Abstract
Antagonism of CXCR4 disrupts the interaction between the CXCR4 receptor on HSCs and the CXCL12 expressed by stromal cells in the bone marrow, which subsequently results in the shedding of hematopoietic stem cells (HSCs) to the periphery. Due to their profound immunomodulatory effects, HSCs have emerged as a promising therapeutic strategy for autoimmune disorders. We sought to investigate the immunomodulatory role of mobilized autologous HSCs, via target of the CXCR4-CXL12 axis, to promote engraftment of islet cell transplantation. Islets from BALB/c mice were transplanted beneath the kidney capsule of hyperglycemic C57BL/6 mice, and treatment of recipients with CXCR4 antagonist resulted in mobilization of HSCs and in prolongation of islet graft survival. Addition of Rapamycin to anti-CXCR4 therapy further promoted HSC mobilization and islet allograft survival, inducing a robust and transferable host hyporesponsiveness, while administration of an ACK2 (anti-CD117) mAb halted CXCR4 antagonist-mediated HSC release and restored allograft rejection. Mobilized HSCs were shown to express high levels of the negative co-stimulatory molecule PD-L1, and HSCs extracted from WT mice, but not from PD-L1 KO, suppressed the in vitro alloimmune response. Moreover, HSC mobilization in PD-L1 KO mice failed to prolong islet allograft survival. Targeting the CXCR4-CXCL12 axis thus mobilizes autologous HSCs and promotes long-term survival of islet allografts via a PD-L1-mediated mechanism.
Keywords: Diabetes, CXCR4-CXCL12 axis, islet transplantation, stem cells
INTRODUCTION
Recent reports of clinical benefits following use of autologous hematopoietic stem cells (HSCs) in type 1 diabetes (T1D) have sparked much interest in the utility and value of HSC therapy as a tool to induce tolerance (1). HSCs have also been used in other debilitating autoimmune diseases with the aim of resetting the patient’s immune system; indeed, HSC-based therapy has yielded success in the treatment of neurological autoimmune diseases, including multiple sclerosis (2), systemic sclerosis (3, 4) and Crohn’s disease (5). The primary objective in using HSCs has been to eliminate autoreactive immune cells, thus ensuring a properly functioning immune system. However, growing evidence suggests that autologous HSCs can induce central and peripheral immunological tolerance per se (6).
Preclinical studies have demonstrated that T cell-depleted bone marrow-resident CD34+ stem cells overcome MHC barriers in sublethally irradiated mice (7) and that murine HSCs may delete effector cells through Fas/FasL interaction or via the TNF-α pathways, which are both present on HSCs (8, 9). Kared et al. (10) have recently demonstrated that murine HSCs may stimulate peripheral FoxP3+ regulatory T cell (Treg) expansion through both cell-cell contact activation of Notch signaling and through soluble factors such as GM-CSF, which is produced at high levels by hematopoietic progenitors (10). With respect to human HSCs, Rachamim et al. (11) have shown that cells within the human CD34+ population are endowed with potent veto activity, referring to the ability of HSCs to neutralize precursors of cytotoxic T lymphocytes in an HLA-restricted and cell contact-dependent fashion (12, 13). HSCs have also been used to improve the outcome of solid organ transplantation, through the induction of mixed hematopoietic chimerism (14). This strategy constitutes a unique approach to generate tolerance in solid organ transplantation without the need for long-term immunosuppressive therapy but also requires intense toxic conditioning strategies. To reduce the burden of these regimens, an attempt has been made to use megadoses of autologous stem cell transplants.
Recent efforts targeting the CXCR4-CXCL12 axis have been aimed at inducing shedding of HSCs to the periphery (15–18). HSCs express high levels of CXCR4, which keeps them anchored to the bone marrow where CXCL12 (or SDF-1α, the ligand for CXCR4) expression is high, particularly in stromal cells (19). We thus aimed to target the CXCR4-CXCL12 axis by blocking the CXCR4 receptor using a novel CXCR4 antagonist (20) to mobilize autologous HSCs in a murine model of islet transplantation. Our goal was to achieve HSC mobilization in our islet transplant recipients to improve allograft survival. This approach could have significant clinical applications, given that CXCR4 antagonists (i.e. Mozobil/Plexifor) are currently under investigation in a Phase III trial to improve engraftment in bone marrow-transplanted patients.
MATERIALS AND METHODS
Mice
C57BL/6, BALB/c, and DBA/1J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and cared for and used in accordance with institutional guidelines. PD-L1−/− mice on a C57BL/6 background were provided by Dr. Arlene Sharp as previously published (21). Protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Interventional animal studies
Mice were treated with a CXCR4 antagonist (NIBR1816, provided by Novartis) or vehicle at 30 mg/kg (1 mg per mouse) i.p. once per day for up to 14 days. Mobilization of murine HSCs, as demonstrated by expression of KLS (c-Kit+Lin−Sca-1+) was then evaluated in the bone marrow and spleen by flow cytometry during CXCR4-CXCL12 targeting. KLS cells were detected by gating on lineage-negative cells (using a cocktail of Gr-1, CD8, CD4, CD11b, and B220 [Miltenyi Biotec, Auburn, CA]) and then evaluating c-Kit/Sca-1 double-positive cells (both from BD Biosciences, San Jose, CA). For functional studies, mobilized HSCs were sorted using the above markers. Anti-CD117 (anti-c-Kit, ACK2 clone, Millipore, Billerica, MA) was used at a dose of 125 μg at days 0 and 5 to ablate HSCs. In some experiments, mice were pre-treated with ACK2 (at days −5 and day 0). We also examined whether Treg depletion with anti-CD25-Ig at the time of transplantation (500 μg at days −6 and −1) was able to abrogate the effect of CXCR4 antagonist treatment.
Islet transplantation
Pancreatic islets were isolated by collagenase digestion, density gradient separation, and handpicking as described previously (22). Islets were transplanted under the renal capsule of mice rendered diabetic with streptozotocin (225 mg/kg, administered i.p.). Islets isolated from MHC-mismatched male BALB/c (H-2d) donors were transplanted under the kidney capsule of recipient C57BL/6 mice (H-2b) (850 islets per recipient). Rejection of islet allografts was defined as blood glucose levels of >250 mg/dl for at least 2 consecutive days.
CXCR4 ligand binding assay
Membranes prepared from the mouse pre-B cell line L1.2 were used as the source of mouse CXCR4. Membranes were prepared from cell homogenates in a buffer containing 20mM HEPES, 1mM EDTA pH=7.4, and protease inhibitors followed by centrifugation at 28,000×g. After a second round of homogenization and centrifugation, membranes were resuspended in 20 mM HEPES, 0.1 mM EDTA pH=7.4, and stored in aliquots at −80 C. Murine CXCL12 was obtained from R&D Systems (Minneapolis, MN) and was 125-I labeled (Amersham, UK). For the assay the following ingredients were added to a 96-well Optiplate: 10 μg of membrane protein in 50 μl assay buffer (25 mM HEPES, 2 mM CaCl2, 5 mM MgCl2, 100 mM NaCl, 0.5% BSA, and protease inhibitors) followed by 0.5 mg of WGA-coated SPA beads (Amersham) followed by 50 μl antagonist (or buffer) or 400 nM unlabeled murine CXCL12. Finally, 50 μl of 125I-labeled murine CXCL12 in assay buffer was added (final concentration 20–25 pM). The plates were incubated for 2 hours at room temperature with continuous shaking. Following incubation, the plates were centrifuged for 10 minutes and measured in a TopCount NXT instrument (Packard Instruments, Boston, MA). Under these experimental conditions murine unlabeled CXCL12 displaced 125I-labeled human CXCL12 equally well (IC50 values 110±35 pM and 167±52 pM respectively).
