Abstract
Clinical studies have established the role of cigarette smoking as a risk factor in the progression of chronic kidney disease (CKD). We have shown that nicotine promotes mesangial cell proliferation and hypertrophy via nonneuronal nicotinic acetylcholine receptors (nAChRs). The α7-nAChR is one of the most important subunits of the nAChRs. These studies were designed to test the hypothesis that nicotine worsens renal injury in rats with 5/6 nephrectomy (5/6Nx) and that the α7-nAChR subunit is required for these effects. We studied five different groups: Sham, 5/6Nx, 5/6Nx + nicotine (Nic; 100 μg/ml dry wt), 5/6Nx + Nic + α7-nAChR blocker methyllicaconitine (MLA; 3 mg·kg−1·day−1 sq), and Sham + Nic. Blood pressure was measured by the tail-cuff method, and urine was collected for proteinuria. After 12 wk, the rats were euthanized and kidneys were collected. We observed expression of the α7-nAChR in the proximal and distal tubules. The administration of nicotine induced a small increase in blood pressure and resulted in cotinine levels similar to those found in the plasma of smokers. In 5/6Nx rats, the administration of nicotine significantly increased urinary protein excretion (onefold), worsened the glomerular injury score and increased fibronectin (∼ 50%), NADPH oxidase 4 (NOX4; ∼100%), and transforming growth factor-β expression (∼200%). The administration of nicotine to sham rats increased total proteinuria but not albuminuria, suggesting direct effects on tubular protein reabsorption. These effects were prevented by MLA, demonstrating a critical role for the α7-nAChR as a mediator of the effects of nicotine in the progression of CKD.
Keywords: extracellular matrix, reactive oxygen species, tobacco
cigarette smoking is an important risk factor for emphysema, atherosclerosis, and cancer and is the most important cause of preventable morbidity and mortality in the United States (3). In addition, epidemiological studies (26, 35) have demonstrated that cigarette smoking is an independent risk factor in the progression of chronic kidney disease (CKD) of different etiologies including hypertension and diabetes.
The mechanisms by which cigarette smoking accelerates the progression of CKD are, however, not well understood. The gas phase of cigarette smoke contains high concentrations of short-lived reactive oxygen species, nitric oxide, and free radicals of organic compounds (31). In addition, it contains varying amounts of more stable substances including nicotine, which, besides its addictive properties (39), plays an important role in the pathogenesis of tobacco-induced disease including atherosclerosis (14) and pulmonary fibrosis (34). Moreover, and as we have previously shown, nicotine worsens glomerular injury in a rat model of acute nephritis (21) and promotes extracellular matrix deposition in a mouse model of diabetic nephropathy (20).
Nicotine mediates its effects via the activation of nicotinic acetylcholine receptors (nAChRs) that function as agonist-regulated Ca2+ channels. The nAChRs are transmembrane oligomers consisting of five subunits and are expressed by neuronal as well nonneuronal cells including human mesangial cells (20), endothelial cells, and vascular smooth muscle cells (15, 24). Of these subunits, the α7-nAchR subunit in particular is critical for several of the cholinergic actions mediated by nAChRs in macrophages, vascular smooth muscle cells, and cancer cell lines (7, 33).
In these studies, we tested the hypothesis that the α7-nAChR plays a major role as mediator of the effects of nicotine in the progression of renal disease. For these studies, we utilized a rat model of 5/6 nephrectomy, a well-characterized, and validated model of CKD that closely mimics CKD in humans (37). In these studies, we demonstrate expression of the α7-nAchR and that nicotine worsens renal injury in this model of CKD that can be prevented by α7-nAchR blockade, suggesting that this receptor subunit is essential as mediator of the actions of nicotine in the progression of CKD. In addition, we demonstrate that the effects of nicotine are accompanied by increases in oxidative stress, NADPH oxidase 4 (NOX4), and transforming growth factor (TGF)-β.
METHODS
Eight-week-old male Sprague-Dawley rats were purchased from Charles River (Boston, MA) and maintained under controlled conditions of light, temperature, and humidity. All rats had baseline blood pressure measurements and urine collections and were subjected to either sham surgery or 5/6 nephrectomy (5/6Nx). The rats were divided in the following groups: group 1 (Sham): sham-operated rats drinking tap water (n = 8); group 2 (5/6Nx): Rats with 5/6Nx on tap water (n = 7); group 3 (5/6Nx + Nic): rats with 5/6Nx and receiving nicotine in the drinking water (21) (100 ug/ml; n = 8); group 4 (5/6Nx + Nic + MLA): rats with 5/6Nx, receiving nicotine in the drinking water (100 ug/ml) and receiving the α7-nAchR blocker methyllicaconitine (23) (MLA; 3 mg·kg−1·day−1 via osmotic minipump; n = 8); group 5 (Sham + Nic): sham-operated rats receiving nicotine in the drinking water (100 ug/ml; n = 8); group 6 (5/6Nx + MLA): rats with 5/6Nx and receiving MLA (3 mg·kg−1·day−1); and group 7 (Sham + Nic + MLA): sham-operated rats receiving nicotine in the drinking water (100 ug/ml) and MLA (3 mg·kg−1·day−1). The rats were maintained in their respective treatment groups for 12 wk after surgery and euthanized under deep anesthesia by exsanguination via cardiac puncture followed by cervical dislocation. Blood was collected for cotinine serum and creatinine measurements and kidneys for Western blot analysis and histology.
Blood pressure was measured biweekly in all groups by the tail-cuff method (CODA-Kent Scientific). Urine collections were also performed biweekly in all groups in metabolic cages. The animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The studies were approved by the Institutional Animal Care and Use Sub-Committee at the University of Alabama at Birmingham.
Nephrectomies.
The 5/6 nephrectomies (5/6Nx) were performed in two stages while the animals were under general anesthesia (isofluorane 2.5%) using the full sterile technique (40). In the first stage, and via a retroperitoneal approach, a subtotal nephrectomy was performed in which both poles of the left kidney were surgically excised and the stumps ligated. Gel-Foam was used for hemostasis. A week later, a total right nephrectomy was performed after ligation of the renal vessels and the ureter.
Western blot analysis.
Western blots were performed as previously described (22). Briefly, 100 mg of kidney cortex were homogenized in 300 μl of homogenization buffer (20 nmol/l Tris·HCl pH 7.4, 140 mmol/l NaCl, 10 mmol/l Na pyrophosphate, 10 mmol/l Na fluoride, 2 mmol/l Na orthovanadate, 3 mmol/l EDTA, and 10% glycerol). Protease inhibitor cocktail (Sigma P8340) was added to the homogenization buffer before use. The resulting lysates were centrifugated for 20 min at 13,200 rpm at 4°C. The supernatants were collected, and protein concentration was quantified by Bio-Rad assay.
