Abstract
Prostasin is a membrane-anchored protease expressed in airway epithelium, where it stimulates salt and water uptake by cleaving the epithelial Na+ channel (ENaC). Prostasin is activated by another transmembrane tryptic protease, matriptase. Because ENaC-mediated dehydration contributes to cystic fibrosis (CF), prostasin and matriptase are potential therapeutic targets, but their catalytic competence on airway epithelial surfaces has been unclear. Seeking tools for exploring sites and modulation of activity, we used recombinant prostasin and matriptase to identify substrate t-butyloxycarbonyl-l-Gln-Ala-Arg-4-nitroanilide (QAR-4NA), which allowed direct assay of proteases in living cells. Comparisons of bronchial epithelial cells (CFBE41o−) with and without functioning cystic fibrosis transmembrane conductance regulator (CFTR) revealed similar levels of apical and basolateral aprotinin-inhibitable activity. Although recombinant matriptase was more active than prostasin in hydrolyzing QAR-4NA, cell surface activity resisted matriptase-selective inhibition, suggesting that prostasin dominates. Surface biotinylation revealed similar expression of matriptase and prostasin in epithelial cells expressing wild-type vs. ΔF508-mutated CFTR. However, the ratio of mature to inactive proprostasin suggested surface enrichment of active enzyme. Although small amounts of matriptase and prostasin were shed spontaneously, prostasin anchored to the cell surface by glycosylphosphatidylinositol was the major contributor to observed QAR-4NA-hydrolyzing activity. For example, the apical surface of wild-type CFBE41o− epithelial cells express 22% of total, extractable, aprotinin-inhibitable, QAR-4NA-hydrolyzing activity and 16% of prostasin immunoreactivity. In conclusion, prostasin is present, mature and active on the apical surface of wild-type and CF bronchial epithelial cells, where it can be targeted for inhibition via the airway lumen.
Keywords: channel-activating protease, cystic fibrosis, epithelial sodium channel, aprotinin glycosylphosphatidylinositol
prostasin is a tryptic, type I transmembrane serine protease encoded by the PRSS8 gene. In mammals, including mice and humans, prostasin is widely expressed in epithelial cells, where it supports barrier integrity (25, 35) and regulates open probability of the epithelial Na+ channel (ENaC1). Prostasin was discovered as a tryptic peptidase of seminal fluid (39). Immunohistochemical studies revealed its presence in epithelial cells of the prostate gland, which inspired naming the enzyme prostasin. Subsequent studies revealed expression by epithelial cells of many organs (36, 38). Sequencing of cDNA revealed a sequence predicting initial synthesis as a membrane-anchored, zymogen with an NH2-terminal prepro-segment and a COOH-terminal transmembrane peptide anchor (38). These predictions have been borne out in several types of epithelia. In the prostate gland, some prostasin is shed via peptide anchor hydrolysis to yield the soluble enzyme (38). However, the peptide anchor is exchanged for a lipid (glycosylphosphatidylinositol, GPI) in some other epithelial cells (9). The catalytic domain of cell surface-expressed, lipid-anchored prostasin can be shed by bacterial lipase or by endogenous GPI-specific phospholipase D (PLD), which mediates a proposed mechanism of downregulation (35).
Major clues regarding the biological functions of prostasin in mammals arose from studies of ENaC function in frogs. Expression cloning strategies identified a channel-activating protease (CAP) (34). Data-mining and phylogenetic analysis identified mammalian prostasin as a likely CAP ortholog (8, 34, 36) alternatively termed CAP1. Among several mammalian epithelial serine proteases with potential to activate ENaC, prostasin/CAP1 is a leading candidate as an endogenous regulator of Na+ transport, as reviewed in Ref. 25. Coexpression of prostasins and ENaC in Xenopus oocytes augments Na+ absorption via ENaC by augmenting channel open probability (1, 37). In cultured mammalian epithelial cells, prostasin inhibitors (like aprotinin and bikunin), reduce amiloride-sensitive (ENaC-mediated) Na+ transport (4, 13). Additionally, siRNA-mediated knockdown of prostasin in wild-type and cystic fibrosis (CF) cells reduces ENaC activity to a degree similar to that produced by nonselective protease inhibitors applied to the cell surface (33). Biochemical studies suggest that hydrolysis of ENaC itself is the basis for augmentation of Na+ transport by prostasin and identify prostasin-sensitive sites in the γ-subunit of ENaC (5). Mutagenesis studies suggest that catalytically active prostasin in its GPI-anchored form is required for effects on ENaC (35).
Further probing of prostasin regulation in mammalian cells predicts activation from its zymogen form by another transmembrane protease, matriptase (22). Although global deletion of prostasin in mouse cells is lethal during embryogenesis, tissue-selective knockouts yield less severe phenotypes. For example, deletion of Prss8 expression in epidermal cells generates mice with skin barrier defects dying within 60 h of birth (19). Skin-specific reduction of matriptase generates a similar phenotype (20, 21), consistent with biochemical evidence that prostasin is activated by matriptase and that the mature form prostasin is required for ENaC stimulation (24). On the other hand, prostasin may activate matriptase in addition to the converse (7), reinforcing the concept that fates and activity of the two enzymes are intertwined. More recently, prostasin catalytic domain mutations were linked to defects in hair and skin development in established strains of mice and rats (28), and skin overexpression caused inflammation and ichthyosis (15). Selective deletion of mouse Prss8 in distal airway epithelial cells reduces alveolar fluid clearance and ENaC-mediated Na+ absorption (26), which is consistent with mounting in vitro evidence that prostasin regulates epithelial Na+ transport.
Although no known genetic defects directly involve prostasin in humans, an inactivating mutation in the catalytic domain of matriptase is associated with autosomal recessive ichthyosis with hypotrichosis (2, 12, 20). Both enzymes are potential targets for therapeutic inhibition in diseases such as CF and systemic hypertension (40). In CF, Na+ hyperabsorption by ENaC is thought to contribute to excessive drying of secretions, ciliary dysfunction, and susceptibility to infection. Work with cultured CF cells suggests that prostasin regulates ENaC (33) and may be overexpressed in CF (23, 32). Furthermore, inhaled, nonspecific inhibitors of prostasin improve mucociliary clearance in sheep (11). In hypertension, retention of Na+ increases blood volume and pressure. Recent evidence suggests that expression of matriptase and prostasin is polarized and that the enzymes are transported vectorially across epithelium (16). Although tryptic activity has been detected on the surface of frog oocytes and cultured epithelial cells (18), little is known concerning relative surface expression of active matriptase and prostasin. The present work tests the hypothesis that prostasin is active on the apical/lumenal surface of airway epithelial cells and overactive in cells with defective CF transmembrane conductance regulator (CFTR).
MATERIALS AND METHODS
Comparison of substrate preferences.
