Abstract
The bladder uroepithelium transmits information to the underlying nervous and musculature systems, is under constant cyclical strain, expresses all four adenosine receptors (A1, A2A, A2B, and A3), and is a site of adenosine production. Although adenosine has a well-described protective effect in several organs, there is a lack of information about adenosine turnover in the uroepithelium or whether altering luminal adenosine concentrations impacts bladder function or overactivity. We observed that the concentration of extracellular adenosine at the mucosal surface of the uroepithelium was regulated by ecto-adenosine deaminase and by equilibrative nucleoside transporters, whereas adenosine kinase and equilibrative nucleoside transporters modulated serosal levels. We further observed that enriching endogenous adenosine by blocking its routes of metabolism or direct activation of mucosal A1 receptors with 2-chloro-N6-cyclopentyladenosine (CCPA), a selective agonist, stimulated bladder activity by lowering the threshold pressure for voiding. Finally, CCPA did not quell bladder hyperactivity in animals with acute cyclophosphamide-induced cystitis but instead exacerbated their irritated bladder phenotype. In conclusion, we find that adenosine levels at both surfaces of the uroepithelium are modulated by turnover, that blocking these pathways or stimulating A1 receptors directly at the luminal surface promotes bladder contractions, and that adenosine further stimulates voiding in animals with cyclophosphamide-induced cystitis.
Keywords: cyclophosphamide-induced cystitis, voiding
adenosine is a ubiquitously occurring nucleoside that is important for the homeostasis of diverse organ systems including the kidneys, heart, lungs, and brain (20, 46, 55). It is found both intracellularly and in the extracellular space, and its concentration in these compartments is dependent on its biogenesis and turnover (43, 44). The intracellular pool of adenosine is synthesized de novo by the hydrolysis of S-adenosylmethionine or is generated from the nucleotidase-dependent breakdown of ATP to ADP to AMP to adenosine. The extracellular pool of adenosine is also formed from the hydrolysis of ATP by ecto-nucleotidases or alkaline phosphatases or by metabolism of cAMPs to AMPs to adenosine. In addition, sodium-coupled concentrative or equilibrative nucleoside transporters shuttle adenosine in a concentration-dependent manner between the intracellular and extracellular compartments (23). The turnover of extracellular and intracellular adenosine is mediated by two enzymes: adenosine deaminase and adenosine kinase, which decrease adenosine by converting it to inosine or AMP, respectively (13, 30, 32, 52). When present, adenosine elicits its effects on tissues by binding to and activating one or more members of a family of four heptahelical G protein-coupled receptors, which have distinct affinities for adenosine where A1 > A2A > A2B > A3 (9, 18, 19, 38). The receptors are further differentiated based on their coupling to downstream heterotrimeric G proteins: A1 and A3 receptors couple to Gi, whereas the A2 receptors couple to Gs.
Adenosine can counteract the effects of excitatory mediators such as ATP and also has a well-known protective effect in several organs (38). An example of the former is observed in the brain, where activation of the A1 receptor has a sedative, anticonvulsant, and locomotor depressing effect, as opposed to the excitatory effects of P2X receptor activation by ATP (17). A prominent example of the protective effect of adenosine is seen in the heart, where during periods of metabolic stress and inflammation the concentration of extracellular adenosine increases by several orders of magnitude (9, 28). Furthermore, when this organ is experimentally stimulated to produce large amounts of adenosine by exposure to short bouts of ischemia and reperfusion, there is a cardio-protective effect that limits tissue damage when the heart is exposed to a subsequent, larger occlusion (36, 41). This effect is mediated downstream of adenosine receptors, which stimulate proteins kinase C and may act to alleviate stress by stimulating nitric oxide (NO) production through induction of inducible NO synthase (18, 28). Likewise, treatment with an A1 receptor agonist such as 2-chloro-N6-cyclopentyladenosine (CCPA) is also cardio-protective and similar roles for adenosine and its receptors have been identified in kidney and airway epithelial cells exposed to acute ischemic injuries (8, 22, 31, 51). In contrast, adenosine can also potentiate events including the stimulation and activation of immune cells. For instance, activation of A2B receptors in T84 cells stimulates secretion of IL-6, resulting in a Ca2+-mediated proinflammatory signaling loop (16, 49).
The role of adenosine in other organs and tissues, such as the bladder uroepithelium, is less well-understood even though this tissue is exposed to high concentrations of waste products and is in a physiologically taxing environment that is in constant flux as the bladder fills and empties. Furthermore, the uroepithelium is not just a high-resistance barrier but can act as a sensory transducer, responding to chemical and mechanical stimuli at its luminal surface by releasing mediators such as ATP, acetylcholine, adenosine, or NO from its basal side (2, 3). In turn, these mediators transmit information to the underlying nervous and muscular systems, altering bladder function. Interestingly, all four adenosine receptors are expressed in the uroepithelium and this tissue is also a site of adenosine production (57), but the mechanism(s) of adenosine turnover at the mucosal or serosal surface of the uroepithelium is unknown. Furthermore, the A1 receptor is prominently expressed at the apical surface of the outermost umbrella cell layer and activation of this receptor by the mucosal addition of adenosine causes the umbrella cells to add apical membrane (57). However, it is not known whether stimulating this lumen-facing receptor has any impact on overall bladder function, nor is there information about whether increasing adenosine concentrations can be used to quell or modulate bladder hyperactivity.
Using isolated rabbit uroepithelial tissues mounted in Ussing stretch chambers, we find that adenosine production is increased during periods of extended stress. The mechanism for modulating adenosine turnover at the mucosal surface of this tissue is adenosine deaminase or equilibrative nucleotide transporters, while adenosine kinase and nucleoside transporters keep adenosine levels in check on the basal surface. We also observe that the A1 receptor agonist CCPA or drugs that impair adenosine turnover significantly lower the threshold pressure needed to trigger voiding in the bladders of rats undergoing cystometry. Furthermore, A1 receptor activation causes an increase in detrusor activity in animals acutely treated with cyclophosphamide, decreasing the cycle time between voids. Our results indicate that the uroepithelium, particularly in response to stress, is an active site of adenosine production and turnover. Furthermore, increasing luminal adenosine by blocking its turnover or stimulating A1 receptors with CCPA lowers the threshold pressure for voiding in normal bladders and increases the voiding frequency of bladders with acute cystitis. The latter effect appears to be independent of changes in A1 receptor expression or distribution in the uroepithelium.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, all chemicals were obtained from Sigma (St. Louis, MO) and were of reagent grade or better. Adenosine, adenosine agonists, and modulators of adenosine turnover were freshly prepared as stocks in the following diluents: a 10-mM stock of CCPA was made in DMSO, 25 mg/ml of cyclophosphamide was prepared in distilled H2O, a 10-mM stock solution of S-(4-nitrobenzyl)-6-thioinosine (NBTI) was prepared in DMSO, 100 μM 5-iodotubericidin (IDT; Tocris, Ellisville, MO), 10-mM stock of erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA; Tocris) was made in DMSO, and a 10-mM stock of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) made in DMSO. Adenosine was freshly prepared and dissolved in Krebs buffer (110 mM NaCl, 5.8 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 2.0 mM CaCl2, 1.2 mM MgSO4, 11.1 mM glucose, pH 7.4). Beuthanasia-D and butorphanol (Torbugesic-SA) were purchased from Butler Schein (Dublin, OH). Lidocaine (LMX4) and isoflurane were purchased from Webster Veterinary (Webster, NY). The polyclonal anti-A1 adenosine receptor rabbit antibody was obtained from Abcam (ab82477; Cambridge, MA), fluorphore- or horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson Immunoresearch (West Grove, PA), and tetramethylrhodamine isothiocyanate-labeled phalloidin and TO-PRO-3 were procured from Molecular Probes/Invitrogen (Grand Island, NY).
Animals.
