Abstract
Objective To investigate whether screening kidney transplant recipients aged over 50 years for colorectal cancer with a faecal immunochemical test for haemoglobin might be justified, by determining the prevalence of advanced colorectal neoplasia and evaluating the diagnostic accuracy of faecal haemoglobin testing compared with colonoscopy in a population of kidney transplant recipients at otherwise average risk.
Design Cross sectional prevalence and diagnostic accuracy study with index test of faecal haemoglobin and reference standard of colonoscopy.
Setting Outpatient clinics in metropolitan and regional hospitals in South Australia.
Participants 229 kidney transplant recipients aged 50 years and over, who were at least 6 months (mean 9.0 (SD 8.4) years) post-transplant and otherwise at average risk of colorectal cancer, completed the study between June 2008 and October 2011.
Interventions Faecal immunochemical testing (Enterix Insure) for human haemoglobin, followed by colonoscopy with histological evaluation of retrieved samples.
Main outcome measures Prevalence of advanced colorectal neoplasia, defined as an adenoma at least 10 mm in diameter, villous features, high grade dysplasia, or colorectal cancer; sensitivity, specificity, and predictive values of faecal haemoglobin testing for advanced neoplasia compared with colonoscopy.
Results Advanced colorectal neoplasia was found in 29 (13%, 95% confidence interval 9% to 18%) participants, including 2% (n=4) with high grade dysplasia and 2% (n=5) with colorectal cancer. Faecal testing for haemoglobin was positive in 12% (n=28); sensitivity, specificity, and positive and negative predictive values for advanced neoplasia were 31.0% (15.3% to 50.8%), 90.5% (85.6% to 94.2%), 32.1% (15.9% to 52.4%), and 90.1% (85.1% to 93.8%). Colonoscopy was well tolerated, with no significant adverse outcomes. To identify one case of advanced neoplasia, 8 (6 to 12) colonoscopies were needed.
Conclusions Kidney transplant recipients aged over 50 years have a high prevalence of advanced colorectal neoplasia. Faecal haemoglobin screening for colorectal neoplasia has similar performance characteristics in transplant recipients to those reported in general population studies, with poor sensitivity but reasonable specificity. Surveillance colonoscopy might be a more appropriate approach in this population.
Trial registration Australian New Zealand Clinical Trials Registry ACTRN12608000154303.
Introduction
Kidney transplantation is the preferred treatment for end stage kidney disease. Short and medium term outcomes for transplant recipients are excellent,1 but mortality is significant in the long term, in large part due to malignancy.2 Transplant recipients need long term immunosuppressive treatment to prevent graft loss, and this has been associated with an increased risk of cancer.3 4 Although the greatest relative increase in risk has been seen for non-melanoma skin cancers and cancers associated with viral infection,3 the risk of more common solid organ cancers, including colorectal cancer, is also significantly increased with a relative risk compared with the general population of between two and three.3 4 5 Transplant recipients who develop colorectal cancer are often younger at diagnosis and have poorer outcomes when compared with the general population.5 6 7 8 9
Colorectal cancer is the third most commonly diagnosed cancer worldwide10; major risk factors include age, male sex, and a family history, and survival is strongly linked to the stage of cancer at diagnosis.11 In the general population, screening with guaiac based faecal occult blood tests followed by colonoscopy in people with a positive test has been shown to reduce the mortality of colorectal cancer.12 Faecal occult blood screening is recommended in people over the age of 50 with average risk.13 14 15 16 Immunochemical tests specific for faecal human haemoglobin have largely replaced the guaiac based tests used in early trials, on the basis of improved acceptability and performance.17 18 The effectiveness of screening depends on the identification and removal of colorectal adenomatous polyps with features associated with an increased risk of malignant transformation and early stage malignant disease, together termed “advanced colorectal neoplasia.”19 Colonoscopy can also been used to screen patients at average risk,20 21 22 23 but this is associated with higher costs, less convenience, and the potential for adverse outcomes in a small number of patients.24 It is, however, accepted as the most appropriate screening test for patients at moderate to high risk of colorectal cancer.13 14 15 25
No studies have been published on the benefits or harms of screening kidney transplant recipients for colorectal cancer. Clinical practice guidelines for the care of kidney transplant recipients have suggested screening from the age of 50 years with faecal haemoglobin and that this might be cost effective.26 27 28 However, the prevalence of advanced colorectal neoplasia is unknown, as is the diagnostic accuracy of faecal haemoglobin testing in kidney transplant recipients. Importantly, faecal haemoglobin might be less specific for colorectal neoplasia owing to the incidence of positive tests from colitis due to cytomegalovirus infection or toxicity of immunosuppressive drugs, for example.26 In addition, no data have been published on the safety of colonoscopy in transplant recipients, which might have increased harms due to the negative influence of immunosuppression.26
To fill this gap in available data, we did a cross sectional study of prevalence and diagnostic accuracy in a kidney transplant recipient population, using both faecal haemoglobin testing and colonoscopy to determine the prevalence and characteristics of advanced colorectal neoplasia and to evaluate the diagnostic accuracy of a faecal immunochemical test for human haemoglobin (the index test) compared with colonoscopy (the reference standard) to detect advanced colorectal neoplasia in this population.
Methods
Study design, setting, and participants
This was a population based, cross sectional study of prevalent kidney transplant recipients, conducted through outpatient clinics at metropolitan and regional hospitals in the state of South Australia. Three tertiary renal services, which collectively care for all adult transplant recipients in the state, were involved. The study took place between June 2008 and October 2011.
