Abstract
Placental insufficiency results in intrauterine growth restriction (IUGR), impaired fetal insulin secretion and less fetal pancreatic β-cell mass, partly due to lower β-cell proliferation rates. Insulin-like growth factors (IGFs) and fibroblast growth factors (FGFs) regulate fetal β-cell proliferation and pancreas development, along with transcription factors, such as pancreatic and duodenal homeobox 1 (PDX-1). We determined expression levels for these growth factors, their receptors and IGF binding proteins in ovine fetal pancreas and isolated islets. In the IUGR pancreas, relative mRNA expression levels of IGF-I, PDX-1, FGF7 and FGFR2IIIb were 64% (P < 0.01), 76% (P < 0.05), 76% (P < 0.05) and 52% (P < 0.01) lower, respectively, compared with control fetuses. Conversely, insulin-like growth factor binding protein 2 (IGFBP-2) mRNA and protein concentrations were 2.25- and 1.2-fold greater (P < 0.05) in the IUGR pancreas compared with controls. In isolated islets from IUGR fetuses, IGF-II and IGFBP-2 mRNA concentrations were 1.5- and 3.7-fold greater (P < 0.05), and insulin mRNA was 56% less (P < 0.05) than control islets. The growth factor expression profiles for IGF and FGF signaling pathways indicate that declines in β-cell mass are due to decreased growth factor signals for both pancreatic progenitor epithelial cell and mature β-cell replication.
Keywords: placental insufficiency, intrauterine growth restriction, islets of Langerhans, β-cell, insulin, fetal programming
Introduction
Several epidemiological and experimental studies have demonstrated a relationship between intrauterine growth restriction (IUGR) and impaired glucose homeostasis in later life, indicating that β-cell dysfunction was established in utero.1–4 IUGR fetuses have lower plasma insulin concentrations and glucose-stimulated insulin secretion,5 which are partially explained by a diminished number of pancreatic β-cells in severe cases.6 Pancreas development in fetal sheep parallels human fetal pancreas development,7,8 and complications of IUGR in human fetuses are similar to those in a comparative sheep model, with placental insufficiency produced by maternal exposure to a high-temperature environment during the bulk of gestation.9–11 Two reasons for decreased β-cell mass in near-term placental insufficiency-induced IUGR (PI-IUGR) sheep fetuses are slower rates of β-cell replication9 and less pancreatic progenitor epithelium at the beginning of the third trimester (SW Limesand et al., unpublished). In normal pancreas development, these processes are regulated by insulin-like growth factors (IGFs) and fibroblast growth factors (FGFs), respectively.12,13 However, the impact of placental insufficiency on pancreatic IGFs and FGFs is not defined.
IGFs regulate cell proliferation, survival and metabolism, while their binding proteins (IGFBPs) can inhibit or potentiate IGF activity, depending on subtypes and target cells.14 –17 Furthermore, pancreatic progenitor epithelial cell proliferation is increased by fibroblast growth factor receptor (FGFR) 2IIIB ligands, FGF7 and 10.13,18–20 Expansion of these pancreatic and duodenal homeobox 1 (PDX-1)-positive progenitor cells will also influence the absolute number of β-cells formed. PDX-1 expression is high in β-cells and regulates insulin and glucose transporter 2 (Glut2), which is required for proper β-cell function.21,22 Therefore, we postulated that reduced expression of IGFs and FGFs or greater expression of IGFBPs in the IUGR fetal pancreas will explain the decline in β-cell mass and insulin secretion in the PI-IUGR sheep fetus. Furthermore, the expression profile for IGFs may occur in fetal islets of Langerhans and reduce other factors required for β-cell function.
Materials and methods
Animals and experimental protocol
Protocols involving animals were approved by the Institutional Animal Care and Use Committee at the University of Arizona and the University of Colorado Anschutz Medical Center. Both laboratories are accredited by the American Association for Accreditation of Laboratory Animal Care. Pregnant Columbia-Rambouillet crossbred ewes (n = 32) carrying singleton fetuses were purchased from Nebekar Ranch (Lancaster, CA, USA). PI-IUGR ewes (n = 16) were housed in environmentally controlled rooms with elevated ambient temperatures (40°C for 12 h and 35°C for 12 h; 30% relative humidity) from 37 ±3 days of gestational age (dGA) to 120 ±3 dGA (0.24–0.82 gestation). Pair-fed control ewes (n = 16) were maintained at thermoneutral conditions (25°C; 25% relative humidity). At 133.4 ±0.7 dGA, ewes were euthanized with an overdose of sodium pentobarbital (10 mmol/L, intravenously; Euthasol, Virbac Animal Health, Fort Worth, TX, USA). Fetal pancreases were removed and islets were isolated from a subset of these fetuses (PI-IUGR, n = 7; controls, n = 6) as previously described.10,23 The head of the pancreas was collected from the remaining PI-IUGR (n = 9) and control (n = 10) fetuses, snap-frozen in liquid nitrogen, and stored at −80°C for RNA and protein extraction.
