Abstract
In pulmonary arterial smooth muscle cells (PASMC), acute hypoxia increases intracellular Ca2+ concentration ([Ca2+]i) by inducing Ca2+ release from the sarcoplasmic reticulum (SR) and Ca2+ influx through store- and voltage-operated Ca2+ channels in sarcolemma. To evaluate the mechanisms of hypoxic Ca2+ release, we measured [Ca2+]i with fluorescent microscopy in primary cultures of rat distal PASMC. In cells perfused with Ca2+-free Krebs Ringer bicarbonate solution (KRBS), brief exposures to caffeine (30 mM) and norepinephrine (300 μM), which activate SR ryanodine and inositol trisphosphate receptors (RyR, IP3R), respectively, or 4% O2 caused rapid transient increases in [Ca2+]i, indicating intracellular Ca2+ release. Preexposure of these cells to caffeine, norepinephrine, or the SR Ca2+-ATPase inhibitor cyclopiazonic acid (CPA; 10 μM) blocked subsequent Ca2+ release to caffeine, norepinephrine, and hypoxia. The RyR antagonist ryanodine (10 μM) blocked Ca2+ release to caffeine and hypoxia but not norepinephrine. The IP3R antagonist xestospongin C (XeC, 0.1 μM) blocked Ca2+ release to norepinephrine and hypoxia but not caffeine. In PASMC perfused with normal KRBS, acute hypoxia caused a sustained increase in [Ca2+]i that was abolished by ryanodine or XeC. These results suggest that in rat distal PASMC 1) the initial increase in [Ca2+]i induced by hypoxia, as well as the subsequent Ca2+ influx that sustained this increase, required release of Ca2+ from both RyR and IP3R, and 2) the SR Ca2+ stores accessed by RyR, IP3R, and hypoxia functioned as a common store, which was replenished by a CPA-inhibitable Ca2+-ATPase.
Keywords: caffeine, norepinephrine, cyclopiazonic acid, store-operated calcium entry, intracellular calcium concentration
accumulating evidence indicates that hypoxic pulmonary vasoconstriction (HPV) is mediated by an increase in intracellular Ca2+ concentration ([Ca2+]i) in pulmonary arterial smooth muscle cells (PASMC) caused by release of Ca2+ from sarcoplasmic reticulum (SR) and secondary Ca2+ entry through sarcolemmal store-operated Ca2+ channels (SOCC). For example, acute hypoxia increased store-operated Ca2+ entry (SOCE), as measured by Mn2+ quenching of fura-2 fluorescence and [Ca2+]i responses to restoration of extracellular Ca2+ in PASMC perfused with Ca2+-free physiologic salt solutions (35, 48). Antagonists of SOCC, such as SKF-96365 and NiCl2, blocked hypoxia-induced increases in SOCE and [Ca2+]i in PASMC and HPV in isolated lungs but not the increases in PASMC [Ca2+]i or pulmonary vasoconstriction induced by KCl (35, 48, 52). Preventing SOCE by removal of extracellular Ca2+ or knockdown of stromal interaction molelcule 1 (STIM1), a 90-kDa transmembrane Ca2+-binding protein found in SR and sarcolemma that activates SOCE upon depletion of SR Ca2+ (3), blocked the sustained but not the initial increase in [Ca2+]i generated by hypoxia in PASMC (28, 34, 42).
In vascular smooth muscle, SR Ca2+ is released through channels in the SR membrane known as ryanodine and inositol 1,4,5-trisphosphate receptors (RyR, IP3R). RyR and IP3R each have three subtypes (RyR1–3; IP3R1–3), all of which are expressed in PASMC (9, 54, 57, 59, 60). Pharmacologically, RyR can be activated by caffeine and inhibited by the polycyclic polyhydroxylic diterpene ryanodine, whereas IP3R can be activated by agonists of sarcolemmal G-protein- or tyrosine kinase-linked receptors, which stimulate production of IP3 by phospholipase C, and inhibited by xestospongin C (XeC), an alkaloid produced by the Australian marine sponge species Xestospongia (13, 20, 22).
The SR Ca2+ stores accessed by RyR and IP3R may be the same or different. Store depletion by cyclopiazonic acid (CPA) or thapsigargin, which inhibit Ca2+-ATPases that pump Ca2+ into the SR, prevented contractile responses to both norepinephrine and caffeine in rat pulmonary arteries (15). In freshly isolated rat PASMC, caffeine prevented increases in [Ca2+]i in response to norepinephrine and, conversely, norepinephrine prevented increases in [Ca2+]i in response to caffeine (50). These results suggested that RyR and IP3R accessed the same SR Ca2+ store in rat PASMC. In contrast, CPA or thapsigargin blocked contractions and increases in [Ca2+]i induced by phenylephrine or angiotensin II but not caffeine in canine freshly isolated PASMC and pulmonary arteries (16, 17). Furthermore, depletion of SR Ca2+ stores with ryanodine and caffeine eliminated [Ca2+]i responses to caffeine but not to angiotensin II (17). These results suggested that RyR and IP3R accessed separate SR Ca2+ stores in canine PASMC and that the SR Ca2+-ATPase inhibited by CPA or thapsigargin in these cells maintained stores accessed by IP3R but not RyR.
There is evidence that Ca2+ release from RyR plays an important role in HPV. Ryanodine or ryanodine plus caffeine strongly inhibited or abolished hypoxia-induced increases in [Ca2+]i in rat PASMC (8, 46) and hypoxic constriction in pulmonary arteries isolated from rats (7–9, 14), pigs (27), and dogs (16). By itself, caffeine blocked the early transient increase and partially inhibited the steady-state increase in [Ca2+]i caused by dithionite-induced hypoxia in rat PASMC (42). Hypoxic pulmonary arterial constriction and increases in PASMC [Ca2+]i were reduced in mice deficient in the RyR1 or RyR3 isoforms of the receptor (25, 60) and enhanced in mice deficient in FK506 binding protein 12.6 (FKBP12.6), which stabilizes RyR in a closed state (58). However, other data suggest that RyR plays a minor role in HPV. Ryanodine or ryanodine plus caffeine had little or no effect on HPV in rat pulmonary arteries (19, 40). High concentrations of ryanodine (≥100 μM) caused incomplete inhibition of [Ca2+]i and contractile responses to hypoxia (∼65 and 50%, respectively) in rat PASMC and pulmonary arterial strips (60) and reversed HPV by only 20–30% in nitric oxide synthase-inhibited isolated rat lungs (32).
