Abstract
We investigated a physiological role for ERK, a member of the MAPK family, in the hypotonic stimulation of epithelial Na+ channel (ENaC)-mediated Na+ reabsorption in renal epithelial A6 cells. We show that hypotonic stress causes a major dephosphorylation of ERK following a rapid transient phosphorylation. PD98059 (a MEK inhibitor) increases dephosphorylated ERK and enhances the hypotonic-stress-stimulated Na+ reabsorption. ERK dephosphorylation is mediated by MAPK phosphatase (MKP). Hypotonic stress activates p38, which in turn induces MKP-1 and to a lesser extent MKP-3 mRNA expression. Inhibition of p38 suppresses MKP-1 induction, preventing hypotonic stress from dephosphorylating ERK. Inhibition of MKP-1 and -3 by the inhibitor NSC95397 also suppresses the hypotonicity-induced dephosphorylation of ERK. NSC95397 reduces both β- and γ-ENaC mRNA expression and ENaC-mediated Na+ reabsorption stimulated by hypotonic stress. In contrast, pretreatment with PD98059 significantly enhances mRNA and protein expression of β- and γ-ENaC even under isotonic conditions. However, PD98059 only stimulates Na+ reabsorption in response to hypotonic stress, suggesting that ERK inactivation by itself (i.e., under isotonic conditions) is not sufficient to stimulate Na+ reabsorption, even though ERK inactivation enhances β- and γ-ENaC expression. Based on these results, we conclude that hypotonic stress stimulates Na+ reabsorption through at least two signaling pathways: 1) induction of MKP-1 that suppresses ERK activity and induces β- and γ-ENaC expression, and 2) promotion of translocation of the newly synthesized ENaC to the apical membrane.
Keywords: epithelium, p38, A6, osmolality
na+ reabsorption in the distal nephron is important for extracellular fluid volume and blood osmolarity. The signals that regulate extracellular fluid volume are determined by changes in blood pressure; however, blood osmolarity itself can act as a signal to regulate directly renal Na+ reabsorption (17, 20, 24, 25, 34). The rate-limiting step in transepithelial Na+ reabsorption in distal nephron principal cells is the apical entry step mediated by epithelial Na+ channels (ENaC) (19). Changes in Na+ reabsorption require either a change in the activity of individual ENaC or a change in ENaC surface expression in the apical membrane (5, 6, 13). The surface expression of ENaC is increased by stimulating translocation of preexisting ENaC from a subapical recycling pool or the insertion of newly synthesized ENaC into the apical membrane (34). At a systemic level, when plasma osmolarity is increased, vasopressin is released to increase water reabsorption in the distal nephron (collecting duct) for antidiuresis. On the other hand, when plasma osmolarity is decreased, vasopressin release is suppressed to reduce water reabsorption, leading to diuresis. In addition to this systemic hormonal response to changes in plasma osmolarity, renal epithelial cells have their own regulatory systems that respond to changes in plasma osmolarity. Our work and that of others have shown that the decrease in basolateral (plasma) osmolarity via several signaling cascades stimulates Na+ reabsorption by increasing Na+ entry across the apical membrane (24, 30, 41). We have previously shown that hypotonic stress increases ENaC-mediated Na+ reabsorption in renal epithelial A6 cells by stimulating both translocation of ENaC to the apical membrane through an EGFR-JNK-phosphatidylinositol 3-kinase-dependent pathway in an early phase (0–3 h) (34) and induction of ENaC mRNA expression via a p38-dependent pathway in a late phase (3–24 h) (23). Although two members of the MAPK family, p38 and JNK, contribute to stimulation of Na+ reabsorption in response to hypotonic stress, a physiological role for a third member of the family, ERK, in hypotonic-stress-stimulated Na+ reabsorption in renal epithelial A6 cells is unclear.
MAPK is activated by phosphorylation on both the threonine and tyrosine residues of a conserved signature T-X-Y motif within the activation loop of the kinase. This process is mediated by a dual-specificity MAPK kinase (MKK or MEK) (12). The requirement for phosphorylation on both threonine and tyrosine residues for MAPK activation means that dephosphorylation of either residue is sufficient to inhibit MAPK kinase activity. This can be achieved by either serine/threonine phosphatases, tyrosine-specific phosphatases, or by dual-specificity phosphatases (7, 31). Among these phosphatases, the dual-specificity MAPK phosphatases (MKPs) specifically regulate the levels of phosphorylation and activity of MAPKs in response to various stimuli and hormones.
On the other hand, the MAPK signaling pathways are evolutionarily highly conserved and are involved in diverse cellular functions, including cell proliferation, cell migration, cell cycle progression, differentiation, and stress responses. The difference in duration and magnitude of MAPK activation determines signaling specificity responsible for diverse responses (31). In other words, controlling a balance between MKK and MKP enables the fine-tuning of MAPK activity for complex cellular regulation. Thus both activation but also inactivation of MAPK plays a pivotal role in various physiological processes.
ERK1 and ERK2 are members of the MAPK signaling cascade that translates external signals into intracellular responses. Accumulating evidence has demonstrated that ERK is a crucial factor in regulating activity, trafficking, and transcription of ENaC. Previous studies have demonstrated that activation of Raf/ERK suppresses GRE-dependent α-ENaC expression at the transcriptional level in rat salivary epithelial cells (45) and human lung epithelial cells (40). On the contrary, ERK- and p38-dependent activation of CREB contributes to the cAMP-induced increase in mRNA expression of α-ENaC in a rat submandibular gland epithelial cell line (15). In normal human middle ear epithelial cells, IL-1β suppresses the β-ENaC expression and the ENaC-dependent Na+ absorption via the PLC-PKC-ERK1/2 pathway (3). Furthermore, the PMA-induced PKC signaling via the ERK1/2 cascade promotes γ-ENaC retrieval and subsequent degradation in aldosterone-treated A6 cells, resulting in decreased Na+ reabsorption (2). These previous studies have shown that activated ERK inhibits ENaC expression and ENaC-mediated Na+ reabsorption and have described different mechanisms for activating ERK. However, mechanisms for reducing ERK activity and stimulating ENaC-mediated Na+ reabsorption especially by MKP have not been described.
In this study, we showed that a major effect of hypotonic stress on transcriptional regulation of ENaC is caused by dephosphorylation (suppression) of ERK. This dephosphorylation is mediated through p38-dependent induction of MKP-1. We conclude that spatiotemporal regulation of the MAPK family such as p38, JNK, and ERK enables fine control of ENaC-mediated Na+ reabsorption in A6 cells.
METHODS
Materials.
Transwell-Clear permeable supports (PET membrane Transwell-Clear, 6.5 and 24 mm) were obtained from Corning (Lowell, MA). NCTC-109 medium, benzamil, 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), ouabain, NSC95397, and nystatin were purchased from Sigma (St. Louis, MO). SB202190 and PD98059 were obtained from Calbiochem (San Diego, CA).
Solutions.
The isotonic test solution contained 120 mM NaCl, 3.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES, adjusted to pH 7.4. The hypotonic challenge solution contained 60 mM NaCl, 3.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES, adjusted to pH 7.4.
Cell culture.
Renal epithelial A6 cells derived from Xenopus laevis were purchased from American Type Culture Collection (ATCC). A6 cells (passages 73–84) were cultured as previously described (33, 35). Namely, A6 cells (passages 73–84) were grown on plastic flasks at 27°C in a humidified incubator with 1.0% CO2 in air in a culture medium which contained 75% (vol/vol) NCTC-109, 15% (vol/vol) distilled water, and 10% (vol/vol) fetal bovine serum. For preparation of total RNA and cell lysate, A6 cells were cultured for 11–14 days on 24-mm Transwell-Clear permeable supports. For measurement of short-circuit current (Isc), A6 cells were cultured for 13 - 14 days on 6.5 mm Transwell-Clear permeable supports.
Measurement of Isc.
Monolayers of A6 cells subcultured on tissue culture-treated Transwell-Clear filter cups were transferred to a modified Ussing chamber (Jim's Instrument Manufacturing, Iowa City, IA) designed to hold the filter cup. Transepithelial potential (PD) was continuously measured by a high-impedance millivoltmeter that could function as a voltage clamp with automatic fluid resistance compensation (VCC-600, Physiologic Instruments, San Diego, CA) with a pair of calomel electrodes that were immersed in a saturated KCl solution and bridged to the modified Ussing chamber by a pair of polyethylene tubes filled with a solution of 2% (wt/vol) agarose in a 2 M KCl solution (18, 19, 22, 38). Isc was measured by the amplifier (VCC-600) with a pair of silver-silver chloride electrodes that were immersed in a 2 M NaCl solution and bridged to the modified Ussing chamber by a pair of polyethylene tubes filled with a solution of 2% (wt/vol) agarose in a 2 M NaCl solution (1, 42–44). When the Isc was measured, the PD was clamped to 0 mV for 1 s by the amplifier. Under a steady-state condition, the Isc was stable and did not change even if the transepithelial voltage was clamped to 0 mV for 1 min. In a non-steady state, the value of Isc measured at 1 s after clamping the PD to 0 mV is shown as Isc in the present study. A positive current represents a net flow of cations from the apical to the basolateral solution (36, 37).
Western blotting.
After pretreatment with various inhibitors for 1 h in an isotonic (normal) culture medium, confluent A6 monolayers grown on Transwell-Clear permeable supports were exposed to a hypotonic (50% diluted) culture medium containing inhibitors for the indicated time period. The A6 monolayers were lysed by lysis buffer (50 mM HEPES, 120 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 100 mM NaF, 10 mM pyrophosphate, 250 μg/ml leupeptin, 0.1 mM phenyl methylsulfonyl fluoride, 100 kallokein inactivator units/ml aprotinin, pH 7.4) after various experimental treatments. Then, cells were homogenized by sonication and centrifuged at 12,000 g for 10 min at 4°C to remove insoluble debris. The cell lysates containing 45 μg protein were boiled in SDS sample buffer [60 mM Tris·HCl, 2% (wt/vol) SDS, 5% (vol/vol) glycerol, pH 6.8] and then subjected to 10% SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes. Nonspecific binding was blocked by incubation in 5% (wt/vol) nonfat milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) at room temperature for 60 min. Membranes were blotted with anti-phospho-p44/42 MAPK (ERK; Thr202/Tyr204), anti-p44/42 MAPK (ERK), anti-MKP-3, anti-β-tublin, and anti-GAPDH antibodies (Cell Signaling Technology, Beverly, MA) in 5% BSA in TBST and also blotted with antibodies for α-, β-, and γ-ENaC subunits (9–11, 32) in 5% milk in TBST at 4°C overnight. The membranes were then washed with TBST and incubated for 60 min at room temperature with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cell Signaling Technology, Danvers, MA) in 5% (wt/vol) nonfat milk in TBST. After washing with TBST, the blots were detected with ECL plus chemiluminescent reagent (GE Healthcare Bio-Sciences, Piscataway, NJ). Each blot presented was performed at least three times.
