Abstract
Red Blood Cell (RBC) transfusion is indicated to improve oxygen delivery to tissue, and for no other purpose. We have come to appreciate that donor RBCs are fundamentally altered during processing and storage, in a fashion that both impairs oxygen transport efficacy and introduces additional risk by perturbing both immune and coagulation systems. The protean biophysical and physiologic changes in RBC function arising from storage are termed the ‘storage lesion’; many have been understood for some time; for example, we know that the oxygen affinity of stored blood rises during the storage period1 and that intracellular allosteric regulators, notably 2,3-bisphosphoglyceric acid (DPG) and ATP, are depleted during storage. Our appreciation of other storage lesion features has emerged with improved understanding of coagulation, immune and vascular signaling systems. Herein we review key features of the ‘storage lesion’. Additionally, we call particular attention to the newly appreciated role of RBCs in regulating linkage between regional blood flow and regional O2 consumption by regulating the bioavailability of key vasoactive mediators in plasma, as well as discuss how processing and storage disturbs this key signaling function and impairs transfusion efficacy.
Goal of Red Blood Cell (RBC) Transfusion
Red Blood Cell (RBC) transfusion is indicated to improve oxygen delivery to tissue, and for no other purpose. We have come to appreciate that donor RBCs are fundamentally altered during processing and storage, in a fashion that both impairs oxygen transport efficacy and introduces additional risk by perturbing both immune and coagulation systems. The protean biophysical and physiologic changes in RBC function arising from storage are termed the ‘storage lesion’; many have been understood for some time; for example, we know that the oxygen affinity of stored blood rises during the storage period1 and that intracellular allosteric regulators, notably 2,3-bisphosphoglyceric acid (DPG) and ATP, are depleted during storage. Our appreciation of other storage lesion features has emerged with improved understanding of coagulation, immune and vascular signaling systems. In this review, we will call particular attention to the newly appreciated role of RBCs in regulating regional blood flow (and thus O2 delivery) as well as discuss how disturbance of this key signaling function (by processing and storage for transfusion) impairs transfusion efficacy (improving O2 delivery).
Alterations to RBCs during Processing and Storage (‘the Storage Lesion’)
Many recent reports summarize the changes that occur with RBC storage2-7 (FIGURE 1). These reports document increased potassium, lactate, and free hemoglobin with increased RBC storage time8, 9. Additionally, RBCs loose deformability with increased duration of storage8, limiting passage through the microcirculation, which is further impaired by increased RBC aggregation and adhesion to endothelium8, 10-12. As noted above, the concentration of 2,3 DPG decreases with storage time8, the resultant increase in oxygen affinity limits oxygen unloading from hemoglobin during systemic perfusion13. RBC storage also impacts recipient immune function. Stored RBCs induce alterations in multiple cytokines after incubation with plasma or whole blood samples, including increased IL-6, IL-8, phospholipase A2, and superoxide anions, and decreased TNF-alpha concentrations9, 14. The clinical consequences of this phenomena was first reported in the 1970's when Opelz et al. reported improved renal allograft success in patients transfused pre-renal transplant15. Subsequently, additional reports indicating an immune suppressive effect of RBC transfusions have documented an association with increased cancer reoccurrence, live births in women with a history of spontaneous abortions, and increased postoperative infection rates16, 17.
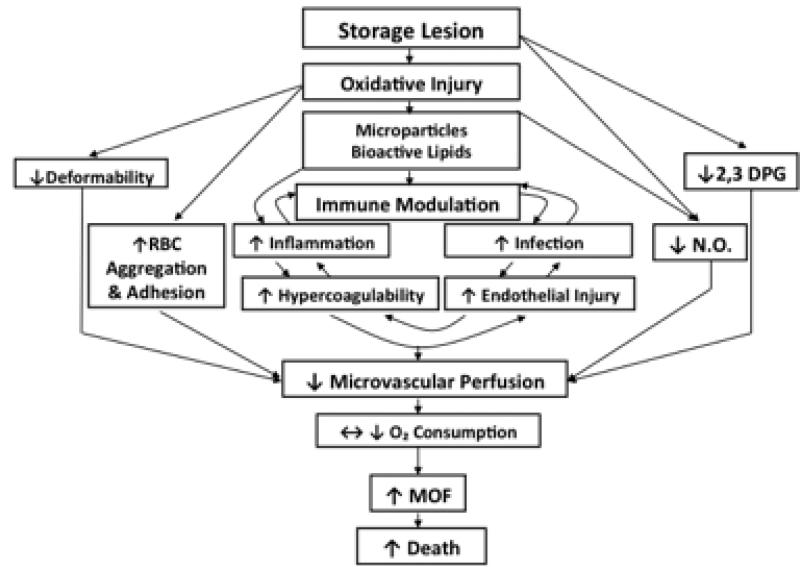
FIGURE 1.
Interaction between donor RBCs and transfusion recipients as a consequence of receiving RBCs altered by processing and storage2. The figure illustrates that the storage lesion impacts overlapping pathways of oxygen delivery, RBC rheology and physiology, as well as immune modulation. MOF = multiple organ failure; NO = nitric oxide.
Of note, the genesis of RBC microparticles during RBC storage may be related to the influence of transfusion upon donor immune and coagulation systems18, 19. The oxidative injury that occurs with storage leads to formation of RBC membrane microparticles and release of bioactive lipids from its membrane20. Such RBC microvesicles contain CD47 antigens associated with macrophage inhibition20. Other data suggests that increased generation of procoagulant phospholipids occurs during RBC storage21, 22. These bioactive lipids, such as lysophosphatidylcholine, have also been demonstrated to be pro-inflammatory and are associated with an increased risk of acute lung injury23. The immune dysfunction secondary to transfusion has been termed Transfusion Related Immune Modulation (TRIM) and has been well described24, 25. Interestingly, the transfusion of RBCs of increased storage duration has also been associated with Transfusion Related Acute Lung Injury (TRALI) via pro-inflammatory mechanisms17.
RBC Transfusion Efficacy as a function of RBC Storage Duration
While transfusion of RBCs increases the hematocrit (Hct) and therefore increases oxygen (O2) content, it does not necessarily follow that O2 delivery to tissue is likewise increased. The purpose of transfusion is not to merely improve O2 content, but instead – to improve O2 delivery. As outlined above, fundamental changes occur to RBCs during storage that alter their biological function and their ability to deliver O27. Beyond their role as O2 carriers, RBCs play a fundamental role in the O2 dependent regulation of vascular tone by exporting vasodilatory signals that regulate regional blood flow. This key RBC regulatory function is modified by processing and storage, resulting in compromised RBC-dependent vasoregulation, which impairs regional O2 delivery8, 26.
In general, animal models evaluating transfusion efficacy demonstrate adverse effects of storage upon O2 delivery27-29. For example, in an animal shock model, human RBCs of decreased storage age improved O2 consumption whereas older RBCs could not29. In humans, several groups have measured O2 delivery and consumption before and after RBC transfusion in critically ill patients and have reported a disconcerting lack of benefit directly attributable to RBC transfusion30-39. Though it is difficult to ascertain the RBC transfusion efficacy and there are many difficulties with measures of global or tissue-specific cellular respiration, available evidence suggests that transfusion of stored RBCs may have adverse effects on microcirculatory flow and oxygen utilization.
Most storage lesion effects appear to worsen with increased storage duration. When older RBCs (21-42 days) have been compared to fresh RBCs (0-4 days of storage): inflammatory response, mediators of oxidative injury, and risk of hyper-coagulation appear increased8, 14, 21, 22, 40, 41.
