1 ml of solubilization buffer as described above. The soluble fraction was then exposed for 25 min to 1 ml of MTSEA-biotin (1 mg/ml), diluted in PBS/CM. The MTSEA-biotin was added directly to the wells containing the solubulized cells. After biotinylation, lysates were dialyzed (10,000 MWCO Slide-A-Lyzer dialysis cassette; Thermo Scientific) to remove unreacted MTSEA-biotin. Cassettes hydrated in dialysis buffer (PBS/CM; containing in mM: 137 NaCl, 2.7 KCl, 8 Na2HPO4, 1.5 KH2PO4, 0.1 CaCl2, and 1 MgCl2, pH 7.0 with HCl) were loaded with 2 ml of lysate and dialyzed for 2 h at room temperature in 600 ml of buffer on a stir-plate with constant cassette rotation. After 2 h the buffer was removed, replaced, and the process was repeated. After the second 2-h period, the buffer was changed and the volume was increased to 800 ml. The final dialysis was completed after an overnight incubation at 4°C. Samples were removed from each cassette, 75 μl of streptavidin-agarose beads were added, and the mixture was incubated overnight at 4°C with constant mixing (as above). After extensive washing with the above PBS/CM buffer, 75 μl of Laemmli sample buffer (wt/5% 2-mercaptoethanol) were added and the proteins were eluted from the beads at 100°C for 5 min.
Crude membrane preparation.
CHO cells grown to confluence in a 10-cm dish were rinsed twice with PBS and scraped from the dish using a cell scraper. The cells were resuspended in 10 ml of PBS and pelleted by centrifugation (230 g) for 10 min at 4°C. The cell pellet was resuspended in 1 ml of lysis buffer (50 mM mannitol, 1 mM tris-base, pH 7.4 with HEPES) by passing it ∼20 times through a 27-gauge needle. Insoluble cellular material was removed by centrifugation at 100 g for 5 min at 4°C. The supernatant was centrifuged for 30 min at 15,800 g (4°C), and the resulting pellet was resuspended (by vortexing) in lysis buffer. Protein concentration was determined by the bicinchoninic acid method. Crude membrane proteins were diluted to 1–2 μg/μl in Laemmli sample buffer.
SDS-PAGE and Western blotting.
Proteins were separated on 8% SDS-PAGE gels and electrophoretically transferred to a polyvinylidene fluoride membrane. The membrane was blocked for 1 h in blocking buffer [5% nonfat dry milk in PBS-T (PBS containing 0.05% Tween-20)] at room temperature, followed by overnight incubation (4°C) with mouse anti-V5 antibody (0.1 μg/ml; Invitrogen, Carlsbad, CA) diluted in blocking buffer. After being extensively washed with PBS-T, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (0.01 μg/ml) diluted in blocking buffer. Following extensive washing with PBS-T, the membrane was incubated in SuperSignal West Femto Maximum Sensitivity Substrate (Pierce), and the secondary antibody was detected on high-performance chemiluminescence film (Amersham Biosciences, Buckinghamshire, UK).
Immunocytochemistry.
CHO cells grown on coverslips in 12-well plates were washed with PBS (137 mM NaCl, 2.7 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.3). All subsequent washes were performed in triplicate at room temperature in PBS. Cells were fixed in ice-cold 100% methanol for 20 min, washed, and incubated for 1 h with mouse anti-V5 antibody (Invitrogen) diluted in PBS (final concentration of 2 μg/ml). The cells were washed and incubated for 1 h in the dark with FITC-conjugated goat anti-mouse IgG (Invitrogen) diluted to 2 μg/ml in PBS. The cells were washed before staining the nuclei with propidium iodide (5 μg/ml in PBS; Sigma) for 10 min. Cells were washed again and the coverslips were mounted onto microscope slides. A confocal microscope (Nikon PCM 2000 scan head fitted to a Nikon E800 microscope) was used for detection of immunoreactivity.
Transport experiments.