It is even less clear whether Ca2+ release from IP3R contributes to HPV. In rat pulmonary arteries, tissue IP3 levels increased during norepinephrine-induced constriction but did not change during hypoxia-induced constriction (18). Thapsigargin or CPA, which were thought to deplete IP3R- but not RyR-linked SR Ca2+ stores, potentiated HPV in canine pulmonary arteries (16) and isolated rat lungs (32) but inhibited HPV in rat pulmonary arteries (7, 14). In canine PASMC, SOCE induced by depletion of RyR- and IP3R-linked Ca2+ stores was not blocked by high concentrations of ryanodine or further increased by hypoxia, and SOCE induced by hypoxia was not further increased by store depletion (35, 36), suggesting that store depletion and hypoxia might induce SOCE by a similar mechanism, such as activation of IP3R. However, the IP3R antagonist XeC inhibited SOCE induced by store depletion but did not alter SOCE induced by hypoxia, indicating that IP3R did not contribute to the effects of hypoxia (36). These interpretations are complicated by the rapid and marked decrease in baseline [Ca2+]i that occurred in canine PASMC upon exposure to Ca2+-free perfusate alone (33, 35, 36). This decrease, which did not occur in rat distal PASMC (28, 29, 47–49), indicates that a large influx of Ca2+ was required to maintain [Ca2+]i at normal levels in canine PASMC under basal conditions. The nature of this influx and its influence on [Ca2+]i responses to hypoxia and evaluation of SOCE in these cells remain unclear.
The present study was performed to clarify the role played by SR Ca2+ release from RyR and IP3R in the [Ca2+]i response of rat distal PASMC to acute hypoxia.
METHODS
Isolation and culture of PASMC.
Animal protocols were approved by the Johns Hopkins Animal Care and Use Committee. As previously described (47), distal (>4th generation) pulmonary arteries were dissected from lungs of male Wistar rats (300–500 g body wt) anesthetized with pentobarbital sodium (65 mg/kg ip). Endothelium was removed by rubbing with a cotton swab. PASMC were obtained by enzymatic digestion, plated on glass coverslips, and either maintained for 1–2 days in basal medium (Ham's F-12 medium with l-glutamine, Mediatech, Manassas, VA) containing 0.5% fetal calf serum, 1% streptomycin, and 1% penicillin (freshly isolated cells) or cultured for 3–4 days in Smooth Muscle Growth Medium 2 (Clonetics, Walkersville, MD) containing 5% serum, 1% streptomycin, and 1% penicillin (cultured cells). In cultured cells, serum concentration was decreased to 0.3% 24 h before an experiment to stop cell growth. Cellular purity was >98%, as assessed by morphological appearance under phase-contrast microscopy and immunofluorescence staining for α-actin (47).
Measurement of [Ca2+]i.
PASMC on coverslips were loaded with 7.5 μM fura-2 (Molecular Probes, Eugene, OR) for 60 min at 37°C under an atmosphere of 5% CO2-95% air and then mounted in a closed polycarbonate chamber clamped in a heated aluminum platform (PH-2; Warner Instruments, Hamden, CT) on the stage of a Nikon TSE 100 Ellipse inverted microscope (Melville, NY). The chamber was perfused at 1 ml/min with Krebs-Ringer bicarbonate solution (KRBS), which contained the following (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 0.57 MgSO4, 1.18 KH2PO4, 25 NaHCO3, and 10 glucose. The KRBS was equilbrated with a normoxic gas mixture (16% O2-5% CO2) at 38°C in heated reservoirs and led via stainless steel tubing and manifold through an in-line heat exchanger (SF-28; Warner Instruments) controlled by a dual channel heater controller (TC-344B, Warner Instruments) set to maintain chamber temperature at 37°C. To allow complete removal of extracellular dye and stabilization of chamber temperature, PASMC were perfused for 15 min before initiation of the experimental protocols described below. In each experiment, fura-2 fluorescence at 510 nm after excitation at 340 and 380 nm (F340, F380) was measured at 6-s intervals in 20–30 PASMC using a collimated light beam from a xenon arc lamp filtered by interference filters at 340 and 380 nm and focused onto cells visualized with a ×20 fluorescence objective (Super Fluor 20; Nikon) and a cooled charge-coupled device imaging camera. Data were collected online with InCyte software (Intracellular Imaging, Cincinnati, OH). [Ca2+]i was estimated from F340/F380 measured in calibration solutions with Ca2+ concentrations of 0–1,350 nM (Molecular Probes, Eugene, OR).
Experimental protocols.
To determine how the absence of the extracellular Ca2+ concentration ([Ca2+]e) affected [Ca2+]i responses of PASMC to hypoxia, cells were perfused with normal (n = 9) or Ca2+-free (n = 4) KRBS and sequentially exposed to normoxia for 5 min and then hypoxia for 10 min. Ca2+-free KRBS contained 0.5 mM EGTA and no CaCl2, but otherwise its composition was the same as normal KRBS. During hypoxia, perfusates were equilibrated with 4% O2-5% CO2.. After hypoxia, all cells were perfused with normoxic normal KRBS.