Recombinant, soluble catalytic domains of human prostasin (R&D Systems, Minneapolis, MN) and matriptase (Enzo Life Sciences, Plymouth Meeting, PA) were used to screen potential peptidyl 4-nitroanilide (4NA) substrates of the two enzymes. Screened substrates included d-Val-l-Leu-Lys-4NA, N-benzoyl-l-Lys-Gly-Arg-4NA, N-benzoyl-l-Val-Gly-Arg-4NA, N-benzoyl-l-Arg-4NA, β-Ala-Gly-l-Arg-4NA, and N-(p-tosyl)-Gly-l-Pro-Lys-4NA from Sigma (St. Louis, MO) and carbobenzoxy-l-Arg-Arg-4NA and t-butyloxycarbonyl-l-Gln-Ala-Arg (QAR)-4NA from Bachem Americas (Torrance, CA). Substrates (1 mM) were incubated with 1.45 μg/ml of human prostasin or 1 ng/ml of human matriptase in PBS at 37°C. Generation of free 4-nitroaniline by enzymatic hydrolysis was monitored at 410 nm in cuvettes using a Genesys 5 spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Comparison of inhibitor susceptibilities.
Recombinant, soluble human prostasin and matriptase were preincubated in PBS at room temperature for 15 min with aprotinin (100 μM; Sigma) or with 4 μM cell surface-active, human single-chain (sc) Fv human matriptase-inactivating MAb (3, 30) and then assayed at 37°C for residual amidolytic activity by addition of QAR-4NA (1 mM) and detection of 4-nitroaniline release as above.
Culture of human bronchial epithelial cell lines at an air-liquid interface.
This study used established subclones of the stable ΔF508-homozygous line CFBE41o−, which originated from bronchial epithelial cells from a subject with CF and was immortalized using origin of replication-deficient SV40 (6, 10). The CFBE41o− subclones were complemented by transfection with an episomal plasmid vector expressing cDNA encoding full-length (6.2 kb) wild-type CFTR (CFBE41o−WT) or transfected with the same vector expressing nonfunctional, truncated (4.7 kb) CFTR (CFBE41o−ΔF508) to control for CFTR overexpression, as described (17). Retention of both plasmids, which contain a gene conferring resistance to toxic effects of hygromycin, was maintained by culturing cells with hygromycin. CFBE41o−WT and CFBE41o−ΔF508 cells were seeded to confluence at 3 × 105 cells/well onto polyester membrane Transwell supports (0.33 cm2, 0.4 μm pores; Corning, Lowell, MA) coated with a mixture of BSA (1 mg/ml; Invitrogen, Carlsbad, CA), human fibronectin (30 μg/ml; BD Biosciences, San Jose, CA), and bovine collagen type II (10 μg/ml; BD Biosciences) in LHC basal medium (Invitrogen) and grown in minimal essential medium with Earle's salt supplemented with 10% FBS, 4 mM l-glutamine, 100 U/ml penicillin G, and 300 μg/ml hygromycin at 37°C in a 5% CO2 incubator. Apical medium was removed 48 h after seeding to initiate air-interface culture. Basolateral medium was changed every 48 h for 12 days. Except as specified, culture media and supplements were from Invitrogen.
Growth of cells in liquid culture.
Alternatively, to obtain larger numbers of cells and to serve as a basis of comparison with cells subjected to air-interface culture, CFBE41o− bronchial epithelial cells were cultured to confluence on polystyrene 6- or 96-well cell culture plates (Corning) coated as above with BSA, fibronectin, and collagen and cultured by immersion of the apical surface in the same medium as described above in connection with air-interface culture.
Measurement of transepithelial electrical resistance and passage of phenol red.
To assess integrity of cell monolayers cultured at an air-liquid interface, ΔF508 and wild-type CFBE41o− cell transepithelial electrical resistance was measured with an epithelial voltmeter (World Precision Instruments, Sarasota, FL). Monolayer integrity also was assessed spectrophotometrically by tracking passage of the small molecule phenol red from apical to basal medium. Basolateral and apical media were replaced, respectively, with 0.8 ml of PBS and 0.2 ml of minimal essential medium containing phenol red (27 μM). After 6 h of incubation at 37°C, absorbance at 559 nm of medium was measured to detect transepithelial passage of phenol red, as reflected by absorbance ratios in basolateral vs. apical medium.
Confocal microscopic imaging of air interface-cultured cells.
After 12 days of culture, cells on inserts were fixed with 4% paraformaldehyde in PBS at 4°C for 10 min, washed with PBS, exposed to 1% Triton X-100 (Sigma) on ice for 10 min, rewashed with PBS, then stained with Alexa Fluor 568 phalloidin (1:1,000; Invitrogen) and mounted onto glass slides using ProLong Gold Antifade Reagent with 4′6-diamidino-2-phenylindole (DAPI; Invitrogen). Cells on inserts were imaged using a LSM 510 META confocal laser scanning module (Carl Zeiss Microscopy, Thornwood, NY) mounted onto an inverted Axio Observer (R&D in collaboration with EMBL Heidelberg, Germany). Images were acquired using a ×40 EC Plan-NEOFLUAR (NA 1.3) oil objective. Excitation wavelengths were 405 nm for DAPI and 543 nm for Alexa Fluor 568 phalloidin. A gallery of 0.4-μm optical sections was collected and processed using LSM 510 software (Carl Zeiss).
Assay of apical and basolateral surface-associated tryptic activity in living epithelial cells.
After 12 days of air-interface culture, CFBE41o−WT and CFBE41o−ΔF508 cells were washed in PBS. In preparation for measuring cell surface tryptic activity, the apical or basolateral side of the monolayer cultured on inserts was immersed in 0.2 ml (apical) or 0.8 ml (basolateral) of PBS containing QAR-4NA (1 mM) alone as control, QAR-4NA plus aprotinin (100 μM), or QAR-4NA plus anti-matriptase single-chain variable fragment (scFv) MAb (4 μM). For measurements of apical activity, the basolateral surface was bathed in PBS alone, whereas for measurements of basolateral activity, the apical surface was bathed in PBS alone. Tryptic activity in aliquots of apical or basolateral conditioned medium was monitored hourly for 4 h at 37°C by spectrophotometry using a Synergy 2 SL Microplate Reader (BioTek Instruments, Winooski, VT). To allow comparison of surface QAR-4NA-hydrolyzing activity with total cellular activity, apical and basolateral activity measurements in cells cultured under liquid culture or air-interface conditions were compared with that of the same number of cells lysed by repeated freeze-thaws or by vigorous pipetting.
Solubilization, separation, and blotting of matriptase, proprostasin, and mature prostasin in whole cell extracts.
