Abstract
Objective
To demonstrate that TNFα, via sphingosine-1-phosphate (S1P) signaling, has the potential to alter cochlear blood flow and thus, cause ischemic hearing loss.
Methods and Results
TNFα induced a pro-constrictive state throughout the cochlear microvasculature, which reduced capillary diameter and cochlear blood flow in vivo. In vitro isolated preparations of the spiral modiolar artery and spiral ligament capillaries confirmed these observations. Antagonizing S1P receptor 2 subtype signaling (1µmol/L JTE013) attenuated the effects of TNFα in all models. TNFα activated Sk1 and induced its translocation to the smooth muscle cell membrane. Expression of a dominant-negative Sk1 mutant (Sk1G82D) eliminated both baseline spiral modiolar artery calcium sensitivity and TNFα effects, while a non-phosphorylatable Sk1 mutant (Sk1S225A) only blocked the effects of TNFα. A small group of etanercept-treated hearing loss patients recovered with a one-phase exponential decay (t½=1.56±0.20 weeks), which matched a kinetic predicted for a vascular origin.
Conclusions
TNFα indeed reduces cochlear blood flow via the activation of vascular S1P signaling. This integrates hearing loss into the family of ischemic microvascular pathologies, with implications for risk stratification, diagnosis and treatment.
Keywords: Signal transduction, transfection, etanercept, sphingosine kinase 1, cochlear microcirculation
INTRODUCTION
Inflammation1,2 and reductions in cochlear blood flow3–5 can induce hearing loss, a condition that affects approximately 36 million Americans6. Most investigations have separated the two etiologic factors (i.e., inflammation versus vascular), under the assumption that the two pathogenic mechanisms are distinct and mutually exclusive. Recent experimental7–9 and clinical10–12 data demonstrate that tumor necrosis factor α (TNFα) sequestration significantly improves auditory function in a large subgroup of idiopathic hearing loss patients: the beneficial clinical outcomes have been widely interpreted as supporting evidence that idiopathic hearing loss is primarily caused by direct inflammatory damage to cochlear cells. The present investigation, however, presents an alternative scenario: since TNFα has the capability to alter vascular reactivity13, loss of hearing could arise from a vascular response that compromises the energy intensive process hearing transduction (i.e., cochlear ischemia)14.
Cochlear blood flow relies exclusively on the spiral modiolar artery (SMA), a functional end artery (i.e., lacking collaterals)15. Fine spatial distribution of blood flow is controlled by the cochlear microvascular networks, which ultimately feed the stria vascularis. This architecture creates a direct relationship between the location of flow restriction and the extent of auditory symptoms (i.e., range of frequencies affected). Thus, more proximal flow restrictions are prone to yield widespread auditory symptoms (pantonal hearing loss), while the symptoms resulting from distal flow restrictions would be more discrete (hearing loss in a narrow frequency range). Despite the vital function of cochlear blood flow, remarkably little is known about the molecular mechanisms that regulate cochlear microvascular tone or its potential connection to hearing loss. We have previously described that tone16 and resistance to blood flow15 in the SMA are principally controlled by the pro-constrictive phospholipid sphingosine-1-phosphate (S1P). The S1P-generating enzyme sphingosine kinase 1 (Sk1) is activated by TNFα17, which, in principle, imbues this cytokine with the potential to profoundly disturb the cochlear microcirculation.
The present study proposes that inflammation, via TNFα-dependent activation of S1P signaling, can reduce cochlear blood flow. To advance this vascular hypothesis, we assessed the effect of TNFα/S1P signaling on cochlear blood flow/arteriolar tone (intravital microscopy of the cochlear microvascular) and capillary diameter (ex vivo spiral ligament preparation). To provide evidence for a vascular-based mechanism in humans, the recovery profiles of a selected, small group of etanercept-treated hearing loss patients were compared to a kinetic predicted for a primary vascular origin.
METHODS
Animal models
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All experimental protocols complied with Canadian, American and German federal animal protection laws and were approved by the respective Institutional Animal Care and Use Committees.