To confirm that hypoxia caused Ca2+ release from RyR and IP3R and to determine whether SR Ca2+ stores accessed by these receptors were the same or different, we measured the effects of preexposure to caffeine (30 mM), norepinephrine (300 μM), or CPA (10 μM) on subsequent responses to caffeine (n = 4, 5, and 5, respectively), norepinephrine (n = 4, 5, and 4, respectively), and hypoxia (n = 4, 4, and 4, respectively) in primary cultures of PASMC perfused with Ca2+-free KRBS. Preexposure to caffeine and norepinephrine occurred at 2–5 min of Ca2+-free perfusion, while preexposure to CPA occurred at 2–15 min. Subsequent exposures to caffeine, norepinephrine, and hypoxia occurred at 10–15 min. Responses to caffeine (n = 11), norepinephrine (n = 9), and hypoxia (n = 12) measured at 10–15 min in PASMC not subjected to preexposures provided control values. To assess the effect of cell culture on compartmentation of SR Ca2+ stores, we also determined the effects of preexposure to CPA on subsequent responses to caffeine (n = 3) and norepinephrine (n = 3) in freshly isolated PASMC. Similarly treated freshly isolated cells not subjected to preexposures served as controls (n = 3 and 3, respectively).
To determine the contribution of RyR and IP3R to hypoxia-induced intracellular Ca2+ release in PASMC, we measured the effects of 5-min exposure to caffeine (30 mM), norepinephrine (300 μM), or hypoxia (4% O2) on [Ca2+]i after 2 min of perfusion with Ca2+-free KRBS followed by 8 min of perfusion with Ca2+-free KRBS containing either ryanodine (10 μM; n = 4, 3, and 5, respectively) or XeC (0.1 μM; n = 4, 4, and 4, respectively). PASMC not exposed to ryanodine or XeC served as controls (n = 11, 9, and 12, respectively). To determine whether Ca2+ release from RyR and/or IP3R was required for the [Ca2+]i response to hypoxia, PASMC perfused with normal KRBS containing ryanodine (10 μM; n = 3) or XeC (0.1 μM; n = 3) were sequentially exposed to normoxia for 10 min and hypoxia for 20 min. Again, cells not exposed to these antagonists served as controls (n = 6).
Statistical analysis.
Data are expressed as means ± SE, and n equals both the number of experiments and the number of animals that provided cells. Statistical analyses were performed using t-tests or ANOVA, as appropriate. When significant F-ratios were obtained with ANOVA, pair wise comparisons of means were performed using least significant differences. Comparisons with control values were made using Dunnett's two-tailed test. Differences were considered significant when P < 0.05.
Solutions and drugs.
Stock solutions of norepinephrine and caffeine were made up in water on the day of the experiment. Stock solutions of ryanodine and CPA were made up in DMSO and stored at −20°C until use. XeC was stored at −20°C and made up in DMSO on the day of the experiment. Ryanodine, XeC, caffeine, and norepinephrine were obtained from CalBiochem (La Jolla, CA). All other reagents were obtained from Sigma Chemical (St. Louis, MO).
RESULTS
Effect of Ca2+-free perfusion on the [Ca2+]i response to hypoxia.
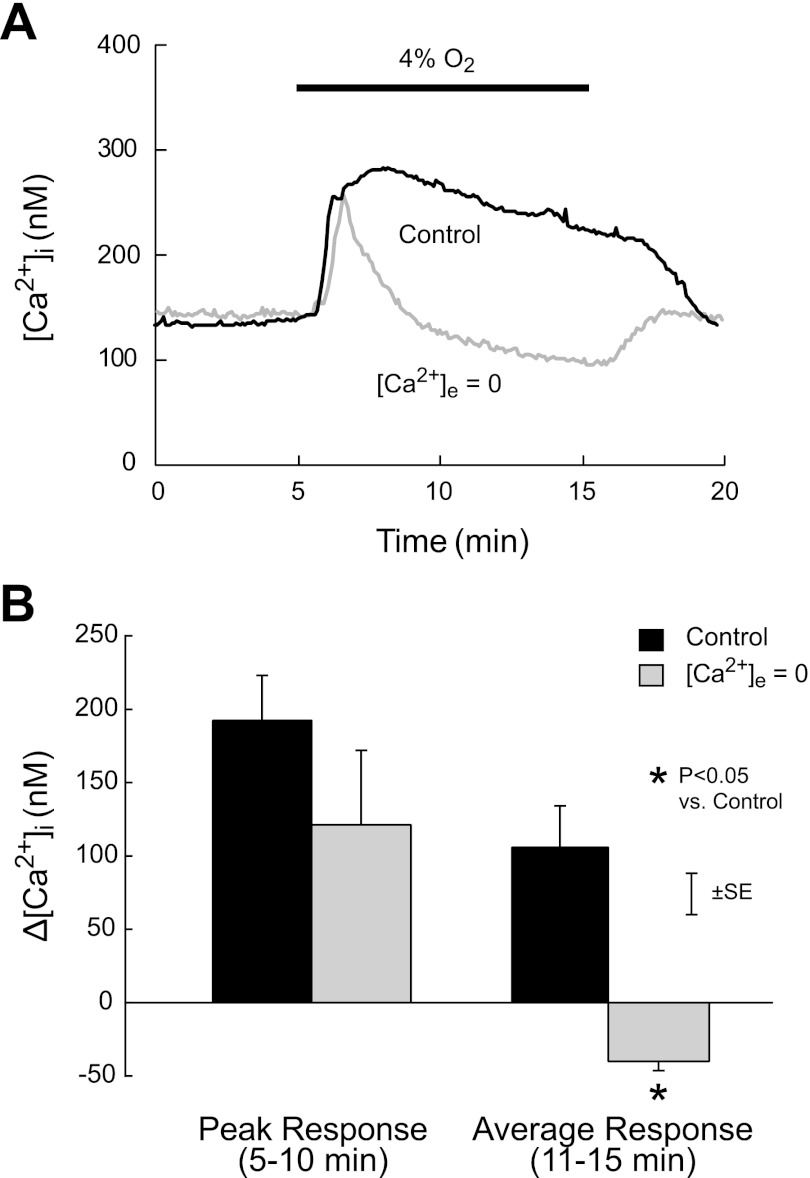
The time course of mean [Ca2+]i in PASMC exposed to hypoxia is shown in Fig. 1A. Normoxic baseline [Ca2+]i was not different in cells perfused with normal and Ca2+-free KRBS (140 ± 31 vs. 147 ± 77 nM; P > 0.9). In PASMC perfused with normal KRBS, exposure to hypoxia for 10 min caused a rapid increase in [Ca2+]i to a maximum of 286 nM after 3.3 min of exposure. [Ca2+]i then slowly declined to 231 nM at the end of exposure and fell rapidly to baseline upon restoration of normoxia. In cells perfused with Ca2+-free KRBS, hypoxia again rapidly increased [Ca2+]i; however, the response (147 to 268 nM) peaked earlier (1.5 min of exposure) and was followed by a rapid fall to baseline (3.8 min of exposure). During the remainder of the exposure, [Ca2+]i declined more slowly and then rose rapidly to baseline upon restoration of normoxia and normal [Ca2+]e. The peak Δ[Ca2+]i induced by hypoxia during the first 5 min of exposure was not different in PASMC perfused with normal and Ca2+-free KRBS (Fig. 1B; 192 ± 30 vs. 121 ± 59 nM, P > 0.1); however, the average Δ[Ca2+]i during the last 5 min of hypoxia was greater in cells perfused with normal KRBS (106 ± 28 vs. −40 ± 6 nM; P < 0.007).