Animals used in this study were female New Zealand white rabbits (3–4 kg; Myrtle's Rabbitry, Thompson Station, TN) and female Sprague-Dawley rats (250–300 g; Harlan Laboratories). Rabbits were euthanized by intravenous injection of 300 mg of pentobarbital sodium (Buthensia D) into the ear vein after the area was numbed using topical lidocaine ointment. After euthanasia, the bladders were rapidly excised and processed as described below. Rats were sedated by inhalation of isoflurane and then 1.2 g/kg urethane dissolved in H2O was injected subcutaneously. After 2 h, the rats were prepared for adenosine measurements or cystometry as detailed below. At the end of the experiments, the rats were euthanized by inhalation of 100% CO2, and a thoracotomy was performed to verify death. All animal studies were carried out with the approval of the University of Pittsburgh Animal Care and Use Committee.
Mounting the uroepithelium in Ussing stretch chambers.
Mounting of tissue in Ussing stretch chambers was performed as described previously (53). Briefly, excised rabbit bladders were cut open longitudinally, washed with Krebs buffer, and then mounted on custom-made Teflon racks, mucosal side down. The smooth muscle layers were removed with sharp scissors and forceps. The remaining mucosal tissue, containing the uroepithelium, was mounted on the pins of a plastic ring with a 2-cm2 opening. The ring, with tissue, was sandwiched between two halves of a modified Ussing stretch chamber. Each hemichamber (mucosal and serosal) was filled with 12.5 ml of Krebs solution and the serosal hemichamber was bubbled with gas containing 5% (vol/vol) CO2-95% (vol/vol) air. The tissue was equilibrated for 30 min before the start of the experiment.
Measurement of adenosine, AMP, and inosine.
After mounting and equilibration, rabbit tissue was isovolumetrically washed three times with 60 ml of Krebs buffer. After a 60-min incubation, the tissue was gradually stretched by infusing Krebs solution into the enclosed mucosal chamber at a rate of 100 μl/min until the chamber was filled (2 ml). Afterwards, the Luer ports providing access to the mucosal hemichamber were sealed and an additional 500 μl of buffer were pumped into the mucosal hemichamber, causing the uroepithelial tissue to completely bow outwards. The fully distended tissue was incubated for an additional 60 min. Samples, taken with replacement from the serosal or mucosal hemichambers at the designated time points, were heat inactivated, flash-frozen in liquid nitrogen, and stored at −80°C.
For in vivo studies, rats were anesthetized and 2-h post urethane administration, a toe/tail pinch, was used to confirm that the animals had reached an appropriate anesthetic depth. Anesthetized rats were placed on their backs and the hind legs were secured to the table to provide easier access to the abdomen. The fur around the urethral opening and along the caudal midline was removed with a surgical grade trimmer (Oster, McMinnville, TN). The hairless area was wiped with 70% ethanol and a 1.5-cm incision was made at the midline. The subcutaneous fat and muscle layer were incised and the bladder was then located and exteriorized. The ureters were located, tied off using a 6–0 silk suture (Coviden, Mansfield, MA), and then cut to prevent urine from entering the bladder. The bladder was placed back in the body cavity and the incision was sutured close. A 22-gauge intravenous catheter was trimmed to a length of 1.5 cm, inserted through the urethra, and then residual urine was removed by gently pressing the abdomen to promote voiding. The bladder was then filled with sterile PBS containing 0.9 mM CaCl2 and 1.0 mM MgCl2 (PBS+) and the bladder was voided. This was repeated two additional times. The bladder was then slowly filled for 60 min with PBS+ at a rate of 6 μl/min. At the end of this incubation, the bladders were voided and the intravesicle fluid was collected for analysis. Subsequently, the bladder was again slow filled for 60 min, and then maintained in its filled state for an additional 60 min. At the end of the treatment, the bladder was voided and fluid was then collected. Samples were treated and stored at −80°C as described above.
Adenosine, AMP, and inosine concentrations in the samples were determined using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra, ThermoFisher Scientific, San Jose, CA) as previously described (26).
Cystometry analysis.
Rats were anesthetized and their bladders were exposed as described above. Upon being exteriorized, the bladder was held in position by placing a plastic dowel behind the bladder. The bladder was moistened with normal saline and shallow purse string sutures were made around the dome using a 6–0 silk suture material. Care was taken to not puncture the bladder. The area within the boundary of the sutures was punctured with an 18-gauge needle, and a flame-flanged PE-50 tube was inserted into the hole. The suture was tightened around the tubing and the tube was gently retracted until the flange was flush with the mucosa. The bladder was returned to the peritoneal cavity, and the surgical incision was closed around the PE-50 tubing in two layers using 6–0 silk suture.
The PE-50 tubing was connected to a three-way port: one branch led to a pressure transducer, while the other two were connected to syringes containing buffered urine substitute (BUS) ± drug. BUS, made in accordance with the published composition of Sprague-Dawley rat urine (48), had the following composition: 26 mM (NH4)2SO4, 149 mM NaCl, 267 mM KCl, 3 mM CaCl2, and 0.4% (wt/vol) MgPO4. The solution was titrated with NaOH until a pH of 6.5 was measured. The pressure transducer was connected to a Quad Bridge Amplifier and Powerlab 4/30 (ADInstruments, Castle Hill, Australia), which was interfaced with an iMac computer (Apple, Cupertino, CA) running the Chart 5.0 program (ADInstruments). The syringes containing the buffers were placed in a syringe pump (New Era Syringe Pump Systems, Farmingdale, NY) and BUS was infused into the bladder at a rate of 50 μl/min for 45 min, until uniform peaks were obtained. BUS ( ± drugs) was then infused for 60–90 min. The effluent was collected and the volume was measured to determine the fluid recovery.
The cystometrograms (CMGs) were recorded using the Chart program (ADInstruments). Following a 45-min period of equilibration, data for 8–12 successive bladder cycles were collected and for each animal the average values for the following parameters were entered in an Excel spread sheet (MS Office, Microsoft, Redmond, WA): 1) the basal pressure was the lowest pressure point recorded immediately following a void, 2) the threshold pressure was the pressure point recorded just before the spike in pressure was observed as the bladder began to void, 3) the peak pressure was the maximum pressure recorded during voiding, 4) the intercontraction interval (ICI) was the time between two consecutive peak pressures, and 5) the percent fluid recovery was the ratio of the volume of fluid released vs. that infused into the bladder. The mean and SE were calculated for each similarly treated experimental group and the data were analyzed for statistical significance using the techniques described below.
Cyclophosphamide treatment.
An acute cyclophosphamide-induced cystitis was induced as described previously (11, 29). In brief, rats were sedated with isoflurane and administered 1 mg/kg butorphenol subcutaneously and 150 mg/kg cyclophosphamide intraperitoneally. Two hours after these injections, the rats were given 1.2 g/kg urethane subcutaneously, and then 2 h later the CMG analysis was performed as detailed above.
Immunofluorescence labeling and image acquisition.
Bladder tissue was fixed and processed as described previously (5, 50). Images were captured using a ×63 1.2 NA glycerol objective and the appropriate laser lines of a Leica TCS SP5 CW-STED confocal microscope (in normal confocal mode; Leica Microsystems GmbH, Mannheim, Germany). The photomultipliers were set at 900–1,200 and images were collected using an average of six line scans. Serial 0.25-μm z-sections were acquired. The images were imported into Volocity four-dimensional software (Perkin Elmers, Waltham, MA) and following image reconstruction and contrast correction were exported as TIFF files. The exported images were opened in Adobe Photoshop CS5, converted to JPEG format, and the composite images were prepared in Adobe Illustrator CS5 (Adobe, San Jose, CA).
Western blot analysis.
Lysates of uroepithelial cells and Western blotting were performed as described previously (50, 57).
Statistical analysis.
Statistical significance between means was determined by Student's t-test or in the case of multiple comparisons by ANOVA. If a significant difference in the means was detected by ANOVA, multiple comparisons were performed using Dunnett's posttest correction. Statistical analyses were performed using Prism 5 software (GraphPad, La Jolla, CA).