Biochemical analysis
Fetuses were instrumented with arterial catheters as described previously.10,23 Fetal blood was collected in syringes lined with ethylenediaminetetraacetic acid or heparin. Plasma glucose concentrations were measured with a YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Blood oxygen content was measured with an ABL 520 (Radiometer, Copenhagen, Denmark).
Quantitative realtime polymerase chain reaction
RNA was isolated and reverse transcribed as described previously.23,24 Synthetic oligonucleotide primers for IGFs, FGFs, PDX-1, insulin and Glut2 (Table 1) were designed from conserved sequences. Polymerase chain reaction (PCR) products were cloned into pCR II (Invitrogen Life Technologies, Carlsbad, CA, USA) and verified by nucleotide sequencing. Quantitative PCR was performed using a master-mix of SYBR® Green JumpStart™ Taq Ready Mix™ (Sigma-Aldrich, St Louis, MO, USA), 0.3 μmol/L 5′ and 3′ primers, and 2 μL of cDNA in a DNA Engine Opticon II™ System (MJ Research, Waltham, MA, USA). The standard thermocycler protocol began with an initial denaturation (96°C) for two minutes, plus 35 cycles of denaturation (96°C) for 45 s, annealing temperature (Table 1) for 1.5 min, extension (72°C) for 45 s and fluorescent detection (72°C). After DNA amplification, melting curve analysis (72–99.9°C with detection every 0.1°C/s) was used to verify the product purity. In addition, a Southern hybridization was performed on a sample of amplified DNA to confirm PCR products, as described previously.25 Results were normalized to a reference gene (β-actin for the pancreas, S15 for the islet) using the comparative change of threshold cycles (ΔCt) method (Ct gene of interest– Ct reference gene) and fold change was determined by the Pfaffl’s and Livak’s method.26,27 Values are presented as means ± SEM.
Table 1.
Quantitative realtime polymerase chain reaction parameters
| Gene | Forward primer (5′ –3′) | Reverse primer (5′ –3′) | Product size (bp) | Annealing temperature (°C) | Accession # |
|---|---|---|---|---|---|
| IGF-I | TCGCATCTCTCTTCTATCTGGCCCTGT | GCAGTACATCTCCAGCCTCCTCAGA | 240 | 62 | M30653 |
| IGF-II | GACCGCGGCTTCTACTTCAG | AAGAACTTGCCCACGGGGTAT | 203 | 62 | X55638 |
| IGFBP-1 | TGATGACCGAGTCCAGTGAG | GTCCAGCGAAGTCTCACA | 248 | 62 | X54979 |
| IGFBP-2 | CAATGGCGAGGAGCACTC | TGGGGATGTGTGTAGGGAAT | 335 | 55 | S44612 |
| IGFBP-3 | GGCATATTTGAGCTCCACA | TCTCAGAGCACAGACACCCA | 337 | 54 | M76478 |
| IGFBP-4 | TGTGCGTGTGTGTTGATG | GAGGGAGCCAAGATGAGT | 229 | 62 | S77394 |
| IGFBP-5 | TCGTGCGGCGTCTACACTGAG | GAGTAGGTCTCCTCCGCCATC | 203 | 61 | EU727460 |
| IGFBP-6 | AGGAGAGTAAGCCCCAAGCAG | GTCACAATTGGGCAGGTAGAG | 221 | 62 | EU862545 |
| IR | CAGCTGAGCTAGAAGCCAAC | CCATTTCAGCAAGATCTTGTC | 416 | 57 | AY157728 |
| IGF-1R | AACTGTCATCTCCAACCTC | CAAGCCTCCCACTATCAAC | 490 | 53 | X54980 |
| IGF-2R | GAAGAGCAACGTGCACGA | TTGTCCAGGGAGATCAGCA | 368 | 54 | AF342811 |
| FGF7 | TCAGGACAGTGGCTGTTGGAA | CAAACATTTCTCCTCCGCTGTG | 195 | 65 | EU626202 |
| FGF10 | CCCTCGATCAGGACATGGTG | AAGGCAAGGAGACGGACGAG | 87 | 65 | AF213396 |
| FGFR2IIIb | CCGAGTGCTTCAGAACCTTGA | AGAGGCGATGTGGAGTTTGTC | 136 | 65 | DQ908953 |
| PDX-1 | TTTCCCGTGGATGAAGTCTAC | CGGTGCGTGTCCGCTTGTTCT | 101 | 60 | JF728303 |