Fig. 1.
A: time course of mean change in intracellular Ca2+ concentration ([Ca2+]i) during acute hypoxia (4% O2) in rat distal pulmonary arterial smooth muscle cells (PASMC) exposed to normal (Control) or zero extracellular Ca2+ concentration ([Ca2+]e = 0). B: mean values of peak and average [Ca2+]i responses (Δ[Ca2+]i) measured, respectively, during the first (5–10 min) and last (11–15 min) 5-min halves of the 10-min period of hypoxia (4% O2) in rat distal PASMC exposed to normal (Control) or zero extracellular Ca2+ concentration ([Ca2+]e = 0).
Compartmentation of SR Ca2+ stores in rat distal PASMC.
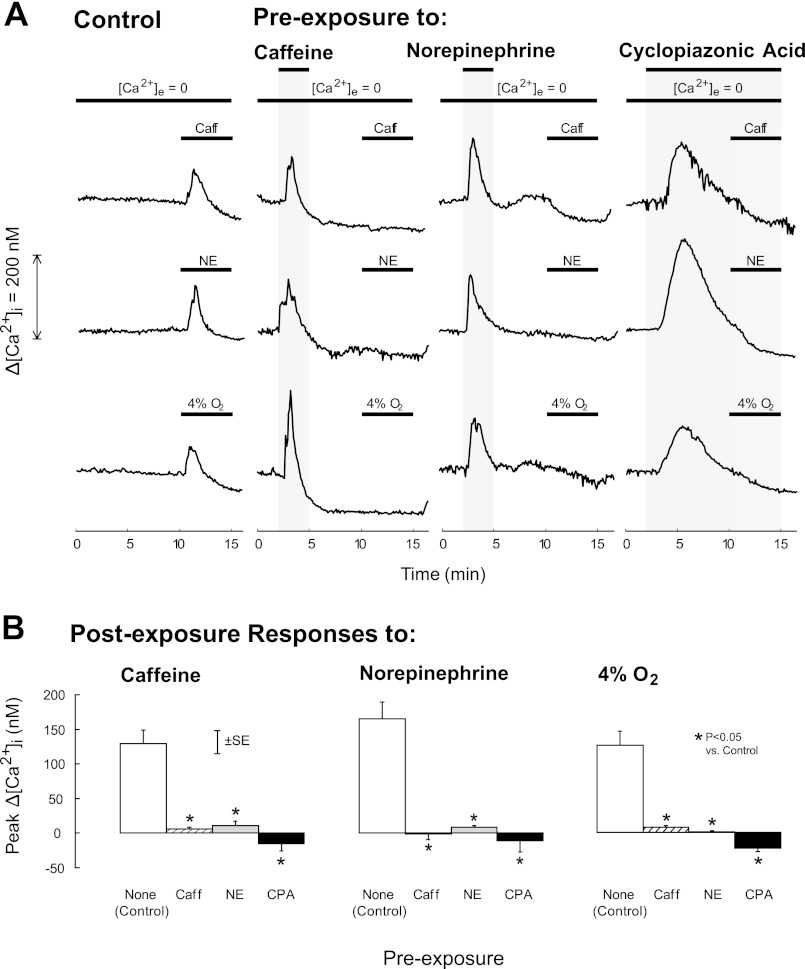
The effects of preexposure to caffeine (RyR agonist), norepinephrine (IP3R agonist), or CPA (SR Ca2+-ATPase pump antagonist) on [Ca2+]i responses to subsequent exposures to caffeine, norepinephrine, and hypoxia in PASMC perfused with Ca2+-free KRBS are shown in Fig. 2. These responses were compared with those obtained in control PASMC, which were not preexposed but were perfused with Ca2+-free KRBS for the same amount of time as preexposed cells. All preexposures caused transient increases in [Ca2+]i (Fig. 2A) and abolished postexposure increases to caffeine (P < 0.001), norepinephrine (P < 0.001), and hypoxia (P < 0.002; Fig. 2B). Preexposure to CPA also abolished increases in [Ca2+]i induced by caffeine and norepinephrine in freshly isolated rat distal PASMC (Table 1).
Fig. 2.
