Rapid signals at the neuromuscular junction and at synapses between neurons are carried by small molecules, called neurotransmitters, that are released from the presynaptic terminal and bind to ligand-gated ion channels in the postsynaptic membrane. When transmitter binds, a small pore opens through which ions flow, resulting in a transient depolarization or hyperpolarization of the membrane and translating the chemical signal into an electrical one. The transmitters at the neuromuscular junction, and at excitatory synapses in the central nervous system, are acetylcholine and glutamate, respectively. Inhibitory signals are carried by two major transmitters: glycine, predominantly in the spinal cord, and γ-aminobutyric acid, or GABA, predominantly in the brain.
Receptors for these transmitters are important targets for drugs used to treat mental disorders, or to modulate sleep and mood. In particular, benzodiazepine-related drugs, such as Valium, Halcion, and Xanax, which are widely used for the treatment of anxiety and insomnia, appear to act by binding directly to a specific site on the GABA type A (GABAA) receptor, the principal GABA-gated ion channel (1, 2). Molecular biologists have spent much productive effort over the last decade working out the molecular structures of receptors for each of the major transmitters, while biophysicists have unraveled the detailed kinetics of transmitter binding and gating (1, 3, 4). The pharmaceutical industry concentrates enormous resources on determining the specificity and physiological consequences of binding of pharmacological agents to these receptors.
In recent years, many researchers have turned to a new issue governing receptor function: What is the immediate protein environment of the receptors, and how does that environment influence receptor function at different synapses? Receptors for the rapid transmitters are not distributed randomly over the surface of the membrane. Rather, they are “targeted” to the postsynaptic membrane and are thus concentrated adjacent to the sites of transmitter release. The acetylcholine receptor is an extreme example of this process, clustering into a near crystalline array at the neuromuscular junction (5). In addition, the receptors are tightly associated with a cytoplasmic meshwork of proteins comprising regulatory enzymes that may modify the receptor itself to alter its kinetics or location, or transmit biochemical signals deeper into the cytoplasm (6–9). The N-methyl-d-aspartate (NMDA) subtype of the glutamate receptors is an extreme example of this situation. It is equipped with unusually long carboxyl-terminal “tails” that extend into the cytoplasm and associate with a variety of scaffold and signaling molecules (10).
Several recent papers, including two in PNAS, by Kneussel et al. (11) and by Chen et al. (12), have begun to clarify the mechanism of clustering of the inhibitory receptors for glycine and GABA, and the nature of the matrix of intracellular proteins associated with these receptors. A possible glycine receptor clustering protein of ≈93 kDa, termed gephyrin (from the Greek word for bridge), originally was identified based on its copurification with the glycine receptor (13). Recent genetic experiments show that gephyrin is required for synaptic clustering of both glycine and GABAA receptors (8, 14). These experiments are puzzling, however, because gephyrin itself shows no significant affinity for GABAA receptors in vitro (8). A partial solution to the puzzle was offered in a set of experiments demonstrating that a small protein of ≈14 kDa, termed GABARAP (GABAA receptor-associated protein) can bind to a subunit of the GABAA receptor (15) and to gephyrin (11), perhaps forming the missing physical link between the two (Fig. 1). GABARAP has an N-terminal tubulin-binding domain and is closely related to the previously described “late-acting intra-Golgi transport factor,” termed p16 (11). These findings suggest the hypothesis that GABARAP may link gephyrin and the GABAA receptor together as they are carried along microtubules and targeted to the proper membrane site (16). The propensity of gephyrin to form multimeric assemblies in vitro may be important for later stabilization of receptor complexes at the postsynaptic site.
Figure 1.
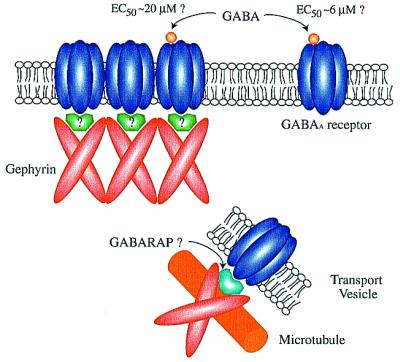
Gephyrin (93 kDa) binds glycine receptors and tubulin in vitro and is required for clustering of glycine and GABAA receptors at synapses; yet it does not associate with GABAA receptors in vitro. GABARAP (14 kDa) binds a subunit of the GABAA receptor, gephyrin, and tubulin in vitro. Furthermore, GABARAP recruits GABAA receptors into clusters at the plasma membrane in heterologous quail fibroblast cells. However, GABARAP does not colocalize with gephyrin or GABAA receptors in primary neurons. Instead it is associated with intracellular vesicles. The data suggest that GABARAP may mediate transport or targeting of gephyrin and GABAA receptors, but another unknown protein may link gephyrin and the GABAA receptor at synapses. Chen et al. (12) also show that clustered receptors recruited to the membrane by GABARAP in quail cells have a 4-fold reduced affinity for GABA. If the GABARAP promoted clustering resembles that at synapses, the implication is that clustering of GABAA receptors and association with the subcellular cytoskeleton can dramatically alter their kinetic properties.