CHO cells grown to confluence in 12-well plates were rinsed twice with Waymouth's buffer (WB; in mM: 135 NaCl, 28 d-glucose, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, 0.8 MgSO4, and 13 HEPES-NaOH, pH 7.4) at room temperature before transport measurement. Transport experiments were conducted using [3H]TEA (∼20 nM) or [3H]MPP (∼10 nM) diluted in WB. [14C]TEA (∼5 μM) was used in the transport experiments with the mutant loop cysteine constructs. In some cases, transport was conducted in the presence of unlabeled substrate. All transport experiments were conducted at initial rates (30 s), as preliminary experiments showed that uptake of the radiolabeled substrates into cells expressing wild-type hOCT2 was linear for at least 30 s (data not shown). To examine the effect of DTT (10 mM), maleimide-PEO2-biotin (1 mM), or DTT and maleimide-PEO2-biotin in combination, cells were treated identically as in the cell surface biotinylation assays and rinsed quickly (three times with 2 ml of WB, <30 s total) before transport measurement. For each of the treatments, including the control, the time spent out of culture media and in WB was identical (40 min). For example, the control cells were put in WB for 40 min before transport measurement, whereas the DTT-treated cells spent 20 min in WB followed by 20 min in WB containing 10 mM DTT before transport measurement. After the uptake period, cells were washed with WB and solubilized in 400 μl of 0.5 N NaOH with 1% SDS (vol/vol), and the resulting lysate was neutralized with 200 μl of 1 N HCl. Accumulated radioactivity was determined by liquid scintillation spectrometry (Beckman model LS3801). Mediated uptake was calculated from the difference in uptake in the absence vs. the presence of a saturating concentration of unlabeled substrate. Individual transport observations were performed in duplicate for each experiment, and observations were usually confirmed several times in separate experiments using cells of a different passage.
Kinetic analysis of transport.
Increasing concentrations of unlabeled substrate reduced the rate of radiolabeled substrate transport by a process adequately described by the Michaelis-Menten equation for competitive interaction of labeled and unlabeled substrate (13): J = [Jmax (S*)]/[Kt + (S*) + (S)]+ C, where J is the rate of [3H]substrate transport from a concentration of labeled substrate equal to (S*), Jmax is the maximum rate of transport, Kt is the substrate concentration that results in half-maximal transport (Michaelis constant), (S) is the concentration of unlabeled substrate, and C is a constant representing the component of total substrate uptake that is not saturable over the concentration range tested. This nonsaturable component likely reflects the combined influence of diffusive flux, nonspecific binding, and/or incomplete rinsing of the cell layer.
Statistics.
All data are expressed as means ± SE, with calculations of SE based on the number of separate experiments conducted on cells at a different passage number. Paired comparison of sample means was done using unpaired Student's t-test. One-way ANOVA was used to test the effect of multiple treatments and was followed by the Student-Newman-Keuls test for pairwise comparisons. All statistical analyses were performed with GraphPad Prism 5.04 (GraphPad Software) and deemed significant when P < 0.05.
RESULTS
The conservation of loop cysteines across OCT family members suggests that they are functionally important. To examine the role of each individual loop cysteine, we constructed several mutant constructs (C51A, C63A, C89A, C103A, and C143S) and measured TEA and MPP transport activity and cell surface expression using biotinylation with maleimide-PEO2-biotin (Fig. 1). Numerous attempts to mutate C122 were unsuccessful. Since C474 is readily accessible to maleimide-PEO2-biotin (18), and each of the mutant constructs tested had C474 in their sequence, the level of precipitation with maleimide-PEO2-biotin was used to approximate cell surface expression. The uptake of MPP and TEA was stimulated 24- and 33-fold, respectively, by expression of wild-type hOCT2 (Fig. 1A). Uptake of MPP by cells expressing the mutant constructs ranged from 0 to 34% of the uptake into cells expressing wild-type hOCT2, with only the C89A and C103A mutants showing a level of uptake significantly higher than that of control CHO cells. Uptake of TEA by cells expressing the mutant constructs was lower than MPP uptake (expressed as the percentage of uptake into cells expressing wild-type hOCT2), and not significantly different than the level of uptake into control CHO cells. The kinetics of MPP transport by the C89A and C103A mutants, and wild-type hOCT2, were also determined. The Kt values for MPP transport by the C89A and C103A mutants were 9.64 ± 3.4 and 10.9 ± 3.7% of the Km value observed for wild-type hOCT2 (n = 3, P < 0.05). The Jmax values for MPP transport by the C89A and C103A mutants were 5.93 ± 2.2 and 3.85 ± 1.6% of the Jmax value observed for wild-type hOCT2 (n = 3, P < 0.05). The reductions in Jmax were, at least in part, accounted for by large reductions in the level of plasma membrane expression of the single-cysteine mutants. Compared with wild-type hOCT2, plasma membrane expression of the mutant constructs was undetectable by cell surface biotinylation with maleimide-PEO2-biotin (Fig. 1B). However, the protein was detected in crude membrane preparations prepared from cells expressing each of the hOCT2 constructs (Fig. 1C). Although wild-type hOCT2 had an apparent molecular mass of ∼85 kDa, the mutant constructs had a lower apparent molecular mass (∼60 kDa; Fig. 1C). In previous work, the lower molecular weight band was shown to reflect immature protein within the cell (17).