Cells were solubilized in lysis buffer (10 mM Tris·HCl, 50 mM EGTA, 0.4% Na+ deoxycholate, 1% NP-40, and 1% Triton X-100, pH 7.4). After centrifugation for 7.5 min at 10,000 g, supernatants were prepared for SDS-PAGE by adding lithium dodecylsulfate sample buffer and reducing agent (50 mM DTT). Samples were denatured at 70°C for 10 min before loading onto NuPAGE Novex 10% bis-Tris gels (1-mm thick; Invitrogen) and resolved with MOPS SDS running buffer (Invitrogen). Proteins then were transferred to a polyvinylidene difluoride membrane (Perkin Elmer, Shelton, CT), which was incubated with anti-human prostasin MAb (diluted 1:360; BD Biosciences), anti-human matriptase rabbit polyclonal IgG (1:2,000; EMD Chemicals, Gibbstown, NJ) or β-tubulin rabbit polyclonal IgG (1:500 in 5% BSA; Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with horseradish peroxidase-conjugated secondary antibodies from Santa Cruz Biotechnology and then with SuperSignal West Dura chemiluminescence substrate from Thermo Fisher Scientific. Densitometry was performed using ImageJ software.
Biotinylation, purification, and blotting of apical and basolateral surface proteases in polarized bronchial epithelial cells.
For cells cultured on porous polyester membranes at an air-liquid interface, inserts were washed three times with cold PBS to remove residual serum proteins. Apical surfaces of the cells then were incubated for 20 min at room temperature in PBS containing N-hydroxysuccinamide-SS-biotin (1 mg/ml; Thermo Fisher Scientific). Biotinylation was quenched by flooding cells with 20 mM Tris·HCl (pH 7.5) containing 150 mM NaCl. Cells then were lysed and proteins solubilized as described above for unbiotinylated cells. Resulting supernatants, after centrifugation, were incubated with streptavidin-agarose resin (Thermo Fisher Scientific), which were rotated gently overnight at 4°C. Biotinylated proteins captured in this manner were released into solution and debiotinylated by incubating PBS-washed resin for 20 min at room temperature with sample buffer containing 50 mM DTT, followed by heating for 3 min at 95°C.
Detection of apically shed matriptase and prostasin.
The apical surface of wild-type and ΔF508-homozygous CFBE41o− cells grown to confluent monolayers on porous membrane inserts or tissue culture plastic as above were exposed for 1 h at 37°C to 1 U/ml Bacillus cereus phosphatidylinositol-specific PLC (PI-PLC; Molecular Probes, Eugene, OR) in PBS (with ambient 5% CO2 in a tissue culture incubator). PI-PLC-conditioned medium was assayed at 37°C for amidolytic activity by addition of QAR-4NA (1 mM) alone or with aprotinin followed by detection of released 4-nitroaniline as above. Conditioned medium then was concentrated 10-fold using a centrifugal filter (5K NMWL; Millipore, Billerica, MA) and subjected to SDS-PAGE and immunoblotting for prostasin and matriptase as above.
Statistical analysis.
Results are reported as means ± SE. Except where otherwise specified, differences between groups were analyzed for significance using two-tailed Student t-tests. For data not normally distributed, differences were compared using Mann-Whitney U-tests. P values of <0.05 were considered significant.
RESULTS
Identification of a peptidic chromogenic substrate preferred by matriptase and prostasin.
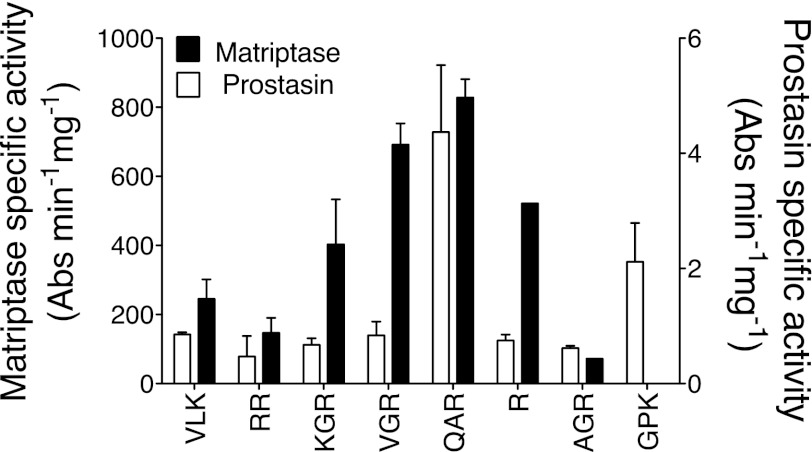
As shown in Fig. 1, the screen of peptidic tryptic substrates identified several substrates hydrolyzed by prostasin and matriptase. Of the substrates screened, QAR-4NA was the substrate preferred by both enzymes and on this basis was selected to use in assays of living cells for prostasin and matriptase activity. Based on specific activity, matriptase was intrinsically more active than prostasin toward all substrates, including QAR-4NA.
Fig. 1.
Comparison of peptidic substrate preferences of human matriptase and prostasin. Amidolytic activity was tested against soluble, recombinant proteases using 8 tryptic 4-nitroanilide (4NA) peptidic substrates, including d-Val-l-Leu-Lys-4NA (VLK), carbobenzoxy-l-Arg-Arg-4NA (RR), N-benzoyl-l-Lys-Gly-Arg-4NA (KGR), N-benzoyl-l-Val-Gly-Arg-4NA (VGR), t-butyloxycarbonyl-l-Gln-Ala-Arg-4NA (QAR), N-benzoyl-l-Arg-4NA (R), β-Ala-Gly-l-Arg-4NA (AGR), and N-(p-tosyl)-Gly-l-Pro-Arg-4NA (GPK). For each enzyme, data obtained with each substrate were normalized to specific activity [Absorbance (Abs)·min−1·mg−1 of protease] toward QAR-4NA, which was the best substrate identified for both matriptase and prostasin (N = 3).
Selective inhibition of matriptase.
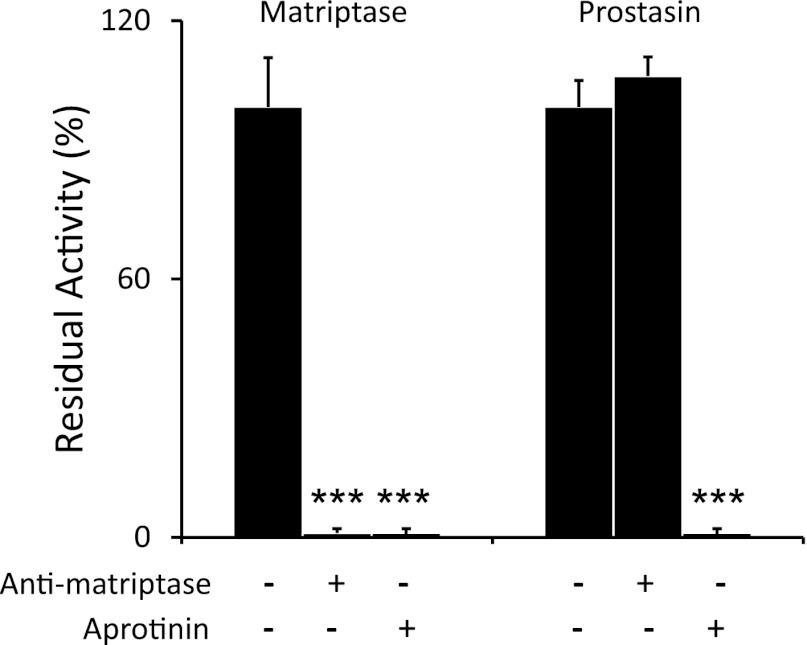
As shown in Fig. 2, QAR-4NA-hydrolyzing activity of soluble, recombinant human matriptase is completely blocked by scFv anti-matriptase at a concentration with no effect on soluble prostasin activity. Both proteases are highly sensitive to inhibition by the Kunitz-class inhibitor aprotinin. Thus scFv anti-matriptase distinguishes matriptase activity from prostasin activity.