RESULTS
Distinct pathways for adenosine biogenesis are found at the mucosal and serosal surfaces of the rabbit uroepithelium.
Isolated rabbit uroepithelium is ideal for studying adenosine biosynthesis and turnover because the tissue can be manipulated in a physiologically relevant manner and samples can be taken from both the mucosal and serosal surfaces of the tissue (54, 57). We previously showed that the adenosine concentrations increased in the fluid bathing isolated rabbit uroepithelium mounted between two halves of an Ussing stretch chamber (57). Furthermore, in response to an abrupt change in stretch, there was a large increase in the amount of adenosine found in both hemichambers of the device (57). To gain a better understanding of the relationship between adenosine production and bladder filling, we again used these isolated rabbit preparations, but employed the following stretch protocol. First, following washing, we incubated the tissue for 60 min in the absence of stretch to measure baseline production of the nucleoside. Then, to mimic the filling phase of the bladder micturition cycle, the mucosal hemichamber was slowly filled for ∼30 min during which time the tissue was in a dynamic state as it slowly bowed outwards. At the 90-min mark, when the tissue had completely bowed outwards, filling was stopped and the tissue was incubated for an additional 60 min under this potentially more stressful “filled” state. At the indicated time points, we took samples and then measured, using ultra performance liquid chromatography-tandem mass spectrometry, the concentration of adenosine, inosine, or AMP in the mucosal (Fig. 1) or serosal (Fig. 2) hemichambers of the stretch device.
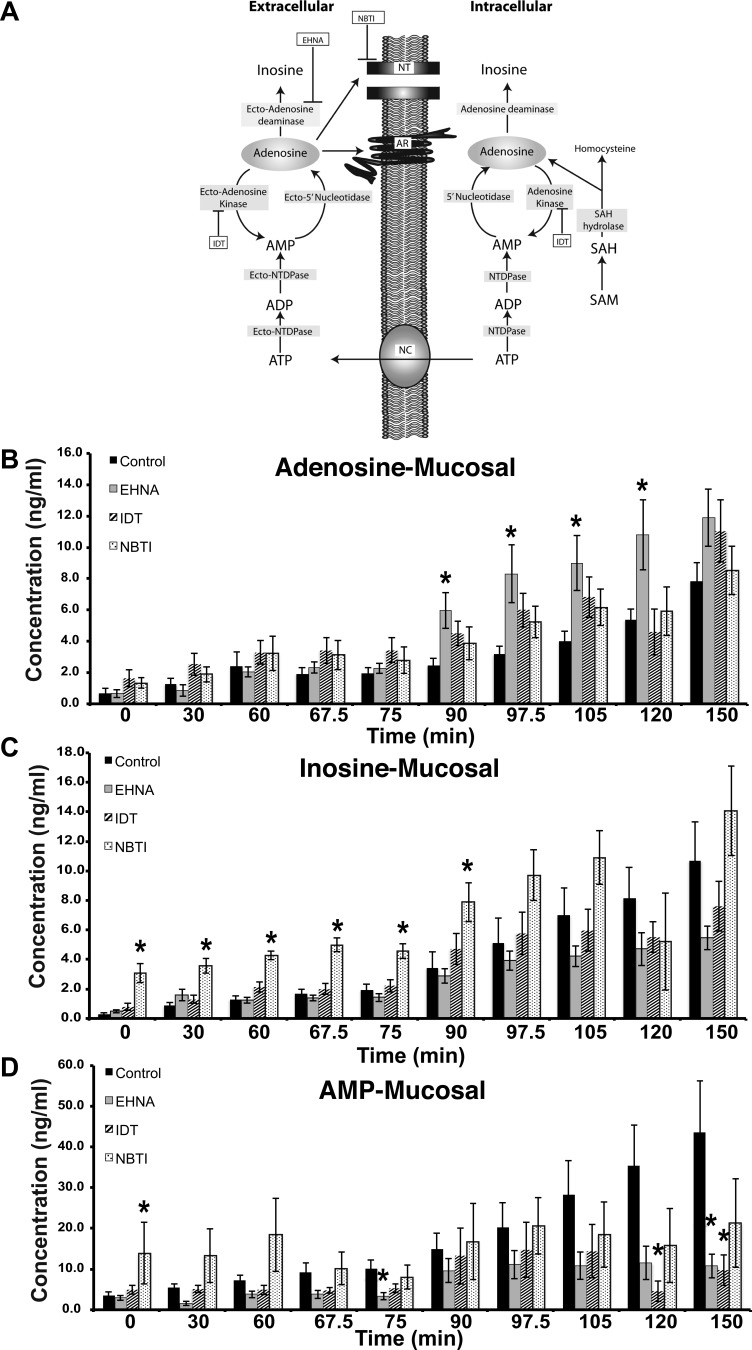
Fig. 1.
Adenosine turnover at the mucosal surface of the uroepithelium. A: schematic of adenosine biosynthesis and turnover in the intracellular and extracellular spaces. Intracellular adenosine is formed either by the conversion of S-adenosylmethionine (SAM) to S-adenosylhomocystine (SAH) to adenosine, or by the hydrolysis of ATP. The latter is expelled from the cells via nucleotide channels (NC). The extracellular ATP is converted to adenosine by the ecto-nucleotide triphosphate diphosphohydrolases (NTDPases), ecto-NTDPase (CD39), and ecto-5′ nucleotidases (CD73). Extracellular adenosine can have multiple fates: 1) transport back into the cell by nucleoside transporters (NT), 2) binding to and activation of adenosine receptors (AR), 3) metabolism to inosine by adenosine deaminase, or 4) conversion to AMP by ecto-adenosine kinase. EHNA, erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride; IDT, 5-iodotubericidin; NBTI, S-(4-nitrobenzyl)-6-thioinosine. B-D: rabbit uroepithelium was mounted in Ussing stretch chambers and pretreated with the indicated drug for 60 min before the start of the experiment. The tissue was left in its quiescent state for 60 min and then stretched by increasing the fluid in the mucosal chamber for 30 min. The tissue was then left in this stretched state for an additional 60 min. Samples were drawn at the indicated times and then analyzed by mass spectrometry to determine the concentration of adenosine, inosine, and AMP. The experiment was performed on 3 separate occasions and the data are expressed as means ± SE (control n = 7, EHNA n = 7, IDT n = 7, NBTI n = 4). *Statistically significant differences (P < 0.05) between control samples and treated ones.
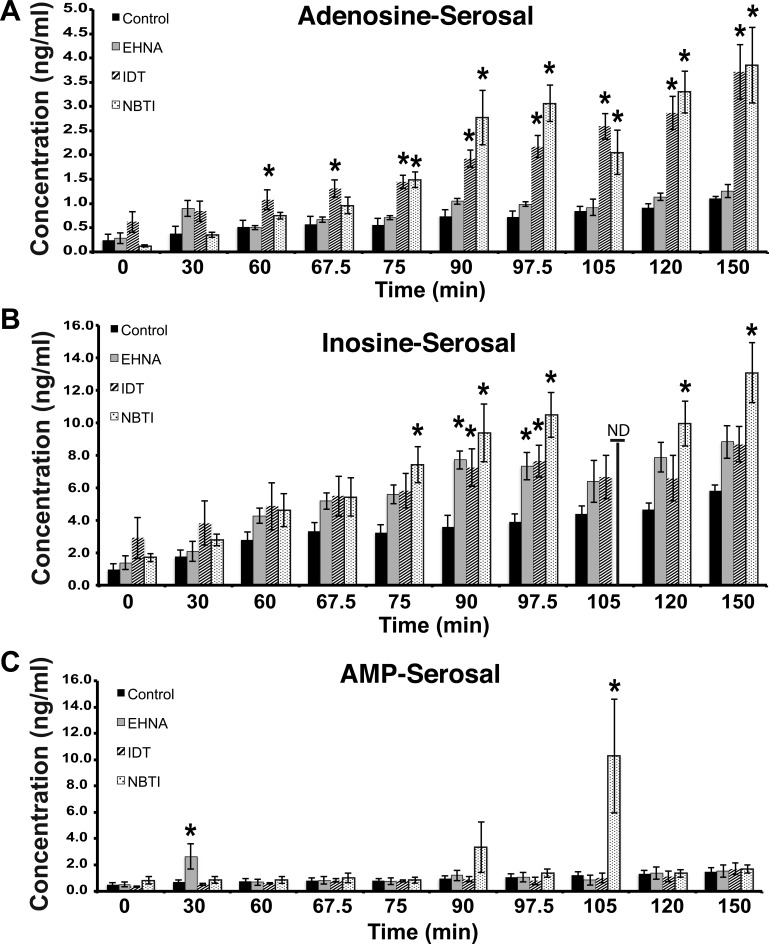
Fig. 2.