Kidney transplant recipients were eligible if they were aged over 50 years, were at least six months post-transplant, and gave written informed consent. Patients were invited to participate during the course of routine follow-up in the outpatient clinic. Exclusion criteria were previous colorectal cancer or adenoma with high grade dysplasia, colonoscopy within 12 months, symptoms or signs suggestive of colorectal cancer or other significant non-neoplastic colorectal disease, known or suspected familial colorectal cancer syndrome (patients with cancer in one close relative were eligible), chronic inflammatory bowel disease, unstable cardiopulmonary disease, bleeding disorder or unacceptable risk of bleeding, terminal illness or life threatening malignancy, or a failed transplant and subsequent return to dialysis.
Patients with a history of previous rectal bleeding attributed to haemorrhoids or other non-neoplastic disease were eligible provided bleeding was not an active problem. Patients receiving anticoagulant treatment were also eligible provided they could discontinue this treatment at the time of screening colonoscopy, if requested by the endoscopist. Patients who had had a previous faecal haemoglobin test, colonoscopy, or both were eligible provided they did not meet any of the other exclusion criteria.
Study procedures
Study participants completed a faecal immunochemical test for human haemoglobin by using the Enterix Insure kit (Enterix Australia). This test uses a brush to obtain each faecal sample, which is then applied to a testing card; two samples are taken from consecutive bowel motions. Participants completed a questionnaire concerning timing of the samples, colorectal symptoms, and details of colorectal disease, previous screening, and family history. A history of anticoagulant or antiplatelet drug use and allergies was also sought. We reviewed medical records to provide additional data.
De-identified test cards were forwarded to a central laboratory (Enterix Australia, Sydney) for processing using methods previously described.18 Study investigators had no involvement in the laboratory analysis of samples.
After faecal testing, and regardless of the result, participants were referred for colonoscopy. To reduce the risk of dropout, participants were informed of faecal haemoglobin results before colonoscopy only on request. Interventional gastroenterologists and colorectal surgeons did colonoscopies; to meet ethical clearance requirements, we could not blind physicians to the faecal haemoglobin result. In the three major participating centres, specific colonoscopy lists were arranged for this study; colonoscopies were also done in regional hospitals and in the private sector.
Participants received bowel preparation according to the endoscopist’s preference—usually a polyethylene glycol based preparation alone or in combination with a sodium picosulfate based preparation. Sodium phosphate preparations were not used. At physicians’ discretion, participants could be admitted for intravenous hydration before colonoscopy. Participants had a serum creatinine measurement to estimate renal function (glomerular filtration rate) before and after colonoscopy.29 Any adverse events were noted.
During colonoscopy, the location and size of all polypoid lesions were recorded, and the presence of any other lesions was noted. We defined location within the colon as either proximal (caecum, ascending or transverse colon), distal (descending or sigmoid colon), or in the rectum. Biopsy forceps were used to estimate the size of each polyp. If the examination of the bowel was incomplete because of failure to reach the caecum or poor bowel preparation, the patient was asked to return for a second attempt at the discretion of the endoscopist; we included combined results in the analysis when this occurred within six months of the date of the original colonoscopy. If a participant had surgery as a result of colonoscopy findings, we included results from surgically resected specimens in the analysis.
Classification of index test and reference standard results
We recorded faecal immunochemical test results as either positive or negative for the presence of human haemoglobin, as reported by the laboratory. Retrieved colorectal biopsy samples were sent to local laboratories for histological examination. Pathologists were not aware of the faecal haemoglobin result or that patients were enrolled in this study. The final diagnosis for each participant was determined on the basis of combined results of colonoscopy and histology of retrieved or resected specimens.
To calculate the prevalence of advanced colorectal neoplasia, as well as other pathology, we classified participants on the basis of their most advanced lesion. For example, we classified a participant with an adenoma with high grade dysplastic changes and a tubular adenoma as having an advanced adenoma with high grade dysplasia. We defined advanced colorectal neoplasia, in accordance with previous studies,20 21 22 as the presence of either cancer or an advanced adenoma—a tubular adenoma of at least 10 mm diameter, a villous or tubulovillous adenoma (that is, at least 25% villous), or an adenoma with high grade dysplasia. We diagnosed and staged cancer according to the American Joint Committee on Cancer’s classification.30
Data analysis
We used Stata software version 11.2 for all statistical analyses. We express data as raw values and percentages for categorical data and as mean (standard deviation) or median (interquartile range) for continuous data. We used Student’s t test to compare continuous variables and logistic regression to determine associations between categorical variables. We set statistical significance for hypothesis testing at 0.05 (that is P≤0.05). All statistical tests were two tailed.
To evaluate the diagnostic accuracy of faecal haemoglobin to detect advanced colorectal neoplasia, we compared results with colonoscopy findings by using two by two tables. We calculated sensitivity, specificity, positive and negative predictive values, likelihood ratios, and diagnostic odds ratios. We present estimates with 95% confidence intervals.
Sample size
We estimated the prevalence of advanced colorectal neoplasia in transplant recipients to be 12%, on the basis of a general population prevalence of approximately 6%23 and transplant registry data suggesting an increased risk of colorectal cancer in transplant recipients of at least twofold. We estimated that a sample size of 218 participants would allow us to detect a prevalence (proportion) of advanced neoplasia of 12% (0.12), with a resulting binomial exact 95% confidence interval that ranged from 7.9% to 17.0% (that is, less than 5% in either direction). Assuming a completion rate of 60-70%, we sought to target between 310 and 360 patients for recruitment.