Intravital fluorescence microscopy measuring blood flow and capillary diameter in the stria vascularis was accomplished via intravenous injection of FITC-labeled dextran into anaesthetized guinea pigs in combination with standard intravital fluorescence microscopy techniques18.
To measure capillary diameter ex vivo, capillary beds from the gerbil spiral ligament were isolated from the cochlear lateral wall and maintained in an organ bath19. Because direct measurement of capillary diameter yields a poor signal-to-noise ratio, an indirect method, based on the measurement of red blood cell (RBC) movement with the capillary lumen, was utilized19,20.
The gerbil spiral modiolar artery (SMA) was isolated from modiolus, cut into ~1.5mm segments, cannulated and hydrostatically pressurized (30cmH2O)15,21; vessels were then cultured for 24hrs before experimentation. Genetic constructs (Sk1G82D, Sk1S225A and Sk1-GFP) were transfected during the culture period. All values of tone represent acute diameter measurements that have been normalized so that the values represent the magnitude of vessel constriction relative to maximal diameter. Variability in baseline calcium sensitivity prevents the direct statistical comparison of data from separate experimental series (described in the online supplement). Sk1-GFP was visualized in SMA smooth muscle cells under live conditions using a Zeiss LSM410 confocal microscope equipped with a Kr/Ar laser and a 63×/1.2W water immersion objective.
Assessment of auditory function in etanercept-treated patients
The use of human subjects in this study was approved by the Human Studies Ethics Committee of the Technische Universität München; Munich, Germany (#2172/08). A detailed description of the selection/exclusion criteria, pre-study assessments and a patient composite is located in the online supplement. A total of 12 adult patients presenting with the typical symptoms of sudden hearing loss, who were non- or only partially responsive to prednisolone treatment, were identified and selected for etanercept treatment. Etanercept (25mg) was self-administered subcutaneously twice a week for 12 weeks
Hearing function was assessed by pure-tone audiometry, speech reception threshold and word recognition. Pure-tone average (PTA) was performed at 9 frequencies between 250 and 6000Hz. Hearing improvement was defined as an improvement of (i) hearing from baseline (ii) ≥20dB in the pure-tone air conduction thresholds in at least 3 of the tested frequencies and (iii) 15dB at 2 consecutive frequencies.
Statistics
Data represent means±SEM for n experiments. Experimental groups were compared using paired or unpaired Student’s t-tests, or an analysis of variance (ANOVA) followed by a t-test with a Bonferroni correction for multiple comparisons, as appropriate. Differences were considered significant at an error probability of p<0.05.
RESULTS
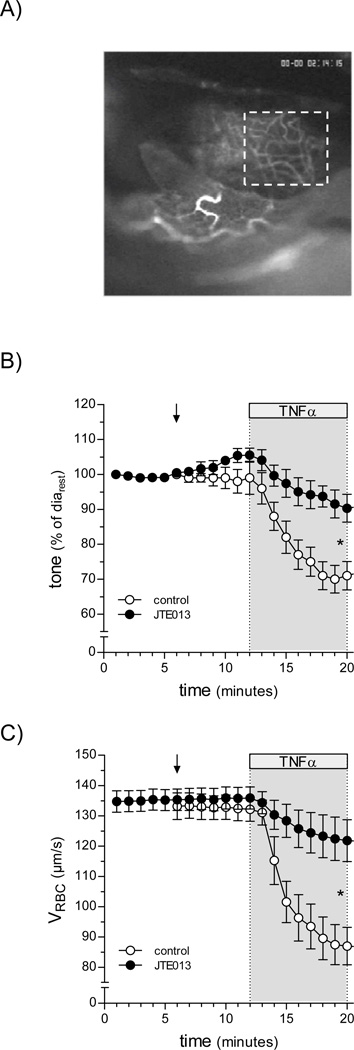
TNFα superfusion (1ng/ml, 20min) rapidly reduced guinea pig cochlear blood flow (determined as red blood cell velocity, VRBC) from 132.9±4.0µm/s to 86.8±6.0µm/s, with a half-maximal effect reached after 7min (Figure 1B). S1P2 receptor blockade (10µmol/L JTE013; 10min superfusion prior to addition of TNFα) fully prevented this response (Figure 1B). The TNFα-stimulated reduction in cochlear blood flow was associated with a decrease in capillary diameter (from 9.0±0.4µm to 6.7±0.2µm), which was also prevented by S1P2 receptor blockade (Figure 1C). Systemic effects of the treatments can be excluded, since arterial pressure remained constant throughout the experiment (data not shown).