Fig. 2.
Comparison of inhibitor susceptibilities of matriptase and prostasin. Amidolytic activity toward colorimetric substrate QAR-4NA was tested in preparations of recombinant soluble human matriptase and prostasin with and without incubation with anti-matriptase single-chain MAb or aprotinin. N = 3; ***P ≤ 0.001.
Bronchial epithelial cells expressing ΔF508 and wild-type CFTR form electrically resistant monolayers in air-interface culture.
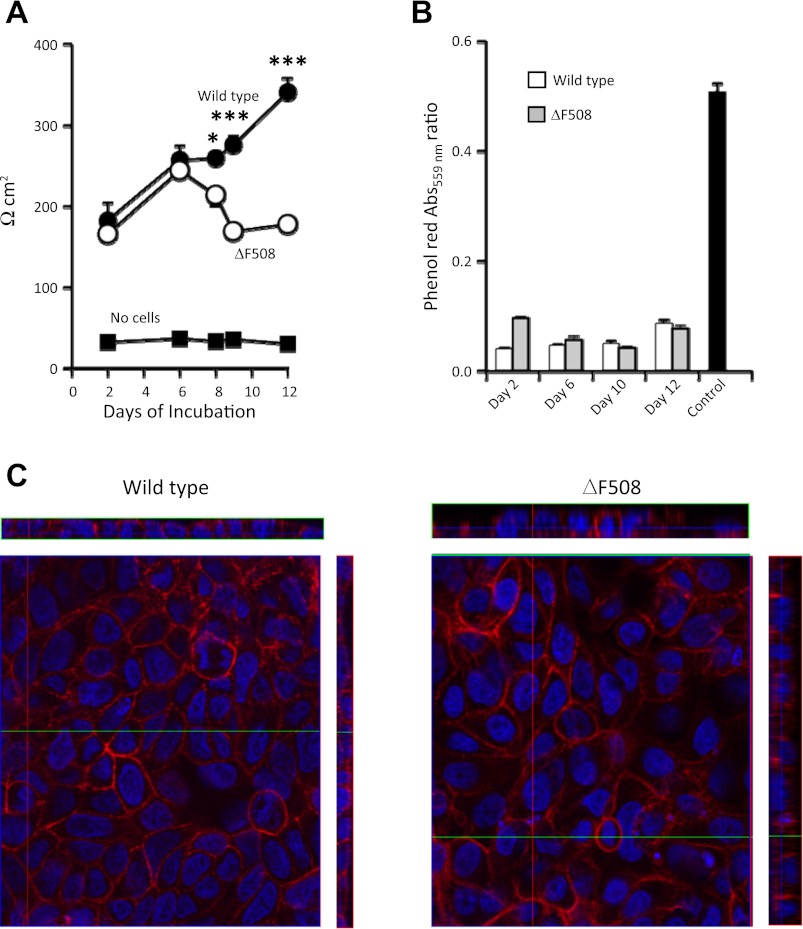
CFBE41o−ΔF508 and CFBE41o−WT cells grown under air-interface conditions both achieved confluence within 2 days of plating. As shown in Fig. 3A, transepithelial electrical resistance of ΔF508-homozygous CFBE41o− cells is significantly higher than that of inserts without cells but remains <250 Ω cm2 without significantly increasing between days 2 and 12 of culture. However, measured resistance of wild-type CFBE41o− cells increases with time in culture and is higher at day 12 than in ΔF508-homozygous CFBE41o− cells. As shown in Fig. 3B, both types of monolayers largely prevented equilibration of phenol red dye from apical to basolateral medium between culture day 2 and 12, during a 6-h incubation mimicking conditions used in the tryptic protease assay of living cells. At confluence, both cell types formed monolayers of similar appearance, as assessed by confocal fluorescence microscopy (see Fig. 3C). Thus, ΔF508-homozygous and wild-type CFBE41o− cells both develop electrically resistant monolayers that hinder passage of small ions although resistance developing in the latter cells is higher with sustained culture.
Fig. 3.
Characteristics of epithelial monolayers in air-interface culture. A: transepithelial electrical resistance in CFBE41o− cell monolayers in air-interface culture. Resistance normalized for monolayer surface area (Ω × cm2) was assessed at intervals during air-interface culture in ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR)-homozygous CFBE41o− cells or in the same line of cells complemented with wild-type CFTR. N = 5–6 for wild-type and ΔF508 cells; *P < 0.05 and ***P < 0.001 (wild-type vs. ΔF508). B: comparison of permeability of wild-type and ΔF508 monolayers to phenol red after 2 to 12 days in air-interface culture, as assessed by apical/basolateral ratios of Abs at 559 nm 6 h after addition of phenol red to basolateral medium. Control permeability was assessed in coated inserts without cells. N = 3–6; no significant difference between wild-type and ΔF508 at days 6–12; P < 0.05 between wild-type and ΔF508 at day 2; P = 0.01 for all monolayers vs. control. C: representative confocal images of wild-type and ΔF508 monolayers cultured on inserts at an air interface. Cells were stained with 4′6-diamidino-2-phenylindole to visualize nuclei and with Alexa Fluor 568 phalloidin to detect F-actin. The main image is a plane in the z-stack; rectangular images are orthogonal reconstructions with the air interface facing outward.
Tryptic serine peptidases are active and inhibitable on both surfaces of polarized, living epithelial cells.