Adenosine turnover at the serosal surface of the uroepithelium. A–C: tissue was treated as in Fig. 1, but samples were taken and analyzed from the serosal surfaces of the tissue. The data are expressed as means ± SE (control n = 7, EHNA n = 7, IDT n = 7, NBTI n = 4). *Statistically significant differences (P < 0.05) between control samples and treated ones.
We found that the concentrations of adenosine, AMP, and inosine slowly increased in the mucosal hemichamber during the first 60 min when the tissue was left unstretched (Fig. 1, B–D). While the levels of adenosine remained somewhat stable during the period of experimental filling (i.e., 60–90 min; Fig. 1B), the concentrations of inosine and AMP increased by ∼100 or ∼50%, respectively (Fig. 1, C–D). By 90 min, and during the subsequent 60-min incubation in the presence of continual stretch, there was a pronounced increase in the concentration of the three nucleosides (Fig. 1, B–D).
Next, we used inhibitors of enzymes known to be critical for adenosine turnover and assessed their impact on the levels of adenosine, AMP, and inosine. Inhibitors employed included: the adenosine deaminase inhibitor EHNA (23), the adenosine kinase inhibitor IDT (42), or the general blocker of the widely expressed equilibrative nucleoside transporter-1 NBTI (14) (Fig. 1A). We used the drugs at concentrations that were previously employed (14, 23, 42). None of these drugs affected the production of adenosine in unstretched tissue or during experimental filling. However, by 90 min, the concentration of adenosine in the EHNA-treated tissues was significantly increased by ∼140% above that observed in the untreated samples (Fig. 1B). The other drugs appeared to have some effect on the amounts of mucosal adenosine, but these effects did not reach statistical significance. A precursor-product relationship between mucosal adenosine and the other two nucleosides we examined was not clear-cut (Fig. 1, C–D). For example, the inosine concentrations trended downwards in tissue treated with the adenosine deaminase inhibitor EHNA but the effect was not significant. Furthermore, treatment with the nucleoside transporter inhibitor NBTI did not alter adenosine concentrations, but did significantly impact inosine concentrations in the absence of stretch or during filling (compare Fig. 1B with 1C).
Similar measurements were made at the serosal surface of the tissue (Fig. 2). We found that the levels of adenosine, AMP, and inosine gradually increased over the length of the experiment; however, in these control samples there was no obvious change in rates of nucleoside production during the varying tissue manipulations (Fig. 2, A–C). In contrast, treatment with the adenosine kinase inhibitor IDT caused a significant increase in serosal adenosine toward the end of experimental filling, raising its concentration by ∼160%, and was also stimulatory after the 90-min time point (Fig. 2A). Furthermore, the nucleoside transporter inhibitor NBTI also caused a large increase in the concentration of serosal adenosine, an effect that was especially enhanced at time points ≥90 min (Fig. 2A). In contrast, we found no evidence that adenosine deaminase plays a significant role in modulating adenosine concentration at the serosal surface of the uroepithelium. In general, there was no obvious correlation between effects of the drug treatments on adenosine vs. its metabolites AMP or inosine (Fig. 2, A–C). However, treatment with the nucleoside transport inhibitor NBTI increased inosine concentrations by ∼130% during the end of the experimental filling phase, continuing into the period of extended stretch (Fig. 2B). The AMP levels were generally unaffected by any of the drugs, except for two time points that showed large variations in values (Fig. 2C).
Adenosine release from the mucosal surface of filled rat bladders is sensitive to inhibitors of adenosine deaminase and concentrative nucleoside transporters.
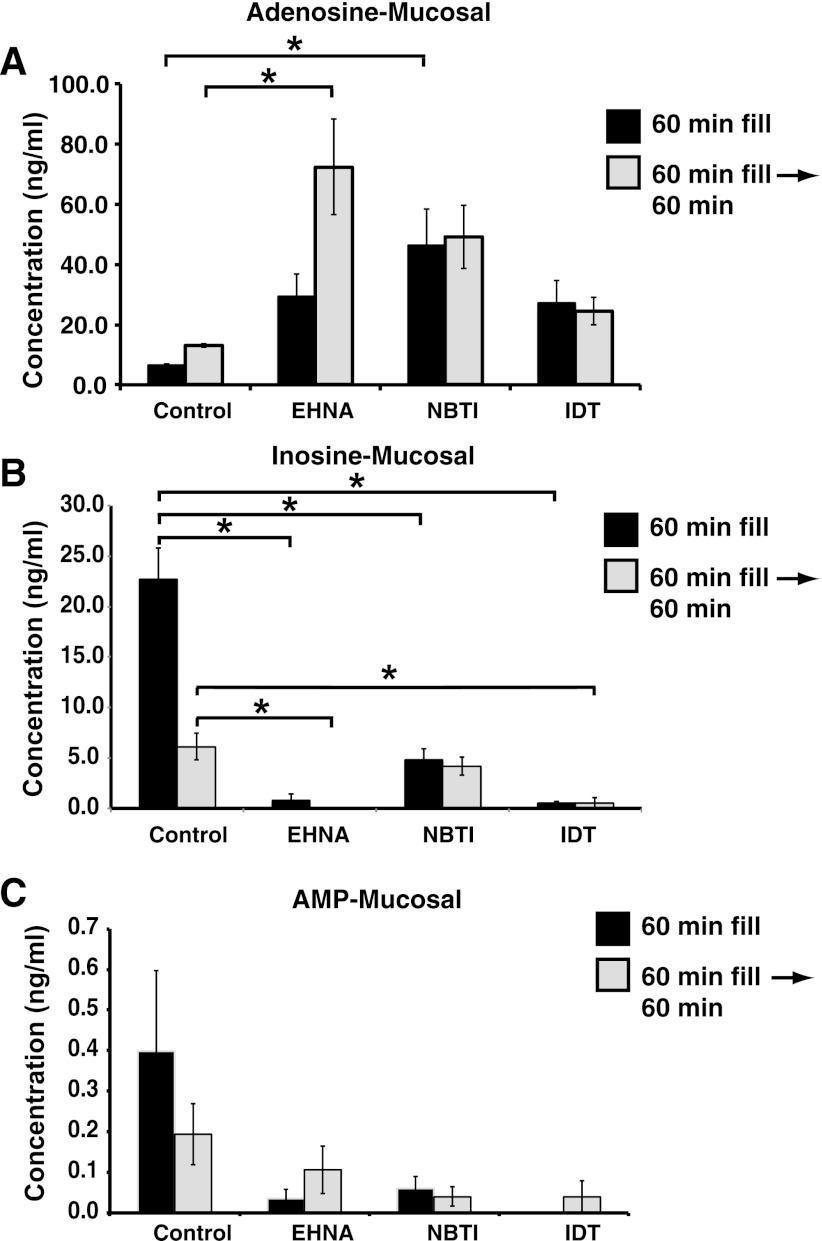
To further confirm that adenosine was released from the uroepithelium in situ, we examined the release of adenosine and its metabolites from the luminal surface of filled rat bladders. Unlike ex vivo tissue, we did not have access to the serosal surface of the uroepithelium in this setting. We observed that adenosine was released into the lumen after slowly filling the bladder for 60 min, and the release was further potentiated by an additional 60 min in the filled state (Fig. 3A). Inosine and AMP were also released during bladder filling, but their levels appeared to decrease as the bladders were left in their filled state (Fig. 3, B-C). The adenosine deaminase inhibitor EHNA caused adenosine concentrations to trend upwards during bladder filling (an effect that did not reach statistical significance), but did result in a significant increase in adenosine levels when the bladder was left filled for 60 min (Fig. 3A). This was coupled with a significant decrease in inosine levels (Fig. 3B). The nucleoside transporter inhibitor NBTI also caused a significant increase in adenosine concentrations during bladder filling and these trended higher upon extended time in the filled state, but did not reach significance (Fig. 3A). NBTI also caused a large decrease in inosine concentrations (Fig. 3B). The adenosine kinase inhibitor IDT significantly decreased inosine concentrations, but had no effect on adenosine levels, even though these values trended higher than control ones. Finally, none of the treatments affected the concentration of AMP in the experimental groups, although the amount of AMP trended downwards in all of the treatment groups (Fig. 3C). In sum, our data indicate that adenosine is released at the luminal surface of the bladder in situ and that like isolated rabbit tissue adenosine release was sensitive to inhibition by adenosine deaminase. However, we also observed that nucleoside transporters played a more active role in rat bladder uroepithelium vs. that of rabbit bladders, possibly indicating a difference between species or a difference in our methods (i.e., ex vivo preparations vs. analysis in situ).