Results
Participants
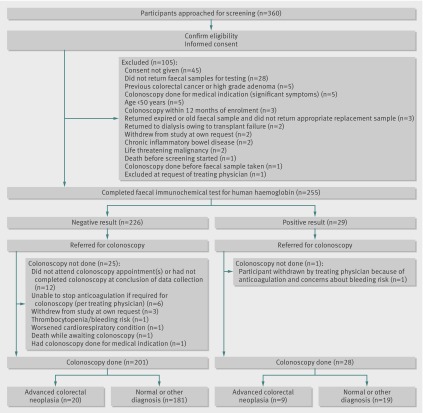
From information available from hospital units’ records, we estimated that the population of eligible transplant recipients in South Australia would be approximately 400 patients. Between 1 June 2008 and 30 June 2011, 360 patients were assessed for initial eligibility and invited to participate. The figure shows the flow of patients through the study, along with the primary outcome of advanced colorectal neoplasia. Patients who were excluded (and reasons for this) or who withdrew from the study are noted. In total, 229 patients completed the study, a completion rate of 64%; table 1 outlines the characteristics of these participants. Twenty-six (10%) patients who completed faecal haemoglobin testing did not have colonoscopy and were excluded from analysis. Table 2 shows details of previous colorectal history and screening. Outcomes for all participants were finalised according to available results as at 31 October 2011.
Fig 1 Flow of participants through study
Table 1.
Characteristics of study participants who completed screening. Values are numbers (percentages) unless stated otherwise
| Characteristic | Value (n=229) |
|---|---|
| Mean (SD) age (years): | |
| At transplant | 52.6 (10.3) |
| At screening | 61.5 (6.9) |
| Male sex | 151 (66) |
| Time since transplant (years): | |
| <5 | 97 (42) |
| 5-10 | 57 (25) |
| 10-15 | 35 (15) |
| 15-20 | 16 (7) |
| >20 | 24 (10) |
| Mean (SD) | 9.0 (8.4) |
| Median | 6.5 |
| Renal function: | |
| Mean (SD) serum creatinine (µmol/L) | 122.2 (47.3) |
| Mean (SD) MDRD eGFR (mL/min/1.73 m2) | 55.1 (19.3) |
| Cause of end stage kidney disease: | |
| Glomerulonephritis | 127 (55) |
| Polycystic kidney disease | 32 (14) |
| Diabetic nephropathy | 20 (9) |
| Hypertension | 8 (3) |
| Other | 36 (16) |
| Unknown | 6 (3) |
| Deceased donor | 189 (83) |
| Second or subsequent transplant | 29 (13) |
| Immunosuppressive drugs at time of screening: | |
| Triple—ciclosporin based (+ MMF/azathioprine and steroids) | 24 (10) |
| Triple—tacrolimus based (+ MMF/azathioprine and steroids) | 53 (23) |
| Triple—mTOR inhibitor based (+ MMF/azathioprine and steroids) | 45 (20) |
| Dual—ciclosporin based (+ MMF/azathioprine) | 35 (15) |
| Dual—tacrolimus based (+ MMF/azathioprine) | 12 (5) |
| Dual—mTOR inhibitor (+ MMF/azathioprine) | 10 (4) |
| Dual—MMF/azathioprine + steroids | 25 (11) |
| Other (including steroid or MMF alone, experimental agents) | 21 (9) |
| Not on immunosuppression at time of screening | 1 (<1) |
| Not recorded | 1 (<1) |
| Current or former smoker | 86 (38) |
| History of other significant medical conditions: | |
| Cardiovascular disease | 93 (41) |
| Diabetes | 81 (35) |
| Chronic lung disease | 23 (10) |
| Previous malignancy: | |
| Non-melanoma skin cancer (SCC or BCC) | 75 (33) |
| Other* | 20 (9) |
| Antiplatelet or anticoagulant drug use: | |
| Aspirin | 69 (30) |
| Clopidogrel | 8 (3) |
| Warfarin | 19 (8) |
BCC=basal cell carcinoma; MDRD eGFR=glomerular filtration rate estimated using four variable modification of diet in renal disease equation; MMF=mycophenolic acid derivative (mycophenolate mofetil or mycophenolic acid sodium); mTOR inhibitor=mammalian target of rapamycin inhibitor (sirolimus or everolimus); SCC=squamous cell carcinoma.
*Melanoma (n=4); prostate (n=4); bladder (n=3); cervical (n=2); head and neck (n=2); renal (n=2); endometrium (n=1); breast (n=1); lymphoma (n=1); leukaemia (n=1).
Table 2.
Reported colorectal history and previous screening of study participants
| Reported participants’ history item | No (%) (n=229) |
|---|---|
| Family history of colorectal cancer in first degree relative* | 24 (10) |
| History of non-neoplastic colorectal disease (not polyps): | |
| Any non-neoplastic disease | 14 (6) |
| Diverticulosis | 11 (5) |
| Other | 3 (1) |
| History of colonic polyps: | |
| Any polyps | 17 (7) |
| Previous low grade tubular adenomas | 8 (3) |
| Polyps—details unknown or unspecified | 9 (4) |
| History of rectal bleeding before study: | |
| Any rectal bleeding | 57 (25) |
| Haemorrhoids | 35 (15) |
| Other non-neoplastic causes (anal fissure, diverticulitis, other) | 22 (10) |
| Rectal bleeding reported initially at time of faecal sample submission: | |
| Within 4 weeks of sampling | 1 (<1) |
| At time of faecal sampling | 0 |
| Previous faecal screening for human haemoglobin/occult blood: | |
| Any | 79 (34) |
| Known positive result in past | 9 (4) |
| Previous colonoscopy (screening or otherwise) at any time | 78 (34) |
| Previous colonoscopy after transplant: | |
| At any time after transplant | 38 (17) |
| Within 1-5 years of screening study | 21 (9) |
| >5 years before screening study | 17 (7) |
| Previous colonoscopy before transplant: | |
| At any time before transplant | 40 (17) |
| Within 1-5 years of screening study | 19 (8) |
| >5 years before screening study | 21 (9) |
*More than one affected first degree relative was an exclusion criterion; no participants reported this.