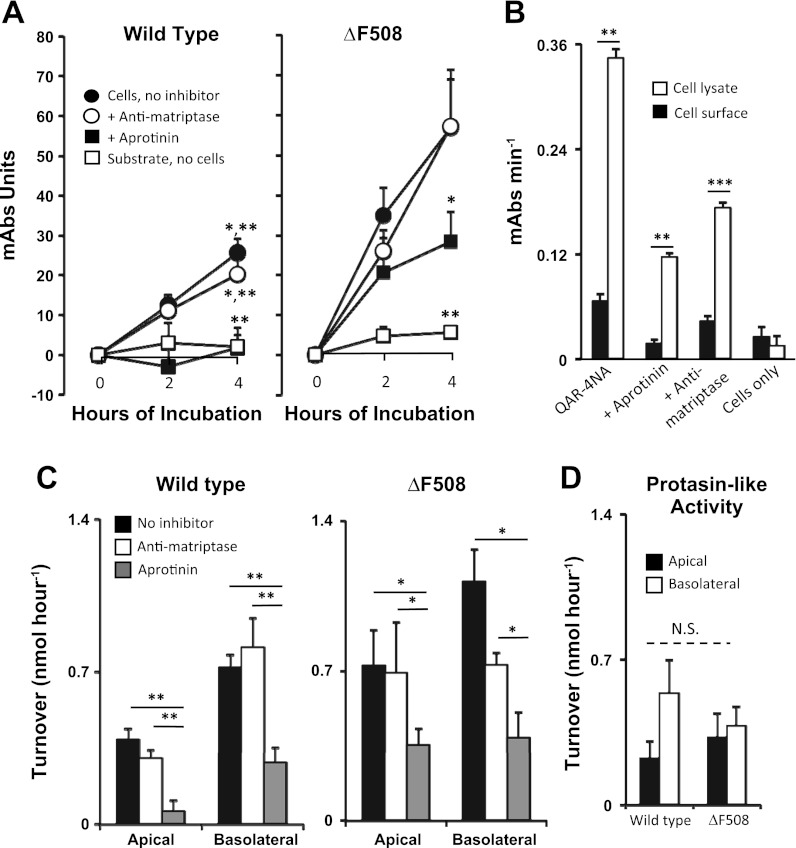
As shown in Fig. 4A, apical tryptic serine protease activity, as reflected by hydrolysis of QAR-4NA, is detected on the surface of ΔF508-homozygous CFBE41o− cells as well as of wild-type CFBE41o− cells. Aprotinin at a concentration 100-fold and 2,000-fold higher than the Ki for prostasin and matriptase, respectively (14, 27), significantly reduced apical tryptic activity compared with activity of cells incubated with substrate alone, indicating that observed activity is due to one or more trypsin-like serine peptidases. A concentration of scFv anti-matriptase 100-fold above the Ki for human matriptase (14) produced either no change or statistically insignificant reduction in surface activity, suggesting that little if any apical activity is due to matriptase. Figure 4A reveals that hydrolysis is roughly constant over the interval of observation, suggesting that the apical tryptic enzymes are in a steady state, which is inconsistent with activity being produced by accumulation of enzyme shed into medium, which would produce increasing activity over time. Compared with wild-type CFBE41o− cells, ΔF508-homozygous CFBE41o− cells had higher QAR-4NA-hydrolyzing activity remaining in cells incubated with aprotinin, suggesting higher levels of tryptic cysteine proteases or aprotinin-resistant serine proteases in the ΔF508-homozygous cells. Similar results (not shown) were obtained in assays of basolateral activity. As depicted by Fig. 3B, QAR-4NA-hydrolyzing activity was much higher in lysates of wild-type CFBE41o− cells than detected on the apical surface, consistent with the assay detecting surface activity rather than activity from intracellular sources. Similar results (not shown) were obtained in comparisons of apical and lysate activity in ΔF508-homozygous CFBE41o− cells and in comparisons of basolateral vs. lysate activity. To facilitate comparisons between the two cell types and both surfaces, activity expressed as substrate turnover is compared in Fig. 4, C and D. These results show that wild-type and ΔF508-homozygous CFBE41o− cells had little if any detectable matriptase activity on the apical or the basolateral surface and that both had prostasin-like activity (defined as activity that is aprotinin-sensitive and anti-matriptase-resistant) that was similar for both cell types on both surfaces.
Fig. 4.
Bronchial epithelial cell surface activity of matriptase and prostasin. A: time course of peptidase activity measured in living cells. Tryptic activity on the apical surface of bronchial epithelial cells (ΔF508 CFTR-homozygous CFBE41o− cells or the same line of cells complemented with wild-type CFTR) was assayed in polarized monolayers of cells that had been cultured at an air interface. Cells were incubated in PBS with substrate QAR-4NA (“Cells, no inhibitor”) or with substrate plus inhibitor (“+ Anti-matriptase” or “+ Aprotinin”). As an additional control, substrate was incubated in PBS alone (“Substrate, no cells”). B: comparison of QAR-4NA-hydrolyzing activity at the apical surface of wild-type cells with total activity in the same number of cells subjected to lysis by repeated freeze thawing. In all conditions in which substrate was present, QAR-4NA-hydrolyzing activity was higher in cell lysates than at the surface. QAR-4NA was omitted from the “Cells only” negative control condition. C: comparison of activity curves derived from apical surface data shown in A, as well as from data obtained similarly from the basolateral surface of cells expressing ΔF508 and wild-type CFTR. D: comparison of apical and basolateral prostasin-like activity (substrate hydrolysis in the presence of anti-matriptase minus activity in the presence of aprotinin). N = 4–6; *P < 0.05; **P < 0.01; ***P < 0.001; N.S. = not significant.
Mature prostasin is enriched on the apical surface of bronchial epithelial cells in air-liquid interface culture.
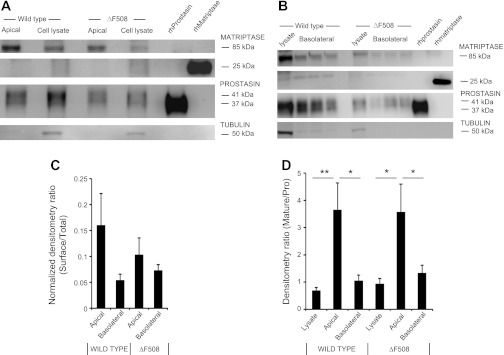
As shown in Fig. 5A by immunoblots of streptavidin-purified, biotin-labeled surface proteins, apical matriptase and prostasin are readily detected in detergent extracts of ΔF508-homozygous and wild-type CFBE41o− cells cultured at an air-liquid interface to mimic in vivo conditions faced by bronchial epithelial cells. Bands corresponding to both proteins also were detected in extracts of corresponding whole-cell lysates. Apical prostasin is a small fraction of total prostasin in cell lysates. However, apical prostasin is enriched in the 37-kDa mature form relative to the 41-kDa proform. Sequential immuno-screening of the same membranes using antibodies recognizing tubulin, which is an intracellular protein, provides a negative control for selective biotinylation of surface proteins as well as a normalization control for protein loading, thereby allowing comparison of levels of matriptase and prostasin expression in ΔF508-homozygous vs. wild-type CFBE41o− cells. Reduced matriptase in the apical preparations appears almost exclusively as a narrow immunoreactive band of ∼85 kDa, consistent with partially processed membrane-bound zymogen, as previously observed in transfected mammalian cells (12). However, prostasin appears as broader bands with ∼38- and ∼41-kDa components [attributed to mature and inactive proprostasin, respectively (16)]. As shown in Fig. 5B, the results of immunoblotting of basolaterally biotinylated proteases were similar, with the exception that a ∼27-kDa band likely corresponding to activated matriptase was more apparent although minor in intensity compared with the band at 85 kDa attributed to unactivated zymogen. The densitometric comparisons of matriptase and total (mature plus pro) prostasin band density in Fig. 5C show no significant differences in expression between ΔF508-homozygous and wild-type CFBE41o− cells or in apical vs. basolateral expression. In extracts of both cell types, however, the ratio of mature to proprostasin was much higher in biotinylated surface preparations than in whole cell extracts, suggesting enrichment of cleaved, active prostasin at the apical surface.