A: time course of mean change in [Ca2+]i from baseline (Δ[Ca2+]i) induced by 5-min exposures to 30 mM caffeine (Caff, first row), 300 μM norepinephrine (NE, second row), or hypoxia (4% O2, third row) in rat distal PASMC perfused with Ca2+-free Krebs Ringer bicarbonate solution (KRBS) ([Ca2+]e = 0) after preexposure to 30 mM caffeine, 300 μM norepinephrine, or 10 μM cyclopiazonic acid (CPA), as indicated by the top horizontal bars. Control PASMC were treated similarly but not subjected to preexposures. B: mean peak postexposure change in [Ca2+]i (Δ[Ca2+]i) induced by caffeine (left), norepinephrine (middle), and 4% O2 (right) in rat distal PASMC perfused with Ca2+-free KRBS after no preexposure [none (Control)] or preexposure to caffeine, NE, or CPA.
Table 1.
Effects of CPA on SR Ca2+ release induced by caffeine and norepinephrine in freshly isolated rat distal PASMC
| Peak Δ[Ca2+]i, nM |
|||
|---|---|---|---|
| Stimulus | Control | CPA (10 μM) | P Value |
| Caffeine (30 mM) | 290 ± 94 | −3 ± 2 | <0.04 |
| Norepinephrine (300 μM) | 249 ± 14 | 3 ± 6 | <0.001 |
Values are means ± SE. PASMC, pulmonary arterial smooth muscle cells; CPA, cyclopiazonic acid; SR, sarcoplasmic reticulum; Δ[Ca2+]i, change in intracellular Ca2+ concentration.
Contribution of SR ryanodine and IP3 receptors to hypoxia-induced Ca2+ release.
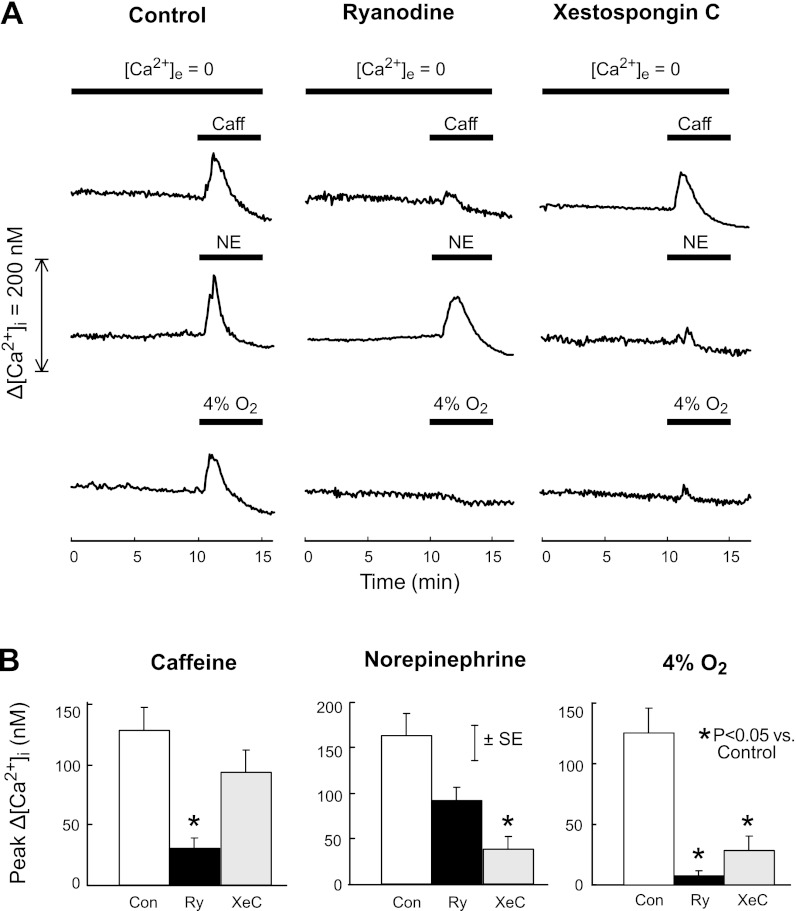
The effects of RyR and IP3R antagonists on Ca2+ release induced by caffeine, norepinephrine, and hypoxia are shown in Fig. 3. In control cells perfused with Ca2+-free KRBS, baseline [Ca2+]i remained stable (111 ± 7 nM during the 1st min vs. 107 ± 7 nM during the 10th min of perfusion; P > 0.2). At 10–15 min, caffeine, norepinephrine, and hypoxia caused rapid transient increases in [Ca2+]i (Fig. 3A) that averaged 129 ± 19, 165 ± 23, and 125 ± 22 nM, respectively (Fig. 3B). Addition of the RyR antagonist ryanodine (10 μM) to the perfusate did not alter baseline [Ca2+]i (83 ± 6 nM during min 1 vs. 81 ± 6 nM during min 10; P > 0.3) or the [Ca2+]i response to norepinephrine (94 ± 14 nM, P = 0.09) but markedly reduced [Ca2+]i responses to caffeine (30 ± 14 nM; P < 0.006) and hypoxia (8 ± 3 nM; P < 0.004). Perfusion with Ca2+-free KRBS containing the IP3R antagonist XeC (0.1 μM) did not alter baseline [Ca2+]i (84 ± 9 nM during minute 1 vs. 78 ± 8 nM during minute 10, P > 0.1) or the [Ca2+]i response to caffeine (94 ± 18 nM; P = 0.28) but markedly reduced [Ca2+]i responses to norepinephrine (36 ± 14 nM; P < 0.003) and hypoxia (28 ± 12 nM; P < 0.02).
Fig. 3.
A: time course of mean change in [Ca2+]i from baseline (Δ[Ca2+]i) caused by 30 mM caffeine (first row), 300 μM NE (second row), or hypoxia (4% O2, third row) after treatment with ryanodine (10 μM) or xestospongin C (0.1 μM) beginning at 2 min in rat distal PASMC perfused with Ca2+-free KRBS ([Ca2+]e = 0). Untreated cells served as controls. B: mean peak changes in [Ca2+]i (Δ[Ca2+]i) induced by caffeine (left), norepinephrine (middle), and 4% O2 (right) in rat distal PASMC perfused with Ca2+-free KRBS after treatment with ryanodine (Ry; 10 μM) or xestospongin C (XeC; 0.1 μM). Untreated cells served as controls (Con).