Figure 1. TNFα-induces reductions in stria vascularis blood flow and capillary diameter by a S1P2 receptor-dependent mechanism.
Using a cochlear window preparation, alterations in guinea pig cochlear blood flow and capillary diameter were assessed in vivo. (A) Capillaries in the convex area of the cochlear second turn were visualized using epi-illumination following intravenous injection of FITC-labeled dextran. The inspection area is outlined by a white box. Superfusion of a cochlear window with TNFα (1ng/ml, 20min) reduced both (B) blood flow (measured as red blood cell velocity) and (C) capillary diameter (n=6). Pre-treatment with the S1P2 receptor antagonist JTE013 (10µmol/L for 10min) significantly attenuated both effects of TNFα.
Consistent with Figure 1C, TNFα constricted gerbil spiral ligament capillaries ex vivo (length=260±23µm; diameter=7.2±0.2µm) in an S1P2 receptor-dependent manner (i.e., inhibited by 10µmol/L JTE013; Figure 2B). Accordingly, S1P also stimulated capillary constriction (Figure 2C) with an EC50 approximated to be 4.7µmol/L (Supplemental Figure 2).
Figure 2. TNFα and S1P stimulate vasoconstriction of spiral ligament capillaries.
Changes in the capillary diameter were measured in an ex vivo preparation of capillary beds isolated from the spiral ligament of the cochlear lateral wall. (A) Capillaries were occluded on one end, opened on the other end and the red blood cells (RBC) trapped inside were visualized by laser-scanning microscopy. The capillary lumen between the occluder and the RBC was assumed to be a cylinder of constant volume: vasoconstriction (assumed to be proportional changes in diameter and length) forces the movement of RBCs towards the open end of the capillary, providing a highly-sensitive measure of vasoconstriction magnitude. (B) TNFα (1ng/ml) stimulated capillary vasoconstriction (open circles; n=7), which could be completely blocked by JTE013 pre-treatment (1µmol/L; closed circles; n=4). (C) S1P (response to 100µmol/L shown) also stimulated capillary vasoconstriction (n=9).
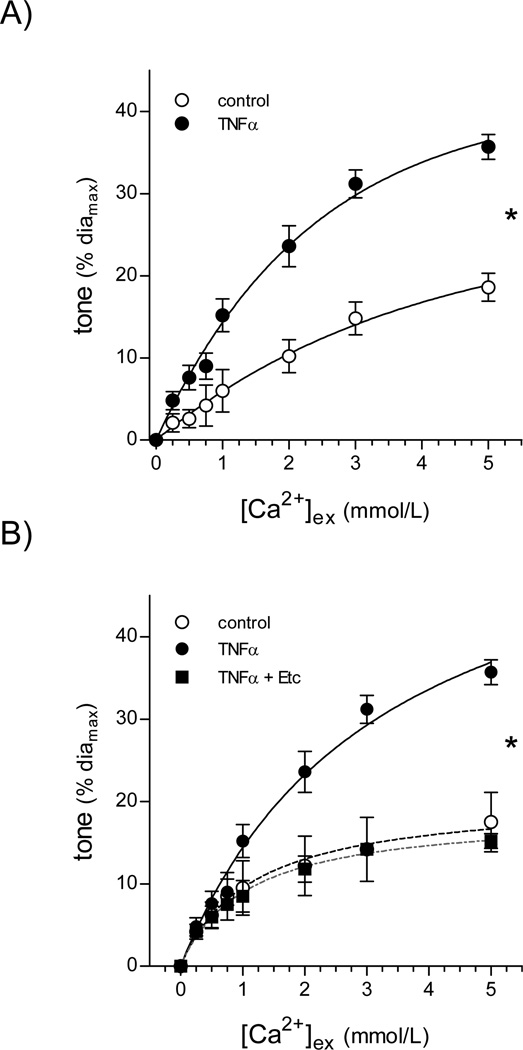
In the isolated gerbil spiral modiolar artery (SMA; a model with the unique advantage that it can be transfected), TNFα (1ng/ml, 2h) significantly increased vasoconstriction in response to calcium under depolarizing conditions (Bmax con:26±1; TNFα:41±4% of diamax; n=5; Figure 3A). TNFα had no effect on resting SMA tone. Etanercept (competitive TNFα inhibitor) co-treatment prevented the TNFα-mediated increase in SMA tone (Bmax con:21±1; TNFα+Etc:19±1% of diamax, n=5; Figure 3B). JTE013 (1µmol/L, 30min) also fully reversed the effect of TNFα (Bmax con:37±5, TNFα:50±5, TNFα+JTE013:33±4% of diamax; Figure 4A); however, unlike etanercept (Figure 3B), JTE013 significantly decreased basal Ca2+ sensitivity (Bmax con:23±3, JTE013:11±2% of diamax; Figure 4B). The 1µmol/L JTE013 concentration appears to specifically inhibit S1P responses (supplemental results).