RBCs stored for a longer time also appear more likely to activate recipient neutrophils that have been primed by recipient disease (such as sepsis or multiple trauma)23, a mechanism posited for transfusion related acute lung injury (TRALI)17, 42. In a study of critically ill adults, transfusion of RBCs stored for > 14 days was associated with a decrease in tissue oxygenation, whereas transfusion of RBCs stored < 14 days did not change tissue O2 saturation43. In a study of rats in shock, human RBCs stored for 3 days increased O2 consumption whereas human RBCs stored for 28 days did not29. RBCs of increased storage age have also been associated with decreased perfusion and O2 consumption in critically ill adults4. These results were not confirmed in a similar study involving 22 patients which did not reveal any improvement in perfusion or oxygen consumption with RBCs of increased or decreased age potentially because it was not adequately powered to detect a difference in O2 consumption44. Conflicting results have also been reported for patients with traumatic brain injury, where one study reports no improvement in the partial pressure of O2 in brain tissue with RBCs > 19 days of storage and another found no relationship between RBC age and cerebral oxygenation45, 46. Other small studies have not measured a decrease in O2 consumption or tissue saturations with the transfusion of RBC stored for longer times46. Of note, evaluation of blood product efficacy and safety is best informed by distinctly separating analysis of outcome in critically ill and non–critically ill populations, since the risk-benefit ratio for blood product administration is dependent on the baseline physiologic state and differs between critically ill and non–critically ill patients47. Specifically, patients with marginally compensated organ failure are less tolerant of additional insult, including adverse effects attributable to transfusion17.
RBC Vasoactivity: Relevance to O2 Delivery and Transfusion Efficacy
Oxygen delivery is a function of blood O2 content and flow, but the latter (flow) is the far more important determinant. Specifically, the ability to increase O2 content is somewhat limited (varying linearly with Hb concentration and % O2 saturation), whereas regional blood flow (a function of vessel radius to the 4th power) may be increased or decreased by several orders of magnitude. In fact, under most circumstances, it is principally the volume and distribution of blood flow that vary to maintain dynamic coupling between O2 delivery and metabolic demand. Of note, classic experiments demonstrate that it is Hb O2 saturation (rather than free plasma or tissue PO2) that is coupled to blood flow to maintain this physiology in vivo48, 49. These and other studies provide evidence that RBCs act as both O2 sensors and transducers of vasoconstrictor and vasodilator responses50-53. RBCs have been demonstrated to recapitulate this response in vitro, actuating graded vasodilation across the physiological O2 gradient52. As such, remarkably, RBCs dilate blood vessels in concert with, and proportional to, O2 delivery54, 55. That this coupling is directly linked to Hb O2 saturation rather than to PO2, indicates that, in addition to service as an O2 transport protein, Hb is a key physiological effector in resolution of tissue hypoxia49, 56-59.
Control of NO transport and bioavailability is fundamental to RBC-based blood flow autoregulation and acts by effecting reflex hypoxic vasodilation (HVD) in the circulation. It should be noted, however, that endothelium-derived NO does not directly mediate the HVD that underlies blood flow regulation51, 60. In fact, because of substrate (O2) limitation, NO production by eNOS is most likely attenuated by hypoxia61-63. Morover, NOS inhibitors do not block the acute change in blood flow that is coupled to Hb desaturation48. Rather, it appears that NO is transported by RBCs to effect HVD at a time and place remote from the original site of NO synthesis (the NO groups deployed by RBCs do, however, originally arise from eNOS54 and perhaps, other NOS isoforms64 or nitrite65). RBCs are therefore vascular control elements (FIGURE 2). Thus, it is increasingly appreciated that the transition in Hb conformation that occurs in the course of O2 loading and delivery during arterio-venous (A-V) transit also governs transactions between RBCs and circulating NO groups in plasma: oxygenated (R-state) Hb sequesters, and deoxygenated (T-state) Hb releases, net NO bioactivity. As such, Hb R- to T-state cycling drives processing of circulating NO groups (through RBCs) to plasma or cellular thiols, to form vasoactive NO adducts known as S-nitrosothiols (SNOs). This system provides functional coupling between regional blood flow and biochemical cues of perfusion insufficiency, which may include hypoxia, hypercarbia and acidosis (FIGURE 3). As described below, the system is vulnerable to disruption during periods of oxidative stress and sustained alterations in PO2.
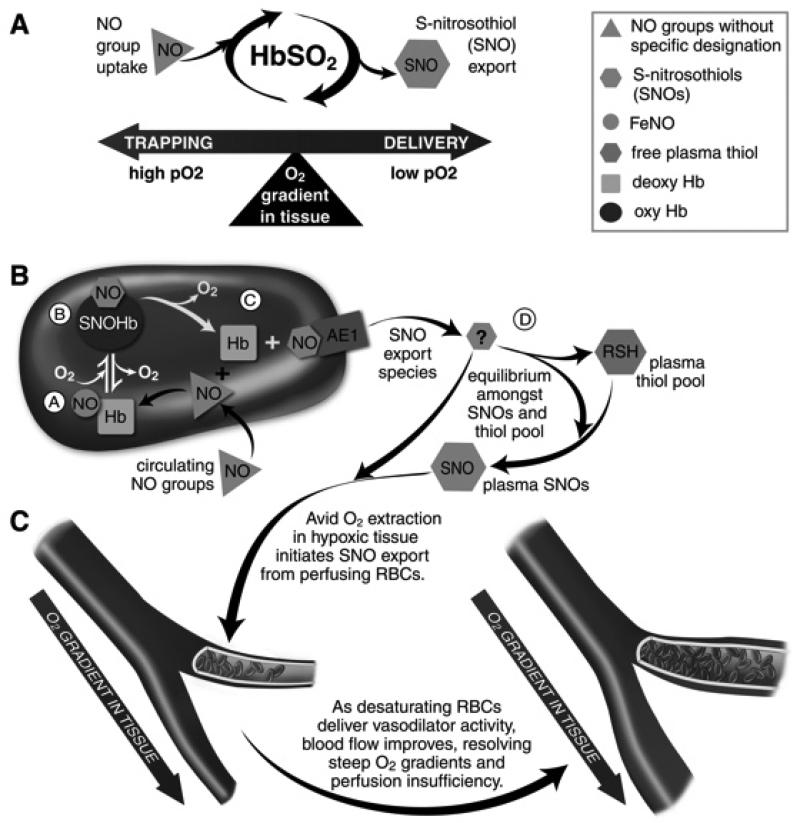
FIGURE 2.
RBCs transduce regional O2 gradients in tissue to control NO bioactivity in plasma by trapping or delivering NO groups as a function of Hb O2 saturation. A) In this fashion, circulating NO groups are processed by Hb into the highly vasoactive (thiol-based) NO congener, S-nitrosothiol (SNO). By exporting SNOs as a function of Hb deoxygenation, RBCs precisely dispense vasodilator bioactivity in direct proportion to regional blood flow lack. B) O2 delivery homeostasis requires biochemical coupling of vessel tone to environmental cues that matches perfusion sufficiency to metabolic demand. Because oxy- and deoxy-Hb process NO differently (see text), allosteric transitions in Hb conformation afford context-responsive (O2 - coupled) control of NO bioavailability, thereby linking the sensor and effector arms of this system. Specifically, Hb conformation governs the equilibria among deoxyHbFeNO (A; NO sink), oxySNOHb (B; NO store), and acceptor thiols including the membrane protein SNO-AE1 (C; bioactive NO source). Direct SNO export from RBCs or S-transnitrosylation from RBCs to plasma thiols (D) yields vasoactive SNOs, which influence resistance vessel caliber and close this signaling loop. Thus, RBCs either trap (A) or export (D) NO groups to optimize blood flow. C) NO processing in RBCs (panels A and B) couples vessel tone to tissue PO2; this system subserves hypoxic vasodilation in the arterial periphery and thereby calibrates blood flow to regional tissue hypoxia.