Ca2+ mobilization assay
CXCL12-induced Ca2+ mobilization from intracellular stores was measured in L1.2 pre-B cells loaded with the Ca2+-sensing fluorochrome Fluo-4 (Molecular Probes, Invitrogen). Cells were incubated with fluorochrome at a concentration of 4 μM for 1 h at 37°C and then washed in assay buffer. Loaded cells were dispensed into microtiter wells, mixed with antagonist, incubated for 2 hours, and subsequently dispensed into 384-well plates in quadruplicate and placed in a Fluorescence Image Plate Reader (FLIPR384, Molecular Devices). Fluorescence of the cells was recorded for 20 seconds, after which CXCL12 was added (final concentration 3 nM), and fluorescence was further recorded for 215 seconds. The minimal (baseline) and maximal fluorescence were used to calculate the inhibitory effect of CXCR4 antagonists. IC50 values were expressed as the inhibitor concentration that yielded 50% inhibition of the Fmax−Fmin/Fmin value measured in the absence of inhibitor.
Chemotaxis assay
Cell migration stimulated by CXCL12 was assessed in Transwell tissue culture inserts with porous polycarbonate membranes (Costar, 5 μm pore size). Target cells (500,000 L1.2 pre-B cells for mouse CXCR4 in 100 μl HBSS, 0.1% BSA) were placed in the upper chamber, and 600 μl of buffer or chemoattractant (recombinant CXCL12, final concentration 10 nM) was placed in the lower chamber. After incubation for 4 hours at 37°C, the migrated cells were counted in a FACSCalibur (BD Biosciences) by acquiring all events for 30 seconds. When inhibitor was tested, the compound was added to both the upper and the lower compartments to give the desired final concentration.
Actin polymerization assay in whole blood
Blood was collected in EDTA-coated tubes. CXCR4 inhibitors were diluted in phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA) to the appropriate final concentration, and typically 3 μl of CXCR4 antagonist solution was mixed with 100 μl of whole blood in a 96-well plate (Costar). After incubation for 10 minutes at room temperature, 3 μl of CXCL12 solution (to achieve a final concentration of 100 nM) was added to the sample and incubated for 25 seconds at room temperature. Ice-cold FACS lysing buffer (BD Biosciences) was added to the samples, followed by incubation for 10 minutes on ice. After several washes with PBS/0.5% BSA on ice, the blood cells were resuspended in 200 μl of PBS containing 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) and incubated for 5 minutes on ice. After centrifugation the cells were resuspended in 100 μl PBS/0.5% BSA, 1% Alexa 488 phalloidin (Invitrogen), and 1% L-α-palmitoyl-lysophosphatidyl-choline (Sigma-Aldrich) and incubated for 20 minutes on ice, protected from light. Cells were washed 3 times with PBS/0.5% BSA, and fluorescence was analyzed with a FACSCalibur (BD Biosciences) by gating on granulocytes on FSC vs. SSC dot plots.
ELISPOT
An ELISPOT assay was used to measure cytokine production by splenocytes extracted from treated or control mice (23). Briefly, Millipore immunospot plates (Millipore Corporation, Bedford, MA) are coated with capture antibodies (BD Biosciences). Plates were blocked with 1% bovine serum albumin (BSA) to prevent non-specific binding. Naive donor splenocytes were used as stimulators, while 5×105 C57BL/6 splenocytes isolated from CXCR4-antagonist-treated or untreated control islet-transplanted C57BL/6 mice were used as responders. After 4 days of culture in RPMI 10% FCS penicillin/streptomycin at 37° C and 5% CO2, plates were washed, and biotinylated antibodies specific for each cytokine were added to wells and incubated at 4° C for 12 hours. Plates were then washed and incubated at room temperature with streptavidin-horseradish peroxidase (HRP) for 2 hours and developed with aminoethyl carbazole (Sigma Aldrich) diluted in N,N-dimethylformamide. Spots were counted on an immunospot analyzer (Cellular Technology Ltd., Cleveland, OH). The supernatant of each culture was used for Luminex analysis.
Mixed lymphocyte reaction (MLR) and anti-CD3/-CD28 stimulation
To measure the alloimmune response in the presence or absence of CXCR4 antagonist, cultured BALB/c dendritic cells (DCs) were used to stimulate C57BL/6 CD4+ T cells isolated from splenocytes by magnetic bead separation (Miltenyi Biotec, Auburn, CA) at a ratio of 1:1 DCs:splenocytes. Proliferation was measured at day 3 of incubation at 37° C and 5% CO2 following pulsing with [3H]TdR (Perkin Elmer, Wellesley, MA) using a liquid scintillation counter. Lymphocytes were also stimulated with con A as a positive control. An anti-CD3/-CD28 stimulation assay was performed as well; briefly, 5×105 splenocytes were plated with 1 μg/ml anti-CD3 and anti-CD28. CXCR4 antagonist was added to both assays at doses of 1, 10 and 100 μg/ml.
Flow cytometry
Rat anti-mouse CD3 PE, CD4 FITC, CD8 PE, CD25 PE, and CD62L APC were purchased from BD Biosciences. FoxP3 APC was purchased from eBioscience (San Diego, CA). Cells recovered from spleens and peripheral lymphoid tissues were subjected to FACS analysis and run on a FACSCalibur. FoxP3 analysis was performed following overnight permeabilization of cells extracted from spleens and peripheral lymphoid tissue with commercially available antibodies and gating on CD4+CD25+ cells. Data were analyzed with FloJo software version 6.3.2 (Treestar, Ashland, OR).
Islet pathology and immunohistochemistry
Immunohistochemistry was performed with 5-micron-thick formalin-fixed, paraffin-embedded tissue sections. Briefly, slides were soaked in xylene, passed through graded alcohols, and immersed in distilled water. Slides were then pretreated with 10 mM citrate, pH 6.0 (Zymed, South San Francisco, CA) or with 1 mM EDTA (pH 8.0) in a steam pressure cooker, followed by washing in distilled water for antigen retrieval. All further steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (DAKO USA, Carpinteria, CA) for 5 minutes to quench endogenous peroxidase activity. The following primary antibodies were used (including company, clone/reference, dilution, and retrieval method, respectively): anti–CD3 (Cell Marque, 1:1500, EDTA), anti–CD45/B220 (BD Biosciences, 1:200, citrate), anti–FoxP3 (eBioscience, 1:25, citrate), and anti-insulin (DAKO, N1542, undiluted, EDTA). All primary antibodies were applied to slides in DAKO diluent for 1 hour. Slides were then washed in 50-mM Tris-Cl, pH 7.4, and the appropriate horseradish peroxidase-conjugated secondary antibody (Envision detection kits, DAKO) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed with a DAB chromogen kit (DAKO) per the manufacturer and counterstained with hematoxylin. Photomicrographs were taken on an Olympus BX41 microscope (Center Valley, PA) at indicated magnifications with an Olympus Q-color5 digital camera and analyzed with Adobe Photoshop Elements 2.0 (San Jose, CA). All photos were taken at 400x original magnification. To detect GFP+HSCs after adoptive transfer, rabbit anti-GFP (Abcam, Cambridge, MA) was used at 1:3000 dilution with EDTA retrieval.
