Abstract
Fertility preservation is an important issue for young women diagnosed with breast cancer. The most well-established options for fertility preservation in cancer patients, embryo and oocyte cryopreservation, have not been traditionally offered to breast cancer patients as estradiol rise during standard stimulation protocols may not be safe for those patients. Potentially safer stimulation protocols using tamoxifen and aromatase inhibitors induce lower levels of estradiol while similar results in terms of number of oocyte and embryo obtained to standard protocols. Cryopreservation of immature oocytes and ovarian cortical tissue, both still experimental methods, are also fertility preservation options for breast cancer patients.
Keywords: fertility preservation, breast cancer, cryopreservation, female, aromatase inhibitors
BREAST CANCER IN WOMEN OF REPRODUCTIVE AGE
Malignancy of the breast affects up to one in eight women in developed countries and is the most common cancer in women of reproductive age. According to SEER Cancer statistics review (1) approximately 2% of breast cancer cases occur in young women between 20–34 years of age and 11% between 35–44 years of age.
Fortunately, most breast cancers are currently diagnosed at an early stage. However, the majority of breast cancers presenting in young women are invasive cancers with a high prevalence of ductal infiltration types and most of these patients are likely to undergo adjuvant systemic chemotherapy which will threaten their future fertility. A five year, or longer, course of adjuvant endocrine therapy with tamoxifen is recommended for patients with endocrine-sensitive tumours. Although tamoxifen therapy is not in itself gonadotoxic, it may delay the possibility of pregnancy in some older women whose fertility is naturally diminished and thus reduce the likelihood that these women will conceive.
More than 80% of women below the age of 40 suffering from breat cancer are successfully treated today. Given this high rate of survival, it is fitting that issues in breast cancer patients should focus on the quality of life including reproductive potential, which is threatened by cancer treatment.
FERTILITY PRESERVATION OPTIONS FOR WOMEN WITH BREAST CANCER
Counseling the patient
Fertility issues have been recognized of great importance for young women diagnosed with breast cancer and other malignancies during their reproductive years (2,3). According to the American Society of Clinical Oncology (ASCO) guidelines for fertility preservation in cancer patients (4), oncologists should be prepared to discuss the implications of chemotherapy on fertility with their patients as early as possible during cancer treatment planning or alternatively refer patients to reproductive specialists to discuss the probability of iatrogenic ovarian failure and possible fertility preservation options. It is imperative that patients receive timely and accurate information but unfortunately only one third of breast cancer patients recall discussions regarding the impact of treatment on their future fertility (5).
The incidence of breast cancer increases with age, therefore delaying childbearing to later in life results in more female cancer patients expressing an interest in fertility preservation options. The most well-established methods for fertility preservation are embryo and oocyte cryopreservation. These procedures both require ovarian stimulation which usually takes two weeks from the beginning of the menstrual cycle to complete. Typically there is a gap of 4–6 weeks between women undergoing breast cancer surgery and the commencing of chemotherapy, therefore often sufficient time is available to undergo ovarian stimulation. There are however, particular issues that need to be highlighted with regard to fertility preservation in breast cancer patients, including the impact of breast cancer therapy on future fertility, the potential deletereous effect of supraphysiological estrogen levels on breast malignancies, the particular difficulties when counseling carriers of BRCA mutations who may need to undergo oophorectomy later in life and the potential risks of subsequent pregnancy in former breast cancer patients.
Impact of breast cancer treatment on fertility
Gonadal toxicity by chemotherapy agents varies largely depending on the age of the patient, the type of agent, number of cycles and cumulative dose. Chemotherapy causes depletion of the primordial follicle pool in a drug- and dose dependent manner. The most cytotoxic group of chemotherapy substances to the female gonad includes alkylating agents such as cyclophosphamide currently used for treatment of breast cancer. Although most chemotherapy agents target dividing cells, cyclophosphamide is not cell cycle specific and can damage the resting primordial follicles which comprise the ovarian reserve.
Amenorrhea rates after therapies including cyclphosphamide vary. For example, in the breast cancer CMF protocol (cyclophosphamide, metotrexate, 5-fluorouracil) 20–70% of women younger than 40 years at the time of treatment experience amenorrhoea but this can rise to as high as 100% if women are older (6).