Fig. 5.
Immunodetection of bronchial epithelial cell matriptase and pro- and mature prostasin. Surface proteins were labeled by biotinylation of polarized epithelial cells, purified from cell lysates using streptavidin beads, and subjected to SDS-PAGE and sequential immunoblotting with antibodies specific for matriptase and prostasin. Results were compared with those of unpurified whole cell lysates. Extracts were also immunoblotted to detect tubulin as a loading control for cell lysates and as a negative control for purified biotinylated surface proteins. 1.5 × 105 cells were plated for each cell type. A and B, respectively, show representative immunoblots obtained after apical and basolateral biotinylation of wild-type and ΔF508 epithelial cells maintained in air-interface culture. C: results of densitometric analysis of repeated blots (N = 5–9) assessing total prostasin (mature + pro) expression on apical and basolateral surfaces relative to expression in whole cell lysates; no significant difference in ratios were found. D: results of densitometric comparison of the ratio of bands corresponding to mature (37 kDa) vs. proprostasin (41 kDa); N = 5–10; *P < 0.05; **P < 0.01.
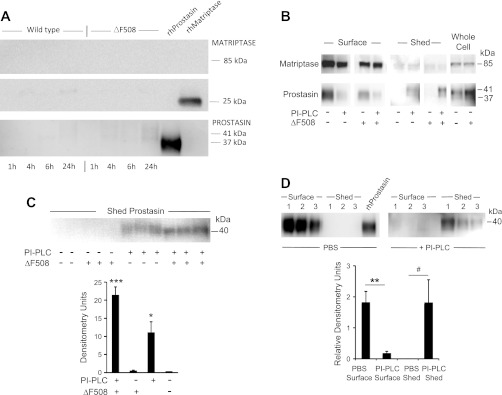
Matriptase and prostasin are shed slowly from the apical surface of cultured bronchial epithelial cells.
As shown in Fig. 6A, immunoreactive bands corresponding to matriptase and prostasin appear in apical medium conditioned by cultured ΔF508-homozygous or wild-type CFBE41o− cells, with band intensity increasing over time and most evident after 24 h. However, release is scant overall and nearly undetectable during the first 4 h, which brackets the interval during which the cells were assayed for surface activity, consistent with most of the measured activity being derived from surface-bound rather than shed enzyme. The mechanism of surface attachment and release was further explored and compared between liquid-cultured ΔF508-homozygous and wild-type CFBE41o− cells by incubating with PI-PLC, which sheds lipid-anchored proteins attached via GPI (9, 35). As revealed by the examples in Fig. 6B, prostasin band intensity in apical medium markedly increases following incubation of either type of cell with PI-PLC, with concomitant marked decrease in surface prostasin immunoreactivity derived from apically biotinylated cells. These results suggest that GPI anchors most apical prostasin to the plasma membrane and that relatively little prostasin sheds spontaneously during short-term culture. The results suggest that matriptase is similar to prostasin in that little surface enzyme sheds; however, matriptase differs in not being GPI anchored or releasable by PI-PLC. Shedding of prostasin was further compared in ΔF508-homozygous and wild-type CFBE41o− cells cultured for 10 days at an air-liquid interface. As shown in Fig. 6C, neither cell type secretes detectable prostasin during short-term (1 h) culture. Marginally more prostasin is released by PI-PLC from ΔF508-homozygous cells than from wild-type CFBE41o− cells. Thus spontaneous release of prostasin and matriptase is slow from the surface of these bronchial epithelial cell lines grown and matured in liquid culture or air-interface conditions, and the amounts released are low compared with protein anchored to the apical surface, from which most of the observed apical activity originates. Furthermore, because nearly all of the biotinylated (apical surface) prostasin is released into medium by PI-PLC (as revealed in Fig. 6D), almost all of the apical membrane-associated prostasin in these cells is lipid anchored via GPI. This is in contrast to apical matriptase (Fig. 6B and data not shown), which is not released into medium by PI-PLC. Spontaneous and GPI-induced shedding of prostasin from apical membrane is similar in ΔF508-homozygous and wild-type CFBE41o− cells and is also similar in cells matured with either liquid or air interfacing with the apical surface of the polarized monolayers (Fig. 6B and data not shown).
Fig. 6.
Spontaneous and induced shedding of matriptase and prostasin from CFBE41o− bronchial epithelial cells. A: results of immunoblotting to detect spontaneous shedding into apical medium by polarized monolayers of ΔF508 CFTR-homozygous (“ΔF508”) CFBE41o− cells or the same line of cells complemented with wild-type CFTR (“Wild type”). Cells were incubated with medium bathing apical and the basolateral surfaces to mimic conditions under which apical protease activity was assayed in living cells (see Fig. 4). Medium harvested at the intervals noted was electrophoresed and immunoblotted using protease-selective antibodies. Immunoblots in B explore apical expression and modes of membrane attachment by proteases in cells cultured under liquid conditions. ΔF508 and wild-type CFBE41o− cells were incubated with PBS or with phosphatidylinositol-specific phospholipase C (PI-PLC) to release glycosylphosphatidylinositol (GPI)-anchored proteins. Apical protease expression with or without exposure to PI-PLC was detected in purified preparations of apically biotinylated surface proteins and compared with expression in unbiotinylated whole cell extracts. The immunoblot in C reveals PI-PLC-induced shedding of prostasin in unbiotinylated ΔF508 and wild-type cells cultured for 10 days at an air interface followed by 1-h exposure to PI-PLC in liquid medium. The graph compares prostasin band density in ΔF508 and wild-type cells with and without exposure to PI-PLC. *P < 0.05, PI-PLC-exposed ΔF508 vs. wild-type; ***P < 0.001, PI-PLC-exposed vs. unexposed ΔF508 cells. D: comparison of apical surface prostasin to prostasin shed into medium by wild-type cells in liquid culture exposed apically to PBS or PI-PLC. Band density is normalized to density of recombinant prostasin band (equal amounts loaded for each gel). **P = 0.01, apical surface prostasin in PBS- vs. PI-PLC-exposed cells; #P = 0.07, shed prostasin in PBS- vs. PI-PLC-exposed cells.