Fig. 3.
Effect of EHNA, NBTI, or IDT on adenosine release in the rat bladder. A–C: rat bladders were filled with PBS containing DMSO (control), EHNA, NBTI, or IDT at a rate of 6 μl/min, and the intravesicle fluid was collected for analysis. Subsequently, the bladder was slow filled for 60 min and then maintained in its filled state for an additional 60 min before collecting samples. The concentration of adenosine (A), inosine (B), or AMP (C) is indicated. Data are means ± SE (n ≥ 4). *Significant differences (P < 0.05).
Stimulation of luminal A1 receptors decreases the threshold pressure of bladders during cystometry.
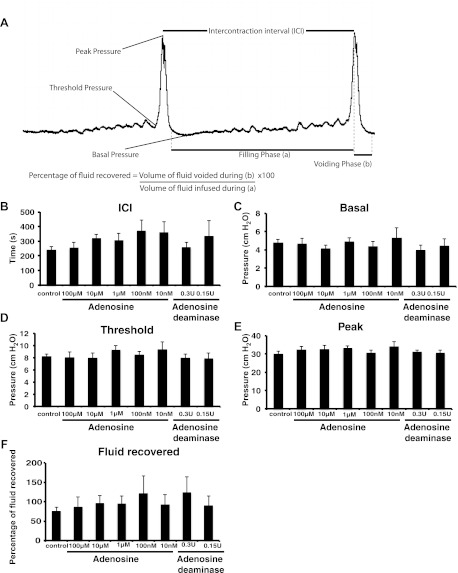
Considering its established role in modulating stress and countering the effects of stimulatory modulators such as ATP (4, 12), we next sought to determine whether the luminal addition of adenosine had any impact on bladder function. To test such a role, we used continuous cystometry, a technique in which a solution is introduced into the bladder lumen (via a catheter inserted into the bladder dome) and the pressure monitored as the bladder fills and then actively empties (35). A representative CMG is shown in Fig. 4A. We measured five parameters: 1) the basal pressure, which is the lowest pressure measured following a void; 2) the threshold pressure, which is that measured just before the detrusor contracts and voiding is initiated; 3) the peak pressure, which is the maximum pressure during detrusor contraction and coincides with voiding; 4) the ICI, which is the distance between two peaks, and correlates with the time between two consecutive voids; 5) the percent fluid recovery, which is a ratio of the volume of fluid released vs. that infused into the bladder. This latter parameter is useful to evaluate whether the bladder voids efficiently or whether there is fluid retention that results from, for example, a spastic urethra that does not allow complete voiding. Average values for these parameters are shown in Fig. 4.
Fig. 4.
Effect of adenosine on bladder function. A: sample cystometrogram. B–F: continuous cystometry was performed using the indicated concentration of adenosine or adenosine deaminase dissolved in buffered urine substitute (BUS). The parameters measured included intercontraction interval (ICI; B), basal pressure (C), threshold pressure (D), peak pressure (E), and fluid recovered (F). Data are expressed as means ± SE (control n = 7, 100 μM adenosine n = 8, 10 μM adenosine n = 8, 1 μM adenosine n = 8, 100 nM adenosine n = 9, 10 nM adenosine n = 6, 0.3 U adenosine deaminase n = 8, 0.15 U adenosine deaminase n = 6). No parameters were significantly different from those of controls.
Cystometry is typically performed using unbuffered normal saline or low-tonicity PBS that has a pH of 7.4. However, normal urine is decidedly more complex and contains electrolytes not found in saline or PBS. In addition, the average pH of rats housed at the University of Pittsburgh animal facility and fed rat chow was 6.5. Thus, we also tested a BUS in addition to normal saline or PBS. We found that BUS had no adverse effects on the bladder and all the five parameters that we studied did not vary significantly between the three solutions (data not shown). Because BUS better reflects the physiological composition of urine, we employed it in our subsequent studies.
We first determined what happened if adenosine was introduced into the bladder lumen during cystometry. A broad range of concentrations was used (10 nM-100 μM), and although there was an upward trend in the ICI as the concentration of adenosine dropped, the difference failed to reach statistical significance (Fig. 4B). Furthermore, there was no significant change in the basal pressure, threshold pressure, peak pressure, or fluid recovery (Fig. 4, C–F). We also explored what happened to the CMG parameters when adenosine levels were decreased by continuously infusing the bladder with exogenous adenosine deaminase, which converts adenosine to inosine. Again, we did not identify any significant difference between the control group parameters and those in which adenosine was depleted (Fig. 4, B–F).
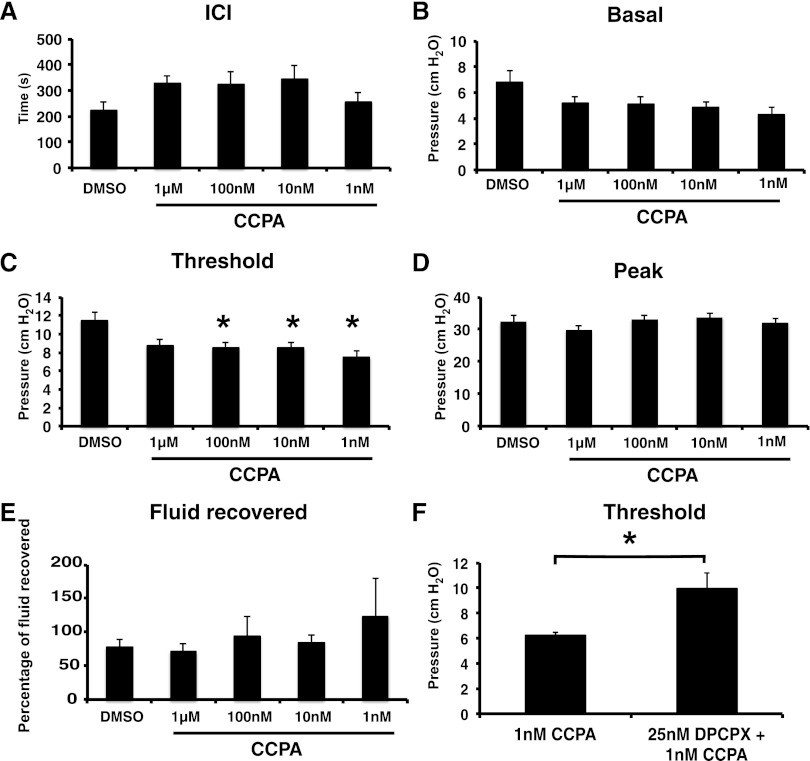
We previously reported that the apical surface of umbrella cells expresses the A1 receptor. Hence, we infused bladders with BUS containing CCPA, which when used at low nanomolar concentrations is an A1-selective agonist (34). In addition to its high affinity, CCPA has other beneficial characteristics including its lack of conversion to AMP or inosine and its failure to be shuttled from the lumen to the cell interior by nucleoside transporters (47). Remarkably, when CCPA was infused at a concentration as low as 1 nM, it decreased the threshold pressure by ∼55% (Fig. 5C). There was no statistically significant effect at 1 μM, possibly because non-A1 receptor targets were activated and counteracted the effects of CCPA-mediated A1 receptor activation at these high concentrations. There was no significant effect on the other parameters measured (Fig. 5, A–B, D–E). To confirm that these effects were a result of A1 receptor activation, we treated bladders with the A1 receptor antagonist DPCPX in addition to CCPA (57). This blocked the effect of CCPA, and it raised threshold levels back to those of controls (Fig. 5, C and F). DPCPX alone had no significant effect on the CMG parameters (data not shown). In summary, activation of luminal A1 receptors appeared to lower the pressure needed to stimulate bladder contractions, but without changes in ICI, basal pressure, peak pressure, or fluid recovery.
