Abstract
The apolipoprotein E4 allele (APOE4) contributes to Alzheimer’s disease (AD) risk and APOE2 is protective, but the relevant cellular mechanisms are unknown. We have used flow cytometry analysis to measure apolipoprotein E (apoE) and amyloid beta peptide (Aβ) levels in large populations of synaptic terminals from AD and aged cognitively normal controls, and demonstrate that modest but significant increases in soluble apoE levels accompany elevated Aβ in AD cortical synapses and in an APP/PS1 rat model of AD. Dual labeling experiments document co-localization of apoE and Aβ in individual synapses with concentration of Aβ in a small population of apoE-positive synapses in both AD and controls. Consistent with a clearance role, the apoE level was higher in Aβ-positive synapses in control cases. In aged targeted replacement mice expressing human apoE, apoE2/4 synaptic terminals demonstrated the highest level of apoE and the lowest level of Aβ compared to apoE3/3 and apoE4/4 lines. In apoE2/4 terminals, the pattern of immunolabeling for apoE and Aβ closely resembled the pattern in human control cases, and elevated apoE was accompanied by elevated free cholesterol in apoE2/4 synaptic terminals. These results are consistent with a role for APOE in Aβ clearance in AD synapses, and suggest that optimal lipidation of apoE2 compared to E3 and E4 makes an important contribution to Aβ clearance and synaptic function.
Keywords: Synaptosome, Flow cytometry, Filipin, Ganglioside GM1, Triple transgenic mouse, Triple transgenic rat
Introduction
Many environmental risk/lifestyle factors and susceptibility genes for Alzheimer’s disease (AD) have been reported. However, for sporadic or late-onset Alzheimer’s disease, which represents the vast majority of cases, apolipoprotein E (APOE) remains the strongest genetic risk factor, with 60–80% of AD cases having at least one apoE4 allele (for review see [31, 37]). Like other apolipoproteins, apoE is a cholesterol chaperone, and a ligand for members of the low-density lipoprotein receptor (LDLR) family. The human gene exists in three alleles, (E2, E3 and E4); while APOE4 confers risk for AD [9], APOE2 is protective [8]. In the brain, most apoE is produced by astrocytes, which are thought to deliver cholesterol and other lipids to neurons via receptor-mediated endocytosis. In normal brain, APOE4 is associated with reduced pre- and postsynaptic proteins, and APOE2 with increases in a key post-synaptic protein, PSD-95 [40]. In addition to cholesterol transport, a number of isoform-dependent functions have been identified for apoE in vitro and in vivo. For example, APOE4 has been linked to accumulation of intraneuronal amyloid beta protein (Aβ; [7]) and to capillary cerebral amyloid angiopathy [65]; however, the mechanism(s) by which APOE4 confers risk for AD remain unclear.
The three isoforms of apoE differ at two amino acids (position 112 and 158), which alter lipid association and receptor binding of apoE. The major apoE receptors in brain are the LDLR and the LDL-related protein (LRP1). A strong body of evidence shows that apoE also binds tightly with the Aβ protein [35, 36, 49], and this interaction is widely hypothesized to influence Aβ deposition and dementia progression in AD. For example, increased neuritic plaques in AD and fibrillar Aβ burden are associated with APOE4 dosage [53, 66]. In vitro experiments show that lipidated apoE4 forms less SDS-stable apoE/Aβ complex compared to apoE2 and apoE3 [35, 76], and our earlier work has demonstrated that complex formation with apoE3 enhances uptake of soluble but not aggregated Aβ into synaptosomes [18]. The impaired ability of apoE4 to bind Aβ has suggested a hypothesis in which the apoE4 allele reduces Aβ clearance in the brain of AD patients by reducing receptor-mediated uptake and cellular metabolism of the apoE/Aβ complex. Isoform-related differences in receptor-mediated uptake have also been shown to occur across the endothelium into the blood, with apoE2-and apoE3-containing Aβ complexes cleared from the brain at a faster rate than apoE4/Aβ complexes [10]. Recent evidence also suggests that complex formation with apoE4 may reduce peripheral Aβ clearance [3, 26]. Despite a large body of in vitro evidence, the cellular location and function of neuronal apoE/Aβ complexes is not clear.
Synaptic dysfunction and loss are thought to be the earliest correlates of cognitive dysfunction, and a large body of evidence indicates an important role for apoE in synaptic integrity and plasticity. For example, apoE is up-regulated in rodents after neuronal injury [74], and human apoE4 target replacement (TR) mice display impaired LTP and decreased dendritic spine density [28]. A role for apoE in plasticity and repair is supported by evidence that APOE4 carriers have poorer outcomes in head injury and stroke [42], as well as higher rates of other neurologic disorders including tauopathies and PD [30, 45]. Based on the evidence linking synaptic plasticity to apoE, and the production of brain apoE by astrocytes that envelope and maintain synapses [67], we hypothesized altered apoE level in AD cortical synapses. We also tested the hypothesis that apoE and Aβ are colocalized, and report here that apoE level is modestly increased in AD compared to control, and that Aβ is concentrated in a small population of apoE-positive synapses in both AD cortex and in TR mice expressing human APOE isoforms. Mice with APOE2 allele demonstrated the highest level of synaptic apoE and the lowest level of synaptic Aβ, and these changes in the synapse were accompanied by elevated free cholesterol.
Methods
Materials
The monoclonal anti-Aβ antibody 10G4 has been described previously [43]. Polystyrene microsphere size standards were purchased from Polysciences, Inc. (Warrington, PA, USA). Zenon mouse IgG Labeling kits for dual labeling were purchased from Molecular Probes (Eugene, OR, USA), and rhodamine-conjugated anti-mouse antibody from Chemicon (San Diego, CA, USA). The following monoclonal antibodies were purchased: anti-SNAP-25 (Sternberger Monoclonals Inc., Lutherville, MD, USA), mouse IgG1 Isotype control (BD Pharmingen, San Diego CA), apoE 2E1 (anti-human, Roche Diagnostics, Mannheim, Germany), and apoE E6D7 (directed against N-terminal human apoE, Abcam, Cambridge, MA, USA). Filipin and biotinylated Cholera Toxin subunit B were purchased from Sigma (St. Louis, MO, USA).
Human brain specimens
Samples of parietal (A39) cortex were obtained from the Alzheimer’s Disease Centers at USC, UCLA, and UC Irvine on the day of autopsy. Immediately upon receipt, samples (~0.3–5 g) were minced in 0.32 M sucrose and slowly frozen for cryopreservation, then stored at −70°C until homogenization. The mean postmortem interval for AD cases was 6.7 h, and for normal and control cases were 8.6 h. Controls included aged cognitively normal controls and neurological control cases with spinocerebellar ataxia and other non-Alzheimer’s disease conditions. Complete case information is presented in Table 1.