Quantitative real-time-PCR.
To compare relative amounts of mRNA for α-, β-, and γ-ENaC subunits, and MKP-1 and MKP-3 in A6 monolayers treated under different conditions, we performed quantitative real-time PCR. Total RNA was extracted from A6 monolayers using an RNeasy kit (Qiagen, Tokyo, Japan) with DNase digestion according to the manufacturer's protocol. RNA was extracted after isotonic or hypotonic challenge for specific times with or without various inhibitors. RT was performed on 1 μg total RNA, calculated from absorbance measured at 260 nm, using a High Capacity Coda transcription kit (Applied Biosystems, Rotkreuz, Switzerland) according to the manufacturer's protocol. Primers (Sigma Genosis) and TaqMan probes (Applied Biosystems) unique for each X. laevis ENaC subunit, X. laevis MKP-1 or MKP-3, and X. laevis β-actin or GAPDH were the following: α-ENaC forward 5′-TAGGGACATTATCCTCTCGCTCAT-3′, reverse 5′-CCTTCTACCTCCATTCTCCTCATAGT-3′, TaqMan probe 5′-CAGCATGCGCAGCAACAGGTCC-3′; β-ENaC forward 5′-CGGCTTTAGATCGCATTCAGT-3′, reverse 5′-TCTGACGTGTTTG GTTGTTGTG-3′, TaqMan probe 5′-TCTAGCCAAAACCAAGGCAACACATT-3′; γ-ENaC forward 5′-GGTGGGAAAATCGCAAAGAG-3′, reverse 5′-TGTCATGCCCAGTCATGGTT-3′, TaqMan probe 5′-ATCAAGCAGAAGACACCCCTGAAATCCC-3′; MKP-1 forward 5′-AGAGGAGGGCGAAAGGCA-3′, reverse 5′-GGCACCTCTGCTCCTCATTG-3′, TaqMan probe 5′-CATGGGCTTGGAGCACATCGTCC-3′; β-actin forward 5′-GATGCTCCCCGTGCTGTTT-3′, reverse 5′-TTCCAACCATGACACCCT GA-3′, TaqMan probe 5′-CCCATCTATTGTGGGTCGCCCAAGA-3′; GAPDH forward 5′-TGACAACAGTCCATGCTTTCACT-3′, reverse 5′-TCTGCCATCTCTCCACAGCTT-3′, TaqMan probe 5′-CACCCAGAAGACAGTGGATGGCCC-3′; and MKP-3 forward 5′-CAGACGGTAGTCCTCTATCCAAC-3′, reverse 5′-GGCACAGCCAAGGTAAAGAT-3′. Quantitative real-time PCR with TaqMan probes using the ABI 7300 Real-time PCR system was performed according to the manufacturer's protocol. Diluted RT samples (100 ng total RNA) were amplified in a final volume of 25 μl. Primers were used at a concentration of 800 nM and probes at a concentration of 200 nM. GAPDH and β-actin were used as housekeeping genes. The thermal cycling conditions were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative amounts of ENaC and MKP-1 mRNA were determined by normalizing with GAPDH or β-actin mRNA expression. In the case of MKP-3, quantitative real-time PCR with sybergreen was performed using an ABI 7300 Real-time PCR system according to the manufacturer's protocol.
Temperature.
All experiments for electrophysiological and other measurements were performed at 24–25°C unless otherwise indicated.
Data presentation.
All data are presented as means ± SE. Where SE bars are not visible, they are smaller than the symbol. Student's t-test and ANOVA were used for statistical analysis as appropriate, and P < 0.05 was considered significant.
RESULTS
The active form of ERK is a negative regulator of hypotonic-stimulated Na+ reabsorption.
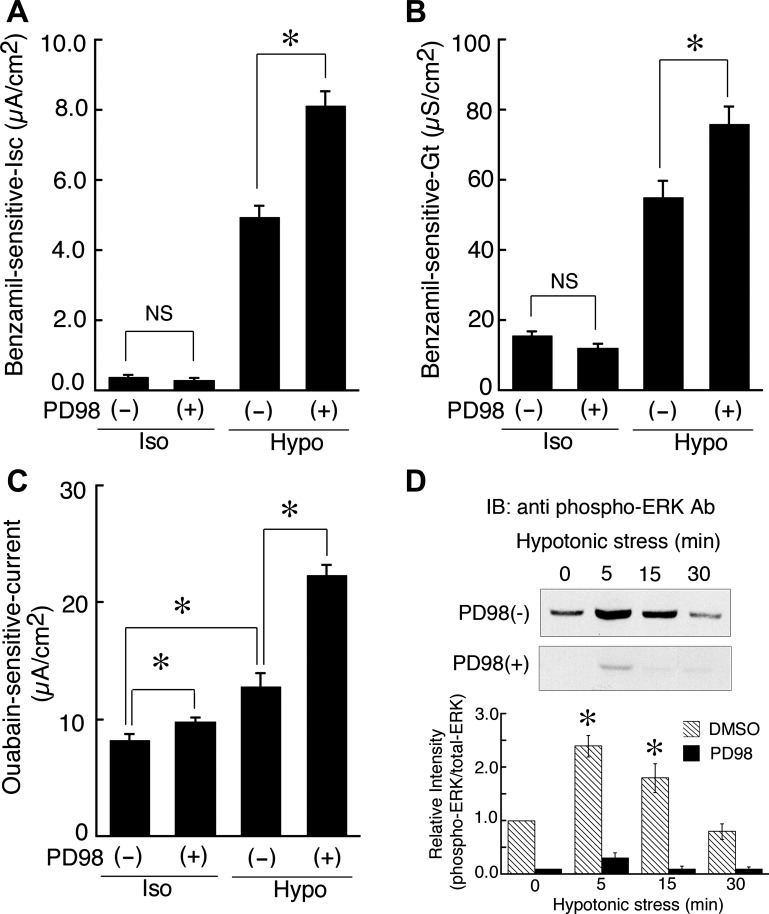
In this study, we first tried to determine the involvement of ERK in the hypotonic stimulation of ENaC-mediated Na+ reabsorption in monolayers of A6 cells by pretreating the cells with 50 μM PD98059, a MEK inhibitor (or 0.1% DMSO as a vehicle control for PD98059), by measuring benzamil (a specific blocker of ENaC)-sensitive Isc and transepithelial conductance (Gt). PD98059 is widely used as an inhibitor of MEK to abolish ERK-dependent signals. Hypotonic stress (exposure to 50% diluted hypotonic culture medium) for 20–24 h increases ENaC-mediated Na+ reabsorption, detected as benzamil-sensitive Isc (17, 23). As shown in Fig. 1, A and B, pretreatment with 50 μM PD98059 significantly enhanced the hypotonic stress-stimulated Na+ reabsorption by increasing apical ENaC-mediated Na+ entry via elevation of ENaC activity detected as benzamil-sensitive Gt, but did not enhance Na+ reabsorption under isotonic conditions. Basolateral Na+ extrusion through the Na+/K+ pump (ATPase) detected as the ouabain (an inhibitor of Na+/K+ ATPase)-sensitive current, was also enhanced by pretreatment with 50 μM PD98059 under hypotonic conditions (Fig. 1C). PD98059 actually reduced ERK phosphorylation under both isotonic (0 min) and hypotonic conditions (Fig. 1D), suggesting that PD98059 does indeed inhibit MEK and thereby prevent ERK phosphorylation. These observations suggest that ERK might be a negative regulator of chronic hypotonic stimulation of Na+ reabsorption and that ERK has inhibitory effects on both the apical Na+ entry step and the basolateral Na+ extrusion step, although the Na+ entry step remains the rate-limiting step for transepithelial Na+ flux in A6 cells pretreated with PD98059.
Fig. 1.
Effects of PD98059 (an MEK inhibitor) on epithelial Na+ channel (ENaC)-mediated Na+ reabsorption [measured as benzamil-sensitive short-circuit current (Isc)], ENaC activity [benzamil-sensitive transepithelial conductance (Gt)], capacity of Na+-K+-ATPase (ouabain-sensitive current), and transient phosphorylation of ERK. After pretreatment with 50 μM PD98059 for 1 h in an isotonic culture medium, A6 monolayers were exposed to an isotonic or a hypotonic culture medium with or without 50 μM PD98059 for 20–24 h. Then, A6 monolayers were mounted in a modified Ussing chamber with 120 mM NaCl (Iso) or 60 mM NaCl (Hypo) solution, after which 10 μM benzamil was applied for 10 min and the change in current was plotted as the benzamil-sensitive Isc (A) and Gt (B), respectively. Subsequently, for measurement of ouabain-sensitive Na+-K+-ATPase (pump) capacity (C), 50 μM nystatin was added to the apical surface of the cells. After incubation for 30 min in the presence of 50 μM nystatin, the ouabain-sensitive current was taken to be the maximum capacity of the pump. Values are means ± SE; n = 7–11 (A and B) and n = 4 (C). *P < 0.05. D: effect of PD98059 on the hypotonic stress-induced transient phosphorylation of ERK is shown in a typical immunoblot with anti-phospho-ERK antibody, and relative changes in phosphorylated ERK by hypotonic stress with or without PD95059 were statistically analyzed; n = 4. *P < 0.05.
Hypotonic stress dephosphorylates ERK by inducing MKP-1.
