Abstract
Mechanical stimulation caused by increasing flow induces nucleotide release from many cells. Luminal flow and extracellular ATP stimulate production of nitric oxide (NO) in thick ascending limbs. However, the factors that mediate flow-induced NO production are unknown. We hypothesized that luminal flow stimulates thick ascending limb NO production via ATP. We measured NO in isolated, perfused rat thick ascending limbs using the fluorescent dye DAF FM. The rate of increase in dye fluorescence reflects NO accumulation. Increasing luminal flow from 0 to 20 nl/min stimulated NO production from 17 ± 16 to 130 ± 37 arbitrary units (AU)/min (P < 0.02). Increasing flow from 0 to 20 nl/min raised ATP release from 4 ± 1 to 21 ± 6 AU/min (P < 0.04). Hexokinase (10 U/ml) plus glucose, which consumes ATP, completely prevented the measured increase in ATP. Luminal flow did not increase NO production in the presence of luminal and basolateral hexokinase (10 U/ml). When flow was increased with the ATPase apyrase in both luminal and basolateral solutions (5 U/ml), NO levels did not change significantly. The P2 receptor antagonist suramin (300 μmol/l) reduced flow-induced NO production by 83 ± 25% (P < 0.03) when added to both and basolateral sides. Luminal hexokinase decreased flow-induced NO production from 205.6 ± 85.6 to 36.6 ± 118.6 AU/min (P < 0.02). Basolateral hexokinase also reduced flow-induced NO production. The P2X receptor-selective antagonist NF023 (200 μmol/l) prevented flow-induced NO production when added to the basolateral side but not the luminal side. We conclude that ATP mediates flow-induced NO production in the thick ascending limb likely via activation of P2Y receptors in the luminal and P2X receptors in the basolateral membrane.
Keywords: mechanosensation, nucleotides, purinergic receptors, nitric oxide synthase
nitric oxide (no) helps regulate kidney function and therefore blood pressure. It increases renal blood flow (28, 34) and glomerular filtration rate (10, 48) and inhibits salt and water reabsorption along the nephron (8, 39, 61), thereby inducing natriuresis and diuresis. NO can be synthesized by several different types of cells in the kidney, among them vascular endothelial (21, 27), the macula densa (29, 65), and the epithelial cells of the nephron (53, 64, 67) including the thick ascending limb of the loop of Henle (16, 17, 43).
The thick ascending limb reabsorbs 20–30% of the filtered load of NaCl, creating the osmotic gradient required for water reabsorption from the cortical collecting duct (4). In thick ascending limb cells, NO produced by NOS 3 (also known as endothelial nitric oxide synthase) acts as an autacoid to inhibit net NaCl (41). Several factors can stimulate NO production in this segment, including endothelin 1 (17), angiotensin II (16), α2-adrenergic receptor activation (42), and extracellular ATP (57). We reported that increasing luminal flow also stimulates NO production in thick ascending limbs (5, 40); however, the factors that mediate flow-induced NO production remain unknown.
ATP is a paracrine/autocrine factor found in the lumen and interstitial space of the nephron (35, 63) which helps regulate several aspects of renal function, including tubuloglomerular feedback (19, 51) and epithelial transport (24, 47, 52, 59). These effects are mediated by two categories of transmembrane purinergic (P2) receptors: P2X and P2Y (20, 62). ATP can be released by many cells in response to different stimuli (9, 23, 26, 58). Similar to endothelial cells, cells of the thick ascending limb release ATP in response to increased luminal flow (22). ATP stimulates NO production in thick ascending limb cells (57, 59). However, it is not known whether ATP mediates flow-induced NO production in thick ascending limbs.
We hypothesized that ATP mediates flow-induced NO production in the thick ascending limb, and our studies demonstrated that 1) increasing luminal flow stimulates ATP release and 2) this ATP mediates flow-induced NO production in the thick ascending limb.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats, weighing 100–150 g (Charles River Breeding Laboratories, Wilmington, MA), were fed a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for a minimum of 5 days before the experiments. All protocols were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Chemicals and solutions.
4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF FM-DA) was purchased from Invitrogen (Eugene, OR). l-Arginine, hexokinase from Saccharomyces cerevisiae, apyrase from potato, NF023, and suramin sodium salt were obtained from Sigma-Aldrich (St. Louis, MO). The physiological saline used to perfuse and bathe the tubules contained (in mmol/l) 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 6 l-alanine, 0.1 l-arginine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C.
The solution we used to dilute the luciferin-luciferase assay mixture when assessing ATP bioluminescence contained (in mmol/l) 50 NaCl, 4 KCl, 2.5 NaH2PO4, 5 MgSO4, 5 glucose, and 10 HEPES, pH 7.4 at 37°C. Mannitol was used to increase the osmolality of the solution. All solutions were adjusted to 290 ± 3 mosmol/kgH2O as measured by freezing-point depression.
Measurement of NO in isolated tubules.
Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip), and medullary thick ascending limbs were isolated and perfused as previously described (5, 11). Briefly, the abdominal cavity was opened and the left kidney was superfused with ice-cold saline, then removed, placed in physiological saline (4°C), and coronal slices were cut. Thick ascending limbs were isolated from the outer medullas under a stereomicroscope at 4–10°C, and tubules ranging from 0.7 to 1.0 mm were transferred to a temperature-regulated chamber and perfused using concentric glass pipettes at 37 ± 1°C. Luminal perfusion rates were 0 or 20 nl/min, and the basolateral flow rate was 0.6 ml/min. Isolated thick ascending limbs were loaded with 4 μmol/l DAF FM-DA for 15 min and washed for 20 min using a dye-free solution. DAF FM was excited at 488 nm, and the emitted fluorescence was measured using a 515-nm long-pass dichroic mirror and a 535/50-nm barrier filter in the regions of interest (ROI). Fluorescence was imaged digitally using an inverted microscope (TE Nikon 2000; Nikon, Japan) with a ×100 immersion oil objective and a Coolsnap HQ digital camera (Photometrics, Tucson, AZ). Data were recorded using Metafluor version 7 imaging software (Universal Imaging, Downington, PA). NO production was measured at the beginning of each period and once every 30 s for 5 min first in the absence of luminal flow and then after an increase in flow to 20 nl/min with physiological saline. In separate experiments, we repeated the procedure but added either hexokinase (10 U/ml) or apyrase (5 U/ml) to both basolateral and luminal solutions.