The more recently introduced adjuvant chemotherapy regimens as AC (anthracycline and cyclophosphamide) appear to have a lower incidence of amenorrhea. It is thought that this is related to a lower cumulative dose of cyclophosphamide reached with this protocol, although data is not yet conclusive. Anthracycline-based regimens present also with a variable incidence of amenorrhea from 0 to 96% depending if patients are younger or older than 30 years, respectively (7). The further addition of taxanes to antracycline regimens (ACT) for the treatment of early stage breast cancer has not demonstrated a further increase in risk of amenorrhea in breast cancer patients when paclitaxel was used but in protocols including docetaxel, doxorubicin and cyclophosphamide (FAC) the risk of amenorrhea increased from 33% to 51 % (8).
As seen in most studies, the risk of ovarian failure following chemotherapy is clearly dependent on a patient's age. Younger patients have a greater follicle reserve in their ovaries than older women and therefore are more likely to retain some ovarian funtion after a chemotherapeutic insult.
Table 1 summarizes the clinical benefits from adjuvant therapy in breast cancer patients and the impact of these therapies on future fertility.
Table 1.
Clinical benefit of current adjuvant therapy in treatment of breast cancer and impact of adjuvant therapies on fertility (35).
| Adjuvant therapy for treatment of breast cancer | Clinical benefit | Impact on fertility |
|---|---|---|
| Six cycles of anthracycline-based combination chemotherapy i.e. FEC (5-fluorouracil, epirubicin, cyclophosphamide or FAC (5-fluorouracil, doxorubicin, cyclophosphamide) | Reduction in mortality of 38%. 5–15% absolute improvement in survival at 15 years follow-up | The doses and the number of cycles can increase the likelihood of amenorrhea and infertility |
| Dose dense chemotherapy (admininstered every 2 weeks rather than every 3 weeks) | Seems to further reduce mortality | The doses and the number of cycles can increase the likelihood of amenorrhea and infertility |
| Addition of taxanes | Seems to further reduce mortality | Dependent on type of drug |
| Adjuvant therapy with trastuzumab | Improvement in relapse-free survival in women with HER2 positive | Unknown |
| Tamoxifen for five years | Reduction of the annual death rate by 31% in women with ER-positive independent of the use of chemotherapy or age. Reduction of absolute recurrence 11.8% and mortality 9.2% | Delay of pregnancy of 5 years and fertility may be reduced due to age-related decline |
Breast cancer susceptibility gene carriers
Most hereditary breast cancers are attributed to autosomal dominant germline mutations in breast cancer susceptibility genes 1 and 2 (BRCA 1 and BRCA 2) and this group of patients accounts for up to 10% of women with breast cancer. It is estimated that, in the general population, one in every 1000 women is a carrier of BRCA mutations with an increased incidence to up to 2.5% in certain ethnic groups, such as people with Jewish-Ashkenazi origin.
Women with BRCA 1 gene mutations have a 50–80% lifetime risk of breast cancer with an increased risk for presenting with bilateral breast cancer and also at a young age. BRCA 1 gene mutations carriers have also a 40–60% risk of ovarian cancer and patients are usually offered prophylactic bilateral mastectomy to reduce the risk of a contralateral breast cancer and prophylactic bilateral salpingo-oophorectomy around the age of 40 to reduce the risk of ovarian cancer but in some cases also to reduce the risk of breast cancer. Carriers of BRCA 2 mutations present with similar features although with a lesser risk for ovarian cancer, which also presents at older age than in BRCA 1 carriers. Hysterectomy is not usually performed in these patients and although there is still the possibility of carrying a pregnancy to term, some may prefer to have a gestational carrier.
Consequently, the presence of a BRCA gene mutation in a breast cancer patient interested in fertility preservation is a complex issue. Investigation of treatments for infertility in a large population series of BRCA mutation carriers has revealed no association between the risk of breast cancer and exposure to fertility drugs which is a reassuring information (9). On the other hand, in a recent study of breast cancer patients undergoing ovarian stimulation for fertility preservation, the presence of a BRCA 1 mutation was associated with a significantly lower response to stimulation and occult primary ovarian insufficiency (10).
Breast cancer patients with BRCA mutations undergoing fertility preservation may transmit increased cancer risks to their offspring. If the patients are concerned by this risk, preimplantation genetic diagnosis after thawing of cryopreserved embryos may be useful for selection of the embryos appropriate for transfer.