DISCUSSION
This work is the first report of a substrate and assay enabling detection of tryptic proteolytic activity on the apical surface of living airway epithelial cells. By screening recombinant, soluble versions of the dominant tryptic membrane (associated epithelial proteases prostasin and matriptase), this work identified tripeptide-based, colorimetric substrates hydrolyzed by both enzymes. The best of these substrates, QAR-4NA, seeded development of a sensitive, microplate-based method to detect and quantify matriptase and prostasin activity on the lumen-facing side of polarized bronchial epithelial cell monolayers. The findings reveal that recombinant matriptase catalytic domain is much more active than recombinant prostasin in hydrolyzing QAR-4NA and that tryptic serine peptidase activity is present and readily detected on the apical as well as basolateral surfaces of cultured human bronchial epithelial cells. Somewhat unexpectedly, the great majority of QAR-4NA-cleaving activity on the cell surfaces was prostasin-like rather than matriptase-like, given that the activity is sensitive to aprotinin but mostly insensitive to matriptase-selective, inhibitory antibodies. However, the suggestion by these data that comparatively little apical matriptase is active fits well with the immunoblots of electrophoresed, membrane-associated matriptase, which is detected principally as an ∼85-kDa band consistent with promatriptase but not with the proteolytically active catalytic domain, which is released as a ∼27-kDa protein in the presence of reducing agents (12). These findings establish that there is much more active prostasin than active matriptase on the apical cell surface and that prostasin is the more obvious target for potential therapeutic inhibition by agents inhaled or otherwise introduced via the airway lumen.
We compared matriptase and prostasin-like expression and activity in a line of cultured human airway cells homozygous for the ΔF508 CFTR mutation with cells from the same line in which the CFTR defect was complemented by expression of normal CFTR. The ΔF508 and complemented lines both expressed immunoreactive matriptase, and prostasin and both exhibited apical surface matriptase- and prostasin-like peptidolytic activity in both liquid and air-liquid interface culture. Although a prior study found expression of prostasin protein to be upregulated in response to increases in airway surface liquid volume in normal bronchial epithelial cells and to be upregulated in CF cells despite Na+ hyperabsorption (23), the present study detected little difference in apical prostasin immunoreactivity and activity between ΔF508-homozygous CF cells and CF cells complemented with wild-type CFTR. Nonetheless, the past and present studies involving cells with and without functioning CFTR suggest that prostasin is expressed, active in regulating ENaC, and inhibitable on the apical surface (4, 23, 33), even though some of the electrophysiological defect in CF airway epithelial cells may be due to failure of CFTR to regulate ENaC via pathways independent of prostasin (29).
The observed preference of matriptase for QAR-4NA among the peptidic substrates surveyed is consistent with observed peptide cleavage preferences, as determined by profiling of matriptase catalytic domain substrate preferences using a combinatorial fluorogenic peptide library or multiple rounds of phage display screening (31), which identified glutamine as one of the residues preferred at P3, alanine as a residue tolerated at P2, and arginine or lysine as preferred at P1. For human prostasin, the peptide sequence of QAR-4NA is optimal only with respect to arginine, which is strongly preferred at the P1 position in a combinatorial peptide library (27), with alanine at P2 and glutamine at P3 being tolerated but not preferred. Nonetheless, our data with the recombinant enzyme establish that prostasin prefers QAR-4NA to several potential tryptic substrates with P1 basic residues and that it also can hydrolyze QAR-4NA while attached to the apical and basolateral surfaces of airway epithelial cells. As a substrate that is fully optimized for neither matriptase nor prostasin but is readily cleaved by both enzymes, QAR-4NA is useful when used in conjunction with selective inhibitors for assaying active forms of both enzymes on epithelial surfaces.
Although endogenous pathways for shedding of prostasin mediated by a tryptic protease (38) or by GPI-specific PLD (35) have been identified in some tissues and cells, little spontaneous shedding was observed in the human bronchial epithelial cells examined in the present study, given that prostasin was detected in conditioned medium only after prolonged incubation but was readily detectable after hydrolysis of the GPI anchor with bacterial PI-PLC. Thus the present study suggests that there are no intrinsic differences in use of endogenous pathways of prostasin shedding between airway epithelial cells with and without functioning CFTR. This does not preclude the possibility that lumenal proteases present in vivo, such as neutrophil and bacterial proteases in infected CF airway, shed prostasin and thereby alter function, including ability to activate ENaC. The results also suggest that little matriptase is shed spontaneously. Furthermore, PI-PLC does not release matriptase from the apical surface, in contrast to PI-PLC-mediated release of prostasin. This difference is expected, in that matriptase, in contrast to prostasin, is a type II transmembrane protease that is anchored to the plasma membrane via an NH2-terminal membrane-spanning peptide segment that is incapable of being exchanged for a GPI lipid anchor (31).
The finding in the present study that most of the tryptic activity on the apical/lumenal surface of airway epithelial cells is prostasin-like is consistent with recent findings in colonic epithelial cells, suggesting that matriptase is activated on the basolateral surface (16). However, the present studies, which differ not only in the type of cell studied but in culture conditions (air-liquid interface), suggest that matriptase was present on the apical surface although largely inactive given lack of inhibition by scFv anti-matriptase, which is capable of inactivating membrane-bound matriptase in its active form (14, 30). Alternatively, a fraction of apical bronchial epithelial cell matriptase is active but is present in small amounts compared with active prostasin.
An additional intriguing finding, shown in Fig. 5C, is that prostasin labeled on the apical or basolateral surface and captured on streptavidin beads is enriched in the lower molecular weight mature form of the enzyme, in contrast to prostasin in whole cell lysates, which contain primarily the larger proenzyme (zymogen) form of prostasin. Whole cell lysates contain prostasin originating from both polarized surfaces and from membrane-bound intracellular organelles, like endoplasmic reticulum and transport vesicles. This finding is consistent with prostasin being activated on the cell surface or being transported promptly to the surface after activation and is also consistent with the recent suggestion that prostasin can be activated by basolateral matriptase and conveyed to the apical surface in transcytotic vesicles (16). These observations regarding surface localization of mature prostasin, in combination with comparisons of surface and whole cell lysate prostasin-like activity, suggest that a substantial pool of activated prostasin lies on each of the membrane surfaces. According to the densitometric results summarized in Fig. 5C, 22% and 17% of total cellular prostasin resides on the surfaces of wild-type and ΔF508 cells, respectively, with no significant difference in apical vs. basolateral distribution. The findings further invite speculation that proteolytic activation of ENaC occurs at the apical surface, a possibility that also fits with the observed loss of ENaC-mediated absorption of Na+ in cultured epithelial monolayers apically exposed to aprotinin or bikunin (4, 13). A less straightforward possibility is that hydrolytic activation of ENaC occurs in a subsurface compartment via mature prostasin taken up into vesicles from the cell surface. The finding in this work that a sizable pool of active prostasin localizes to the basolateral surface, which is thought to lack ENaC, raises the possibility of targets and roles for prostasin apart from activating ENaC. Roles for prostasin in epithelial monolayer preservation and function not clearly related to regulation of ENaC have been predicted by several studies of engineered and naturally occurring prostasin variants in cultured cells and rodent models of epithelial function (15, 19, 28, 35).