Fig. 1.
A: 30-s uptakes of [14C]tetraethylammonium (TEA; ∼5 μM) or [3H]1-methyl-4-phenypyridinium (MPP; ∼10 nM) by Chinese hamster ovary (CHO) cells (control), CHO cells stably expressing wild-type human organic cation transporter 2 (hOCT2; WT), or CHO cells stably expressing one of the mutant loop cysteine constructs of hOCT2. Data are expressed as a percentage of substrate uptake by cells expressing WT hOCT2. *Significantly different from control (n = 3, P < 0.05; Student-Newman-Kuels test). B: Western blot showing the maleimide-PEO2-biotin precipitation of WT hOCT2 (WT) or the mutant loop cysteine constructs of hOCT2 expressed at the plasma membrane. Note, C474 was previously found to be readily accessible to biotinylation with maleimide-PEO2-biotin, and all constructs tested here contain C474 in their sequence. C: Western blot showing the expression of hOCT2 in crude membranes prepared from cells expressing the various constructs.
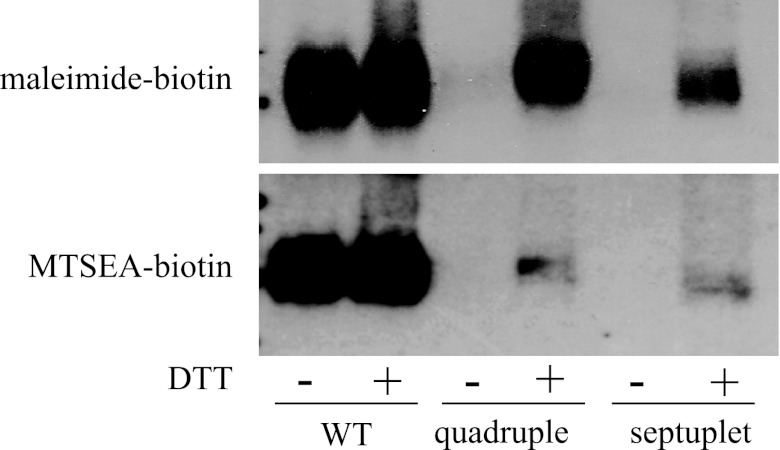
Previous studies showed that of the 13 cysteine residues in the hOCT2 sequence, only C474 (TMH 11) is accessible to maleimide-PEO2-biotin, whereas both C451 and C474 are accessible to MTSEA-biotin (16, 18). The refractoriness of the loop cysteines to interaction with the membrane-impermeant, thiol-reactive biotinylation reagents led our group and others to suggest that some or all of the loop cysteines form disulfide bridges (16, 23). To test this hypothesis, we examined thiol reactivity in a mutant of hOCT2 (septuplet mutant) in which all seven of the cysteine residues residing in the TMHs were mutated to alanines, leaving only the six loop cysteines. We also tested thiol reactivity in a mutant (quadruple mutant) that was previously shown to be unreactive toward MTSEA-biotin and malemide-PEO2-biotin; the four cysteines in TMH 10 and TMH 11 were converted to alanines in the quadruple mutant (16). The septuplet mutant was functional, supporting blockable TEA and MPP transport (Fig. 2), and the quadruple mutant was previously shown to be functional (16). Both MTSEA-biotin and maleimide-PEO2-biotin reacted with wild-type hOCT2 in biotinylation experiments, whereas the quadruple and septuplet mutants appeared unreactive (Fig. 3). However, pretreatment of the cells with the reducing agent DTT rescued at least some of the thiol reactivity, and from the observed level of immunoreactivity, the reduced loop cysteines were more accessible to maleimide-PEO2-biotin than MTSEA-biotin (Fig. 3). To provide further evidence for the presence of disulfide bridges in the long extracellular loop, an additional cysteine was added to the loop at amino acid position 144 (alanine to cysteine mutation at position 144; A144C). This amino acid is adjacent to the loop cysteine C143. Indeed, the A144C mutation on the septuplet background led to hOCT2 being reactive toward both thiol-reactive biotinylation reagents in the absence of DTT pretreatment (Fig. 4). Similar to reduced cysteines in the loop, cysteine at position 144 was more accessible to maleimide-PEO2-biotin than MTSEA biotin. DTT pretreatment enhanced thiol reactivity in the septuplet/A144C mutant.