When suramin, luminal or basolateral hexokinase, and luminal or basolateral NF023 were tested, we first measured NO production for 5 min in the absence of luminal flow and then after increasing flow to 20 nl/min with physiological saline. At this point, flow was stopped and either suramin (300 μmol/l) was added to both luminal and basolateral solutions, hexokinase (10 U/ml) was added to either luminal or basolateral solutions, or NF023 (200 μmol/l) was added to either luminal or basolateral solutions. After 15 min, NO was measured again for 5 min in the absence of luminal flow and then after an increase in flow to 20 nl/min. In all cases, the slope of emitted fluorescence over time was taken as NO production.
In a separate group of experiments, we tested the response to lower flow rates (5 and 10 nl/min). Because these experiments were conducted at a different time, a correction factor for the basal NO production under 0 nl/min was generated.
Measurement of ATP in isolated tubules.
ATP released to the luminal side of isolated and perfused thick ascending limbs was measured using an ATP bioluminescence assay (luciferin-luciferase; Sigma-Aldrich). A 1:3 dilution of the ATP assay mixture was prepared according to the manufacturer's instructions, and emitted light was collected using a photomultiplier tube adapted to the perfusion system. Released ATP was quantified using the area under the curve for 10 min in the absence of luminal flow and 10 min after an increase in luminal flow to 20 nl/min.
Statistical analysis.
Statistical analysis was performed by the Department of Biostatistics and Epidemiology at Henry Ford Hospital. Results are expressed as means ± SE. Paired and unpaired Student's t-tests were used, taking P < 0.05 as significant.
RESULTS
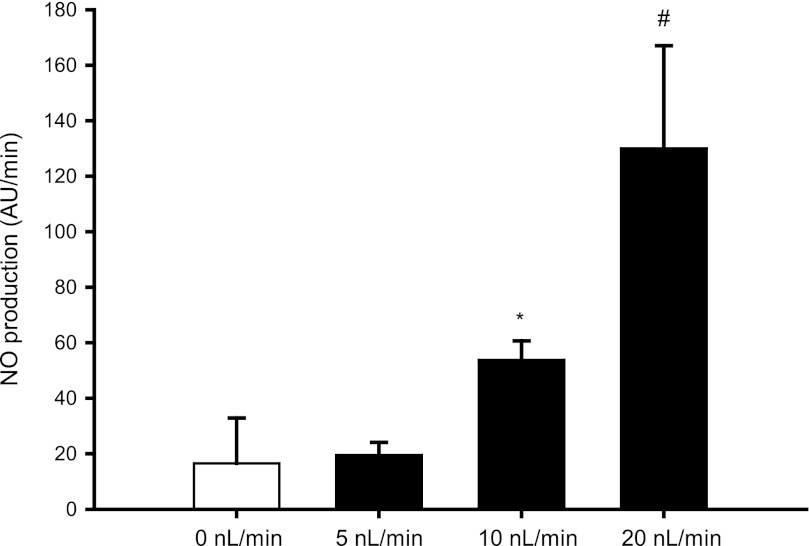
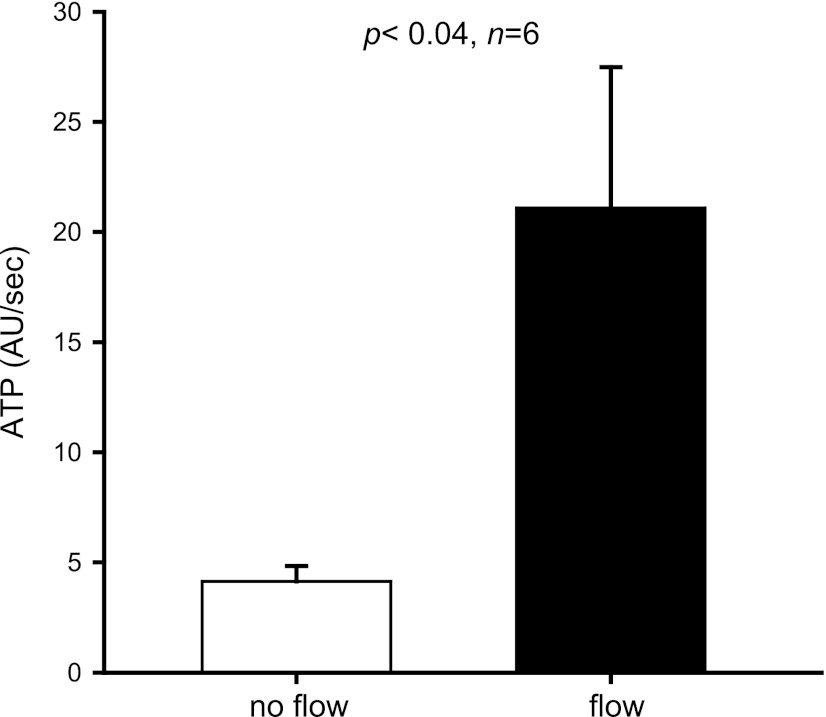
To determine whether ATP mediates flow-induced NO production in medullary thick ascending limbs, we first measured NO production in the absence (0 nl/min) and presence of different luminal flow rates (5, 10, and 20 nl/min). Under 0 and 5 nl/min, NO production was 17 ± 16 and 19 ± 5 arbitrary units (AU)/min, respectively (n = 5–7). After we increased luminal flow to 10 and 20 nl/min, NO rose to 54 ± 7 and 130 ± 37 AU/min, respectively (5 vs. 10 nl/min = P < 0.002; n = 5; 0 vs. 20 nl/min = P < 0.02; n = 7) (Fig. 1), confirming that luminal flow enhances NO production in medullary thick ascending limbs. Next, we tested whether luminal flow enhances ATP release from isolated thick ascending limbs. Increasing luminal flow from 0 to 20 nl/min stimulated luminal ATP release from 4 ± 1 to 21 ± 6 AU/min (P < 0.04; n = 6) (Fig. 2). These experiments indicate that ATP is released from isolated thick ascending limb cells when luminal flow is increased to 20 nl/min.