Potential risk of a subsequent pregnancy in breast cancer survivors
Pregnancy after breast cancer does not seem to increase the risk of recurrence or to adversely affect maternal outcome. Moreover, no increased risk for congenital malformations in children conceived after completion of chemotherapy has been found.
Pregnancy is associated with a reduced risk for death in women who had breast cancer previously (11). However, breast cancer survivors who become pregnant may be a self-selected group and may not be representative of the larger population of women as those having a high risk of tumour recurrence may have chosen not to get pregnant. Timing a pregnancy after a diagnosis of breast cancer is also a complex issue but in general patients are advised to delay pregnancy attempts for at least two years after the diagnosis, mainly because the risk of reccurrence is greatest during this period. For patients prescribed tamoxifen, pregnancy is usually considered safe after completion of the 5-year treatment period. Tamoxifen has a similar structure of diethylstilbestrol and pregnancy while on tamoxifen treatment is discouraged due to the potential teratogenic effects of this drug during pregnancy.
Although a pregnancy is considered safe for most breast cancer survivors, gestational surrogacy may be suitable for breast cancer survivors with high risk for recurrence or who have to be on life-long therapy with tamoxifen or aromatase inhibitors. In women with BRCA 1 or 2 gene mutations the risk of pregnancy is less well established. A gestational carrier may be indicated in patients who have undergone salpingo-oophorectomy with the aim of reducing endogenous estrogen production and therefore the risk of cancer recurrence.
Fertility preservation options for breast cancer patients
For women with a partner or those wishing to use a sperm donnor, embryo cryopreservation after IVF is considered an established fertility preservation method. It is routinely used worldwide for surplus embryos after infertilty treatments and it has been used for more than 20 years. Live birth rates after transfer of embryos which are intact after thaw have the same implantation potential as fresh embryos and they can lead to a 59% pregnancy rate and a 26% live birth rate (12). For single cancer patients without a partner and those not wishing to use a sperm donor, freezing mature or immature oocytes, although still experimental, is the only option (13).
To obtain mature oocytes ovarian stimulation must be performed. and therefore a delay of 2–6 weeks may be necessary. Cryopreservation of mature oocytes using vitrification techniques to avoid ice crystal formation has become a very effective method to store oocytes in recent years and the success rates of fertilization post- thaw and pregnancy rates have increased significantly close to those of current IVF with fresh oocytes (14). The clinical outcome of oocyte cryopreservation depends on many factors including the original quality of the retrieved oocytes, the freezing protocol and each stage of handling of the oocytes.
When there is not enough time or the patient does not wish to undergo an ovarian stimulation treatment, alternative approaches include the retrieval of oocytes without ovarian stimulation and the cryopreservation of ovarian cortex. Retrieved immature oocytes may be cryopreserved at an immature stage or after in vitro maturation (13). This option is limited as only a few fertility centers worldwide offer treatments by using this technique.. The cryopreservsation of immature oocytes is still experimental and requires optimisation however this method has resulted in live births (15). Encouragingly, it has been demonstrated that immature oocytes survive cryopreservation better than mature oocytes; after thawing these oocytes can be successfully matured in vitro and fertilized.
Ovarian tissue cryopreservation for future transplantation is a new promising method for fertility preservation, which is discussed in a separate chapter of this issue. With regard to breast cancer patients, the risk for metastatic ovarian involvement is extremely unlikely in early stages of the disease and the procedure is considered today as safe for breast cancer patients (16,17). In two recent reports, the histological evaluation of fresh or frozen-thawed cortical tissue of breast cancer patients undergoing cryopreservation of ovarian tissue did not evidence any metastases (18,19)
Stimulation protocols for oocyte or embryo cryopreservation in women with breast cancer
Epidemiological and experimental data indicates that estrogen plays a central role in the initiation and promotion of breast tumorigenesis. Circulating estradiol levels are elevated during conventional ovulation induction for in vitro fertilization (IVF). The rise in estradiol is directly proportional to number of follicles recruited to grow, therefore alternative and potentially safer protocols have been introduced for fertility preservation for breast cancer patients including natural cycle IVF, stimulation protocols with tamoxifen alone or combined with gonadotropins and stimulation protocols with aromatase inhibitors to reduce the estrogen production. Natural cycle IVF do not give more than one oocyte or embryo per cycle and has a high rate of cycle cancellation. The cycle may thus result ineffective when a chemotherapy treatment is imminent and the patient may not have a chance for a second cycle of IVF treatment.