Fig. 2.
Thirty-second uptakes of either [3H]MPP (∼10 nM; A) or [3H]TEA (∼20 nM; B) by CHO cells expressing WT hOCT2 (WT) or the septuplet mutant. Uptake was conducted in the absence or presence of 1 mM unlabeled substrate (n = 2).
Fig. 3.
Western blots showing the precipitation of WT hOCT2 (WT), the quadruple mutant, and the septuplet mutant with maleimide-PEO2-biotin and MTSEA-biotin. In some instances, the cells were pretreated (+) with 10 mM dithiothreitol (DTT) for 20 min before biotinylation. Cell surface biotinylation was carried out as described in materials and methods.
Fig. 4.
Western blot showing the precipitation of WT hOCT2 (WT) and the septuplet/A144C mutant with maleimide-PEO2-biotin and MTSEA-biotin. In some instances, the cells were pretreated (+) with DTT for 20 min before biotinylation. Cell surface biotinylation was carried out as described in materials and methods.
The effect of DTT pretreatment and adding an additional cysteine to the loop was consistent with the presence in the loop of at least one disulfide bridge. To determine whether any of the six loop cysteines do not form bridges, we performed the biotinylation experiments with MTSEA-biotin either before (pre) or after (post) solubilizing the CHO cells containing either wild-type hOCT2 or the septuplet mutant with solubilization buffer (Fig. 5). As anticipated, MTSEA-biotin reacted with wild-type hOCT2 both pre- and postsolubilization. MTSEA-biotin, however, did not react with the septuplet mutant presolubilization, but did when the biotinylation experiment was carried out postsolubilization. These data suggest that at least two cysteines within the loop have thiol groups that do not contribute to disulfide bridge formation. Two bands (∼85 and ∼60 kDa) corresponding to wild-type hOCT2 and the septuplet mutant were apparent on the Western blots when the biotinylation was carried out postsolubilization.
Fig. 5.
Western blot showing the precipitation of WT hOCT2 (WT) and the septuplet mutant with MTSEA-biotin either before (pre) or after (post) solubilization of CHO cells expressing the respective transport proteins with lysis buffer. Biotinylation was carried out as described in materials and methods.
The long extracellular loop is a feature common to all SLC22A members. Studies using amino acid substitutions (as in Fig. 1) and chimeric substitutions to examine the function of the loop have met limited success since many of these manipulations cause intracellular sequestration of the transport protein in living cells (e.g., Refs. 10, 23). Thus, to examine whether alterations to the loop would influence transport function, we took advantage of the fact that disulfide bridge(s) in the long extracellular loop could be reduced and loop cysteine(s) labeled with maleimide-PEO2-biotin. The functional significance (TEA transport) of disrupting disulfide bridge(s) and labeling the loop cysteines with maleimide-PEO2-biotin was determined in both wild-type hOCT2 and the septuplet mutant (Fig. 6). DTT by itself had no effect on TEA transport mediated by the wild-type protein or the septuplet mutant. Consistent with these data, the Kt (25.0 ± 1.0 vs. 32.2 ± 4.2 μM, P > 0.05, n = 3) and Jmax (74.8 ± 14 vs. 79.9 ± 10 pmol·cm−2·min−1, P > 0.05, n = 3) values associated with TEA transport by wild-type hOCT2 were not changed by DTT treatment (20 mM for 20 min). Maleimide-PEO2-biotin by itself caused a ∼50% reduction in TEA transport by wild-type hOCT2, but did not affect the septuplet mutant. These data are consistent with our previous report showing that maleimide-PEO2-biotin covalently modifies C474 to cause a ∼50% reduction in TEA transport activity (18); the septuplet mutant has an alanine instead of a cysteine at position 474. Covalent modification of loop cysteine(s) in wild-type hOCT2 (DTT/MB) caused a slight reduction in TEA transport compared with treatment with MB alone (Fig. 6). However, the effect of labeling loop cysteine(s) was more pronounced (and the effect significant) in the septuplet mutant, where TEA transport was reduced 44% compared with control (Fig. 6).
Fig. 6.