Fig. 1.
Effect of increasing luminal flow on nitric oxide (NO) production by isolated thick ascending limbs . There was no difference between 0 and 5 nl/min (n = 5). *P < 0.002 compared with 5 nl/min. #P < 0.02 compared with 0 nl/min (n = 5–7).
Fig. 2.
Effect of increasing luminal flow on luminal ATP release by isolated thick ascending limbs (n = 6).
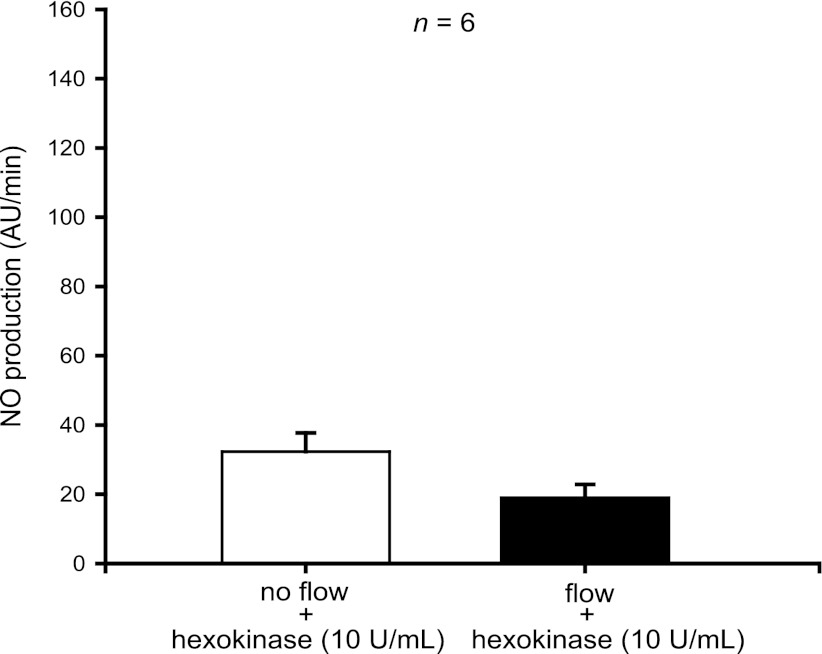
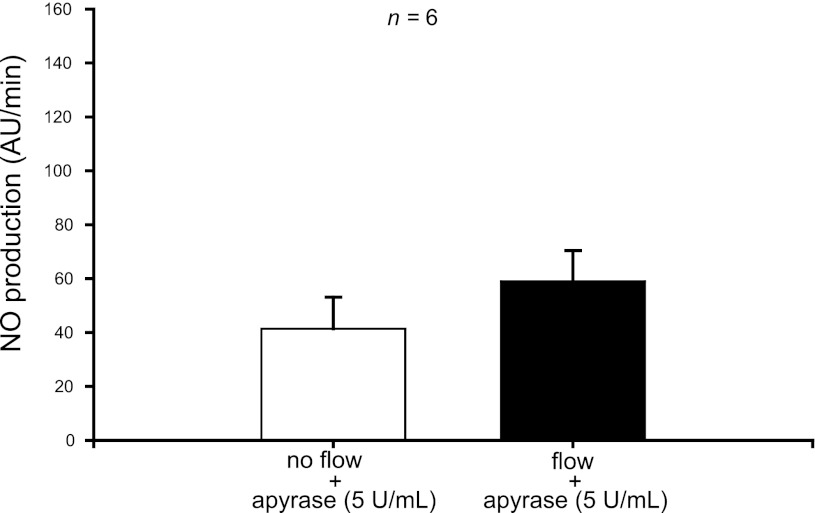
To test the role of ATP in flow-induced NO production, we used enzymes that degrade ATP. When we treated thick ascending limbs with hexokinase (10 U/ml), which degrades ATP in the presence of glucose, increasing luminal flow from 0 to 20 nl/min did not enhance NO production (from 32 ± 5 to 19 ± 4 AU/min; n = 6) (Fig. 3). To make sure the hexokinase was not interfering with the method we used to measure NO, we instead treated thick ascending limbs with the ATPase apyrase (5 U/ml) and once again saw no significant increase in NO with increased flow (from 41 ± 12 to 59 ± 11; n = 6) (Fig. 4). Together, these data suggest that ATP mediates flow-induced NO production.
Fig. 3.
Effect of increasing luminal flow in the presence of hexokinase (10 U/ml) on NO production by isolated thick ascending limbs. Luminal flow did not increase NO production in the presence of hexokinase (n = 6).
Fig. 4.
Effect of increasing luminal flow in the presence of the ATPase apyrase (5 U/ml) on NO production by isolated thick ascending limbs. Luminal flow did not increase NO production significantly in the presence of apyrase (n = 6).