For immunoblotting, 15–20 μl of homogenate were separated by SDS-PAGE (10–15% acrylamide gel) and transferred to a nitrocellulose membrane (Bio-Rad; 0.2 μm). The blots were incubated with antibodies against fibronectin (Sigma, St Louis, MO), nitrotyrosine (Millipore Billerica, MA, ), α-7 (Abcam, Boston, MA), and NOX 4 (Novus, Littleton, CO). β-Actin (Sigma) was used to control for loading. The blots were washed and incubated with goat anti-rabbit antibody (Jackson), and the signal was detected by luminol chemoluminescence (Millipore, Millipore Billerica, MA).
Immunofluorescence.
Formalin-fixed, paraffin-embedded rat kidney cortex sections (5 μm) were deparaffinized and antigen retrieval performed on all sections using Vector antigen retrieval solution. Sections used to identify specific tubules were incubated for 15 min. each with Avidin blocking reagent and Biotin blocking reagent. Sections used to identify proximal tubules were incubated with biotinylated Lotus tetragonolobus lectin (1:200) in PBS for 30 min. Sections used to identify distal tubules and collecting duct were incubated with biotinylated peanut agglutinin (1:1,000) in PBS for 30 min (4). All sections were then washed and incubated with streptavidin Texas red (1:200) in PBS for 30 min. Biotinylated reagents and streptavidin TxR were purchased from Vector Laboratories (Burlingame, CA). Sections were blocked with PBS + 1% bovine serum and 5% goat serum for 1 h at room temperature then incubated with primary antibody to fibronectin (Sigma), α-7 (Abcam), or NOX4 (Novus) at a 1:200 dilution in blocking buffer, overnight at 4°C in a humidified chamber. Sections were then washed three times for 5 min with PBS and incubated with secondary antibody, Alexa Fluor 488-labeled goat anti-rabbit (Molecular Probes, Portland. OR) at 1:400 dilution in blocking buffer, 1 h at room temperature. Sections were then washed three times for 5 min with PBS and mounted with coverslips. Image acquisition was performed on a Leica DM6000 epifluorescence microscope (Leica Microsystems, Bannockburn, IL) with a Hamamatsu ORCA ER cooled CCD camera and SimplePCI software (Compix, Cranberry Township, PA). Images were adjusted appropriately to remove background fluorescence. Identical sections used as controls were processed, either without primary or secondary antibodies present during identical incubation conditions.
Proteinuria.
Proteinuria was measured by the Lowry method and adjusted by urinary creatinine (Cayman, Ann Arbor, MI). Urinary albumin concentrations were measured using a rat albumin ELISA quantitation kit from Bethyl (Montgomery, TX) and adjusted for urinary creatinine.
Serum creatinine.
Serum creatinine was measured by mass spectrometry at the O'Brien Kidney Center Core Facilities at the University of Alabama at Birmingham.
Glomerular injury score.
Glomerular injury score was measured in trichrome-stained kidney slides by one of us, an experienced pathologist purposely blinded to the different experimental conditions and utilizing a 0+ to 4+ scale as previously described (32). All glomeruli available in each slide (n = 32–263) were analyzed, and the data are expressed as the percentage of glomeruli injured at every level.
TGF-β1.
TGF-β1 was measured in kidney cortex lysates by ELISA (R&D Systems, Minneapolis, MN) following the manufacturer's instructions and adjusted for protein content (Lowry).
Urinary isoprostanes.
Urinary F2-isoprostranes were measured by EIA (Cayman, Ann Arbor, MI) following the manufacturer's instructions and adjusted by urinary creatinine.
Cotinine.
The serum and urinary concentrations of cotinine, a stable metabolite of nicotine, were determined using a mouse/rat cotinine ELISA kit (Calbiotech, San Diego, CA) following the manufacturer's instructions.
Statistical analysis.
All data are expressed as the means ± SE. Statistical analysis was performed by ANOVA (Statistix 8 Analytical Software, Tallahassee, FL). Significance was considered when P < 0.05.
RESULTS
Renal expression of nicotinic α7 receptors.
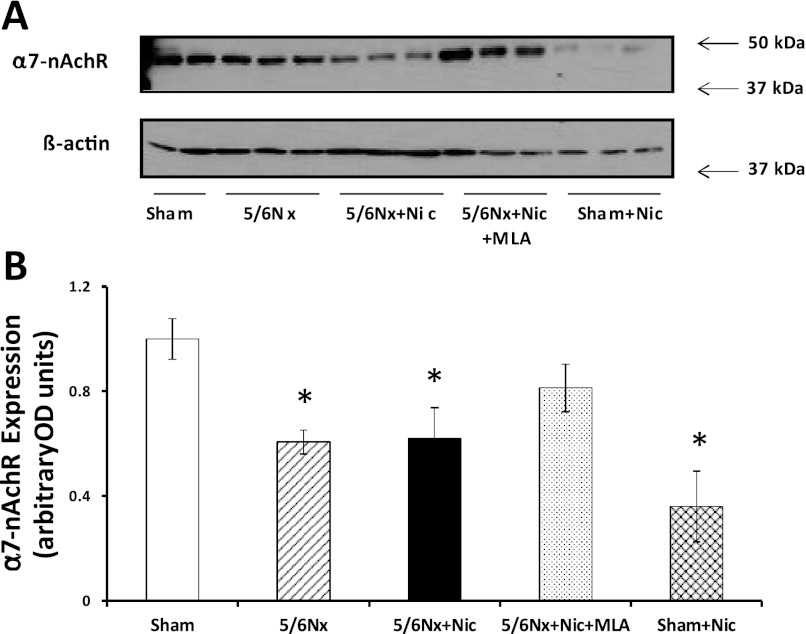
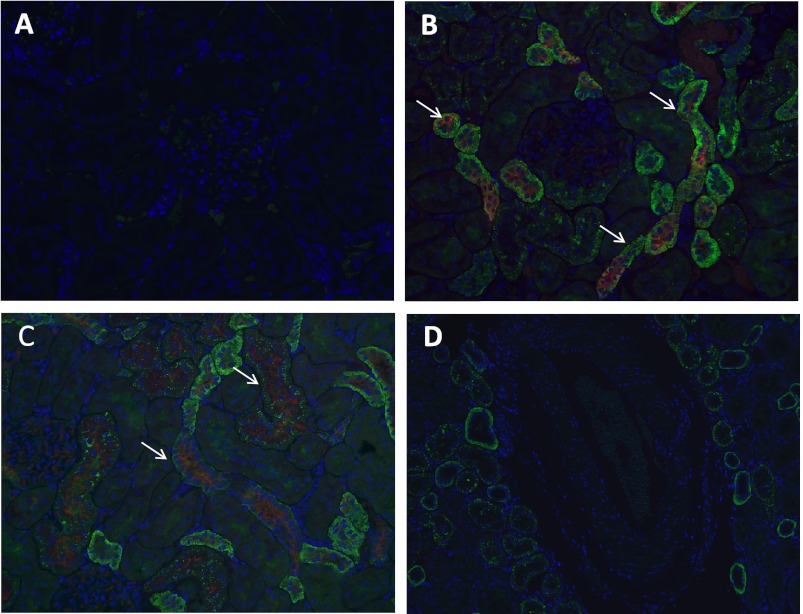
As shown in Fig. 1, we observed strong expression of the α7-nAchR in sham rats as assessed by Western blot. The expression of the α7-nAchR was reduced in 5/6Nx rats drinking tap water or on nicotine compared with sham rats. The administration of the α7-nAchR inhibitor MLA resulted in significant increases in the expression of α7-nAChR while the administration of nicotine to sham animals led to a twofold reduction (Fig. 1). By immunofluorescence, we observed strong expression of this receptor in the proximal tubules (Fig. 2B) and at a lower degree in the distal tubules (Fig. 2C). We did not observe significant expression of this receptor subunit in the glomerulus or intrarenal vasculature in any of the groups (Fig. 2D).