| Glut2 | CTTTGCAGTTGGTGGAATGAT | GCTGATGAAGAGCACCGATAG | 272 | 60 | AJ318925 |
| Insulin | TCAGCAAACAGGTCCTCGCAAG | GGGCCAGGTCTAGTTACAGTAG | 352 | 60 | U00659 |
| β-Actin | GGGACCTGACCGACTACC | GACAGCGAGGCAGGATGG | 502 | 62 | U39357 |
| S15 | ATCATTCTGCCCGAGATGGTG | TGCTTTACGGGCTTGTAGGTG | 134 | 60 | AY949774 |
Glut2, glucose transporter 2; FGF, fibroblast growth factor; FGFR2IIIb, fibroblast growth factor receptor 2IIIB; IGF-I, insulin-like growth factor I; IGF-II, insulin-like growth factor II; IGFBP, insulin-like growth factor binding protein; IGF-1R, insulin-like growth factor type 1 receptor; IGF-2R, insulin-like growth factor type 2 receptor; IR, insulin receptor; PDX-1, pancreatic and duodenal homeobox 1
Protein immunoblot analysis
Immunoblots were performed for IGF-I and IGFBP-2 in fetal pancreatic tissue (sc-9013 and sc-13096, respectively; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Protein was extracted, separated by polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane as previously described.28 Non-specific binding was blocked in phosphate-buffered saline with 0.05% Tween-20 (PBST) with 5% non-fat dry milk for one hour, and then membranes were incubated with either IGF-I or IGFBP-2 antibody diluted (1:1000) in PBST with 5% non-fat dry milk at 4°C overnight. Immunocomplexes were detected with anti-goat immunoglobulin G horseradish peroxidase-conjugated secondary antibody (1:10,000; Bio-Rad Laboratories, Hercules, CA, USA) using enhanced chemiluminesence-Plus (Amersham Pharmacia Biotech, Arlington Heights, IL, USA) and exposed to X-ray film. IGF-I or IGFBP-2 immunocomplexes were removed with a 62.5 mmol/L Tris, pH 6.8, 2% sodium dodecyl sulfate and 100 mmol/L β-mercaptoethanol solution for 30 min at 50°C. Western blot analysis for β-actin (1:40,000; ICN Biomedicals, Burlingame, CA, USA) was used to normalize for loading differences. Densitometry value was measured with ImageJ software version 1.41 (National Institutes of Health, Bethesda, MD, USA) and the analysis was completed by comparing individual density value against mean value of control blots. Data are presented as means ± SEM.
Statistical analysis
One-way analysis of variance was performed using the general linear model procedure of SAS (SAS v. 9.1; SAS Institute Inc, Cary, NC, USA), and pair-wise comparisons were made using Student’s t-test. Analysis of quantitative realtime PCR data was performed on ΔCt values.
Results
Fetal parameters
Mean fetal body weight was lower (1.7 ±0.2 kg versus 3.4 ±0.1 kg, P < 0.01) in PI-IUGR fetuses (n = 16) compared with controls (n = 16). Gestation ages were similar (134 ±1 days in PI-IUGRs versus 134 ±1 days in controls). Mean plasma glucose concentrations were lower in IUGR fetuses compared with controls (0.65 ±0.04 mmol/L versus 1.11 ±0.04 mmol/L; P < 0.01). Mean blood oxygen contents were also lower in IUGR than control fetuses (1.26 ±0.20 mmol/L versus 3.00 ±0.13 mmol/L; P < 0.01). An equal number of fetal pancreases from each treatment group (control and PI-IUGR) were randomly collected for either pancreatic RNA and protein extraction or isolation of islets of Langerhans. Fetal characteristics (weight, glucose and oxygen) within treatments were not different between collection assignments.