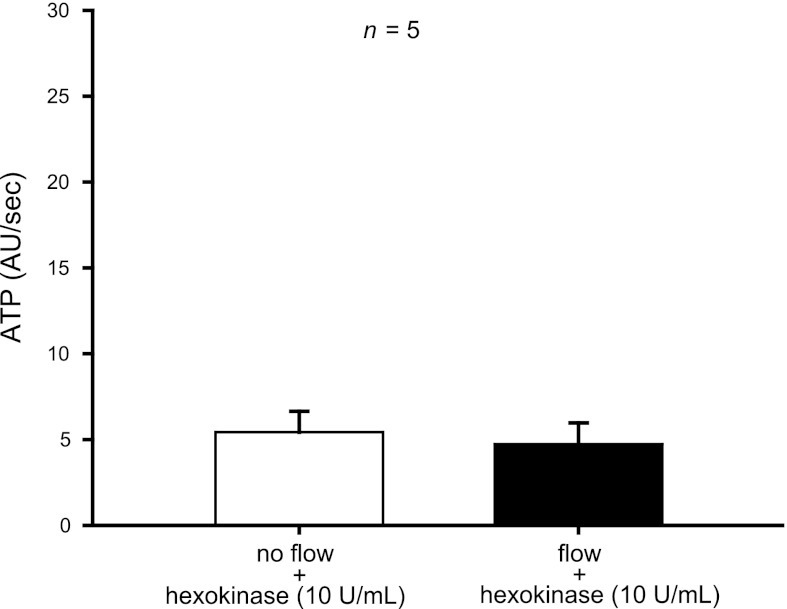
To show that hexokinase blocked flow-induced NO production by degrading ATP, we measured luminal ATP in the presence of hexokinase. The presence of hexokinase (10 U/ml) in the perfusate completely prevented the measured flow-induced increase in ATP (5 ± 1 vs. 5 ± 1 AU/min; n = 5) (Fig. 5).
Fig. 5.
Effect of increasing luminal flow in the presence of hexokinase (10 U/ml) on luminal ATP release by isolated thick ascending limbs. The presence of hexokinase in the perfusate completely prevented the measured flow-induced increase in ATP (n = 5).
Because we added hexokinase simultaneously with the agents used to measure ATP bioluminescence, we needed to be sure it was not interfering with the ATP bioluminescence assay mixture. We therefore generated two standard curves using ATP at 10–80 nM with and without hexokinase in the absence of glucose to avoid ATP degradation. Luminescence was measured using a luminometer (model FB12/Sirius, Zylux, Oak Ridge, TN). We observed no differences between the first and second standard curves. These data indicate that hexokinase itself does not interfere with the ATP bioluminescence assay.
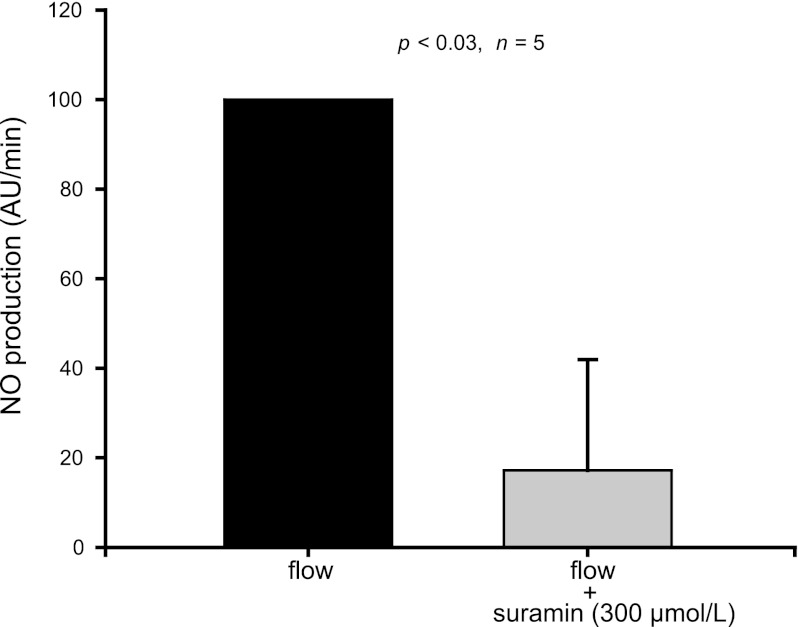
Extracellular ATP stimulates NO production by activating purinergic P2 receptors in other cells. Thus we tested whether these receptors are involved in flow-induced NO production in thick ascending limbs using the P2 receptor antagonist suramin and found that it reduced flow-induced NO production by 83 ± 25% (P < 0.03; n = 5) (Fig. 6). These data suggest that ATP mediates flow-induced NO production by activating purinergic P2 receptors.
Fig. 6.
Effect of the P2 receptor antagonist suramin (300 μmol/l) on flow-induced NO production by isolated thick ascending limbs. Suramin reduced flow-induced NO production by 83 ± 25% (n = 5).
