Abstract
Epithelial sodium channels (ENaC) are critically important in the regulation of ion and fluid balance in both renal and respiratory epithelia. ENaC functional polymorphisms may contribute to alterations in blood pressure in the general population. We previously reported that the A663T polymorphism in the C terminus of the α-subunit altered ENaC functional and surface expression in Xenopus laevis oocytes (Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. J Biol Chem 279: 23900–23907, 2004). We examined whether sites in the vicinity of 663 influenced channel activity by performing scanning Ala mutagenesis. Interestingly, only αT663/G667Aβγ channels exhibited increased currents compared with αT663βγ. This increase in channel activity reflected an increase in channel open probability and not an increase in channel surface expression. In contrast, decreases in channel activity were observed with both αT663/C664Aβγ and αT663/C664Mβγ channels. The decrease in functional expression of αT663/C664Mβγ channels correlated with decreased surface expression, suggesting that the αC664M mutation altered the intracellular trafficking of the channel. While cytoplasmic Cys residues may be modified by the addition of palmitate, we did not observe palmitoylation of αC664. Our results suggest that multiple residues in the distal part of the cytoplasmic C terminus have roles in modulating channel activity.
Keywords: gating, polymorphism, scanning mutagenesis, trafficking
in the principal cells of the late distal convoluted, connecting, and collecting tubules of the kidney, the epithelial sodium channel (ENaC) serves as a final site for reabsorption of Na+ from the glomerular ultrafiltrate. Renal tubular Na+ reabsorption is modulated by a number of volume-regulatory hormones, including aldosterone, which directly regulate the functional expression of ENaC at the apical plasma membrane (14). ENaC is also present on the apical surface of airway epithelia, where ENaC hyperfunction is hypothesized to contribute to the pathophysiology of impaired mucociliary clearance and predilection to chronic respiratory infections in cystic fibrosis (4, 8, 21).
ENaCs are members of the ENaC/degenerin family of ion channels and are composed of three structurally related subunits, termed α, β, and γ. These three subunits share a common topology of two membrane-spanning domains and intracellular N and C termini but have limited (∼30–40%) sequence identity (6, 23, 29). The recently resolved structure of an acid-sensing ion channel (ASIC1), an ENaC/degenerin family member, revealed that ASIC1 is a homotrimer (16). This observation strongly suggests that ENaC likewise assembles as an αβγ heterotrimer.
Some ENaC mutations that change functional expression are associated with alterations in blood pressure (14, 15). For example, mutations that reduce ENaC expression have been described in individuals with pseudohypoaldosteronism type I, which is characterized by volume depletion, hypotension, and hyperkalemia (7, 26), as well as profuse respiratory secretions and increased mucociliary clearance (19). In contrast, ENaC gain-of-function mutations are described in individuals with Liddle syndrome, a disorder characterized by volume expansion, hypokalemia, and hypertension (28). Interestingly, individuals with Liddle syndrome have little apparent pulmonary phenotype (3). ENaC polymorphisms may subtly alter functional channel expression and contribute to the development of hypertension in the general population. In fact, some common polymorphisms of human ENaC (hENaC) segregate with changes in blood pressure in selected populations (i.e., βT594M) (2).
αA663T is a common hENaC polymorphism that is located in the distal cytoplasmic C terminus of the α-subunit. While there are conflicting reports regarding the influence of this polymorphism on blood pressure (1, 31), we have previously shown that this polymorphism influences hENaC functional expression and trafficking in vitro. Xenopus laevis oocytes expressing αT663βγ channels had significantly greater amiloride-sensitive currents than did oocytes expressing αA663βγ. These higher αT663βγ-mediated currents correlated with higher levels of expression of channels at the cell surface (25), which resulted in part from a decreased rate at which channels were removed from the plasma membrane (36).
The distal C terminus of the α-subunit encompassing residue 663 is not well conserved between human and mouse α-subunits, and we previously demonstrated that the context of residue 663 is both required for the functional differences observed with the A663T polymorphism and may influence ENaC's interactions with the CFTR (34). With regard to the context of residue 663 and ENaC functional expression, we demonstrated that the αA692T mutation in mouse ENaC (mENaC), corresponding to human αA663T, was not associated with differences in functional αβγ mENaC expression. However, replacement of the distal C terminus of the mouse α-subunit with the distal C terminus of the human α-subunit restored the functional differences that were observed with the human αA663T polymorphism (26). These data suggested that multiple residues within the distal C terminus of the human α-subunit have a role in modulating channel activity. We have used scanning Ala mutagenesis to identify two sites within the vicinity of human αT663 that influence channel activity. We found sites where substitutions either increased or decreased channel activity.
MATERIALS AND METHODS
Materials.
All reagents were purchased from Fisher Chemical unless otherwise noted.
Expression of ENaC in oocytes.
hENaC cDNAs (α, β, and γ) were from M. J. Welsh (University of Iowa). All mutants were generated using a PCR-based mutagenesis technique as we have done previously (25, 34). All sequences were confirmed by automated DNA sequence analyses performed at sequencing facilities of the Children's Hospital of Philadelphia and the University of Pittsburgh School of Medicine.
cRNAs for wild-type and mutant α-, wild-type β-, and wild-type γ-subunits were synthesized from linearized plasmids using appropriate RNA polymerases (mMessage mMachine, Ambion, Austin, TX) and stored at −80°C. cRNA concentration was determined spectroscopically. Stage V-VI oocytes were surgically harvested from female X. laevis (NASCO, Fort Atkinson, WI, or Xenopus Express, Plant City, FL) and pretreated with 2 mg/ml collagenase (type IV, Sigma), as previously described (27). Oocytes were injected with 2 ng/subunit of hENaC cRNAs in 50 nl of H2O. After injection, oocytes were incubated at 18°C in modified Barth's saline [MBS; 88 mM NaCl, 1 mM KCl, 2.4 mM Na HCO3, 15 mM HEPES, 0.3 mM Ca (NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, pH 7.2] supplemented with 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamycin sulfate. The Children's Hospital of Philadelphia and the University of Pittsburgh Institutional Animal Care and Use Committees approved the animal protocol.
Two-electrode voltage clamp.
Two-electrode voltage clamp (TEV) was performed 24–48 h after cRNA injection at room temperature using a DigiData 1320 interface and Axon Geneclamp 500B Amplifier (Axon Instruments, Foster City, CA). Data were acquired at 200 Hz, and analyses were performed using pClamp 8.0 or 8.1 software (Axon Instruments) on 833-MHz Pentium III or 3-GHz Pentium 4 Personal Computers (Dell Computer, Austin, TX). Pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) with a Micropipette Puller (Sutter Instrument, Novato, CA) and had resistances of 0.5–5 MΩ when filled with 3 M KCl and inserted into the bath solution. Oocytes were maintained in a recording chamber with 1 ml of bath solution and continuously perfused with bath solution at a flow rate of 4–5 ml/min. The bath solution contained either 110 mM NaCl, 2 mM KCl, 2 mM CaCl2, 10 mM HEPES, pH 7.4, or 100 mM Na gluconate, 2 mM KCl, 1.8 mM CaCl2, 3 mM BaCl2, 10 mM tetraethylammonium Cl, and 10 mM HEPES, pH 7.4 (25). Similar results were obtained with either bath solution. ENaC-mediated current was defined as the difference in whole oocyte current at either −100- or −60-mV holding potential (adjusted for resting membrane potential) before and after addition of 10 μM amiloride-HCl (Sigma) to the bath solution.
Whole oocyte and cell surface expression.