Fig. 1.
Cortical expression of the α7-nicotinic acetylcholine receptors (nAChRs). A: representative Western blot for the α7-nAChR and β-actin, which was used to control for loading. B: densitometry data analysis for cortical α7-nAChR expression (n = 6–7 per group; *P < 0.05 vs. Sham). 5/6Nx, 5/6 nephrectomy; Nic, nicotine; MLA, methyllicaconitine; OD, optical density.
Fig. 2.
Cortical localization of the α7-nAChR. A: control slide incubated with primary antibody but without secondary antibody (×20). B: representative photomicrograph demonstrating expression of α7-nAChR in the proximal tubules as identified by Lotus tetragonolobus lectin (×20). C: representative photomicrograph of a slide showing expression of α7-nAChR in the distal tubules as identified by peanut agglutinin (×20). D: representative photomicrograph demonstrating lack of expression of the α7-nAChR in the intrarenal vasculature (×20).
Hemodynamic and metabolic effects of nicotine.
The administration of nicotine in the drinking water at the concentrations used for these studies resulted in serum concentrations of cotinine, a stable metabolite of nicotine, similar to those found in the plasma of active smokers (16; Table 1). The serum levels of cotinine in rats with 5/6Nx were, however, slightly higher compared with those obtained in sham animals. The urinary excretion of cotinine was lower in rats with 5/6Nx compared with sham rats, indicating impaired cotinine excretion in rats with reduced renal function. The administration of nicotine to either sham or 5/6Nx rats did not result in significant changes in final body weight (Table 1). However, the administration of MLA to 5/6Nx rats resulted in lower final weights especially in rats concomitantly receiving nicotine (Table 1).
Table 1.
Weight, systolic blood pressure, and serum and urinary cotinine
| Sham | 5/6Nx | 5/6Nx + Nic | 5/6Nx + Nic + MLA | Sham + Nic | Sham + Nic + MLA | 5/6Nx + MLA | |
|---|---|---|---|---|---|---|---|
| Weight at death, g | 431.5 ± 30.0 | 424.3 ± 23.1 | 407.4 ± 20.0 | 327.3.1 ± 12.8*‡ | 398.4 ± 17.0 | 468.4 ± 9.0 | 368.6 ± 11.3*† |
| Systolic blood pressure, mmHg | 135 ± 8.6 | 151 ± 7.1 | 159 ± 7.8* | 148 ± 5.4 | 150 ± 6.8 | 132 ± 11.0‡ | 151 ± 7.1 |
| Serum cotinine, ng/ml | Nondetectable | Nondetectable | 67.2 ± 2.7 | 73.3 ± 2.0 | 55.4 ± 1.0§ | 54.4 ± 0.8§ | Nondetectable |
| Urinary cotinine, μg/day | Nondetectable | Nondetectable | 285.5 ± 31 | 228.6 ± 23 | 719.4 ± 267§ | 532.8 ± 124§ | Nondetectable |
Values are means ± SE.
P < 0.05 vs. Sham;
P < 0.05 vs. 5/6 nephrectomy (5/6Nx);
P < 0.05 vs. 5/6Nx + nicotine (Nic);
P < 0.05 vs. 5/6Nx + Nic and 5/6Nx + Nic + methyllicaconitine (MLA).
Rats with 5/6Nx had a small and nonsignificant increase in systolic blood pressure compared with sham rats. The administration of nicotine to either sham or 5/6Nx rats resulted in further increases in blood pressure (Table 1). The administration of the α7-nAChR receptor blocker MLA induced a small reduction in blood pressure in sham or 5/6Nx rats on nicotine but not in rats on tap water (Table 1).
Effects of nicotine on proteinuria and renal injury.