Fig. 5.
Activation of apical A1 receptors with 2-chloro-N6-cyclopentyladenosine (CCPA) lowers the threshold pressure for voiding. Continuous cystometry was performed using the indicated concentration of drug dissolved in BUS. A–E: cystometry parameters were measured as described in Fig. 4. Data are expressed as means ± SE (DMSO control n = 10, 1 mM CCPA n = 7, 100 nM CCPA n = 13, 10 nM CCPA n = 12, 1 nM CCPA n = 9). *Statistically significant differences (P < 0.05) between DMSO-treated bladders and CCPA-treated ones. F: specificity of the effect of CCPA was tested by including 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) in the cystometry buffer. Data are expressed means ± SE (CCPA n = 7, DPCPX + CCPA n = 8). DPCPX significantly antagonized the effects of CCPA alone.
Blocking pathways for adenosine turnover reveals an adenosine and A1 receptor-like response.
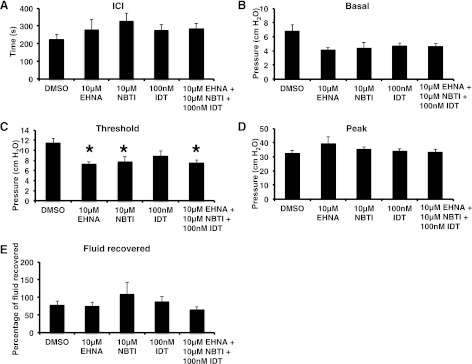
The ability of CCPA to elicit responses, but not adenosine, led us to examine whether adenosine turnover could account for the differences. We infused EHNA, IDT, or NBTI, individually or in combination, and measured the effects on bladder function. Consistent with our in situ rat bladder experiments, we observed that the adenosine deaminase inhibitor EHNA and the nucleoside transporter inhibitor NBTI decreased the threshold pressure for voiding (Fig. 6C), presumably because they increased luminal adenosine concentrations. These drugs did not alter the other parameters (Fig. 6, A–B and D–E). The adenosine kinase inhibitor IDT was without effect (Fig. 6). As expected, treatment with all three inhibitors also decreased threshold pressure (Fig. 6C). We also assessed whether the inhibitors of adenosine turnover would unmask a further sensitivity to exogenously added adenosine. However, we found no such effect (data not shown).
Fig. 6.
Inhibitors of adenosine turnover lower the threshold pressure for voiding. Continuous cystometry was performed using the indicated concentration of drug dissolved in BUS. A–E: comparison of cystometrogram (CMG) parameters for bladders treated with EHNA, NBTI, IDT, or a cocktail containing all 3 inhibitors. The DMSO values in Fig. 5 are reproduced in A–E to aid in making comparisons (DMSO control n = 10, 10 μM EHNA n = 7, 10 μM NBTI n = 8, 100 nM IDT n = 8, drug cocktail n = 7). *Statistically significant differences (P < 0.05) between DMSO-treated bladders and those treated with drugs.
A1 receptor activation stimulates bladder activity in bladders with cyclophosphamide-induced cystitis.
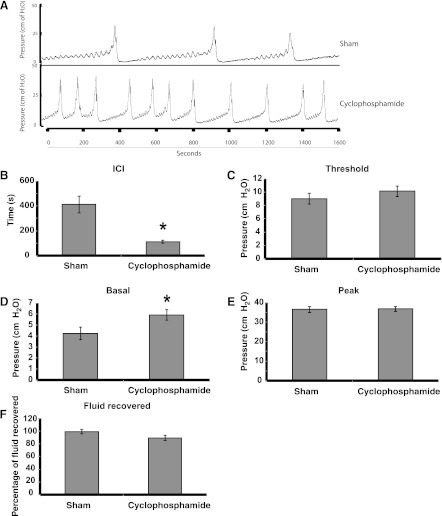
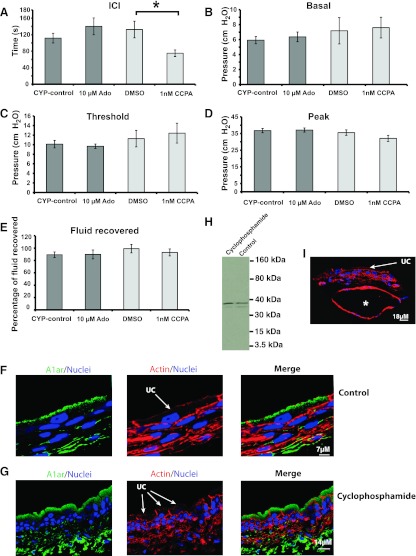
Finally, we determined whether adenosine modulated the hyperactivity found in bladders with acute cyclophosphamide-induced cystitis. Cyclophosphamide is a chemotherapeutic agent that is converted to acrolein and can cause a hemorrhagic cystitis within a short period of time (11). After a 4-h treatment with cyclosphosphamide, we observed that this drug caused a marked decrease in ICI and an increase in the basal pressure (Fig. 7, A–B, D). The threshold pressure, peak pressure, and fluid recovery were not significantly altered (Fig. 7, C, E–F). We then treated the inflamed bladders with 10 μM adenosine, but did not find any significant difference between control and test samples (Fig. 8, A–E). In contrast, 1 nM CCPA exacerbated the effect of cystitis by further reducing the ICI compared with animals treated with cyclosphosphamide and DMSO diluent (Fig. 8A). However, no other parameters were altered in this setting (Fig. 8, B–E).
Fig. 7.
Cyclophosphamide treatment induces urinary bladder cystitis. Rats were either injected with saline (sham treatment) or with 150 mg/kg cyclophosphamide. Four hours later, BUS was intravesically infused at a constant rate of 50 μl/min for a period of 90 min. A: representative images of CMGs obtained from sham- or cyclophosphamide-treated bladders. B–F: CMG parameters for sham- or cyclophosphamide-treated bladders. Data are expressed as means ± SE (sham n = 8, cyclophosphamide n = 9). *Statistically significant differences (P < 0.05).
Fig. 8.
CCPA exacerbates the hyperactive bladder phenotype. A–E: rats were treated with cyclophosphamide for 4 h to induce an acute cystitis and then cystometry was performed. Data are expressed as means ± SE (Cyp-control n = 9, 10 μM adenosine n = 8, DMSO control n = 7, 1 nM CCPA n = 7). *Statistically significant differences (P < 0.05). F–G, I: cross sections of frozen uroepithelial tissues obtained from sham-treated animals or cyclophosphamide-treated ones. Images were captured with a confocal microscope and the z-series was projected. The A1 receptor is shown in green, actin staining is shown in red, and nuclei are shown in blue. The location of umbrella cells (UCs) is indicated. The * in I shows a region of cyclophosphamide-treated bladders where the submucosal tissue is expanded, most likely a result of edema. H: Western blot showing A1 receptor expression in sham (control) or cyclophosphamide-treated animals. Epithelial lysates from at least 2 rats were pooled for this analysis.