Tamoxifen
Tamoxifen is a nonsteroidal triphenylethylene compound related to clomiphene. Tamoxifen was initially demonstrated to be as effective as clomiphene in ovarian stimulation of anovulatory patients in the early 1970's. However, tamoxifen was later identified as a selective estrogen receptor modulator (SERM) as it has demonstrable antiestrogenic actions on breast tissue with inhibition of growth of breast tumours by competitive antagonism of estrogen at its receptor site. Tamoxifen has been used in treatment and prevention of estrogen receptor positive breast cancer for nearly three decades and it is accepted as first line drug in hormonal treatment of breast cancer (20).
Tamoxifen may be used for ovulation induction alone starting on day 2–3 or 5 of the menstrual cycle in doses of 20–60 mg/day, or in combination with low dose gonadotropins, similar to the use of clomiphene when given alone or combined with gonadotropins for IVF. It has been demonstrated that ovarian stimulation using tamoxifen for fertility preservation in cancer patients can increase the mature oocyte and embryo yield when compared to natural cycle IVF (1.6 vs 0.7 P=0.03 and 1.6 vs 0.6 P=0.02, respectively) and reduce cycle cancellations (21).
The combined protocol with tamoxifen and gonadotropins for IVF has shown to further increase the number of oocytes and embryos for cryopreservation (5.1 vs 1.5 P=0.001 and 3.8 vs 1.3 P=0.001, respectively (22).
Studies with tamoxifen demonstrated that its short use for ovulation induction does not adversely affect oocyte and embryo development (23). Even though peak estradiol levels in ovarian stimulation with tamoxifen on day of hCG admininstration may be significantly higher than in women undergoing natural cycle IVF, the effects of estrogen on breast tissue are blocked at the receptor sites.
Aromatase Inhibitors
Aromatase inhibitors have being used in the treatment of breast cancer in postmenopausal women for more than 30 years. Aromatase, a product of CYP 19 gene, is a cytochrome P450 enzyme complex that catalyses the conversion of androstendione and testosterone to their respective estrogenic products estrone and estradiol. CYP19 is highly expressed in granulosa cells of ovarian follicles, where its expression depends on cyclical gonadotropin stimulation. Aromatase is not only present in the ovary and many other extragonadal tissues including adipose tissue and breast have also aromatization of androgens to estrogens.
The third generation aromatase inhibitors developed in the 1990's, anastrozole and letrozole, are drugs of choice for the treatment of breast cancer in women with receptor-positive metastatic breast cancer. Their use has also been introduced as new treatment option for ovulation induction. They are potent and highly selective by competitively binding the active site of the enzyme complex. Letrozole suppresses plasma estradiol, estrone and estrone sulphate levels significantly at doses of 0.1–5 mg/day.
Inhibition of the aromatase system increases gonadotropin production by releasing the hyphotalamic-pituitary axis from estrogenic negative feed-back.
Ovulation induction with aromatase inhibitors has been described in clomiphene-resistent patients with polycystic ovarian syndrome (24). Aromatase inhibitors have a relatively short life of about 40 hours, therefore the effects on the pituitary are not long-lasting and down-regulation of the pituitary does not occur when their administration is discontinued. Consequently, the adverse effects observed with clomiphene on endometrial growth and cervical secretion are avoided with aromatase inhibitors. Available data indicates a significantly higher pregnancy and delivery rates in anovulatory women stimulated with aromatase inhibitors than with clomiphene (25).
Use of letrozole alone for ovarian stimulation has been associated with lower estrogen levels than those of the natural cycle (26). Data on the use of anastrozole for ovarian stimulation in anovulatory women however is more limited and studies so far do not support its use over clomiphene (25).
Combined stimulation protocols with letrozole and gonadotropins
Combined stimulation protocols with letrozole and gonadotropins have produced comparable results to standard IVF while decreasing gonadotropin requirements, which appears to be cost-effective. Combined letrozole and gonadotropin protocols have also demonstrated significantly lower peak estradiol levels than standard IVF (278.9 vs 483.4 pg/ml, P=<0.001) which may be more suitable for breast cancer patients (27).