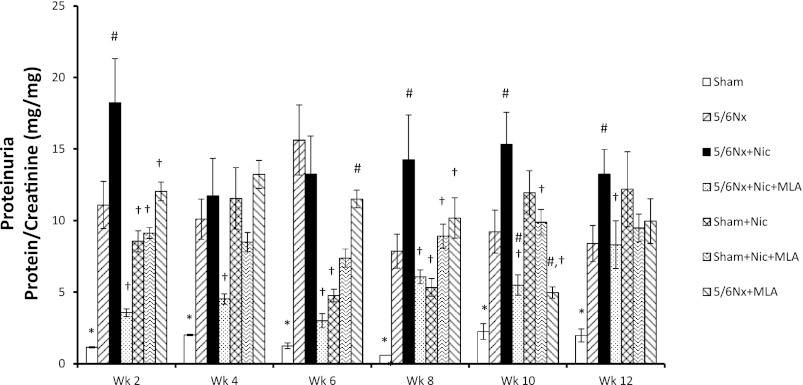
As previously described by others, 5/6Nx resulted in significant increases in urinary protein excretion compared with sham rats (40). The administration of nicotine to 5/6Nx rats induced further and significant increases in proteinuria as expressed as protein/creatinine ratio (Fig. 3), which were evident as early as 2 wk after 5/6Nx. Blockade of the α7-nAchR with MLA in rats with 5/6Nx and receiving nicotine resulted in significantly lower urinary protein excretions. Interestingly, the administration of nicotine to sham rats also resulted in significant increases in urinary protein excretion that were more evident in the last 2 wk of the experimental protocol (Fig. 3). As shown in Table 2, these effects of nicotine on protein/creatinine ratios were not due to changes in the urinary excretion of creatinine. In addition, we measured albumin excretion in the last urine collection. As shown in Table 2, the urinary excretion of albumin was increased in 5/6Nx rats, further increased by the administration of nicotine and reduced by MLA. However, and in contrast with the measurements of total protein excretion, the administration of nicotine to sham rats did not modify the urinary excretion of albumin, indicating that the proteinuria observed in these rats was tubular in origin. In light of these results, we performed additional experiments in two extra groups: Sham + Nicotine + MLA and 5/6Nx + MLA. As shown in Table 2, the administration of MLA did not modify the urinary excretion of either total protein or albumin in Sham rats on nicotine or 5/6Nx rats on tap water.
Fig. 3.
Urinary protein excretion. Urinary protein excretion at baseline and during the 12 wk following surgery. Urine was collected biweekly, and protein excretion was adjusted against urinary creatinine excretion (*P < 0.05 vs. all other groups; #P < 0.05 vs. 5/6Nx; †P < 0.05 vs. 5/6Nx + Nic).
Table 2.
Serum creatinine, creatinine clearance, and urinary creatinine, protein excretion, and albumin
| Sham | 5/6Nx | 5/6Nx + Nic | 5/6Nx + Nic + MLA | Sham + Nic | Sham + Nic + MLA | 5/6Nx + MLA | |
|---|---|---|---|---|---|---|---|
| Serum creatinine, mg/dl | 0.26 ± 0.01 | 0.62 ± 0.19* | 0.51 ± 0.06* | 0.26 ± 0.03†‡ | 0.14 ± 0.02†‡ | 0.32 ± 0.01# | 0.56 ± 0.06* |
| Creatinine clearance, mg·kg−1·min−1 | 7.1 ± 0.9 | 2.7 ± 0.40* | 3.8 ± 0.4* | 10.3 ± 1.0*†‡ | 10.6 ± 2.4*†‡ | 6.2 ± 0.4§ | 2.7 ± 0.4 |
| Urinary creatinine week 12, mg/day | 13.6 ± 1.2 | 7.7 ± 1.8* | 9.6 ± 0.96* | 12.9 ± 1.0# | 9.8 ± 1.8 | 11.3 ± 0.8 | 11.2 ± 1.2 |
| Urinary protein excretion week 12, mg/day | 39.1 ± 11.9 | 122.7 ± 13.6* | 162.3 ± 30.3* | 91 ± 10*#‡ | 101 ± 31*‡ | 131 ± 18* | 123.7 ± 22* |
| Urinary albumin, mg/day | 0.54 ± 0.3 | 3.29 ± 2.0*‡ | 36.67 ± 13* | 13.31 ± 5.6*‡ | 0.1 ± 0.02‡ | 0.65 ± 0.3‡ | 13.5 ± 4.8*‡ |
Values are means ± SE.
P < 0.05 vs. Sham;
P < 0.05 vs. 5/6Nx;
P < 0.05 vs. 5/6Nx + Nic;
P < 0.05 vs. Sham + Nic.
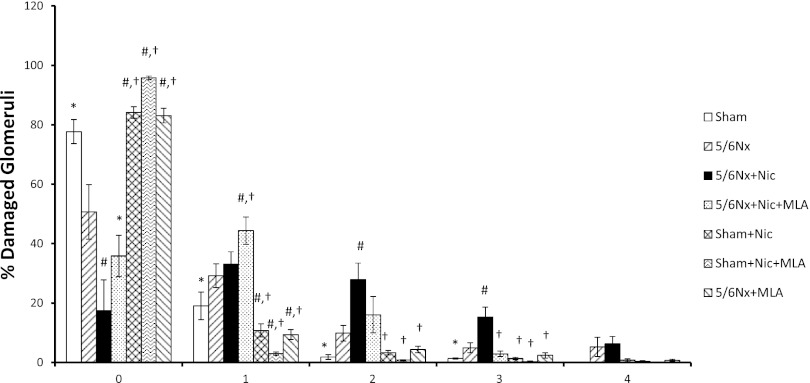
The glomerular injury score was increased in rats with 5/6Nx compared with sham rats and significantly reduced by MLA. As shown in Fig. 4, there were more injured glomeruli with scores of 2+ and 3+ in rats with 5/6Nx+Nic compared with 5/6Nx. The administration of MLA resulted in a significant improvement in glomerular injury as shown by a reduction in the number of glomeruli with 2+ and 3+ scores and an increase in the number of glomeruli with 0+ and 1+ scores. Although the administration of nicotine to sham animals resulted in significant increases in urinary protein excretion, the injury score of these rats was similar to the sham rats. Even though the administration of MLA did not significantly modify the urinary protein excretion in 5/6Nx rats on tap water, it did improve glomerular injury score in these rats (Fig. 4).
Fig. 4.
Glomerular injury score. Glomerular injury score was assessed in all groups and the results presented as percent and severity of damaged glomeruli on a 0+ to 4+ scale where 0 is no damage [*P < 0.05 vs. 5/6Nx, 5/6Nx + Nic, and 5/6Nx + Nic + MLA; #P < 0.05 vs. 5/6Nx; †P < 0.05 vs. 5/6Nx + Nic].
As expected, rats with 5/6Nx had significant increases in serum creatinine that were not further increased by nicotine, in spite of a worse injury score, but were normalized by MLA (Table 2). The administration of nicotine to sham rats resulted in lower serum creatinines. Treatment with MLA in this group normalized serum creatinines but had no effect on 5/6Nx rats on tap water (Table 2). To better assess renal function, we measured creatinine clearance (CrCl) in all groups. As shown in Table 2, 5/6Nx rats had lower CrCl that was not significantly modified by nicotine. In 5/6Nx rats on nicotine, treatment with MLA resulted in significantly higher CrCl but had no effect on CrCl in 5/6Nx rats on tap water. Sham rats on nicotine had significantly higher CrCl that were normalized by MLA.
Effects of nicotine on fibronectin expression.