Adoptive and co-adoptive cell transfer
To assess the robustness of tolerance towards alloantigen and the presence of infectious tolerance or hyporesponsiveness of the immune system, two different adoptive transfer experiments were performed. In the first experiment, the ability of splenocytes obtained from long-term tolerant-treated mice to reject islets in immunodeficient mice was evaluated. We adoptively transferred 10×106 splenocytes extracted from Rapamycin+CXCR4 antagonist-treated long-term tolerant C57BL/6 islet-transplanted mice into C57BL/6-RAG immunodeficient mice previously transplanted with BALB/c islets. Splenocytes extracted from C57BL/6 mice transplanted with BALB/c islets that had been rejected were used as controls for the adoptive transfer experiments, as previously reported (24). In a second experiment, we evaluated the ability of splenocytes obtained from long-term Rapamycin+CXCR4 antagonist-treated mice to control rejection mediated by splenocytes obtained from naïve mice. We co-adoptively transferred 10×106 splenocytes extracted from Rapamycin+CXCR4 antagonist-treated long-term tolerant C57BL/6 islet-transplanted mice and 10×106 splenocytes extracted from naïve mice into C57BL/6-RAG immunodeficient mice previously transplanted with BALB/c islets. In another set of experiments, tolerant mice or controls (C57BL/6 [H-2b]) were transplanted with islet donor skin (BALB/c [H-2d]) or from a 3rd party donor (DBA/1J [H-2q]).
Statistical analyses
Data are expressed as mean±standard error of mean. Kaplan-Meier analysis was used for survival analysis. To compare the two groups, we used a two-sided unpaired Student t-test (for parametric data) or Mann-Whitney test (for non-parametric data) according to distribution. A P value of less than 0.05 (by two-tailed testing) was considered an indicator of statistical significance. Data were analyzed with an SPSS statistical package for Windows (SPSS Inc., Chicago, Illinois).
RESULTS
NIBR1816 is a potent antagonist of murine CXCR4
We first evaluated the ability of the recently described small molecule CXCR4 antagonist (NIBR1816) to block murine CXCR4 in vitro in various assays, as previously demonstrated for rat and human CXCR4 (20). The binding of 125I-labeled CXCL12 to CXCR4 (from murine L1.2 pre-B cell membranes) was inhibited by the presence of CXCR4 antagonist (IC50=106.6±9.1 nM). Ca2+ mobilization, from murine L1.2 pre-B cells loaded with the Ca2+-sensing fluorochrome Fluo-4, following the addition of CXCL12 was inhibited by CXCR4 antagonist (IC50=15.7±3.1 nM). CXCL12-stimulated migration of leukocytes in a chemotaxis assay was also inhibited by CXCR4 antagonist (IC50=31.1±5.5 nM). The antagonist was almost equally potent in inhibiting the CXCL12-induced actin polymerization in blood granulocytes (IC50=11±5 nM). We then evaluated the concentration and half-life in vivo in mice and found that the peak concentration of CXCR4 antagonist, after the i.p. injection of 1 mg, is reached at 6 hours and that the half-life is approximately 12 hours.
CXCR4 antagonist mobilizes HSCs
HSC mobilization was evaluated in naïve C57BL/6 mice after daily injections of 30 mg/kg/day for up to 14 days. Bone marrow and spleen were obtained from treated C57BL/6 mice at different time points (baseline, 6, 12, 18, 24 hours and 7, 14, 28 days post-injection), and HSC percentage was evaluated by FACS analysis (n=at least 3 mice/time point) by gating on Lin−c-Kit+Sca-1+ (KLS) cells.
6 hours after a single injection of 30 mg/kg (approximately 1 mg), an increase in HSCs was evident in the bone marrow (Figures 1A and 1C). HSCs increased from 2.28±0.70% at baseline to 8.88±2.65% 6 hours after injection (p=0.01); thereafter the number of HSCs was generally decreased compared to the 6-hour peak (Figures 1A and 1C). At days 7 and 14 of treatment, a trend demonstrating a slight decrease in HSC percentage was noted in the bone marrow (p=ns), while a recovery of HSCs was found at day 28 (2 weeks following discontinuation of treatment), (Figures 1A and 1C). The frequency of HSCs was also examined in the spleen of treated mice. We noted an increase in the percentage of HSCs at days 7 and 14 post-treatment, but not at 6 hours, as compared to baseline (baseline=1.48±0.08% vs. day 7=3.49±0.93% [p=0.004], and vs. day 14=2.86±0.97%, [p=0.03]), (Figures 1B and 1D).
Figure 1.
HSC percentage (Lin−cKit+Sca1+ cells) was evaluated by FACS analysis in bone marrow (BM) and spleen of CXCR4 antagonist-treated and untreated mice (n=3–6 mice/time point). 6h after a single injection of CXCR4 antagonist, an increase in the percentage of HSCs (KLS cells) in the BM was evident (*p=0.01) (A, C). At day 7 of treatment, a trend toward a slight decrease in HSC percentage (ns) was noted in the BM, which lasted until day 14, while a recovery of HSCs was found at day 28 (2 weeks; recovery in HSC percentage was observed at day 28 (A, C). HSC percentage increased in spleen at days 7 and 14 post-treatment (baseline vs. day 7 and vs. day 14, *p=0.004 and *p=0.03, respectively), (B, D). In an MLR assay, where bone marrow-derived BALB/c DCs were incubated with fully mismatched C57BL/6 CD4+ T cells, addition of increasing doses anti-CXCR4 (1, 10 and 100 μg/ml) significantly reduced lymphocyte proliferation, as assessed by 3H-thymidine incorporation compared to control (*control vs. all, p<0.001), (E). When anti-CXCR4 was added to an anti-CD3/-CD28 stimulation assay, a similar dose-dependent reduction of proliferation was observed (*control vs. 10 and 100 μg/ml, p<0.001), (F).
When CXCR4 antagonist was administered for more than 14 days (i.e. 21 days), no further HSC mobilization was noted in the spleen; this prolonged CXCR4 administration, however, induced a sustained reduction of HSC number in the bone marrow (data not shown).
CXCR4 antagonist diminishes the in vitro alloimmune response
To evaluate the effect of CXCR4 antagonist on the alloimmune response, we used increasing doses of CXCR4 antagonist in vitro in both an MLR and in an anti-CD3/-CD28 stimulation assay. Stimulator BALB/c DC and responder C57BL/6 CD4+ T cells were incubated with or without CXCR4 antagonist at doses of 1, 10 and 100 μg/ml. Compared to control, treatment with CXCR4 antagonist reduced lymphocyte proliferation, as assessed by 3H-thymidine incorporation in an MLR, with a dose-dependent effect (n=3, control vs. all p<0.001), (Figure 1E) and in an in vitro anti-CD3/-CD28 stimulation assay (n=3, control vs. 10 and 100 μg/ml p<0.001), (Figure 1F).
CXCR4 antagonist prolongs islet allograft survival and mobilizes recipient HSCs
Fully allogeneic islets from BALB/c donors were transplanted under the kidney capsule of C57BL/6 mice chemically rendered diabetic using streptozotocin. CXCR4 antagonist treatment was initiated on the day of transplantation and was sustained for 14 days, with a single daily dose of 30 mg/kg (n=11 mice). Islet allograft survival was prolonged compared to vehicle-treated control mice (n=15 mice) (MST of 21 vs. 12 days for CXCR4 antagonist-treated vs. controls, p<0.0001), (Figure 2A).
Figure 2.
Prolongation of islet allograft survival was observed in CXCR4 antagonist-treated (21 days) (n=11) compared with untreated control mice (12 days) (n=15) (*p<0.0001), (A). ACK2 (anti-CD117 mAb) abrogated the effect of CXCR4 antagonist on islet allograft survival and restored islet allograft rejection (n=5 mice), (A). 10-day ACK2 treatment alone (n=5 mice) or 14-day ACK2 pre-treatment (n=4 mice) did not affect islet allograft survival (n=5 mice), (A). In CXCR4 antagonist-treated islet transplanted mice, 14-day ACK2 treatment (but not 31-day ACK2 pre-treatment) abrogated the effect of CXCR4 antagonist by diminishing islet allograft survival (#p=0.005), (A). Anti-CD25-treatment in CXCR4 antagonist-treated islet-transplanted mice did not abrogate the effect of CXCR4 antagonist on islet allograft survival (18 days) (A). HSCs increased in CXCR4 antagonist-treated islet-transplanted mice at day 7 in the spleen compared with naïve mice and controls (*p<0.001) (B). The injection of ACK2 reduced the HSC mobilization observed at day 7 with CXCR4 antagonist treatment (B). ACK2 treatment alone did not affect HSC percentage (B). Rapamycin alone (0.1 mg/kg) administered every other day (at days 0, 2, 4, 6, 8, and 10) prolonged islet allograft survival (26 days) compared to controls (12 days) (*p<0.001), (C). 18.5-day ACK2 treatment (but not 24-day ACK2 pre-treatment) abrogated the effect of Rapamycin on islet allograft survival (#p=0.05), (C).