ENaC surface expression (see Fig. 5) was determined using a cell surface biotinylation assay as we have previously described (25, 34). To facilitate detection of biotinylated hENaC subunits, these experiments used a β-subunit with a C-terminal V5 epitope tag (β-V5), also as previously described (13). For the experiment in Fig. 2, a β-subunit with N-terminal HA and C-terminal V5 tags was used (provided by Michael Myerburg, University of Pittsburgh). Briefly, ENaC cRNAs (2 ng/subunit) were coinjected into X. laevis oocytes. Forty-eight hours after injection, oocytes were mechanically stripped of their vitelline membranes in hypertonic media (300 mM sucrose in MBS) and then washed sequentially with MBS and 10 mM triethylamine in MBS (pH 9.0) (see Fig. 5). Surface proteins were labeled with 1.5 mg/ml sulfo-NHS-Biotin (Pierce) in triethylamine/MBS for 30 min on ice. The biotinylation reaction was quenched with 5 mM glycine in MBS (4 separate 5-min incubations on ice). Oocytes (10/group) were subsequently washed with MBS, lysed in 0.15 M NaCl, 0.01 M Tris·Cl, pH 8.0, 0.01 M EDTA, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 1.0 mM phenylmethanesulfonyl fluoride, 0.1 mM Na-αtosyl-l-lysine chloromethyl ketone, 0.1 mM l-1-tosylamide-2-phenylethyl-chloromethyl ketone and 2 μg/ml aprotinin for 1 h at 4°C, and centrifuged at 13,000 g for 15 min at 4°C.
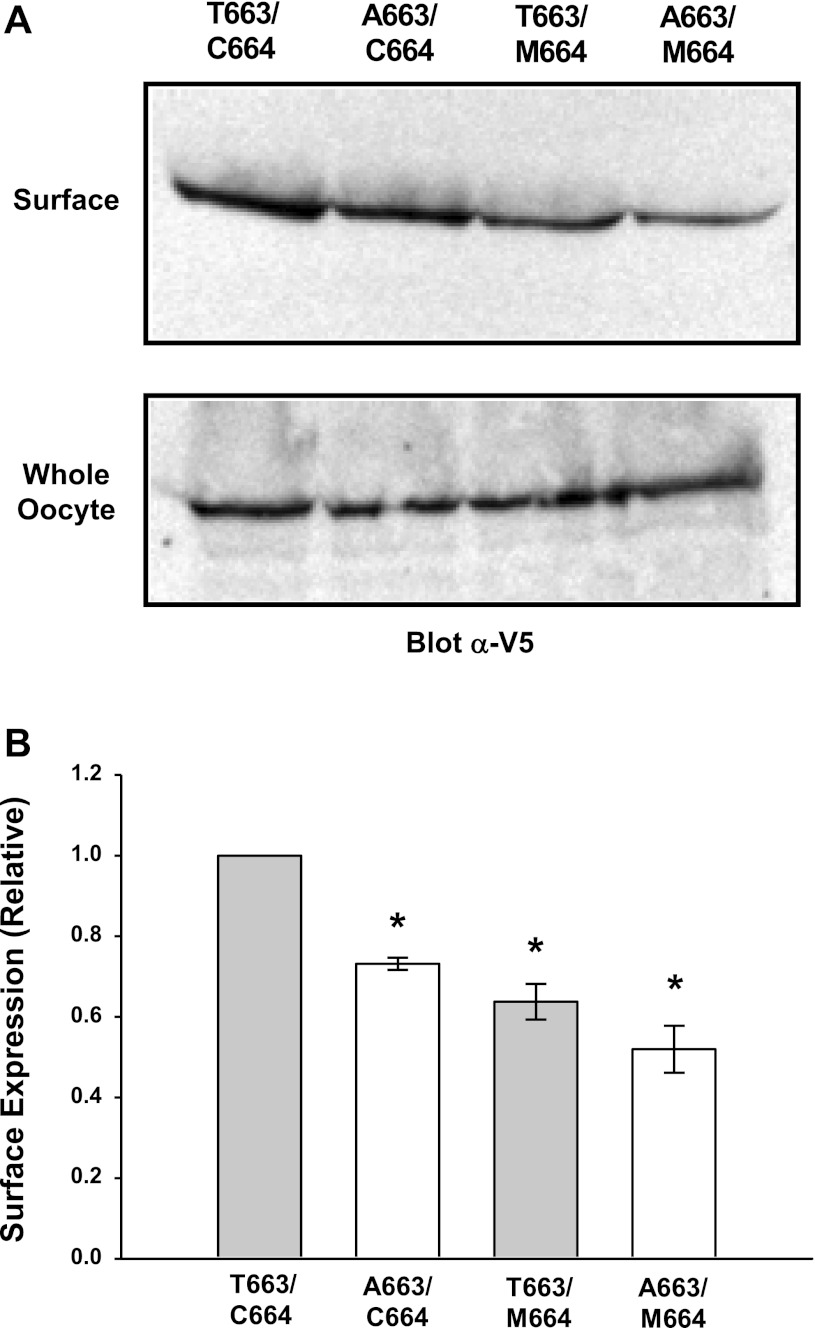
Fig. 5.
αC664M mutant reduces surface expression of the αT663 variant. A: αT663 and αA663 variants were expressed in oocytes with or without the αC664M mutation (C-terminal V5 epitope tag on the β-subunit). Cell surface biotinylation was performed 24–48 h after injection, and surface proteins were recovered by streptavidin-agarose precipitation. Surface channels were detected by immunoblotting with an anti-V5 antibody. A representative immunoblot (n = 5 independent experiments; 10 oocytes/group) is shown. A representative immunoblot of whole oocyte lysates demonstrating similar expression of β-V5 is also shown and is similarly representative of n = 6 independent experiments. B: densitometric analyses of the surface biotinylation experiments were performed as described in materials and methods and are presented as means ± SE; n = 6 independent experiments, normalized to values obtained with oocytes expressing the αT663 variant. *P < 0.05 vs. αT663 variant, as determined by ANOVA.