Thirty-second uptakes of [3H]TEA (∼20 nM) by WT hOCT2 or the septuplet mutant immediately following treatment with DTT, maleimide-PEO2-biotin (MB), or both treatments in combination (DTT/MB). Control cells were placed in Waymouth's buffer (WB) for 20 min, the WB was replaced, and the cells were incubated for an additional 20 min before transport measurement. Cells treated with DTT were incubated in WB for 20 min followed by 20-min incubation in WB containing 10 mM DTT. Cells treated with MB were incubated in WB for 20 min followed by 20-min incubation in WB containing 1 mM MB. Cells treated with MB and DTT in combination were treated with WB containing 10 mM DTT for 20 min followed by 20-min incubation in WB containing 1 mM MB. For each treatment, the cells were rinsed 3× rapidly (3 ml each rinse) with WB between the 20-min incubations and just before transport measurement. *Significantly different from control (n = 3, P < 0.05; Student-Newman-Keuls test).
The previous observation that C474 in TMH 11 of hOCT2 is accessible from the hydrophilic cleft and that occupation of the binding surface with substrate renders C474 refractory to interaction with membrane-impermeant, thiol-reactive reagents led to the suggestion that C474 may be part of or close to a substrate binding surface (18). To test the hypothesis that C474 may influence substrate binding, we mutated C474 to an alanine (C474A) or a phenylalanine (C474F) and examined MPP and TEA transport. The C474A and C474F mutants were expressed at the plasma membrane as assessed by immunocytochemistry (Fig. 7A). However, TEA and MPP transport were differentially affected by modifications to C474 (Fig. 7B). The C474A mutant supported both TEA and MPP transport, albeit at a reduced level compared with wild-type hOCT2. Compared with wild-type hOCT2, the Kt value for the interaction of TEA with the C474A mutant was reduced sevenfold, whereas the Kt value associated with MPP transport was unchanged (Table 1). The C474F mutation also exerted markedly different effects on the transport of MPP and TEA; it had no effect on the Kt for MPP transport, but abolished TEA transport. Furthermore, covalent modification of C474 significantly reduced TEA transport (∼50%), but had no appreciable effect on MPP transport (Fig. 8).
Fig. 7.
A: immunolocalization of WT hOCT2, the C474A mutant, and the C474F mutant in CHO cells expressing the respective transport proteins. The hOCT2 constructs are labeled in green and nuclei are stained red with propidium iodide. B: 30-s uptakes of [3H]MPP (∼10 nM) and [3H]TEA (∼20 nM) by cells expressing WT hOCT2, the C474A mutant, or the C474F mutant (n = 2).
Table 1.
Effect of mutating C474 on transport kinetics
| MPP |
TEA |
|||
|---|---|---|---|---|
| Kt | Jmax | Kt | Jmax | |
| WT | 1.76 ± 0.27 | 20.6 ± 3.8 | 54.0 ± 13 | 98.6 ± 17 |
| C474A | 1.55 ± 0.87 | 12.3 ± 6.0* | 7.74 ± 2.4* | 12.0 ± 6.8* |
| C474F | 1.78 ± 0.37 | 5.66 ± 1.1* | No transport | No transport |
Significantly different from wild-type (WT), P < 0.05; n = 3 or 4, 2-tailed t-test for tetraethylammonium (TEA) and 1-way ANOVA followed by Student-Newman-Keuls for 1-methyl-4-phenypyridinium (MPP). Kt units are μM and Jmax units are pmol·cm−2·min−1.
Fig. 8.
Thirty-second uptakes of [3H]MPP (∼10 nM) and [3H]TEA (∼20 nM) by CHO cells expressing WT hOCT2 without (control) or with 20-min pretreatment with 1 mM malmide-PEO2-biotin. *Significantly different from control (n = 3, P < 0.05; 2-tailed t-test).
DISCUSSION
The hOCT2 sequence contains 13 cysteine residues. Six are in the long extracellular loop while the other seven cysteines reside in putative TMHs. The long extracellular loop is a feature common to all OCT family members, and the cysteines within the loop are highly conserved, suggesting that the loop and the cysteines contained within it are important for OCT2 function. The long extracellular loop may well be expected to influence transport activity given that it resides between TMHs (TMH1 and TMH2) suspected of contributing to the substrate translocation pathway (20, 21, 27). In fact, data presented here as well as elsewhere suggest that this is the case. In the present study, mutation of C51, C63, and C143 completely abolished MPP and TEA transport. At least some MPP transport was retained following mutation of C89 and C103, whereas TEA uptake was not significantly different from control CHO cells following mutation of these residues. Although the observed reduction in plasma membrane expression was most likely responsible for the general decline in transport activity observed for all loop cysteine mutants, the differential effects on TEA and MPP transport of cysteine modification in the long extracellular loop suggest that the loop can influence substrate binding and/or translocation, as well. Indeed, mutation of C89 and C103 resulted in considerable changes in the Kt value for MPP transport (∼10-fold different compared with wild-type hOCT2). Additionally, TEA transport by the septuplet mutant was reduced considerably by labeling cysteine(s) in the long extracellular loop with maleimide-PEO2-biotin. Chimeric replacement of the long extracellular loop of rat Oct1 with the loop of rat Oat1 caused a decreased affinity of rat Oct1 for MPP (10). Also, there are three sites of N-glycosylation in the long extracellular loop of OCT2 (N71, N96, and N112), and the Kt value associated with TEA transport was about twofold lower following removal of N-glycosylation at each individual site (17). Moreover, removal of N-glycosylation at N96 caused a threefold reduction in the maximal rate of TEA transport, but did not influence plasma membrane expression, suggesting that N-glycosylation at this site influences transporter turnover number (17). Together, these data provide support for the contention that the long extracellular loop can influence substrate binding as well as the translocation event.