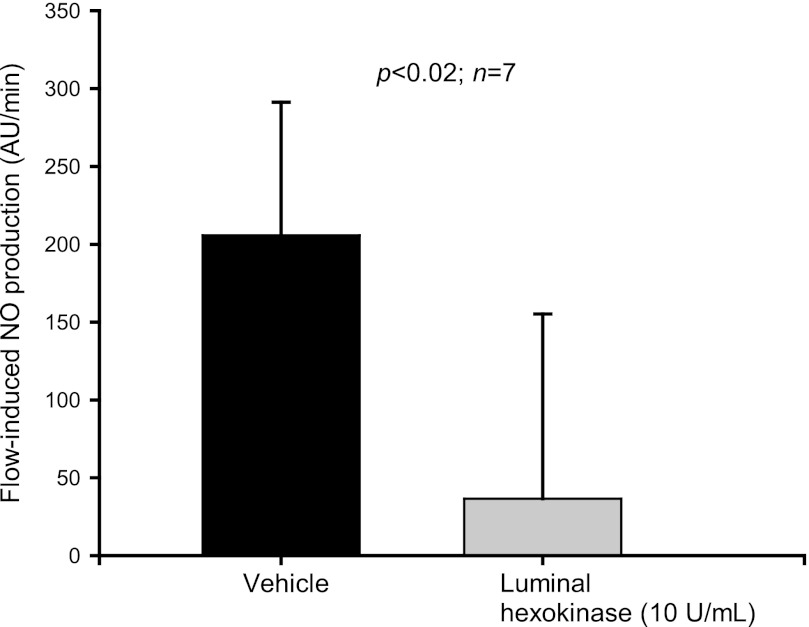
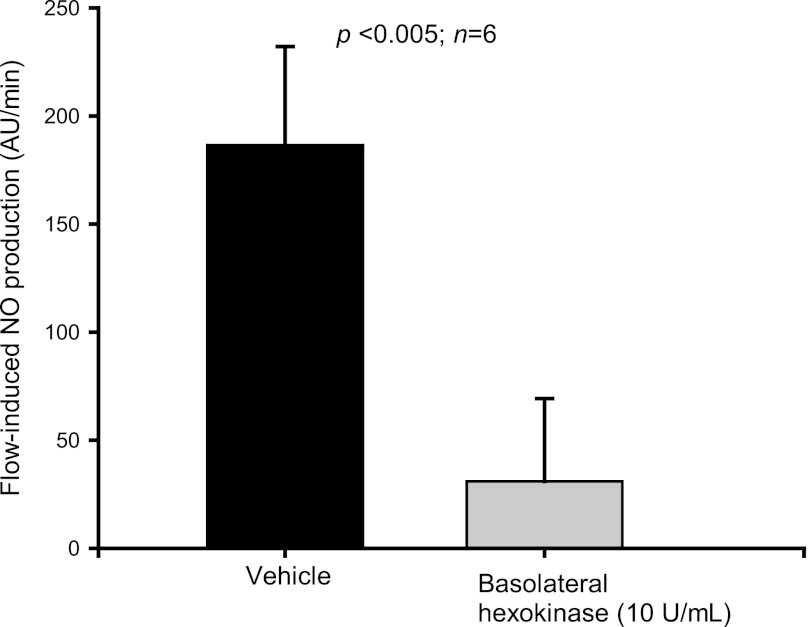
In the previous experiments, all compounds used to degrade or antagonize the effects of ATP were added to both the luminal and basolateral sides at the same time. Therefore, to study whether luminal and/or basolateral receptors mediate this effect, hexokinase (10 U/ml) was added to either the luminal or basolateral side. Luminal hexokinase decreased flow-induced NO production from 205.6 ± 85.6 to 36.6 ± 118.6 AU/min (P < 0.02; n = 7) (Fig. 7). When hexokinase was added only to the basolateral side, flow-induced NO production was reduced from 186.4 ± 45.7 to 30.9 ± 38.4 AU/min (P <0.005; n = 6) (Fig. 8). These findings suggest that both luminal and basolateral ATP mediate flow-induced NO production.
Fig. 7.
Effect of increasing luminal flow in the presence of luminal hexokinase (10 U/ml) on NO production by isolated thick ascending limbs. Luminal hexokinase reduced flow-induced NO production (n = 7).
Fig. 8.
Effect of increasing luminal flow in the presence of basolateral hexokinase (10 U/ml) on NO production by isolated thick ascending limbs. Basolateral hexokinase blunted flow-induced NO production (n = 6).
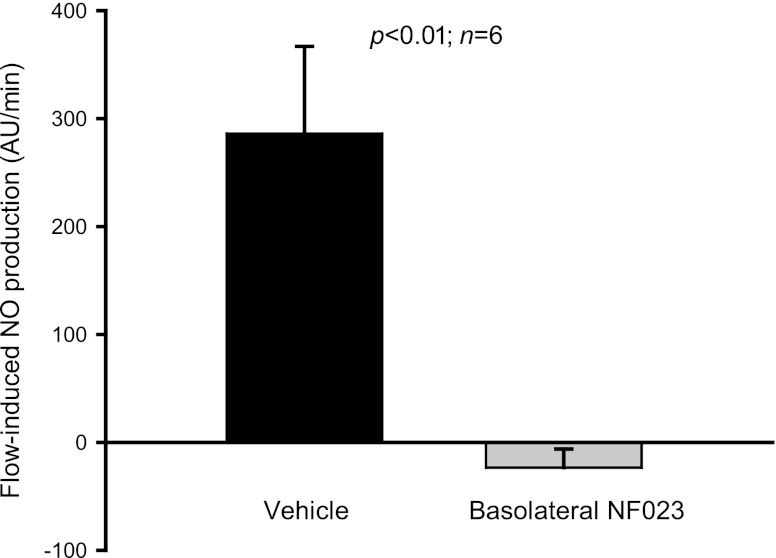
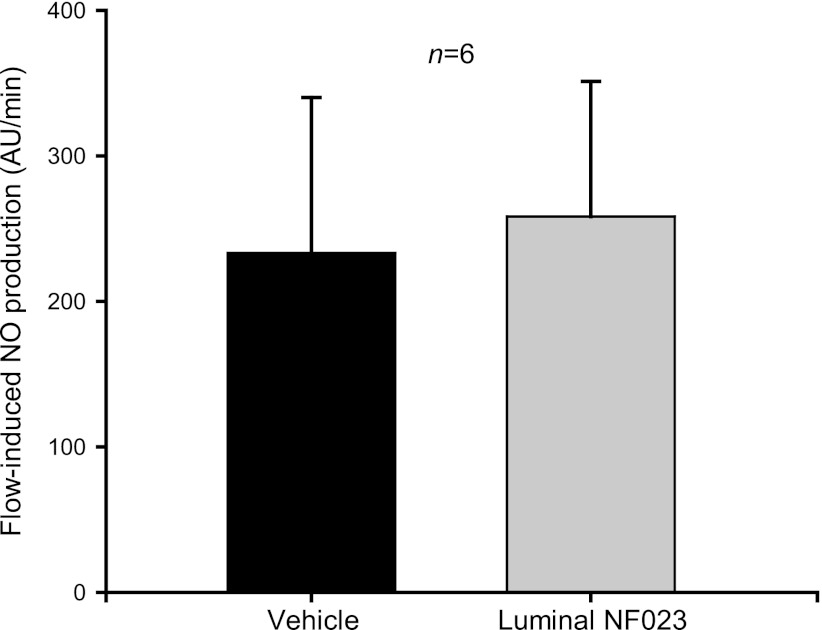
Finally, we studied the P2 receptor subtype responsible for this effect. To our knowledge, there is no selective antagonist for P2Y receptors. Consequently, we used the P2X-selective antagonist NF023 to determine whether a P2X- or P2Y-dependent differential contribution exists. The P2X receptor-selective antagonist NF023 (200 μmol/l) prevented flow-induced NO production when added to the basolateral side (from 286.1 ± 81 to −23.5 ± 17.3 AU/min; P < 0.01; n = 6) (Fig. 9). However, luminal NF023 did not affect flow-induced NO production (from 233.2 ± 106.8 to 258.2 ± 93 AU/min; n = 6) (Fig. 10). These data indicate that ATP mediates flow-induced NO production in the thick ascending limb likely via activation of P2Y receptors at the luminal and P2X receptors at the basolateral side.
Fig. 9.
Effect of increasing luminal flow in the presence of basolateral NF023 (200 μmol/l) on NO production by isolated thick ascending limbs. Basolateral NF023 prevented flow-induced NO production (n = 6).
Fig. 10.
Effect of increasing luminal flow in the presence of luminal NF023 (200 μmol/l) on NO production by isolated thick ascending limbs. Luminal NF023 did not affect flow-induced NO production (n = 6).
DISCUSSION
We previously found that luminal flow (5, 40) and extracellular ATP (57) both stimulate NO production in the thick ascending limb. Based on these findings, we hypothesized that ATP mediates flow-induced NO production in the thick ascending limb. We first found that increasing luminal flow from 0 to 20 nl/min stimulated NO production as we reported before (5, 40). We then tested whether ATP released from thick ascending limbs mediates flow-induced NO production. We found that enhancing luminal flow stimulated luminal ATP release. When combined with our earlier data showing that ATP can stimulate thick ascending limb NO production (57), these results suggest that ATP mediates flow-induced NO production; however, such a conclusion would be based solely on a correlation from separate studies and do not show cause and effect. Consequently, we next tested whether we could block flow-induced NO production