Quality of faecal haemoglobin testing and colonoscopy
Faecal testing cards were developed a mean of 9.0 (SD 4.4) days after the first faecal sample was taken and 8.2 (4.1) days after the second sample. The median interval between the faecal haemoglobin result and completion of colonoscopy was 82 (interquartile range 47-135) days.
The caecum was intubated in 219 (96%) of 229 screening colonoscopies. Colonoscopy was incomplete in six cases; screening was supplemented with computed tomographic colonography (n=2), barium enema (n=2), or a repeat colonoscopy 12 months later (n=1). One participant did not attend the appointment for computed tomographic colonography. In four cases, no data on caecal intubation was recorded in the colonoscopy report.
Views of the bowel were considered at least partly reduced because of suboptimal preparation in 36 (16%) participants. Seven (3%) participants returned for a follow-up colonoscopy with improved preparation and had composite results from both procedures included in study outcomes.
Primary outcomes
Table 3 shows the final diagnoses after colonoscopy, classified according to the most advanced lesion. Overall, 29 (13%, 95% confidence interval 9% to 18%) participants had advanced colorectal neoplasia; 4 (2%) participants had a high grade dysplastic lesion, and 5 (2%) had previously undiagnosed colorectal cancer. The appendix gives further details of the location and staging of the cancer and the surgical procedure performed. In addition to the participants who had surgery for cancer, one participant with a large adenoma had a right hemicolectomy.
Table 3.
Final diagnosis in study participants, according to most advanced lesion
| Diagnosis | No (%) (n=229) |
|---|---|
| Normal or non-neoplastic disease | 157 (69) |
| Non-advanced adenoma | 43 (19) |
| Advanced adenoma: | 24 (10) |
| Tubular adenoma ≥10 mm | 11 (5) |
| Villous/tubulovillous adenoma (regardless of size) | 9 (4) |
| High grade dysplasia (regardless of size) | 4 (2) |
| Colorectal cancer | 5 (2) |
| Prevalence of advanced colorectal neoplasia | 29 (13, 95% CI 9 to 18) |
Faecal haemoglobin was positive in 28 (12%, 8% to 17%) participants. We found no association between a previous history of visible rectal bleeding or the use of aspirin, warfarin, or clopidogrel and the faecal haemoglobin result (P>0.05). Tables 4 and 5 show analyses of the diagnostic accuracy of faecal haemoglobin to detect advanced colorectal neoplasia. Among the five participants ultimately diagnosed as having cancer, three had positive faecal haemoglobin (sensitivity of 60%); the tumour’s location was not associated with the faecal haemoglobin result (P>0.05).
Table 4.
Two by two table of faecal haemoglobin (index test) results versus colonoscopy (reference standard) for detection of advanced colorectal neoplasia
| Advanced colorectal neoplasia | Faecal haemoglobin result | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Present | 9 | 20 | 29 |
| Absent | 19 | 181 | 200 |
| Total | 28 | 201 | 229 |
Table 5.
Diagnostic accuracy estimates of faecal haemoglobin screening for detection of advanced colorectal neoplasia
| Parameter | Point estimate (95% CI) |
|---|---|
| Sensitivity (%) | 31.0 (15.3 to 50.8) |
| Specificity (%) | 90.5 (85.6 to 94.2) |
| Positive predictive value (%) | 32.1 (15.9 to 52.4) |
| Negative predictive value (%) | 90.1 (85.1 to 93.8) |
| Positive likelihood ratio | 3.27 (1.64 to 6.52) |
| Negative likelihood ratio | 0.762 (0.595 to 0.977) |
| Diagnostic odds ratio | 4.29 (1.75 to 10.60) |
Of the 72 participants with neoplasia, 18 (8%) had two or more adenomas and seven (3%) had three or more. One participant with colorectal cancer also had a tubulovillous adenoma. In addition to colorectal neoplasia, non-neoplastic disease was frequently detected; the most common diagnoses were diverticular disease (n=76; 33%) and haemorrhoids (n=23; 10%). The appendix gives further details of the location of lesions and of non-neoplastic disease.
Safety of colonoscopy and adverse events
No significant adverse events occurred as a result of colonoscopy. Four (2%) patients had minor bleeding in association with polypectomy that was controlled endoscopically. Other minor adverse events are noted in the appendix.
Renal function results were available before and after colonoscopy for 226 participants (three had data missing). Mean estimated glomerular filtration rate did not change (55.1 (SD 19.3) versus 54.5 (19.4) mL/min/1.73 m2; P>0.05, paired t test).
Twenty-one (9%) participants were admitted to hospital for intravenous hydration (mean creatinine 171.0 (80.6) μmol/L) before colonoscopy. No adverse outcomes were noted in this group.