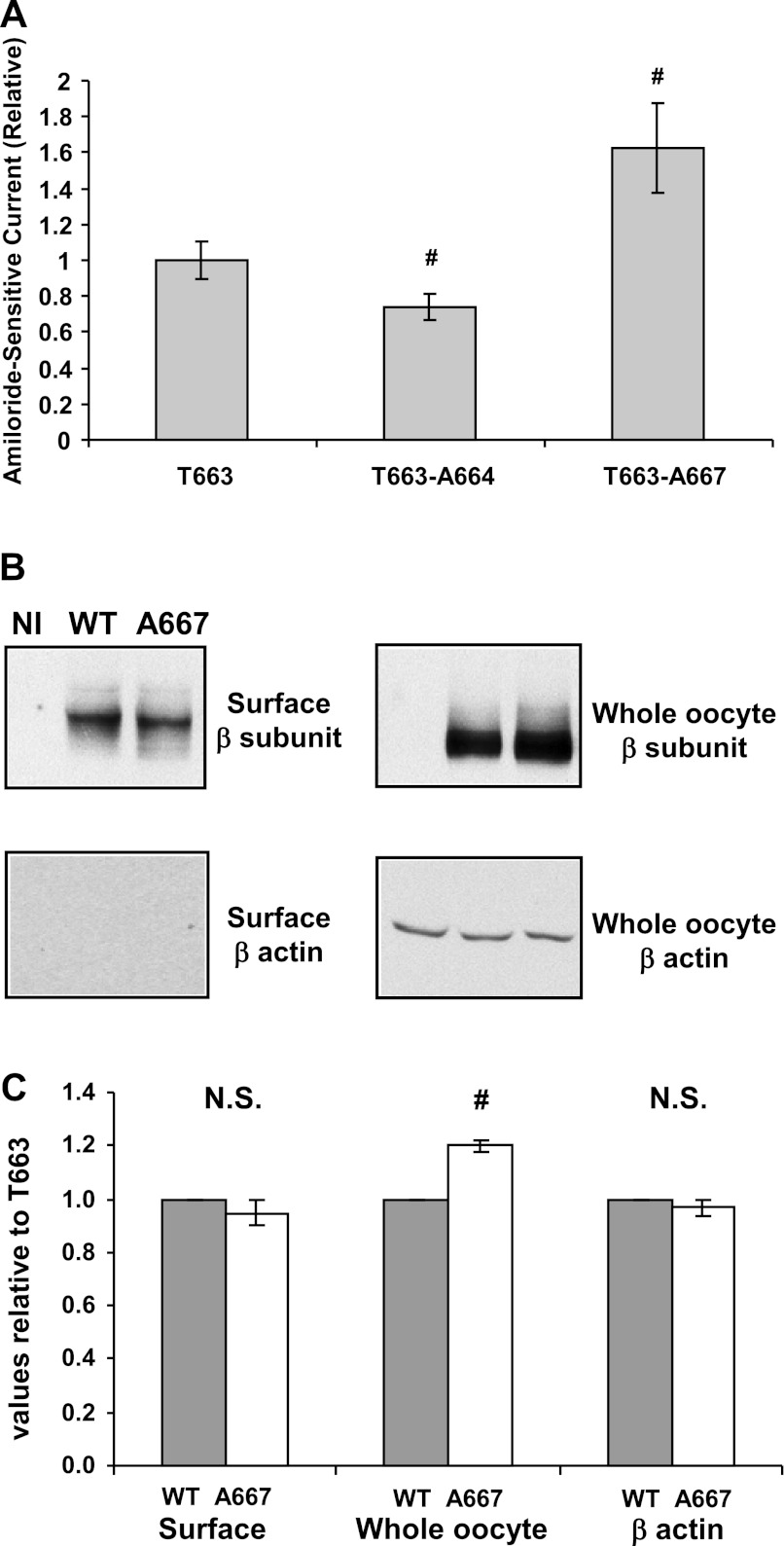
Fig. 2.
Selected Ala mutants alter the activity of the human αT663 variant. A: αT663βγ (wild-type), αA664βγ, and αA667βγ channels were expressed in oocytes. Amiloride-sensitive whole cell currents were determined by 2-electrode voltage clamp (TEV) at −100 mV and were expressed relative to currents in oocytes expressing wild-type channels. Values are means ± SE, and P values were determined by 1-way ANOVA. Mean currents were −1.28 ± 0.15 μA (n = 25 oocytes, n = 4 frogs) for wild-type; −0.94 ± 0.11 μA (n = 31 oocytes, n = 4 frogs) for the A66 mutant; and −1.98 ± 0.28 μA (n = 24 oocytes, n = 4 frogs) for the A667 mutant. B: surface expression of wild-type (WT) and αA667βγ channels (C-terminal V5 epitope tag on the β-subunit) was determined as described in materials and methods. Following surface biotinylation, surface proteins were recovered with streptavidin-conjugated beads and subjected to immunoblotting with an anti-V5 antibody to detect the β-subunit or with a β-actin antibody (control). A representative blot is shown (NI, not injected). Whole cell lysates were also subjected to immunoblotting and probed for V5 or β-actin (loading control). C: densitometric quantification of data presented in B. Data from n = 3 frogs with 16–30 oocytes/group are presented. There was no significant (N.S.) difference in levels of surface expression between wild-type and αA667βγ channels. Whole cell β-actin expression was also similar between the 2 groups (loading control). There was a modest, but significant increase in whole cell ENaC (β-subunit) protein expression with the αG667A mutant compared with wild-type (#P < 0.05).
Surface proteins were also labeled with 1.5 mg/ml sulfo-NHS-Biotin (Pierce) in triethylamine/MBS for 20 min on ice (see Fig. 2). Oocytes were lysed in 50 mM Tris, 100 mM NaCl, pH 7.4 plus protease inhibitors (protease inhibitor cocktail set III, EMD Millipore, Darmstadt, Germany) with an insulin syringe. Yolk pellets were removed with two 10-min, 200-g centrifugations at 4°C. Triton X-100 was then added to the low-speed supernatants to a final concentration of 1%. After 2 h, solubilized proteins were recovered following a centrifugation for 15 min at 14,000 g, 4°C (5, 11, 32).
Biotinylated proteins were precipitated with streptavidin-agarose (Pierce) and subjected to SDS-PAGE. Biotinylated β-subunits were detected on immunoblots probed with an anti-V5 antibody. Densitometry was performed using a Bio-Rad Versadoc (Bio-Rad) and Bio-Rad Quantity One software (see Fig. 2) or an AlphaImager 2200 system (AlphaInnotech, San Leandro, CA) (see Fig. 5). As a control for the integrity of the plasma membrane, immunoblots of streptavidin precipitates were probed for β-actin. Whole oocyte expression of β-V5 or β-actin was assessed by immunoblotting of the whole oocyte lysate.
Assessing Cys palmitoylation of ENaC subunits.
Fatty acid-exchange chemistry, whereby S-palmitate is selectively removed by treatment of immunoprecipitates with hydroxylamine and exchanged for biotin, was performed as previously described to assess Cys palmitoylation of ENaC subunits (20). Briefly, αβγ hENaC was transiently expressed in Madin-Darby canine kidney (MDCK) cells, where the α-, β-, or γ-subunit had both an N-terminal HA tag and a C-terminal V5 tag, and the other two subunits lacked epitope tags. Following solubilization in a detergent buffer (50 mM Tris·HCl, pH 8, 4 mg/ml deoxycholate, 1% NP-40, protease inhibitor cocktail set III, EMD Millipore) containing 50 mM N-ethyl maleimide to alkylate-free Cys residues, ENaC subunits were immunoprecipitated and then resuspended for 2 h in either 1 M hydroxylamine-HCl (pH 7.4, 150 mM NaCl, 0.2% Triton X-100) to remove S-palmitate or 1 M Tris·HCl (pH 7.4, 150 mM NaCl, 0.2% Triton X-100) as a control. Pellets were washed with 1% Triton X-100 in HEPES buffer and resuspended in 0.5 ml of 0.2 mg/ml EZ-Link (+)-biotinyl-3-maleimidopropionamidyl-3,6-dioxaoactanediamine (maleimide-PEO2-biotin; Thermo Scientific, Rockford, IL) in 50 mM Tris·HCl, pH 7, 150 mM NaCl, and 0.2% Triton X-100 to label newly exposed Cys sulfhydryls. Ten percent of each treated immunoprecipitate was reserved to quantify total ENaC within the sample, and the remainder was incubated with streptavidin-conjugated beads to recover biotinylated (i.e., palmitoylated) subunits and subjected to SDS-PAGE and immunoblotting with anti-V5 antibodies conjugated to horseradish peroxidase. The percentage of biotinylated subunit was calculated for each sample based on the total amount of the immunoprecipitated ENaC subunit. The difference in the percentage of biotinylated subunit following hydroxylamine treatment, relative to the percentage of biotinylated subunit following Tris treatment (i.e., control), represents the percentage of the subunit that was palmitoylated.
Assessment of hENaC delivery to and removal from the oocyte plasma membrane.