Figure 3. TNFα increases Ca2+ sensitivity in the spiral modiolar artery.
(A) TNFα (1ng/ml, 2h) increased the Ca2+ sensitivity in isolated SMAs (diamax=89±5µm, n=5 paired observations). (B) Co-incubation with etanercept (Etc; 1µg/ml), a TNFα inhibitor, prevented the TNFα-stimulated increase in SMA Ca2+ sensitivity (diamax=91±7µm, n=5 paired observations). * denotes a significant difference (p<0.05) in the dose-response relationships.
Figure 4. The S1P2 receptor subtype is critical for TNFα-mediated Ca2+ sensitivity increase in the spiral modiolar artery.
(A) Treatment of SMAs with 1µmol/L JTE013 (30min) following TNFα stimulation (1ng/ml, 2h), which increased Ca2+ sensitivity, reversed the effect of TNFα (diamax=91±4µm, n=9 paired observations). (B) In the absence of TNFα, JTE013 inhibitor treatment resulted in reduced Ca2+ sensitivity (i.e., a rightward shift in the Ca2+/tone relationship), compared to control SMAs (diamax=97±9µm, n=5 paired observations). * denotes a significant difference (p<0.05) in the dose-response relationships.
In SMAs expressing GFP-tagged Sk1 (GFP-Sk1) within their smooth muscle cells, TNFα (1ng/ml for 2h) stimulated translocation of GFP-Sk1 from a primarily cytosolic localization (Figure 5A) to the plasma membrane (Figure 5B). This translocation serves as a marker of Sk1 activation22,23.
Figure 5. TNFα activates sphingosine kinase 1 to enhance spiral modiolar artery Ca2+ sensitivity.
(A) Under resting conditions, GFP-Sk1 was homogenously distributed throughout the cytosol. (B) TNFα (1ng/mL; 2hrs) stimulated a redistribution of GFP-Sk1 to plasma membrane. (C) Expression of a dominant inactive Sk1 mutant dramatically reduced the resting SMA Ca2+ sensitivity (i.e., a rightward shift in the Ca2+/tone relationship), compared to controls. TNFα augmented Ca2+ sensitivity in control SMAs, but not those expressing the dominant-negative Sk1 mutant Sk1G82D (diamax Control= 90±7µm, n=7; diamax Sk1G82D=92±7µm, n=7). (D) The effects of the chemical inhibition of sphingosine kinase (dimethyl-sphingosine; DMS; 3µmol/L, 30min) were similar to that of Sk1G82D expression: it reduced resting Ca2+ sensitivity and prevented the Ca2+ sensitivity increase following subsequent application of TNFα (diamax: 87±9µm, n=7; paired observations). (E) DMS also reversed the TNFα-stimulated enhancement of SMA Ca2+ sensitivity (diamax: 86±4µm, n=5; paired observations). (F) TNFα failed to increase Ca2+ sensitivity in SMAs expressing the non-phosphorylatable, but catalytically active Sk1 mutant Sk1S225A. * denotes a significant difference (p<0.05) in the dose-response relationships.