Contribution of SR ryanodine and IP3 receptors to the [Ca2+]i response to hypoxia.
The effects of RyR and IP3R antagonists on the [Ca2+]i response to hypoxia in PASMC perfused with normal KRBS are shown in Fig. 4. Similar to control cells shown in Fig. 1, exposure of control PASMC to 4% O2 for 10 min rapidly increased mean [Ca2+]i from a baseline of 117 nM to a maximum of 243 nM at 3.5 min of exposure, followed by a slow decline to 212 nM at the end of exposure, and then a rapid fall to baseline upon restoration of normoxia (Fig. 4A). In PASMC treated with ryanodine (10 μM) or XeC (0.1 μM), this response was abolished except for an initial transient increase that was much smaller than the initial increase observed in control cells (Fig. 4B: 15 ± 5 and 32 ± 10 nM in cells treated with ryanodine and XeC, respectively, vs. 160 ± 33 nM in control cells; P < 0.02).
Fig. 4.
A: time course of the mean change in [Ca2+]i from baseline (Δ[Ca2+]i) induced by hypoxia (4% O2) after treatment with ryanodine (10 μM) or xestospongin C (0.1 μM) beginning at 2 min in rat distal PASMC perfused with normal KRBS. Untreated cells served as controls. B: mean peak changes in [Ca2+]i (Δ[Ca2+]i) induced by hypoxia (4% O2) in rat distal PASMC perfused with Ca2+-free KRBS after treatment with ryanodine (10 μM) or xestospongin C (0.1 μM). Untreated cells served as controls.
DISCUSSION
In rat distal PASMC, acute hypoxia caused a rapid increase in [Ca2+]i that was transient when [Ca2+]e was zero but sustained when [Ca2+]e was normal (Fig. 1), consistent with previous observations in this preparation (48, 49) and rat main PASMC exposed to dithionite-generated hypoxia (42). These results confirm that acute hypoxia caused transient release of Ca2+ from intracellular stores followed by sustained influx of Ca2+ from extracellular fluid.
We previously found that sustained [Ca2+]i responses of rat distal PASMC to acute hypoxia were completely blocked by knockdown of STIM1 (28), the protein that activates SOCC in response to decreased [Ca2+] in the SR lumen (3), or by SKF-96365 or NiCl2, pharmacological antagonists of SOCC used at concentrations that did not alter [Ca2+]i responses to KCl (48). In contrast, nifedipine, an antagonist of L-type voltage-operated Ca2+ channels (VOCC) used at concentrations that abolished [Ca2+]i responses to KCl, only partially inhibited sustained [Ca2+]i responses to hypoxia (48). These results suggested that acute hypoxia released Ca2+ from the SR, leading to activation of SOCC and SOCE, which in turn led to activation of VOCC and voltage-operated Ca2+ entry (VOCE). The mechanisms by which SOCE might activate VOCE are unknown, but possibilities include PASMC depolarization secondary to activation of Ca2+-dependent Cl− channels (5, 12, 23), Ca2+-dependent inhibition of voltage-dependent K+ channels (38), and/or influx of Na+ (2) through SOCC, which are likely to be nonspecific cation channels with reversal potentials near 0 mV (37). The present study focuses on hypoxia-induced release of Ca2+ from SR, which initiates these downstream events.
To determine whether hypoxia released Ca2+ from SR stores accessed by RyR or IP3R and whether these stores were the same or independent, we measured the effects of preexposure to the RyR agonist, caffeine, the IP3R agonist norepinephrine and the SR Ca2+-ATPase inhibitor CPA on subsequent [Ca2+]i responses to caffeine, norepinephrine, or hypoxia in rat distal PASMC perfused with Ca2+-free KRBS. As shown in Fig. 2, all preexposures prevented all postexposure responses. The simplest explanation for these results is that caffeine-induced activation of RyR and norepinephrine-induced activation of IP3R caused Ca2+ release from, and depletion of, a common SR Ca2+ store, which was maintained by a CPA-inhibitable Ca2+-ATPase and provided the Ca2+ released by hypoxia. Alternatively, RyR and IP3R could access separate stores that were in sufficient proximity to allow the activation of one receptor to cause heterologous desensitization of the other via cytoplasmic signaling pathways (31). For example, Ca2+ released by activation of IP3R could stimulate Ca2+ release from RyR, or vice-versa, leading to depletion of the separate stores and subsequent desensitization to both caffeine and norepinephrine. In this case, anatomically separate stores would function as a common store. In theory, it is also possible that signaling pathways between separate stores in close proximity could be Ca2+ independent and/or cause heterologous receptor desensitization via mechanisms other than Ca2+ store depletion; however, the nature of such pathways and whether they exist in smooth muscle is unknown (31).
The results shown in Fig. 2 are consistent with previous observations in rat pulmonary arteries (15) and freshly isolated rat PASMC exposed to normal [Ca2+]e, where caffeine prevented contractile or Ca2+ release responses to norepinephrine or IP3 and norepinephrine or IP3 prevented responses to caffeine (50, 60). They are not consistent, however, with results obtained in canine pulmonary arteries (16) and freshly isolated canine PASMC exposed to normal [Ca2+]e (17), where CPA blocked contractile and [Ca2+]i responses to phenylephrine or angiotensin II but not caffeine, suggesting that RyR- and IP3R-linked stores were independent and maintained by different mechanisms. Subsequent experiments from the same laboratory in cultured canine PASMC (33) indicated that CPA blocked responses to both caffeine and 5-hydroxytryptamine, which was thought to activate IP3R. Since CPA blocked responses to 5-hydroxytryptamine but not caffeine in freshly isolated canine PASMC (33), these results suggested that culture of canine PASMC caused reorganization of the SR into a common Ca2+ store accessed by both RyR and IP3R.