One possible explanation for the altered effects of CCPA on cyclophosphamide-treated vs. control animals is that cyclophosphamide changed the expression or distribution of A1 receptors in the uroepithelium. However, we found that A1 receptor expression was largely limited to the apical surfaces and subapical region of the umbrella cells in both control and cyclophosphamide-treated tissues (Fig. 8, F–G). Furthermore, there was no obvious effect on the amount of A1 receptor expressed in the uroepithelium (Fig. 8H). However, we did note that the submucosal region of the cyclophosphamide-treated tissue was edematous in some regions, which appeared as large dilated regions (compare submucosa in Fig. 8F with 8I).
DISCUSSION
We undertook this study to determine the mechanism(s) of adenosine turnover associated with the uroepithelium and to understand whether adenosine would exert a quieting effect when its concentration was increased in the normal or inflamed bladder. Our analysis revealed that there were distinct mechanisms of adenosine turnover operating at each surface of the uroepithelium, which limited the amount of available adenosine. Furthermore, and contrary to our expectations, we observed that adenosine, likely acting through A1 receptors, decreased the threshold pressure needed to stimulate bladder contractions and increased bladder hyperactivity in a model of cyclophosphamide-induced cystitis.
Distinct mechanisms of adenosine turnover at either surface of the uroepithelium.
Stresses such as hypoxia promote the breakdown of extracellular ATP, leading to a sharp increase in tissue adenosine levels (38). Whereas free adenosine binds to its receptors and imparts an organ-protective function (9), chronic elevation of extracellular adenosine is harmful to the tissue (58). Hence, the half-life of extracellular adenosine is kept short (seconds to minutes, depending on species) through the action of enzymes and transporters. This may be particularly important at the luminal surface of the bladder, which is not only a site of adenosine biosynthesis, but it is also impacted by adenosine produced by the kidney and present in the urine (25).
We found that adenosine was released from mucosal surface of rat bladders in response to filling or from the luminal surface of isolated rabbit uroepithelium, particularly when the epithelial tissue was subjected to a prolonged stress. Blocking adenosine deaminase with EHNA further potentiated adenosine levels, indicating that adenosine concentrations are likely kept in check by ecto-adenosine deaminase, which converts the released adenosine to inosine. The importance of the adenosine deaminase pathway for turnover was further supported by our observation that EHNA reduced the threshold pressure for contractions in rat bladders undergoing cystometry. In rat bladders the EHNA-induced increase in adenosine release was coupled to a decrease in inosine levels. However, in isolated rabbit tissue the mucosal inosine levels trended downwards, but the effect was not significant. Thus, a simple precursor-product relationship was not immediately apparent. However, inosine can be phosphorylated to generate IMP and extracellular inosine levels are governed, in part, by nucleotide transporters (39). As a result, we may have been unable to detect correlative changes between adenosine and inosine levels in rabbit tissues. We further observed that when rat bladder equilibrative nucleoside transporters were blocked with NBTI, there was an increase in adenosine. This rise likely explains why NBTI mimicked the effects of CCPA and lowered the threshold pressure for contractions. In contrast, NBTI caused inosine levels to increase in rabbit tissue, but there was no effect on adenosine. This may indicate species differences, or differences in the models employed, but it is also possible that extracellular adenosine was increasing in the NBTI-treated rabbit tissue, but it was rapidly converted to inosine by ecto-adenosine deaminase.
While adenosine deaminase was important at the mucosal surface, adenosine kinase and nucleoside transporters played a more significant role at the serosal side of uroepithelial tissue. These results are in agreement with previous studies showing that the basolateral surface of polarized epithelial cells shows robust equilibrative nucleoside activity (33). Like the mucosal surface, we observed no simple precursor-product relationship between adenosine and its metabolites. For example, treatment with the adenosine kinase inhibitor IDT led to an increase in adenosine levels, but did not cause a corresponding decrease in AMP. The conversion of ATP, which is released from the serosal surface of the uroepithelium (54), to AMP could explain this anomaly. Equilibrative nucleoside transporters were of particular significance at the basal surface of the uroepithelium; their inhibition led to a significant increase in adenosine and inosine levels. We also observed that the levels of inosine increased in the presence of the adenosine deaminase inhibitor as the uroepithelial tissue reached its fully stretched state. Because there was no corresponding loss of adenosine, the source of this inosine is unknown but could be via the pathways described above. As a final note, while the uroepithelium is likely the predominant source of serosally released adenosine in our studies, the preparations we used contain scattered rests of endothelial cells, fibroblasts, interstitial cells, and smooth muscle cells, and that may also contribute to adenosine production and turnover at the serosal surface of this tissue.
Luminal adenosine, acting through A1 receptors, modulates threshold pressure.
The uroepithelium was long thought of as a passive barrier, but it is now realized to play a more active role in regulating bladder function (2, 6). Receptors and channels, sensitive to a broad array of chemicals, mediators, and noxious stimuli, are found on the apical surface of the outermost umbrella cell layer, making it possible to detect small changes in the luminal milieu of the bladder (3). In the case of adenosine, A1 receptors are prominently expressed at this surface of the rat bladder (57), and we showed that luminally added CCPA, used at low nanomolar concentrations (Kd for CCPA is in the 1- to 5-nM range), was sufficient to trigger a change in threshold pressure. This effect was blocked when tissue was simultaneously treated with the highly selective A1 receptor antagonist DCPCX (18). While we cannot rule out a function for other adenosine receptors, our results indicate that A1 receptors play a critical role in this response. Intriguingly, adenosine cannot pass the high-resistance barrier imparted by the apical plasma membrane and tight junctions of the umbrella cell (40). Thus, activation of the A1 receptor most likely initiates a signaling cascade that results in the release of mediators from the uroepithelium. Although known mediators include acetylcholine, adenosine, ATP, NO, and prostaglandins (6), we do not know which of these, if any, is responsible for transduction of the adenosine signal.
On the receiving end are interstitial cells, which are subjacent to the uroepithelium and may interface with nerves and smooth muscle cells (37). In addition, there are neuronal processes in close proximity to the uroepithelium that transmit information from the lower urinary tract to the lumbosacral spinal cord (27, 56). The afferent processes of these nerves are predominantly myelinated Aδ fibers and unmyelinated C-fibers, and subclasses of these processes intercalate deep into the uroepithelium where they can abut the basolateral surfaces of the umbrella cells (7). Interestingly, the effect of adenosine was highly specific and this nucleoside had no effect on basal or peak pressure, the ICI, or fluid retention, but did decrease the threshold pressure. The latter was likely the result of sensitizing the sensory afferent nerve processes so that they responded to lower pressure stimuli. The nature of this sensitization is unknown but could be due to altered release of acetylcholine (15). However, we cannot rule out that adenosine also affected urethral function, possibly by stimulating NO synthesis and release resulting in urethral muscular relaxation (45).
Adenosine as a stimulatory factor in bladder function.
In other organs, such as the heart, kidney, lungs, and brain, adenosine has a well-established protective and anti-inflammatory effect, especially during stress and pathological insult. Thus, A1 receptor-dependent stimulation of micturition by lowering the threshold pressure could be viewed as a protective mechanism to accelerate the elimination of substances that may be injurious to the uroepithelium. However, we also observed that A1 receptor activation exacerbated the bladder hyperactivity seen in rats with cyclophosphamide-induced cystitis. Interestingly, only the ICI was affected in the cystitis model, and not the threshold pressure. This difference did not appear to be the result of altered A1 receptor expression or distribution. However, it is possible that cyclophosphamide-induced inflammation could alter adenosine turnover or the signaling downstream of the A1 receptor, which could change the amount of, or types of, mediators released in response to adenosine. Furthermore, some classes of C-fibers, which are normally quiescent, become activated during cystitis (21), and it is possible that the effects of adenosine were a result of activating these nerve fibers. Thus, adenosine (or other mediators) released from a damaged or inflamed uroepithelium could contribute to bladder hyperactivity by affecting the activity of other cell types and tissues in the bladder proper.