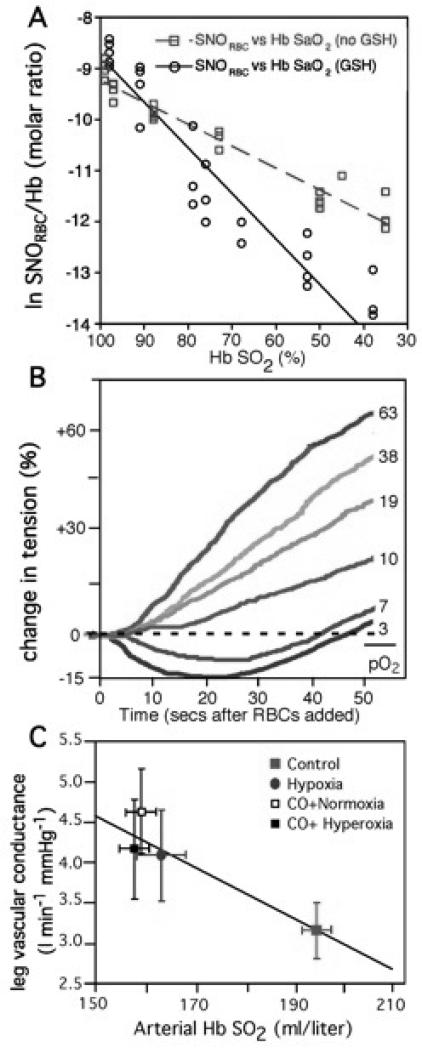
FIGURE 3.
Hb O2 saturation (Hb SO2%) exerts coordinated governance of RBC SNO content, RBC vasoactivity and human peripheral blood flow. A) Human blood gas measurements of SNO and O2. RBCs with (black) or without (red) added extracellular glutathione (GSH) were deoxygenated under inert gas50. The natural logarithm of SNO content in RBCs (SNORBC) is linearly related to Hb O2 saturation. GSH accelerated SNORBC decay, consistent with O2 -linked export of NO groups to extra-erythrocytic thiols. B) RBCs induce graded relaxation of systemic arteries (aortic ring bioassay) that is inversely related to Hb O2 saturation across the physiological range, recapitulating hypoxic vasodilation52. Hb O2 saturations spanned from red (oxy, > 90% Hb SO2) to blue (deoxy, < 40% Hb SO2). C) Leg vascular conductance increases as blood O2 content falls (hypoxic vasodilation)51. Hb O2 saturation and thus arterial blood O2 content were manipulated by CO exposure ± varying FiO2 (fraction of inspired O2; hypoxia versus hyperoxia). Neither vascular conductance nor blood flow correlated with blood PO2 per se.
Evidence for RBC Vasoactivity
Multiple lines of evidence indicate that NO is transported by RBCs to distal tissues. This RBC paracrine function is governed by O2-linked transitions in Hb conformation, and Hb O2 saturation thereby couples export of vasodilatory signals to tissue PO2. Importantly, the only known endogenous NO compounds that retain bioactivity in the presence of Hb are S-nitrosothiols (SNOs). The data corroborating this biology is extensive. For example, Stamler et al.52, 55 and Datta et al.66 demonstrated that RBCs dilate pre-constricted aortic rings at low PO2 (1% O2) but constrict at high PO2 (95% O2). More broadly, vasoconstriction and vasodilation by RBCs is graded across the physiological range of PO2 encountered in the microcirculation52. In addition, Kubes’ group67-69 has demonstrated bioactivity of inhaled NO in the mesenteric circulation that is commensurate with SNO production, and Cannon et al.70 observed that NO gas inhalation lowers forearm vascular resistance in human volunteers who had undergone pharmacological NOS inhibition. McMahon and colleagues71 identified inhaled NO-based activity, at least in part, with SNO-Hb. Moreover, Reynolds and colleagues72 and Diesen and colleagues73 have shown that NO added to RBCs potentiates vasodilatory activity to the extent that SNO-Hb is generated. Sonveaux et al.74 have reported that the lifetime of infused SNO-Hb is increased by high PO2 and destabilized at low PO2, in direct contrast to NO itself. Additional studies support the conclusion that SNOs are impervious to inactivation by RBCs73, 75. It therefore appears that NO bioactivity is preserved by RBCs in the form of stable SNOs that can be ‘exported’ to peripheral tissues on demand. Notably, hypoxic vasodilation by RBCs is entirely independent of endothelium54, 73, 76-78 and is facilitated by the presence of extra-erythrocytic low-molecular-weight thiols.
Expanding the NO Paradigm: RBCs Control NO Bioactivity in Blood
Originally, the “classic” NO paradigm was based on several tenets: 1) NO• is the primary bioactive product of NOS; 2) NO life-time and fate is explained by the diffusion of NO• “gas” in solution and by its terminal reactions with Hb (and superoxide); and 3) the heme center of guanyl cyclase is the primary NO• receptor. More recent evidence suggests that NO-based signaling is significantly more complex. In particular, the landscape has been fundamentally altered by appreciation that NO acts principally through covalent binding to cysteine thiols to form S-nitrosothiols (SNOs), rather than by signaling exclusively via sGC. Approximately one thousand SNO-proteins have now been identified in cells and tissues, indicating the central role of SNOs in NO-based signaling 79-81.
It is now understood that through context-responsive reactions between NO and Hb (that is, reactions coupled to PO2 and influenced by CO2 and pH), RBCs exert functional control over NO group bioavailability in blood, and thereby perform crucial roles as both sensors of O2 supply and effectors in vascular signaling at local, organ, and system levels 54-56, 82. Thus, RBCs shuttle 3 gases in the respiratory cycle (NO/O2/CO2) to control delivery of O2. Both the complexity of the biological chemistry of NO and the specificity of NO-group reactions in signaling are well exemplified in the case of Hb. Through tightly regulated reactions at its heme center and a reactive cysteine thiol (βcys93), in the course of circulatory transit, Hb captures (binds NO at β-hemes), activates (converts β-heme-NO into βcys93-SNO) and deploys NO groups (transnitrosylates receptor thiols, see details below). Heme-redox coupled activation of the NO group, a requirement for the oxidative chemistry that subserves conversion of heme-NO into βcys93-SNO, is governed by O2-coupled allosteric transitions in Hb54, 82-84. The O2-linked change in Hb conformation also initiates export of SNO from RBCs by promoting NO transfer to receptor thiols, including those associated with the erythrocytic membrane protein AE-1 (Band 3)85 and thence to extra-erythrocytic thiols50, 75 to form plasma or other cellular SNOs, which are vasoactive at concentrations as low as 1 to 5 nM54, 55. By governing the bioavailability of NO in the microcirculation through regulated sequestration, transport and delivery of NO groups, RBCs maintain appropriate coupling between regional blood flow and both O2 availability in the lung and O2 need in the periphery.
S-nitrosylation (SNO synthesis) in RBCs
The chemical basis of S-nitrosylation reactions and the molecular determinants of targeting specificity are reviewed in detail elsewhere56, 80, 86. In brief, hemoglobin processes NO•, low-molecular-weight SNO or nitrite (NO -2) into S-nitroso-Hb (SNO-Hb), and thus has been analogized to a SNO reactor87-92. As described below, the locus of NO binding to the Hb globin chain is the highly conserved βcys93, which conforms to both acid-base and hydrophobic motifs for S-nitrosylation87, 89, 92-95. Importantly, as a result of conformation-dependent variation in the βcys93 microenvironment, the likelihood of NO ligation to βcys93 thiol is increased in R-structure (oxygenated Hb) and diminished in T-structure (deoxygenated Hb)54, 55, 82. Therefore, S-nitrosylation is favored upon Hb oxygenation, whereas NO group (NO/NO+) release is promoted upon Hb deoxygenation89. By contrast, NO binding to heme (at physiological ratios of NO/Hb) is favored in T state and disfavored in R. Subsequent binding of O2 to heme as RBCs transit the lung, which promotes Hb R structure thus facilitates NO group transfer from heme to thiol (auto-S-nitrosylation).