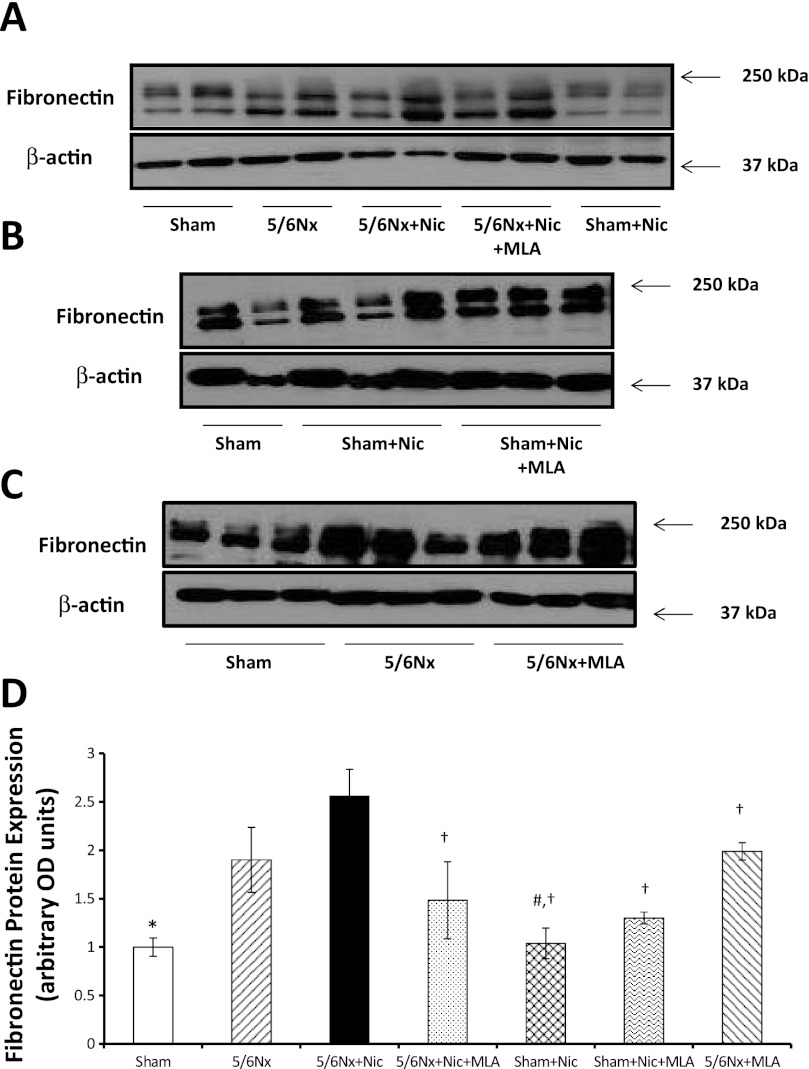
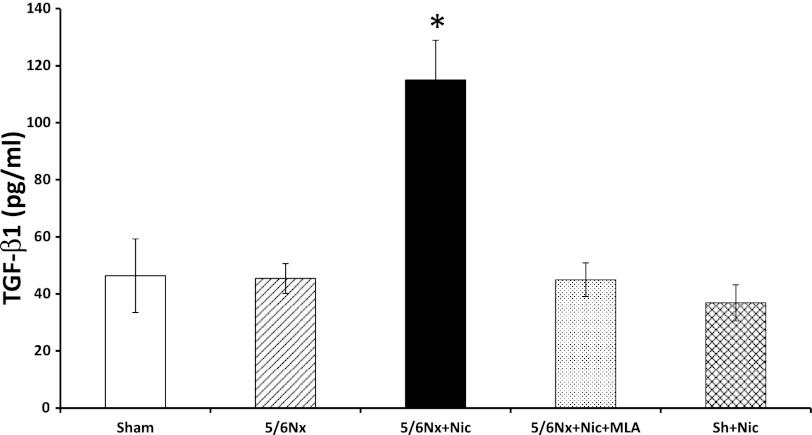
To determine the effects of nicotine on the production of extracellular matrix, we measured fibronectin protein expression in renal cortex homogenates. As shown in Fig. 5A, 5/6Nx resulted in a significant increase in fibronectin expression as assessed by Western blot that was further increased by the administration of nicotine and significantly ameliorated by MLA. In sham rats, the administration of nicotine did not cause significant changes in fibronectin expression (Fig. 5A). In addition, we determined that the administration of MLA had no significant effects on fibronectin expression in sham rats on nicotine (Fig. 5B) or 5/6Nx rats on tap water (Fig. 5C). By immunofluorescence (Fig. 6), we determined that most of the fibronectin expression was interstitial and perivascular although the glomeruli of 5/6Nx + nicotine rats also had increased glomerular expression of fibronectin (Fig. 6D). In addition we measured the cortical expression of TGF-β a well-recognized mediator of fibrosis and extracellular matrix production (42). As shown in Fig. 7, 5/6Nx alone did not result in significant increases in TGF-β expression but the administration of nicotine resulted in a twofold increase in TGF-β that was prevented by MLA. The administration of nicotine to sham rats did not have any effect on cortical TGF-β expression.
Fig. 5.
Cortical fibronectin expression. A, B, and C: representative Western blot for fibronectin and β-actin, which was used to control for loading. D: densitometry data analysis for cortical fibronectin expression (n = 6–7 per group; *P < 0.05 vs. 5/6 Nx and 5/6Nx + Nic; #P < 0.05 vs. 5/6Nx; †P < 0.05 vs. 5/6Nx + Nic).
Fig. 6.
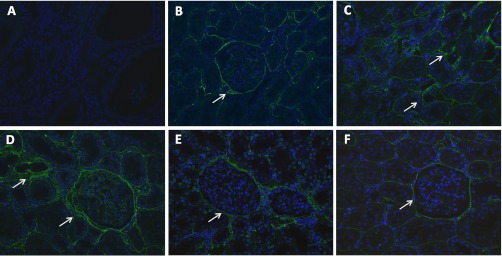
Fibronectin expression by immunofluorescence. A: control negative slide incubated with primary antibody but without secondary antibody (×20). B: representative photomicrograph of a sham-operated rat showing low interstitial expression of fibronectin (×20). C: representative photomicrograph from a 5/6Nx rat showing increased interstitial expression of fibronectin (×20). D: representative photomicrograph from a 5/6Nx + Nic rat showing increased interstitial and glomerular expression of fibronectin (×20). E: representative photomicrograph from a 5/6Nx + Nic + MLA rat showing reduced expression of fibronectin compared with 5/6Nx + Nic (D; ×20). F: representative photomicrograph from a Sham + Nic rat showing no change in the expression of fibronectin (×20).