Expression of a dominant-negative Sk1 mutant (Sk1G82D) in SMA smooth muscle cells, which would be expected to reduce endogenous S1P levels, virtually abolished Ca2+ sensitivity (Bmax con:26±2; Sk1G82D:4±1% of diamax; p<0.05) and eliminated the pro-constrictive effect of TNFα (Bmax TNFα:32±2; Sk1G82D+TNFα:6±2%, n=7; p<0.05 comparing Sk1G82D+TNFα to TNFα; p>0.05 comparing Sk1G82D to Sk1G82D+TNFα; Figure 5C). Since the long term inhibition of endogenous S1P synthesis potentially impacts smooth muscle cell homeostasis24, these experiments were complemented with a series utilizing a competitive chemical inhibitor, dimethyl-sphingosine (DMS; 3µmol/L for 30min). The effects of DMS were virtually identical to those of Sk1G82D-mediated inhibition of Sk1 (Figure 5D). Additionally, the application of DMS following exposure to TNFα fully reversed the TNFα-mediated enhancement on Ca2+ sensitivity (Figure 5E).
An Erk phosphorylation site at serine 225 is critical for Sk1 activation and translocation in response to several stimuli, including TNFα23. Expression of a mutant Sk1 that cannot be phosphorylated at serine 225 (Sk1S225A) prevented the TNFα-dependent increase in Ca2+ sensitivity (Figure 4F; Bmax Sk1S225A:25±3; BmaxSk1S225A+TNFα:23±2%, n=5). Unlike SMAs expressing Sk1G82D, SMAs expressing Sk1S225A retained a significant level of Ca2+ sensitivity (compare Figure 5C and 5F).
In HEK293T cells, TNFα signals via TNFα Receptor Associated Factor 2 (TRAF2) to activate Sk125. In the SMA, however, expression of a Sk1 mutant lacking its TRAF2 binding motif (TB2-Sk1) failed to prevent the TNFα-dependent increase in Ca2+ sensitivity (BmaxTB2-Sk1:22±5; BmaxTB2-Sk1+TNFα: 29±4; n=6, P<0.05).
To translate our rodent data into a human background, we recruited a small number of otherwise non-responsive sudden hearing loss patients for an off-label treatment with Enbrel® (a TNFα inhibitor). Patients were subcategorized into “acute” (symptoms present <3 months) and “chronic” (symptoms present for >3 months) hearing loss cases. Etanercept treatment improved 6 of the 7 acute hearing loss cases, but only 2 of the 7 chronic hearing loss cases. The kinetic data from the responding acute cases of hearing loss were perfectly fit by a one-phase exponential decay (calculated half-life: t1/2=1.56±0.20 weeks; r2=0.9916, Figure 6); after 12 weeks of treatment, etanercept reduced the responding patients’ auditory threshold from 55.7±2.0 to 25.36±1.2dB).
Figure 6. Kinetics of etanercept-mediated improvement of auditory function in hearing loss patients.
Displayed is the recovery kinetic from 6 clinical cases of hearing loss following off-label treatment with etanercept (Enbrel®); all patients sought treatment within 3 months of developing symptoms. In 6 out of 7 ears treated (1 patient had bilateral hearing loss), etanercept improved auditory function. Of these 6 responding ears, 50% of the maximal effect achieved after 1.5 weeks. The kinetic almost perfectly fits a one-phase exponential decay function (r2=0.9916).