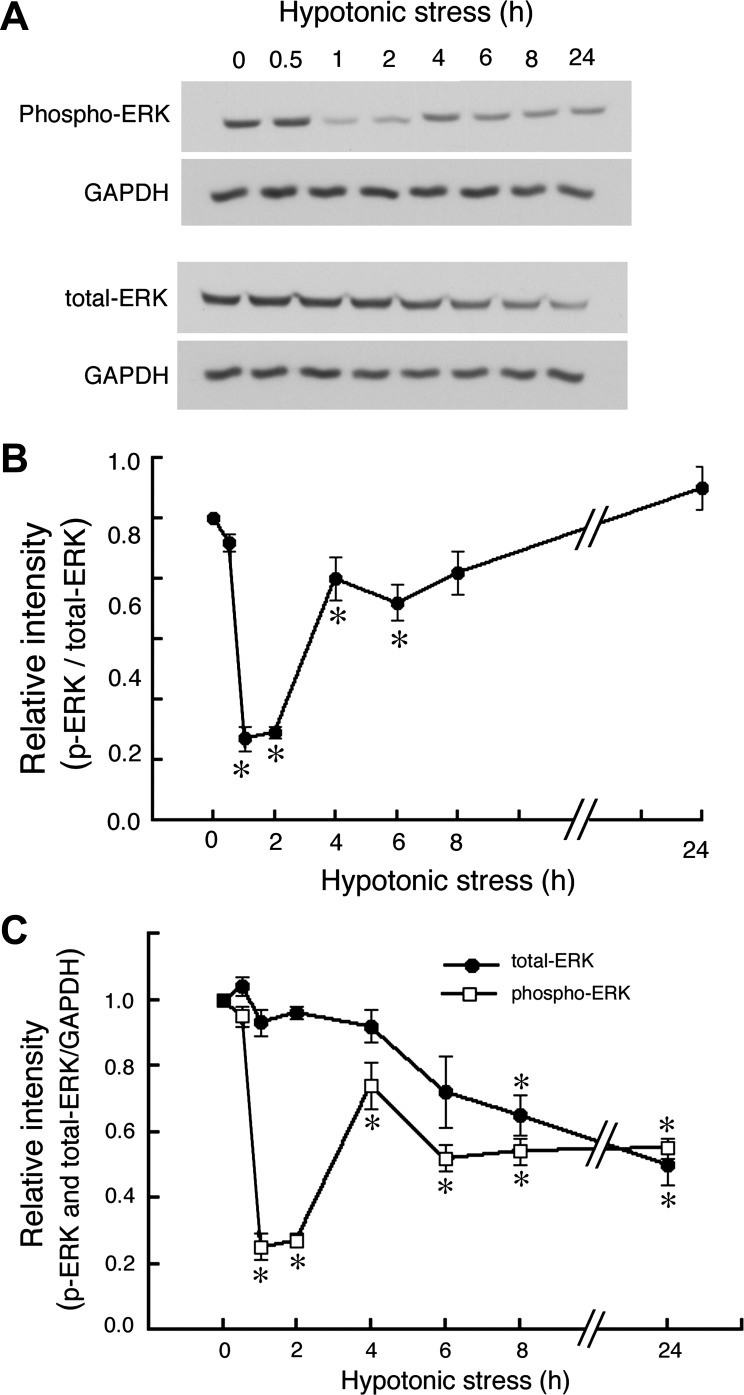
Our previous studies indicate that hypotonic stress causes transient phosphorylation of all MAPKs including ERK (34), p38, and JNK (21) in A6 cells; the phosphorylation of ERK, p38, and JNK reached their peaks at ∼5–15 min, returning to the basal levels within 30 min after application of hypotonic stress (21, 34). If, as others have shown, phosphorylated ERK inhibits Na+ reabsorption, the hypotonicity-induced phosphorylation of ERK should reduce the stimulatory action of chronic hypotonic stress on Na+ reabsorption. To confirm this point, we examined how hypotonic stress modulates phosphorylation/dephosphorylation of ERK for longer time periods by using a specific antibody to recognize activated (phosphorylated) ERK. Generally, ERK is activated by MEK (MKK1/2)-mediated combined phosphorylation of both threonine 202 and tyrosine 204 and is inactivated by protein phosphatase-mediated dephosphorylation of either of these residues. After exposure to a hypotonic culture medium, transiently phosphorylated ERK returned to the basal levels of phosphorylation within 30 min (Figs. 1D and 2). Surprisingly, further exposure (∼24 h) to a hypotonic culture medium caused significant additional dephosphorylation of ERK, which is likely to be involved in hypotonic stimulation of Na+ reabsorption (Fig. 2). The observed dephosphorylation of ERK consisted of two phases; the rapid and profound dephosphorylation in the early phase between 1–2 h after exposure to hypotonic medium and a moderate dephosphorylation in the late phase between 6 and 24 h after hypotonic exposure. The maximum dephosphorylation of ERK was observed between 1 and 2 h after hypotonic stress.
Fig. 2.
Effects of hypotonic stress on dephosphorylation of ERK. A6 monolayers were exposed to a hypotonic culture medium for the indicated times, and then ERK and phospho-ERK were detected by immunoblotting with anti-ERK and anti-phospho-ERK antibodies. A: typical immunoblots containing 45 μg whole-cell lysates. These blots were initially blotted with either anti-phospho-ERK or anti-ERK antibodies and then stripped and reprobed with GAPDH antibody. B: time-dependent changes in relative amounts of phosphorylated and total ERK proteins in response to hypotonic stress are shown. Equal loading of each well was confirmed by measuring GAPDH as an internal control (C). Values are means ± SE; n = 3. *P < 0.05.
Generally, the dephosphorylation (inactivation) of ERK is due to serine/threonine phosphatases, tyrosine-specific phosphatases, or phosphatases with dual specificity. Rapid dephosphorylation (on the order of minutes) in which phosphorylation returns to the basal level after the initial hypotonic-induced transient ERK phosphorylation is likely mediated by preexisting serine/threonine phosphatases and/or tyrosine-specific phosphatases. On the other hand, further dephosphorylation of ERK in the subsequent phase (1–2 h) is more likely mediated by newly transcribed/translated MKPs, especially MKP-1 and MKP-3. To clarify the involvement of MKP-1 and MKP-3 in the dephosphorylation of ERK in the early phase (1–2 h) of hypotonic stress, we examined the effect of the MKP-1/3 inhibitor NSC95397 (39) on the hypotonic-stress-induced dephosphorylation of ERK (Fig. 3). Monolayers of A6 cells were pretreated with different concentrations of NSC95397 and then exposed for 2 h to a hypotonic culture medium containing NSC95397. Pretreatment with 20 and 40 μM NSC95397 abolished the hypotonic stress-induced dephosphorylation of ERK, and in fact induced more phosphorylation of ERK compared with the basal level (under isotonic conditions) (Fig. 3). On the other hand, under isotonic conditions, 20 and 40 μM NSC95397 had much smaller effects on the ERK phosphorylation (data not shown). These observations suggest that hypotonic stress-induced dephosphorylation of ERK in the early phase (between 1 and 2 h) is mainly mediated by MKP-1 and/or MKP-3 and that MKP-1 and/or MKP3 also slightly contributed to dephosphorylation of ERK even under isotonic conditions.
Fig. 3.
Effects of NSC95397 [an MAPK phosphatase (MKP-1/3) inhibitor] on hypotonic-stress-induced ERK dephosphorylation. A: after pretreatment with NSC95397 of several concentrations for 1 h, A6 monolayers were exposed to a hypotonic culture medium containing NSC95397 at the same concentrations for 2 h. Then, phosphorylated ERK and total ERK were detected by immunoblotting with anti-phospho-ERK and anti-ERK antibodies. B: relative amounts of phosphorylated and total ERK proteins are shown in Fig. 2A. Equal loading of cell lysate to each well was confirmed by measuring GAPDH as an internal control. Values are means ± SE; n = 3. *P < 0.05.
MKP-1 is induced by hypotonic stress through a p38-dependent pathway.
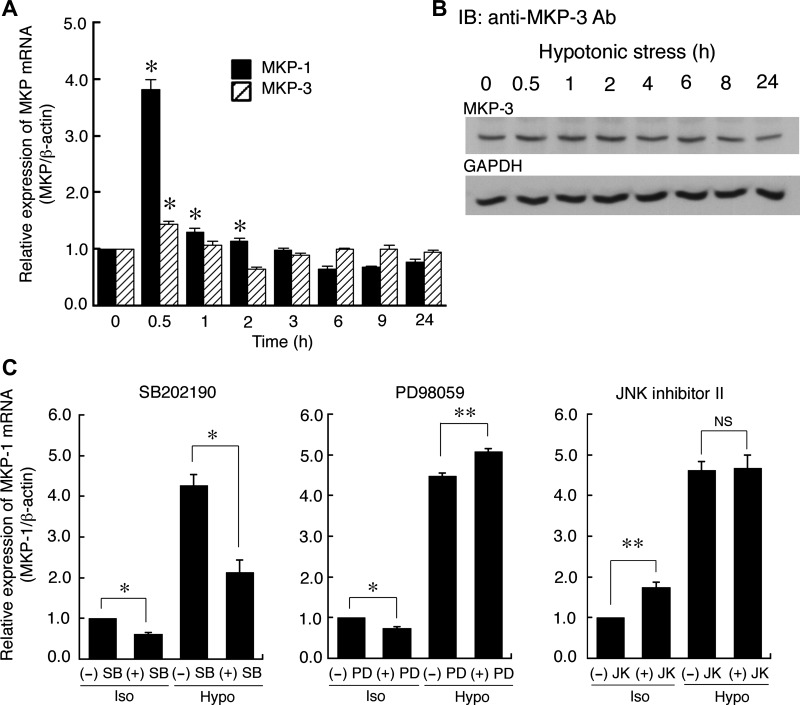
Since MKP-1 is generally considered to be transcriptionally regulated as an early response gene, we next examined whether hypotonic stress induces mRNA expression of MKP-1 and MKP-3 by using quantitative real-time PCR. Hypotonic stress transiently increased mRNA expression of both MKP-1 and MKP-3 (Fig. 4A), although the relative hypotonic-induced increase in MKP-1 (at least 4-fold) was much larger than that of MKP-3 (<1.5-fold). The mRNA expression of MKP-1 and MKP-3 reached its peak at around 0.5 h after hypotonic stress and then quickly returned to the basal level at around 3 h (Fig. 4A). This change seems to coincide with the appearance of significant ERK dephosphorylation 1–2 h after hypotonic stress (Fig. 2). Furthermore, MKP-3 protein expression did not change in response to hypotonic stress until 24 h (Fig. 4B). These observations suggest that MKP-1 is the primary phosphatase that inactivates ERK in the early phase of hypotonic stress, although MKP-3 might also contribute to ERK dephosphorylation (see discussion).