No islet allografts were found in untreated mice (day 14) (D1, D2), while CXCR4 antagonist-treated mice showed preserved islets and strong insulin staining (E1, E2). In the CXCR4 antagonist-treated group, few CD3+ cells were infiltrating the islet allograft at day 14 post transplantation (E3), some of which were FoxP3+ regulatory T cells (E4). In the control group, CD3+ cells were infiltrating the remnants of the graft (D3), with almost none of the CD3+ cells expressed FoxP3 (D4). CXCR4 antagonist+ACK2-treated mice displayed islets infiltrated by few CD3+ FoxP3− cells (F1–F3–F4), with a total lack of insulin staining (F2). All panels are representative of at least 3 mice/group (magnification 200X).
We evaluated the levels of peripheral HSCs after islet transplantation in CXCR4 antagonist-treated and control C57BL/6 mice (n=at least 5 mice/group). While islet transplantation per se did not increase HSCs in the spleen as evaluated at day 7 by flow cytometry (Figure 2B), at day 7 following daily treatment with CXCR4 antagonist, an increase in HSC percentage was noted in the spleen of islet-transplanted C57BL/6 mice (KLS percentage at day 7 in CXCR4 antagonist-treated islet-transplanted mice=2.17±0.30 vs. controls=0.70±0.05%, p<0.001), (Figure 2B).
Islets were recovered from CXCR4 antagonist-treated and control mice at 14 days after transplantation for histological studies. Control-rejecting mice displayed graft infiltration (Figure 2D1), absent insulin staining with disrupted islet morphology (Figure 2D2), and some CD3+ cells (Figure 2D3), which appeared to be FoxP3+, infiltrating the graft (Figure 2D4). Conversely, CXCR4 antagonist-treated mice appeared to have some extent of infiltration (Figure 2E1), but maintained well-preserved islet structure and insulin staining (Figure 2E2) with fewer CD3+ cells infiltrating the graft (Figure 2E3), which were found to be FoxP3+ cells (Figure 2E4).
To address the central issue of whether HSCs traffic to the graft in our model, we treated transgenic GFP-C57BL/6 mice (in which the β-actin promoter drives GFP expression in essentially all tissues) with CXCR4 antagonist. After 7 days of CXCR4 antagonist treatment, when HSC mobilization peaked in the spleen, we isolated mobilized GFP+HSCs from splenocytes by sorting for Linnegc-Kit+ cells, thus obtaining a population of highly enriched GFP+HSCs. We then injected 1×106 sorted GFP+HSCs i.v. into immunocompetent C57BL/6 mice, which had received an islet transplant 1 day prior to injection. 24h after the injection of GFP+HSCs, mice were sacrificed and islet allografts and spleens were harvested for histological examination. Clusters of GFP+HSCs were detected in the spleen and islet grafts (Figures 3A and 3B), thus confirming that HSCs do indeed traffic to islet grafts. With the aim of finding a potential pathway to account for HSC recruitment to the graft, we evaluated the expression of CXCL12 (a chemoattractant for HSCs). CXCL12 expression in the graft was detected at immunohistochemistry at day 7 (Figure 3C), which may confer the ability to recruit HSCs to islet grafts. We then evaluated HSC kinetics in CXCR4 antagonist-treated islet-transplanted and untransplanted mice in spleen, pancreatic lymph nodes, pancreata and islet grafts. HSC were mobilized in the spleen at days 7 and 14 during CXCR4 antagonist treatment (Figure 3D) in transplanted and untransplanted mice (naïve untreated p<0.01 vs. all). HSCs were detected in the lymph nodes and pancreata of treated mice and increased in both sites only after 14 days of treatment in islet-transplanted mice (day 14 transplanted mice vs. naive untreated p<0.01), (Figures 3E and 3F). Some HSCs were detected in the islet grafts of untreated mice, with a decrease in HSC percentage at day 7 and a return to values similar to baseline at day 14 (Figure 3G).
Figure 3.
To evaluate whether HSCs traffic to graft or host lymphoid organs, GFP+HSCs (Linnegc-Kit+) cells sorted from GFP+C57BL/6 mice treated with CXCR4 antagonist for 7 days, were injected into immunocompetent C57BL/6 mice that had received islet transplants (n=3 mice). Clusters of GFP+HSCs were detected in the spleen and in islet grafts (A and B). CXCL12 expression was detected in islet allografts (C). We then evaluated the kinetics of HSC mobilization in CXCR4 antagonist-treated islet-transplanted and untransplanted mice in various organs (spleen, pancreatic lymph nodes, pancreata and islet grafts). HSCs were present in the spleen at days 7 and 14 (D) in treated transplanted and untransplanted mice (*naïve untreated p<0.01 vs. all). HSCs were also detected in lymph nodes and pancreata of treated mice and increased only after 14 days of treatment in transplanted mice (*day 14 transplanted mice vs. naive untreated p<0.01), (E and F). Few HSCs can be detected in the islet graft of untreated mice, with a decrease in HSC percentage at day 7 and a return to values similar to baseline at day 14 (G). All panels are representative of at least 3 mice/group (magnification 200X).
Rapamycin synergizes with CXCR4 antagonist treatment
Establishing a safer immunosuppressive protocol is highly indicated in the current era of islet cell transplantation (25). Here, we determined whether CXCR4 antagonist could synergize with a clinically applicable immunosuppressive drug such as Rapamycin. Islet allograft recipient mice were treated with Rapamycin at a low clinical-grade dose (0.1 mg/kg) administered every other day (D0, D2, D4, D6, D8, and D10), alone or in combination with CXCR4 antagonist as described above (30 mg/kg/day for 14 days). While C57BL/6 islet-transplanted mice treated with Rapamycin experienced a prolongation of islet allograft survival compared with controls (MST of 26 vs. >12 days, Rapamycin [n=7 mice] vs. controls [n=15 mice], p<0.0001), (Figure 4A), but ultimately rejected their islet grafts, mice that received a combination of Rapamycin and CXCR4 antagonist experienced a considerable delay in allograft rejection (MST of 26 vs. >150 days, Rapamycin [n=7 mice] vs. Rapamycin+CXCR4 antagonist-treated [n=5 mice], p=0.01), (Figure 4A). Indeed, 75% of C57BL/6 islet-transplanted mice treated with the combination of CXCR4 antagonist and Rapamycin enjoyed indefinite allograft survival (>150 days), (Figure 4A).
Figure 4.
As compared to untreated controls (12 days), (n=15) and Rapamycin alone-treated mice (26 days), (n=7), the mean survival time for the CXCR4 antagonist+Rapamycin group (n=5) was >150 days (Rapamycin and CXCR4 antagonist+Rapamycin vs. untreated, #p<0.001; Rapamycin vs. CXCR4 antagonist+Rapamycin, *p=0.01), (A). ACK2 abrogated the effect of CXCR4 antagonist and CXCR4 antagonist+Rapamycin on islet allograft survival and restored islet allograft rejection (n=5) (A). Rapamycin synergized with CXCR4 antagonist in the induction of HSC mobilization (B). HSCs increased in CXCR4 antagonist+Rapamycin-treated islet-transplanted mice at day 7 in the spleen compared with naïve, untreated mice and Rapamycin alone-treated mice (B). The injection of ACK2 reduced the HSC mobilization observed at day 7 with CXCR4 antagonist treatment+Rapamycin (*p<0.001), (B). Rapamycin alone induces a slight increase in HSC peripheral levels (*p<0.01), (B). Islet allografts from Rapamycin-plus-CXCR4-antagonist–treated mice showed barely infiltrated islets with maintained insulin staining (C1, C2), with few CD3+ cells (C3) and numerous FoxP3+ cells (C4). All panels are representative of at least 3 mice/group (magnification 200X).