In addition to its protective function, adenosine can also have a stimulatory effect and a strong proinflammatory role, which is cell type, adenosine concentration, and receptor dependent (9). For example, low concentrations of adenosine act through A1 receptors to promote neutrophil adherence to endothelial cells as well as their chemotaxis and tissue infiltration (10). In contrast, high concentrations of adenosine (e.g., at sites of inflammation) activate A2B receptors (EC50 = 25 μM), which are thought to suppress these responses (1). Furthermore, adenosine preexposure can affect the function of T-cells and dendritic cells (24). In the case of the umbrella cell, adenosine, acting through A1 receptors, stimulates bladder function by lowering the threshold for micturition. Such a response may be important as the bladder fills, which our data indicate would stimulate adenosine release and may aid micturition by modulating the threshold pressure. Adenosine-stimulated responses could also play a role in pathological conditions such as bacterial infections and other forms of cystitis, which are often accompanied by increased micturition and pain. Interestingly, gut strains of Escherichia coli may release adenosine in response to enterocyte-released β-defensins (16). If adenosine were produced by uropathogenic E. coli, even at low levels, it could be sensed by the umbrella cell A1 receptors, which could promote the clearance of the bacterial infection by lowering the threshold for micturition.
In conclusion, we identified likely mechanisms of adenosine turnover on the mucosal and serosal surfaces of the uroepithelium, which we show can govern the amount of free adenosine found within the bladder lumen. Furthermore, we established that adenosine has a selective modulatory effect and can promote bladder function by lowering the threshold for micturition. The latter role may be important during bladder filling and may also play a role during pathological conditions such as cystitis.
GRANTS
This work was supported by National Institutes of Health Grants R37-DK54425 and R01-DK077777 (to G. Apodaca) and R01-DK091190 and R01-DK068575 (to E. K. Jackson) and by the Imaging and Metabolomics Cores of the Pittsburgh Center for Kidney Research (P30-DK079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S.P., H.H., J.R.R., E.K.J., and G.A. conception and design of research; H.S.P., H.H., and E.K.J. performed experiments; H.S.P., H.H., and E.K.J. analyzed data; H.S.P., H.H., J.R.R., E.K.J., and G.A. interpreted results of experiments; H.S.P. and H.H. prepared figures; H.S.P. drafted manuscript; H.S.P., H.H., J.R.R., E.K.J., and G.A. edited and revised manuscript; H.S.P., H.H., J.R.R., E.K.J., and G.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Lori Birder and Puneet Khandelwal for the thoughtful comments and suggestions.
REFERENCES
- 1. Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, Laubach VE. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 140: 871–877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apodaca G. The uroepithelium: not just a passive barrier. Traffic 5: 117–128, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107: 603–613, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Balestreire EM, Apodaca G. Apical epidermal growth factor receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell 18: 1312–1323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA. Urothelial signaling. Hand Exp Pharmacol 202: 207–231, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Birder LA, De Groat WC, Apodaca G. Physiology of the urothelium. In: Textbook of the Neurogenic Bladder, edited by Corcos J, Schick E. London; New York: Martin Dunitz, 2008, p. 19–39 [Google Scholar]
- 8. Caruso M, Holgate ST, Polosa R. Adenosine signaling in airways. Curr Opin Pharmacol 6: 251–256, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 103: 203–215, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol 148: 2201–2206, 1992 [PubMed] [Google Scholar]
- 11. Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurol 99: 49–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology 64: 7–11, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Deussen A. Metabolic flux rates of adenosine in the heart. Naunyn Schmiedebergs Arch Pharmacol 362: 351–363, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Dhalla AK, Dodam JR, Jones AW, Rubin LJ. Characterization of an NBTI-sensitive equilibrative nucleoside transporter in vascular smooth muscle. J Mol Cell Cardiol 33: 1143–1152, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Drake MJ, Turner WH. Physiology of the smooth muscles of the bladder and urethra. In: Textbook of the Neurogenic Bladder: Adults and Children. London; New York: Martin Dunitz, 2004, p. xviii, 779 [Google Scholar]
- 16. Estrela AB, Abraham WR. Adenosine in the inflamed gut: a janus faced compound. Curr Med Chem 18: 2791–2815, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Fields RD, Burnstock G. Purinergic signaling in neuron-glia interactions. Nat Rev Neurosci 7: 423–436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharm Rev 53: 527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- 19. Gorlach A. Control of adenosine transport by hypoxia. Circ Res 97: 1–3, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, Nakano K, de Groat WC, Yoshimura N. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 α-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol 296: R1661–R1670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA 92: 4666–4670, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry 46: 10373–10383, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hofer S, Ivarsson L, Stoitzner P, Auffinger M, Rainer C, Romani N, Heufler C. Adenosine slows migration of dendritic cells but does not affect other aspects of dendritic cell maturation. J Invest Dermatol 121: 300–307, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol 301: F565–F573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res 67: 87–114, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A1 receptor mediates late preconditioning via activation of PKC-δ signaling pathway. Am J Physiol Heart Circ Physiol 283: H296–H301, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res 105: 220–232, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Law WR, Conlon BA, Ross JD. The extracellular cardiac purine metabolome in sepsis. Shock 28: 259–264, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Liang BT. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J 336: 337–343, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int 26: 387–395, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol 27: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lohse MJ, Klotz KN, Schwabe U, Cristalli G, Vittori S, Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 337: 687–689, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Maggi CA, Conte B, Furio M, Santicioli P, Giuliani S, Meli A. Further studies on mechanisms regulating the voiding cycle of the rat urinary bladder. Gen Pharm 20: 833–838, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA 94: 6541–6546, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCloskey KD. Interstitial cells in the urinary bladder–localization and function. Neurourol Urodyn 29: 82–87, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res 52: 25–39, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Naydenova Z, Rose JB, Coe IR. Inosine and equilibrative nucleoside transporter 2 contribute to hypoxic preconditioning in the murine cardiomyocyte HL-1 cell line. Am J Physiol Heart Circ Physiol 294: H2687–H2692, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Fluid Electrolyte Physiol 271: F886–F894, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Parratt JR. Protection of the heart by ischaemic preconditioning: mechanisms and possibilities for pharmacological exploitation. Trends Pharmacol Sci 15: 19–25, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Phillis JW, Smith-Barbour M. The adenosine kinase inhibitor, 5-iodotubercidin, is not protective against cerebral ischemic injury in the gerbil. Life Sci 53: 497–502, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem 279: 20234–20241, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 278: 13468–13479, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Pinna C, Eberini I, Puglisi L, Burnstock G. Presence of constitutive endothelial nitric oxide synthase immunoreactivity in urothelial cells of hamster proximal urethra. Eur J Pharmacol 367: 85–89, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schaddelee MP, Read KD, Cleypool CG, Ijzerman AP, Danhof M, de Boer AG. Brain penetration of synthetic adenosine A1 receptor agonists in situ: role of the rENT1 nucleoside transporter and binding to blood constituents. Eur J Pharm Sci 24: 59–66, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Shevock PN, Khan SR, Hackett RL. Urinary chemistry of the normal Sprague-Dawley rat. Urol Res 21: 309–312, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallon V, Osswald H. Adenosine receptors and the kidney. Hand Exp Pharmacol 193: 443–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Linden A, Eltzschig HK. Role of pulmonary adenosine during hypoxia: extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin Biol Ther 7: 1437–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Wang E, Truschel S, Apodaca G. Analysis of hydrostatic pressure-induced changes in umbrella cell surface area. Methods 30: 207–217, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta 1808: 1358–1379, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol 4: 111–125, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291: C254–C265, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLos One 5: e9224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]