To evaluate the influence of cell culture in our preparation, we determined the effects of CPA on Ca2+ release induced by caffeine and norepinephrine in freshly isolated rat distal PASMC. In these cells, CPA prevented Ca2+ release to both caffeine and norepinephrine (Table 1), as it did in primary cultures of rat distal PASMC (Fig. 2). Thus cell culture did not account for the discrepancies between the results that we (Fig. 2) and others (50, 60) obtained in rat distal PASMC and those obtained in canine PASMC (17, 33). These discrepancies could be due to differences in vessels providing the PASMC, species, or other factors; however, the effects of SOCC and VOCC antagonists on vasoconstrictor responses to hypoxia and KCl in isolated rat lungs (52) were similar to their effects on [Ca2+]i responses to hypoxia and KCl in primary cultures of rat distal PASMC (48), supporting the physiological relevance of our cell preparation.
Although the results shown in Fig. 2 suggest that caffeine, norepinephrine, and hypoxia released Ca2+ from a common SR store, they do not indicate whether hypoxic Ca2+ release involved RyR, IP3R, or some other Ca2+ release pathway. To evaluate these possibilities, we determined the effects of the RyR antagonist ryanodine and the IP3R antagonist XeC on [Ca2+]i responses to caffeine, norepinephrine, and hypoxia in rat distal PASMC perfused with Ca2+-free KRBS. As shown in Fig. 3, ryanodine inhibited Ca2+ release to caffeine but not norepinephrine, and XeC inhibited Ca2+ release to norepinephrine but not caffeine, confirming that ryanodine and XeC had their intended actions. In contrast, Ca2+ release to hypoxia was virtually abolished by either antagonist. These results suggest that hypoxic Ca2+ release required activation of both RyR and IP3R. Furthermore, Ca2+ release pathways other than RyR and IP3R were not required.
The validity of these conclusions depends on the absence of nonspecific actions of ryanodine and XeC that could prevent Ca2+ release to hypoxia. For example, low concentrations of ryanodine can lock RyR in an open low-conductance state (4, 22, 43) and high concentrations of XeC can inhibit SR Ca2+-ATPase (4, 6). Such actions might deplete a common SR Ca2+ store and thereby prevent Ca2+ release to hypoxia; however, this possibility is unlikely because, as noted above, high concentrations of ryanodine did not prevent Ca2+ release to norepinephrine, and low concentrations of XeC did not prevent Ca2+ release to caffeine (Fig. 3).
The mechanisms by which hypoxia may have activated RyR and IP3R in PASMC remain unclear. With respect to RyR, one possibility is cyclic ADP ribose (cADPR), which causes activation by binding to the channel or FKBP12.6, an associated regulatory protein (24, 44). Hypoxia increased cADPR concentration in PASMC, possibly by increasing cADPR synthesis via activation of AMP kinase (10, 11, 41) or decreasing cADPR catabolism via inhibition of ADP-ribosyl hydrolase (7, 8, 53). Another possibility is reactive O2 species, which increased in the cytosol of hypoxic PASMC (51) and caused dissociation of FKBP12.6 from RyR, thereby activating the channel (26). With respect to IP3R, PASMC are able to generate their own agonists, such as endothelin (1, 30, 45, 55, 56). Although hypoxia did not increase endothelin levels in PASMC (30) as it did in endothelial cells (21), normoxic levels of this agonist might generate enough IP3 to activate IP3R. In this case, basal Ca2+ release from IP3R could facilitate stimulated Ca2+ release from nearby RyR, which could then cause Ca2+-induced Ca2+ release from IP3R, leading to continued Ca2+ release from both RyR and IP3R and eventual store depletion. Consistent with this possibility, exogenous endothelin was found to cause cross-activation of RyR and IP3R in rat PASMC, where the channels appeared to be colocalized (57). Cross-activation would also explain why hypoxic Ca2+ release required both RyR and IP3R. More investigation is needed to evaluate these and other possible mechanisms of hypoxic Ca2+ release in PASMC.
As noted above, we previously found that knockdown of STIM1 prevented the late sustained but not the early transient [Ca2+]i response to hypoxia in rat PASMC perfused with normal KRBS (28). Since STIM1 senses SR [Ca2+] and transduces SOCC activation (3), these results imply that the direct contribution of Ca2+ release to the sustained hypoxic [Ca2+]i response was activation of SOCE, and not some other effect, such as Ca2+-dependent alteration of sarcolemmal K+ or Cl− channel activity (39, 57). If this is correct, and both RyR and IP3R are required for hypoxic Ca2+ release (Fig. 3), then blocking either RyR or IP3R should prevent the sustained [Ca2+]i response to hypoxia. We tested this possibility by measuring the effects of ryanodine and XeC on the [Ca2+]i response to hypoxia in rat distal PASMC perfused with normal KRBS. As shown in Fig. 4, both antagonists abolished the sustained increase in [Ca2+]i caused by hypoxia in these cells. These results provide further support for the conclusion that Ca2+ release from RyR and IP3R, depletion of SR Ca2+ stores, and SOCE are essential components of the [Ca2+]i response to acute hypoxia in PASMC.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-51912 and HL-75113 (to J. T. Sylvester), HL-67191 (to L. A. Shimoda), and HL-093020 (to J. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W., L.A.S., and J.T.S. conception and design of research; J.W. performed experiments; J.W., L.A.S., and J.T.S. analyzed data; J.W., L.A.S., and J.T.S. interpreted results of experiments; J.W. and J.T.S. drafted manuscript; J.W., L.A.S., and J.T.S. edited and revised manuscript; J.W., L.A.S., and J.T.S. approved final version of manuscript; J.T.S. prepared figures.