Fig. 4.
Hypotonic stress-induced mRNA expression of MAPK phosphatases MKP-1 and MKP-3. A: A6 monolayers were exposed to a hypotonic culture medium for the indicated times, and then MKP-1 and MKP-3 mRNA expression was detected by quantitative real-time PCR. B: A6 monolayers were exposed to hypotonic culture medium for the indicated time periods, and then MKP-3 protein expression was detected by Western blotting with an anti-MKP-3 antibody. A typical result was obtained in 3 similar experiments. C: effects of p38, ERK, and JNK inhibitors on mRNA expression of MKP-1 induced by hypotonic stress for 0.5 h. A6 monolayers were pretreated with SB202190 (a p38 inhibitor), PD98059 (a MEK inhibitor), or JNK inhibitor-II (a JNK inhibitor) for 60 min in an isotonic culture medium, and then the monolayers were exposed to an isotonic or a hypotonic culture medium in the presence and absence of each inhibitor for 0.5 h. After this procedure, mRNA expression of MKP-1 was detected. Relative amounts of MKP-1 mRNA expression were calculated as the ratio of the MKP expression to the expression of β-actin. Values are means ± SE; n = 4. *P < 0.05.
As described above, dephosphorylation of ERK in the early phase might be caused by induced MKP-1. After the initial ERK dephosphorylation, ERK phosphorylation returned toward the basal phosphorylation level around 4 h after hypotonic stress, after which dephosphorylation of ERK was again observed in the late phase up to at least 24 h. Therefore, we also examined an effect of NSC95397 on the subsequent dephosphorylation of ERK in the late phase. In the late phase (4–24 h after hypotonic stress), NSC95397 did not increase phosphorylated ERK at 24 h after hypotonic stress (data not shown). Furthermore, a gradual decrease in the total amount of ERK protein started around 6 h after hypotonic stress (Fig. 2). These observations strongly suggest that dephosphorylation of ERK in the late phase (between 6 and 24 h) is not mediated by MKP-1, but that the reduction of the total amount of ERK protein in the late phase causes a proportional decrease in the amount of phosphorylated ERK (Fig. 2).
We next addressed the question of how hypotonic stress induces MKP-1 expression. One of the main mechanisms inducing expression of MKP-1 is feedback regulation by activated MAPKs via several stimuli. To clarify the involvement of MAPK-dependent feedback regulation of MKP-1, we examined the effects of inhibitors for p38, ERK, and JNK. Pretreatment with 30 μM SB202190 (a specific p38 inhibitor) significantly suppressed hypotonic stress-induced MKP-1 mRNA expression (Fig. 4C), whereas 50 μM PD98059 or 20 μM JNK inhibitor II (a specific JNK inhibitor) did not reduce MKP-1 expression (Fig. 4C). We have already shown that 50 μM PD98059 (Fig. 1D) or 20 μM JNK inhibitor II (34) is a sufficient concentration to inhibit MEK or JNK in A6 cells exposed to hypotonic stress. These results with inhibitors indicate that 1) the MEK- or JNK-dependent pathway did not contribute to the hypotonic induction of MKP-1 expression, and 2) a p38-dependent pathway is one of the main mechanisms for hypotonic stress-induced mRNA expression of MKP-1.
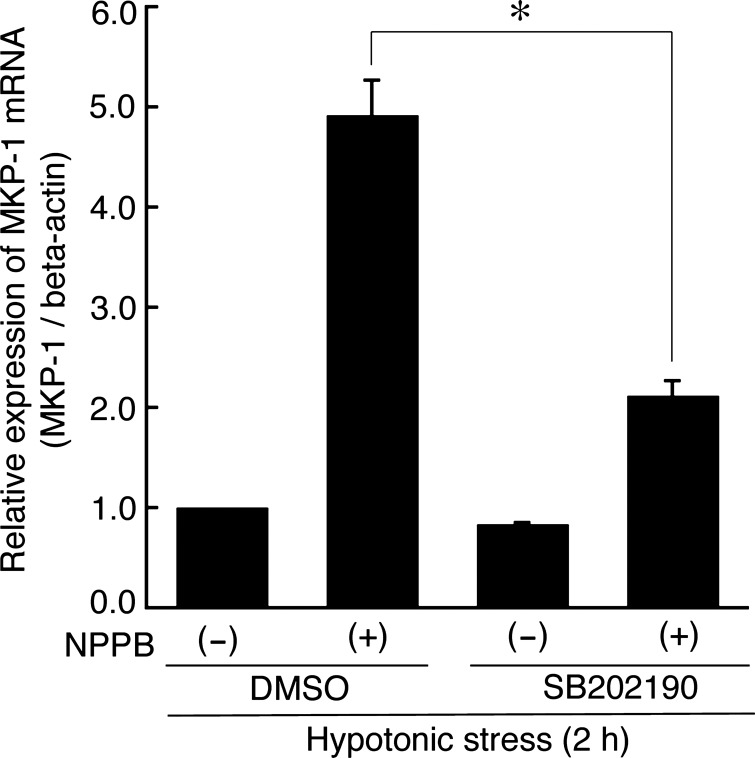
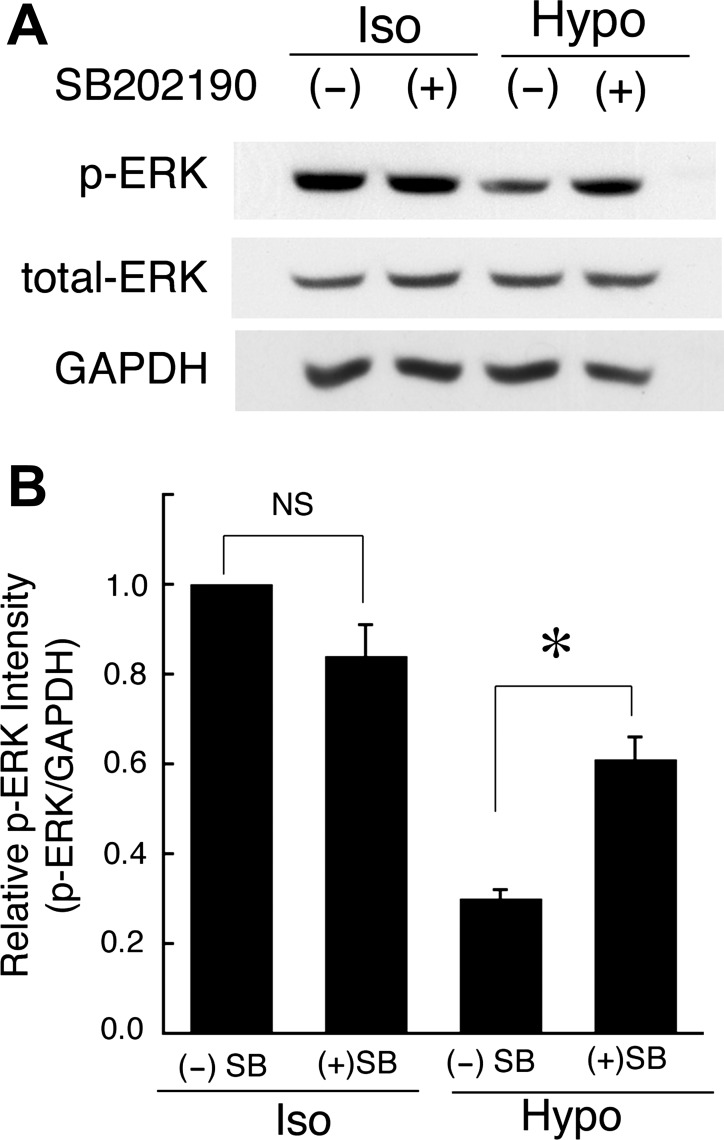
Generally, hypotonic stress causes transient cell swelling followed by a regulatory volume decrease (RVD) by which KCl release restores initial cell volume. Our previous report (21) indicates that in A6 cells hypotonic stress produces transient cell swelling that transiently activates p38. The transient swelling is followed by RVD. When we abolished the RVD following hypotonicity-induced cell swelling by blocking KCl release with NPPB (a Cl− channel blocker) or quinine (a K+ channel blocker), we observed sustained cell swelling associated with sustained activation of p38, rather than only a transient activation of p38 (21). These observations indicate that cell swelling causes activation of p38. To confirm the relationship between cell swelling-induced activation of p38 and MKP-1 mRNA expression, we tried to assess the effects of NPPB and quinine on the MKP-1 mRNA expression induced by hypotonic stress. Apical and basolateral pretreatment with 100 μM NPPB (Fig. 5) or 1 mM quinine (data not shown) caused a sustained increase in the MKP-1 mRNA expression which seemed to be parallel to the sustained cell swelling (21). Furthermore, pretreatment with 30 μM SB202190 suppressed the cell swelling-dependent sustained increase in MKP-1 mRNA expression caused by NPPB (Fig. 5). The observations suggest that a p38-dependent pathway actually contributes to the MKP-1 mRNA expression induced by hypotonic stress in a cell swelling-dependent manner. We further studied whether p38-dependent expression of MKP-1 mRNA really causes the dephosphorylation of ERK observed after application of hypotonic stress. Pretreatment with SB202190 inhibited dephosphorylation of ERK in the early phase (at 2 h after hypotonic stress) (Fig. 6), suggesting that the hypotonic stress-induced dephosphorylation of ERK is mediated by the p38-dependent induction of MKP-1 in renal A6 cells.
Fig. 5.
Effects of 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB) on MKP-1 mRNA expression. NPPB (a Cl− channel blocker) sustained the hypotonic stress-induced cell swelling by blocking KCl release. After pretreatment with 30 μM SB202190 for 60 min and 100 μM NPPB for 15 min in an isotonic culture medium, A6 monolayers were exposed to the hypotonic culture medium for 2 h in the presence and absence of NPPB and SB202190. Then, mRNA expression of MKP-1 was detected by real-time PCR. Values are means ± SE; n = 4. *P < 0.05.
Fig. 6.