The dramatic reduction in transport activity following mutation of individual loop cysteines in hOCT2 appeared to be primarily caused by a defect in targeting of the transport protein to the plasma membrane, as cell surface expression was not apparent in the mutant constructs. Consistent with the near loss of membrane expression, the Jmax value associated with MPP transport by the C89A and C103A mutants, both of which supported modest MPP transport, was reduced ∼15- to 25-fold. These data are in agreement with those of Brast et al. (2), who used a green fluorescent protein-tagged construct of hOCT2 to show by immunocytochemistry that single loop cysteine mutants are largely retained in the cytosol of HeLa SS6 cells. Plasma membrane expression of mouse Oat1 at the plasma membrane of HeLa cells was also reduced following mutation of conserved cysteine residues in its long extracellular loop (24). The apparent lack of cell surface expression of the C89A and C103A mutants was not expected given their ability to transport MPP, albeit modestly; the immunoreactive signals were apparently lower than the limit of detection on our Western blots. Interestingly, the mutant constructs of hOCT2 from crude membrane preparations migrated to a lower apparent molecular mass (∼60 kDa) on SDS-PAGE gels than wild-type hOCT2. This band has been noted in other studies, but has not been detected by cell surface biotinylation in intact cells, suggesting that it is not expressed at the plasma membrane, but rather, retained in an intracellular compartment (16–18). The observation of an immunoreactive band at ∼60 kDa in biotinylation studies performed on solubilized CHO cells containing wild-type hOCT2 supported this contention. The immunoreactive band with a lower apparent molecular mass likely represents misfolded OCT2 protein (17).
Previous studies showed that the loop cysteines in hOCT2 are refractory to interaction with maleimide-PEO2-biotin and MTSEA-biotin, leading to the hypothesis that they participate in disulfide bridges (16, 18). Thus, in the present study we tested thiol reactivity in a mutant of hOCT2 (septuplet mutant) that contained only the six cysteines in the long extracellular loop; the other seven cysteines in the sequence were mutated to alanines. Consistent with at least one pair of loop cysteines participating in disulfide bridges, the septuplet mutant was reactive toward maleimide-PEO2-biotin and MTSEA-biotin only after pretreatment with DTT. Moreover, thiol reactivity of the septuplet mutant was present when an additional cysteine was placed in the loop (septuplet/A144C mutant). Two recent studies (2, 10) showed that either mutation of cysteines in the long extracellular loop or DTT treatment prevents homo-oligomerization of rat Oct1 and hOCT2, suggesting that disulfide bridges are necessary for homo-oligomerization. Whereas our data as well as that of Brast et al. (2) and Keller et al. (10) indicate that one or more disulfide bridges exist in the loop of OCTs, data have not been presented as to whether all six cysteines within the loop form bridges. Data presented here indicate that at least two cysteine residues within the long extracellular loop of hOCT2 are not bridged. That is, solubilization of CHO cells rendered the septuplet mutant reactive toward MTSEA-biotin (in the absence of DTT treatment). This was unlikely to reflect breaking of existing disulfide bridges as the bridges involved in homo-dimerization of BCRP are stable in the presence of detergents (2% SDS and 1% Triton X-100) (8) similar to those used in our study (1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS). The three-dimensional structure of hOCT2 apparently places steric hindrance on the accessibility of free thiol groups within the loop to MTSEA-biotin and maleimide-PEO2-biotin in intact cells. The loss of membrane expression associated with mutation of individual loop cysteines, along with the corresponding intracellular retention of hOCT2 protein with a lower apparent molecular mass, implies that cysteines in the long extracellular loop are important for stabilizing hOCT2 structure and for targeting of the transport protein to the plasma membrane.