Table 1.
Case information for postmortem samples
| Number | Sex | Age | Postmortem interval (h) | ApoE | Diagnosis/Braak and Braak score |
|---|---|---|---|---|---|
| Normal/control cases | |||||
| 726ab | F | 97 | 8.5 | 3/4 | N |
| 758b | M | 93 | 8.5 | 3/4 | N |
| 774a | M | 93 | 6 | 2/3 | N |
| 789a | F | 105 | 9 | 3/3 | N |
| 810b | M | 83 | 5 | 4/4 | Spinocerebellar ataxia type 2 |
| 824ab | F | 86 | 12.5 | 3/4 | N |
| 07-09abc | F | 63 | 6.5 | 3/3 | Pick’s disease |
| 12-09ac | M | 85 | 6.75 | 3/4 | N |
| 15-09bc | M | 82 | 14.9 | 3/3 | Hippocampal sclerosis |
| AD cases | |||||
| 716a | F | 86 | 8.5 | 3/4 | VI |
| 718ab | M | 83 | 11 | 3/4 | V |
| 731ab | F | 87 | 10 | nd | VI |
| 737a | F | 76 | 5 | 3/4 | III |
| 745b | F | 92 | 7.5 | 3/4 | V |
| 788a | M | 82 | 9.5 | 3/3 | VI |
| 796b | M | 76 | 9 | 3/4 | V |
| 809ac | M | 65 | 4.5 | 4/4 | V |
| 813ac | M | 79 | 5.75 | 3/4 | V |
| 814c | F | 98 | 6 | 3/3 | V |
| 835a | F | 78 | 6.5 | 3/4 | III; PD |
| 809c | M | 70 | 5 | nd | VI; trisomy 21 |
| 9-09ac | M | 87 | 6 | 3/4 | V |
| 10-09ac | F | 82 | 4.5 | 3/4 | VI |
| 11-09ac | M | 77 | 5.5 | 2/4 | VI |
| 133a | F | 92 | 6 | 3/4 | IV–V |
| 159a | F | 96 | 5 | 3/3 | VI |
| 163b | M | 82 | 5 | 4/4 | VI |
ApoE, flow cytometry
ApoE, ELISA
ApoE/amyloid beta (Aβ) dual labeling, flow cytometry
Animals
ApoE TR mice
Human APOE genomic fragments were used to replace the mouse apoE gene via homologous recombination. All three lines of apoE TR mice contain chimeric genes consisting of mouse 5′ regulatory sequences continuous with mouse exon 1 (non-coding) followed by human exons (and introns) 2–4 [62]. Thus, all three lines of apoE TR mice regulate gene expression in the same fashion. Mice have been backcrossed to C57Bl6/J mice 8 times and are therefore 99.6% C57Bl6/J. Animals were genotyped using an allele-specific PCR approach based on [23]. All mice were maintained on a normal chow diet and handled under protocols approved by the Duke Institutional Animal Care and Use Committee. Experiments were performed on 18-month-old male animals that were homozygous apoE 3/3, apoE 4/4 or heterozygous 2/4 mice. Rodent samples were cryopreserved as described above immediately after killing.
APP/PS1 transgenic rat
The transgenic APP/PS1 rats overexpress two human APP mutations (K670N/M671L and V717F) and one PS1 mutation (M146V). By 19–20 months of age, extensive amyloid plaque deposition is seen in hippocampal and cortical regions. The neuropathological characterization of these animals has previously been described in detail [16].
P-2 preparation
The P-2 (crude synaptosome) fraction was prepared as described previously [18]; briefly, tissue was homogenized in ice-cold buffer (0.32 M sucrose, 10 mM TRIS pH 7.5, plus protease inhibitors: pepstatin (4 μg/ml), aprotinin (5 μg/ml), trypsin inhibitor (20 μg/ml), EDTA (2 mM), EGTA (2 mM), PMSF (0.2 mM), Leupeptin (4 μg/ml). The homogenate was first centrifuged at 1000g for 10 min; the resulting supernatant was centrifuged at 10,000g for 20 min to obtain the crude synaptosomal pellet. Aliquots of P-2 are routinely cryopreserved in 0.32 M sucrose and banked at −70°C until the day of the experiment.
Immunolabeling of P-2 fraction
P-2 aliquots were immunolabeled for flow cytometry analysis according to a method for staining of intracellular antigens [55]. Pellets were fixed in 0.25% buffered paraformaldehyde (1 h, 4°C) and permeabilized in 0.2% Tween20/PBS (15 min, 37°C). Antibodies were labeled directly with Alexa Fluor 488 or 647 reagents according to kit directions. This mixture was added to P-2 aliquots and incubated at RT for 30 min. Pellets were washed twice with 1 ml 0.2% Tween20/PBS, resuspended in PBS buffer (0.75 ml) for flow cytometry analysis. The synaptosomal pellet was dispersed for all washes and for incubations with fixative, detergent, and antibody, then collected by centrifugation (1,310g at 4°C).
Flow cytometry
Data was acquired using an BD-FACSCalibur analytic flow cytometer (Becton–Dickinson, San Jose, CA, USA) equipped with argon 488 nm, helium–neon 635 nm, and helium–cadmium 325 nm lasers. 5,000 particles were collected and analyzed for each sample. Debris was excluded by establishing a size threshold set on forward light scatter. Alexa 488 and Alexa 647 fluorochromes were detected by the LSR’s FL1, Ssc-W, photomultiplier tube detectors, respectively. Analysis was performed using FCS Express software (DeNovo Software, Ontario, Canada).
ApoE ELISA
ApoE levels were measures in P-2 samples using a sandwich ELISA using the protocol of Sullivan et al. [59]. Briefly, P-2 homogenate samples (20 μg protein) were loaded into a 96-well plate coated with an anti-apoE antibody (goat anti-human apoE; 1–200 dilution; Millipore, San Francisco, CA, USA). Following overnight incubation at 4°C, the samples were removed and the plate washed thrice with PBS-T (1× PBS, pH 7.4, 0.05% tween-20 and 0.4% glycine) prior to incubation with a biotinylated anti-apoE antibody (goat anti-human apoE; 1:5,000; Meridian Life Science, Saco, ME, USA) for 1 h at room temperature. The plates were then washed again thrice with PBS-T and incubated with streptavidin-HRP for an additional 1 h at room temperature. The immunocomplex was reacted with TMB substrate and detected using a Versamax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Levels of apoE were normalized to a standard curve generated using recombinant human apoE (Meridian Life Science, Saco, ME, USA).