Our stimulation protocol for embryo and oocyte cryopreservation with letrozole in combination with gonadotropins (controlled ovarian stimulation treatment with letrozole supplementation study, COST-LESS) (22) has not show any detrimental effects in breast cancer recurrence after short-term follow-up (28). Combined anastrozole and gonadotropins protocols have demonstrated comparable results to COST-LESS regarding number of oocytes and embryos obtained, but significantly higher peak estradiol levels than in the COST-LESS (1325.89 vs 427.78 pg/ml) (29). Therefore we currently prefer the COST-LESS protocol for women with breast cancer undergoing fertility preservation for embryo and oocyte cryopreservation.
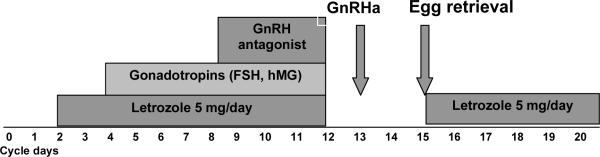
Figure 1 illustrates the COST-LESS protocol. Letrozole is commenced orally on the second or third day of the menstrual cycle at a dose of 5 mg/day. After two days of letrozole administration 150–300 IU/day of recombinant FSH or hMG are added depending on patient's age and antral follicle counts on day 2 or 3. A GnRH antagonist is added when estradiol levels exceed 250 pg/ml (918 pmol/L) or the leading follicle reached 14 mm in diameter to prevent premature LH surge. Patients are monitored with ultrasound and E2, FSH and LH measurements every 1 to 2 days until oocyte retrieval. To obtain a high oocyte maturation rate, oocyte maturation is triggered when at least two leading follicles reach a diameter of 20 mm (27) and oocyte retrieval is performed 36 hours after the trigger. In the original protocol we used human chorionic gonadotropin (hCG) to trigger the final maturation of oocytes. However, hCG has a longer half life compared to endogenous luteinizing hormone (LH), and it may further increase estradiol production even after egg retrieval. We have recently improved the COST-LESS protocol by triggering egg maturation with a GnRH analogue (GnRHa), which resulted in significantly decreased estradiol levels on the day of retrieval and a faster drop of estradiol levels in the subsequent days (30). In that study, the GnRHa trigger was also associated with lower incidence of ovarian hyperstimulation syndrome, indicating that GnRHa had a suppressive effect on estrogen production after trigger (30).
Figure 1. COST-LESS protocol (Controlled Ovarian Stimulation with Letrozole Supplementation) and GnRHa trigger to reduce estrogen exposure in breast cancer patients.
Letrozole starts on the 2nd day of the cycle and is discontinued the day of trigger. Gonadotropins are initiated on the 4th day of the cycle. A GnRH antagonist is administered when tle leading follicle has reached 14 mm in size or estradiol levels reach ≥ 250 pg/ml (918 pmol/L). The trigger is induced with a GnRH analogue (GnRHa) administered when at least two follicles have reached 20 mm in diameter. Letrozole is reinitiated after egg retrieval and continued until estradiol levels fall below 50 pg/ml (183 pmol/L).
Letrozole is reinitiated after oocyte retrieval to prevent a rebound increase of estradiol levels (Figure 1). Monitoring of estradiol levels is repeated three days after oocyte retrieval and if the levels are higher than 250 pg/ml, letrozole is continued until estradiol levels fall below 50 pg/ml (183.5 pmol/L) and then discontinued.
COST-LESS protocols in breast cancer patients are associated with a 44% reduction in gonadotropin requirements and treatment costs and with similar fertility outcomes than after high dose gonadotropin treatment for IVF in terms of oocyte and embryo yield and of fertilization rates (27). However, up to 12–20% of oocytes for IVF treatments may be expected to be immature. To further increase the yield of mature oocytes and embryos for fertility preservation in breast cancer patients we have recently introduced in vitro maturation (IVM) as a complementary strategy when immature oocytes were also retrieved in stimulated cycles with aromatase inhibitors combined with gonadotropins (31).