The rate of delivery of channels to the oocyte membrane was assessed as previously described (36), with hENaC mutants bearing a Cys at the amiloride binding site (γG536C). These channels are irreversibly blocked by [2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET). The time-dependent recovery of benzamil-sensitive whole cell currents reflects delivery of unmodified channels to the cell surface. Benzamil (100 μM) is used for these studies, as channels containing the amiloride binding site mutant are relatively amiloride insensitive (6, 30). Oocytes were injected with ENaC cRNAs as described above. Twenty-four to thirty-six hours after injection, whole oocyte currents were determined by TEV before application of MTSET, after two applications of MTSET (1 mM, 5 min each), and every 2 min for 10 min after removal of MTSET and washing of the oocyte. Benzamil (100 μM) was then added, and the remaining whole oocyte current that was insensitive to benzamil inhibition was determined. The benzamil-sensitive current at a given point was the difference between the whole oocyte current at that point and the whole oocyte current remaining after the addition of benzamil.
To assess the rate of removal of channels from the oocyte membrane, oocytes were injected with cRNAs as described above. Twenty-four to thirty-six hours after injection, amiloride-sensitive currents were determined by TEV before (t = 0) and following 2.5, 4.5, and 6.5 h of incubation with brefeldin A (5 μM) to block delivery of new channels to the oocyte membrane. Amiloride (10 μM) was added at the end of the experiment to determine the amiloride-insensitive component of the whole cell current. Amiloride-sensitive currents were expressed relative to the initial amiloride-sensitive current (t = 0), and pseudo-first-order rate constants for the decline of amiloride-sensitive current were determined for each individual oocyte (SigmaPlot 2000).
Single-channel recordings.
Patch clamping was performed in oocytes expressing wild-type or mutant hENaCs as previously described (20). Both pipette and bath solutions were 110 mM NaCl, 2 mM KCl, 2 mM CaCl2, 10 mM HEPES, pH 7.4. Patch-clamp recordings were performed with a cell-attached configuration using a PC-One Patch Clamp amplifier (Dagan, Minneapolis, MN) and a DigiData 1322A interface connected to a PC. Patches were clamped at membrane potentials (negative value of pipette potentials) of either −80 or −100 mV. pClamp 8 or 10 software (Molecular Devices/MDS Analytical Technologies) was used for data acquisition and analyses. Single-channel recordings were acquired at 5 kHz, filtered at 300 Hz by using a built-in, four-pole, low-pass Bessel filter. Channel open probability (Po) was estimated by the single-channel search function of pClamp 10 from recordings that were a minimum of 5 min in length and contained a maximum of two current levels (open and closed states). Unitary currents were determined by averaging multiple cursor measurements at each voltage.
Statistical analyses.
Whole cell amiloride-sensitive current data were expressed relative to that of the control condition, usually αT663βγ. To decrease the influence of batch-to-batch variability in ENaC expression, data were normalized to the mean amiloride-sensitive current for the control condition within a batch of oocytes before combining data of multiple independent batches for statistical analyses. Data are presented as means ± SE. P values were determined by a two-tailed t-test or ANOVA as appropriate. When a Poisson distribution, rather than a Gaussian distribution, best described these combined data, P values were determined by a two-tailed t-test or ANOVA after a square root transformation to better approximate a Gaussian distribution (37). A P value ≤0.05 was considered significant. Other data that were normally distributed are expressed as means ± SE with P values determined by a two-tailed t-test or paired t-test as appropriate. All statistical data analyses were performed with SigmaStat version 2.03.
RESULTS
We and others have previously shown that the T663 variant of the hENaC A663T polymorphism located within the distal C terminus of the α-subunit has increased channel activity, compared with A663 (25, 33). The increase in channel activity with the T663 variant was associated with an increase in surface expression, reflecting a reduced rate of channel retrieval from the plasma membrane compared with αA663 (25, 36). We performed scanning Ala mutagenesis of nine residues in the tract αG658 to αG668 flanking αT663 (Fig. 1 and Table 1) to determine whether residues adjacent to αT663 influenced channel activity. Within this tract, an endogenous Ala (αA659) was not mutated. Two Ala mutants (G667A and C664A) exhibited significantly altered ENaC functional expression (Table 1 and Fig. 2A).
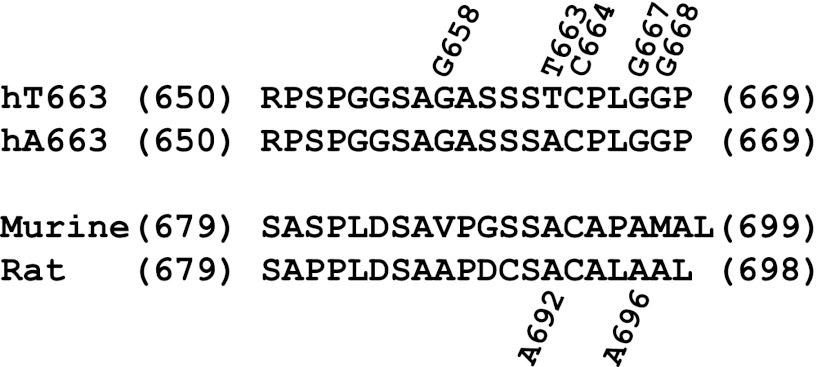
Fig. 1.
Sequences of the C termini of the human, murine, and rat α-subunit. Amino acid residue numbers at the beginning and end of each sequence are indicated. Residues 663 (T/A), C664, and G667 in the human α-subunit are indicated. A692 in the murine and rat α-epithelial sodium channel (ENaC) corresponds to 663 in the human α-subunit. A696 in murine and rat α-ENaC correspond to G667 in the human α-subunit. G658 and G668 mark the beginning and end of the scanned residues in the human α-subunit.
Table 1.
Scanning Ala mutagenesis of C terminus of αT663βγ
| Whole Cell Current Relative to αT663 | n | P Value | |
|---|---|---|---|
| αT663 Mutants | |||
| G658A | 1.207 ± 0.272 | 12 | NS |
| S660A | 1.017 ± 0.189 | 15 | NS |
| S661A | 1.081 ± 0.196 | 15 | NS |
| S662A | 1.090 ± 0.073 | 19 | NS |
| C664A | 0.737 ± 0.135 | 31 | <0.05 |
| P665A | 1.229 ± 0.143 | 17 | NS |
| L666A | 1.100 ± 0.264 | 16 | NS |
| G667A | 1.624 ± 0.154 | 24 | <0.05 |
| G668A | 0.976 ± 0.154 | 22 | NS |
| C664M | 0.648 ± 0.071 | 27 | <0.05 |
| Other variants/mutants | |||
| A663 | 0.676 ± 0.058 | 29 | <0.05 |
| A663,C664M | 0.612 ± 0.084 | 27 | <0.05 |
Values are means ± SE. NS, not significant. Whole cell amiloride-sensitive currents were determined by 2-electrode voltage clamp and are expressed relative to αT663βγ currents. The response of mutant channels was always examined with αT663βγ within the same batch of oocytes to account for the variability in whole cell currents within different batches of oocytes. P values were determined by t-test, as only 1–3 mutants (and αT663βγ control) were tested in an individual batch of oocytes. Whole cell currents of oocytes expressing αA663βγ and αA663,C664Mβγ are also shown, relative to αT663βγ. Data represent results from multiple oocyte preparations.
G667A increases ENaC functional expression.