Fig. 7.
Transforming growth factor (TGF)-β expression in kidney lysates. Administration of nicotine to 5/6Nx rats resulted in a twofold increase in TGF-β as assessed by ELISA in kidney cortex lysates. MLA prevented the increase in TGF-β suggesting that these effects are mediated by the α7-nAChR (*P < 0.05 vs. all other groups).
Effects of nicotine on oxidative stress.
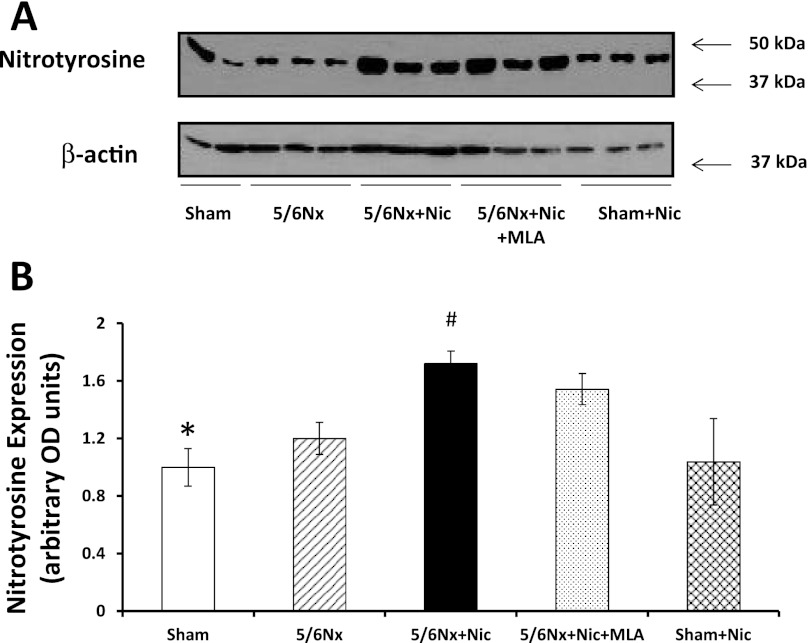
Renal injury in models of CKD such as 5/6Nx is associated with increases in oxidative stress (40). To determine whether nicotine administration results in further oxidative stress, we measured the levels of nitrotyrosine, a well-validated marker of oxidative stress, in the cortex of the different experimental groups. In our studies, 5/6Nx resulted in a small, albeit significant, increase in nitrotyrosine expression as assessed by Western blot (Fig. 8). The administration of nicotine to 5/6Nx rats resulted in further increases in nitrotyrosine expression that, however, was only partially and not significantly reduced by MLA. The administration of nicotine to sham rats did not induce any significant changes in nitrotyrosine.
Fig. 8.
Nitrotyrosine expression. A: representative western blot for nitrotyrosine and β-actin, which was used to control for loading. B: densitometry data analysis for cortical nitrotyrosine expression (n = 6–7 per group; *P < 0.05 vs. 5/6Nx + Nic and 5/6Nx + Nic + MLA; #P < 0.05 vs. 5/6Nx).
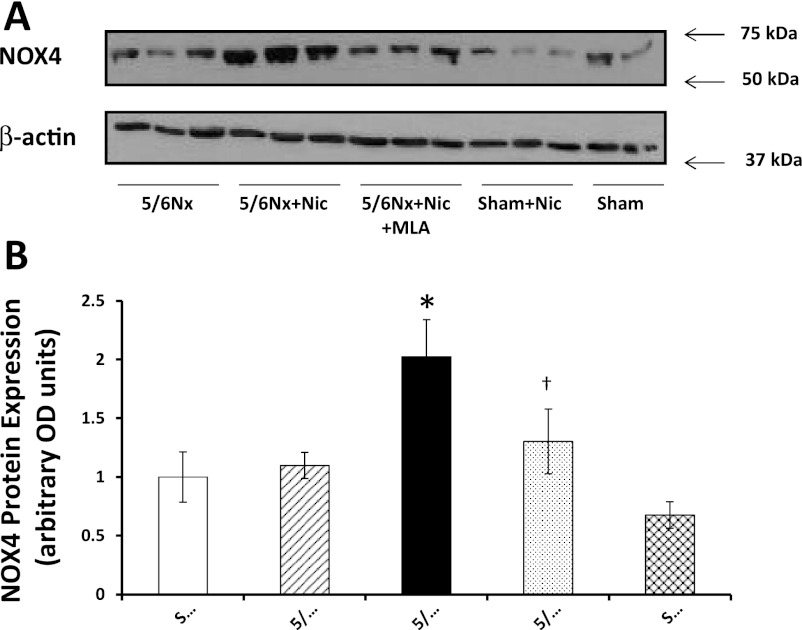
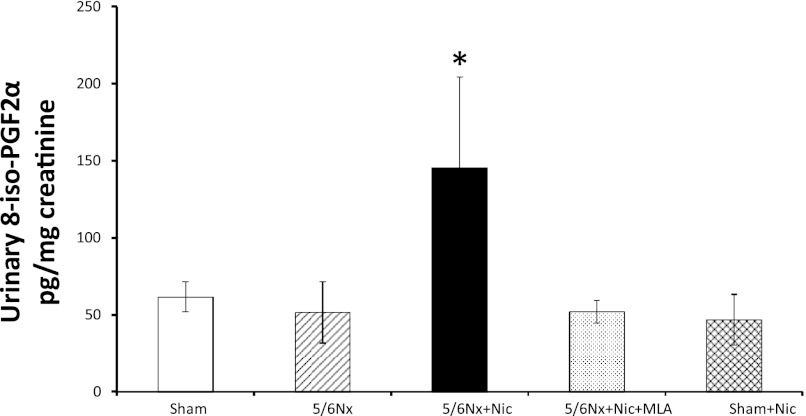
NOX4 is one of the most important sources of reactive oxygen species in the kidney cortex, and its expression and activity are increased in diverse models of kidney injury (12, 18). We observed high basal levels of NOX4 in sham rats, which were not significantly different in rats with 5/6Nx but markedly upregulated by nicotine and normalized by α7nAChR blockade (Fig. 9). The administration of nicotine to sham rats did not modify the cortical expression of NOX4. In addition, we measured the urinary excretion of F2-isoprostanes as markers of oxidative stress. The 5/6Nx did not result in a significant increase in the urinary excretion of isoprostanes; however, the administration of nicotine resulted in large increases in urinary isoprostanes that were normalized by MLA (Fig. 10). The administration of nicotine to sham-operated animals had no effect on the urinary excretion of isoprostanes.