Effects of SB202190 (a p38 inhibitor) on dephosphorylation of ERK caused by hypotonic stress. A: after pretreatment with 30 μM SB202190 for 1 h in an isotonic culture medium, A6 monolayers were exposed to an isotonic or a hypotonic culture medium with or without SB202190 for 2 h, and then phosphorylated and total ERK proteins were detected by immunoblotting. B: relative amounts of phosphorylated ERK are shown. Equal loading of cell lysate to each well was confirmed by measuring GAPDH as an internal control. Values are means ± SE; n = 4. *P < 0.05.
MKP-1 is crucial for β- and γ-ENaC expression induced by hypotonic stress.
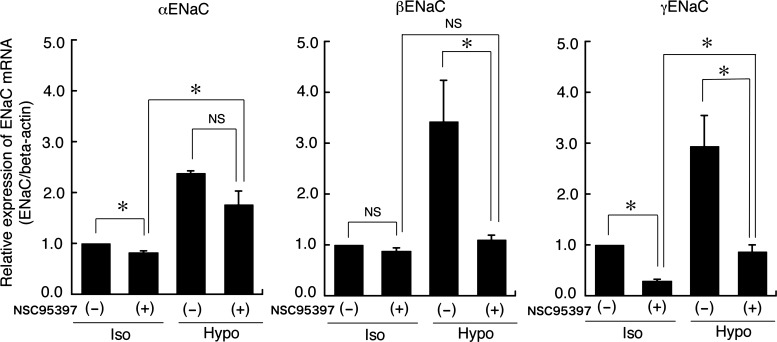
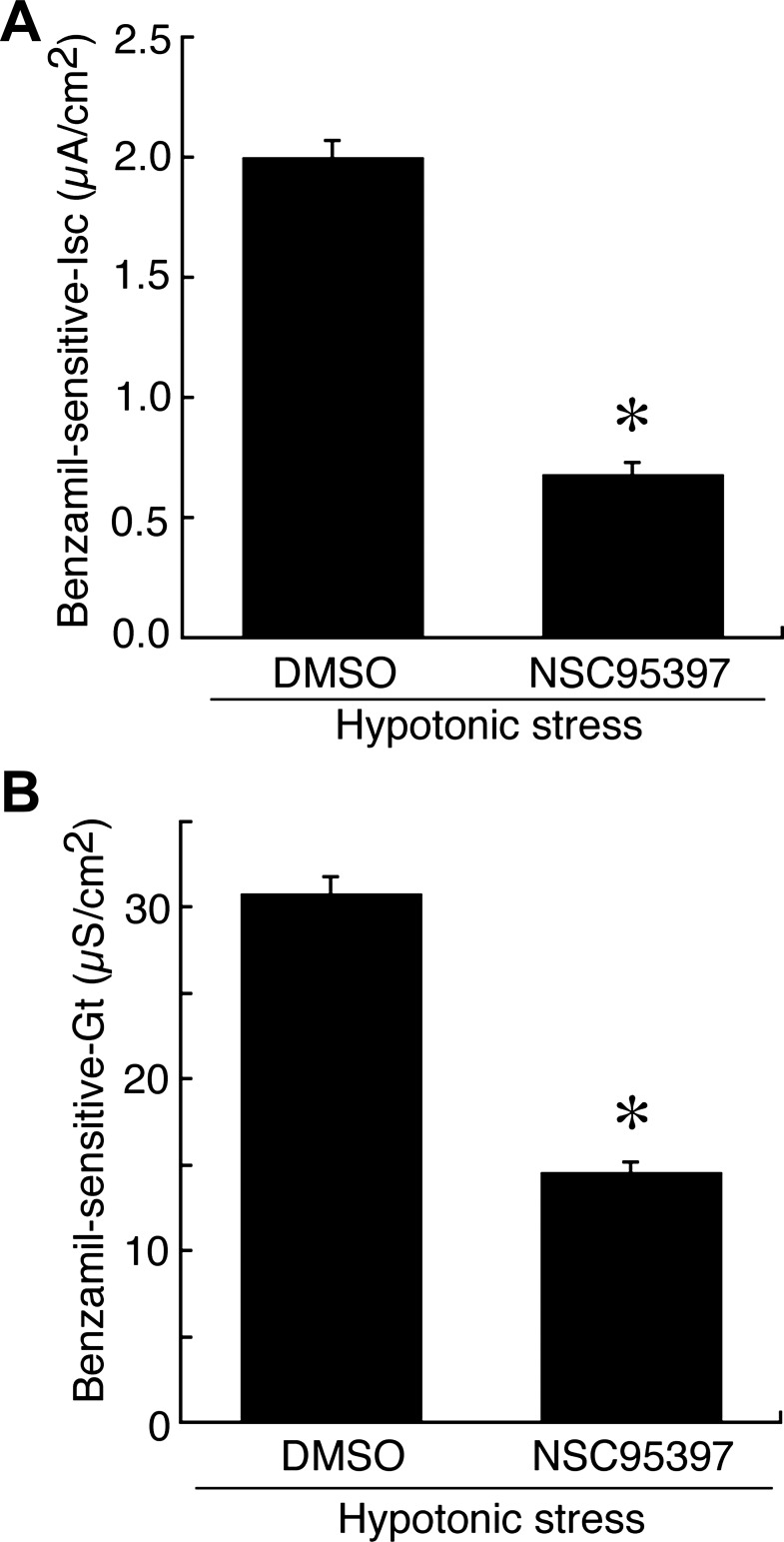
To further investigate the correlation between the hypotonicity-induced, MKP-1-dependent dephosphorylation of ERK and hypotonic stimulation of ENaC mRNA expression, we examined the effect of NSC95397 (an MKP-1/3 inhibitor) on the ENaC mRNA expression induced by hypotonic stress. After pretreatment with 40 μM NSC95397 or 0.1% DMSO alone for 1 h, A6 monolayers were exposed to a hypotonic culture medium containing 40 μM NSC95397 or 0.1% DMSO alone for 6 h. Then, total RNA was extracted, and mRNA expression of α-, β-, and γ-ENaC was detected by quantitative real-time PCR. Pretreatment with NSC95397 abolished the hypotonic stress-induced mRNA expression of β- and γ-ENaC (Fig. 7). This implies that the NSC95397-induced reduction of ERK dephosphorylation in the early phase of hypotonic stress (i.e., an increase in ERK phosphorylation; Fig. 3) suppressed the hypotonic induction of β- and γ-ENaC mRNA expression. These results suggest that dephosphorylation of ERK caused by MKP-1 in the early phase is essential for hypotonic induction of β- and γ-ENaC mRNA expression in A6 cells. We next examined the effects of NSC95397 on the hypotonic stimulation of Na+ reabsorption measured as benzamil-sensitive Isc. Pretreatment with 40 μM NSC95397 significantly decreased the benzamil-sensitive Isc and Gt in A6 cells incubated in a hypotonic culture medium (Fig. 8).
Fig. 7.
Effects of NSC95397 on ENaC mRNA expression induced by hypotonic stress. After pretreatment with 40 μM NSC95397 for 1 h in an isotonic culture medium, A6 monolayers were exposed to an isotonic or a hypotonic culture medium with or without 40 μM NSC95397 for 6 h. The mRNA expression of α-, β-, and γ-ENaC was detected by real-time PCR. Relative amounts of ENaC mRNA expression were normalized to β-actin as an internal control. Values are means ± SE; n = 4. *P < 0.05.
Fig. 8.
Effects of NSC95397 on ENaC-mediated Na+ reabsorption (benzamil-sensitive Isc; A) and ENaC activity (benzamil-sensitive Gt; B). After pretreatment with 40 μM NSC95397 for 1 h in an isotonic culture medium, A6 monolayers were exposed to a hypotonic culture medium with or without 40 μM NSC95397 for 6 h. Then, A6 monolayers were mounted in modified Ussing chambers with 60 mM NaCl solution, and benzamil-sensitive Isc (A) and Gt (B) were measured after application of 10 μM benzamil for 10 min. Values are means ± SE; n = 4. *P < 0.05.
Blockade of ERK phosphorylation by PD98059 increases ENaC expression.