In conclusion, this report reveals for the first time that there is much more active prostasin than active matriptase on the apical and basolateral surfaces of airway epithelial cells, that cells with and without functioning CFTR are similar in this regard, that apical prostasin is an airway-accessible target for potential therapeutic inhibition by inhaled antagonists, and that the identification of active prostasin on basolateral surfaces suggests the possibility of important targets for prostasin in addition to ENaC.
GRANTS
This work supported by grant P01 HL024136 (S. Nimishakavi, M. Besprozvannaya, W. Raymond, and G. Caughey) and R01CA128765 (C. C Craik) from the National Institutes of Health, by the Northern California Institute for Research and Education, and by funds from Pennsylvania Cystic Fibrosis, and Cystic Fibrosis Research (D. Gruenert).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.N., M.B., W.W.R., C.S.C., D.C.G., and G.H.C. conception and design of research; S.N. and M.B. performed experiments; S.N., M.B., W.W.R., and G.H.C. analyzed data; S.N., M.B., W.W.R., C.S.C., and G.H.C. interpreted results of experiments; S.N. and G.H.C. prepared figures; S.N., M.B., W.W.R., C.S.C., D.C.G., and G.H.C. edited and revised manuscript; S.N., M.B., W.W.R., C.S.C., D.C.G., and G.H.C. approved final version of manuscript; G.H.C. drafted manuscript.
ACKNOWLEDGMENTS
Present address of M. Besprozvannaya: 16 Divinity Ave., Biological Laboratories 3036, Harvard University, Cambridge, MA 02138.
REFERENCES
- 1. Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114– 1121, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet 80: 467– 477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA 104: 5771– 5776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39–9437. Am J Physiol Lung Cell Mol Physiol 281: L16– L23, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma subunit. J Biol Chem 282: 6153– 6160, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther 9: 683– 685, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, Cornelissen I, Darragh MR, Hussain A, Zheng YW, Srinivasan Y, Brown C, Xu SM, Regard JB, Lin CY, Craik CS, Kirchhofer D, Coughlin SR. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell 18: 25– 38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caughey GH, Raymond WW, Blount JL, Hau LWT, Pallaoro M, Wolters PJ, Verghese GM. Characterization of human γ-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol 164: 6566– 6575, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chen LM, Skinner ML, Kauffman SW, Chao J, Chao L, Thaler CD, Chai KX. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem 276: 21434– 21442, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Colosimo A, Goncz KK, Novelli G, Dallapiccola B, Gruenert DC. Targeted correction of a defective selectable marker gene in human epithelial cells by small DNA fragments. Mol Ther 3: 178– 185, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Coote K, Atherton-Watson H, Sugar R, Young A, Mackenzie-Beevor A, Gosling M, Bhalay G, Bloomfield G, Dunstan A, Bridges RJ, Sabater JR, Abraham WM, Tully D, Pacoma R, Schumacher A, Harris JL, Danahay HL. Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J Pharmacol Exp Ther 329: 764– 774, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Desilets A, Beliveau F, Vandal G, McDuff FO, Lavigne P, Leduc R. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem 283: 10535– 10542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 277: 8338– 8345, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Farady CJ, Sun J, Darragh MR, Miller SM, Craik CS. The mechanism of inhibition of antibody-based inhibitors of membrane-type serine protease 1 (MT-SP1). J Mol Biol 369: 1041– 1051, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, Charles RP, Beermann F, Stehle JC, Breiden B, Sandhoff K, Rotman S, Haftek M, Wilson A, Ryser S, Steinhoff M, Coughlin SR, Hummler E. PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat Commun 2: 161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, Bugge TH, Lin CY, Vogel LK. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem 286: 5793– 5802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem 22: 57– 68, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazrak A, Nita I, Subramaniyam D, Wei S, Song W, Ji HL, Janciauskiene S, Matalon S. Alpha(1)-antitrypsin inhibits epithelial Na+ transport in vitro and in vivo. Am J Respir Cell Mol Biol 41: 261– 270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol 170: 487– 496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem 282: 36714– 36723, 2007 [DOI] [PubMed] [Google Scholar]
- 21. List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 21: 3765– 3779, 2002 [DOI] [PubMed] [Google Scholar]
- 22. List K, Hobson JP, Molinolo A, Bugge TH. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol 213: 237– 245, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Myerburg MM, McKenna EE, Luke CJ, Frizzell RA, Kleyman TR, Pilewski JM. Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L932– L941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem 281: 32941– 32945, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol 78: 23– 46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, Soler P, Clerici C, Rossier BC, Hummler E. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med 2: 26– 37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shipway A, Danahay H, Williams JA, Tully DC, Backes BJ, Harris JL. Biochemical characterization of prostasin, a channel activating protease. Biochem Biophys Res Commun 324: 953– 963, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Spacek DV, Perez AF, Ferranti KM, Wu LK, Moy DM, Magnan DR, King TR. The mouse frizzy (fr) and rat ‘hairless’ (frCR) mutations are natural variants of protease serine S1 family member 8 (Prss8). Exp Dermatol 19: 527– 532, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847– 850, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Sun J, Pons J, Craik CS. Potent and selective inhibition of membrane-type serine protease 1 by human single-chain antibodies. Biochemistry 42: 892– 900, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275: 26333– 26342, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591– 604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 287: L928– L935, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607– 610, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Verghese GM, Gutknecht MF, Caughey GH. Prostasin regulates epithelial monolayer function: cell-specific Gpld1-mediated secretion and functional role for the GPI anchor. Am J Physiol Cell Physiol 291: C1258– C1270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verghese GM, Tong ZY, Bhagwandin V, Caughey GH. Mouse prostasin gene structure, promoter analysis, and restricted expression in lung and kidney. Am J Respir Cell Mol Biol 30: 519– 529, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel- activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191– 201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu JX, Chao L, Chao J. Molecular cloning, tissue-specific expression, and cellular localization of human prostasin mRNA. J Biol Chem 270: 13483– 13489, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem 269: 18843– 18848, 1994 [PubMed] [Google Scholar]
- 40. Zhu H, Guo D, Li K, Yan W, Tan Y, Wang X, Treiber FA, Chao J, Snieder H, Dong Y. Prostasin: a possible candidate gene for human hypertension. Am J Hypertens 21: 1028– 1033, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]