Fig. 9.
NADPH oxidase 4 (NOX4) expression. A: representative Western blot for NOX4 and β-actin, which was used to control for loading. B: densitometry data analysis for cortical NOX4 expression (n = 6–7 per group; *P < 0.05 vs. Sham, 5/6Nx, and 5/6Nx + Nic; †P < 0.05 vs. 5/6Nx + Nic).
Fig. 10.
Urinary isoprostanes. Administration of nicotine to 5/6Nx rats resulted in a twofold increase in TGF-β as assessed by ELISA in kidney cortex lysates. MLA prevented the increase in TGF-β, suggesting that these effects are mediated by the α7-nAChR (*P < 0.05 vs. all other groups).
DISCUSSION
Epidemiologic studies suggest that cigarette smoking accelerates the rate of progression of CKD of diverse etiologies including diabetes mellitus (26, 35), hypertension (2, 17), lupus nephritis (41), polycystic kidney disease, IgA nephropathy, and postkidney transplantation (27). Moreover, in experimental studies we and others have demonstrated that the exposure to environmental tobacco smoke worsens renal injury in mouse models of renal injury such as diabetic nephropathy (5) and aging (30). However, and in spite of the clinical and experimental evidence the mechanisms responsible for these effects have not been identified.
Nicotine is one of the biologically active and stable compounds present in large concentrations in tobacco that can be acquired through active and passive smoking (6). In addition to its addictive properties, nicotine promotes atherosclerosis and angiogenesis (14) and as we have previously demonstrated nicotine worsens glomerular injury in the anti-Thy1 model of acute proliferative glomerulonephritis (21) and worsens proteinuria and extracellular matrix expansion in db/db mice, a well-known model of diabetic nephropathy (36). In the current studies, we have demonstrated that activation of the α7-nAChR is responsible in large part for the harmful effects of nicotine in renal injury. In these studies, we utilized a model of renal ablation (5/6Nx) that closely mimics CKD in humans and is characterized by progressive decline in renal function, glomerulosclerosis, interstitial fibrosis, and proteinuria (40).
We determined that the administration of nicotine in the drinking water and at concentrations that result in serum levels similar to those found in the plasma of smokers (16), resulted in increased proteinuria, increased glomerular injury and interstitial fibronectin and TGF-β production, and increased oxidative stress in rats with 5/6Nx. The administration of nicotine to sham and 5/6Nx rats resulted in small increases in blood pressure as assessed by the tail-cuff method. Although these increases in blood pressure were small, we cannot rule out that some of the effects of nicotine on renal injury are at least in part mediated by changes in blood pressure.
Moreover, and although the administration of nicotine worsened renal injury, it did not result in a significant change in renal function as assessed by either serum creatinine or by CrCl. Of interest, the administration of nicotine to sham rats resulted in a significant increase in CrCl, which was prevented by MLA. These results suggest that nicotine may have hemodynamic effects on the glomerular microcirculation that result in glomerular hyperfiltration. Indeed, large epidemiological studies (13) have shown that smokers have a higher glomerular filtration rate and an elevated risk for proteinuria compared with nonsmokers. We hypothesize that the chronic administration of nicotine is associated with abnormal autoregulation of the glomerular microcirculation and that the resultant hyperfiltration may be playing a role in the accelerated progression of renal injury. In 5/6Nx rats receiving nicotine and treated with MLA, we also observed significant increases in glomerular filtration rate. These rats had significantly better injury scores, proteinuria, and fibronectin but also significantly lower body weights, which could potentially skew the determination of CrCl.
The nAChRs receptors are a family of ligand-gated pentameric ion channels. In humans 16 different subunits (α1–7, α9–10, β1–4, δ, ε, and γ) have been identified that form a large number of homo- and heteropentameric receptors with distinct structural and pharmacological properties (8). Several studies have highlighted the particular importance of the α7-nAchR subunit, which can form homomeric receptors or be part of heteromeric receptors (8) and is required for several of the biological effects nicotine (10, 34). In our current studies, we observed strong expression of the α7-nAchR in proximal tubules and at some degree in distal tubules. Although α7-nAChR expression has been described in the systemic vasculature (14, 15), we did not observe α7-nAChR expression in the intrarenal vasculature suggesting regional differences in the vascular expression of the α7-nAChR. We (20) have previously shown that cultured human mesangial cells express several nAChRs subunits including the α7-nAchR, and that nicotine promotes mesangial cell proliferation and fibronectin production in these cells. In the current studies, however, we did not detect glomerular expression of α7-nAchR expression, suggesting differences in expression according to the species or alternatively that the in vitro cell culture conditions favor the expression of this receptor subunit.
In our current studies, we utilized the compound MLA to block the α7-nAchR. MLA binds potently (KD around 2 nM) to α-bungarotoxin-binding sites (9) and has been classified as a competitive antagonist of α7-containing nAChRs (23). For these studies, we utilized similar concentrations as reported by others that result in specific blockade of the α7-nAChR and without any evident toxicity (38). In our studies, the administration of this inhibitor was well tolerated, did not result in significant changes in blood pressure, and resulted in significant reductions in proteinuria, glomerular injury score, fibronectin expression, and TGF-β production. These results therefore suggest that activation of the α7-nAchR plays a major role as mediator of the deleterious effects of nicotine in this model of CKD. Although we observed predominantly tubular expression of the α7-nAChR, blockade of the α7-nAChR with MLA reduced both glomerular injury as well as interstitial fibronectin expression. Based on these results and the experimental evidence supporting the role for the proximal tubules as a source of profibrotic and proinflammatory cytokines (11), we hypothesize that tubular activation of the α7-nAChR might results in increased production of these mediators resulting in increased glomerular and interstitial injury. The administration of MLA, however, especially to 5/6Nx rats on nicotine, resulted in lower body weights. We hypothesize that in these rats MLA may be having an effect on metabolic rate and/or caloric intake and will be investigated in future studies in our laboratory.
Renal ablation alone was associated with reduced α7-nAChR expression as assessed by Western blot. Although the mechanisms for this reduction are unclear, we speculate that they may be related, at least in part, to the reduction in total tubular mass a result of the surgical ablation of kidney mass. The administration of nicotine to sham rats was also associated with reductions in the expression of the α7-nAChR, while the administration of MLA increased its expression, suggesting a negative feedback by nicotine on the expression of this receptor subunit.