either by 1) scavenging ATP or 2) treating the tubules with a purinergic type 2 receptor antagonist at both the luminal and basolateral sides. We found that hexokinase in the presence of glucose rendered flow unable to augment NO production, nor could we detect any flow-induced ATP release into the luminal solution. This was not unique to hexokinase/glucose, as a different ATP scavenger, apyrase, had the same effect. The P2 receptor antagonist suramin prevented flow-induced NO production. The presence of hexokinase in only the luminal or basolateral solution blocked flow-induced NO production. When the P2X-selective antagonist NF023 was present at the basolateral side, flow did not increase NO production. However, luminal NF023 did not affect flow-induced NO production. Thus we concluded that 1) luminal flow enhances ATP release and 2) this ATP mediates flow-induced NO production by the thick ascending limb likely via activation of P2Y receptors at the luminal and P2X receptors at the basolateral side.
Our finding that flow augments thick ascending limb NO production agrees with our earlier reports (5, 40). Furthermore, luminal flow has been shown to increase NO production in inner medullary collecting duct (6) and macula densa cells (68) similar to in thick ascending limbs. Finally, flow also stimulates production of endothelin (30), a key regulator of NO production by renal epithelial cells (17, 55). Together, these data indicate that luminal flow of the forming urine is an important regulator of NO synthesis along the nephron and thus renal function.
In our experiments, raising the luminal flow rate to 20 nl/min in thick ascending limbs enhanced luminal ATP release. Similarly, Jensen et al. (22) reported that luminal and basolateral scavenging of ATP in mouse medullary thick ascending limbs prevented flow-induced increases in intracellular calcium, indicating that flow-induced ATP release is necessary for calcium to increase. In addition, flow has been shown to induce ATP release from other cells. Praetorius et al. (46) showed that increased fluid flow stimulated ATP release in Madin-Darby canine kidney cells, and Sipos et al. (60) showed similar results in isolated, perfused mouse cortical collecting ducts, reporting that increasing flow to 20 nl/min stimulated luminal ATP release (60).
ATP acts primarily via a class of receptors known as purinergic type 2 receptors. P2 receptors are composed of two major subtypes, P2X and P2Y (2, 49), both of which are expressed in the thick ascending limb (56, 62). Here, we found that flow-induced NO production could be blocked by suramin, a nonselective P2 antagonist. We believe these are the first reported data showing that ATP released in response to luminal flow acts via activation of P2 receptors to stimulate NO production in thick ascending limbs. In agreement with our findings, regulation of NO by nucleotides and their receptors has been described in other systems. Exogenous ATP stimulated NO release by aortic (15) and human umbilical vein endothelial cells (50). ATP-mediated vasodilatation could be blocked by the NOS inhibitor NG-nitro-l-arginine methyl ester hydrochloride and P2 receptor antagonists in cochlear capillaries (66), indicating that vasodilatation was due to activation of NO synthase by P2 receptors activation. P2 receptors are also reportedly involved in NO production by microglial cells (12).