Despite the presence of one (or two) disulfide bridge(s) in the long extracellular loop, and evidence that the long extracellular loop can influence transport function, reduction of the disulfide bridges with DTT (10 mM for 20 min) had no apparent effect on TEA transport by wild-type hOCT2 or the septuplet mutant. Accordingly, the kinetics of TEA transport by wild-type hOCT2 were not affected by DTT treatment, in agreement with the observation of Brast et al. (2), that DTT treatment (20 mM for 10 min) does not affect TEA binding to hOCT2. Interestingly, they observed a dramatic reduction in hOCT2-mediated Asp+ transport following DTT treatment (2). Keller et al. (10) observed a reduction in the Kt value associated with MPP transport by rat Oct1 following DTT treatment (10 mM for 6 min). Together, these data suggest that the long extracellular loop has an influence on substrate binding/translocation and that the effects are substrate specific, further supporting the contention that ligand interaction with OCTs involves a complex binding surface, rather than a common binding site.
Cysteine at position 474 in TMH11 of hOCT2 was previously shown to be accessible to several membrane-impermeant, thiol-reactive reagents (16, 18). Accordingly, C474 was found to be hydrated by the aqueous environment of the hydrophilic cleft in a three-dimensional homology model of hOCT2 structure (16, 18). Additionally, saturating concentrations of substrate in the hOCT2 binding surface rendered hOCT2 almost entirely refractory to interaction with maleimide-PEO2-biotin (18). These data led to the suggestion that C474 may be close to or part of a substrate binding surface in hOCT2 (18). Indeed, C474 is adjacent to D475, a residue known to have a profound influence on substrate binding (7). Highlighting the potential importance of C474 in transport function, this particular cysteine is conserved at a homologous position in all cloned orthologs of OCT1, OCT2, and OCT3. Thus, we mutated C474 to determine its potential functional significance. The cysteine-to-alanine mutation was relatively conservative (the side chains are similar in size), whereas the phenylalanine conversion was less so, as phenylalanine has a bulkier side-chain (it contains an aromatic ring) than either alanine or cysteine. Although the cysteine-to-alanine and cysteine-to-phenylalanine mutations had no effect on the Kt value associated with MPP transport, the same mutations markedly influenced TEA binding. Compared with wild-type hOCT2, the Kt value for TEA transport was reduced sevenfold in the C474A mutant, and there was no blockable TEA transport in the C474F mutant. To further examine the differential effect on TEA and MPP transport of modifications to C474, we took advantage of the fact that C474 is the only cysteine in the hOCT2 sequence that is accessible to maleimide-PEO2-biotin. Consistent with the mutational analysis, covalent modification of C474 with maleimide-PEO2-biotin significantly reduced TEA transport, but not MPP transport. These data indicate that the binding of TEA and MPP occurs at different regions within the hydrophilic cleft. The differential effects on TEA and MPP transport of mutation of C89 and C103 in the long extracellular loop provide additional support for this contention. Mutation of the adjacent residue, D475, was associated with a reduction in the Kt value for TEA transport by rat Oct1, but did not influence MPP transport (7). Kinetically, however, TEA and MPP behave as competitive inhibitors of hOCT2 and rat OCT1, implying that they share a common binding site (25). A rational explanation for these apparent discrepancies is that the binding of TEA and MPP to hOCT2 occurs at distinct regions, which are overlapping in space, and, thus, the binding of TEA and MPP to hOCT2 is mutually exclusive. The effect of C474 modification on TEA binding suggests that it may participate (along with D475) in forming the TEA binding surface. Alternatively, C474 may not directly contribute to forming the TEA binding surface, but rather, influences the ability of TEA to access this region of the hydrophilic cleft. Regardless, these data show that C474 has a profound influence on the binding of select substrates to hOCT2. In conclusion, the data presented here show that the long loop of OCT2, and select cysteines within it, influences membrane expression and substrate binding and that C474 in transmembrane helix 11 contributes to a substrate translocation pathway. These data also highlight that the OCT substrate binding surface contains multiple sites of substrate interaction, which helps explain the multiselective behavior of this clinically relevant transport protein.