Control of NO Entry into RBCs
Although free Hb readily inactivates NO•, Hb packaging in RBCs limits NO• consumption by a factor of ~1,00096-99. Specifically, four successive barriers separate eNOS-generated NO• from intra-erythrocytic Hb: 1) the endothelial cell membrane, 2) an abluminal RBC-free zone, created by velocity gradients at the vascular edge of the migrating RBC column99-101, 3) an unstirred layer of plasma surrounding moving RBCs102, and 4) the RBC membrane and cytoskeleton – which together provide a dynamically responsive diffusion barrier to NO• 97, 103, 104. It is generally accepted that, due to its solubility properties, the neutral and (relatively) nonpolar NO• radical freely permeates membranes. However, it has been recently reported that dynamic chemical and physical variation of the RBC membrane alters, and in fact may control, the rate of NO• uptake by RBCs97, 104. This variation appears to arise from conformation- dependent interactions between Hb and key RBC membrane proteins that modulate NO• uptake (FIGURE 4), rather than from an O2-linked change in the physical nature of the lipid bilayer itself.
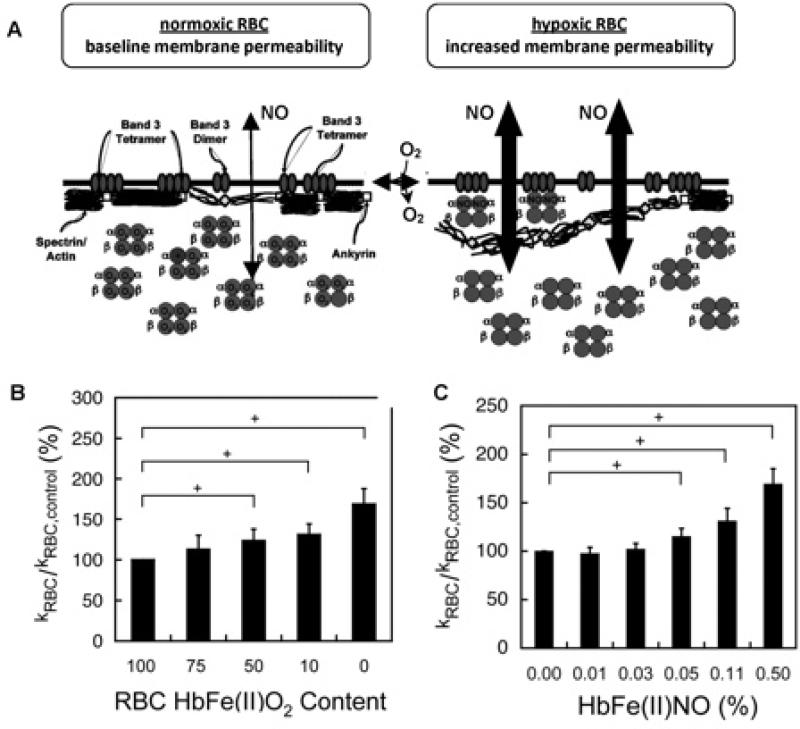
FIGURE 4.
Conformation-specific binding between Hb and the RBC membrane protein AE-1 affords O2 responsive control of NO trapping103. A) Under oxygenated conditions (normoxic RBC, at left), the RBC membrane constitutes a significant barrier to NO• entry via tight association between the sub-membrane cytoskeleton and the cytoplasmic domain of the Band 3 (AE-1) membrane protein (ankyrin successfully competes with oxyHb for binding to AE-1). Upon RBC deoxygenation (hypoxic RBC), oxyHb R-state is converted to deoxyHb T-state, which now successfully competes with ankyrin for the AE-1 cytoplasmic domain (affinity for AE-1 ranks as follows: deoxyHb > ankryn > oxyHb). Proximal apposition of heme to the membrane and diminished cytoskeleton-membrane interaction allows increased NO entry and affords intraerythrocytic Hb greater access to extraerythrocytic NO. Moreover, if deoxyHb encounters high concentrations of NO•, “super-T” αFe(II)NOHb may form (in which NO bound to the α subunit disrupts normal heme-globin linkage, locking Hb in the deoxy or T conformation; see text for details). B) Increasing the proportion of intra-erythrocytic T-state Hb (by forming either deoxyHb or αFe(II)NO, both of which are T-state tetramers and bind avidly to AE-1) increases NO consumption by intact RBCs as measured by a competition assay. In this plot, increased consumption in treated vs control RBCs appears as an increased KRBC/KRBCcontrol ratio (y-axis). C) Increased NO uptake by NOpretreated hypoxic RBCs correlates with the formation of αFe(II)NO (i.e. “super-T” Hb).
NO Group Processing in RBCs
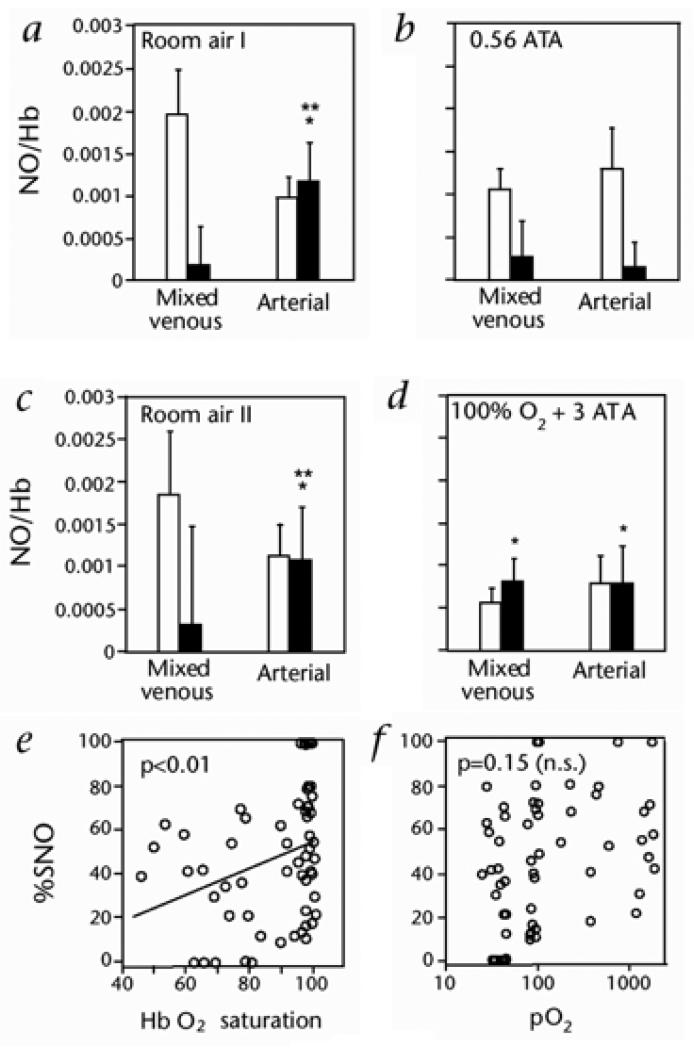
As described above, after formation of HbFeNO, the O2-linked transition from T- to R- structure promotes intra-molecular transfer of the NO group from β-heme to β-thiol (Cysβ93), thereby forming HbCysβ93SNO (SNO-Hb)65, 84. Once formed in R-state Hb, Cysβ93SNO is stable and protected from solvent through confinement in a hydrophobic pocket54, 55, 105-107. Additionally cycling of NO between Cysβ93 and heme in vivo is supported by measurements of FeNO and SNO as a function of PO2 (FIGURE 5)50, 52, 55.
FIGURE 5.