Confocal microscopy
Crude P-2 aliquots were immunolabeled as described above and washed, then dispersed with a pipette and spread on slides. Slides were dried, coverslipped with Prolong Antifade (Molecular Probes, Eugene, OR, USA), and stored at 4°C. The Alexa 647 fluor usually used to label SNAP-25 was not compatible with the filter system on the microscope; therefore, samples were first incubated with 488-labeled 10G4 antibody and then anti-SNAP-25 antibody (1:1,000), followed by secondary antibody (rhodamine-conjugated anti-mouse, 1:200). Confocal fluorescence and differential interference contrast images of synaptosomes and plastic bead standards were taken using a 100×/1.4 Planapo objective lens on a Leica TCS-SP Confocal Inverted Microscope (Heidelberg, Germany) equipped with argon (488 nm excitation: blue) and helium–neon (633 nm excitation: red).
Statistics
Student’s t tests were calculated using the Vassarstat interactive statistical website (http://faculty.vassar.edu/lowry/VassarStats.html; Richard Lowry, Poughkeepsie, NY, USA).
Results
Synaptic terminal ApoE is elevated in AD cortex and in a transgenic rat model of AD
Previous work has demonstrated elevated Aβ in cortical regions early in AD [48], and we have previously demonstrated synapse-associated pathology including Aβ oligomers and p-tau, reduced PSD-95, and dilated pre-synaptic terminals in multiple regions of AD cortex [14, 20, 21, 58]. The present experiments used synaptosomes prepared from crypreserved, short postmortem interval AD samples with clinical and histopathological confirmation of disease in order to measure apoE and Aβ. Synaptosomes are formed as membranes instantaneously reseal when fresh tissue is gently homogenized in isotonic sucrose. Synaptosomes contain calcium and the presynaptic exocytotic apparatus, along with attached postsynaptic structures [13], and the preparation has been widely used to study neurotransmitter regulation and release. Flow cytometry analysis of synaptosomes permits focus on a large and highly pure population (3,000–5,000 analyzed/case) of human synaptic terminals. Using an analysis gate including only particles between 0.50 and 1.5 μ in size, the analysis is focused on a synaptosome population that is ~95% pure; in comparison, gradient-purified synaptosomes are ~80% pure [19, 72]. Figure 1 illustrates flow cytometry analysis for representative samples, with fluorescence plotted against the forward scatter parameter, which is proportional to particle size. The rectangular analysis gate is drawn to include only particles ~1 micron and to include only immunolabeling above background. Aβ fluorescence is plotted against the forward scatter parameter, which is proportional to particle size. Background labeling was determined in the presence of an isotype-specific IgG control (Fig. 1a), and SNAP-25, an abundant presynaptic docking protein, was used as a positive control (Fig. 1b). As previously reported, [14, 20, 21], little Aβ is detected in synapses from cognitively normal aged control cases (Fig. 1c), but marked synaptic Aβ accumulation is observed in AD parietal cortex, with ~65% of terminals immunolabeled for Aβ (Fig. 1d). We have recently shown that the N-terminal epitope of the 10G4 antibody resembles 6E10 but more prominently labels Aβ42 monomer, multiple oligomers and aggregates in synaptosomes with a pattern distinct from an N-terminal APP antibody [58]. Figure 1e, f illustrates the generally low level of apoE-associated immunofluorescence measured with flow cytometry analysis for AD parietal cortex synaptosomes; however, the apoE-positive fraction in synaptosomes is larger in the AD case (Fig. 1f) compared to a normal case (Fig. 1e). Case information for all human samples is presented in Table 1; control comparisons include neurologic controls (Pick’s disease, hippocampal sclerosis, and spinocerebellar degeneration) in addition to cognitively normal aged controls. Neurologic controls were included to determine the degree to which synaptic changes are specific for AD. Moreover, a large fraction of probable AD and MCI diagnoses are mixed dementia on neuropathological exam [56].
Fig. 1.
Flow cytometry analysis of amyloid beta (Aβ) and apolipoprotein E (apoE) in Alzheimer’s disease (AD) synaptosomes. Representative dot plots show a background in the presence of a nonspecific isotype control antibody, and b SNAP-25 as a positive control and indicator of synaptosomal purity. c Synaptosomal Aβ labeling is shown for a representative aged normal control case and d for a representative AD case. e Synaptosomal apoE labeling for a representative aged normal control case, and f for a representative AD case. Dot plots show fluorescence plotted against forward scatter (FSC), which is proportional to particle size; the 10G4 antibody was used for Aβ, and the E6D7 antibody was used for apoE, data were collected from 5,000 synaptosomes/sample
Flow cytometry was used to quantify synaptic apoE levels in a larger sample; Fig. 2a confirms the larger apoE-positive fraction in AD compared to normal synaptosomes, suggesting increased synaptic apoE associated with or in response to synaptic pathology. A similar modest but significant increase in synaptic apoE was measured by flow cytometry in hippocampal synaptosomes from 20-month old APP/PS1 transgenic rats ([16]; Fig. 2b). Similar increases were observed when PBS-soluble apoE was measured by ELISA using synaptosome-enriched fractions [58] from cortex of human (n = 5 each for AD and control, Fig. 2c) and aged APP/PS1 rats.
Fig. 2.
ApoE level is elevated in AD cortical synapses. a Flow cytometry measure of apoE-associated immunofluorescence in AD (n = 14) versus control synaptosomes (n = 12; *p < 0.05; RFU, relative fluorescent intensity). b Flow cytometry measure of apoE-associated immunofluorescence in 3XTg rat synaptosomes (n = 6 each group, *p < 0.05). c, d ApoE level in synaptosome-enriched fractions measured by ELISA (c) for AD versus control (n = 5 each group) and d for 3XTg rat versus wt littermates (n = 6 each group; *p < 0.05)
Aβ is concentrated in a small population of apoE-positive synapses with more Aβ/synapse in AD cases
To test the hypothesis that apoE is increased in parietal cortex synapses with Aβ pathology, synaptosomes were dual labeled for apoE and for Aβ; flow cytometry data were collected (5,000 particles/sample) for apoE-positive synaptosomes as shown in the representative dot plot in Fig. 3a. In AD cases, apoE-positive synaptosomes were highly labeled for Aβ as seen in the representative dot plots in Fig. 3b, c which illustrate the flow cytometry analysis for the same dual-labeled AD sample shown in Fig. 3a. Additional flow cytometry controls and representative dual labeling for a normal case are shown in Supplemental Figure 1. In Fig. 3b, the fluorescence associated with Aβ is plotted against apoE fluorescence, and dual-labeled synaptosomes are in the upper right quadrant. Figure 3c shows Aβ fluorescence in the apoE-positives as a function of size (forward scatter). When the size of the positive fraction is plotted (Fig. 3d), relatively high levels of Aβ in apoE-positives were observed in both normal (47 ± 10.9%) and AD cases (62 ± 3.5% positive), and Aβ was markedly increased in apoE-positive compared to apoE-negative synapses for both AD (p < 0.0001) and control cases (p < 0.01). These results indicate that much of the total Aβ is concentrated within the small population of apoE-containing synapses.