We then investigated whether adding Rapamycin to our CXCR4 antagonist therapy would enhance HSC mobilization. Indeed, Rapamycin synergized with CXCR4 antagonist in mobilizing HSCs (Figure 4B). At day 7 after islet transplantation during Rapamycin-plus-CXCR4-antagonist treatment, HSC percentage in the spleen was significantly increased compared to controls or in mice treated with CXCR4 antagonist alone (CXCR4 antagonist=2.17±0.30% vs. CXCR4 antagonist+Rapamycin=3.27±0.19%, p=0.03), (Figure 4B).
Islets harvested from mice treated with Rapamycin and CXCR4 antagonist (at >150 days), showed intact and barely infiltrated islets (Figure 4C1) with preserved insulin staining (Figure 4C2). Scarce CD3+ (Figure 4C3) cells were detected within the grafts, and the infiltrate consisted primarily of FoxP3+ cells (Figure 4C4).
We then evaluated the robustness of CXCR4 antagonist treatment. Spleens were harvested at day 150 in long-term normoglycemic combination-treated mice and in control rejecting mice. No difference was evident in the absolute number of Tregs or T effector cells between the 2 groups (data not shown), while a reduction in IFN-γ production was observed in our ELISPOT assay in which recipient splenocytes (from C57BL/6 mice) are stimulated with donor alloantigen (BALB/c naïve splenocytes). Splenocytes harvested from long-term–tolerant CXCR4 antagonist-treated mice produced less IFN-γ upon donor alloantigen challenge compared to splenocytes obtained from control rejecting mice (92.3±7.9 vs. 173.2±16.4, p=0.0008), (Figure 5A). While the percentage of Tregs has not always consistently predicted the outcomes of tolerogenic mechanisms, transferable tolerance has been the determinant index of the presence of such mechanisms (26). We therefore recovered splenocytes from long-term–tolerant combination-treated mice 150 days after transplant and adoptively transferred 10×106 cells into immunodeficient C57BL/6 RAG mice that were transplanted 2 weeks prior with islets from BALB/c donors. Splenocytes from untreated C57BL/6 naïve mice, which served as controls, induced islet allograft rejection within 50 days when adoptively transferred into previously-islet-transplanted C57BL/6 RAG mice (MST of 35 days, n=5 mice), (Figure 5B, Group 3). However, more than 75% of C57BL/6 RAG islet-transplanted mice that received splenocytes from long-term tolerant combination-treated mice in fact did not reject BALB/c islets, (MST >150 days, n=5 mice, p=0.02 vs. control mice), (Figure 5B, Group 1). When 10×106 splenocytes from long-term–tolerant C57BL/6 mice were co-adoptively transferred with 10×106 naïve splenocytes into immunodeficient C57BL/6 RAG mice previously transplanted with islets from BALB/c donors, more than 50% of adoptively transferred mice also did not reject islet allografts, (MST >150 days, n=5 mice, ns vs. mice receiving adoptive transfer from naïve splenocytes), (Figure 5B, Group 2). These results strongly suggest that in an in vivo setting, the combination of CXCR4 antagonist and Rapamycin induces a robust and transferable tolerance. As further proof of the tolerance induced by our combination treatment, CXCR4 antagonist+Rapamycin-treated mice (85% tolerant in the long-term) or control rejecting mice were re-transplanted with donor (BALB/c) or 3rd party (DBA/1J) skin grafts. Both control (MST: 3rd party=13 days; donor=12 days), and CXCR4 antagonist+Rapamycin-treated mice (MST: 3rd party=12 days; donor=14 days) promptly rejected donor and 3rd party skin graft, thus confirming the presence of a graft-specific tolerance.
Figure 5.
Splenocytes obtained from CXCR4 antagonist+Rapamycin-treated mice showed a reduction in IFN-γ production when challenged with alloantigens (*p=0.0008), (n=3 mice/group performed in triplicate) (A). Splenocytes were recovered from long-term tolerant, combination-treated mice, and 10×106 cells were adoptively transferred into immunodeficient C57BL/6 RAG mice previously transplanted 24h before with islets from BALB/c donors (n=5 mice/group) (B). Splenocytes from untreated naïve mice that served as controls induced rejection of islet allografts within 50 days after the adoptive transfer (B, Group 3). More than 75% of RAG-islet-transplanted mice that received splenocytes from long-term tolerant mice did not reject islets (*p=0.02 vs. naïve), (B, Group 1). When splenocytes from long-term-tolerant mice were co-adoptively transferred with naïve splenocytes into immunodeficient C57BL/6 RAG mice previously transplanted with islets from BALB/c donors, more than 50% of mice did not reject islet allografts (B, Group 2).
CD25 depletion has no effect on CXCR4 antagonist-induced allograft survival
To confirm that the prolongation of islet allograft survival observed in CXCR4 antagonist-treated mice was Treg-dependent, we depleted Tregs using an anti-CD25 mAb. Treg ablation did not induce a loss of CXCR4 antagonist-mediated graft protection, (MST=18 days) (Figure 2A).
Target of c-Kit and HSC ablation abrogates the effects of CXCR4 antagonist
To establish a link between mobilized HSCs and prolongation of islet allograft survival observed following treatment with CXCR4 antagonist, we performed islet transplantation using CXCR4 antagonist treatment combined with treatment with ACK2, an antibody that targets c-Kit (also known as CD117, the receptor for stem-cell factor). ACK2 has been shown to halt bone marrow HSC mobilization in various models and to function by fully inhibiting c-Kit signaling and SCF-dependent stem cell proliferation, resulting in temporary HSC depletion (27, 28). When co-injected with CXCR4 antagonist, ACK2 abrogated the positive effect on islet allograft survival observed following target of the CXCR4-CXCL12 axis (MST of 21 vs. 13 days, ACK2+CXCR4 antagonist-treated [n=5] vs. CXCR4 antagonist-treated [n=11] p<0.0001), (Figure 2A). Of note, ACK2 treatment alone did not result in any change in survival compared to control mice (MST of 11.5 days, n=5), (Figure 2A). Injection of ACK2 also reduced CXCR4 antagonist-mediated HSC mobilization (HSCs at day 7 after islet transplantation: CXCR4 antagonist-treated mice=2.17±0.30% and CXCR4 antagonist+ACK2-treated mice=0.41±0.07%; p<0.001), (Figure 2B), while ACK2 treatment alone had no effect on HSC mobilization (Figure 2B). CXCR4 antagonist+ACK2-treated mice displayed islets infiltrated by few CD3+ FoxP3− cells (Figures 2F1–2F3–2F4), with a total lack of insulin staining (Figure 2F2) confirming that the appearance of Tregs in the graft was HSC-mediated. We then tested if ACK2 treatment and HSC ablation prior to islet transplantation could affect islet allograft survival in untreated, CXCR4 antagonist-treated and Rapamycin-treated islet-transplanted mice. ACK2 pre-treated mice displayed islet allograft survival (MST=14 days, n=4) comparable to untreated controls (ns). In CXCR4 antagonist-treated islet transplanted mice, ACK2 treatment (14 days) (but not ACK2 pre-treatment [31 days]) abrogated the effect of CXCR4 antagonist by diminishing islet allograft survival (p=0.005), (Figure 2A). Rapamycin alone (0.1 mg/kg) administered every other day (at days 0, 2, 4, 6, 8, and 10) prolonged islet allograft survival (26 days) compared to controls (12 days) (p<0.001), (Figure 2C). ACK2 treatment (18.5 days) (but not ACK2 pre-treatment [24 days]) abrogated the effect of rapamycin on islet allograft survival (p=0.05), (Figure 2C).