O2-dependent variation in SNO–Hb (■) and Hb[FeNO] (□) demonstrate the association between Hb conformation and intramolecular heme → thiol migration of NO groups in RBCs. A - D)52, Moles NO per mole Hb tetramer in arterial and mixed venous blood from humans breathing either 21% O2 at 1 ATA (absolute atmospheres) (A and D), 21% O2 at 0.56 ATA (equivalent to ~ 12% O2 at 1 ATA) (B) or 100% O2 at 3 ATA (D). Total Hb-bound NO equals the sum of the 2 bars for each condition. These data demonstrate O2 - dependent shuttling of Hb-bound NO groups between heme and Cys-ß93. (E and F), SNO content of blood Hb, presented as the fraction of Hb-NO (% SNO), correlates with Hb O2 saturation (E), but not with pO2 (F).
NO Group Delivery by RBCs
The described arterio-venous gradient of SNO-Hb50, 52, 54, 66, 108-110 is consistent with dynamic processing of NO by circulating RBCs, in which O2 loading in the lung is accompanied by Hb S-nitrosylation and NO-group export is facilitated by O2 delivery when steep O2 gradients are encountered in the periphery (hypoxic vasodilation)54. The R → T transition in Hb conformation that occurs with O2 delivery shifts Cysβ93 SNO from its sequestered hydrophobic niche to exposure in aqueous solvent54, 55, 106, making the NO group available for transnitrosylation of target protein or small-molecular-weight thiol54, 75, 85, 111. Importantly, NO is transferred in this reaction as a nitrosonium ion (NO+)54, 56 and is therefore protected from (Fe(II)) heme recapture and/or inactivation (as well as from pharmacological NO• chelators)112.
Hb deoxygenation (and/or heme oxidation) enables release of NO bioactivity by RBCs, which results in vasodilation 50, 54, 55, 66, 85, 113. Export of NO bioactivity from RBCs occurs, at least in part, via low-mass thiols in plasma50, 52, 73, 75 (FIGURE 6), although a role for direct cell-cell contact in the microcirculation may be envisaged. In either case, signaling is believed to proceed via a transnitrosylation cascade; the RBC membrane protein AE-1, with which Hb is known to undergo conformation-dependent docking114, 115, appears to initiate this cascade. SNO-Hb docking on AE-1 is followed by NO-group transfer from SNO-Cysβ93 to cysteine thiol(s) of the cytoplasmic domain of AE-1; moreover, this transfer is required for RBC-induced vasodilation85. The identity of the acceptor thiols that ferry the NO signal in vivo from AE-1 to the vascular wall is unknown.
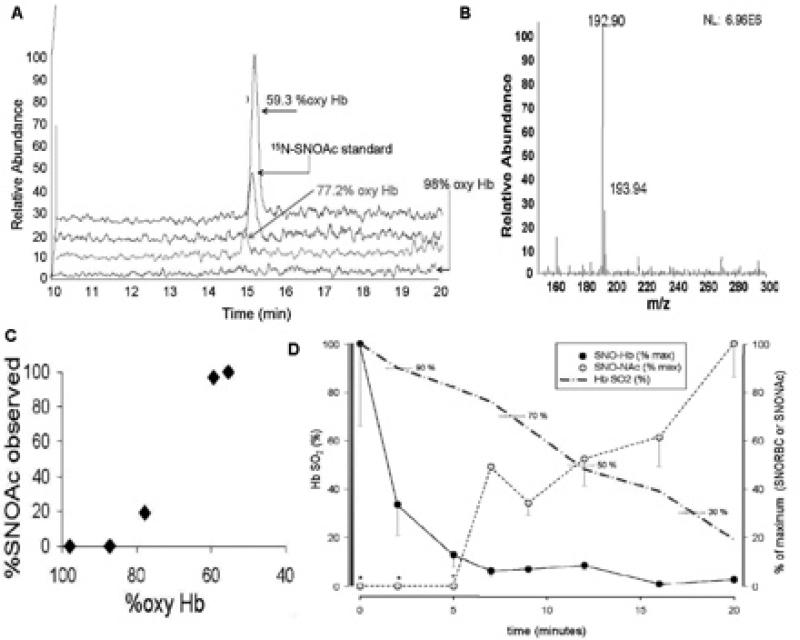
FIGURE 6.
PO2 - regulated export of NO groups from RBCs can occur via NO group transfer from SNO-Hb to an extra-erythrocytic thiol reactant75. Circulatory transit was simulated for human whole blood in a thin-film tonometer (under 5% CO2, balance N2, pH 7.4) after spiking the sample with the non-native, low-mass thiol, N-acetyl cysteine (NAC, 100 M in plasma). The concentration of S-nitroso-N-acetyl cysteine (SNOAC) that formed in plasma was measured by mass spectrometry, and confirmed by mass labeling with 15N. The conversion of extra-erythrocytic NAC to SNOAC correlated with RBC O2 and SNO content. Serum SNOAC formed as a function of Hb SO2. A) Liquid chromatogram demonstrating co-elution of RBC-generated SNOAC with the 15N-labeled SNOAC standard. B) Mass spectrum demonstrating paired signals from RBC-generated SNOAC and the 15N-labeled SNOAC standard (m/z 194). C) Extra-erythrocytic SNOAC concentration follows oxyHb desaturation (by co-oximetry). D) RBC SNO content (black) decreased in tandem with HbO2 desaturation (dashed blue). Note that, as SNO content in RBCs fell, extra-erythrocytic SNOAC (yellow symbols) accumulated. Note also that SNOAC levels were below the limits of detection when the HbO2 saturation was above 80%.
Vasodilation by SNO-Hb is readily detected at < 10–8 M, which suggests that the endogenous SNO-Hb pool (10–7 – 10–5.5 M) dispenses NO bioactivity in a tightly regulated fashion and with significant potency. By exploiting the Hb conformation-based NO delivery system, RBCs actuate graded dilation of blood vessels over rapid time scales - that differ greatly from the slow relaxation of native vascular smooth muscle under hypoxia (a response that occurs over minutes, and which may set background vascular tone). Thus, baseline tone appears to be regulated further by RBC-based HVD (acute changes, over seconds)51, 60, 66, 73. Hypercarbia and acidosis are additional effectors of the T-conformation in Hb, and thereby also promote vasodilation by RBCs116. Furthermore, oxidation of hemes can elicit a T-like conformation in SNO-Hb to promote NO release54, 113. Thus, ischemia and oxidative stress may provide multiple biochemical cues that can be employed by RBCs to correct perfusion insufficiency.
Dysregulated RBC Vasoactivity Arising from the Storage Lesion
Strong evidence is mounting in support of a causal relationship between acquired, NO-related RBC dysfunction and a host of morbidities that complicate critical illness117-124. Recently, it has been observed that levels of SNO-Hb are altered in several disease states characterized by disordered tissue oxygenation66, 71, 74, 77, 78, 125-127. In addition, where examined, RBCs from such patients exhibit impaired vasodilatory capacity50, 71, 78, 127. These data suggest that altered RBC-derived vasoactivity contributes to human pathophysiology. Specifically, alterations in NO metabolism by RBCs have been reported in congestive heart failure66, diabetes78, 126, pulmonary hypertension52, 71 and sickle cell disease127, 128, all of which are conditions characterized by inflammation, oxidative stress and dysfunctional vascular control.
RBCs are progressively altered by processing and storage for transfusion (referred to as the ‘‘storage lesion’’, as described above)7, and the interval between RBC donation and administration appears to be an independent risk factor for transfusion-associated morbidity and mortality3, 129-131. Altered O2 loading/unloading by stored RBCs is well documented132-134. It has been shown more recently that, in blood that has been collected, processed, and stored using blood-banking industry standard operating procedures, RBC SNO-Hb levels and RBC-dependent vasodilatory activity are profoundly depressed, and that these defects can be reversed by repletion of RBC SNO8, 72 (FIGURE 7). These observations provide mechanistic insight into clinical data that suggest impaired vasoregulation by administration of banked blood4, 132, 135-137.