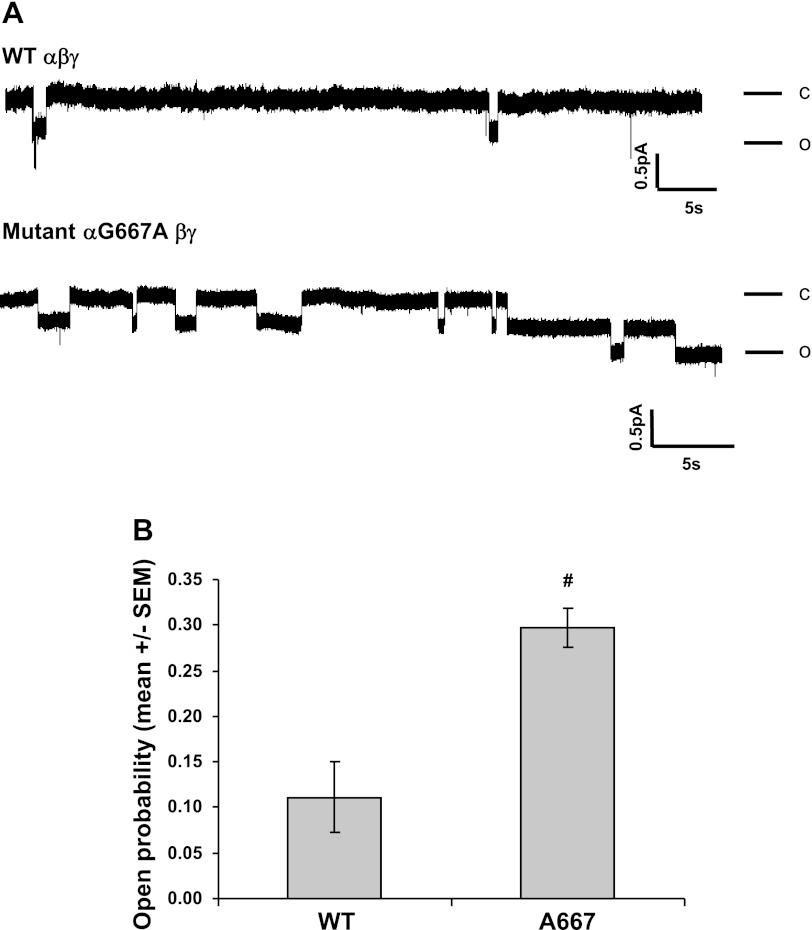
Oocytes expressing channels with the αT663 variant, αG667A increased ENaC currents by 62 ± 15% compared with wild-type (αT663) channels (Fig. 2A). This increase in channel activity was not associated with a change in ENaC (β-subunit) protein expression at the oocyte surface, as assessed by surface biotinylation (Fig. 2B, representative experiment; Fig. 2C, densitometric quantification). As a control, β-actin was not detected in blots of streptavidin-precipitated proteins. We noted a modest, but significant increase in whole cell ENaC (β-subunit) protein expression with the αG667A mutant compared with wild-type. However, the G667A mutant altered channel Po (Fig. 3). The Po of αG667Aβγ (0.30 ± 0.02) was significantly higher than that determined for wild-type αβγ (0.11 ± 0.04, P = 0.01, n = 5). Single-channel conductances, based on currents measured at a single holding potential, were 4.09 ± 0.40 pS for wild-type and 3.92 ± 0.43 pS for the αG667Aβγ mutant.
Fig. 3.
αG667A mutation increases channel open probability (Po). A: cell-attached patch-clamp recordings were obtained from oocytes expressing αβγ or αG667Aβγ channels. Patches were clamped at −100 mV (membrane potential) for wild-type channels or −80 mV for αG667Aβγ channels. Both bath and pipette solutions contained 110 mm Na+. Recordings containing single channels lasting for at least 5 min were selected for analysis. Representative recordings of single-channel currents are shown. C and O represent closed and open states, respectively. The scale bars indicate current amplitude (pA) and time (s). B: Po was determined as described under materials and methods and presented for multiple experiments (n = 5) and as means ± SE. The Po of αG667Aβγ (0.30 ± 0.02) was significantly greater than that of wild-type αβγ (0.11 ± 0.04). #P = 0.01; n = 5.
αC664 mutants reduce ENaC functional expression in the αT663 variant.
In contrast to the effect of αG667A, the αC664A mutant decreased relative Na+ currents by ∼26% in oocytes expressing the αT663 variant (Fig. 2A). Cys at this site is conserved in the human and rodent α-subunit (Fig. 1). ENaCs are known to be regulated by methylation (24), palmitoylation (20), and by the intracellular redox state (18), suggesting that modifications of cytoplasmic Cys residues influence channel activity. To further test this hypothesis, we mutated αC664 to Met (C664M) and assessed the influence of this mutation on the αA663T polymorphism. While the C664M substitution eliminates the free thiol group at this position, it should least perturb the α-subunit's structure.
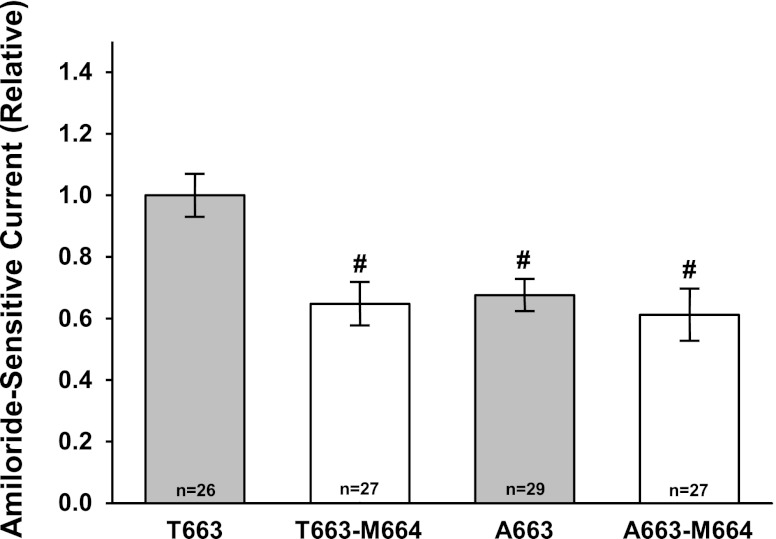
We assessed the influence of the αC664M mutant on the functional expression of the αT663 and αA663 variants in oocytes (Fig. 4). With the αT663 variant, oocytes expressing the αC664M mutant exhibited a 35 ± 7% reduction in amiloride-sensitive current compared with oocytes expressing wild-type channels. Consistent with our previous observations, the αA663 variant showed reduced functional ENaC expression compared with the αT663 variant by 33 ± 5%. However, in the presence of the αA663 variant, the αC664M mutation did not alter channel activity.
Fig. 4.
αC664M mutation selectively reduces functional expression of the αT663 variant. αT663 and αA663 variants were expressed in oocytes with or without the αC664M mutation. Amiloride-sensitive whole cell currents were determined at −100-mV holding potential and were expressed relative to the mean whole oocyte current for wild-type channels with the αT663 variant (n = 26–29 oocytes, 1-way ANOVA). The average whole cell current for the αT663 variant was −1.10 ± 0.20, n = 26. Values are means ± SE. #P < 0.05 vs. αT663 variant, 1-way ANOVA.
αC664M mutation reduces surface expression of channels with the αT663 variant.