Fig. 3.
ApoE co-localization with amyloid beta (Aβ) in individual synaptosomes from AD cortex. a–c Flow cytometry analysis of a synaptosome sample dual labeled for apoE and for Aβ; dot plots are shown for a representative AD sample; forward scatter (FSC) is proportional to size and data was collected from 2,000 apoE-positive synaptosomes. a positive fraction for apoE, b Aβ immunofluorescence in apoE-positive synaptosomes; particles labeled for apoE only are in upper left quadrant, and dual positives are in upper right quadrant. c Aβ immunolabeling in the same 2,000 apoE-positive synaptosomes. d–f Flow cytometry analysis of synaptosomes dual labeled for apoE and Aβ in control (n = 3) versus AD (n = 7) samples. d size of positive fraction in control versus AD, e brightness of immunolabeling (RFU, relative fluorescence units) in control versus AD. f ApoE labeling in Aβ-positive synaptosomes. Data represent *p < 0.05, **p < 0.02, ***p < 0.005 for AD versus control comparison
Flow cytometry quantifies brightness of fluorescence in addition to size of positive fraction; when the brightness of fluorescence (relative fluorescence units, RFU) is plotted (Fig. 3e), a large difference is observed between the Aβ fluorescence associated with the apoE-positive terminals in normal (22.13 ± 2.6 RFU) compared to AD cases (105.64 ± 20 RFU). An increase in brightness when the size of the positive fraction is not significantly different translates to an increased level of Aβ per apoE-containing synapse in AD cortex, indicating much more Aβ/apoE-positive synaptic terminal in AD cortex. Similar to results for the percent positive parameter, Aβ level was higher in apoE-positive compared to apoE-negative terminals for control (p < 0.03) and AD cases (p < 0.004), confirming concentration of Aβ in apoE-positive synaptosomes.
In Aβ-positive synapses, apoE level is higher for control cases
The converse analytic strategy was also performed, with collection and analysis of 5,000 Aβ-positive synapses dual labeled for apoE. Figure 3f demonstrates that, in Aβ-positive terminals, apoE is higher in non-AD control cases (50.29 vs. 35.84 RFU, p < 0.005). The increase in apoE per Aβ-bearing synapse in aged control cases is consistent with a role for the level of synaptic apoE in Aβ clearance. Taken together, dual-labeling results suggest that apoE and Aβ are concentrated in a relatively small fraction of apoE-positive synaptic terminals in both AD and normal cases. This result suggests that each is targeted to the same synaptic terminals, possibly via direct apoE/Aβ binding and receptor-mediated entry into synaptic terminals, which has been shown to occur in vitro [35, 44] and in vivo [49]. Confirmation of apoE/Aβ colocalization by confocal analysis in representative AD synaptosomes is illustrated in Fig. 4.
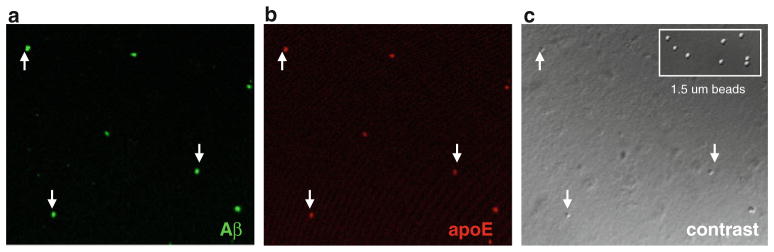
Fig. 4.
Confocal analysis of Aβ and apoE colocalization. a Aβ immunolabeling in washed P-2 fraction from an 87-year-old AD case (parietal cortex). b ApoE immunolabeling, and c differential contrast image of same field; inset shows contrast image of 1.53-μm size standard beads; arrowheads indicate dual-labeled synaptosomes
Synaptic Aβ and apoE in TR mice expressing human APOE
To examine for isoform-dependent apoE effects, synaptosomes were prepared from cryopreserved cortex samples from 18-month-old apoE TR mice expressing human apoE 2/4, 3/3 and 4/4 [32, 61, 62]. ApoE TR mice are a well-characterized in vivo model for the study of human APOE; each apoE TR line expresses human apoE under control of mouse regulatory elements, and TRE4 mice display reduced plasma, CSF, and brain levels of apoE compared to 3/3 and 2/2 [1, 59]. Heterozygous apoE2/4 mice were used because type III hyperlipoproteinemia has been demonstrated in homozygous animals [63]. Synaptosomes were dual labeled for apoE and Aβ and analyzed by flow cytometry as described above for human cortex samples. A majority of synaptic terminals (~50–80%, 5,000 events/ sample) demonstrate widespread low levels of synaptic Aβ immunoreactivity in aged TR mice (Fig. 5), despite the absence of AD-related mutations. This observation is consistent with previous observation of parenchymal diffuse Aβ deposits in aged TR animals [60]. The widespread diffuse Aβ immunoreactivity in aged TR mice is in contrast to the intense Aβ immunreactivity and larger Aβ-positive fraction measured in two rodent models of AD, the 3XTg mouse ([51]; Fig. 4c, d), and an APP/PS1 rat model that expresses two APP mutations and one PS1 mutation ([16]; Fig. 4e, f). We have also observed very similar bright synaptic Aβ labeling in aged Tg2576 animals [14]. The frequent but low level of synaptic Aβ in TR mice likewise contrasts with bright synaptic Aβ labeling in human AD synapses (Fig. 1), but resembles the intermediate levels of synaptic Aβ seen in some cognitively normal aged controls and in PD cases [58].
Fig. 5.