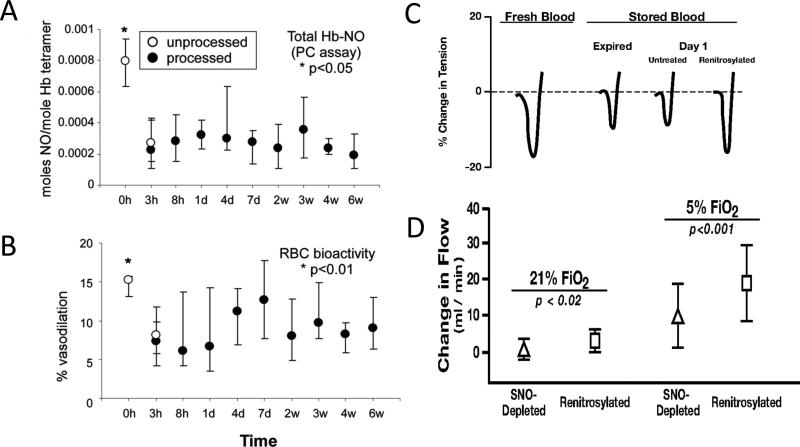
FIGURE 7.
Impact of processing and storage upon RBC NO content and vasoactivity (both in isolated vascular rings8 and in a whole-animal cardiac preparation72). In these projects, blood from healthy volunteers was leukofiltered, processed into standard additive solution and stored according to AABB standards. (A) RBC NO content was significantly depressed, (B) as was vasoactivity in a rabbit vascular ring preparation. (C) Representative tracings from a similar project demonstrate the degree of vasorelaxation as percent change in tension in rabbit aortic rings produced by fresh, stored (expired and day 1), or renitrosylated (day 1) RBCs. (D) Hypoxic vasodilation by stored and renitrosylated RBCs in vivo. Shown are changes in canine coronary artery bloodflow(mean ± SD; n7) produced by infusion of SNO-depleted or renitrosylated RBCs. Increases in flow elicited by renitrosylated RBCs were significantly greater than those produced by SNO-depleted RBCs, and the degree of change was greater under hypoxic (5% FiO2) than normoxic (21% FiO2) conditions.
Summary
Trapping, sequestration, processing and delivery of NO groups by RBCs, which are coupled to Hb allostery, links regional blood flow to biochemical cues of perfusion insufficiency (metabolic demand). The respiratory cycle may thus be broadly viewed as comprised of three gases, NO, O2 and CO2, which are coordinately or covalently bound to Hb. RBCs therefore actively regulate regional blood flow (and thus, O2 delivery) by coupling O2 sensing by Hb to the formation and release of vasodilator SNO, conveying a PO2-linked signal in the setting of perfusion lack. In addition to the multiple components of the ‘stroage lesaion’, which impact every aspect of RBC physiology, malfunction of this precise RBC vascular signaling system may undermine transfusion efficacy. Understanding of such should inform improvements in blood processing and storage, which are necessary to ameliorate the ‘storage lesion’.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Azarow K, Holcomb JB. Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit Care Med. 2007;35:2576–2581. doi: 10.1097/01.CCM.0000285996.65226.A9. [DOI] [PubMed] [Google Scholar]
- 3.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: When is it not safe? Crit Care Med. 2003;31:S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Sibbald WJ. Effect of stored blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. [PubMed] [Google Scholar]
- 5.Napolitano LM, Corwin HL. Efficacy of blood transfusion in the critically ill: Does age of blood make a difference? Crit Care Med. 2004;32:594–595. doi: 10.1097/01.CCM.0000110676.93531.05. [DOI] [PubMed] [Google Scholar]
- 6.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295–307. doi: 10.1378/chest.127.1.295. [DOI] [PubMed] [Google Scholar]
- 7.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 8.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored rbcs. Proceedings of the National Academy of Sciences. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karam O, Tucci M, Toledano BJ, Robitaille N, Cousineau J, Thibault L, Lacroix J, Le Deist F. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009;49:2326–2334. doi: 10.1111/j.1537-2995.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 10.Luk CS, Gray-Statchuk LA, Cepinkas G, Chin-Yee IH. Wbc reduction reduces storage-associated rbc adhesion to human vascular endothelial cells under conditions of continuous flow in vitro. Transfusion. 2003;43:151–156. doi: 10.1046/j.1537-2995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–281. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 12.Yedgar S, Hovav T, Barshtein G. Red blood cell intercellular interactions in oxidative stress states. Clin Hemorheol Microcirc. 1999;21:189–193. [PubMed] [Google Scholar]
- 13.Apstein CS, Dennis RC, Briggs L, Vogel WM, Frazer J, Valeri CR. Effect of erythrocyte storage and oxyhemoglobin affinity changes on cardiac function. Am J Physiol. 1985;248:H508–515. doi: 10.1152/ajpheart.1985.248.4.H508. [DOI] [PubMed] [Google Scholar]
- 14.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release il-8 and secretory pla2. Shock. 2000;13:29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 16.Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: A meta-analysis. J Trauma. 2003;54:908–914. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 17.Silliman CC, Moore EE, Johnson JL, Gonzalez RJ, Biffl WL. Transfusion of the injured patient: Proceed with caution. Shock. 2004;21:291–299. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: Role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Z, Cavaretta J, Qu L, Stolz DB, Triulzi D, Lee JS. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–621. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Rbc-derived vesicles during storage: Ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 21.Cardo LJ, Hmel P, Wilder D. Stored packed red blood cells contain a procoagulant phospholipid reducible by leukodepletion filters and washing. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2008;38:141–147. doi: 10.1016/j.transci.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney J, Kouttab N, Kurtis J. Stored red blood cell supernatant facilitates thrombin generation. Transfusion. 2009;49:1569–1579. doi: 10.1111/j.1537-2995.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- 23.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil nadph oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 24.Blajchman MA. Immunomodulation and blood transfusion. American journal of therapeutics. 2002;9:389–395. doi: 10.1097/00045391-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Blumberg N. Deleterious clinical effects of transfusion immunomodulation: Proven beyond a reasonable doubt. Transfusion. 2005;45:33S–39S. doi: 10.1111/j.1537-2995.2005.00529.x. discussion 39S-40S. [DOI] [PubMed] [Google Scholar]
- 26.Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, de Korte D, Ince C. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med. 2005;33:39–45. doi: 10.1097/01.ccm.0000150655.75519.02. discussion 238-239. [DOI] [PubMed] [Google Scholar]
- 27.d'Almeida MS, Gray D, Martin C, Ellis CG, Chin-Yee IH. Effect of prophylactic transfusion of stored rbcs on oxygen reserve in response to acute isovolemic hemorrhage in a rodent model. Transfusion. 2001;41:950–956. doi: 10.1046/j.1537-2995.2001.41070950.x. [DOI] [PubMed] [Google Scholar]
- 28.van Bommel J, de Korte D, Lind A, Siegemund M, Trouwborst A, Verhoeven AJ, Ince C, Henny CP. The effect of the transfusion of stored rbcs on intestinal microvascular oxygenation in the rat. Transfusion. 2001;41:1515–1523. doi: 10.1046/j.1537-2995.2001.41121515.x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald RD, Martin CM, Dietz GE, Doig GS, Potter RF, Sibbald WJ. Transfusing red blood cells stored in citrate phosphate dextrose adenine-1 for 28 days fails to improve tissue oxygenation in rats. Crit Care Med. 1997;25:726–732. doi: 10.1097/00003246-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Shah DM, Gottlieb ME, Rahm RL, Stratton HH, Barie PS, Paloski WH, Newell JC. Failure of red blood cell transfusion to increase oxygen transport or mixed venous po2 in injured patients. J Trauma. 1982;22:741–746. doi: 10.1097/00005373-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Steffes CP, Bender JS, Levison MA. Blood transfusion and oxygen consumption in surgical sepsis. Crit Care Med. 1991;19:512–517. doi: 10.1097/00003246-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Conrad SA, Dietrich KA, Hebert CA, Romero MD. Effect of red cell transfusion on oxygen consumption following fluid resuscitation in septic shock. Circ Shock. 1990;31:419–429. [PubMed] [Google Scholar]
- 33.Dietrich KA, Conrad SA, Hebert CA, Levy GL, Romero MD. Cardiovascular and metabolic response to red blood cell transfusion in critically ill volume-resuscitated nonsurgical patients. Crit Care Med. 1990;18:940–944. doi: 10.1097/00003246-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert EM, Haupt MT, Mandanas RY, Huaringa AJ, Carlson RW. The effect of fluid loading, blood transfusion, and catecholamine infusion on oxygen delivery and consumption in patients with sepsis. The American review of respiratory disease. 1986;134:873–878. doi: 10.1164/arrd.1986.134.5.873. [DOI] [PubMed] [Google Scholar]
- 35.Lorente JA, Landin L, De Pablo R, Renes E, Rodriguez-Diaz R, Liste D. Effects of blood transfusion on oxygen transport variables in severe sepsis. Crit Care Med. 1993;21:1312–1318. doi: 10.1097/00003246-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–2707. [PubMed] [Google Scholar]