mRNA expression in fetal pancreatic tissue
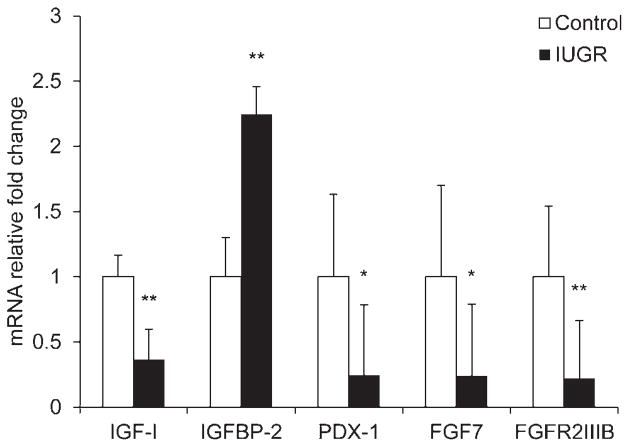
Relative IGF-I mRNA concentrations were 64% lower (P < 0.01) in PI-IUGR pancreases compared with control pancreases (Figure 1). IGFBP-2 mRNA was 2.25-fold higher (P < 0.01) in PI-IUGR pancreases. IGFBP-1 mRNA transcripts were not detected in the pancreas by PCR-Southern blot hybridization procedures, but were detected in fetal liver (data not shown29). No differences were observed in the expression of IGF-II, IGFBP-3, -4 and -5, insulin receptor (IR), IGF type 1 receptor (IGF-1R) or IGF type 2 receptor (IGF-2R) between PI-IUGR and control fetuses (data not shown). IGFBP-6 mRNA concentrations tended to be 46% lower (P = 0.07) in PI-IUGR pancreases than controls (data not shown). Expression levels of PDX-1 were 76% lower (P < 0.05) in PI-IUGR pancreases (Figure 1). FGF7 and FGFR2IIIb mRNA concentrations were 76% (P < 0.05) and 78% (P < 0.01) lower in PI-IUGR pancreases compared with controls, but FGF10 concentrations were not different (data not shown).
Figure 1.
Pancreas expression profiles for IGF-I, IGFBP-2, PDX-1, FGF7 and FGFR2IIIB. Relative mRNA concentrations for the genes listed on the abscissa are normalized to a reference gene (β-actin) and expressed as the fold change from control calculated by the 2−ΔΔCt in pancreatic tissue collected from control (□) and IUGR (■) fetuses. Mean ± SEM values for animals are presented. Significance is indicated by *P < 0.05 and **P < 0.01. IGF-I, insulin-like growth factor I; IGFBP-2, insulin-like growth factor binding protein 2; PDX-1, pancreatic and duodenal homeobox 1; FGF7, fibroblast growth factor; FGFR2IIIB, fibroblast growth factor receptor 2IIIB; IUGR, intrauterine growth restriction
mRNA expression in isolated fetal pancreatic islets
Islets of Langerhans isolated from PI-IUGR fetuses had higher (P < 0.05) IGF-II (1.5-fold) and IGFBP-2 (3.7-fold) mRNA concentrations than controls (Figure 2). IGF-I and IGFBP-6 were not different between groups (data not shown). Insulin expression was 66% lower (P < 0.05) in PI-IUGR islets than control islets (Figure 2). However, PDX-1 and Glut2 islet expression levels were not different between groups (data not shown).
Figure 2.