The follow-up of 79 breast cancer patients (mean age 36 ± 3.8) having undergone fertility preservation with COST-LESS protocols has not shown to increase the hazard ratio recurrence when compared to controls who did not undergo fertility preservation after a mean follow-up of 2-years (28). Although the time lapse between surgery and chemotherapy in patients who underwent fertility preservation was significantly longer (45 vs. 33 days, P=0.01) and one of the concerns with ovarian stimulation before breast cancer chemotherapy is the delay in the initiation of treatment, there was no difference in terms of recurrence rate and relapse free survival between breast cancer patients who underwent ovarian stimulation with COST-LESS and controls (28). Furthermore, data from large studies have demonstrated no effect on survival or recurrence if chemotherapy is initiated within 12 weeks after surgery (32,33).
Although peak estradiol levels in the COST-LESS protocol are maintained close to the levels of the natural cycle (405.9 pg/ml ± 256.6 or 1486.7 ± 942.1 pmol/L) (28), longer follow-up of breast cancer patients having undergone fertility preservation with COST-LESS, tamoxifen and other stimulation protocols are are still needed.
Aromatase inhibitors are contraindicated during pregnancy. However, data indicates that fertility treatment with letrozole is safe and its use before conception does not seem to have increased risks for the fetus. In a retrospective analysis of data from five centers in Canada, the overall incidence of congenital malformations and chromosomal abnormalities was similar among children born after fertility treatments with clomiphene (4.8%) or letrozole (2.4%). In fact, the incidence of major malformations was significantly higher after fertility treatments with clomiphene than with letrozole (3% vs. 1.2%, respectively) (34).
Of note, when aromatase inhibitors are used for fertility preservation and IVF, the embryos are never exposed to the drug as they are not transferred in that treatment cycle.
Summary
In conclusion, fertility preservation is often possible for most breast cancer patients. There is still a need to increase the awareness with fertility preservation techniques so that patients are referred as early as possible after the intial diagnosis is made. For those undergoing oocyte or embryo freezing, potentially safer stimulation protocols have been proposed.
Acknowledgements
Doctor Rodriguez-Wallberg is supported by research grants from The Swedish Society of Medical Research and The Swedish Society of Medicine. Prof Kutluk Oktay is supported by NIH grant HD053112A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: [Accessed August 11,2009]. based on November 2008 SEER data submission. Available at: http://seer.cancer.gov/statfacts/html/breast.html. [Google Scholar]
- 2.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004 Oct 15;22(20):4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 3.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer. 1999 Aug 15;86(4):697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. American Society of Clinical Oncology. J Clin Oncol. 2006 Jun 20;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 5.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005 Feb 1;23(4):766–73. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 6.Gadducci A, Cosio S, Genazzani AR. Ovarian function and childbearing issues in breast cancer survivors. Gynecol Endocrinol. 2007 Nov;23(11):625–31. doi: 10.1080/09513590701582406. Review. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Buzdar AU, Marcus CE, Smith TL. Immediate and long-term toxicity of adjuvant chemotherapy regimens containing doxorubicin in trials at M.D. Anderson Hospital and Tumor Institute. NCI Monogr. 1986;(1):105–9. [PubMed] [Google Scholar]
- 8.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006 May;11(5):422–34. doi: 10.1634/theoncologist.11-5-422. Review. [DOI] [PubMed] [Google Scholar]
- 9.Kotsopoulos J, Librach CL, Lubinski J, Gronwald J, Kim-Sing C, Ghadirian P, Lynch HT, Moller P, Foulkes WD, Randall S, Manoukian S, Pasini B, Tung N, Ainsworth PJ, Cummings S, Sun P, Narod SA, Hereditary Breast Cancer Clinical Study Group Infertility, treatment of infertility, and the risk of breast cancer among women with BRCA1 and BRCA2 mutations: a case-control study. Cancer Causes Control. 2008 Dec;19(10):1111–9. doi: 10.1007/s10552-008-9175-0. [DOI] [PubMed] [Google Scholar]
- 10.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010 Jan 10;28(2):240–4. doi: 10.1200/JCO.2009.24.2057. Epub 2009 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroman N, Jensen MB, Wohlfahrt J, Ejlertsen B, Danish Breast Cancer Cooperative Group Pregnancy after treatment of breast cancer--a population-based study on behalf of Danish Breast Cancer Cooperative Group. Acta Oncol. 47(4):545–9. doi: 10.1080/02841860801935491. [DOI] [PubMed] [Google Scholar]