Furthermore, treatment with ACK2 abrogated the beneficial effect of the combination therapy of CXCR4 antagonist+Rapamycin on islet allograft survival (MST of 21 vs. >150 days, CXCR4 antagonist+Rapamycin+ACK2-treated [n=5] vs. CXCR4 antagonist+Rapamycin-treated [n=5] p<0.0001), (Figure 4A). We also tested the effect of ACK2 administration on HSC mobilization in the CXCR4 antagonist+Rapamycin-treated group. When ACK2 was co-injected, a clear abrogation of HSC mobilization was evident (KLS at day 7 after islet transplantation: CXCR4 antagonist+Rapamycin-treated mice=3.27±0.19% and CXCR4 antagonist+Rapamycin+ACK2-treated mice=0.80±0.10%; p<0.001), (Figure 4B). Rapamycin alone induced a slight increase in the peripheral percentage of HSCs (p<0.01 compared to controls), (Figure 4B).
Role of PD-L1 in HSC-mediated effect
We evaluated the characteristics of CXCR4 antagonist-mobilized HSCs by FACS analysis. Linneg cells were isolated from splenocytes by microbead-negative selection using biotinylated Lineage beads, followed by FACS sorting for c-Kit+ cells. Cells were isolated from islet-transplanted or naïve CXCR4 antagonist-treated mice, after 7 and 14 days of CXCR4 antagonist treatment. As reported previously, these Linnegc-Kit+ cells are 50% positive for Sca-1 (Figure 6A), indicative of murine HSCs (29). HSCs were then stained for specific positive and negative costimulatory molecules that have been shown to exert significant immunoregulatory roles in the alloimmune response. Interestingly, while most positive costimulatory molecules were found to be negative or scarcely expressed (CD40, CD80, CD86, PD-L2, ICOS, OX40, OX40L) (Figure 6B), PD-L1 (a negative costimulatory molecule) was highly expressed by mobilized HSCs (58.0±7.1%). Extracted HSCs also expressed CXCR4 (38.4±4.2%), (Figure 6B). The profiling of costimulatory molecules on HSCs obtained from CXCR4 antagonist-treated islet-transplanted and untransplanted treated mice at days 7 and 14 after transplantation confirmed that PD-L1 is constitutively expressed by HSCs (Figure 6C), while most other receptors are absent or scarcely expressed (Figure 6C). OX40L expression on HSCs increased transiently in untransplanted mice at day 7 (p<0.05 vs. all) and PD-1 expression on HSCs increased at day 7 in transplanted and untransplanted mice (p<0.01 vs. all), (Figure 6C).
Figure 6.
Lin−-Kit+ cells extracted from splenocytes of CXCR4 antagonist-treated mice after 7 days of treatment and were 50% positive for Sca-1 (n=3 mice), (A). While most positive costimulatory molecules were absent or expressed at low levels (CD40, CD80, CD86, PD-L2, ICOS, OX40, OX40L), (B); PD-L1 was highly expressed in these extracted HSCs (*p<0.001 compared to isotype), (B). Extracted HSCs also expressed CXCR4 (*p<0.001 compared to isotype), (B). The costimulatory molecule expression profile on HSCs obtained from islet-transplanted and untransplanted treated mice at days 7 and 14 after transplantation confirmed that PD-L1 is constitutively expressed by HSCs (C), while most other receptors are absent or scarcely expressed (C). OX40L expression on HSCs increased transiently in untransplanted mice at day 7 (*p<0.05 vs. all) and PD-1 expression on HSCs increased at day 7 in transplanted and untransplanted mice (#p<0.01 vs. all), (C). We then evaluated the in vivo percentage of Kit+PD-L1+ cells during CXCR4 antagonist treatment. Kit+PD-L1+ cells increased in BM 6h after initiating CXCR4 antagonist treatment, confirming that HSCs express PD-L1 (#p=0.04), (D).
We then evaluated the in vivo percentage of c-Kit+PD-L1+ cells during CXCR4 antagonist treatment. c-Kit+PD-L1+ cells increased in bone marrow from C57BL/6 islet-transplanted mice 6 hours after the initiation of CXCR4 antagonist treatment (30 mg/kg/day), but this increase was no longer evident at 12 and 24 hours (baseline=10.3±4.4, 6 hours=31.5±5.4, 12 hours=17.2±5.1 and 24 hours=18.6±5.8%; baseline vs. 6 hours, p=0.04), (Figure 6D).
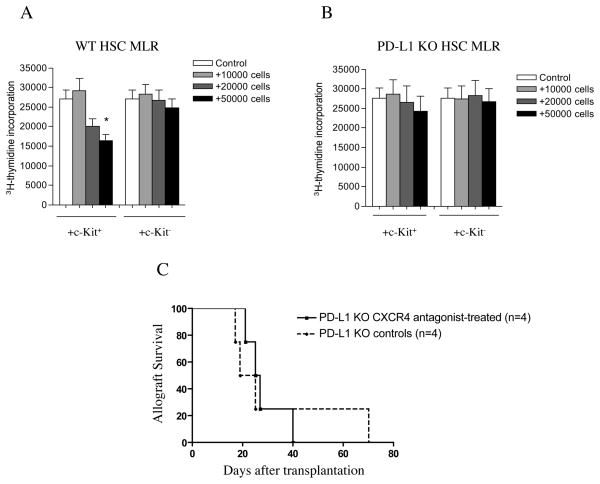
To evaluate the immunoregulatory role of the PD-1 pathway in murine HSCs, we investigated the effect of mobilized HSCs from WT and PD-L1 KO mice on the alloimmune response in vitro. A standard MLR assay was performed in which HSCs (from WT C57BL/6 or PD-L1 KO mice) were syngeneic to responder cells (CD4+ cells from C57BL/6 mice) but allogeneic to bone marrow-derived DCs (from BALB/c mice), as in our in vivo setting. While HSCs from WT C57BL/6 mice were capable of significantly abrogating the MLR response when added to culture (control=27,000±2,374 vs. +50,000 HSCs=16,329±1,641, p=0.003), HSCs from PD-L1 KO mice failed to do so (Figures 7A and 7B).
Figure 7.
A standard MLR assay was performed in which HSCs (from WT C57BL/6 or PD-L1 KO mice) were syngeneic to responder cells (CD4+ cells from C57BL/6 mice) but allogeneic to bone marrow-derived DCs (from BALB/c mice), as in our in vivo setting. Lin−c-Kit− cells were used as a control. HSCs were obtained from WT C57BL/6 mice were capable of significantly abrogating the MLR response (A) (*p=0.003 for +50,000 HSCs compared to control), while HSCs from PD-L1 KO mice failed to have such an effect (B). Targeting the CXCR4-CXCL12 axis was ineffective in delaying islet allograft rejection in PD-L1 KO mice (C).
To examine the in vivo functional role of PD-L1 on HSCs in the prolongation of islet allograft survival, we performed islet transplantation in PD-L1 KO mice with or without CXCR4 antagonist treatment. Untreated PD-L1 KO C57BL/6 mice rejected BALB/c islet allografts (MST of 22 days, n=4 mice), albeit with a delay compared to WT C57BL/6 mice (MST of 12 days, n=15 mice; p=0.001), (Figures 7C and 2A). In contrast to the prolongation observed in C57BL/6 WT mice, CXCR4 antagonist treatment failed to further delay islet allograft rejection in PD-L1 KO C57BL/6 mice (MST of 26 days, n=4 mice, p=0.001 vs. WT C57BL/6 mice and ns vs. PD-L1 KO untreated), (Figure 7C).