We next examined whether the αC664M mutation affected surface expression of channels with the αT663 or αA663 variants. As shown in Fig. 5, channel expression at the cell surface was significantly reduced with the αC664M mutation in oocytes expressing the αT663 variant [surface expression of 0.67 ± 0.05 relative to wild-type (αT663), P = 0.005, n = 6]. In agreement with our previous observations, channel surface expression was significantly reduced with the αA663 variant compared with the αT663 variant [surface expression of 0.75 ± 0.03 (αA663) relative to αT663, P = 0.04, n = 6]. In contrast, levels of surface expression were not significantly further reduced with the C664M mutation in the presence of the αA663 variant (αA663 vs. αA663,C664M, P = not significant, n = 6). Surface expression of αA663,C664Mβγ was 0.62 ± 0.11 of the level observed with wild-type αT663. These data suggest that the αC664M mutation affects the trafficking of the αT663 variant, but does not affect the trafficking of the αA663 variant.
Influence of C664M on hENaC trafficking in oocytes.
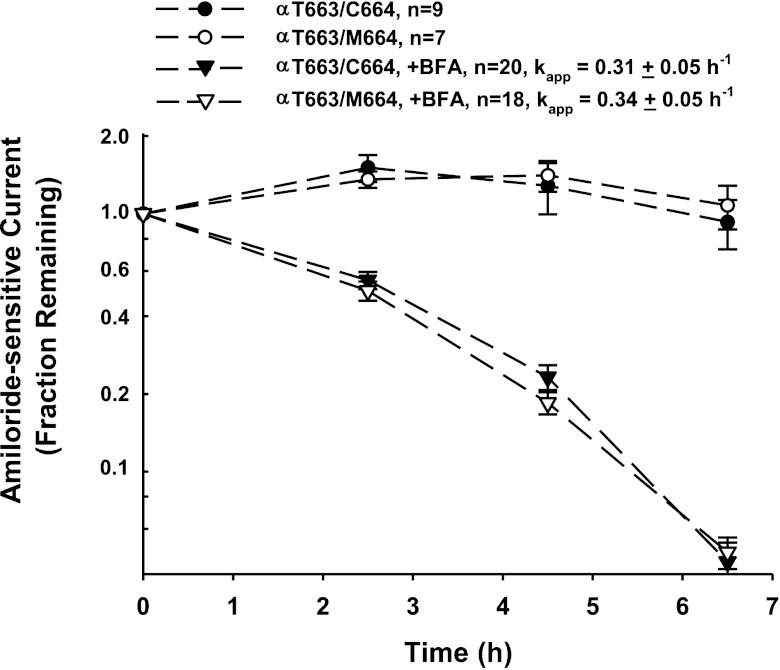
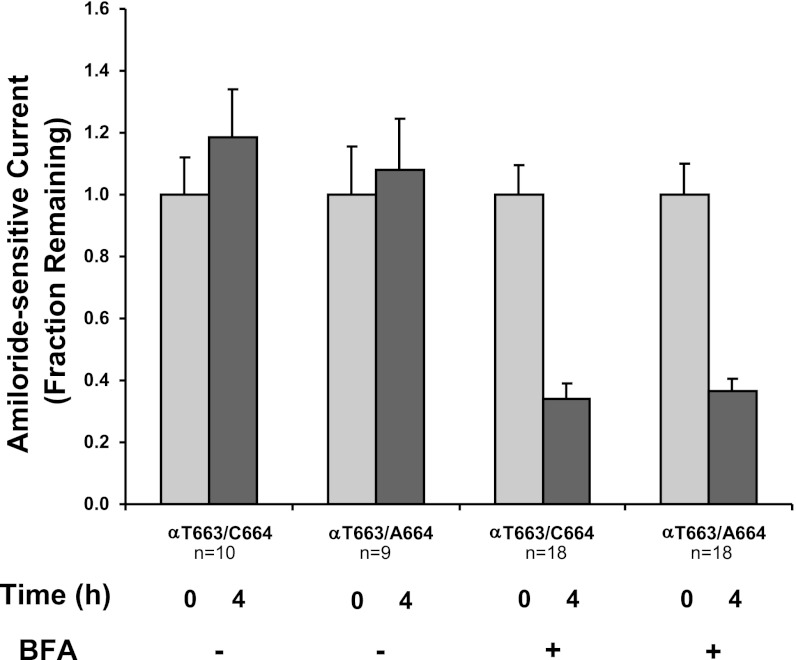
We previously demonstrated that the increase in functional and surface expression observed with αT663 channels in oocytes, compared with αA663, reflected in part a reduced rate of removal of channels from the plasma membrane (36). We therefore examined whether the αC664M mutation in the setting of the αT663 variant led to a more rapid removal of channels from the cell surface. In the absence of brefeldin A, relative amiloride-sensitive currents for wild-type channels and the αC664M mutant were fairly stable over a 6.5-h period (Fig. 6). When brefeldin A was added to block the delivery of new channels to the oocyte membrane, the apparent first-order rate constants for loss of αβγ (kapp = −0.31 ± 0.05 h, n = 20)- or αC664Mβγ (kapp=−0.34 ± 0.05 h, n = 18)- dependent currents were similar (Fig. 6, triangles). These apparent rate constants for channel retrieval were also similar to those we have previously observed in similar experiments (35, 36). We also tested whether the αC664A mutation altered the retrieval of channels with the αT663 variant. αT663/C664Aβγ channels were compared with αβγ channels before and 4 h after brefeldin A addition (Fig. 7). Mutant and wild-type channels exhibited similar reductions of current after 4 h of treatment with brefeldin A. These data suggest that neither the αC664M nor the αC664A mutations influence the retrieval of the αT663 variant from the plasma membrane.
Fig. 6.
αC664M mutation does not alter the rate of removal of the αT663 variant from the oocyte plasma membrane. Oocytes were injected with either wild-type channels (αT663 variant, closed symbols) or the αC664M mutant (open symbols). After 24–36 h, amiloride-sensitive currents at −60-mV holding potential were determined by TEV, and the oocytes were subsequently incubated in NaCl bath solution without (circles) or with 5 μM brefeldin A (BFA; triangles). Amiloride-sensitive currents were again determined by TEV after 2.5, 4.5, and 6.5 h of incubation without or with BFA. Amiloride-sensitive currents were expressed relative to the amiloride-sensitive current before incubation with BFA (means ± SE), and apparent first-order rate constants were fit as described in materials and methods and compared by a 2-tailed t-test. These data were derived from 2 different batches of oocytes (n = 7–9 oocytes no addition, 18–20 oocytes with BFA).
Fig. 7.
αC664A mutation does not alter the removal of the αT663 variant from the oocyte plasma membrane. Oocytes were injected with either wild-type channels (αT663/C664) or the αC664A mutant (αT663/C664A). After 24–36 h, amiloride-sensitive currents at −60-mV holding potential were determined by TEV, and the oocytes were subsequently incubated in a NaCl bath solution without or with 5 μM BFA as indicated. Amiloride-sensitive currents were again determined by TEV after 4 h of incubation without or with BFA. Amiloride-sensitive currents were expressed relative to the amiloride-sensitive current before incubation with BFA (means ± SE). Data were derived from 2 different batches of oocytes, (n = 9–10 oocytes no addition, 18 oocytes with BFA). After 4-h incubation with BFA, there was no significant difference in the reduction of current between wild-type and mutant channels.