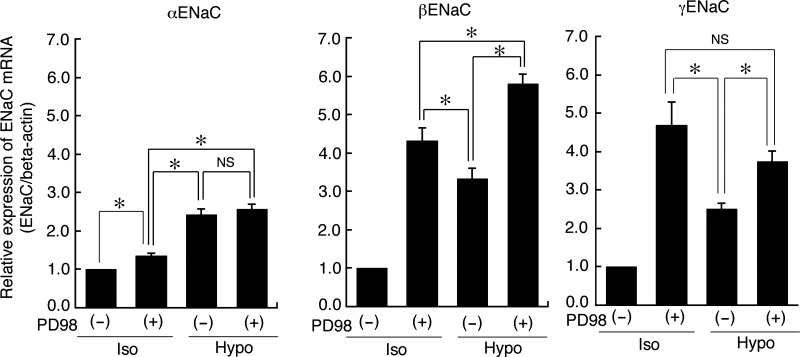
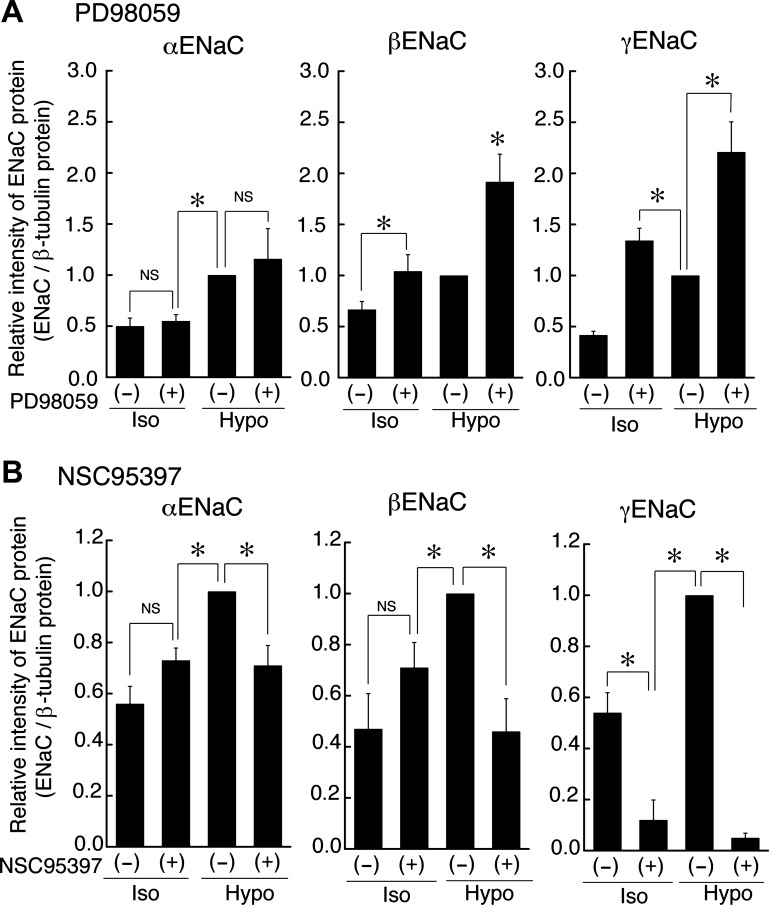
As described above, suppression of MKP-1 decreased mRNA expression of β- and γ-ENaC (Fig. 7) and Na+ reabsorption (Fig. 8) by increasing the phosphorylated (activated) ERK. This observation leads us to hypothesize that reduction of phosphorylated ERK increases ENaC mRNA expression. To confirm this hypothesis, we next examined effects of PD98059 on ENaC mRNA expression under isotonic and hypotonic conditions. We pretreated monolayers of A6 cells with 50 μM PD98059 or 0.1% DMSO alone for 1 h before exposure to an isotonic or a hypotonic culture medium containing 50 μM PD98059 or 0.1% DMSO alone for 20–24 h, respectively. Then, we detected α-, β-, and γ-ENaC mRNA expression by quantitative real-time-PCR. As expected, treatment with PD98059 significantly increased β- and γ-ENaC mRNA expression even under isotonic conditions and enhanced (further increased) the β- and γ-ENaC mRNA expression under hypotonic conditions (Fig. 9). The expression level of β- and γ-ENaC as a result of ERK inactivation with PD98059 under both the isotonic and hypotonic conditions was significantly higher than that induced by hypotonic stress alone. However, the expression level of β-ENaC under hypotonic conditions in the presence of 50 μM PD98059 was significantly higher than that under isotonic conditions in the presence of 50 μM PD98059. In contrast, there was no difference between the expression level of γ-ENaC under either isotonic or hypotonic conditions in the presence of 50 μM PD98059. PD98059 inhibited ERK phosphorylation to a much greater extent than application of hypotonic stress alone. This observation suggests that different durations and magnitudes of ERK inactivation would cause differing expression levels of β- and γ-ENaC mRNA due to differing sensitivity of β- and γ-ENaC mRNA expression to ERK activity. Next, we characterized the correlation between ENaC mRNA expression and ENaC protein expression under isotonic and hypotonic conditions with or without PD98059 or NSC95397. Pretreatment with 50 μM PD98059 enhanced β- and γ-ENaC protein expression under both isotonic and hypotonic conditions, in contrast, pretreatment with 40 μM NSC95397 suppressed β- and γ-ENaC protein expression (Figs. 10 and 11). Changes in ENaC protein expression under isotonic and hypotonic conditions with or without PD98059 or NSC95397 seem to correlate with changes in mRNA expression, although quantitative changes in ENaC protein were not completely same as the quantitative changes in mRNA expression.
Fig. 9.
Effects of PD98059 on ENaC mRNA expression induced by hypotonic stress. After pretreatment with 50 μM PD98059 for 1 h in an isotonic culture medium, A6 monolayers were exposed to an isotonic or a hypotonic culture medium with or without 50 μM PD98059 for 20–24 h. The mRNA expression of α-, β-, and γ-ENaC was detected by real-time PCR. Relative amounts of ENaC mRNA expression were normalized to β-actin. Values are means ± SE; n = 4–7. *P < 0.05.
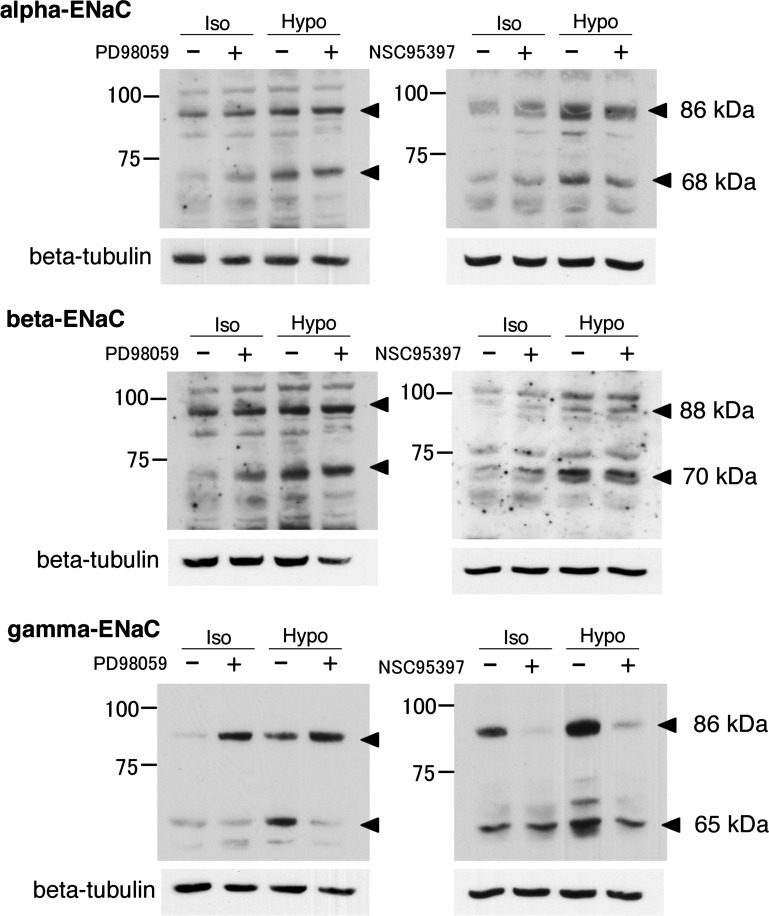
Fig. 10.
Typical Western blottings of ENaC protein expression under isotonic and hypotonic conditions with or without PD98059 or NSC95397. After pretreatment with 50 μM PD98059 (A) or 40 μM NSC95397 (B) for 1 h in isotonic culture medium, A6 monolayers were exposed to isotonic or hypotonic culture medium with or without 50 μM PD98059 (A) for 20–24 h or 40 μM NSC95397 (B) for 6 h. ENaC protein expression was detected by Western blotting with anti-α-, β-, or γ-ENaC antibodies.
Fig. 11.
Statistical results of typical Western blottings of ENaC protein expression under isotonic and hypotonic conditions with or without PD98059 or NSC95397. After pretreatment with 50 μM PD98059 (A) or 40 μM NSC95397 (B) for 1 h in isotonic culture medium, A6 monolayers were exposed to isotonic or hypotonic culture medium with or without 50 μM PD98059 (A) for 20–24 h or 40 μM NSC95397 (B) for 6 h. ENaC protein expression was detected by Western blotting with anti-α-, β-, or γ-ENaC antibodies. The cleaved forms of α-ENaC (68 kDa) and β-ENaC (70 kDa) subunits and the full-length form of the γ-ENaC (86 kDa) subunit were analyzed statistically. Values are means ± SE; n = 4–5. *P < 0.05.
Moreover, as shown in Fig. 1, A and B, under hypotonic conditions pretreatment of A6 cells with PD98059 dramatically enhanced benzamil-sensitive (ENaC-mediated) Na+ reabsorption by increasing benzamil-sensitive apical Gt (a measure of apical membrane ENaC activity). In contrast, under isotonic conditions, pretreatment with PD98059 did not increase benzamil-sensitive Isc (Fig. 1A) or Gt (Fig. 1B). We observed much higher benzamil-sensitive Isc just after application of hypotonic stress than that in A6 cells without pretreatment with PD98059 (Fig. 12). Even under isotonic conditions, pretreatment of A6 cells with PD98059 significantly increased mRNA expression of β- and γ-ENaC (Fig. 9). These observations show that the increase in β- and γ-ENaC mRNA expression caused by PD98059-induced inactivation of ERK is not sufficient by itself to increase ENaC-mediated Na+ reabsorption or ENaC activity at the apical membrane.
Fig. 12.
Effects of PD98059 on ENaC-mediated Na+ reabsorption (benzamil-sensitive Isc) and ENaC activity (benzamil-sensitive Gt). Pretreatment with PD98059 under isotonic conditions increased ENaC mRNA expression but not Na+ reabsorption. After A6 monolayers were pretreated with 0.1% DMSO or 50 μM PD98059 for 20–24 h in an isotonic culture medium, A6 monolayers were mounted in modified Ussing chambers with 120 mM NaCl (Iso) solution without inhibitors. Then, the extracellular solution was changed from an isotonic to a hypotonic (60 mM NaCl) one without any inhibitors at time 0. A: time course of Isc. B: benzamil-sensitive Isc and Gt. After incubation for 120 min, 10 μM benzamil was applied to the apical side and incubated for 10 min for measurement of benzamil-sensitive Isc and G. Values are means ± SE; n = 4. *P < 0.05.
DISCUSSION
In the present study, we show that phosphorylated ERK (active form) is a negative regulator for β- and γ-ENaC mRNA expression through changes in extracellular osmolarity and that hypotonic stress produces its stimulatory action on Na+ reabsorption by suppressing phosphorylated ERK via induction of MKPs. Dephosphorylation of MAPK is a crucial event in determining the duration and magnitude of MAPK activity. So far, 10 dual-specificity MAPK phosphatases (MKPs) have been identified and categorized into three groups (26): 1) MKPs categorized into the first group, including MKP-1, DUSP2/PAC-1, DUSP4/MKP-2, and DUSP5, are all inducible nuclear phosphatases that usually dephosphorylate p38 and JNK rather than ERK; 2) MKPs in the second group, including MKP-3, are cytoplasmic phosphatases that preferentially dephosphorylate ERK1/2; and 3) MKPs categorized into the third group dephosphorylate p38 and JNK selectively. In the present study, we show that 1) NSC95397 (a specific inhibitor for MKP-1/3) completely prevented hypotonic stress-induced ERK inactivation; and 2) hypotonic stress mainly induced MKP-1 rather than MKP-3 at the transcriptional level, suggesting that MKP-1, but not MKP-3, mainly contributes to ERK dephosphorylation and inactivation in A6 cells. In A6 cells, hypotonic stress slightly induced mRNA expression of MKP-3; however, the protein level detected by a specific antibody did not increase (Fig. 4), suggesting that MKP-3 does not significantly contribute to ERK dephosphorylation in response to hypotonic stress. On the other hand, MKP-1 is an inducible nuclear MKP and is induced by many stimuli that activate MAPKs. Although MKP-1 is considered to act mostly on JNK and p38 rather than ERK1/2, it is also known to inactivate ERK in several cell types (4, 16). These reports support our idea that MKP-1 is involved in ERK dephosphorylation in the early phase of hypotonic stress in A6 cells, although we cannot rule out some small contribution of MKP-3 to ERK dephosphorylation.