Sensitivity and subgroup analyses
We did a sensitivity analysis to determine if the diagnostic accuracy of faecal haemoglobin testing would be significantly affected by verification bias due to the exclusion of 26 patients who did not have colonoscopy. To estimate the effect of missing reference standard data, we assumed hypothetical best case and worst case scenarios from the perspective of faecal testing and added the results to the observed data: best case—positive = advanced neoplasia present, negative = prevalence of advanced neoplasia same as observed data for negative faecal haemoglobin results (10%); worst case—positive = no advanced neoplasia, negative = double prevalence advanced neoplasia (20%). Under these scenarios, sensitivity reduced slightly (range 26.5-30.3%); specificity was largely unchanged (91.0-91.4%).
To take account of “suboptimal” colonoscopy (that is, caecum not intubated or poor preparation, without a follow-up procedure) or a long interval between faecal sampling and colonoscopy (>6 months), we estimated diagnostic accuracy separately with these participants excluded. Faecal haemoglobin performed slightly better with optimal colonoscopy (n=188 participants), with an increase in sensitivity (36.0%, 18.0% to 57.5%), specificity (92.0%, 86.7% to 95.7%), and the diagnostic odds ratio (6.49, 2.46 to 17.3). No improvement occurred when we excluded longer time intervals (n=196; diagnostic odds ratio 3.71, 1.5 to 9.24).
Predictors of colorectal neoplasia
In univariate logistic regression analyses, the only characteristic of participants that was significantly associated with an increased risk of cancer was age (unadjusted odds ratio 1.16 (95% confidence interval 1.01 to 1.33) per year). We found similar results when we analysed age in 5 year bands (odds ratio 2.07 (1.04 to 4.13) for every 5 years above 55). All patients diagnosed as having cancer were male; none had had previous screening colonoscopy or a family history of cancer. No identifiable characteristics of patients were significantly associated with advanced neoplasia.
Previous screening for colorectal cancer
Seventy-eight (34%) participants had had one or more colonoscopies before enrolment in this study (table 2). A family history of colorectal cancer (odds ratio 2.37, 1.10 to 5.10) and a history of rectal bleeding (2.00, 1.09 to 3.68) were both associated with a previous colonoscopy. We did not find any significant differences in the rates of advanced and non-advanced neoplasia between participants who had had previous screening in a post hoc analysis (data not shown).
Discussion
In a population of asymptomatic kidney transplant recipients aged over 50 years at otherwise average risk for colorectal cancer, we found a high prevalence of advanced colorectal neoplasia (13%, 95% confidence interval 9% to 18%). To detect one case of advanced colorectal neoplasia, 8 (6 to 12) participants needed to have colonoscopy. Faecal haemoglobin had poor sensitivity (31.0%, 15.3% to 50.8%) but reasonable specificity (90.5%, 85.6% to 94.2%) for advanced neoplasia. If colonoscopy had been done only after a positive faecal haemoglobin test (as in population screening), three participants would have needed to have colonoscopy for each case identified, but 20 (69%) cases of advanced neoplasia would have gone undetected.
This is the first study to evaluate faecal haemoglobin screening for colorectal neoplasia in kidney transplant recipients, and it is the only study of colonoscopy surveillance among transplant recipients of a similar age and risk profile to the general population included in trials of screening that showed reduced mortality from colorectal cancer. This study presents for the first time prospective data on the safety of routine surveillance colonoscopy in kidney transplant recipients and shows that this can be implemented without adverse effects in a population with a wide range of renal function and comorbidity.
Strengths and limitations of study
This study has several important strengths. We recruited a high proportion of potentially eligible transplant recipients in South Australia, reaching the target sample size in just over three years. Of 360 patients who were identified, 71% enrolled and submitted a faecal sample, and 64% completed the study—a high rate for a study involving an intervention such as colonoscopy, reducing the potential for selection bias. Quality of performance of colonoscopy was high (96% caecal intubation rate); the diagnostic accuracy of faecal haemoglobin was not significantly affected when suboptimal colonoscopies were excluded. A sensitivity analysis indicated that the 10% of participants excluded because of not having a colonoscopy would not have introduced a significant risk of verification bias.
Because we included a broad range of patients, from both metropolitan and regional settings (not just those cared for at the transplanting centre), who had colonoscopies done in a variety of setting by a variety of operators, these results are more likely to be generalisable to other transplant populations, although the high participation rate achieved could be argued to reflect a cohort of patients that is more compliant and cooperative than might be found in other jurisdictions. However, the demographic and comorbidity profiles of the participants in this study are similar to those of the transplant population aged over 50 years in Australia as a whole and those reported in international comparisons.1 2 31 We chose an age of eligibility of 50 years on the basis that current guidelines recommend starting screening from this age onwards.13 14 16 Although evidence shows that the relative risk of colorectal cancer is highest in younger transplant patients,5 the absolute risk in those aged under 50 remains low. Sex, family history, and a history of rectal bleeding due to non-neoplastic disease were not discriminating factors, and screening was offered from six months after transplantation, although the median time post-transplant was 6.5 years.
A potential criticism of our study is that we used a cross sectional design without a control (that is, non-transplant recipient) group, which limits our ability to directly compare the findings with the general population. However, this study was designed to fill the gaps in evidence for screening of transplant recipients, rather than as a comparison with the general population. Doing a large scale colonoscopy study in a comparable general population cohort truly at average risk would have been ethically and logistically difficult, given established screening guidelines and that a population screening programme has been implemented in Australia, similar to those in other countries.14 32 33 Such a study of participants at average risk selected from the general population by using electoral (voter) registrations encountered difficulties in recruiting participants to have colonoscopy and produced limited results.34
This study has limited statistical power to identify predictors of risk of neoplasia or to compare subgroups owing to the relatively small number of cases of advanced colorectal neoplasia and cancer. Larger studies in multiple jurisdictions would be needed to identify sufficient cases to investigate the relations between factors such as immunosuppressive drugs or other transplant related factors and colorectal neoplasia.