Both P2 receptor subtypes are expressed differentially at the basolateral and apical sides of the thick ascending limb plasma membrane (22, 62). Therefore, in theory ATP released in response to flow could bind P2 receptors at both sites. We found that hexokinase, apyrase, and suramin blocked flow-induced NO production when added to both the luminal perfusate and basolateral bath. Furthermore, degrading extracellular ATP using hexokinase in only the luminal perfusate or basolateral bath prevented flow-induced NO production. The P2X-selective antagonist NF023 added only to the basolateral bath also blocked flow-induced NO production. On the other hand, flow-induced NO production was not affected when this compound was added only to the luminal perfusate. Thus we can conclude from these data that P2 receptors present at both sides mediate flow-induced NO production. Even more, these findings likely suggest that P2X receptors in the basolateral and P2Y receptors in the luminal membrane are involved in this effect. Comparable to our results, Jensen et al. (22) showed that degrading ATP either at the luminal or basolateral side prevented flow-induced increases in intracellular calcium. Additionally, they showed that flow-induced increases in intracellular calcium was mediated by bilateral P2Y activation and presumably by activation of basolateral P2X receptors.
It could be argued that increases in luminal flow may directly activate P2 receptors rather than flow-induced release of ATP with subsequent activation of P2 receptors. However, this seems to be unlikely for several reasons: 1) our findings showed that degrading extracellular ATP with apyrase and hexokinase completely blunted flow-induced NO production; 2) Jensen et al. (22) showed that degrading ATP prevented flow-induced increases in intracellular calcium; and 3) there are currently no data showing that flow directly activates P2 receptors.
Detailed information regarding the expression and function of the different subtypes of P2 receptors in the thick ascending limb is lacking. Overall, it has been suggested that the P2Y receptor subtype is functionally expressed in the luminal and basolateral membranes and the P2X subtype only in the latter. However, the functional expression of each subtype could vary in different species (3, 22, 62). In this regard, we previously reported that exogenously added ATP stimulated NO production in rat thick ascending limb suspensions in a dose-dependent manner via activation of P2X receptors (57). However, the P2Y agonist UTP stimulated NO production to a lesser extent. This could be due to limited access of UTP to the luminal side. Very recently, Marques et al. (31) presented evidence indicating that basolateral P2X activation reduces NaCl transport in mouse thick ascending limbs. Similar to those findings, Silva et al. (59) showed that P2X activation reduces the rate of Na-related oxygen consumption in a preparation of rat thick ascending limb suspension. Particularly, it has been shown that rat thick ascending limbs challenged with basolateral nucleotides respond with weaker elevations of intracellular calcium compared with those seen in mouse thick ascending limbs (3, 22).
Previous studies indicate that ATP can be released from the apical and basolateral sides of thick ascending limb cells (22, 35, 36). Based on direct ATP measurements, our results clearly indicate that ATP is released into the luminal perfusate. Even though we were unable to measure ATP release into the basolateral bath due to the nature of the protocol, our findings indicate the involvement of nucleotide release from both membranes and subsequent P2 receptor activation in flow-induced NO production, as suggested before by other authors (22).
Luminal flow in the thick ascending limb varies over a wide range under physiological conditions. Flow through the early distal nephron can drop close to zero or be as high as ≈30 nl/min during volume expansion or induced diuresis (1, 13). In isolated and perfused rat thick ascending limbs, the release of NO displays a linear response up to 50 nl/min (40), and we found here a threshold at 5 nl/min. Therefore, the flow rates we used to evaluate the rate of increase in NO production were within physiological values. However, it could be argued that 5 nl/min as a threshold might represent a limitation of the technique used to measure NO production since at this flow rate concentrations of l-arginine in the micromolar range were demonstrated to inhibit NaCl transport in the thick ascending limb (38). Nevertheless, the results depicted in this report represent changes in the rate of NO production and not absolute levels of NO.
It has been demonstrated that flow through the nephron is oscillatory due to tubuloglomerular feedback, which can alter luminal flow by ≈2.5 nl/min (18). More importantly, luminal flow can be stopped by cyclic peristaltic constrictions of the renal pelvis (7, 54). In a previous report, we have shown in isolated and perfused thick ascending limbs that after 10 min of stopping luminal flow, the rate of NO production decreased to basal levels. NO production then increased when luminal flow was elevated again (5).These findings in addition to data coming from endothelial cells suggest that rapid responses and changes in the rate of NO production can be seen in the presence of acute variations in luminal flow rate. However, further studies will be needed to clarify how these oscillations impact NO synthesis in the thick ascending limb.
We did not directly test which aspect(s) of luminal flow might mediate ATP release by thick ascending limbs: enhanced shear stress, pressure, cellular stretch, and/or ion delivery could be involved. Shear stress (23, 32), stretch (9, 58), and pressure (14, 37) have all been shown to stimulate ATP release in other cells; however, based on our previous results showing that shear stress is the mechanical component involved in flow-induced NO production (5), it most likely also mediates ATP release in the thick ascending limb.