- 37.Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. Svo2 collaborative group. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- 38.Mink RB, Pollack MM. Effect of blood transfusion on oxygen consumption in pediatric septic shock. Crit Care Med. 1990;18:1087–1091. doi: 10.1097/00003246-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 40.Biffl WL, Moore EE, Offner PJ, Ciesla DJ, Gonzalez RJ, Silliman CC. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: Abrogation by poststorage washing but not prestorage leukoreduction. J Trauma. 2001;50:426–431. doi: 10.1097/00005373-200103000-00005. discussion 432. [DOI] [PubMed] [Google Scholar]
- 41.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2008;38:117–125. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 43.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 44.Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, McClelland DB. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 45.Leal-Noval SR, Munoz-Gomez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Current opinion in critical care. 2008;14:156–162. doi: 10.1097/MCC.0b013e3282f57577. [DOI] [PubMed] [Google Scholar]
- 46.Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, Le Roux PD. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–1108. doi: 10.1097/01.ccm.0000162685.60609.49. [DOI] [PubMed] [Google Scholar]
- 47.Spinella PC, Doctor A, Blumberg N, Holcomb JB. Does the storage duration of blood products affect outcomes in critically ill patients? Transfusion. 2011;51:1644–1650. doi: 10.1111/j.1537-2995.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 48.Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with n g -nitro-l-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. AJP - Legacy. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 50.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Hemoglobin conformation couples erythrocyte s-nitrosothiol content to o2 gradients. Proceedings of the National Academy of Sciences. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. The Journal of Physiology Online. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat.Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc.Natl.Acad.Sci.U.S.A. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 55.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by s-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 56.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: Role of nitric oxide and s-nitrosohemoglobin. Annual Review of Physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 57.Singel DJ, Stamler JS. Blood traffic control. Nature. 2004;430:297–297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 58.Duling BR, Berne RM. Oxygen and the local regulation of blood flow; possible significance of longitudinal gradients in arterial blood oxygen tension. Circulation Research. 1971;28(Suppl 1):65–69. [PubMed] [Google Scholar]
- 59.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circulation Research. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: Role of erythrocyte count and oxygenation state of haemoglobin. The Journal of Physiology. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kantrow SP, Huang YC, Whorton AR, Grayck EN, Knight JM, Millington DS, Piantadosi CA. Hypoxia inhibits nitric oxide synthesis in isolated rabbit lung. AJP - Lung Cellular and Molecular Physiology. 1997;272:L1167–L1173. doi: 10.1152/ajplung.1997.272.6.L1167. [DOI] [PubMed] [Google Scholar]
- 62.Leone AM, Palmer RM, Knowles RG, Francis PL, Ashton DS, Moncada S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. Journal of Biological Chemistry. 1991;266:23790–23795. [PubMed] [Google Scholar]
- 63.Rengasamy A, Johns RA. Determination of km for oxygen of nitric oxide synthase isoforms. Journal of Pharmacology and Experimental Therapeutics. 1996;276:30–33. [PubMed] [Google Scholar]
- 64.Mamone G, Sannolo N, Malorni A, Ferranti P. In vitro formation of s-nitrosohemoglobin in red cells by inducible nitric oxide synthase. FEBS Lett. 1999;462:241–245. doi: 10.1016/s0014-5793(99)01527-6. [DOI] [PubMed] [Google Scholar]
- 65.Angelo M, Singel DJ, Stamler JS. An s-nitrosothiol (sno) synthase function of hemoglobin that utilizes nitrite as a substrate. Proceedings of the National Academy of Sciences. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Datta B, Tufnell-Barrett T, Bleasdale RA, Jones CJ, Beeton I, Paul V, Frenneaux M, James P. Red blood cell nitric oxide as an endocrine vasoregulator: A potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 67.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled no as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. Journal of Clinical Investigation. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox-Robichaud A, Payne D, Kubes P. Inhaled no reaches distal vasculatures to inhibit endothelium- but not leukocyte-dependent cell adhesion. AJP - Lung Cellular and Molecular Physiology. 1999;277:L1224–L1231. doi: 10.1152/ajplung.1999.277.6.L1224. [DOI] [PubMed] [Google Scholar]
- 69.Kubes P, Payne D, Grisham MB, Jourd'heuil D, Fox-Robichaud A. Inhaled no impacts vascular but not extravascular compartments in postischemic peripheral organs. American Journal of Physiology. 1999;277:H676–H682. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 70.Cannon RO, III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin.Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, Califf RM, Singel DJ, Piantadosi CA, Tapson VF, Stamler JS. A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of s-nitrosohemoglobin in pulmonary hypertension. Proceedings of the National Academy of Sciences. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proceedings of the National Academy of Sciences. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an s-nitrosothiol-based signal. Circulation Research. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonveaux P, Kaz AM, Snyder SA, Richardson RA, Cardenas-Navia LI, Braun RD, Pawloski JR, Tozer GM, Bonaventura J, McMahon TJ, Stamler JS, Dewhirst MW. Oxygen regulation of tumor perfusion by s-nitrosohemoglobin reveals a pressor activity of nitric oxide. Circulation Research. 2005;96:1119–1126. doi: 10.1161/01.RES.0000168740.04986.a7. [DOI] [PubMed] [Google Scholar]
- 75.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S -nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat.Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 77.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of no-bioactivity by the red blood cell in sepsis: Novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 78.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: Reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circulation Research. 2004;94:976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 79.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am.J.Physiol Lung Cell Mol.Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 80.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein s-nitrosylation: Purview and parameters. Nature Reviews Molecular Cell Biology. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 81.Xue Y, Liu Z, Gao X, Jin C, Wen L, Yao X, Ren J. Gps-sno: Computational prediction of protein s-nitrosylation sites with a modified gps algorithm. PLoS ONE. 5:e11290. doi: 10.1371/journal.pone.0011290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 83.Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. The oxyhemoglobin reaction of nitric oxide. Proceedings of the National Academy of Sciences. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to s-nitrosohemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proceedings of the National Academy of Sciences. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 86.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends in Molecular Medicine. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 87.Stamler JS. S-nitrosothiols and the bioregulatory actions of nitrogen oxides through reactions with thiol groups. Current Topics in Microbiology & Immunology. 1995;196:19–36. doi: 10.1007/978-3-642-79130-7_4. [DOI] [PubMed] [Google Scholar]
- 88.Stamler JS. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 89.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 90.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proceedings of the National Academy of Sciences. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stamler JS, Lamas S, Fang FC. Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 92.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (s)no signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 93.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiological Reviews. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 94.Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nature Structural Biology. 1998;5:247–249. doi: 10.1038/nsb0498-247. [DOI] [PubMed] [Google Scholar]