REFERENCES
- 1. Barker S, Khan NQ, Wood EG, Corder R. Effect of an antisense oligodeoxynucleotide to endothelin-converting enzyme-1c (ECE-1c) on ECE-1c mRNA, ECE-1 protein and endothelin-1 synthesis in bovine pulmonary artery smooth muscle cells. Mol Pharmacol 59: 163–169, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bergofsky EH, Holtzman S. A study of the mechanisms involved in the pulmonary arterial pressor response to hypoxia. Circ Res 20: 506–519, 1967 [DOI] [PubMed] [Google Scholar]
- 3. Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol 11: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castonguay A, Robitaille R. Xestospongin C is a potent inhibitor of SERCA at a vertebrate synapse. Cell Calcium 32: 39–47, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Clapp LH, Turner JL, Kozlowski RZ. Ca2+-activated Cl− currents in pulmonary arterial myocytes. Am J Physiol Heart Circ Physiol 270: H1577–H1584, 1996 [DOI] [PubMed] [Google Scholar]
- 6. De Smet P, Parys JB, Callewaert G, Weidema AF, Hill E, De Smedt H, Erneux C, Sorrentino V, Missiaen L. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium 26: 9–13, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res 89: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 281: L318–L325, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Du W, Frazier M, McMahon TJ, Eu JP. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 128: 556S-–558S., 2005 [DOI] [PubMed] [Google Scholar]
- 10. Evans AM. AMP-activated protein kinase underpins hypoxic pulmonary vasoconstriction and carotid body excitation by hypoxia in mammals. Exp Physiol 91: 821–827, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem 280: 41504–41511, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells. Adv Exp Med Biol 661: 31–55, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Gelband CH, Gelband H. Ca2+ release from intracellular stores is an initial step in hypoxic pulmonary vasoconstriction of rat pulmonary artery resistance vessels. Circulation 96: 3647–3654, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez De La Fuente P, Savineau JP, Marthan R. Control of pulmonary vascular smooth muscle tone by sarcoplasmic reticulum Ca2+ pump blockers: thapsigargin and cyclopiazonic acid. Pflügers Arch 429: 617–624, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 122: 21–30, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 280: C22–C33, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Jin N, Packer CS, English D, Rhoades RA. Inositol trisphosphate is involved in norepinephrine- but not in hypoxia-induced pulmonary arterial contraction. Am J Physiol Lung Cell Mol Physiol 264: L160–L164, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Jin N, Packer CS, Rhoades RA. Pulmonary arterial hypoxic contraction: signal transduction. Am J Physiol Lung Cell Mol Physiol 263: L73–L78, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49: 157–230, 1997 [PubMed] [Google Scholar]
- 21. Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 88: 1054–1057, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev 56: 439–513, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Large WA, Piper AS, Yuan JX. Ca2+-activated Cl− channels and pulmonary vascular tone. In: Ion Channels in the Pulmonary Vasculature, edited by Claude L. Boca Raton, FL: Taylor & Francis, 2005, p. 311–334 [Google Scholar]
- 24. Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev 77: 1133–1164, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflügers Arch 457: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liao B, Zheng YM, Yadav VR, Korde AS, Wang YX. Hypoxia induces intracellular Ca2+ release by causing reactive oxygen species-mediated dissociation of FK506-binding protein 12.6 from ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal 14: 37–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Q, Sham JS, Shimoda LA, Sylvester JT. Hypoxic constriction of porcine distal pulmonary arteries: endothelium and endothelin dependence. Am J Physiol Lung Cell Mol Physiol 280: L856–L865, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L17–L25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Markewitz BA, Farrukh IS, Chen Y, Li Y, Michael JR. Regulation of endothelin-1 synthesis in human pulmonary arterial smooth muscle cells. Effects of transforming growth factor-beta and hypoxia. Cardiovasc Res 49: 200–206, 2001 [DOI] [PubMed] [Google Scholar]
- 31. McGeown JG. Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two? Cell Calcium 35: 613–619, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Morio Y, McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol 92: 527–534, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR. Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 294: C313–C323, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng LC, Wilson SM, Hume JR. Mobilization of SR stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol 152: 101–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 77: 131–139, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L433–L444, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525: 669–680, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robertson TP, Mustard KJ, Lewis TH, Clark JH, Wyatt CN, Blanco EA, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase and hypoxic pulmonary vasoconstriction. Eur J Pharmacol 595: 39–43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L323–L328, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev 49: 53–98, 1997 [PubMed] [Google Scholar]
- 44. Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol 282: H1304–H1310, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Tchekneva E, Lawrence ML, Meyrick B. Cell-specific differences in ET-1 system in adjacent layers of main pulmonary artery. A new source of ET-1. Am J Physiol Lung Cell Mol Physiol 278: L813–L821, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol Lung Cell Mol Physiol 265: L591–L597, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Wang J, Weigand LA, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Wang YX, Zheng YM, Abdullaev I, Kotlikoff MI. Metabolic inhibition with cyanide induces calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol Cell Physiol 284: C378–C388, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. A primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem 276: 11180–11188., 2001 [DOI] [PubMed] [Google Scholar]
- 54. Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L338–L348, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Yi SL, Kantores C, Belcastro R, Cabacungan J, Tanswell AK, Jankov RP. 8-Isoprostane-induced endothelin-1 production by infant rat pulmonary artery smooth muscle cells is mediated by Rho-kinase. Free Radic Biol Med 41: 942–949, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zamora MR, Stelzner TJ, Webb S, Panos RJ, Ruff LJ, Dempsey EC. Overexpression of endothelin-1 and enhanced growth of pulmonary artery smooth muscle cells from fawn-hooded rats. Am J Physiol Lung Cell Mol Physiol 270: L101–L109, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKB12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium 35: 345–355, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Zheng YM, Wang QS, Liu QH, Rathore R, Yadav V, Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res 45: 469–479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia- but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]