Flow cytometry analysis of synaptic amyloid beta (Aβ) immunolabeling in mouse models. a Background labeling in the presence of a nonspecific isotype control antibody. b–f Aβ immunolabeling in cortical synaptosomes from representative samples: b aged apolipoprotein E targeted replacement (apoE TR) 3/3 mouse, and c aged WT mouse, and d 3XTg mouse model of AD. e Aβ immunolabeling in aged WT rat and b 3XTg rat model of AD
Synaptic apoE and Aβ levels in apoE2 TR mice resemble non-AD control cases
As in human synapses, apoE-positive synaptosomes were highly labeled for Aβ as illustrated by the representative plots in Fig. 6a–c, which illustrate the flow cytometry analysis for the same dual-labeled apoE TR 3/3 sample. The fluorescence associated with Aβ is plotted against apoE fluorescence in Fig. 6b, in which dual-labeled synaptosomes are in the upper right quadrant. Aβ fluorescence in the apoE-positives as a function of size (forward scatter) is plotted in Fig. 6c. Similar to relatively low apoE levels measured by flow cytometry in human and APP/PS1 synaptosomes, the apoE-positive fraction was fairly small; the highest apoE-positive fraction was measured in apoE2/4 animals (16.0 ± 0.6), and the smallest positive fraction was in apoE4/4 animals (9.7 ± 1.0; p < 0.05). In contrast to previous results showing reduced spine density in apo-ETR4 mice [12], pre-synaptic (SNAP-25) and post-synaptic (PSD-95) were not reduced in aged 4/4 animals (data not shown). Also similar to the results above in AD and normal cases, relatively high levels of Aβ in apoE-positives were observed in all three TR lines (~50–80% positive), and Aβ levels were higher in apoE-positives compared to apoE-negatives for all three TR lines (p < 0.001), consistent with targeting of apoE and Aβ to the same small population of synapses. When the brightness of fluorescence is plotted (Fig. 6e) for the apoE TR lines, the Aβ fluorescence associated with the apoE-positive terminals is much lower in apoE2/4 compared to apoE3/3 and 4/4 animals. As for human results, the Aβ RFU was markedly higher in apoE-positive compared to apoE-negative terminals for 3/3 and 4/4 TR lines (Fig. 6e; p < 0.001). The pattern of synaptic apoE/Aβ colocalization in aged TR2/4 in comparison to the TR3/3 and TR 4/4 mice therefore closely resembles the pattern of normal controls compared to AD synaptosomes (Fig. 3), with concentration of Aβ in a small population of apoE-containing synaptic terminals, and increased Aβ per apoE-containing synapse in 3/3 and 4/4 lines compared to 2/4. For direct comparison of parameter values, flow cytometry results for human versus TR mouse experiments are presented in Supplementary Table 1.
Fig. 6.
ApoE co-localization with amyloid beta (Aβ) in individual synaptosomes from apolipoprotein E targeted replacement (apoE TR) mouse cortex. a–c Flow cytometry analysis of a synaptosome sample dual labeled for apoE and for Aβ; dot plots are shown for a representative aged apoE TR 3/3 sample; forward scatter (FSC) is proportional to size and data were collected from 2,000 apoE-positive synaptosomes. a the positive fraction for apoE, b Aβ immunofluorescence in apoE-positive synaptosomes; particles labeled for apoE only are in upper left quadrant, and dual positives are in upper right quadrant. c Aβ immunolabeling in the same 2,000 apoE-positive synaptosomes. d–f Flow cytometry analysis of synaptosomes dual labeled for apoE and Aβ in control (n = 3) versus AD (n = 7) samples. d Size of positive fraction in control versus AD. e Brightness of immunolabeling (RFU, relative fluorescence units) in control versus AD. f ApoE labeling in Aβ-positive synaptosomes. g Ganglioside GM1 (GM1) and free cholesterol in cortical synaptosomes from apoE TR mice. Data represent mean ± SEM, n = 7–9/group, *p < 0.05, **p < 0.01, ***p < 0.003 for 2/4 versus 4/4 comparison
Synaptic apoE and free cholesterol levels are elevated in the presence of ApoE2
Again resembling AD versus control results, when the converse analytic strategy was performed with collection and analysis of Aβ-positive synapses dual labeled for apoE, the apoE level/Aβ-containing synapse was highest in TR 2/4 compared to 4/4 mice (26.5 vs. 15.8; p < 0.003).
Reasoning that apoE changes may alter synaptic lipids, we next labeled aged TR synaptosomes with filipin, a fluorescent polyene antibiotic that binds unesterified cholesterol [47], and with cholera toxin B subunit to label GM1 gangliosides (GM1) for flow cytometry analysis (Supplemental Figure 2). Gangliosides are membrane glycosphingolipids with a hydrophilic sialic acid moiety and a hydrophobic ceramide core; free cholesterol and GM1 are major component of lipid rafts and both play important roles in signaling and particularly for Aβ interaction with membranes [73, 75]. Figure 6d shows that, in TR2/4 compared to 4/4 animals, ganglioside GM1 was decreased (139.74 vs. 358.4 RFU; p < 0.05) and free cholesterol was strongly increased (1,801 vs. 1,016 RFU; p < 0.01), consistent with increased lipidation of apoE2 and delivery of cholesterol along with apoE to the synaptic compartment.
Discussion
Interaction between apoE and Aβ is well-documented in vitro, but little is understood about the cellular mechanisms by which APOE4 confers risk and APOE2 confers protection fr om AD. In both aged cognitive normals and AD cases, apoE and Aβ are highly co-localized in a small population of apoE-positive synaptic terminals, consistent with uptake of an apoE/Aβ complex, or ta rgeting of apoE to Aβ-positive synapses. Consistent with clearance of Aβ, the Aβ level per synaptic terminal is markedly lower in normal control cases compared to AD. Similarly, with respect to isoform, the lowest level of synaptic Aβ and the highest level of apoE are associated with APOE2 in aged TR animals without cognitive deficits or pathology. Higher synaptic free cholesterol suggests that increased apoE lipidation in the presence of E2 delivers cholesterol to the synapse.
Reduced levels of plasma apoE in E4 compared to E2 and E3 carriers have long been documented [68]; moreover, a reduction in total plasma apoE and apoE4 has recently been demonstrated in AD versus controls and to correlate with AD PiB-PET pathology [17]. In TR mice expressing human apoE, reduced apoE has been shown in plasma, brain and CSF in E4 compared to E2 and E3 [52, 54, 59], and this reduction is likewise seen in the PDAPP mouse model of AD when crossed with TR line expressing human apoE2, 3 and 4 [1]. The present results extend this finding to the synaptic compartment, where the earliest cognitive deficits are hypothesized to occur [57], suggesting that soluble synaptic apoE level may make a direct contribution to synaptic dysfunction in E4 carriers. A modest degree of difference in isoform-dependent apoE protein levels was observed in the present results and has been consistently observed by others. This result is consistent with the extended time required for expression of the increased AD risk conferred by E4 and the protection conferred by E2.