DISCUSSION
Using in vivo and in vitro models, this investigation demonstrates that TNFα induces a pro-constrictive state throughout the cochlear microcirculation. In accordance with our overall hypothesis, TNFα causes a rapid reduction of blood flow within the guinea pig stria vascularis, which was associated with decreased capillary diameter. TNFα has been shown to elicit vasoconstriction in both human mammary arteries26 and mouse cerebral arteries27: our in vitro measurements in spiral ligament capillaries confirm that the TNFα-stimulated reduction in capillary diameter is, in fact, an active constriction (rather than a passive collapse of the capillaries) 20,28. It is therefore tempting to speculate that micro-regional TNFα production could compromise blood flow in specific regions along the length of the cochlea, which could give rise to hearing loss in distinct frequency ranges.
In addition to stimulating vasoconstriction within the distal cochlear microcirculation, TNFα also augmented Ca2+ sensitivity (i.e., a pro-constrictive state) in the more proximal gerbil spiral modiolar artery (SMA). Etanercept was able to block the TNFα-stimulated augmentation of Ca2+ sensitivity in the SMA, which may, in part, explain the beneficial clinical effects of the drug in a subgroup of hearing loss patients10,11. A common feature of all parameters studied in the experimental models (i.e., blood flow, capillary constriction and SMA Ca2+ sensitivity) is the susceptibility of the TNFα effects to the S1P2 antagonist JTE013. This links the effects of TNFα to microvascular S1P signaling and is consistent with the known role of TNFα as an activator of Sk124. Indeed, we were able to visualize TNFα-stimulated translocation of Sk1 (an activation marker) and demonstrate that the tone-enhancing effect of TNFα critically depends on a functional Sk1 enzyme (Figure 5).
In addition to blocking the effects of TNFα, the inhibition of Sk1 catalytic activity with a dominant-negative mutant (Sk1G82D) also profoundly reduced basal SMA Ca2+ sensitivity; JTE013 displayed similar effects. These are important observations, since the SMA serves as a resistance artery and thus, protects the cochlear microcirculation from systemic pressures16. The indiscriminate interruption of S1P signaling could, therefore, compromise this important function and lead to excessive pressure within the inner ear microcirculation: such a mechanism may explain why the S1P2 receptor knockout mouse is deaf as a result of structural damage to the cochlear microcirculation and strial tissue16. Since the SMA’s resistance artery function appears to be dependent on constitutive S1P synthesis, interventions that target the S1P2 receptor (JTE013) and/or S1P synthesis (DMS) may not represent viable clinical strategies to counter the tone-enhancing effects of TNFα.
We therefore assessed the effect of a catalytically active Sk1 mutant that lacks the ERK1/2 phosphorylation motif (Sk1S225A) necessary for TNFα-stimulated activation of the enzyme23. Remarkably, expression of Sk1S225A abolished the effect of TNFα but retained a significant degree of Ca2+ sensitivity. This identifies the phosphorylation-dependent activation of Sk1 as a more precise molecular target for selective therapeutic approaches aiming to reduce the deleterious vascular effects of TNFα. The fact that ERK1/2-dependent Sk1 phosphorylation integrates several inflammatory cytokine inputs (e.g., IL1β, IL6)29,30 highlights the additional potential of this suggested therapeutic approach as a more general means to interfere with the microvascular effects of inflammatory processes.
Pitson and co-workers have proposed that TNFα-mediated Sk1 activation requires direct multiplexing with the TNFα receptor, via an interaction with TRAF225. The lack of effect of TB2-Sk1 expression in the SMA, however, suggests that TNFα-mediated increase in Ca2+ sensitivity relies on ERK1/2 activation downstream of the TNFα receptor.
Patients who responded to etanercept displayed a functional hearing recovery time-course that was tightly fit with a single exponential function possessing an average time constant of 1.5 weeks. We acknowledge that the patient group is small in size; however the time-course, combined with a low inter-patient variability, suggests a single common mechanism underlying the benefit of etanercept treatment (i.e., sequestration of TNFα). The time course of hearing recovery, approximately 10–15 days, is consistent with the combined time for etanercept to reach effective plasma levels (~2 days)31, the normalization of blood flow (rather immediate, once therapeutically relevant concentrations of etanercept are established within the vascular wall) and for the endocochlear potential to be restored following oxygen deprivation (~7 days)32. Although none of these individual clinical observations directly links hearing loss to reductions in cochlear blood flow, the cumulative evidence provided from the human and animal data in this investigation supports a strong vascular component in the pathogenesis of hearing loss. While we cannot exclude direct effects of TNFα on hair cells (e.g., apoptosis) 33, the reversibility shown in Figure 6 is more consistent with a vascular-based mechanism.