Purinergic signaling along the nephron has been recognized as a physiological regulator of sodium and water homeostasis (47, 52). We reported that extracellular ATP inhibits transport in medullary thick ascending limbs via a mechanism dependent on NO production (59). In the distal nephron, ATP decreases ENaC activity (44, 45). Also, it has been shown that the basolateral P2Y2 receptors in the collecting duct block AVP-stimulated water transport (24, 25). In addition, Mironova et al. (33) reported in C57BL/6J mice that increased urinary flow due to a high-salt diet was positively correlated to urinary ATP excretion.
We believe this is the first report showing that ATP mediates NO production in response to increased luminal flow in the thick ascending limb. Understanding the effect of changes in luminal flow on these important regulators of sodium and water handling could be important in physiopathological conditions such as hypertension or a high-salt diet in which luminal flow through the nephron is increased.
GRANTS
This work was supported in part by grants to J. L. Garvin from the National Heart, Lung and Blood Institute of the National Institutes of Health (HL 070985; HL 090550-Project 1 and HL 028982-Project 5) and to P. D. Cabral from the American Heart Association (11POST7490010).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.D.C. and J.L.G. provided conception and design of research; P.D.C., N.J.H., and J.L.G. performed experiments; P.D.C. and J.L.G. analyzed data; P.D.C., N.J.H., and J.L.G. interpreted results of experiments; P.D.C. and J.L.G. prepared figures; P.D.C. and J.L.G. drafted manuscript; P.D.C. and J.L.G. edited and revised manuscript; P.D.C., N.J.H., and J.L.G. approved final version of manuscript.
REFERENCES
- 1. Arendshorst WJ, Beierwaltes WH. Renal tubular reabsorption in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 237: F38–F47, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst 81: 264–270, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Burg MB. Thick ascending limb of Henle's loop. Kidney Int 22: 454–464, 1982 [DOI] [PubMed] [Google Scholar]
- 5. Cabral PD, Hong NJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185–F1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci 18: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Eitle E, Hiranyachattada S, Wang H, Harris PJ. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am J Physiol Cell Physiol 274: C1075–C1080, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Feranchak AP, Roman RM, Schwiebert EM, Fitz JG. Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. J Biol Chem 273: 14906–14911, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Gabbai FB, Blantz RC. Role of nitric oxide in renal hemodynamics. Semin Nephrol 19: 242–250, 1999 [PubMed] [Google Scholar]
- 11. Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, Gonzalez FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem 87: 344–352, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979 [DOI] [PubMed] [Google Scholar]
- 14. Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum 43: 1571–1579, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Guns PJ, Korda A, Crauwels HM, Van AT, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol 146: 288–295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT2 receptors and Akt1-dependent nitric-oxide synthase 3 (NOS3) activation. J Biol Chem 285: 14932–14940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1448–F1459, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280: F927–F944, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol Renal Physiol 274: F718–F727, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Ito S, Carretero OA, Abe K. Role of nitric oxide in the control of glomerular microcirculation. Clin Exp Pharmacol Physiol 24: 578–581, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 23. John K, Barakat AI. Modulation of ATP/ADP concentration at the endothelial surface by shear stress: effect of flow-induced ATP release. Ann Biomed Eng 29: 740–751, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054–F1058, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Lahera V, Salom MG, Fiksen-Olsen MJ, Raij L, Romero JC. Effects of NG-monomethyl-l-arginine and l-arginine on acetylcholine renal response. Hypertension 15: 659–663, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J. Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL). Am J Physiol Renal Physiol 302: F487–F494, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Milner P, Bodin P, Loesch A, Burnstock G. Increased shear stress leads to differential release of endothelin and ATP from isolated endothelial cells from 4- and 12-month-old male rabbit aorta. J Vasc Res 29: 420–425, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naess PA, Kirkeboen KA, Christensen G, Kiil F. Inhibition of renal nitric oxide synthesis with NG-monomethyl-l-arginine and NG-nitro-l-arginine. Am J Physiol Renal Fluid Electrolyte Physiol 262: F939–F942, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Nishiyama A, Majid DS, Walker M, III, Miyatake A, Navar LG. Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37: 753–759, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. Am J Physiol Renal Physiol 297: F341–F349, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39: 688–697, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Plato CF, Garvin JL. α2-Adrenergic-mediated tubular NO production inhibits thick ascending limb chloride absorption. Am J Physiol Renal Physiol 281: F679–F686, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 197: 241–251, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Raij L, Baylis C. Glomerular actions of nitric oxide. Kidney Int 48: 20–32, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 50. Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca2+ mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49: 240–248, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roczniak A, Zimpelmann J, Burns KD. Effect of dietary salt on neuronal nitric oxide synthase in the inner medullary collecting duct. Am J Physiol Renal Physiol 275: F46–F54, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Silva GB, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47: 563–567, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Silva GB, Garvin JL. Extracellular ATP inhibits transport in medullary thick ascending limbs: role of P2X receptors. Am J Physiol Renal Physiol 297: F1168–F1173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 62. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Wang T, Inglis FM, Kalb RG. Defective fluid and HCO3− absorption in proximal tubule of neuronal nitric oxide synthase-knockout mice. Am J Physiol Renal Physiol 279: F518–F524, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu T, Dai M, Shi XR, Jiang ZG, Nuttall AL. Functional expression of P2X4 receptor in capillary endothelial cells of the cochlear spiral ligament and its role in regulating the capillary diameter. Am J Physiol Heart Circ Physiol 301: H69–H78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu XC, Harris PJ, Johns EJ. Nitric oxide and renal nerve-mediated proximal tubular reabsorption in normotensive and hypertensive rats. Am J Physiol Renal Physiol 277: F560–F566, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Zhu X, Lu D, Fu Y, Liu H, Lu Y, Juncos LA, Liu R. Shear stress at the macula densa blunts tubuloglomerular feedback via primary cilia-dependent increases in MD nNOS activity. Hypertension 56: e50–e166 2010 [Google Scholar]