PD98059 preferentially inhibits MEK1 much more than MEK2, although U0126 inhibits both MEK1 and MEK 2 equally. Therefore, we also checked the effect of U0126 (2 μM; Calbiochem; MEK1 IC50 = 72 nM, MEK2 IC50 = 58 nM) on ENaC mRNA expression. Although pretreatment with U0126 also increased β- and γ-ENaC mRNA expression (data not shown), the increase in β- and γ-ENaC mRNA expression was only slightly more than PD98059. This lack of an additional increase in response to U0126 implies that most of the effect of MEK inhibition is due to inhibition of MEK1 with little effect of MEK2. Therefore, inhibition of MEK1 is necessary to upregulate β- and γ-ENaC mRNA expression.
Although phosphorylated ERK is a crucial, negative regulator for ENaC mRNA expression in A6 cells, the ability of hypotonic stress to activate ENaC by dephosporylation and inactivation of ERK (or by PD98059 pretreatment) varies between different ENaC subunits. Hypotonic stress increased α-ENaC mRNA 2.5-fold in the presence or absence of PD98059, suggesting that ERK inactivation does not play a major role in the changes in α-ENaC expression following hypotonic stress. Our previous work shows that a calcium-dependent signal plays a role in α-ENaC mRNA expression in response to hypotonic stress (35).
Our previous reports (24, 34) indicate that hypotonic stress stimulates translocation of ENaC protein to the apical membrane. Therefore, we speculate that the increase in mRNA expression of β- and γ-ENaC caused by PD98059-induced inactivation of ERK leads to increased synthesis of new ENaC proteins, which we have confirmed by showing that ENaC protein expression increased in parallel to increases in mRNA expression (Fig. 9); however, the newly synthesized ENaC proteins are apparently stored in a cytosolic compartment rather than being translocated to the apical membrane. If newly synthesized ENaC proteins are stored in the cytosol, hypotonic stress should induce much larger benzamil-sensitive Isc in A6 cells pretreated with PD98059 under isotonic conditions compared with that in untreated A6 cells in which expression of ENaC protein synthesis has not been stimulated. Indeed, in A6 cells pretreated with PD98059, we observed much higher benzamil-sensitive Isc just after application of hypotonic stress than that in A6 cells without pretreatment with PD98059 (Fig. 12). In the presence of 10 μM benzamil in the apical solution, we did not observe the hypotonic stress-induced increase in Isc regardless of PD98059 treatment, except for a transient increase in Isc due to Cl− secretion in the 5 min immediately after application of hypotonic stress (19), suggesting that hypotonic stress induces an increase in ENaC-mediated Na+ reabsorption within 10 min after application of hypotonic stress. Therefore, these observations support our speculation that the increase in mRNA expression of β- and γ-ENaC caused by PD98059-induced inactivation of ERK leads to more synthesis of new ENaC proteins (Figs. 10–12), but the newly synthesized ENaC proteins are stored in a cytosolic compartment. This implies that inactivation of ERK by itself is not sufficient to increase ENaC translocation to the apical membrane from a cytosolic store, and that hypotonic stress must regulate an additional signal stimulating ENaC translocation to the apical membrane in addition to inactivation (dephosphorylation) of ERK. Based on these results, we suggest that 1) active ERK (phosphorylated ERK) suppresses mRNA and protein expression of β- and γ-ENaC; 2) hypotonic stress induces MKP-1 expression via activation of p38 MAPK; this suppresses ERK activity (by dephosphorylation); and the reduction in ERK activity induces expression of β- and γ-ENaC but ERK inactivation alone is insufficient to promote ENaC insertion in the membrane; and 3) hypotonic stress-induced translocation of ENaC from a cytosolic pool to the apical membrane is regulated differently from the p38-dependent ERK dephosphorylation that promotes ENaC β- and γ-subunit expression.
α- and γ-ENaC subunits are cleaved (8, 14, 27–29). Therefore, we should consider the effects of hypotonic stress on cleavage of α- and γ-ENaC subunits. Antibodies for α- and γ-ENaC subunits used in the present study recognize the cleaved forms of α- and γ-ENaC subunits in addition to the full-length forms of both subunits (9–11, 32). The α-ENaC subunit was detected as full-length (86 kDa) and cleaved (68 kDa) forms (Fig. 10), and hypotonic stress increased expression of both forms of the α-ENaC subunit (Fig. 10). Expression of both forms of the α-ENaC subunit was not affected by inhibition of ERK [PD98059 (−)/(+) in Fig. 10], while expression of the cleaved form of the α-ENaC subunit was slightly decreased by MKP-1 inhibitor [NSC95397 (−)/(+) in Fig. 10]. Unlike α-ENaC, expression of full-length γ-ENaC (86 kDa) increased in response to hypotonic stress (Iso/Hypo in Fig. 10) or inhibition of ERK [PD 98059 (−)/(+) in Fig. 10]. On the other hand, the cleaved form of the γ-ENaC subunit (65 kDa) increased in response to hypotonic stress (Iso/Hypo in Fig. 10) like the full-length form, but not in response to inhibition of ERK [PD 98059 (−)/(+) in Fig. 10] unlike the full-length form. These different responses of cleaved and full-length forms of the γ-ENaC subunit to hypotonic stress and inhibition of ERK might be due to secretion of proteases cleaving the γ-ENaC subunit (8); namely, secretion of proteases might be stimulated by hypotonic stress, but not by inhibition of ERK, although further studies are required to confirm this point.
Besides not necessarily leading to more ENaC in the membrane, inactivation of ERK alone with PD98059 led to differing effects on total cellular expression of ENaC subunit mRNA and protein. Under isotonic conditions, PD98059 significantly increased β- and γ-ENaC mRNA expression to higher levels than that induced by hypotonic stress alone (Fig. 9). γ-ENaC expression was not increased further by hypotonic stress. On the other hand, β-ENaC expression in the presence of PD98059 was increased to levels significantly above that under isotonic conditions [compare PD98059 (+) in ISO with PD98059 (+) in HYPO in Fig. 9]. These observations suggest that dephosphorylation of ERK alone is sufficient to promote increased γ-ENaC expression whether the dephosphorylation is induced by hypotonicity or PD98059. However, β-ENaC mRNA and protein expression can be increased by PD98059-induced dephosphorylation of ERK, but hypotonicity induces an additional increase that is independent of ERK dephosphorylation.
We have previously reported that chronic hypotonic stress induces mRNA expression of β- and γ-ENaC and stimulation of ENaC-mediated Na+ reabsorption in a p38-dependent manner (23). In this study, we showed that the p38-dependent regulation of β- and γ-ENaC mRNA expression is at least partly due to hypotonic stress-mediated induction of MKP-1 that inactivates (dephosphorylates) ERK. In A6 cells, hypotonic stress transiently activated p38 via cell swelling (21). To reveal the correlation between p38 activation and MKP-1 mRNA expression, we produced sustained activation of p38 by blocking the RVD with a Cl− channel blocker (NPPB), leading to sustained MKP-1 mRNA expression (Fig. 5). This observation suggests that activation of p38 is a key factor for MKP-1 mRNA expression and that continuous activation of p38 keeps mRNA expression of MKP-1 at a high level for an extended period. Generally, in the acute (early) phase of hypotonic stress, preexisting ENaC is translocated to the apical membrane. Then, ENaC mRNA expression is upregulated and the newly synthesized ENaC is continuously translocated to the apical membrane to maintain Na+ reabsorption at a stimulated level in the late phase. In other words, both translocation and gene expression are required to continuously maintain Na+ reabsorption at a stimulated level in the late phase, although the acute regulation of Na+ reabsorption in the early phase is mediated by only ENaC translocation. In renal epithelial A6 cells, the JNK-dependent pathway has been reported to contribute to translocation of ENaC in the early phase of hypotonic stress (34), and the present study indicates a p38/ERK-dependent pathway via MKP contributes to ENaC mRNA and protein expression in the late phase of hypotonic stress. Thus spatiotemporal regulation of MAPKs such as ERK, JNK, and p38 provides fine, harmonized control in the stimulation of ENaC-mediated Na+ reabsorption via both translocation-dependent early and gene expression-dependent late responses to hypotonic stress.
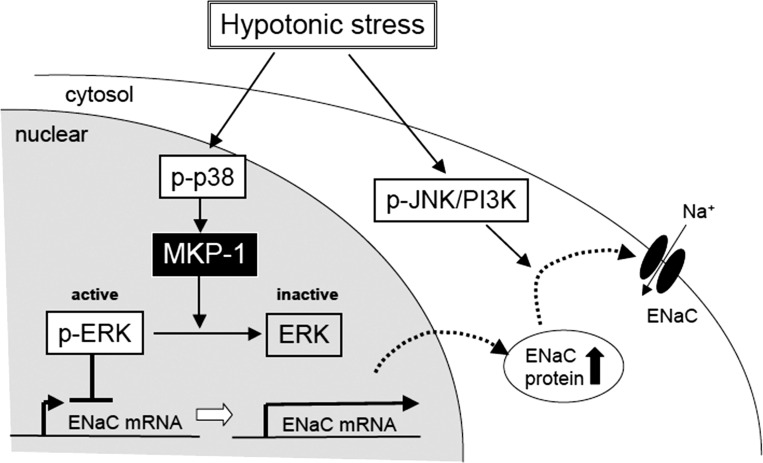
In conclusion (Fig. 13), we show that 1) a renal epithelium in the distal nephron itself has the ability to respond to changes in plasma osmolarity in addition to hormonal and nervous regulatory systems; 2) hypotonic stress causes a MKP-1-dependent ERK inactivation that in a physiological context of a whole animal would promote recovery of normal plasma osmolarity by stimulating Na+ reabsorption; and 3) cell swelling is a crucial signal inducing p38-mediated expression of MKP-1 mRNA, promoting a hypotonic stress-induced increase in β-and γ-ENaC mRNA expression and Na+ reabsorption. Thus each member of the MAPK family, ERK, JNK, and p38, plays an important role in the concerted regulation of Na+ reabsorption following hypotonic stress that enables fine, complex regulation of Na+ reabsorption for maintenance of the constant body Na+ content.