Although laboratory analysis of faecal samples and the interpretation of histology were done in a blinded fashion, we were not able to blind endoscopists to the faecal haemoglobin result. This was because of ethical requirements to ensure that abnormal test results were made available. This may have introduced “expectation” bias through greater effort being made to identify neoplastic disease at colonoscopy after a positive result and might potentially have increased the specificity of faecal haemoglobin. However, in clinical practice this information would always be available to a physician doing colonoscopy.
In our study, we included participants who had had a previous colonoscopy, as long as it was more than a year before enrolment in the study. This creates a potential for “conservative” bias and implies that the prevalence of neoplasia in a population of participants naive to screening might be higher than our findings suggest. However, we judged it inappropriate to exclude such participants on the basis that little has been known about the development or progression of pre-malignant colorectal neoplasia in transplant recipients, whereas the poorer outcomes from cancers in this group have been reported for some time.6 7 9 None of the cancers identified in this study was found in a pre-screened patient, although no differences existed in the rates of advanced and non-advanced adenomas between previously screened and screen naive participants.
Comparisons with other studies
In a meta-analysis of studies of screening with colonoscopy in the general population, with a similar age, sex, and family history profile to this study, the prevalence of colorectal cancer was 0.78% (0.13% to 2.97%) and that of advanced neoplasia was 5.0% (4.0% to 6.0%).23 The finding of a prevalence estimate of advanced colorectal neoplasia (the precursor to colorectal cancer) in this study of 13% (that is, approximately twice the prevalence in the general population) is consistent with other studies showing a standardised incidence ratio of colorectal cancer of approximately two to three in transplant recipients.3 4 5 The increased risk of many types of cancers in transplant recipients has been associated with immunosuppression3; however, we were not able to show an association between the duration or type of immunosuppression and neoplasia, perhaps because of the relatively small number of cases. Other as yet unrecognised factors may also have contributed to the increased risk of colorectal cancer in these patients, and larger studies of transplant populations will be needed to determine these.
In line with current clinical practice, we chose a faecal immunochemical test (Insure, Enterix Australia) as a means to screen for colorectal neoplasia. The sensitivity and predictive value of this test were poor for advanced colorectal neoplasia, although specificity was better. Although several stools were sampled, testing was done on a one-off basis, and sensitivity could potentially be improved with serial testing.12 35 The Insure faecal immunochemical test has been found to have superior diagnostic accuracy for the detection of advanced colorectal neoplasia compared with sensitive guaiac faecal occult blood tests (such as Hemoccult II SENSA),18 and it can speed detection of interval cancers when used in a colonoscopy surveillance programme.35 In addition, it had the highest level of acceptability to patients in a randomised trial of participation in screening that included several different immunochemical tests,36 and it had a high screening uptake rate and acceptability to patients in the large Australian bowel cancer screening pilot.37
The performance characteristics of immunochemical faecal haemoglobin tests to identify advanced colorectal neoplasia have been variable across a range of studies.38 39 40 41 However, a large study of a one-off faecal haemoglobin versus colonoscopy found a sensitivity and specificity for advanced neoplasia of 27.1% and 95.1%,40 comparable to our findings. Faecal haemoglobin thus seems to have similar diagnostic accuracy to detect advanced colorectal neoplasia in both the transplant and general populations. Factors such as sub-clinical colitis from cytomegalovirus infection or drug toxicity, or potentially microscopic haematuria, which might be present in some transplant recipients, most commonly in the early post-transplant period, do not seem to have resulted in any significant adverse effect on specificity in this predominantly long term post-transplant cohort.
In a case-control study of colonoscopy surveillance done as “usual care” in 315 kidney transplant recipients compared with 630 general population controls referred for screening colonoscopy (and therefore unlikely to be truly at “average risk” owing to referral bias), Park and colleagues reported prevalences of 7.0% for advanced adenomas and 1.9% for cancers.42 The odds ratios were 3.5 for advanced adenomas and 12.0 for cancers in screened transplant recipients. However, most of the transplant cohort were aged under 50, and such a population would be expected to have low rates of advanced colorectal neoplasia, compared with the older population included in our study.
Conclusions, policy implications, and questions for future research
The findings of this study have important implications for the development of guidelines on screening for colorectal cancer in kidney transplant recipients. Although the sensitivity of the immunochemical faecal haemoglobin test is poor, its performance as a screening test for neoplasia in transplant recipients is comparable to that reported in studies of population screening, without any apparent loss of specificity. Colonoscopy seems to be safe to use as a surveillance tool in transplant recipients.
Given the difficulties and costs of doing studies of this type in transplant recipient populations, or indeed randomised interventional trials, definitive data to determine whether the benefits of screening outweigh the harms are unlikely to be available. Our study provides novel data that can be used in decision analyses and cost effectiveness studies to inform clinical practice guidelines, which to date have relied on extrapolations of general population data.26 28 In addition, long term follow-up of this cohort compared with an unscreened transplant population should provide comparative data on the outcomes of screening transplant recipients for colorectal cancer. From a payer’s perspective, a simplistic analysis of our data would suggest that the estimated cost of detecting one advanced neoplasia through surveillance colonoscopy of transplant recipients, at an estimated cost of $A1192 (£786; €990; $1219) per procedure,43 would be $A9536 (95% confidence interval $A7152 to $A14 304), but the cost effectiveness of such an approach is unknown. How transplant patients might regard surveillance colonoscopy is unclear, but the high participation rate here suggests that many are comfortable with this approach.