In summary, our data indicate that TNFα induces a pro-constrictive state at all levels of the cochlear microcirculation, via the activation of S1P signaling: therefore, any pathology linked to the release of TNFα (infection, autoimmune disorders, systemic inflammatory responses, etc.) has the potential to cause vascular-based, ischemic hearing loss. In this scenario, failure of hearing transduction is secondary to ischemia in the inner ear, events that result from increased resistance to cochlear blood flow due to vasoconstriction of the SMA and cochlear microcirculation. As a result, this investigation integrates a subgroup of hearing loss cases into the family of ischemic vascular pathologies, with immediate implications related to risk stratification, diagnosis and treatment.
Supplementary Material
Acknowledgments
Acknowledgements and Funding Page:
FUNDING
This work was supported by research and infrastructure grants from the Canadian Institutes of Health Research (MOP-84402 to SSB); National Institutes of Health (NIH-R01-DC04280 to PW) Canadian Foundation for Innovation (11810 to SSB); Ontario Research Fund (11810 to SSB); Canadian Stroke Network (to SSB); University of Toronto Start-up Funding (to SSB); Kansas State University Core Facility Support (NIH-P20-RR017686 to PW) and the Rechts der Isar Clinical Research Fund, Technische Universität München, Munich, Germany (8758155 to EQS). This work was also received salary support from Heart and Stroke Foundation of Ontario New Investigator (SSB) and Career Investigator (PHB) Awards; a PhD scholarship from the Boehringer Ingelheim Fund (CDD); and a stipend from the Deutscher Akademischer Austauschdienst (KI). SSB and JVB wish to dedicate this study to their mothers, who suffer with SHL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None declared.
REFERENCES
- 1.Merchant SN, Durand ML, Adams JC. Sudden deafness: is it viral? ORL J Otorhinolaryngol Relat Spec. 2008;70:52–60. doi: 10.1159/000111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrocal JR, Ramirez-Camacho R. Sudden sensorineural hearing loss: supporting the immunologic theory. Ann Otol Rhinol Laryngol. 2002;111:989–997. doi: 10.1177/000348940211101107. [DOI] [PubMed] [Google Scholar]
- 3.Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Rev Bras Otorrinolaringol (Engl Ed) 2006;72:554–561. doi: 10.1016/S1808-8694(15)31004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou J, Pyykko I, Sutinen P, Toppila E. Vibration induced hearing loss in guinea pig cochlea: expression of TNF-alpha and VEGF. Hear Res. 2005;202:13–20. doi: 10.1016/j.heares.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Suckfull M. Fibrinogen and LDL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lancet. 2002;360:1811–1817. doi: 10.1016/S0140-6736(02)11768-5. [DOI] [PubMed] [Google Scholar]
- 6.Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngol Clin North Am. 1996;29:393–405. [PubMed] [Google Scholar]
- 7.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol. 2003;4:139–147. doi: 10.1007/s10162-002-3025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope. 2002;112:1627–1634. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Truong T, Billings PB, Harris JP, Keithley EM. Blockage of immune-mediated inner ear damage by etanercept. Otol Neurotol. 2003;24:52–57. doi: 10.1097/00129492-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Matteson EL, Choi HK, Poe DS, Wise C, Lowe VJ, McDonald TJ, Rahman MU. Etanercept therapy for immune-mediated cochleovestibular disorders: a multi-center, open-label, pilot study. Arthritis Rheum. 2005;53:337–342. doi: 10.1002/art.21179. [DOI] [PubMed] [Google Scholar]