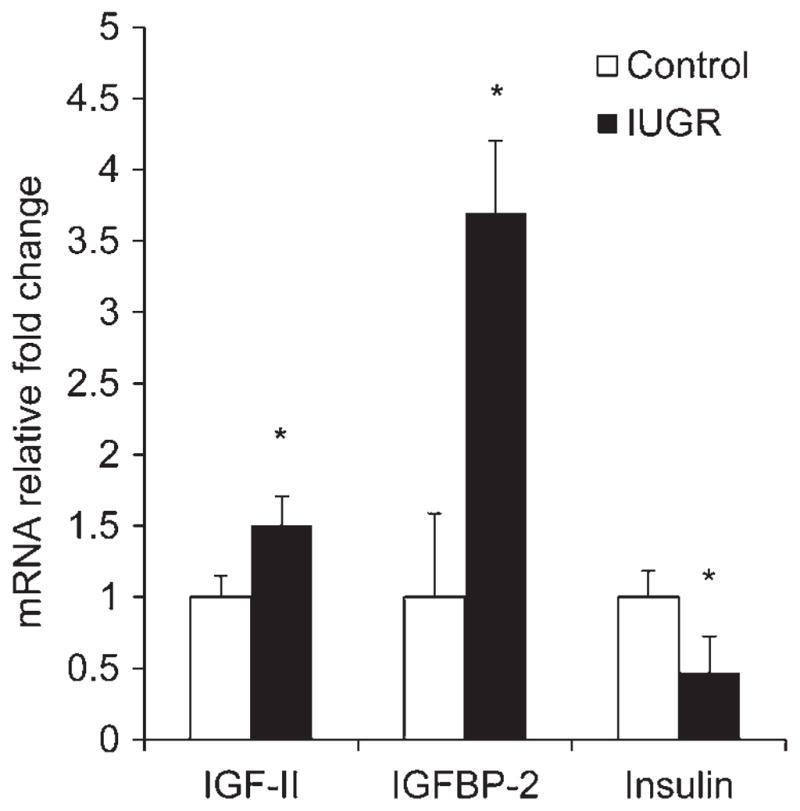
Pancreatic islet expression profiles for IGF-II, IGFBP-2 and insulin. Relative mRNA concentrations for the genes listed on the abscissa are normalized to a reference gene (S15) and expressed as the fold change from control calculated by the 2−ΔΔ in pancreatic tissue collected from control (□) and IUGR (■) fetuses. Mean ± SEM values for animals are presented. Significance is indicated by *P < 0.05 and **P < 0.01. IUGR, intrauterine growth restriction. IGF-11, insulin-like growth factor II; IGFBP-2, insulin-like growth factor binding protein 2
IGF-I and IGFBP-2 protein concentrations in pancreatic tissue
IGF-I protein content was not different between PI-IUGR and control pancreases. However, IGFBP-2 protein content was 1.2-fold greater (P < 0.01) in PI-IUGR pancreases compared with controls (Figure 3).
Figure 3.
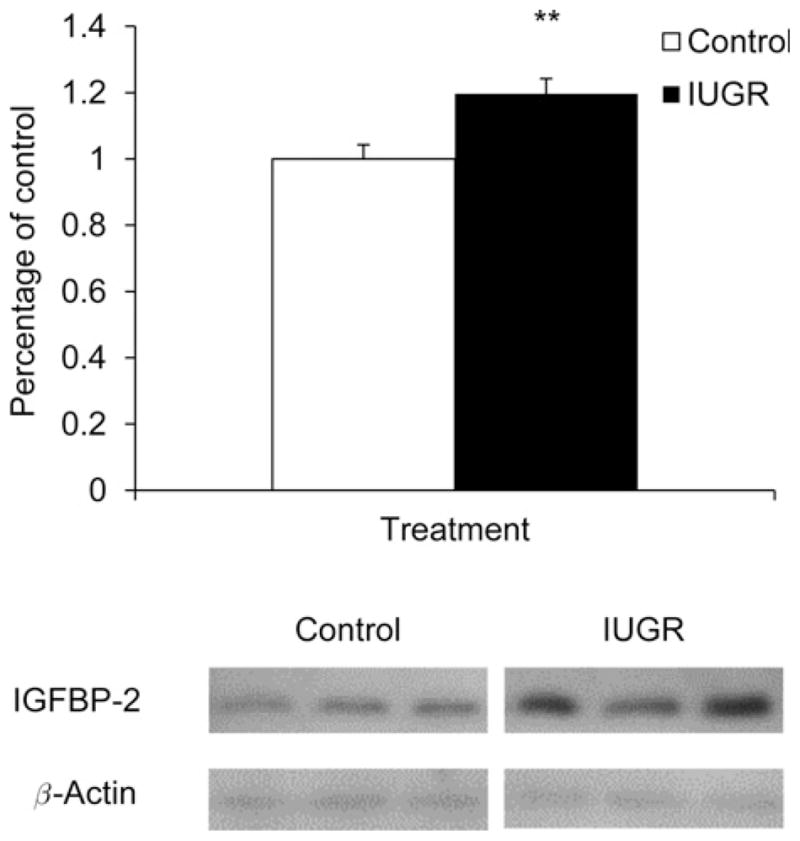
Immunoblot analysis for IGFBP-2 in pancreas tissue. IGFBP-2 protein concentrations were measured in control (□) and IUGR (■) pancreatic tissue. The graph summarizes band intensities from each treament group. Significance from the Student’s t-test analyses are depicted by * *P < 0.01. Representative immunoblots are shown for three control and three PI-IUGR pancreas samples from both IGFBP-2 and β-actin. IUGR, intrauterine growth restriction; IGFBP-2, insulin-like growth factor binding protein 2
Discussion
Two scenarios could explain how the IGF and FGF signaling pathways act independently to reduce β-cell mass in IUGR fetuses following placental insufficiency. First, greater IGFBP-2 mRNA and protein concentrations can slow β-cell replication rates by antagonizing IGF action. Second, decreased FGF7 and FGFR2IIIb signaling can lower PDX-1 expression which, along with decreased IGF signaling, will contribute to reduced pancreatic progenitor epithelial cells and lower the proportion of cells available for differentiation into mature β-cells.13,30,31 Factors influencing β-cell mass are replication of mature β-cells, neoformation from pancreatic progenitor epithelium and apoptosis.32,33 Previously, we showed that mitotic rates, but not apoptotic rates, of β-cells are lower in IUGR sheep fetuses at 0.9 of gestation, which is consistent with IGFBP-2 inhibition of IGF-induced proliferation.9 We also showed that rates of β-cell neoformation are similar between IUGR and control fetuses.9 Contrary to reduced pancreatic IGF-II, isolated PI-IUGR islets have greater IGF-II expression. This may represent a local compensatory mechanism to sustain a minimal β-cell mass by inhibiting apoptosis. Together, these findings begin to identify several mechanisms that define how reductions in selected growth factors in the PI-IUGR pancreas contribute to decreased β-cell mass in response to placental insufficiency.
Plasma IGF-I concentrations are lower in the IUGR fetus;34 however, IGF regulation of somatic cell growth may not be dependent on circulating IGF-I concentrations but instead on local autocrine/paracrine action. This has been shown in mice with a liver-specific IGF-I mutation, where circulating IGF-I concentrations were reduced but growth and development were unaffected.35,36 Thus, IGF production within the pancreas most likely regulates pancreatic β-cell development, expansion and survival by local autocrine/paracrine actions.15,16,30,37 Increased expression of IGF-I coincides with increased rates of β-cell mitosis during regeneration,38 whereas disruption of pancreatic IGF signaling decreases β-cell mass and pancreas development,30 which might explain the reduction in fetal β-cell mass in the IUGR pancreas.
The greater expression of IGF-II in our IUGR fetal pancreatic islets contradicts reports in fetal rat models of IUGR, including the experimental model of IUGR produced by a maternal low protein diet and the genetic β cell-deficient Goto-Kakizaki rat model, which exhibit reduced pancreatic IGF-II mRNA, lower β-cell proliferation rates and greater apoptotic rates.39,40 However, elevated IGF-II expression supports the survival of β-cells, which is consistent with our observations of similar apoptotic rates between control and IUGR β-cells.9,15,41