- 95.Stamler JS. S-nitrosothiols in the blood: Roles, amounts, and methods of analysis. Circulation Research. 2004;94:414–417. doi: 10.1161/01.RES.0000122071.55721.BC. [DOI] [PubMed] [Google Scholar]
- 96.Carlsen E, Comroe JH. The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J Gen Physiol. 1958;42:83–107. doi: 10.1085/jgp.42.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proceedings of the National Academy of Sciences. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. Diffusion-limited reaction of free nitric oxide with erythrocytes. Journal of Biological Chemistry. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 99.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. Journal of Biological Chemistry. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 100.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proceedings of the National Academy of Sciences. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. American Journal of Physiology. 1998;274:H1705–H1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 102.Barry PH, Diamond JM. Effects of unstirred layers on membrane phenomena. Physiological Reviews. 1984;64:763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- 103.Han TH, Qamirani E, Nelson AG, Hyduke DR, Chaudhuri G, Kuo L, Liao JC. Regulation of nitric oxide consumption by hypoxic red blood cells. Proceedings of the National Academy of Sciences. 2003;100:12504–12509. doi: 10.1073/pnas.2133409100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han TH, Liao JC. Erythrocyte nitric oxide transport reduced by a submembrane cytoskeletal barrier. Biochimica et Biophysica Acta (BBA) - General Subjects. 2005;1723:135–142. doi: 10.1016/j.bbagen.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 105.Brunori M, Taylor JF, Antonini E, Wyman J, Rossi-Fanelli A. Studies on the oxidation-reduction potentials of heme proteins. Vi. Human hemoglobin treated with various sulfhydryl reagents. Journal of Biological Chemistry. 1967;242:2295–2300. [PubMed] [Google Scholar]
- 106.Chan NL, Rogers PH, Arnone A. Crystal structure of the s-nitroso form of liganded human hemoglobin. Biochemistry. 1998;37:16459–16464. doi: 10.1021/bi9816711. [DOI] [PubMed] [Google Scholar]
- 107.Chiancone E, Currell DL, Vecchini P, Antonini E, Wyman J. Kinetics of the reaction of the “masked” and “free” sulfhydryl groups of human hemoglobin with p-mercuribenzoate. Journal of Biological Chemistry. 1970;245:4105–4111. [PubMed] [Google Scholar]
- 108.Funai EF, Davidson A, Seligman SP, Finlay TH. S-nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochemical & Biophysical Research Communications. 1997;239:875–877. doi: 10.1006/bbrc.1997.7565. [DOI] [PubMed] [Google Scholar]
- 109.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO., III Role of circulating nitrite and s-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. Journal of Biological Chemistry. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 111.Mayer B, Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Brunner F. A new pathway of nitric oxide.Cyclic gmp signaling involving s-nitrosoglutathione. Journal of Biological Chemistry. 1998;273:3264–3270. doi: 10.1074/jbc.273.6.3264. [DOI] [PubMed] [Google Scholar]
- 112.Crawford JH, White CR, Patel RP. Vasoactivity of s-nitrosohemoglobin: Role of oxygen, heme, and no oxidation states. Blood. 2003;101:4408–4415. doi: 10.1182/blood-2002-12-3825. [DOI] [PubMed] [Google Scholar]
- 113.Pawloski JR, Swaminathan RV, Stamler JS. Cell-free and erythrocytic s-nitrosohemoglobin inhibits human platelet aggregation. Circulation. 1998;97:263–267. doi: 10.1161/01.cir.97.3.263. [DOI] [PubMed] [Google Scholar]
- 114.Barvitenko NN, Adragna NC, Weber RE. Erythrocyte signal transduction pathways, their oxygenation dependence and functional significance. Cellular Physiology and Biochemistry. 2005;15:1–18. doi: 10.1159/000083634. [DOI] [PubMed] [Google Scholar]
- 115.Chu H, Breite A, Ciraolo P, Franco RS, Low PS. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: Implications for o2 regulation of erythrocyte properties. Blood. 2008;111:932–938. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shimazutsu K, Uemura K, Auten K, Baldwin M, Belknap S, La Banca F, Jones M, McClaine D, Eubanks W, Stamler J, Reynolds J. Inclusion of a nitric oxide congener in the insufflation gas repletes s-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci. 2009;2:405–412. doi: 10.1111/j.1752-8062.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Besarab A. The effects of normal as compared to low hematocrit values in patients with cardiac disease who are recieving hemodialysis and epoetin. New England Journal of Medicine. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 118.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. New England Journal of Medicine. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 119.Hart RG, Kanter MC. Hematologic disorders and ischemic stroke. Stroke. 1990;21:1111–1121. doi: 10.1161/01.str.21.8.1111. [DOI] [PubMed] [Google Scholar]
- 120.Cirillo M, Laurenzi M, Trevisan M, Stamler J. Hematocrit, blood pressure, and hypertension. The gubbio population study. Hypertension. 1992;20:319–326. doi: 10.1161/01.hyp.20.3.319. [DOI] [PubMed] [Google Scholar]
- 121.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284:2611–2617. doi: 10.1001/jama.284.20.2611. [DOI] [PubMed] [Google Scholar]
- 122.Hebert PC, Wells G, Blajchamn MA, Marshall J. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New England Journal of Medicine. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 123.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Noillet G, Peres-Bota D, Investigators AAaBTiCC Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 124.Ketcham EM, Cairns CB. Hemoglobin based oxygen carriers: Development and clinical potential. Annals of Emergency Medicine. 1999;33:326–337. doi: 10.1016/s0196-0644(99)70370-7. [DOI] [PubMed] [Google Scholar]
- 125.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of s-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 126.Milsom AB, Jones CJ, Goodfellow J, Frenneaux M, Peters JR, James P. Abnormal metabolic fate of nitric oxide in type i diabetes. Diabetologia. 2002;45:1515–1522. doi: 10.1007/s00125-002-0956-9. [DOI] [PubMed] [Google Scholar]
- 127.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proceedings of the National Academy of Sciences. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hammerman SI, Klings ES, Hendra KP, Upchurch GR, Jr., Rishikof DC, Loscalzo J, Farber HW. Endothelial cell nitric oxide production in acute chest syndrome. AJP - Heart and Circulatory Physiology. 1999;277:H1579–H1592. doi: 10.1152/ajpheart.1999.277.4.H1579. [DOI] [PubMed] [Google Scholar]
- 129.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, Marin-Niebla A, Herruzo-Aviles AMD, Camacho-Larana P, Loscertales J. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 130.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic icu patients. Canadian Journal of Anesthesia. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 131.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. American Journal of Surgery. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 132.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 133.Valeri CR, Hirsch NM. Restoration in vivo of erythrocyte adenosine triphosphate 2,3-diphosphoglycerate potassium ion and sodium ion concentrations following the transfusion of acid-citrate-dextrose stored human red blood cells. J Lab Clin Med. 1969;73:722–733. [PubMed] [Google Scholar]
- 134.Valtis DJ. Defective gas transport function of stored red blood cells. Lancet. 1954;266:119–124. doi: 10.1016/s0140-6736(54)90978-2. [DOI] [PubMed] [Google Scholar]
- 135.Fortune JB, FeustelP J, Saifi J, Stratton HH, newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. J Trauma. 1987;27:243–249. doi: 10.1097/00005373-198703000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, Marin-Caballos A, Amaya-villar R, Marin A, Puppo-Moreno A, Ferrandiz-millon C, Flores-cordero JM, Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 137.Sugerman HJ, Davidson DT, Vibul S, Delivoria-Papadopoulos M, Miller LD, Oski FA. The basis of defective oxygen delivery from stored blood. Surg Gynecol Obstet. 1970;131:733–741. [PubMed] [Google Scholar]