- 11.Van Wijk F, Staecker H, Keithley E, Lefebvre PP. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neurootol. 2006;11:357–365. doi: 10.1159/000095897. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MU, Poe DS, Choi HK. Etanercept therapy for immune-mediated cochleovestibular disorders: preliminary results in a pilot study. Otol Neurotol. 2001;22:619–624. doi: 10.1097/00129492-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 14.Thalmann R, Miyoshi T, Thalmann I. The influence of ischemia upon the energy reserves of inner ear tissues. Laryngoscope. 1972;82:2249–2272. doi: 10.1288/00005537-197212000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Scherer EQ, Lidington D, Oestreicher E, Arnold W, Pohl U, Bolz SS. Sphingosine-1- phosphate modulates spiral modiolar artery tone: A potential role in vascular-based inner ear pathologies? Cardiovasc Res. 2006;70:79–87. doi: 10.1016/j.cardiores.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 17.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- 18.Canis M, Arpornchayanon W, Messmer C, Suckfuell M, Olzowy B, Strieth S. An animal model for the analysis of cochlear blood flood disturbance and hearing threshold in vivo. Eur Arch Otorhinolaryngol. 2010;267:197–203. doi: 10.1007/s00405-009-1036-2. [DOI] [PubMed] [Google Scholar]
- 19.Wangemann P, Liu J. Osmotic water permeability of capillaries from the isolated spiral ligament: new in-vitro techniques for the study of vascular permeability and diameter. Hear Res. 1996;95:49–56. doi: 10.1016/0378-5955(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 20.Sadanaga M, Liu J, Wangemann P. Endothelin-A receptors mediate vasoconstriction of capillaries in the spiral ligament. Hear Res. 1997;112:106–114. doi: 10.1016/s0378-5955(97)00121-4. [DOI] [PubMed] [Google Scholar]
- 21.Wangemann P, Gruber DD. The isolated in vitro perfused spiral modiolar artery: pressure dependence of vasoconstriction. Hear Res. 1998;115:113–118. doi: 10.1016/s0378-5955(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 22.Lidington D, Peter BF, Meissner A, Kroetsch JT, Pitson SM, Pohl U, Bolz SS. The phosphorylation motif at serine 225 governs the localization and function of sphingosine kinase 1 in resistance arteries. Arterioscler Thromb Vasc Biol. 2009;29:1916–1922. doi: 10.1161/ATVBAHA.109.194803. [DOI] [PubMed] [Google Scholar]
- 23.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine Kinase Modulates Microvascular Tone and Myogenic Responses Through Activation of RhoA/Rho Kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 25.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D'Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 26.Iversen PO, Nicolaysen A, Kvernebo K, Benestad HB, Nicolaysen G. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflugers Arch. 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- 27.Vecchione C, Frati A, Di PA, Cifelli G, Carnevale D, Gentile MT, Carangi R, Landolfi A, Carullo P, Bettarini U, Antenucci G, Mascio G, Busceti CL, Notte A, Maffei A, Cantore GP, Lembo G. Tumor necrosis factor-alpha mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension. 2009;54:150–156. doi: 10.1161/HYPERTENSIONAHA.108.128124. [DOI] [PubMed] [Google Scholar]
- 28.Dai M, Nuttall A, Yang Y, Shi X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear Res. 2009;254:100–107. doi: 10.1016/j.heares.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Li QF, Wu CT, Duan HF, Sun HY, Wang H, Lu ZZ, Zhang QW, Liu HJ, Wang LS. Activation of sphingosine kinase mediates suppressive effect of interleukin-6 on human multiple myeloma cell apoptosis. Br J Haematol. 2007;138:632–639. doi: 10.1111/j.1365-2141.2007.06711.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawai S, Sekino H, Yamashita N, Tsuchiwata S, Liu H, Korth-Bradley JM. The comparability of etanercept pharmacokinetics in healthy Japanese and American subjects. J Clin Pharmacol. 2006;46:418–423. doi: 10.1177/0091270006286435. [DOI] [PubMed] [Google Scholar]
- 32.Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear Res. 2009;255:22–32. doi: 10.1016/j.heares.2009.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.