We identified pancreatic IGFBP-2–6 transcripts in the present study, but IGFBP-1 expression was not found, comparable to previous observations in the fetal rat pancreas.35 The six high-affinity IGF binding proteins positively or negatively influence IGF actions, adding an additional level of regulation.14,42,43 Transgenic mice that globally over-express IGFBP-2 have lighter carcass weights, showing that IGFBP-2 negatively influences IGF growth actions.17 Therefore, as a negative regulator, greater IGFBP-2 levels in the pancreas and islets would act to reduce IGF signaling despite normal IGF-I and increased IGF-II concentrations. Additionally, maximal IGF stimulation of β-cell proliferation requires adequate glucose concentrations, which play an important and specific role in β-cell development by activating the endocrine progenitors.16,44 Therefore, due to placental insufficiency, fetal hypoglycemia may further inhibit IGF-potentiated β-cell proliferation.9
In sheep and humans, β-cells differentiate from the pancreatic progenitor epithelium from at least 0.24 of gestation until term.7 During this period, pancreatic progenitor epithelial cells also proliferate, which is regulated by FGF signaling.19,20,45 Although FGF10 mRNA was not different, lower pancreatic FGF7 and FGFR2IIIb mRNA concentrations would act to reduce pancreatic progenitor epithelial cell expansion and subsequent β-cell neoformation in PI-IUGR fetuses.19,20,45 In genetically engineered mice, transient ablation of PDX-1-positive pancreatic progenitor cells was shown to be a critical and limiting determinant of pancreas size and β-cell area.47 Decreased FGFR2IIIb signaling also lowers PDX-1 expression in pancreatic epithelial cells.13,46 Thus, while decreased pancreatic PDX-1 mRNA concentrations in PI-IUGR fetuses may be partially due to decreased numbers of β-cells,9 it may also be due to the lower expression of pancreatic FGF7 and FGFR2IIIb (Figure 1). Together, these conditions will reduce the pancreatic progenitor cell pool, and lower β-cell mass in PI-IUGR fetuses. In the β-cells, PDX-1 transactivates insulin and Glut2 genes.19,48,49 In isolated pancreatic islets in the present studies, PDX-1 and Glut2 mRNA concentrations were similar between IUGR and control fetuses (Figure 2). This normal expression of PDX-1 mRNA, however, was not associated with normal insulin mRNA (Figure 2), which was also reduced by 66% in the IUGR pancreas.9 These findings reflect differential regulation of insulin and Glut2 expression by factors other than PDX-1. For example, normal glucose concentrations are required for the normal regulation of insulin gene expression and so fetal hypoglycemia may be responsible for decreased islet insulin mRNA.50
In summary, we showed reductions in IGF-I, FGF7, FGFR2IIIB and PDX-1 mRNA expression in PI-IUGR fetal pancreatic tissue, and increased IGFBP-2 mRNA and protein in both pancreatic tissue and isolated islets in PI-IUGR fetuses. Reductions in IGF and FGF signaling in the PI-IUGR pancreas act to decrease expansion of the pancreas progenitor epithelium, leading to fewer cells differentiating into mature β-cells.13,30,31 Furthermore, decreased IGF signaling will also reduce β-cell replication. Decreased differentiation of new β-cells and reduced replication of mature β-cells will result in less overall β-cell mass, consistent with our previous observations.9 These findings are the first to define how growth factor signaling pathways reduce β-cell mass in fetuses with placental insufficiency-induced IUGR and provide a mechanistic foundation for the development of type 2 diabetes. In addition, the new data point out that the response of the pancreas in the IUGR fetus is complex and potentially treatable.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK084842, SWL, Principle Investigator and HD42815, WWH Jr, Principle Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Miranda Anderson and Megan Wyckoff for their technical assistance with this work.