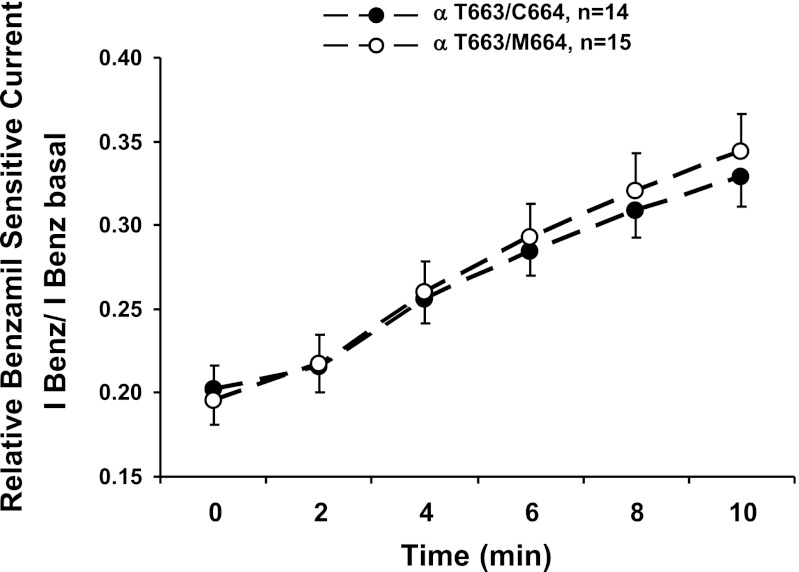
We next examined whether the decreased functional and surface expression of the αC664M mutant was due to a decreased rate of delivery of channels with the αT663 variant to the membrane (Fig. 8). To assess rates of delivery, we introduced a Cys residue into the amiloride binding site of the γ-subunit (G536C). This mutant allows for irreversible channel block after treatment with MTSET (30). The rate of recovery of the benzamil-sensitive current is a measure of the rate of delivery of nonmodified channels to the plasma membrane. We did not observe a difference in the rates of recovery of the benzamil-sensitive current over a 10-min period (Fig. 8). These data suggest that the αC664M mutation does not significantly influence the rate at which channels with the αT663 variant are delivered to the oocyte plasma membrane.
Fig. 8.
Trafficking of αT663 variant to the plasma membrane is not altered by the αC664M mutation. Oocytes expressing either wild-type channels (αT663 variant, ●) or the αC664M mutant (○) with a γG536C mutation were treated with MTSET to covalently label and inhibit surface channels. The recovery of benzamil-sensitive currents at −60 mV was plotted as a function of time over 10 min. Benzamil (100 μM) was added at the end of the experiment. Currents were normalized to the benzamil-sensitive current before MTSET treatment. There was no significant difference in the rates of current recovery after MTSET treatment between wild-type and αC664M channels (n = 14–15 oocytes/group from 2 different batches of oocytes).
αC664 is not palmitoylated.
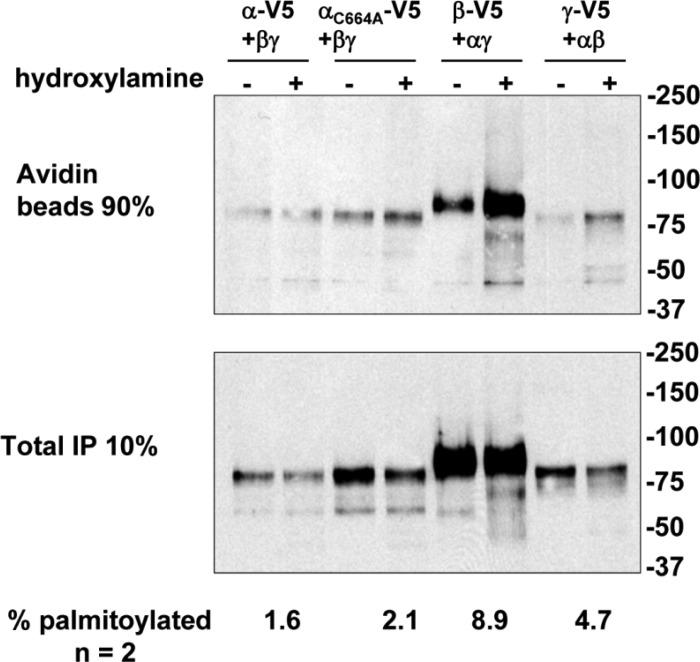
Palmitoylation of cytoplasmic Cys residues have been reported to influence the gating or trafficking of ion channels (10, 12, 17, 22). We previously reported that the β- and γ-subunits of mouse ENaC are palmitoylated and that β-subunit palmitoylation is associated with an increase in channel Po (20). Given the sequence divergence in the distal C terminus of the rodent and human α-subunit (Fig. 1), and our observation that αC664 mutants affect channel surface expression, we examined whether the human α-subunit was modified by palmitoylation.
We employed fatty acid-exchange chemistry to determine the extent of palmitolyation of human ENaC subunits, as we have previously described to assess Cys palmitoylation of mouse ENaC subunits (20). In these experiments, palmitate is selectively removed from Cys residues by treatment with hydroxylamine and the freed Cys residues are subsequently labeled with biotin. Treatment with Tris (control) will not release palmitate from Cys residues. The difference in subunit biotinylation following hydroxylamine vs. Tris treatment represents the amount of subunit palmitoylation, which is expressed relative to the total amount of the subunit in the cell lysate (Fig. 9). We observed more β- and γ-subunit biotinylation after treatment with hydroxylamine vs. Tris, consistent with Cys palmitoylation of the β- and γ-subunits; 8.9% of β-subunits and 4.7% of γ-subunits were palmitoylated (n = 2 experiments). In contrast, only 1.6% of αT633 subunits were palmitoylated, which is similar to the 2.1% α-subunit palmitoylation observed with the αC664A mutant. These data suggest that αC664 is not palmitoylated.
Fig. 9.
Human ENaC β- and γ subunits are Cys palmitoylated. Human αβγ-subunits with one subunit bearing N- and C- terminal epitope tags (N-terminal HA and C-terminal V5) were expressed in Madin-Darby canine kidney cells and analyzed for Cys palmitoylation by fatty-acid exchange chemistry, where Cys palmitate was replaced with biotin after treatment with hydroxylamine. Ninety percent of the sample was used to recover the biotinylated ENaC subunit from anti-V5 IPs with streptavidin beads, and 10% of the sample was used to assess the total amount of ENaC present in the sample. The percentage of each subunit that was biotinylated was calculated after quantitative immunoblotting for V5. The percent palmitoylated was calculated by subtracting percent biotinylated after treatment with Tris (control) from that after treatment with hydroxylamine. A representative immunoblot is shown. Data from 2 experiments are presented.
DISCUSSION
Our previous work demonstrated that the αA663T polymorphism in the C terminus of the human α-subunit was associated with altered channel functional and surface expression in X. laevis oocytes. We found that the αT663 variant had higher levels of functional and surface expression than the αA663 variant, that the context of the C-terminal 20 residues of the α-subunit was critical for this effect (25), and that the increased functional and surface expression of the αT663 variant reflected, in part, a decreased rate of channel retrieved from the oocyte surface (25, 36).
We previously tested a number of hypotheses regarding the underlying molecular basis for this polymorphism's functional effect. We examined whether targeted phosphorylation of αT663 might alter channel trafficking. Two kinases were predicted to potentially phosphorylate αT663, PKCδ (36) and casein kinase I (35). Both kinases altered the trafficking of the αT663 variant, but neither influenced trafficking in a manner that explained the differences we observed with the αA663T polymorphism. PKCδ specifically decreased the functional and surface expression of the αT663 variant (36). In contrast, casein kinase I, and in particular the δ-isoform, appeared to enhance functional expression of the αT663 variant. It did so by enhancing αT663 delivery to the oocyte plasma membrane rather than by inhibiting its retrieval (35). Furthermore, we did not find evidence that either PKCδ or casein kinase I directly phosphorylated αT663 (35, 36).