- 12.Marrs RP, Greene J, Stone BA. Potential factors affecting embryo survival and clinical outcome with cryopreserved pronuclear human embryos. Am J Obstet Gynecol. 2004 Jun;190(6):1766–71. doi: 10.1016/j.ajog.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Wallberg KA, Keros V, Hovatta O. Clinical aspects of fertility preservation in female patients. Pediatr Blood Cancer. 2009 Aug;53(2):254–60. doi: 10.1002/pbc.21995. [DOI] [PubMed] [Google Scholar]
- 14.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009 Jun;18(6):769–76. doi: 10.1016/s1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 15.Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, Buckett WM, Tulandi T, Tan SL. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009 Jun;91(6):2391–8. doi: 10.1016/j.fertnstert.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Oktay K, Buyuk E. Ovarian transplantation in humans: indications, techniques and the risk of reseeding cancer. Eur J Obstet Gynecol Reprod Biol. 2004 Apr 5;113(Suppl 1):S45–7. doi: 10.1016/j.ejogrb.2003.11.010. Review. [DOI] [PubMed] [Google Scholar]
- 17.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010 Feb;93(3):762–8. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Serrano M, Novella-Maestre E, Roselló-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod. 2009 Sep;24(9):2238–43. doi: 10.1093/humrep/dep196. [DOI] [PubMed] [Google Scholar]
- 19.Azem F, Hasson J, Ben-Yosef D, Kossoy N, Cohen T, Almog B, Amit A, Lessing JB, Lifschitz-Mercer B. Histologic evaluation of fresh human ovarian tissue before cryopreservation. Int J Gynecol Pathol. 2010 Jan;29(1):19–23. doi: 10.1097/PGP.0b013e3181ad1c52. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31 000 recurrences and 24 000 deaths among 75 000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 21.Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003 Jan;18(1):90–5. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 22.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005 Jul 1;23(19):4347–53. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Fisk NM, Templeton AA, Papadopoulos GC, Matlin SA, Wu ZY. Lack of effect of high-dose antioestrogen on the maturation and in-vitro fertilization of human oocytes. Hum Reprod. 1989 Jul;4(5):584–7. doi: 10.1093/oxfordjournals.humrep.a136947. [DOI] [PubMed] [Google Scholar]
- 24.Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001 Feb;75(2):305–9. doi: 10.1016/s0015-0282(00)01705-2. [DOI] [PubMed] [Google Scholar]
- 25.Polyzos NP, Tzioras S, Badawy AM, Valachis A, Dritsas C, Mauri D. Aromatase inhibitors for female infertility: a systematic review of the literature. Reprod Biomed Online. 2009 Oct;19(4):456–71. doi: 10.1016/j.rbmo.2009.06.008. Review. [DOI] [PubMed] [Google Scholar]
- 26.Fisher SA, Reid RL, Van Vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril. 2002 Aug;78(2):280–5. doi: 10.1016/s0015-0282(02)03241-7. [DOI] [PubMed] [Google Scholar]
- 27.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, Bang H. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006 Oct;91(10):3885–90. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 28.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008 Jun 1;26(16):2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 29.Azim AA, Costantini-Ferrando M, Lostritto K, Oktay K. Relative potencies of anastrozole and letrozole to suppress estradiol in breast cancer patients undergoing ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab. 2007 Jun;92(6):2197–200. doi: 10.1210/jc.2007-0247. [DOI] [PubMed] [Google Scholar]
- 30.Oktay K, Türkçüoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online. 2010 doi: 10.1016/j.rbmo.2010.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oktay, K, Buyuk E, Rodriguez-Wallberg KA, Sahin G. IVM improves oocyte or embryo cryopreservation outcome in breast cncer patients undergoing ovarian stimulation for fertility preservation. Reprod Biomed Online. 2010 in press; doi: 10.1016/j.rbmo.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Cold S, Düring M, Ewertz M, Knoop A, Møller S. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG) Br J Cancer. 2005 Sep 19;93(6):627–32. doi: 10.1038/sj.bjc.6602734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, Olivotto IA. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006 Oct 20;24(30):4888–94. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 34.Tulandi T, Martin J, Al-Fadhli R, Kabli N, Forman R, Hitkari J, Librach C, Greenblatt E, Casper RF. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006 Jun;85(6):1761–5. doi: 10.1016/j.fertnstert.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Hickley M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Human Reproduction Update. 2009;15(3):323–339. doi: 10.1093/humupd/dmn064. [DOI] [PMC free article] [PubMed] [Google Scholar]