Kneussel et al. (11) have found a fly in the hypothetical ointment, however. In cortical neurons in culture, where GABARAP previously had been shown to colocalize with GABAA receptors (15), they found that GABARAP is primarily in intracellular vesicles and does not colocalize with gephyrin. Furthermore, in the retina, GABAA receptors and gephyrin are tightly colocalized, but GABARAP shows no significant colocalization with either of them. Thus, in neurons GABARAP is not usually clustered at inhibitory synaptic sites; thus, its interaction with the GABAA receptor and gephyrin may be important for cellular functions other than receptor anchoring. For example, GABARAP may be involved in intraneuronal receptor sorting and targeting that precede and/or initiate receptor clustering at the synapse (16).
In light of these findings, it is curious that Chen et al. (12) find that GABARAP is targeted to the plasma membrane when expressed in heterologous cells, specifically quail fibroblast (QF6) cells in culture. Furthermore, they show that GABARAP increases the fraction of clustered GABAA receptors at the plasma membrane and colocalizes with the receptors, when they are expressed together in the same cells. Kneussel et al. (11) found that GABARAP and gephyrin become colocalized in clusters at the plasma membrane when expressed heterologously in PC12 cells. The apparent contradiction between the behavior of GABARAP in neurons and that in QF6 cells and PC12 cells illustrates the danger of extrapolating too literally to neurons from results in heterologous cells overexpressing a foreign protein.
Taken together, the data suggest that GABARAP is likely important for early steps in movement and sorting of GABAA receptors through the Golgi apparatus and along microtubules. Genetic modification of GABARAP is needed to test this hypothesis. On the other hand, gephyrin is clearly necessary for stable clustering of GABAA receptors at the synapse, as shown by genetic experiments and by its colocalization with the receptor in synaptic clusters; but, its mode of association with the GABAA receptor remains unclear.
Even more intriguing, however, is the finding by Chen et al. (12) in QF6 cells that clustered GABAA receptors have a significantly reduced affinity for GABA (from an EC50 of ≈6 μM to ≈20 μM) and altered kinetics of inactivation and desensitization compared with diffuse GABAA receptors. If the clustering of the receptors in heterologous cells is similar to that at synapses, the data imply that physical clustering and accompanying association with the cytoskeleton can dramatically modulate kinetic properties of receptors. Earlier work had indicated that the kinetic behavior of synaptic GABAA receptors differs from that of nonsynaptic receptors (17). However, these data were interpreted to suggest that certain combinations of GABAA receptor subunits are selectively concentrated at synapses. Indeed, this might well be the case. GABAA receptors are heteropentameric and are composed of various combinations of six α subunits, four β, four γ, one δ, and two ρ subunits (2). Most contain at least two copies of α and β subunits and at least one γ subunit. Hence, the number of distinct GABAA receptors is very large. Each combination can have slightly different kinetic properties. It is plausible that certain subunit combinations with characteristic kinetics might be more readily localized at synapses. The data of Chen et al. suggest the additional possibility that the physical clustering of receptors can itself alter receptor properties significantly. The change in affinity for GABA is in a range that could have functional consequences. A lower affinity and faster dissociation rate might permit faster channel closing at the synapse after release of one vesicle's worth of GABA.
The brain continues to show us that its range and subtlety of function are based on highly tunable properties of each processing unit. The next decade will be exciting indeed as we begin to learn how these processing units (synapses) are tuned during development, and moment by moment in adults, so that they work together to produce appropriate behavioral output.
Footnotes
References
- 1.Macdonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 2.Smith G B, Olsen R W. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 3.Seeburg P H. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 4.Satish P R, Balasubramanian A S. Curr Sci. 1995;69:336–342. [Google Scholar]
- 5.Salpeter M M. Science. 1999;286:424–425. doi: 10.1126/science.286.5439.424. [DOI] [PubMed] [Google Scholar]
- 6.Kornau H-C, Seeburg P H, Kennedy M B. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 7.Sheng M. Nature (London) 1997;386:221–223. doi: 10.1038/386221a0. [DOI] [PubMed] [Google Scholar]
- 8.Betz H. Nat Neurosci. 1998;1:541–543. doi: 10.1038/2777. [DOI] [PubMed] [Google Scholar]
- 9.Craven S E, Bredt D S. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy M B. Brain Res Rev. 1998;26:243–257. doi: 10.1016/s0165-0173(97)00043-x. [DOI] [PubMed] [Google Scholar]
- 11.Kneussel M, Haverkamp S, Fuhrmann J C, Wang H, Wassle H, Olsen R W, Betz H. Proc Natl Acad Sci USA. 2000;97:8594–8599. doi: 10.1073/pnas.97.15.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Wang H, Vicini S, Olsen R W. Proc Natl Acad Sci USA. 2000;97:11557–11562. doi: 10.1073/pnas.190133497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, et al. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 14.Essrich C, Lorez M, Benson J A, Fritschy J M, Luscher B. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Bedford F K, Brandon N J, Moss S J, Olsen R W. Nature (London) 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 16.Kneussel M, Betz H. Trends Neurosci. 2000;23:429–435. doi: 10.1016/s0166-2236(00)01627-1. [DOI] [PubMed] [Google Scholar]
- 17.Brickley S G, Cull-Candy S G, Farrant M. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