Footnotes
Author contributions: All authors participated in the experimental design, interpretation of the findings and manuscript review. XC, PJR and SWL conducted the experiments and analyzed the data; XC wrote the manuscript; and PJR, WWH Jr and SWL contributed constructive suggestions to the writing of the manuscript.
References
- 1.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav. 2006;88:234–43. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–86. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 4.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res. 2001;2:139–43. doi: 10.1155/EDR.2001.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–30. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- 6.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–3. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 7.Cole L, Anderson M, Antin PB, Limesand SW. One process for pancreatic beta-cell coalescence into islets involves an epithelial–mesenchymal transition. J Endocrinol. 2009;203:19–31. doi: 10.1677/JOE-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–24. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 10.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–97. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 11.Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies – a review. Placenta. 2002;23(Suppl A):S119–29. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- 12.Hill DJ, Petrik J, Arany E. Growth factors and the regulation of fetal growth. Diabetes Care. 1998;21(Suppl 2):B60–9. [PubMed] [Google Scholar]
- 13.Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–17. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 14.Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Petrik J, Pell JM, Arany E, McDonald TJ, Dean WL, Reik W, Hill DJ. Overexpression of insulin-like growth factor-II in transgenic mice is associated with pancreatic islet cell hyperplasia. Endocrinology. 1999;140:2353–63. doi: 10.1210/endo.140.5.6732. [DOI] [PubMed] [Google Scholar]
- 16.Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273:17771–9. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- 17.Hoeflich A, Wu M, Mohan S, Foll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–96. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- 18.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 19.Edlund H. Pancreatic organogenesis – developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–32. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 20.Attali M, Stetsyuk V, Basmaciogullari A, Aiello V, Zanta-Boussif MA, Duvillie B, Scharfmann R. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–58. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- 21.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–9. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–8. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–8. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Fahy AL, Green AS, Anderson MJ, Rhoads RP, Limesand SW. Beta2-adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J Physiol. 2010;588:3539–49. doi: 10.1113/jphysiol.2010.192310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang R, Limesand SW, Anthony RV. Structure and transcriptional regulation of the ovine placental lactogen gene. Eur J Biochem. 1999;265:883–95. doi: 10.1046/j.1432-1327.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2007;293:E1716–25. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 29.de Vrijer B, Davidsen ML, Wilkening RB, Anthony RV, Regnault TR. Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy. Pediatr Res. 2006;60:507–12. doi: 10.1203/01.PDR.0000242364.78002.71. [DOI] [PubMed] [Google Scholar]