In previous studies, we (18) determined that the administration of nicotine to wild type C57BL/6 mice had no significant effects on albuminuria or renal injury. In the current studies, the administration of nicotine for 12 wk to sham-operated rats, resulted in significant increases in urinary protein excretion without concomitant changes in glomerular injury score, fibronectin, or TGF-β expression. In light of these results, we measured urinary albumin excretion in the last urine collection from all groups. As shown in Table 2, 5/6Nx resulted in a 10-fold increase in urinary albumin excretion that was further increased 10-fold by the administration of nicotine. However, the administration of nicotine to sham rats did not modify the urinary excretion of albumin. Given the strong expression of the α7-nAChR in the proximal tubules and the role of the proximal tubule in megalin-mediated protein reabsorption (25), we hypothesize that the administration of nicotine may be affecting the tubular reabsorption of filtered proteins (i.e., via megalin) resulting in increases in low-molecular weight proteinuria but not albuminuria in sham animals and at the same contributing in part to the increases in protein excretion and albuminuria in 5/6Nx rats. Interestingly, the administration of MLA did not prevent the increases in proteinuria induced by nicotine in sham animals, suggesting that the α7-nAChR does not mediate these effects. The study of the potential mechanisms involved is the subject of ongoing studies in our laboratory.
NOX is considered to be the most important source of reactive oxygen species in the kidney. Three different NOX isoforms are expressed in the kidney cortex (NOX4, NOX2, and NOX1). Of these, NOX4 is the most abundant NOX in the kidney (1). Induction of NOX4 mRNA expression is observed in response to inflammation (28), endoplasmic reticulum stress (29), diabetes mellitus (12) and activation of the renin angiotensin system (19). In our studies the administration of nicotine increased oxidative stress in 5/6Nx as demonstrated by increases in NOX4 expression and nitrotyrosine expression and urinary isoprostanes. The increases in NOX4 and urinary isoprostanes induced by nicotine in 5/6Nx rats were significantly reduced by α7-nAChr blockade; however, nitrotyrosine was only partially and nonsignificantly reduced suggesting the presence of other NOX4-independent mechanisms that lead to increased oxidative stress in these rats.
In conclusion, in these studies, we have identified the role of the a7-nAChR as an essential mediator of the effects of nicotine in renal injury. The administration of nicotine to rats with 5/6Nx resulted in increased proteinuria and a worse glomerular injury score. In addition, we determined that these effects are accompanied by increased oxidative stress, increased NOX4 and TGF-β expression. Most of the effects of nicotine were reversed by blockade of the α7-nAChR with MLA. These studies unveil novel mechanisms that mediate the deleterious effects of smoking in the progression of CKD and may result in the development of novel therapeutic strategies in the treatment and prevention of CKD in patients unsuccessful in their efforts to quit tobacco smoking.
GRANTS
These studies were funded by a Research Grant from the National Institutes of Health (R01-ES-014948 to E. A. Jaimes) and (P30-DK-079337 to G. P. Siegal) and a Clinical Innovator Grant from the Flight Attendant Research Institute (to E. A. Jaimes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.R., P.C., W.F., P.H., and G.S. performed experiments; G.R., P.C., G.S., and E.A.J. analyzed data; G.R. and P.C. prepared figures; G.R. and E.A.J. drafted manuscript; G.R., P.C., W.F., P.H., G.S., and E.A.J. approved final version of manuscript; G.S. and E.A.J. edited and revised manuscript; E.A.J. conception and design of research; E.A.J. interpreted results of experiments.
REFERENCES
- 1. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG. Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int 57: 2072–2079, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Boyle P. Cancer, cigarette smoking and premature death in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer 17: 1–60, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Cachat F, Meagher-Villemure K, Guignard JP. Lymphomatoid granulomatosis in a renal transplant patient. Pediatr Nephrol 18: 838–842, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Carmona R. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, 2006, p. iii. [PubMed] [Google Scholar]
- 6. Celermajer D, Adams M, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield J. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, Raggenbass M, Feuerbach D, Bertrand D, Fuhrer C. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J Neurosci 25: 9836–9849, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen CY, Chow D, Chiamvimonvat N, Glatter KA, Li N, He Y, Pinkerton KE, Bonham AC. Short-term secondhand smoke exposure decreases heart rate variability and increases arrhythmia susceptibility in mice. Am J Physiol Heart Circ Physiol 295: H632–H639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest 116: 2208–2217, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Halimi JM, Giraudeau B, Vol S, Caces E, Nivet H, Lebranchu Y, Tichet J. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int 58: 1285–1292, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 7: 833–839, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest 110: 527–536, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heinrich J, Holscher B, Seiwert M, Carty CL, Merkel G, Schulz C. Nicotine and cotinine in adults' urine: The German Environmental Survey 1998. J Expo Anal Environ Epidemiol 15: 74–80, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Horner D, Fliser D, Klimm HP, Ritz E. Albuminuria in normotensive and hypertensive individuals attending offices of general practitioners. J Hypertens 14: 655–660, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Hua P, Feng W, Ji S, Raij L, Jaimes EA. Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol 299: F732–F739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–232, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Jaimes E, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 292: H76–H82, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Jaimes EA, Tian RX, Joshi M, Raij L. Nicotine worsens renal injury in nephritis. Am J Nephrol 29: 319–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int 68: 2143–2153, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther 287: 435–439, 1998 [PubMed] [Google Scholar]
- 25. Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, Ichikawa I. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74: 1262–1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orth SR. Smoking–a renal risk factor. Nephron 86: 12–26, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Orth SR, Viedt C, Ritz E. Adverse effects of smoking in the renal patient. Tohoku J Exp Med 194: 1–15, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prevention CfDCa National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 31. Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci 686: 12–27; discussion 27–18, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med 79: 37–41, 1985 [DOI] [PubMed] [Google Scholar]
- 33. Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol 179: 2889–2898, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J 18: 1436–1438, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 25: 859–864, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 284: F1138–F1144, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Shimamura T, Morrison AB. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol 79: 95–106, 1975 [PMC free article] [PubMed] [Google Scholar]
- 38. Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci 27: 5615–5620, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stedman R. The chemical composition of tobacco and tobacco smoke. Chem Rev 68: 153–207, 1968 [DOI] [PubMed] [Google Scholar]
- 40. Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol 292: F1404–F1410, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med 152: 2082–2088, 1992 [PubMed] [Google Scholar]
- 42. Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]