In conclusion, the main findings of this study are that a high prevalence of advanced colorectal neoplasia exists in kidney transplant recipients over the age of 50 and that, compared with when it is used in the general population, faecal haemoglobin screening of transplant recipients has similarly low sensitivity although acceptable specificity for neoplasia. Given the high prevalence of neoplasia, screening with faecal haemoglobin testing alone will miss significant lesions with the potential to develop into colorectal cancer. Taken together with previously reported increased risk of colorectal cancer and poor outcomes, these findings lead us to conclude that surveillance with colonoscopy may be the most appropriate approach to reduce the risk of colorectal cancer in kidney transplant recipients. Further studies are needed to assess the outcomes and costs of such an approach.
What is already known on this topic
Faecal haemoglobin screening in people over the age of 50 years reduces mortality from colorectal cancer in the general population
Kidney transplant recipients, compared with the general population, have an increased risk of developing colorectal cancer and have inferior outcomes once cancer develops
Data on the prevalence of colorectal neoplastic lesions and the diagnostic accuracy of faecal haemoglobin tests have been insufficient to enable guidelines to be developed on screening for colorectal cancer in kidney transplant recipients
What this study adds
Kidney transplant recipients aged over 50, at otherwise average risk, have a high prevalence of advanced colorectal neoplasia (13%), the precursor to most colorectal cancers
Faecal haemoglobin screening for colorectal neoplasia in transplant recipients has a similar poor sensitivity (31.0%) to that reported in general population studies but reasonable specificity (90.5%)
Colonoscopy surveillance rather than faecal haemoglobin screening may be a better option to reduce the risks of colorectal cancer in kidney transplant recipients
We thank the physicians, surgeons, nurses, and kidney transplant recipients who took part in this study. We also thank Enterix Australia for providing faecal immunochemical testing at no cost and Nancy Briggs (from the Australian and New Zealand Dialysis and Transplant Registry) who provided statistical advice.
Investigators and participating centres were as follows. Nephrologists: Queen Elizabeth Hospital and Royal Adelaide Hospital—Michael Collins, P Toby Coates, Stephen McDonald, and Graeme Russ (all principal investigators); Kym Bannister, Robert Carroll, Ghee Chew, Duncan Cooke, Susan Crail, Alex Disney, Tony Elias, Randall Faull (co-investigator), Lisa Jeffs, Shilpa Jesudason, Chen Au Peh, Natasha Rogers, and Shaundeep Sen; Flinders Medical Centre—Jeffrey Barbara, Jonathan Gleadle, Rajiv Juneja (co-investigator), Caroline Milton, and George Passaris. Gastroenterologists/colorectal surgeons: Queen Elizabeth Hospital—Edward Teo (principal investigator, 104 colonoscopies); Flinders Medical Centre and Tennyson Cancer Centre—Peter Bampton (principal investigator, 48); Mt Gambier Hospital—Matthias Wichmann (co-investigator, 14); Royal Adelaide Hospital—Mark Schoeman (co-investigator, 13); others who performed or assisted at two or more colonoscopies—Judith Gapasin (8), Kate Muller (6), John Bate (4), M Yee (4), Basile Alexander (3), Michael Damp (3), Nam Nguyen (3), Sam Hall (2), Peter Hewett (2), Richard Holloway (2), Matthew Lawrence (2), Kenneth Lim (2), Venkat Mahesh (2), Michelle Thomas (2), and Daniel Van Langenberg (2). In addition, another 31 gastroenterologists/surgeons performed or assisted at a single colonoscopy (not listed here).
Contributors: MGC, PTC, PAB, SPMcD, GRR, SRC, and GPY contributed to the research design. MGC, GPY, PAB, and PTC obtained funding for the study. MGC, C-YC, and PTC coordinated the study; C-YC provided administrative and technical support. PTC, GRR, SPMcD, MGC, and other nephrologists listed above as investigators were responsible for recruitment. SRC and GPY provided access to faecal haemoglobin screening. ET and PAB had primary responsibility for colonoscopy performance and data acquisition; investigators listed above also did colonoscopies. MGC analysed the data, including statistical analysis; all authors contributed to the interpretation of the data. MGC drafted the manuscript; all authors reviewed it and approved the final manuscript. PAB and PTC are joint senior authors of this study; MGC and PTC are the guarantors.
Funding: This study was funded by the Queen Elizabeth Hospital Research Foundation and also by Roche Products Pty Australia through the CellCept Australia Research Grants (CARG). Enterix Australia generously supplied the Insure kits and processed the faecal samples at no cost. Most colonoscopy procedures were done through the public healthcare system as “standard of care,” where such procedures are available for colorectal cancer screening of patients thought to be at increased risk. None of the sponsors had any role in study design, data collection, analysis, interpretation, or the writing of the manuscript. This research was conducted completely independently from funders.
Competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: all authors received support from Roche Products Australia, Queen Elizabeth Hospital Research Foundation, and Enterix Australia for the submitted work as described above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by human research ethics committees at the Queen Elizabeth Hospital, Royal Adelaide Hospital, and Flinders Medical Centre. All participants provided written informed consent.
Data sharing: No additional data available.
Cite this as: BMJ 2012;345:e4657
Web Extra. Extra material supplied by the author
References
- 1.McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991-2001. Nephrol Dial Transplant 2002;17:2212-9. [DOI] [PubMed] [Google Scholar]
- 2.McDonald SP, Excell L, Livingstone B. ANZDATA registry report. Australia and New Zealand Dialysis and Transplant Registry, 2009.