- 30.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 31.Miralles F, Czernichow P, Ozaki K, Itoh N, Scharfmann R. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci USA. 1999;96:6267–72. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swenne I. Pancreatic beta-cell growth and diabetes mellitus. Diabetologia. 1992;35:193–201. doi: 10.1007/BF00400917. [DOI] [PubMed] [Google Scholar]
- 33.Bonner-Weir S, Smith FE. Islet cell growth and the growth factors involved. Trends Endocrinol Metab. 1994;5:60–4. doi: 10.1016/1043-2760(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 34.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–30. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogg J, Hill DJ, Han VK. The ontogeny of insulin-like growth factor (IGF) and IGF-binding protein gene expression in the rat pancreas. J Mol Endocrinol. 1994;13:49–58. doi: 10.1677/jme.0.0130049. [DOI] [PubMed] [Google Scholar]
- 36.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141:4436–41. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 37.George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest. 2002;109:1153–63. doi: 10.1172/JCI12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith FE, Rosen KM, Villa-Komaroff L, Weir GC, Bonner-Weir S. Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc Natl Acad Sci USA. 1991;88:6152–6. doi: 10.1073/pnas.88.14.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serradas P, Goya L, Lacorne M, Gangnerau MN, Ramos S, Alvarez C, Pascual-Leone AM, Portha B. Fetal insulin-like growth factor-2 production is impaired in the GK rat model of type 2 diabetes. Diabetes. 2002;51:392–7. doi: 10.2337/diabetes.51.2.392. [DOI] [PubMed] [Google Scholar]
- 40.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–73. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 41.Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology. 1998;139:2994–3004. doi: 10.1210/endo.139.6.6042. [DOI] [PubMed] [Google Scholar]
- 42.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre HD, Serek R, Crane DI, Veveris-Lowe T, Parry A, Johnson S, Leung KC, Ho KK, Bougoussa M, Hennen G, Igout A, Chan FY, Cowley D, Cotterill A, Barnard R. Placental growth hormone (GH), GH-binding protein, and insulin-like growth factor axis in normal, growth-retarded, and diabetic pregnancies: correlations with fetal growth. J Clin Endocrinol Metab. 2000;85:1143–50. doi: 10.1210/jcem.85.3.6480. [DOI] [PubMed] [Google Scholar]
- 44.Filhoulaud G, Guillemain G, Scharfmann R. The hexosamine biosynthesis pathway is essential for pancreatic beta cell development. J Biol Chem. 2009;284:24583–94. doi: 10.1074/jbc.M109.025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Landsman L, Nijagal A, Whitchurch TJ, Vanderlaan RL, Zimmer WE, Mackenzie TC, Hebrok M. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 2011;9:e1001143. doi: 10.1371/journal.pbio.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–91. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 48.Brissova M, Blaha M, Spear C, Nicholson W, Radhika A, Shiota M, Charron MJ, Wright CV, Powers AC. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am J Physiol Endocrinol Metab. 2005;288:E707–14. doi: 10.1152/ajpendo.00252.2004. [DOI] [PubMed] [Google Scholar]
- 49.Kaneto H, Miyatsuka T, Kawamori D, Matsuoka TA. Pleiotropic roles of PDX-1 in the pancreas. Rev Diabet Stud. 2007;4:209–25. doi: 10.1900/RDS.2007.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macfarlane WM, McKinnon CM, Felton-Edkins ZA, Cragg H, James RF, Docherty K. Glucose stimulates translocation of the homeodomain transcription factor PDX1 from the cytoplasm to the nucleus in pancreatic beta-cells. J Biol Chem. 1999;274:1011–6. doi: 10.1074/jbc.274.2.1011. [DOI] [PubMed] [Google Scholar]