DISCUSSION
Islet transplantation holds great promise as a potential cure for T1D (25, 30–32), yet long-term survival of islet grafts remains problematic (20% at 5 years) (33). Therefore, there exists an immense need for new therapeutic options to aid in prolonging islet allograft survival (22, 34, 35). Here, we propose a novel approach to promote tolerance in an islet transplantation model by inducing the shedding of HSCs, using a CXCR4 antagonist (NIBR1816), with the goal of mobilizing the immunomodulatory ability of autogolous HSCs to dampen the alloimmune response and to prolong islet allograft survival. CXCL12-CXCR4 signaling effectively traps HSCs in the bone marrow (19). Targeting the CXCR4-CXCL12 axis with our novel small molecule NIBR1816, a CXCR4 antagonist, we were able to induce the shedding of HSCs to the periphery similar to others (20). We also show that mobilization of HSCs induced by our strategy prolongs islet allograft survival in a fully mismatched model of islet transplantation. Notably, the tolerogenic effect of CXCR4 antagonist can be abrogated in large part by blocking HSC mobilization induced by the CXCR4 antagonist through use of ACK2 (an anti-CD117 mAb), which targets c-Kit (the receptor of stem-cell factor) (27, 28).
We then tested the synergistic effect of CXCR4 antagonist on a commonly used immunosuppressive drug, Rapamycin. Our combined treatment significantly prolonged islet allograft survival; notably, 75% of islet-transplanted mice did not reject islet grafts until beyond 100 days post transplantation. Interestingly, Rapamycin enhanced the mobilization of HSCs. The extent to which this mobilization of HSCs contributes to the prolongation of survival of allografts is important to address and requires future further investigation. Long-term tolerant mice (treated with a combination of Rapamycin at clinical dose plus CXCR4 antagonist) developed robust hyporesponsiveness toward alloantigens, as demonstrated by our adoptive transfer experiments in C57BL/6-RAG immunodeficient mice, which did not reject BALB/c islet allografts when reconstituted with splenocytes from long-term-tolerant mice treated with Rapamycin plus CXCR4 antagonist. Furthermore, among C57BL/6 RAG immunodeficient mice co-reconstituted with splenocytes from long-term tolerant mice treated with Rapamycin plus CXCR4 antagonist as well as with splenocytes from naïve mice, more than 50% of mice did not reject islet grafts. This robust and transferable tolerance, taken together with the reduced production of IFN-γ in response to alloantigen in vitro, suggests the existence of an active hyporesponsiveness and a regulatory process. Unfortunately, tolerance was graft-specific; when islet-tolerant mice were transplanted with skin, they rejected both donor and 3rd party skin grafts. Our trafficking experiments using isolated GFP+HSCs injected into islet-transplanted mice confirmed that autologous HSCs traffic to the lymphoid organs, to the pancreas and to the graft, possibly exerting their immunomodulatory role locally. While it is difficult to determine precisely which cell types are involved in the transfer of tolerance, HSCs and Tregs appear to be the main suspects; however, myeloid and other progenitor cells may also be involved.
To better elucidate the mechanisms by which HSCs were able to induce this prolongation, we characterized mobilized HSCs for their expression of immunoregulatory molecules. Mobilized HSCs were shown to express the negative co-stimulatory molecule PD-L1 at substantial levels, while they appeared to be negative for most positive co-stimulatory molecules. While HSCs from WT mice inhibited the alloimmune response in our MLR assay in vitro, HSCs extracted from PD-L1 KO mice failed to exert an inhibitory effect in our MLR. CXCR4 antagonist treatment also appeared ineffective in delaying graft rejection in PD-L1 KO mice. PD-L1 has been shown to be expressed on resting and activated B, T, myeloid, and dendritic cells, to play a role in regulating the alloimmune response in vivo by inducing apoptosis of T effector cells, and to delay allograft rejection (36–41). We postulate that PD-L1 expression on HSCs plays a principle role in the tolerogenic effect of HSCs. Precise evaluation of trafficking of mobilized HSCs is problematic, as HSC may differentiate into myeloid or lymphoid lineages and alter their phenotype. Moreover, the half-life of HSCs has not yet been determined, and without a GFP+HSC-specific mouse, accurate tracking of HSCs is difficult to achieve. Our data indicate that HSCs traffic to the spleen and appear to be present to a lower extent in the pancreatic lymph nodes, pancreas and islet graft.
Using a CXCR4 antagonist, available for human use (i.e. Mozobil/Plexifor), to elicit shedding of HSCs has become an appealing strategy to mobilize HSCs and to facilitate HSC engraftment following bone marrow transplantation (16). HSCs have recently emerged as the key regulators of immune responses (42). Our strategy is highly clinically relevant, as it would eliminate the need for bone marrow transplantation, which usually requires institution of chemo and radiation therapy. In conclusion, we have demonstrated that targeting the CXCR4-CXCL12 axis with a CXCR4 antagonist can mobilize autologous HSCs, resulting in increased survival of islet allografts. This effect synergized with Rapamycin, and HSCs likely exert their tolerogenic effect in part through the PD-L1 pathway.
Interestingly, we observed synergism between Rapamycin and CXCR4 antagonist in mobilizing HSCs; as Rapamycin is a well-known anti-proliferative drug, this result was unexpected (43). Of note, recent works have shown that Rapamycin is capable of halting and reverting age-related decline in HSC function (44). Investigators showed that mammalian target of rapamycin (mTOR) activity is increased in HSCs from old mice compared to those from young mice (44). In old mice, Rapamycin increased life span and restored the self-renewal and hematopoiesis of HSCs (44), thus suggesting that mTOR signaling may be dangerous for HSCs and demonstrating the potential of mTOR inhibitors for restoring full competent hematopoiesis. Whether HSC mobilization has any utility in patients with oncogene/tumor suppressor mutations remains to be explored (45), and caution must be taken when considering mobilizing HSCs in patients with disorders of hematopoiesis.
It should be noted that CXCR4 blockade and HSC-mediated immunomodulation are not mutually exclusive. CXCR4 antagonist treatment may also affect several arms of effector and regulatory pathways, which are also important for transplant outcome. For instance, CXCR4 signaling could play an important role in the trafficking of Tregs and DCs, both of which are central to the pathogenesis of alloimmunity. Furthermore, c-kit expressed on DCs has been shown to regulate the differentiation of Th cells (46, 47), so that anti-c-Kit treatment could also affect transplant outcome. While our data shed light on the implication of HSC release in organ transplantation and mechanisms by which HSC exert their function in prolonging graft survival, examining the relative contribution of these pathways is beyond the scope of this study but is nevertheless important to fully explore in future studies. ACK2 treatment may also affect cells other than HSCs.
Our data could ultimately help in the design of tolerogenic strategies in human islet cell transplantation, which is in distinct need of improvement.
Acknowledgments
Grant support: AST-JDRF Faculty grant (P.F.), JDRF-CDA (P.F.), ASN-CDA (P.F.), AST-JDRF fellowship (A.V.), JDRF Grant 4-2007-1065 (R.A and M.H.S.) and JDRF Regular grant (R.A).
We are grateful to Novartis for providing the drug. Paolo Fiorina is the recipient of an AST-JDRF Faculty Grant and a JDRF-Career Development Award and ASN Career Development Award. Andrea Vergani is the recipient of an AST-JDRF fellowship grant. Paolo Fiorina acknowledges the support from a pilot and feasibility award from the Boston Area Diabetes Endocrinology Research Center (5P30DK57521). This work is supported by Juvenile Diabetes Research Foundation (JDRF) Grant 4-2007-1065 and a JDRF Regular grant (R.A) Grant.