We have now examined whether mutations of nine residues flanking αT663 influence the channel. Interestingly, only αG667A increased ENaC currents. This increase did not correlate with increased surface expression. Instead, we observed an increased channel Po with this mutant (Fig. 3). Given the location of this residue in the distal C terminus of the α-subunit, the increase in Po was unexpected. Our results suggest that this region has a role in modulating both channel Po as well as trafficking.
αG667 is not conserved across species. In contrast, αC664 is conserved in the human, mouse, and rat α-subunit. Ala scanning mutagenesis of the αT663 variant revealed that the αC664A mutation reduced ENaC functional expression. We also examined the αC664M mutation, as this substitution should minimally perturb the α-subunit C terminus while blocking potential redox reactivity, methylation, and/or thioesterification. We found that the αC664M mutation also reduced channel functional expression in oocytes and that this decrease was associated with reduced channel expression at the cell surface. αC664M had no significant effect on the activity or surface expression of the αA663 variant, consistent with the notion that C664 has a role in modulating channel trafficking of the αT663 variant.
Experiments examining rates of loss of channel expression at the plasma membrane (Figs. 6 and 7) and rates of channel delivery to the plasma membrane (Fig. 8) did not identify a mechanism by with the αC664M mutation altered the trafficking of the αT663 variant. As we have noted in previous work, such as in our studies of the influence of the 70-kDa heat shock proteins Hsc70 and Hsp70 on ENaC trafficking in oocytes (9), these assays may not be sufficiently sensitive to yield conclusive mechanistic insights. For example, our trafficking assays do not directly address channel recycling. ENaC endocytosis was examined in the presence of brefeldin A that prevents delivery of newly synthesized channels to the plasma membrane, but may still allow channel recycling.
Recent data suggest that ENaC can be regulated by covalent modification. Methylation was demonstrated for the mouse β-subunit (24), and, more recently palmitoylation of specific Cys residues in both the β- and γ-subunits of mouse ENaC (20). While palmitoylation of the mouse α-subunit was not observed, it was possible that α-subunit palmitoylation could occur in a species-specific manner, given that the C terminus in the region of the αCys664 residue is not well conserved. However, we found that only the human β- and γ-subunits are palmitoylated (Fig. 9).
In summary, we have identified two sites in the distal C terminus of the human α-subunit of ENaC where selected mutations affect the activity of the αT663 variant of the channel. While mutations at one site (αC664) affect levels of surface expression, mutations at the second site (αG667) affect channel gating.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK054354 (T. R. Kleyman and R. C. Rubenstein), DK065161 (T. R. Kleyman and R. P. Hughey), DK058046 and DK073185 (R. C. Rubenstein), and DK079307 (Pittsburgh Center for Kidney Research). R. C. Rubenstein was an Established Investigator of the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.M.M., T.R.K., and R.C.R. provided conception and design of research; G.M.M., W.Y., L.C., S.J., Z.W., C.L.K., and M.A.T. performed experiments; G.M.M., W.Y., L.C., S.J., Z.W., C.L.K., R.P.H., T.R.K., and R.C.R. analyzed data; G.M.M., W.Y., Z.W., R.P.H., T.R.K., and R.C.R. interpreted results of experiments; G.M.M., Z.W., R.P.H., and R.C.R. prepared figures; G.M.M., T.R.K., and R.C.R. drafted manuscript; G.M.M., R.P.H., T.R.K., and R.C.R. edited and revised manuscript; G.M.M., Z.W., C.L.K., R.P.H., T.R.K., and R.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Marcelo Carattino for providing helpful suggestions.
REFERENCES
- 1. Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension 34: 631–637, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND, MacGregor GA. Association of hypertension with T594M mutation in beta subunit of epithelial sodium channels in black people resident in London. Lancet 351: 1388–1392, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Baker EH, Portal AJ, McElvaney TA, Blackwood AM, Miller MA, Markandu ND, MacGregor GA. Epithelial sodium channel activity is not increased in hypertension in whites. Hypertension 33: 1031–1035, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma subunit. J Biol Chem 282: 6153–6150, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest 132: 1631–1636, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, Rubenstein RC. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci USA 103: 5817–5822, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gubitosi-Klug RA, Mancuso DJ, Gross RW. The human Kv1.1 channel is palmitoylated, modulating voltage sensing: identification of a palmitoylation consensus sequence. Proc Natl Acad Sci USA 102: 5964–5968, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282: 58–64, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Heneghan JF, Mitra-Ganguli T, Stanish LF, Liu L, Zhao R, Rittenhouse AR. The Ca2+ channel beta subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J Gen Physiol 134: 369–384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hummler E. Epithelial sodium channel, salt intake, and hypertension. Curr Hypertens Rep 5: 11–18, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol Gastrointest Liver Physiol 276: G567–G571, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Jindal HK, Folco EJ, Liu GX, Koren G. Posttranslational modification of voltage-dependent potassium channel Kv1.5: COOH-terminal palmitoylation modulates its biological properties. Am J Physiol Heart Circ Physiol 294: H2012–H2021, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Kellenberger S, Gautschi I, Pfister Y, Schild L. Intracellular thiol-mediated modulation of epithelial sodium channel activity. J Biol Chem 280: 7739–7747, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med 341: 156–162, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Mueller GM, Maarouf AB, Kinlough CL, Sheng N, Kashlan OB, Okumura S, Luthy S, Kleyman TR, Hughey RP. Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel. J Biol Chem 285: 30453–30462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB. Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol 43: 712–719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc Natl Acad Sci USA 95: 4690–4695, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renard S, Lingueglia E, Voilley N, Lazdunski M, Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem 269: 12981–12986, 1994 [PubMed] [Google Scholar]
- 24. Rokaw MD, Wang JM, Edinger RS, Weisz OA, Hui D, Middleton P, Shlyonsky V, Berdiev BK, Ismailov I, Eaton DC, Benos DJ, Johnson JP. Carboxylmethylation of the beta subunit of xENaC regulates channel activity. J Biol Chem 273: 28746–28751, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem 279: 23900–23907, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Saxena A, Hanukoglu I, Saxena D, Thompson RJ, Gardiner RM, Hanukoglu A. Novel mutations responsible for autosomal recessive multisystem pseudohypoaldosteronism and sequence variants in epithelial sodium channel alpha-, beta-, and gamma-subunit genes. J Clin Endocrinol Metab 87: 3344–3350, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. J Biol Chem 275: 8572–8581, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 269: 24379–24383, 1994 [PubMed] [Google Scholar]
- 30. Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na+ channels. J Biol Chem 274: 28484–28490, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Sugiyama T, Kato N, Ishinaga Y, Yamori Y, Yazaki Y. Evaluation of selected polymorphisms of the Mendelian hypertensive disease genes in the Japanese population. Hypertens Res 24: 515–521, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1A. J Biol Chem 286: 16297–16307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the α-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol 290: F821–F827, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Yan W, Samaha FF, Ramkumar M, Kleyman TR, Rubenstein RC. Cystic fibrosis transmembrane conductance regulator differentially regulates human and mouse epithelial sodium channels in Xenopus oocytes. J Biol Chem 279: 23183–23192, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Yan W, Spruce L, Rosenblatt MM, Kleyman TR, Rubenstein RC. Intracellular trafficking of a polymorphism in the COOH terminus of the α-subunit of the human epithelial sodium channel is modulated by casein kinase 1. Am J Physiol Renal Physiol 293: F868–F876, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Yan W, Suaud L, Kleyman TR, Rubenstein RC. Differential modulation of a polymorphism in the COOH terminus of the α-subunit of the human epithelial sodium channel by protein kinase Cδ. Am J Physiol Renal Physiol 290: F279–F288, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984 [Google Scholar]