GRANTS
R. Pelis was supported throughout a portion of this work by a Ruth L. Kirschstein National Research Service Award (DK752422) from the National Institutes of Health (NIH). This work was also supported in part by NIH Grants DK058251, HL07249, and ES06694, and in part by start-up funds from the Department of Pharmacology, Dalhousie University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M.P., Y.D., and S.H.W. conception and design of research; R.M.P., Y.D., Y.C., X.Z., and J.T. performed experiments; R.M.P., Y.D., Y.C., X.Z., and J.T. analyzed data; R.M.P., Y.D., and S.H.W. interpreted results of experiments; R.M.P. prepared figures; R.M.P. drafted manuscript; R.M.P. and S.H.W. edited and revised manuscript; R.M.P., Y.D., Y.C., X.Z., J.T., and S.H.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Faculty of Medicine at Siriraj Hospital, Mahidol University for the support of Y. Dangprapai during the course of this work.
Present address of Y. Dangprapai: Dept. of Physiology, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Present address of Y. Cheng: Bristol Myers Squibb, PO Box 4000, Princeton, NJ.
REFERENCES
- 1. Astorga B, Wunz TM, Morales M, Wright SH, Pelis RM. Differences in the substrate binding regions of renal organic anion transporters 1 (OAT1) and 3 (OAT3). Am J Physiol Renal Physiol 301: F378–F386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brast S, Grabner A, Sucic S, Sitte HH, Hermann E, Pavenstadt H, Schlatter E, Ciarimboli G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J 26: 976–986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem 275: 29413–29420, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271: 32599–32604, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov 9: 215–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorboulev V, Shatskaya N, Volk C, Koepsell H. Subtype-specific affinity for corticosterone of rat organic cation transporters rOCT1 and rOCT2 depends on three amino acids within the substrate binding region. Mol Pharmacol 67: 1612–1619, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Gorboulev V, Volk C, Arndt P, Akhoundova A, Koepsell H. Selectivity of the polyspecific cation transporter rOCT1 is changed by mutation of aspartate 475 to glutamate. Mol Pharmacol 56: 1254–1261, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem 280: 36926–36934, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem 273: 15971–15979, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, Koppatz S, Dotsch V, Hunte C, Sitte HH, Koepsell H. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem 286: 37874–37886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koepsell H. Polyspecific organic cation transporters: their functions and interactions with drugs. Trends Pharmacol Sci 25: 375–381, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Malo C, Berteloot A. Analysis of kinetic data in transport studies: new insights from kinetic studies of Na+-d-glucose cotransport in human intestinal brush-border membrane vesicles using a fast sampling, rapid filtration apparatus. J Membr Biol 122: 127–141, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui KI. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13: 866–874, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Hand Exp Pharmacol 201: 105–167, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Pelis RM, Dangprapai Y, Wunz TM, Wright SH. Inorganic mercury interacts with cysteine residues (C451 and C474) of hOCT2 to reduce its transport activity. Am J Physiol Renal Physiol 292: F1583–F1591, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Pelis RM, Suhre WM, Wright SH. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol 290: F1118–F1126, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Pelis RM, Zhang X, Dangprapai Y, Wright SH. Cysteine accessibility in the hydrophilic cleft of human organic cation transporter 2. J Biol Chem 281: 35272–35280, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pelis RM, Wright SH. Renal transport of organic anions and cations. In: Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, 2011. [Google Scholar]
- 20. Perry JL, Dembla-Rajpal N, Hall LA, Pritchard JB. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. J Biol Chem 281: 38071–38079, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popp C, Gorboulev V, Muller T, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol 67: 1600–1611, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Pritchard JB, Miller DS. Renal secretion of organic anions and cations. Kidney Int 49: 1649–1654, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Sturm A, Gorboulev V, Gorbunov D, Keller T, Volk C, Schmitt BM, Schlachtbauer P, Ciarimboli G, Koepsell H. Identification of cysteines in rat organic cation transporters rOCT1 (C322, C451) and rOCT2 (C451) critical for transport activity and substrate affinity. Am J Physiol Renal Physiol 293: F767–F779, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka K, Zhou F, Kuze K, You G. Cysteine residues in the organic anion transporter mOAT1. Biochem J 380: 283–287, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Umehara KI, Iwatsubo T, Noguchi K, Kamimura H. Comparison of the kinetic characteristics of inhibitory effects exerted by biguanides and H2-blockers on human and rat organic cation transporter-mediated transport: insight into the development of drug candidates. Xenobiotica 37: 618–634, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Wright SH. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol Appl Pharmacol 204: 309–319, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Shirahatti NV, Mahadevan D, Wright SH. A conserved glutamate residue in transmembrane helix 10 influences substrate specificity of rabbit OCT2 (SLC22A2). J Biol Chem 280: 34813–34822, 2005 [DOI] [PubMed] [Google Scholar]