- 3.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823-31. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant 2004;4:905-13. [DOI] [PubMed] [Google Scholar]
- 5.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant 2007;7:2140-51. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EE, Leverson GE, Pirsch JD, Heise CP. A 30-year analysis of colorectal adenocarcinoma in transplant recipients and proposal for altered screening. J Gastrointest Surg 2007;11:272-9. [DOI] [PubMed] [Google Scholar]
- 7.Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 2009;87:1347-59. [DOI] [PubMed] [Google Scholar]
- 8.Papaconstantinou HT, Sklow B, Hanaway MJ, Gross TG, Beebe TM, Trofe J, et al. Characteristics and survival patterns of solid organ transplant patients developing de novo colon and rectal cancer. Dis Colon Rectum 2004;47:1898-903. [DOI] [PubMed] [Google Scholar]
- 9.Buell JF, Papaconstantinou HT, Skalow B, Hanaway MJ, Alloway RR, Woodle ES. De novo colorectal cancer: five-year survival is markedly lower in transplant recipients compared with the general population. Transplant Proc 2005;37:960-1. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Cancer: fact sheet number 297. WHO, 2011.
- 11.Lieberman DA. Clinical practice: screening for colorectal cancer. N Engl J Med 2009;361:1179-87. [DOI] [PubMed] [Google Scholar]
- 12.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541-9. [DOI] [PubMed] [Google Scholar]
- 13.Colorectal cancer screening. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ 2001;165:206-8. [PMC free article] [PubMed] [Google Scholar]
- 14.Guidelines for the prevention, early detection and management of colorectal cancer. Cancer Council Australia and Australian Cancer Network, 2005.
- 15.Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627-37. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med 2008;149:638-58. [DOI] [PubMed] [Google Scholar]
- 17.Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010;59:62-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006;107:2152-9. [DOI] [PubMed] [Google Scholar]
- 19.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 2002;12:1-9. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G; Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med 2000;343:162-8. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005;352:2061-8. [DOI] [PubMed] [Google Scholar]
- 22.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006;355:1863-72. [DOI] [PubMed] [Google Scholar]
- 23.Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci 2008;53:3049-54. [DOI] [PubMed] [Google Scholar]
- 24.Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc 2002;55:307-14. [DOI] [PubMed] [Google Scholar]
- 25.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [DOI] [PubMed] [Google Scholar]
- 26.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(suppl 3):S1-155. [DOI] [PubMed] [Google Scholar]
- 27.Kiberd BA, Keough-Ryan T, Clase CM. Screening for prostate, breast and colorectal cancer in renal transplant recipients. Am J Transplant 2003;3:619-25. [DOI] [PubMed] [Google Scholar]
- 28.Wong G, Howard K, Craig JC, Chapman JR. Cost-effectiveness of colorectal cancer screening in renal transplant recipients. Transplantation 2008;85:532-41. [DOI] [PubMed] [Google Scholar]
- 29.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, et al. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 2006;6:100-8. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, eds. AJCC cancer staging manual. 7th ed. Springer, 2010.
- 31.Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) study. Am J Transplant 2010;10:338-53. [DOI] [PubMed] [Google Scholar]
- 32.Flitcroft KL, St John DJ, Howard K, Carter SM, Pignone MP, Salkeld GP, et al. A comparative case study of bowel cancer screening in the UK and Australia: evidence lost in translation? J Med Screen 2011;18:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570-95. [DOI] [PubMed] [Google Scholar]
- 34.Forbes GM, Mendelson RM, Edwards JT, Foster NM, Pawlik JZ, Bampton PA, et al. A comparison of colorectal neoplasia screening tests: a multicentre community-based study of the impact of consumer choice. Med J Aust 2006;184:546-50. [DOI] [PubMed] [Google Scholar]
- 35.Lane JM, Chow E, Young GP, Good N, Smith A, Bull J, et al. Interval fecal immunochemical testing in a colonoscopic surveillance program speeds detection of colorectal neoplasia. Gastroenterology 2010;139:1918-26. [DOI] [PubMed] [Google Scholar]
- 36.Cole SR, Young GP, Esterman A, Cadd B, Morcom J. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen 2003;10:117-22. [DOI] [PubMed] [Google Scholar]
- 37.Commonwealth of Australia. The Australian bowel cancer screening pilot progam and beyond: final evaluation report. Commonwealth of Australia, 2005.
- 38.Burch JA, Soares-Weiser K, St John DJ, Duffy S, Smith S, Kleijnen J, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. J Med Screen 2007;14:132-7. [DOI] [PubMed] [Google Scholar]
- 39.Loganayagam A. Faecal screening of colorectal cancer. Int J Clin Pract 2008;62:454-9. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005;129:422-8. [DOI] [PubMed] [Google Scholar]
- 41.Rosman AS, Korsten MA. Effect of verification bias on the sensitivity of fecal occult blood testing: a meta-analysis. J Gen Intern Med 2010;25:1211-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JM, Choi MG, Kim SW, Chung IS, Yang CW, Kim YS, et al. Increased incidence of colorectal malignancies in renal transplant recipients: a case control study. Am J Transplant 2010;10:2043-50. [DOI] [PubMed] [Google Scholar]
- 43.Tran B, Keating CL, Ananda SS, Kosmider S, Jones I, Croxford M, et al. A preliminary analysis of the cost-effectiveness of the National Bowel Cancer Screening Program—demonstrating the potential value of comprehensive real world data. Intern Med J 2011; published online 1 September. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.