Clearance rather than production deficits have been suggested to predominate in the majority of typical late-onset AD cases [2], which is consistent with the results of a trial measuring human CSF Aβ clearance and production in AD and control patients [46]. Also confirming the importance of clearance, the recent in vivo microdialysis experiments in PDAPP/TR mice showed longer Aβ half-life in PDAPP/E4 mice compared to PDAPP/E3 and PDAPP E2 animals [6]. In the present results, consistent and significant reductions in synaptic Aβ were seen only in the TR2/4 line, with no major E3 versus E4 differences; in fact the total Aβ-positive fraction was modestly larger in 3/3 compared to 4/4 terminals (Fig. 5d). This main effect of the E2 allele therefore likely follows from a positive effect on clearance mechanisms since Aβ production is not altered in these animals. The sharp reduction of synaptic Aβ in apoE2 terminals (compared to E3 and E4-positives; Fig. 5e), greatly resembles the same comparison in aged normals versus AD (Fig. 3e), and serves to illustrate the importance of Aβ clearance processes in sporadic AD. This similarity of the apoE/Aβ results in sporadic AD and aged TR mice also highlights the usefulness of aged TR mice for study of Aβ clearance without confounding of excess or mutated Aβ production. The lack of an E4 effect in the apoE TR animals may indicate that E4-dependent reduced apoE levels may be required for a period longer than the mouse lifespan. On the other hand, increased Aβ production or Aβ42/40 ratio may also be necessary for full expression of enhanced Aβ deposition by E4.
In apoE2 TR synapses, Aβ and apoE are still co-localized but there is less Aβ. In vitro work has shown that lipidated apoE forms an SDS-stable complex more abundant with apoE2 and E3 compared to E4, which led to an early hypothesis that complex formation between apoE and Aβ facilitates clearance [35, 36]. On the other hand, in vivo results suggest that apoE4/Aβ complex formation may affect binding to the low-density lipoprotein receptor-related protein (LR) and slow clearance of Aβ across the endothelium into the blood [3, 10]. Insoluble apoE/Aβ complexes with a fibrillar structure have been extracted from AD brain [49], and evidence also supports co-deposition of apoE with Aβ in plaques in human disease, particularly in newly formed plaques [38, 50, 64]. In old 5xFAD mouse, both Aβ42 and apoE extraction shifts from the detergent-soluble fraction to buffer-soluble and insoluble fractions, consistent with a shift from association with lipids (detergent soluble) to localization with Aβ in deposits (insoluble) and with oligomers ([77]; buffer soluble). This extraction pattern suggests that apoE may form complexes with multiple Aβ assembly states, which has been observed in vitro [44]. Along this line, our finding that the Aβ level per terminal in AD is higher than in normals, despite similar-sized positive fractions (Fig. 3e), is consistent with a model in which apoE associates with multimeric Aβ assemblies that might be oligomers, aggregates or fibrils. Indeed, in vitro complex formation has used a molar ratio of apoE to Aβ of that varies from 1:30 [44] to 1:100–200 [41, 71]. Alternatively, complex formation may not be required, and colocalization of Aβ and apoE within a cellular compartment may be sufficient for E2-enhanced clearance.
In PDAPP/apoE TR lines, co-localization of apoE and Aβ42 was significantly increased in E4 compared to E3 and E2 lines; nearly all apoE was associated with Aβ in old PDAPP/E4 animals, with only 25% co-localization in PDAPP/E2 animals [1]. This is in contrast to the present experiments where a high degree of co-localization was seen in all three apoE TR lines (77–92%, Fig. 5d). We also observed relatively high co-localization in both normal and AD samples, 47 and 62%, respectively (Fig. 3d). Co-localization was measured by Bales and colleagues at the light microscope level and therefore includes deposited Aβ, while our flow cytometry analysis was focused on synaptic terminals in animals without pathology or deposits. Importantly, in both studies, the E2 allele was associated with the highest level of apoE and the lowest level of Aβ. Both results are consistent with E2-mediated enhanced clearance of Aβ, possibly via the formation of more apoE/Aβ complex due to the E2-related increased apoE level, or more efficient clearance of a complex containing apoE2. Considered together with the dual-labeling results, the increased synaptic apoE level observed in AD cases would be expected to follow over time from less efficient synaptic clearance of apoE/Aβ complex in E3 and E4 carriers, since apoE2 alleles are found in only 15% of the population and are rare in AD postmortem cases, while the E4 allele is overrepresented in human AD [5, 56].
Our previous results have shown increased free cholesterol and ganglioside GM1 in Aβ-positive synaptosomes in AD, and a large body of results has shown a reciprocal relationship between cholesterol and Aβ production [37]. Therefore, we hypothesized that E4 would increase cholesterol, and the striking increase in free cholesterol by E2 was surprising. The contribution of apoE to brain cholesterol levels is not well understood; in general the total cholesterol levels are either unchanged or decreased in AD, likely due to the abundance of cholesterol-rich myelin in brain, and to the difficulty of measuring cholesterol in different cell populations and in different subcellular compartments. To date, our experiments measuring synaptic free cholesterol in AD versus control cases have shown variable results (not shown). It seems likely that these results in human postmortem samples are confounded by the intracellular and extracellular accumulation of Aβ over the decades, and by overrepresentation of E3 and E4 alleles. However, the present results are consistent with a hypothesis that isoform-dependent lipidation status may be important in AB deposition and AD via the liver X-receptor (LXR)-ABCA1-APOE regulatory axis [33, 34]. The ATP-binding cassette transporter A1 (ABCA1) transfers cholesterol from cells to lipid-poor apoE, forming high-density lipoprotein (HDL) particles. Emphasizing the importance of apoE level and lipidation, ABCA1 deficiency reduces soluble apoE levels and lipidation, resulting in either unchanged or increased Aβ levels in AD mouse models [22, 34, 69]. ABCA1 and apoE levels are induced by treatment with liver X-receptor agonists, which reduce amyloid deposition in APP/PS1 animals [11, 29] and also diminish high-fat diet-induced pathology and memory deficits in APP23 mice [15]. Particularly relevant to the present experiments, overexpression of ABCA1 in PDAPP mice reduced Aβ deposition via increased lipidation of apoE [69], and in vitro experiments have demonstrated more efficient Aβ degradation by IDE in the presence of highly lipidated apoE [29].