Fig. 13.
Scheme for MKP-1-dependent regulation of Na+ reabsorption by hypotonic stress in renal epithelial A6 cells. Under isotonic conditions, active (phosphorylated) ERK suppresses β- and γ-ENaC mRNA expression. Hypotonic stress activates p38. Active p38 promotes MKP-1 mRNA expression and MKP-1 dephosphorylates ERK. Dephosphorylated ERK is inactive and, therefore, ERK no longer inhibits β- and γ-ENaC mRNA expression, leading to increased accumulation of ENaC in a subapical, cytosolic pool. The final hypotonic stress-mediated translocation of newly synthesized ENaC in this subapical pool to the apical membrane is mainly regulated through other signaling pathways, in particular, the JNK-phosphatidylinositol 3-kinase (PI3K) pathway, which is also activated by hypotonicity.
GRANTS
This work was supported by Grants-in-Aid from the Japan Society of The Promotion of Science (20390060), Research Conference for Cell Function, Fuji Foundation for Protein Research, and The Salt Science Research Foundation (1035, 1235) to Y. Marunaka and National Institute of Diabetes and Digestive and Kidney Diseases Grant R37 DK037963 to D. C. Eaton.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.N. and Y.M. provided conception and design of research; N.N. and M.O. performed experiments; N.N. and M.O. analyzed data; N.N., D.C.E., and Y.M. interpreted results of experiments; N.N. prepared figures; N.N., D.C.E., and Y.M. drafted manuscript; N.N., D.C.E., and Y.M. edited and revised manuscript; N.N., M.O., D.C.E., and Y.M. approved final version of manuscript.
REFERENCES
- 1. Asano J, Niisato N, Nakajima K, Miyazaki H, Yasuda M, Iwasaki Y, Hama T, Dejima K, Hisa Y, Marunaka Y. Quercetin stimulates Na+/K+/2Cl− cotransport via PTK-dependent mechanisms in human airway epithelium. Am J Respir Cell Mol Biol 41: 688–695, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Choi JY, Choi YS, Kim SJ, Son EJ, Choi HS, Yoon JH. Interleukin-1beta suppresses epithelial sodium channel beta-subunit expression and ENaC-dependent fluid absorption in human middle ear epithelial cells. Eur J Pharmacol 567: 19–25, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Datta NS, Kolailat R, Fite A, Pettway G, Abou-Samra AB. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal 22: 457–466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12: 186–192, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-α and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Malik B, Yue Q, Yue G, Chen XJ, Price SR, Mitch WE, Eaton DC. Role of Nedd4–2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits. Am J Physiol Renal Physiol 289: F107–F116, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 4: 82–89, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Marunaka Y, Niisato N, Taruno A, Ohta M, Miyazaki H, Hosogi S, Nakajima K, Kusuzaki K, Ashihara E, Nishio K, Iwasaki Y, Nakahari T, Kubota T. Regulation of epithelial sodium transport via epithelial Na+ channel. J Biomed Biotechnol 2011: 978196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller GM, Kashlan OB, Bruns JB, Maarouf AB, Aridor M, Kleyman TR, Hughey RP. Epithelial sodium channel exit from the endoplasmic reticulum is regulated by a signal within the carboxyl cytoplasmic domain of the alpha subunit. J Biol Chem 282: 33475–33483, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Mustafa SB, Castro R, Falck AJ, Petershack JA, Henson BM, Mendoza YM, Choudary A, Seidner SR. Protein kinase A and mitogen-activated protein kinase pathways mediate cAMP induction of alpha-epithelial Na+ channels (alpha-ENaC). J Cell Physiol 215: 101–110, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Newton R, King EM, Gong W, Rider CF, Staples KJ, Holden NS, Bergmann MW. Glucocorticoids inhibit IL-1beta-induced GM-CSF expression at multiple levels: roles for the ERK pathway and repression by MKP-1. Biochem J 427: 113–124, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Niisato N, Eaton DC, Marunaka Y. Involvement of cytosolic Cl− in osmoregulation of α-ENaC gene expression. Am J Physiol Renal Physiol 287: F932–F939, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Niisato N, Hasegawa I, Tokuda S, Taruno A, Nakajima K, Miyazaki H, Iwasaki Y, Marunaka Y. Action of neltenexine on anion secretion in human airway epithelia. Biochem Biophys Res Commun 356: 1050–1055, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Niisato N, Marunaka Y. Activation of the Na+-K+ pump by hyposmolality through tyrosine kinase-dependent Cl− conductance in Xenopus renal epithelial A6 cells. J Physiol 518: 417–432, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niisato N, Marunaka Y. Sensing mechanism of extracellular osmolality in renal epithelium. J Physiol Sci 61, Suppl 1: 32, 2011 [Google Scholar]
- 21. Niisato N, Post M, Van Driessche W, Marunaka Y. Cell swelling activates stress-activated protein kinases, p38 MAP kinase and JNK, in renal epithelial A6 cells. Biochem Biophys Res Commun 266: 547–550, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Niisato N, Taruno A, Marunaka Y. Aldosterone-induced modification of osmoregulated ENaC trafficking. Biochem Biophys Res Commun 361: 162–168, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Niisato N, Taruno A, Marunaka Y. Involvement of p38 MAPK in hypotonic stress-induced stimulation of beta- and gamma-ENaC expression in renal epithelium. Biochem Biophys Res Commun 358: 819–824, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Niisato N, Van Driessche W, Liu M, Marunaka Y. Involvement of protein tyrosine kinase in osmoregulation of Na+ transport and membrane capacitance in renal A6 cells. J Membr Biol 175: 63–77, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Ohta M, Niisato N, Marunaka Y. The essential role of p38 in aldosterone action on ENaC-mediated Na+ transport. J Physiol Sci 60, Suppl 1: 124, 2010 [Google Scholar]
- 26. Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26: 3203–3213, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 299: F854–F861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens 19: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Passero CJ, Mueller GM, Myerburg MM, Carattino MD, Hughey RP, Kleyman TR. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the gamma-subunit distal to the furin cleavage site. Am J Physiol Renal Physiol 302: F1–F8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pearce D. SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem 13: 13–20, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Saxena M, Mustelin T. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin Immunol 12: 387–396, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Taruno A, Marunaka Y. Analysis of blocker-labeled channels reveals the dependence of recycling rates of ENaC on the total amount of recycled channels. Cell Physiol Biochem 26: 925–934 2010 [DOI] [PubMed] [Google Scholar]
- 34. Taruno A, Niisato N, Marunaka Y. Hypotonicity stimulates renal epithelial sodium transport by activating JNK via receptor tyrosine kinases. Am J Physiol Renal Physiol 293: F128–F138, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Taruno A, Niisato N, Marunaka Y. Intracellular calcium plays a role as the second messenger of hypotonic stress in gene regulation of SGK1 and ENaC in renal epithelial A6 cells. Am J Physiol Renal Physiol 294: F177–F186, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Tokuda S, Miyazaki H, Nakajima K, Yamada T, Marunaka Y. Hydrostatic pressure regulates tight junctions, actin cytoskeleton and transcellular ion transport. Biochem Biophys Res Commun 390: 1315–1321, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Tokuda S, Miyazaki H, Nakajima K, Yamada T, Marunaka Y. NaCl flux between apical and basolateral side recruits claudin-1 to tight junction strands and regulates paracellular transport. Biochem Biophys Res Commun 393: 390–396, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Tokuda S, Niisato N, Nagai T, Taruno A, Nakajima K, Miyazaki H, Yamada T, Hosogi S, Ohta M, Nishio K, Iwasaki Y, Marunaka Y. Regulation of paracellular Na+ and Cl− conductances by hydrostatic pressure. Cell Biol Int 33: 949–956, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Vogt A, McDonald PR, Tamewitz A, Sikorski RP, Wipf P, Skoko JJ, 3rd, Lazo JS. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol Cancer Ther 7: 330–340, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Wang HC, Zentner MD, Deng HT, Kim KJ, Wu R, Yang PC, Ann DK. Oxidative stress disrupts glucocorticoid hormone-dependent transcription of the amiloride-sensitive epithelial sodium channel alpha-subunit in lung epithelial cells through ERK-dependent and thioredoxin-sensitive pathways. J Biol Chem 275: 8600–8609, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Yamada T, Niisato N, Marunaka Y. Effects of extracellular chloride ion on epithelial sodium channel (ENaC) in arginine vasotocin (AVT)-stimulated renal epithelial cells. Biomed Res 30: 193–198, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Yamada T, Takemura Y, Niisato N, Mitsuyama E, Iwasaki Y, Marunaka Y. Action of N-acylated ambroxol derivatives on secretion of chloride ions in human airway epithelia. Biochem Biophys Res Commun 380: 586–590, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Yasuda M, Niisato N, Miyazaki H, Hama T, Dejima K, Hisa Y, Marunaka Y. Epithelial ion transport of human nasal polyp and paranasal sinus mucosa. Am J Respir Cell Mol Biol 36: 466–472, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Yasuda M, Niisato N, Miyazaki H, Iwasaki Y, Hama T, Dejima K, Hisa Y, Marunaka Y. Epithelial Na+ channel and ion transport in human nasal polyp and paranasal sinus mucosa. Biochem Biophys Res Commun 362: 753–758, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Zentner MD, Lin HH, Deng HT, Kim KJ, Shih HM, Ann DK. Requirement for high mobility group protein HMGI-C interaction with STAT3 inhibitor PIAS3 in repression of alpha-subunit of epithelial Na+ channel (alpha-ENaC) transcription by Ras activation in salivary epithelial cells. J Biol Chem 276: 29805–29814, 2001 [DOI] [PubMed] [Google Scholar]