References
- 1.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simoes BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. Jama. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 2.Dazzi F, van Laar JM, Cope A, Tyndall A. Cell therapy for autoimmune diseases. Arthritis Res Ther. 2007;9:206. doi: 10.1186/ar2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohgaki T, Atsumi T, Bohgaki M, Furusaki A, Kondo M, Sato-Matsumura KC, Abe R, Kataoka H, Horita T, Yasuda S, Amasaki Y, Nishio M, Sawada K, Shimizu H, Koike T. Immunological reconstitution after autologous hematopoietic stem cell transplantation in patients with systemic sclerosis: relationship between clinical benefits and intensity of immunosuppression. J Rheumatol. 2009;36:1240–1248. doi: 10.3899/jrheum.081025. [DOI] [PubMed] [Google Scholar]
- 4.Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, Mach E, Zaprjagaeva M, Guseva N, Nassonov E. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford) 2009;48:61–64. doi: 10.1093/rheumatology/ken407. [DOI] [PubMed] [Google Scholar]
- 5.Burt RK, Traynor A, Oyama Y, Craig R. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood. 2003;101:2064–2066. doi: 10.1182/blood-2002-07-2122. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulin-encoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54:434–442. doi: 10.2337/diabetes.54.2.434. [DOI] [PubMed] [Google Scholar]
- 7.Bachar-Lustig E, Rachamim N, Li HW, Lan F, Reisner Y. Megadose of T cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat Med. 1995;1:1268–1273. doi: 10.1038/nm1295-1268. [DOI] [PubMed] [Google Scholar]
- 8.Gur H, Krauthgamer R, Bachar-Lustig E, Katchman H, Arbel-Goren R, Berrebi A, Klein T, Nagler A, Tabilio A, Martelli MF, Reisner Y. Immune regulatory activity of CD34+ progenitor cells: evidence for a deletion-based mechanism mediated by TNF-alpha. Blood. 2005;105:2585–2593. doi: 10.1182/blood-2002-11-3463. [DOI] [PubMed] [Google Scholar]
- 9.George JF, Sweeney SD, Kirklin JK, Simpson EM, Goldstein DR, Thomas JM. An essential role for Fas ligand in transplantation tolerance induced by donor bone marrow. Nat Med. 1998;4:333–335. doi: 10.1038/nm0398-333. [DOI] [PubMed] [Google Scholar]
- 10.Kared H, Leforban B, Montandon R, Renand A, Layseca Espinosa E, Chatenoud L, Rosenstein Y, Schneider E, Dy M, Zavala F. Role of GM-CSF in tolerance induction by mobilized hematopoietic progenitors. Blood. 2008;112:2575–2578. doi: 10.1182/blood-2008-02-140681. [DOI] [PubMed] [Google Scholar]
- 11.Rachamim N, Gan J, Segall H, Krauthgamer R, Marcus H, Berrebi A, Martelli M, Reisner Y. Tolerance induction by “megadose” hematopoietic transplants: donor-type human CD34 stem cells induce potent specific reduction of host anti-donor cytotoxic T lymphocyte precursors in mixed lymphocyte culture. Transplantation. 1998;65:1386–1393. doi: 10.1097/00007890-199805270-00017. [DOI] [PubMed] [Google Scholar]
- 12.Fink PJ, Shimonkevitz RP, Bevan MJ. Veto cells. Annual review of immunology. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [DOI] [PubMed] [Google Scholar]
- 13.Muraoka S, Miller RG. Cells in bone marrow and in T cell colonies grown from bone marrow can suppress generation of cytotoxic T lymphocytes directed against their self antigens. The Journal of experimental medicine. 1980;152:54–71. doi: 10.1084/jem.152.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin C, Bridger GJ, Rankin SM. Structural analogues of AMD3100 mobilise haematopoietic progenitor cells from bone marrow in vivo according to their ability to inhibit CXCL12 binding to CXCR4 in vitro. Br J Haematol. 2006;134:326–329. doi: 10.1111/j.1365-2141.2006.06181.x. [DOI] [PubMed] [Google Scholar]
- 16.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 17.Flomenberg N, DiPersio J, Calandra G. Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells. Acta Haematol. 2005;114:198–205. doi: 10.1159/000088410. [DOI] [PubMed] [Google Scholar]
- 18.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, Bridger G, Dunbar CE, Hematti P. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Thoma G, Streiff MB, Kovarik J, Glickman F, Wagner T, Beerli C, Zerwes HG. Orally bioavailable isothioureas block function of the chemokine receptor CXCR4 in vitro and in vivo. J Med Chem. 2008;51:7915–7920. doi: 10.1021/jm801065q. [DOI] [PubMed] [Google Scholar]
- 21.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. The Journal of experimental medicine. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdi R, Smith RN, Makhlouf L, Najafian N, Luster AD, Auchincloss H, Jr, Sayegh MH. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes. 2002;51:2489–2495. doi: 10.2337/diabetes.51.8.2489. [DOI] [PubMed] [Google Scholar]
- 23.Makhlouf L, Kishimoto K, Smith RN, Abdi R, Koulmanda M, Winn HJ, Auchincloss H, Jr, Sayegh MH. The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes. 2002;51:3202–3210. doi: 10.2337/diabetes.51.11.3202. [DOI] [PubMed] [Google Scholar]
- 24.Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol. 2002;169:4831–4839. doi: 10.4049/jimmunol.169.9.4831. [DOI] [PubMed] [Google Scholar]
- 25.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 26.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG. Anti-LFA-1 therapy induces long-term islet allograft acceptance in the absence of IFN-gamma or IL-4. J Immunol. 2000;164:3627–3634. doi: 10.4049/jimmunol.164.7.3627. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105:2757–2763. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 28.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science (New York, NY. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M. Pretransplant Infusion of Mesenchymal Stem Cells Prolongs the Survival of a Semiallogeneic Heart Transplant through the Generation of Regulatory T Cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 30.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8:1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 31.Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 32.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. Jama. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. The New England journal of medicine. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 34.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nature reviews. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 35.Fiorina P, Jurewicz M, Tanaka K, Behazin N, Augello A, Vergani A, von Andrian UH, Smith NR, Sayegh MH, Abdi R. Characterization of donor dendritic cells and enhancement of dendritic cell efflux with CC-chemokine ligand 21: a novel strategy to prolong islet allograft survival. Diabetes. 2007;56:912–920. doi: 10.2337/db06-1445. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annual review of immunology. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 37.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. The Journal of experimental medicine. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. European journal of immunology. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 39.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O’Keefe T, Duong T, Smith T, Gutierrez-Ramos JC, Rottman JB, Coyle AJ, Hancock WW. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 40.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, Yagita H, Azuma M, Turka LA, Sayegh MH. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr, Sayegh MH, Najafian N. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 42.Steptoe RJ, Ritchie JM, Harrison LC. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. J Clin Invest. 2003;111:1357–1363. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valantine H. Is there a role for proliferation signal/mTOR inhibitors in the prevention and treatment of de novo malignancies after heart transplantation? Lessons learned from renal transplantation and oncology. J Heart Lung Transplant. 2007;26:557–564. doi: 10.1016/j.healun.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Klumpp S, Amin HM, Liang H, Li J, Estrov Z, Zweidler-McKay P, Brandt SJ, Agulnick A, Nagarajan L. SSBP2 is an in vivo tumor suppressor and regulator of LDB1 stability. Oncogene. 29:3044–3053. doi: 10.1038/onc.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, Ray A, Ray P. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray P, Krishnamoorthy N, Oriss TB, Ray A. Signaling of c-kit in dendritic cells influences adaptive immunity. Ann N Y Acad Sci. 1183:104–122. doi: 10.1111/j.1749-6632.2009.05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]