In the model system used, apoE TR mice expressing human APOE, apoE4 expression has been associated with synaptic and spatial memory deficits even in the absence of pathology [4, 70], and E4-dependent deficits were attenuated in apoE2/4 mice [32]. Confirmation of our cholesterol results is provided by the observation that brain lathosterol, a cholesterol precursor and indicator for cholesterol synthesis, is increased in E2 compared to E4 animals in the same apoE TR model [27]. Therefore, the high level of synaptic free cholesterol observed in 2/4 animals is consistent with the previous evidence of increased apoE lipidation. The observed APOE-related changes in free cholesterol are also supported by a number of previous results; for example, alterations in lipid raft cholesterol have been shown with with age and genotype in synaptic plasma membranes [25]. The importance of cellular cholesterol pools is also supported by evidence of abnormal cholesterol accumulation in the setting of apoE deficiency and environmental stimulation [39], and by evidence that reduction of cholesterol esters by inhibition of acylcoenzymeA:cholesterol acyltransferase (ACAT) reduces amyloid pathology in vivo [24]. In addition to enhanced Aβ clearance, improved lipidation and higher synaptic free cholesterol may contribute to improved synaptic function in these animals.
In synaptic terminals, large isoform-related differences in lipidation level/synaptic cholesterol are accompanied by relatively small differences soluble apoE levels. However, the nature of APOE-dependent risk for AD, like the increased risk for atherosclerosis, and poor outcome in closed head injury and stroke, represent relatively small differences in risk that take years or decades for full expression, consistent with a minor shift in function. The observed changes in apoE level and Aβ clearance pathways operating in or near synapses highlight the potential of diet and apoE-related therapeutics in AD protection. It will be important in the future studies to examine for isoform effects in human cases, and for apoE and Aβ colocalization and levels in glial cells, particularly in the astrocyte processes that surround and nourish synapses.
Supplementary Material
Acknowledgments
This work was supported by NIH AG27465 to KHG, by NIH NS43946 to GMC, by NIA AG18879 to CAM. HVV is supported by the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine. Tissue was obtained from the the Alzheimer’s Disease Research Center Neuropathology Cores of USC (NIA 050 AG05142), UCLA (NIA P50 AG 16970), and UC Irvine (NIA P50 AG016573). Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility supported by NIH CA16042 and AI 28697, and by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine and the Chancellor’s Office at UCLA. ET is supported by K08 AG-34628 (jointly sponsored by NIA, AFAR, the John A. Hartford Foundation and the Atlantic Philanthropies, the Starr Foundation, and an anonymous donor).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-011-0892-1) contains supplementary material, which is available to authorized users.
Contributor Information
Stephen Arold, School of Nursing and Mary S. Easton Center for Alzheimer’s Research, UCLA, Box 956919 Factor Bldg, Los Angeles, CA 90095-6919, USA.
Patrick Sullivan, Department of Medicine, Duke University Medical Center, Durham VAMC GRECC, Durham, NC 27710, USA.
Tina Bilousova, School of Nursing and Mary S. Easton Center for Alzheimer’s Research, UCLA, Box 956919 Factor Bldg, Los Angeles, CA 90095-6919, USA.
Edmond Teng, Department of Neurology, UCLA School of Medicine, Los Angeles, USA. Sepulveda VAMC GRECC, Los Angeles, CA 90095, USA.
Carol A. Miller, Departments of Pathology, Neurology, and Program in Neuroscience, Keck USC School of Medicine, Los Angeles, CA 90033, USA
Wayne W. Poon, Institute for Memory Impairments and Neurological Disorders, UC Irvine, Irvine, CA 92697, USA
Harry V. Vinters, Department of Neurology, UCLA School of Medicine, Los Angeles, USA. Department of Pathology and Laboratory Medicine, UCLA School of Medicine, Los Angeles, USA
Lindsey B. Cornwell, Institute for Memory Impairments and Neurological Disorders, UC Irvine, Irvine, CA 92697, USA
Tommy Saing, Institute for Memory Impairments and Neurological Disorders, UC Irvine, Irvine, CA 92697, USA.
Gregory M. Cole, Department of Neurology, UCLA School of Medicine, Los Angeles, USA. Department of Medicine, UCLA School of Medicine, Los Angeles, USA. Sepulveda VAMC GRECC, Los Angeles, CA 90095, USA
Karen Hoppens Gylys, Email: kgylys@sonnet.ucla.edu, School of Nursing and Mary S. Easton Center for Alzheimer’s Research, UCLA, Box 956919 Factor Bldg, Los Angeles, CA 90095-6919, USA.
References
- 1.Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN. Clearance mechanisms of Alzheimer’s amyloid-beta peptide: implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2009;14:469–486. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]
- 3.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellano JM, Kim J, Stewart FR, Jiang H, Demattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-{beta} peptide clearance. Sci Transl Med. 2011;3:57–89. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen DZ, Schneider-Axmann T, Lucassen PJ, Bayer TA, Wirths O. Accumulation of intraneuronal Abeta correlates with ApoE4 genotype. Acta Neuropathol. 2010;119:555–566. doi: 10.1007/s00401-010-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donkin JJ, Stukas S, Hirsch-Reinshagen V, Namjoshi D, Wilkinson A, May S, Chan J, Fan J, Collins J, Wellington CL. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285:34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3:1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- 14.Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, Gylys KH. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172:1683–1692. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitz NF, Cronican A, Pham T, Fogg A, Fauq AH, Chapman R, Lefterov I, Koldamova R. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci. 2010;30:6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flood DG, Lin YG, Lang DM, Trusko SP, Hirsch JD, Savage MJ, Scott RW, Howland DS. A transgenic rat model of Alzheimer’s disease with extracellular Abeta deposition. Neurobiol Aging. 2009;30:1078–1090. doi: 10.1016/j.neurobiolaging.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Gupta VB, Laws SM, Villemagne VL, Ames D, Bush AI, Ellis KA, Lui JK, Masters C, Rowe CC, Szoeke C, Taddei K, Martins RN. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76:1091–1098. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 18.Gylys KH, Fein JA, Tan AM, Cole GM. Apolipoprotein E enhances uptake of soluble but not aggregated amyloid-beta protein into synaptic terminals. J Neurochem. 2003;84:1442–1451. doi: 10.1046/j.1471-4159.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 19.Gylys KH, Fein JA, Yang F, Cole GM. Enrichment of presynaptic and postsynaptic markers by size-based gating analysis of synaptosome preparations from rat and human cortex. Cytometry A. 2004;60:90–96. doi: 10.1002/cyto.a.20031. [DOI] [PubMed] [Google Scholar]
- 20.Gylys KH, Fein JA, Yang F, Miller CA, Cole GM. Increased cholesterol in Abeta-positive nerve terminals from Alzheimer’s disease cortex. Neurobiol Aging. 2007;28:8–17. doi: 10.1016/j.neurobiolaging.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–1817. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, Poirier J, Van Nostrand W, Wellington CL. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- 23.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 24.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113, 818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Igbavboa U, Eckert GP, Malo TM, Studniski AE, Johnson LN, Yamamoto N, Kobayashi M, Fujita SC, Appel TR, Muller WE, Wood WG, Yanagisawa K. Murine synaptosomal lipid raft protein and lipid composition are altered by expression of human apoE 3 and 4 and by increasing age. J Neurol Sci. 2005;229–230:225–232. doi: 10.1016/j.jns.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 27.Jenner AM, Lim WL, Ng MP, Wenk MR, Shui G, Sharman MJ, Gandy SE, Martins RN. The effect of APOE genotype on brain levels of oxysterols in young and old human APOE epsilon2, epsilon3 and epsilon4 knock-in mice. Neuroscience. 2010;169:109–115. doi: 10.1016/j.neuroscience.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61:1579–1584. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Basak JM, Holtzman DM. The role of apolipo-protein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein RC, Mace BE, Moore SD, Sullivan PM. Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience. 2010;171:1265–1272. doi: 10.1016/j.neuroscience.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koldamova R, Fitz NF, Lefterov I. The role of ATP-binding cassette transporter A1 in Alzheimer’s disease and neurodegeneration. Biochim Biophys Acta. 2010;1801:824–830. doi: 10.1016/j.bbalip.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 35.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 36.LaDu MJ, Lukens JR, Reardon CA, Getz GS. Association of human, rat, and rabbit apolipoprotein E with beta-amyloid. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
- 37.Leduc V, Jasmin-Belanger S, Poirier J. APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med. 2010;16:469–477. doi: 10.1016/j.molmed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 39.Levi O, Lutjohann D, Devir A, von Bergmann K, Hartmann T, Michaelson DM. Regulation of hippocampal cholesterol metabolism by apoE and environmental stimulation. J Neurochem. 2005;95:987–997. doi: 10.1111/j.1471-4159.2005.03441.x. [DOI] [PubMed] [Google Scholar]
- 40.Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006;27:797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 42.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mak K, Yang F, Vinters HV, Frautschy SA, Cole GM. Polyclonals to beta-amyloid(1–42) identify most plaque and vascular deposits in Alzheimer cortex, but not striatum. Brain Res. 1994;667:138–142. doi: 10.1016/0006-8993(94)91725-6. [DOI] [PubMed] [Google Scholar]
- 44.Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ. ApoE and Abeta1–42 interactions: effects of isoform and conformation on structure and function. J Mol Neurosci. 2004;23:235–246. doi: 10.1385/JMN:23:3:235. [DOI] [PubMed] [Google Scholar]
- 45.Martinez M, Brice A, Vaughan JR, Zimprich A, Breteler MM, Meco G, Filla A, Farrer MJ, Betard C, Singleton A, Hardy J, De Michele G, Bonifati V, Oostra BA, Gasser T, Wood NW, Durr A. Apolipoprotein E4 is probably responsible for the chromosome 19 linkage peak for Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:72–74. doi: 10.1002/ajmg.b.30196. [DOI] [PubMed] [Google Scholar]
- 46.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller CP, Stephany DA, Winkler DF, Hoeg JM, Demosky SJ, Jr, Wunderlich JR. Filipin as a flow microfluorometry probe for cellular cholesterol. Cytometry. 1984;5:42–54. doi: 10.1002/cyto.990050108. [DOI] [PubMed] [Google Scholar]
- 48.Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 49.Naslund J, Thyberg J, Tjernberg LO, Wernstedt C, Karlstrom AR, Bogdanovic N, Gandy SE, Lannfelt L, Terenius L, Nordstedt C. Characterization of stable complexes involving apolipo-protein E and the amyloid beta peptide in Alzheimer’s disease brain. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 50.Nishiyama E, Iwamoto N, Ohwada J, Arai H. Distribution of apolipoprotein E in senile plaques in brains with Alzheimer’s disease: investigation with the confocal laser scan microscope. Brain Res. 1997;750:20–24. doi: 10.1016/s0006-8993(96)01329-7. [DOI] [PubMed] [Google Scholar]
- 51.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 52.Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on apolipoprotein E levels in mouse brain. J Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid I, Uittenbogaart CH, Giorgi JV. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12:279–285. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- 56.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokolow S, Henkins KM, Bilousova T, Miller CA, Vinters HV, Poon W, Cole GM, Gylys KH. AD synapses contain abundant Abeta monomer and multiple soluble oligomers, including a 56-kDa assembly. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.05.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Koger D, Paul S, Bales KR. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. 2011;32:791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan PM, Mace BE, Estrada JC, Schmechel DE, Alberts MJ. Human apolipoprotein E4 targeted replacement mice show increased prevalence of intracerebral hemorrhage associated with vascular amyloid deposition. J Stroke Cerebrovasc Dis. 2008;17:303–311. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thal DR, Capetillo-Zarate E, Schultz C, Rub U, Saido TC, Yamaguchi H, Haass C, Griffin WS, Del Tredici K, Braak H, Ghebremedhin E. Apolipoprotein E co-localizes with newly formed amyloid beta-protein (Abeta) deposits lacking immunoreactivity against N-terminal epitopes of Abeta in a genotype-dependent manner. Acta Neuropathol. 2005;110:459–471. doi: 10.1007/s00401-005-1053-1. [DOI] [PubMed] [Google Scholar]
- 65.Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kolsch H, Del Tredici K, Attems J, Ghebremedhin E. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:169–183. doi: 10.1007/s00401-010-0707-9. [DOI] [PubMed] [Google Scholar]
- 66.Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 67.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 68.Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980;32:339–347. [PMC free article] [PubMed] [Google Scholar]
- 69.Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- 70.Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipo-protein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf ME, Kapatos G. Flow cytometric analysis of rat striatal nerve terminals. J Neurosci. 1989;9:94–105. doi: 10.1523/JNEUROSCI.09-01-00094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood WG, Igbavboa U, Muller WE, Eckert GP. Cholesterol asymmetry in synaptic plasma membranes. J Neurochem. 2011;116:684–689. doi: 10.1111/j.1471-4159.2010.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochim Biophys Acta. 2007;1768:1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Yang DS, Smith JD, Zhou Z, Gandy SE, Martins RN. Characterization of the binding of amyloid-beta peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J Neurochem. 1997;68:721–725. doi: 10.1046/j.1471-4159.1997.68020721.x. [DOI] [PubMed] [Google Scholar]
- 77.Youmans KL, Leung S, Zhang J, Maus E, Baysac K, Bu G, Vassar R, Yu C, LaDu MJ. Amyloid-beta42 alters apolipoprotein E solubility in brains of mice with five familial AD mutations. J Neurosci Methods. 2011;196:51–59. doi: 10.1016/j.jneumeth.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.